Abstract
Aims
Insulin like growth factor binding protein 7 (IGFBP7) is a marker of senescence secretome and a novel biomarker in patients with heart failure (HF). We evaluated the prognostic value of IGFBP7 in patients with heart failure and examined associations to uncover potential new pathophysiological pathways related to increased plasma IGFBP7 concentrations.
Methods and results
We have measured plasma IGFBP7 concentrations in 2250 subjects with new‐onset or worsening heart failure (BIOSTAT‐CHF cohort). Higher IGFBP7 plasma concentrations were found in older subjects, those with worse kidney function, history of atrial fibrillation, and diabetes mellitus type 2, and in subjects with higher number of HF hospitalizations. Higher IGFBP7 levels also correlate with the levels of several circulating biomarkers, including higher NT‐proBNP, hsTnT, and urea levels. Cox regression analyses showed that higher plasma IGFBP7 concentrations were strongly associated with increased risk of all three main endpoints (hospitalization, all‐cause mortality, and combined hospitalization and mortality) (HR 1.75, 95% CI 1.25–2.46; HR 1.71, 95% CI 1.39–2.11; and HR 1.44, 95% CI 1.23–1.70, respectively). IGFBP7 remained a significant predictor of these endpoints in patients with both reduced and preserved ejection fraction. Likelihood ratio test showed significant improvement of all three risk prediction models, after adding IGFBP7 (P < 0.001). A biomarker network analysis showed that IGFBP7 levels activate different pathways involved in the regulation of the immune system. Results were externally validated in BIOSTAT‐CHF validation cohort.
Conclusions
IGFPB7 presents as an independent and robust prognostic biomarker in patients with HF, with both reduced and preserved ejection fraction. We validate the previously published data showing IGFBP7 has correlations with a number of echocardiographic markers. Lastly, IGFBP7 pathways are involved in different stages of immune system regulation, linking heart failure to senescence pathways.
Keywords: IGFBP7, heart failure, HFpEF, HFrEF, senescence, BIOSTAT‐CHF
Introduction
Heart failure (HF) is a common and lethal condition, with various aetiologies and precipitants. Prevalent HF is associated with a substantial burden of disease, characterized by high morbidity, especially hospitalizations, and premature death. As a result, a large number of diagnostic and prognostic tools have been studied, including physical signs, imaging modalities, and circulating biomarkers. The combination of various parameters typically provides incremental information. With regard to biomarkers, the natriuretic peptides are the cornerstone of HF diagnosis and management. 1 However, numerous new biomarkers have been detected and evaluated, of which several are entering the clinical arena. 2 For newer biomarkers to be of use, they should provide cumulative value on top of existing diagnostic and prognostic models. Furthermore, new biomarkers should also be actionable, meaning that an elevated biomarker value should prompt the physician to take action, either order additional tests to ascertain a specific aetiology, to change the management plan, for example, more frequent follow‐ups, or to revise the existing treatment approach. Specific biomarker may also signify specific underlying pathophysiology in need of distinct treatment, so that the result is relevant.
Insulin‐like growth factor‐binding protein 7 (IGFBP7) is a protein of the senescence secretome. Senescent cells secrete a wide spectrum of proteins, which are further involved in not only different benevolent processes like aging but also pathological processes like cancer, atherosclerotic disease, and diabetes. 3 , 4 Through its binding to insulin growth factor, IGFBP7 has been shown to exert protective roles in the cardiovascular (CV) system, by enhancing anti‐oxidative effects, supporting the migration of myocardial cells in damaged hearts, enabling myocardial regeneration, 5 , 6 and preventing impaired cardiac contractility and endothelial dysfunction. 7 Its competitive relationship with insulin and potential to block insulin receptors is directly responsible for the modulation of fuel storing metabolism. 8 Unlike other binding proteins, IGFBP7 also can directly inhibit cell‐cycle in situations of uncontrolled cell proliferation or cell injury by inhibiting TGF pathways. 7 At the same time, it is also responsible for normal cell growth and proliferation in healthy tissues, and activation of adaptive and differentiation of innate immune system. 9
Previous studies have reported on the value of IGFBP7 in HF. IGFBP7 was first found to be up‐regulated in mouse HF models and in patients with HF and cardiac hypertrophy. 10 , 11 One study reported IGFBP7 as a possible biomarker of acute HF, improving the diagnosis in patients presenting with acute dyspnoea. 12 , 13 Gandhi et al. reported that low IGFBP7 levels were predictive of event‐free survival in patients with reduced ejection fraction. 14 Furthermore, IGFBP7 also presented as a potential marker of HF with preserved ejection fraction (HFpEF). 15 , 16 We herein report detailed associations between IGFBP7 and HF parameters in a multicentre, multi‐national, observational study that is composed of two different cohorts: an index (discovery) cohort with 2516 subjects and an independent, multi‐centre Scottish (validation) cohort, which included 1738 subjects. The aim of our study was to demonstrate if IGFBP7 has independent prognostic value in HF, when fully adjusted with an extensive list of parameters. Furthermore, we aimed to explore the correlates of IGFBP7 with correlates of HFpEF. Finally, making use of the extensive biomarker measurements present in the cohort, we also aimed to unveil intricate pathophysiological mechanisms linking IGFBP7 and HF.
