Abstract
Infection with Chlamydia pneumoniae, a human respiratory pathogen, has been implicated as a potential risk factor in atherosclerosis, possibly because the pathogen can exist in a persistent form similar to that described for Chlamydia trachomatis. The present study investigated whether gamma interferon (IFN-γ) can induce indoleamine 2,3-dioxygenase (IDO) activity in aortic smooth muscle cells, leading to a marked inhibition of C. pneumoniae growth. Our data indicate a stimulation of IDO mRNA expression and dose-dependent enzymatic activity following IFN-γ treatment. IDO-mediated increase in tryptophan catabolism resulted in a dose-dependent marked inhibition of C. pneumoniae replication.
Chlamydia pneumoniae is an obligate intracellular organism that causes acute respiratory diseases such as pneumonia (10, 17, 27) and chronic respiratory diseases, accompanied by symptoms of asthmatic bronchitis with continuous pulmonary inflammation (21). More recently, the role of C. pneumoniae as a bacterial pathogen participating in or initiating an inflammatory process in atherosclerosis has been extensively considered (18). The detection of C. pneumoniae DNA or antigens within atheromatous plaques of patients with coronary disease correlates with seroepidemiologic studies supporting a physical association of C. pneumoniae and atherosclerosis (5, 11, 16). Our laboratory detected and cultivated C. pneumoniae which was localized within the intimal layer of the coronary artery wall corresponding to the area of atherosclerosis (23).
The earliest recognizable lesion of atherosclerosis is considered to be the “fatty streak,” which is composed of smooth muscle foam cells and contributes to an occlusive lesion called a fibrous plaque. Smooth muscle cell accumulation is a key event in the development of advanced lesions of atherosclerosis (25), where intercellular interactions cause migratory activities. C. pneumoniae has been shown to replicate, in vitro, in human endothelial cells, macrophages, and aortic smooth muscle cells (ASMC), all of which are components of the arterial wall (7–9, 15). Soluble factors from endothelial cells infected with C. pneumoniae have recently been shown to stimulate smooth muscle cell proliferation (6). Inflammatory mediators, such as gamma interferon (IFN-γ), may define a link between C. pneumoniae infection and the exacerbation of atherosclerosis. The pathophysiological role of C. pneumoniae within atheromatous plaques is not understood; however, the organism would need to viably persist within cellular components of the arterial wall to support an active role in atherosclerosis.
Chlamydial persistence is described as a long-term association of the bacteria with a host in which the organism remains in a viable but culture-negative state (24). For the related pathogen Chlamydia trachomatis, a persistent altered life cycle in which the organism exists as an aberrant body can be induced in vitro through indoleamine 2,3-dioxygenase (IDO) activity, which deprives the pathogen of tryptophan (2). Previous studies in our laboratory have established that low levels of IFN-γ induced an altered life cycle of C. pneumoniae, with subsequent recovery of an infectious organism through the addition of excess tryptophan (19). A recent study examined C. pneumoniae infection in HeLa cells by transmission electron microscopy and found aberrant bodies which were larger than typical reticulate bodies in the presence of ampicillin (28). With the current focus on C. pneumoniae and its association with atherosclerosis, the purpose of the present study was to determine if ASMC express IDO enzymatic activity and examine effects on the replication of C. pneumoniae.