Methods
Study population
This is a retrospective study of BIOlogy Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT‐CHF). Details of BIOSTAT‐CHF have been described elsewhere. 17 In short, BIOSTAT‐CHF consists of two independent cohorts. The index (discovery) cohort is a multi‐centre, multi‐national, observational cohort that included 2516 subjects from 69 centres across 11 European countries. The validation cohort is a Scottish cohort for an independent validation, which included 1738 subjects from six centres in Scotland. In brief, patients were included on basis of worsening signs/symptoms and suboptimal treatment of HF. Both cohorts included subjects that were ≥18 years of age, having symptoms of new‐onset or worsening HF, as confirmed by a left ventricular ejection fraction (LVEF) of ≤40%. In the index cohort, patients with LVEF >40% were enrolled in the BIOSTAT‐CHF study but were required to have a B‐type natriuretic peptide >400 pg/mL or an N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) > 2000 pg/mL. The validation cohort had no NT‐proBNP threshold. Patients in both index and validation cohorts were required to be sub‐optimally treated on either angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (ACEi/ARBs) and/or beta‐blockers, or they received ≤50% of ACEi/ARB and/or beta‐blockers at the time of inclusion and anticipated initiation/up‐titration of ACEi/ARBs and beta‐blockers. For our analysis we excluded subjects with missing IGFBP7 measurements, leaving in total 2250 subjects from the index cohort, and 1648 subjects from the validation cohort that were included in our analysis. The study complied with the Declaration of Helsinki and was approved by the medical ethics committees of participating centres, and patients provided written informed consent.
Study definitions
Medical history, medication use, and physical examination were recorded at baseline. Blood sampling was performed at admission before the administration of the study drug and after 24 h. Changes in ACEi/ARB and beta‐blocker usage were recorded. Standard echocardiography was strongly recommended, although not mandatory for study inclusion. Echocardiographic assessment of LVEF was performed at admission or within 6 months before admission. HF with a reduced ejection fraction (HFrEF) was defined as an LVEF ≤40%, HF with a mid‐range ejection fraction (HFmrEF) was defined as an LVEF of 41%–49%, and HF with a preserved ejection fraction (HFpEF) was defined as an LVEF ≥50%. Co‐morbidities were identified by chart review from medical history.
Outcomes
For the study purposes, we defined primary outcomes as HF hospitalizations, all‐cause mortality, and combined outcome of both all‐cause mortality and HF hospitalizations. The secondary outcome was to determine significance of IGFBP7 for three primary outcomes, according to HF category by LVEF: HF with preserved or mildly reduced ejection fraction (HFpEF and HFmEF) or HF with reduced ejection fraction (HFrEF). Lastly, the third objective of the study was to determine the pathophysiological processes linking IGFBP7 and heart failure, by performing network analysis using the rick wealth of biomarkers available in BIOSTAT‐CHF.
Biomarker measurements
Blood was collected at baseline and stored at −80°C. Before measuring, samples were centrifuged for 60 s at 12 000 rpm to remove any cellular debris. IGFBP7 was measured using an Elecsys assay (Roche Diagnostics, Penzberg, Germany). Measurement of IGFBP7 was performed in Roche Diagnostics by laboratory personnel blinded to clinical information. IGFBP7 was measured using a preclinical research‐use only assay on an automated platform blinded to clinical information (Roche Diagnostics GmbH, Penzberg, Germany). The detection method for IGFBP7 was a sandwich immunoassay developed on the Elecsys® platform for electro‐chemiluminescence detection (Roche Diagnostics GmbH, Mannheim, Germany). Mouse monoclonal antibodies were generated and screened for specific detection of IGFBP7. Precision within‐run coefficient of variation for IGFBP7 was 2%, and the limit of detection was 0.01 ng/mL. A large, previously measured biomarker panel of over 363 different biomarkers (CVD‐II/‐III, immune and oncology panels; Olink Proteomics) was used for analysis. Each panel included 92 biomarkers, and details of the panel have been reported previously. 18
Statistical analyses
Normally distributed variables are presented as means (with standard deviations, SD). Non‐normally distributed continuous variables are presented as medians (with interquartile ranges, IQR), and categorical variables as absolute numbers (with percentages). Levels of IGFBP7 were divided into tertiles. Baseline characteristics of the study population of the tertiles were compared using one‐way analysis of variance (ANOVA), Kruskal–Wallis test or χ 2 test where appropriate. Because IGFBP7 values were transformed to log2 scale, each 1‐unit increase is equivalent to a doubling in biomarker levels. Multiple linear regression and Spearman correlation were used to determine independent associations with different clinical predictors and IGFBP7. Survival in subgroups defined by IGFBP7 tertiles for all three primary endpoints was evaluated using the Kaplan–Meier method. Cox regression analysis was used to investigate the association between IGFBP7 and predetermined clinical outcomes: hospitalization, mortality, and combined hospitalization and mortality outcome. Adjusted hazard ratios (HRs) are reported with 95% confidence intervals (CIs) and P‐values for the individual outcomes. Regression splines were used to evaluate the linearity assumption. Schoenfield residuals were checked to assess the proportional hazard assumption. When necessary, non‐proportionality was accounted for by adding a time‐covariate interaction. In a stepwise manner, we corrected for age and gender, BIOSTAT risk score, as published before, 19 and our own risk model based on independent predictive variables according to the linear regression analysis and published literature. To avoid aggregation bias, our risk model was individually adjusted, depending on the variables in the specific BIOSTAT risk model. The BIOSTAT risk model for hospitalization included age, hospitalization in a year previous to inclusion, the extent of peripheral oedema, SBP, and eGFR. In our model, we additionally corrected IGFBP7 for gender, presence of atrial fibrillation, diabetes mellitus, GDF15, Hb, NTproBNP, and BMI. The BIOSTAT risk model for all‐cause mortality endpoint included age, blood urea nitrogen, NT‐proBNP, Hb, and beta‐blocker use at the baseline. Our mortality risk model for IGFBP7 included gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI. Finally, the BIOSTAT risk model for the combined hospitalization and mortality endpoint included age, NT‐proBNP, Hb, beta‐blocker use at baseline, HF hospitalization in a year prior to inclusion, extent of peripheral oedema, SBP, HDL, and serum sodium levels. Again, to avoid aggregation bias, IGFBP7 was in our combined hospitalization and mortality risk model C corrected for gender, presence of atrial fibrillation, diabetes mellitus, GDF15 levels, and BMI. We then validated our results in an independent BIOSTAT CHF cohort. Finally, we divided subjects of our primary cohort into two groups, depending on their LVEF; patients with LVEF ≥40% were separated from subjects with LVEF ≤40%. We then repeated Cox analysis for all endpoints, according to our endpoint‐specific correction models (Models A–C).
Next to receiver operating characteristic curve (ROC) and area under the ROC curve (AUC), we used net reclassification improvement (NDI), integrated discrimination improvement (IDI) indices, and likelihood ratio test to investigate whether IGFBP7 improves published risk prediction models for this cohort. Three selected cut‐off point categories are 0% to 20%, 20%–40%, and 40%–60%.