C. pneumoniae strain A-03 (ATCC VR-1452) was isolated from an atheroma of a patient with coronary artery disease (23). Stock cultures and infection assays were performed as described by Molestina et al. (20). Human ASMC (Clonetics Corporation) were seeded at a density of 1.0 × 105 cells/ml in SMGM-2 (Clonetics bullet kit 2) and allowed to reach confluency. Expression of IDO mRNA in ASMC was examined using reverse transcription (RT)-PCR analysis. ASMC were treated with 500 U of IFN-γ per ml, and RNA was isolated at 12 and 24 h using the RNeasy mini kit (Qiagen, Santa Clarita, Calif.). RT-PCR was performed with 1.0 μl of RNA for first-strand cDNA synthesis (reverse transcription system; Promega, Madison, Wis.). Samples were incubated for 60 min at 42°C, followed by two 5-min incubations at 99 and 4°C, respectively. This was followed by PCR using a reaction mixture (50-μl final volume) containing 10× PCR buffer, 25 mM MgCl2, 2.0 mM concentrations of each deoxynucleoside triphosphate dissolved in water (pH 7.0), AmpliTaq DNA polymerase, and IDO-specific forward and reverse oligonucleotide primers with the following sequences: IDO forward primer, 5′-CCTGACTTATGAGAACATGGACGT-3′; IDO reverse primer, 5′-ATACACCAGACCGTCTGATAGCTG-3′ (13). The positive control was generated from a 1.4-kb IDO cDNA cloned into pUC19 (gift from Joe Carlin, Miami University, Oxford, Ohio); the size of the amplified IDO fragment was 321 bp. For tryptophan catabolism, ASMC were treated with IFN-γ followed by pulse-labeling monolayers with 0.025 mM l-Trp and 1.0 μCi of [5-3H]Trp (New England Nuclear) per ml in Hanks balanced salt solution for 4 h at 37°C. Tryptophan catabolism was measured using paper chromatography as described previously (22). Percent specific catabolism of tryptophan to its metabolites, N-formylkynurenine and kynurenine, was calculated as described by Mehta et al. (19). 1-Methyl-dl-tryptophan (1-MT; Aldrich, Milwaukee, Wis.) was used as a competitive inhibitor of IDO (4); ASMC were pretreated for 1 h with increasing concentrations of 1-MT prior to IFN-γ stimulation. To determine the effects of IFN-γ-mediated IDO activity, ASMC were infected with C. pneumoniae (multiplicity of infection, 5:1) in the presence or absence of 1-MT and treated with increasing concentrations of IFN-γ. Infected monolayers were stained by immunofluorescence (Pathfinder; Sanofi Diagnostics, Chaska, Minn.) and examined for inclusion formation at magnifications under ×400. Statistical analysis was made on raw data from a minimum of three experiments using one-way analysis of variance followed by Tukey's multiple comparison test. All experiments were performed using duplicate wells in triplicate experiments. A P value of <0.05 was used to determine statistical significance for all analyses.
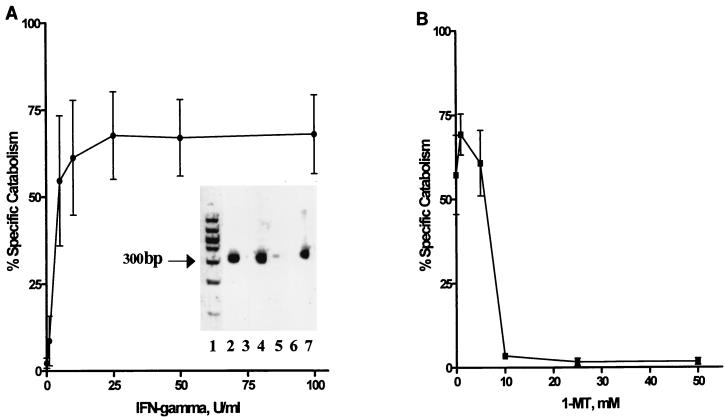
As shown in Fig. 1A (inset), following ASMC stimulation with 500 U of IFN-γ per ml, IDO mRNA was increased at 12 and 24 h compared to untreated controls. Specificity of the RT-PCR product was confirmed by digestion using PvuII (13) to produce 94- and 227-bp specific band fragments of the 321-bp product of IDO (data not shown). Demonstration of IDO enzyme activity showed that IFN-γ had a dose-dependent effect on tryptophan catabolism, with maximal activity (67.6%) when ASMC were treated with a concentration of 25 U of IFN-γ per ml (Fig. 1A). As shown in Fig. 1B, IFN-γ-mediated IDO activity was blocked in a dose-dependent manner through treatment of ASMC with the specific IDO inhibitor 1-MT, causing a significant decrease in tryptophan catabolism, to less than 2% (P < 0.05).
FIG. 1.