Finally, to investigate specific pathways connecting IGFBP7 to HF, we used biomarker network analysis, described elsewhere. 20 In short, using multiple linear regression we tested IGFBP7 correlation with 363 biomarkers (CVD‐II/‐III, immune and oncology panels; Olink Proteomics) in our study population. Pairwise correlations were extracted that passed a P‐value cut‐off point corrected for multiple comparisons. The first 75 most significant genes and their proteins were included in the protein network analysis. Networks were then visualized using Cytoscape, 21 and their functional and biological roles were analysed using ClueGo. 22
P‐value of <0.05 is considered statistically significant. All statistical analysis was done using STATA SE 14.2. and R v.3.2.3.
Results
Baseline characteristics of study population
Characteristics of the study subjects as a function of IGFBP7 tertiles are described in Table 1 . Subjects with higher IGFBP7 levels are older, with a more often history of atrial fibrillation and diabetes mellitus type 2, more often have had a HF hospitalization in a year prior to inclusion, worse extent of peripheral oedema, and worse kidney function. Higher IGFBP7 levels also correlate with several circulating biomarkers, including higher NT‐proBNP, hsTnT, and urea values, and lower Hb and HDL values. Different echocardiographic parameters, including mitral valve regurgitation, posterior and intra‐ventricular wall thickness, E/A ratio, and left atrium diameter also showed statistical correlations with IGFBP7 levels ( Table S1 ).
Table 1.
Baseline characteristics of the study population by IGFBP7 tertiles
| Factor | IGFBP7 1 | IGFBP7 2 | IGFBP7 3 | P‐value |
|---|---|---|---|---|
| N | 750 | 750 | 750 | |
| IGFBP7 (ng/mL), median IQR | 99.4 (89.9, 107.2) | 133.2 (124.7, 143.7) | 195.1 (170.2, 235.7) | <0.001 |
| Age, median (IQR) | 74.2 (65.7, 83.2) | 81.6 (73.3, 88.3) | 83.7 (74.7, 89.7) | <0.001 |
| Male gender (%) | 521 (69.5) | 554 (73.9) | 577 (76.9) | 0.004 |
| BMI, median (IQR) | 27 (24, 30) | 27 (24, 30) | 26 (23, 30) | 0.23 |
| SBP (mmHg), median IQR | 120 (110, 136) | 122.5 (110, 140) | 120 (110, 135) | <0.001 |
| NTproBNP (pg/mL), median IQR | 1351 (533.5, 2861) | 2732 (1353, 5165) | 4853 (2560, 9990) | <0.001 |
| hsTnT (pg/mL), median IQR | 21 (13.0, 38.8) | 31.2 (20.5, 49.6) | 43.7 (28.5, 69.4) | <0.001 |
| GDF‐15 (ng/mL), median IQR | 2.9 (2.3, 3.6) | 3.4 (2.8, 4.1) | 4.1 (3.3, 5.1) | <0.001 |
| Haemoglobin (g/dL), median IQR | 13.7 (12.5, 14.8) | 13.3 (12.1, 14.5) | 12.6 (11.3, 14) | <0.001 |
| Urea (mmol/L), median (IQR) | 9 (6.3, 13.8) | 11 (7.4, 16.1) | 15.1 (9.6, 24.6) | <0.001 |
| eGFR (mL/min/1.73 m2), median IQR | 74.9 (60.0, 88.3) | 58.3 (44.5, 73.2) | 46.9 (32.3, 62.2) | <0.001 |
| Sodium (mmol/L), median IQR | 140 (137, 142) | 140 (138, 142) | 139 (136, 141) | <0.001 |
| HDL (mmol/L) median IQR | 1.1 (0.9, 1.3) | 1.0 (0.8, 1.3) | 0.9 (0.7, 1.3) | <0.001 |
| Use of beta‐blocker at baseline (%) | 644 (85.9) | 631 (84.1) | 604 (8.5) | 0.018 |
| HF hospitalization in previous year (%) | 198 (26.4) | 212 (28.3) | 300 (40) | <0.001 |
| Extent of peripheral oedema (%) | <0.001 | |||
| Not present | 349 (57.7) | 242 (40.1) | 160 (24.2) | |
| Ankle | 176 (29.1) | 196 (32.5) | 179 (27.1) | |
| Below knee | 72 (11.9) | 128 (21.2) | 227 (34.3) | |
| Above knee | 8 (1.3) | 38 (6.3) | 95 (14.4) | |
| Atrial fibrillation present (%) | 226 (30.1) | 362 (48.3) | 435 (58.0) | <0.001 |
| Diabetes mellitus present (%) | 192 (25.6) | 254 (33.9) | 286 (38.1) | <0.001 |
Abbreviations: N, number; IGFBP7, insulin‐like growth factor binding protein 7; BMI, body mass index; SBP, systolic blood pressure; NTproBNP, N‐terminal pro–B‐type natriuretic peptide; hsTnT, high‐sensitive troponin T; GDF‐15, growth/differentiation factor 15; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; HF, heart failure; IQR, interquartile range.
Outcome analysis
During a median follow‐up of 21 months, 527 subjects were hospitalized for worsening HF and 526 subjects died. Higher IGFBP7 levels were significantly associated with higher hospitalization and mortality rates ( Table S2 ) Kaplan–Meier curves for all three primary endpoints show that the mortality risk was increased for patients in the higher IGFBP7 tertiles (Figure 1 ).
Figure 1.
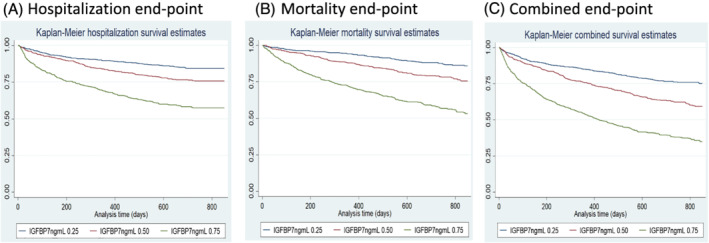
Kaplan–Meier survival curves per IGFBP7 tertiles. (A) Hospitalization end‐point; (B) all‐cause mortality end‐point; (C) combined end‐point. IGFBP7, insulin‐like growth factor binding protein 7.
Cox regression analyses showed significant correlations with IGFBP7 levels and all three main endpoints (hospitalization, all‐cause mortality, and combined hospitalization and mortality), also after adjustment with appropriate correction models. When correcting for previously defined BIOSTAT risk‐model (Model B), higher IGFBP7 levels are independently associated with all three endpoints: hospitalization, all‐cause mortality, and combined hospitalization and mortality endpoint (Table 2 ).
Table 2.
Cox regression models for the prediction of hospitalization, all‐cause mortality, and combined outcome, by each doubling of IGFBP7
| Model | Hospitalization | All‐cause mortality | Combined | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| A | 2.95 (2.35–3.70) | <0.001 | 2.92 (2.49–3.41) | <0.001 | 2.52 (2.23–2.86) | <0.001 |
| B | 1.72 (1.29–2.28) | <0.001 | 1.66 (1.39–2.00) | <0.001 | 1.42 (1.22–1.65) | <0.001 |
| C | 1.75 (1.25–2.46) | 0.001 | 1.71 (1.39–2.11) | <0.001 | 1.44 (1.23–1.70) | <0.001 |
Note: Model A: Age, sex. Model B: Hospitalization: age, hospitalization in a year previous to inclusion, the extent of peripheral oedema, SBP, and eGFR. All‐cause mortality: age, blood urea nitrogen, NT‐proBNP, Hb, and beta‐blocker use at the baseline. Combined: age, NT‐proBNP, Hb, beta‐blocker use at baseline, HF hospitalization in a year prior to inclusion, extent of peripheral oedema, SBP, HDL, and serum sodium levels. Model C: Hospitalization: age, HF hospitalization in previous year, extent of peripheral oedema, SBP, eGFR, gender, atrial fibrillation, diabetes mellitus, GDF15, Hb, NTProBNP, and BMI. All‐cause mortality: age, BUN, NTproBNP, Hb, beta‐blocker at baseline, gender, atrial fibrillation, diabetes mellitus, GDF15, BMI. Combined: age, NTproBNP, Hb, beta‐blocker at baseline, hospitalization in previous year, extent of peripheral oedema, SBP, HDL, sodium, gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI.
Abbreviations: IGFBP7, insulin‐like growth factor binding protein; HF, hazard ratio; CI, confidence interval.