Induction of IFN-γ-mediated IDO mRNA expression and measurement of IDO activity in ASMC with the effects of the inhibitor 1-MT. (A) ASMC were treated with increasing concentrations of IFN-γ for 48 h and pulse-labeled with [3H]tryptophan for 4 h. IDO activity was expressed as the percentage of pulse-labeled [3H]tryptophan converted to metabolites, determined by paper chromatography. (Inset) RT-PCR analysis of IDO mRNA expression in ASMC treated with 500 U of IFN-γ per ml or left untreated. Lane 1, molecular weight standard; lane 2, 12-h IFN-γ treatment; lane 3, 12 h, untreated; lane 4, 24-h IFN-γ treatment; lane 5, 24 h, untreated; lane 6, negative control (distilled H2O); lane 7, positive control (IDO cDNA cloned into pUC19 as described in the text). (B) ASMC monolayers were pretreated for 1 h with 1-MT, followed by IFN-γ (25 U/ml) stimulation for 48 h. Monolayers were pulse-labeled with [3H]tryptophan for 4 h, and IDO activity was measured by [3H]tryptophan catabolism using paper chromatography and calculated as the percentage converted to metabolite fractions.
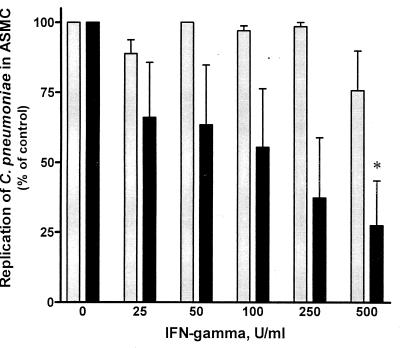
The effect of IFN-γ-mediated IDO induction on C. pneumoniae replication was examined in ASMC that were previously stimulated with increasing concentrations of IFN-γ (0 to 500 U/ml) before infection. Figure 2 shows that IFN-γ had a dose-dependent effect on inclusion formation which decreased from 100% of control (0 U of IFN-γ/ml) to 27.5% with increasing concentrations of IFN-γ. The number of inclusions in ASMC showed a significant decrease with 500 U of IFN-γ/ml (P < 0.05). The specific role of IDO activity in tryptophan catabolism and inhibition of C. pneumoniae inclusion formation was determined through the use of 1-MT followed by treatment with increasing concentrations of IFN-γ for 48 h. The presence of 1-MT reversed the IFN-γ-mediated reduction of C. pneumoniae inclusion formation (Fig. 2).
FIG. 2.
Replication of C. pneumoniae in IFN-γ-treated ASMC and competitive inhibition of IDO activity using 1-MT. Infected ASMC were treated with increasing concentrations of IFN-γ for 48 h followed by quantitation of immunofluorescently stained inclusions (■). ASMC were pretreated with 20 mM 1-MT, infected with C. pneumoniae, and stimulated with increasing concentrations of IFN-γ ( ). To normalize results, data are expressed as a percentage of the number of inclusions from ASMC without IFN-γ treatment (100% is 24.8 ± 6.5 inclusions per 100 ASMC at a magnification of ×400. ∗, P < 0.05.
). To normalize results, data are expressed as a percentage of the number of inclusions from ASMC without IFN-γ treatment (100% is 24.8 ± 6.5 inclusions per 100 ASMC at a magnification of ×400. ∗, P < 0.05.
The present study determined the effects of IFN-γ-mediated IDO activity on C. pneumoniae replication in ASMC and demonstrated a direct role of specific IDO enzymatic activity through the use of 1-MT. To our knowledge, there have been no previous reports as to whether ASMC express IDO activity upon IFN-γ stimulation. Our studies found that expression of IDO mRNA is increased in ASMC treated with 500 U of IFN-γ/ml, followed by production of an active enzyme as measured through tryptophan catabolism. Through the use of 1-MT, IDO was shown to be specific for tryptophan degradation when catabolism was inhibited in a dose-dependent manner. Additionally, it was determined that IFN-γ-mediated IDO activity had a dose-dependent effect on the marked inhibition of C. pneumoniae replication in ASMC.