After further correction for BIOSTAT hospitalization risk‐model and gender, atrial fibrillation, diabetes mellitus, GDF15, Hb, NTProBNP, and BMI (Model C), IGFBP7 remained a strong, independent predictor of HF hospitalizations (Table 2 ). In addition to correction of BIOSTAT mortality risk‐model (Model B), additional correction for gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI (Model C), IGFBP7 preserved independent association with all‐cause mortality (Table 2 ). Lastly, in addition to full correction for BIOSTAT risk‐model for hospitalization and mortality endpoint (Model B), and gender, atrial fibrillation, diabetes mellitus, GDF‐15, and BMI (Model C), IGFBP7 showed, again, an independent association in predicting the combined outcome (Table 2 ). Results mentioned for all three primary endpoints were externally validated in an independent BIOSTAT CHF validation cohort ( Table S3 ).
In total, 546 subjects in our study population presented with an LVEF above 40%, leaving 1630 subjects with LVEF ≤40%. Baseline characteristics of both subject groups are described in Tables S4 and S5. In general, subjects with higher ejection fraction were older, more often women, with higher blood pressure, higher hs‐TnT and GDF‐15 values, lower blood urea nitrogen levels, and higher prevalence of atrial fibrillation. Subjects with higher LVEF also had less often prescribed medication, and higher prevalence of peripheral oedema. After full correction for BIOSTAT risk models (Model B) and additional correction for relevant clinical parameters (Model C), IGFBP7 levels remain an independent predictor all three primary endpoints in both subjects groups (Table 3 ).
Table 3.
Cox regression model by LVEF for the prediction of hospitalization, all‐cause mortality, and combined outcome, by each doubling of IGFBP7
| Model | HFp(m)EF | HFrEF | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Hospitalization | 3.07 (1.69–5.57) | <0.001 | 1.53 (1.03–2.29) | 0.035 |
| All‐cause mortality | 1.88 (1.25–2.84) | <0.001 | 1.49 (1.16–1.92) | 0.002 |
| Combined | 2.41 (1.688–3.46) | <0.001 | 1.27 (1.04–1.55) | 0.015 |
Note: Hospitalization: age, HF hospitalization in previous year, extent of peripheral edema, SBP, eGFR, gender, atrial fibrillation, diabetes mellitus, GDF15, Hb, NTProBNP, and BMI. All‐cause mortality: age, BUN, NTproBNP, Hb, beta‐blocker at baseline, gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI. Combined: age, NTproBNP, Hb, beta‐blocker at baseline, hospitalization in previous year, extent of peripheral oedema, SBP, HDL, sodium, gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI.
Abbreviations: LVEF, left ventricular ejection fraction; IGFBP7, insulin‐like growth factor binding protein 7; HFp(m)EF, heart failure with preserved (medium) ejection fraction; HFrEF, heart failure with reduced ejection fraction; HF, hazard ratio; CI, confidence interval.
Reclassification indices
To confirm the clinical value of IGFBP7 as a relevant HF biomarkers and to estimate its role in the risk prediction for all three primary endpoints, we conducted the ROC/AUC analysis, followed by category‐free net reclassification improvement (NRI), the integrated discrimination improvement (IDI) indices, and likelihood ratio test. While adding IGFBP7 to the known BIOSTAT risk models did not significantly improved risk prediction according to the ROC/AUC (Figure 2 ), NRI, IDI, and likelihood ratio test showed significantly improved risk prediction with IGFBP7, of all three established BIOSTAT risk models. (Table 4 ). Likelihood ratio test, as a most reliable test, showed significant improvement after adding IGFBP7, with P‐value <0.001 for all three models.
Figure 2.
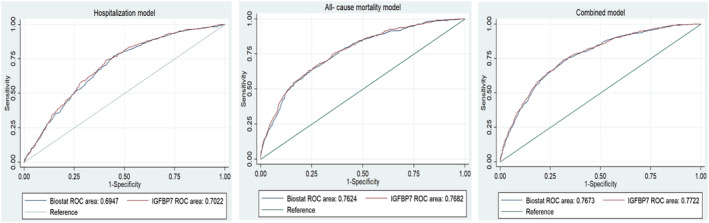
ROC/AUC analysis of Biostat risk models, and combined Biostat risk models with IGFBP7. (A) Hospitalization end‐point; (B) all‐cause mortality end‐point; (C) combined end‐point. IGFBP7, insulin‐like growth factor binding protein 7.
Table 4.
Performance metrics of IGFBP7 in established BIOSTAT‐CHF risk models
| Model | NRI | IDI | Likelihood ratio test | |||
|---|---|---|---|---|---|---|
| Estimate (SD) | P‐value | Estimate (SD) | P‐value | Estimate | P‐value | |
| Hospitalization + IGFBP7 | 0.038 (0.018) | 0.030 | 0.006 (0.002) | 0.002 | 16.14 | <0.001 |
| All‐cause mortality + IGFBP7 | 0.055 (0.020) | 0.007 | 0.014 (0.002) | <0.001 | 32.07 | <0.001 |
| Combined + IGFBP7 | 0.030 (0.014) | 0.042 | 0.010 (0.002) | <0.001 | 26.25 | <0.001 |
Note: Hospitalization: age, HF hospitalization in previous year, extent of peripheral edema, SBP, eGFR, gender, atrial fibrillation, diabetes mellitus, GDF15, Hb, NTProBNP, and BMI. All‐cause mortality: age, BUN, NTproBNP, Hb, beta‐blocker at baseline, gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI. Combined: age, NTproBNP, Hb, beta‐blocker at baseline, hospitalization in previous year, extent of peripheral oedema, SBP, HDL, sodium, gender, atrial fibrillation, diabetes mellitus, GDF15, and BMI.
Abbreviations: IGFBP7, insulin‐like growth factor binding protein 7; NRI, net reclassification index; IDI, integrated discrimination improvement; SD, standard deviation.
Network analysis
To identify prominent pathophysiological pathways associated with IGFBP7 and HF, we have correlated the levels of 363 biomarkers associated with inflammation, and various cardiovascular and oncological conditions (the CVD‐II/‐III, immune and oncology panels; Olink Proteomics) with IGFBP7. Of 363 biomarkers, IGFBP7 statistically correlated with 301 of them. The 75 biomarkers with lowest P‐values most significant biomarkers and their genes ( Table S6 ) were included in the pathway analysis. Figure 3 shows that more than half of the presented pathways are involved in the regulation of the immune system, specifically regulation of T‐cell activity, innate and adaptive immunity, whereas another half of the processes included cell survival, apoptosis, proliferation, and connective tissue formation, Ephrin signalling, and angiogenesis.
Figure 3.
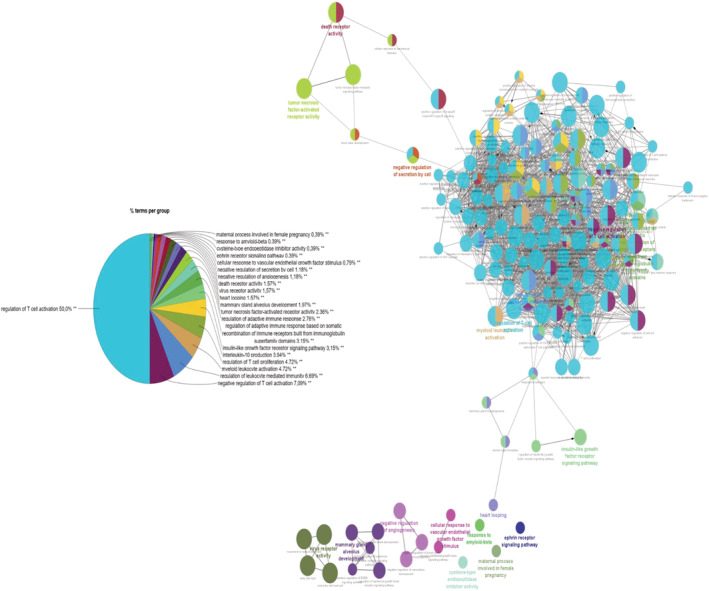
IGFBP7 network analysis. Presented pathways are most strongly correlated with IGFBP7 levels in HF population.