During an immune response to infection, IFN-γ produced by T lymphocytes and natural killer cells regulates the synthesis of IDO (27), catabolizing tryptophan to its two metabolites, which results in a depletion of tryptophan pools within the host cell (12). When tryptophan levels are depleted, C. pneumoniae growth is arrested. Previous studies in our laboratory demonstrated the complete inhibition of C. pneumoniae replication when HEp-2 cells were treated with increasing concentrations of IFN-γ due to tryptophan catabolism (26). However, upon the addition of excess tryptophan, replication was restored (19), suggesting a role for IFN-γ-mediated IDO activity in the inhibition of C. pneumoniae replication due to insufficient tryptophan availability.
IFN-γ induced the synthesis of IDO mRNA, followed by the production of an active enzyme, which then led to a rapid increase in tryptophan catabolism. Studies with C. trachomatis have shown that when cells are exposed to low levels of IFN-γ, the reduced tryptophan pools force the pathogen to be morphologically and biochemically altered. This altered organism is described as a persistent form capable of maintaining intracellular viability for long periods of time but unable to infect other host cells (1, 3). As shown recently (28), C. pneumoniae has the ability to undergo similar morphological alterations in the presence of ampicillin. These abnormal inclusions were found to be noninfectious in the presence of ampicillin; however, infectious elementary bodies were regenerated once the antibiotic was removed. It is unknown whether C. pneumoniae undergoes similar morphological and biological alterations upon exposure to IFN-γ. We demonstrated a reduced number of C. pneumoniae inclusions when ASMC were treated with increasing concentrations of IFN-γ, suggesting that a dose-dependent exposure to IFN-γ may induce an alteration in the replicative growth cycle, similar to that described for C. trachomatis. These results provide evidence that IFN-γ has a direct role in mediating IDO activity, resulting in a significantly reduced ability of C. pneumoniae to replicate in ASMC. The use of 1-MT, a competitive inhibitor of IDO (4), relieved tryptophan catabolism to its metabolites, creating sufficient tryptophan pools for bacterial growth. It remains to be determined whether the restricted growth of C. pneumoniae, due to IFN-γ-mediated IDO activity, is morphologically and biochemically altered from the normal biphasic life cycle.
Isolation of C. pneumoniae from tissues of human carotid and coronary atheromatous plaques (14, 23) provides evidence of an association with atherosclerosis; however, the role of C. pneumoniae remains controversial, specifically with regard to its contribution to the pathogenesis of disease. Inflammatory mediators, such as IFN-γ, may define a link between infection and atherogenesis by promoting persistence in atherosclerotic tissue. This inflammatory process, which stems from initial endothelial cell injury, accumulates leukocytes within the inflamed tissue, followed by increased endothelial permeability within the lumen of the arterial wall. In accelerated forms of atherosclerosis, smooth muscle foam cells accumulate along the luminal margin (25); this emphasizes the importance of determining the effects of C. pneumoniae replication in an essential cell involved in the atherosclerotic process. Long-term survival of C. pneumoniae within associated cells of the vascular wall may provide continuous immunogenic stimuli, creating a cascade of events during the initial inflammatory process described for atherosclerosis. Our observations provide a basis of evidence that demonstrates a restriction in the replicative process of C. pneumoniae inclusion formation in ASMC due to exposure to IFN-γ. Although in vitro studies have provided evidence of possible chlamydial persistence, further investigations are needed to establish a more definitive description of persistence by focusing on morphologic and metabolic alterations, in conjunction with determining an in vivo association with atherosclerosis.
REFERENCES
- 1.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty W L, Morrison R P, Byrne G I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect Immun. 1995;63:199–205. doi: 10.1128/iai.63.1.199-205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cady S G, Sono M. 1-Methyl-DL-tryptophan, β-(3-benzofuranyl)-DS-alanine (the oxygen analog of tryptophan), and β-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 5.Campbell L S, O'Brien E R, Cappuccio A L, Kuo C-C, Wang S-P, Stewart D, Patton D L, Cummings P K, Grayston J T. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 6.Coombs B K, Mahony J B. Chlamydia pneumoniae infection of human endothelial cells induces proliferation of smooth muscle cells via an endothelial cell-derived soluble factor(s) Infect Immun. 1999;67:2909–2915. doi: 10.1128/iai.67.6.2909-2915.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fryer R H, Woods M L, Rodgers G M. Chlamydia species infect human vascular endothelial cells and induce procoagulant activity. J Invest Med. 1994;45:168–174. [PubMed] [Google Scholar]
- 8.Gaydos C A, Summersgill J T, Sahney N N, Ramirez J A, Quinn T C. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Godzik K L, O'Brien E R, Wang S K, Kuo C-C. In vitro susceptibility of human vascular wall cells to infection with Chlamydia pneumoniae. J Clin Microbiol. 1995;33:2411–2414. doi: 10.1128/jcm.33.9.2411-2414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grayson J T. C. pneumoniae strain TWAR pneumonia. Annu Rev Med. 1992;43:317–323. doi: 10.1146/annurev.me.43.020192.001533. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Camm J. Chronic infection in the etiology of atherosclerosis—the case for Chlamydia pneumoniae. Clin Cardiol. 1997;20:829–836. doi: 10.1002/clc.4960201008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S L, Carlin J M, Pyati P, Dai W, Pfefferkorn E R, Murphy M J. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu B, Hissong B D, Carlin J M. Interleukin-1 enhances indoleamine 2,3-dioxygenase activity by increasing specific mRNA expression in human mononuclear phagocytes. J Interferon Cytokine Res. 1995;15:617–624. doi: 10.1089/jir.1995.15.617. [DOI] [PubMed] [Google Scholar]
- 14.Jackson L A, Campbell L A, Kuo C-C, Rodriguez K I, Lee A, Grayston J T. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 15.Kaukoranta-Tolvanen S S, Laitinen K, Saikku P, Leinonen M. Chlamydia pneumoniae multiplies in human endothelial cells in vitro. Microb Pathog. 1994;16:313–319. doi: 10.1006/mpat.1994.1032. [DOI] [PubMed] [Google Scholar]
- 16.Kuo C-C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 17.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindholt J S, Fasting H, Henneberg E W, Ostergaard L. A review of Chlamydia pneumoniae and atherosclerosis. Eur J Vasc Endovasc. 1999;17:283–289. doi: 10.1053/ejvs.1998.0757. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S J, Miller R D, Ramirez J A, Summersgill J T. Inhibition of Chlamydia pneumoniae replication in HEp-2 cells by interferon-γ: role of tryptophan catabolism. J Infect Dis. 1998;177:1326–1331. doi: 10.1086/515287. [DOI] [PubMed] [Google Scholar]
- 20.Molestina R E, Miller R D, Ramirez J A, Summersgill J T. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peeling R W, Brunham R C. Chlamydiae as pathogens: new species and new issues. Emerg Infect Dis. 1996;2:307–319. doi: 10.3201/eid0204.960406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfefferkorn E R. Interferon-γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramirez J A, Ahkee S, Summersgill J T, Ganzel B L, Ogden L L, Quinn T C, Gaydos C A, Bobo L L, Hammerschlag M R, Roblin P M, LeBar W, Grayston J T, Kuo C-C, Campbell L A, Patton D L, Dean D, Schachter J. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 24.Rasmussen S J, Timms P, Beatty P R, Stephens R S. Cytotoxic-T-lymphocyte-mediated cytolysis of L cells persistently infected with Chlamydia spp. Infect Immun. 1996;64:1944–1949. doi: 10.1128/iai.64.6.1944-1949.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz C J, Valente A J, Sprague E A, Kelley J L, Nerem R M. The pathogenesis of atherosclerosis: an overview. Clin Cardiol. 1991;14:I1–I16. doi: 10.1002/clc.4960141302. [DOI] [PubMed] [Google Scholar]
- 26.Summersgill J T, Sahney N N, Gaydos C A, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolf K, Fischer E, Hackstadt T. Ultrastructural analysis of developmental events in Chlamydia pneumoniae-infected cells. Infect Immun. 2000;68:2379–2385. doi: 10.1128/iai.68.4.2379-2385.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]