Discussion
Our study validates previously published data on IGFBP7 and its role in the heart failure patients with both reduced and preserved ejection fraction. 11 , 12 , 14 , 15 Our results support the notion that IGFBP7 levels strongly correlate with the presence of atrial fibrillation, diabetes mellitus, NT‐proBNP levels, and worse clinical status; increased number of hospitalizations and the extent of peripheral oedema. We further demonstrate that IGFBP7 independently predicts hospitalizations and all‐cause mortality in the HF population, even after ‘extreme’ adjustment for known HF and IGFBP7 covariates. We validated our results in an independent HF cohort. Using the extensive biomarker database, we are first to identify several immunological processes associated with IGFBP7. Collectively, our data support the hypothesis that IGFBP7‐related pathophysiological processes are included in both HF with preserved and reduced ejection fraction and have an independent role in the HF syndrome.
Although in our study IGFBP7 emerges as a relevant marker of prognosis in both HF with preserved and reduced ejection fraction, its levels seemed to correlate particularly strong with several echocardiographic parameters that can be used in assessing diastolic dysfunction. IGFBP7 levels are strongly associated with increased wall thickness, mitral valve regurgitation, and LA diameter, which are considered as hallmarks of HF. 23 Furthermore, as a senescence biomarkers, with a strong correlation with aging, it is fair to presume that IGFBP7 prognostic role is pronounced not only in HFrEF but also in HFpEF.
A second step in our analysis was to determine specific pathophysiological pathways between those with high versus low IGFBP7 levels in patients with HF. Using network analysis, we were able to determine the most relevant proteins, and their pathways related to IGFBP7 in HF. As mentioned, IGFBP7 is a known protein of senescence secretome. 9 One of the most important triggers of cell senescence is oxidative stress. 24 Oxidative stress leading to the state of chronic inflammation is also known as complement of a variety of CV risk factors and is considered a crucial trigger of CV events. 25 , 26 , 27 Inflammatory processes play a key role in HFrEF, and particularly in HFpEF. 20 Although neutrophils and ‘sterile’ inflammation seem to have an important impact in HFrEF, ‘metabolic induced’ inflammation, through chronic up‐regulation of adaptive immune system and circulation of inflammation markers, plays an important role in HFpEF. 28 Chronic inflammation in HFpEF is triggered by non‐cardiac comorbidities and results in the systemic activation of the immune system, eventually leading to micro‐vascular endothelial dysfunction and cardiac fibrosis. 23 Through its strong association to the regulation of the immune system, especially T‐cell regulation, cell survival, and apoptosis, cell proliferation, connective tissue formation, and negative regulation of angiogenesis, our data firmly support the notion of IGFBP7 presenting as a robust, prognostic biomarker in HF, especially HFpEF, by showing strong link between regulation of the immune system, specifically T‐cell regulation, as well as the regulation of cell apoptosis, proliferation, connective tissue formation, and negative regulation of angiogenesis.
Furthermore, we are first that observed an interesting correlation between IGFBP7 and Ephrin signalling. Ephrin ligands and Eph receptors are expressed in vascular and inflammatory cells, where they are responsible for fast, intracellular signalling. Although their function in myocardial fibrosis and remodelling is still unknown, ephrin signalling has been shown to hold a critical role in early heart development. 29 , 30 In addition, ephrin signalling attenuates the remodelling process after myocardial infarction, preserving cardiac function. 31 It may be possible that Eph receptors, through different inflammatory mechanisms, play a role in cardiac remodelling in HF. The link between Eph receptors and heart failure warrants further study.
In conclusion, we present IGFPB7 as a robust prognostic biomarker in patients with HF. We validate the previously published data that IGFBP7 has correlations with a number of markers of diastolic dysfunction. We extend these findings by demonstrating independent prognostic value of IGFBP7 in HFrEF and HFpEF. And finally, we present a comprehensive pathophysiological network, linking IGFBP7 to different HF pathophysiological pathways.
Limitations
The following limitations should be considered. First, due to study design and lack of detailed assessment of echocardiographic parameters, diastolic dysfunction parameters could not fully be included in our analyses. Second, due to inclusion criteria, these results do not apply to patients with stable, chronic HF. Third, because the study population is predominantly Caucasian, we cannot extend the results to other races. Lastly, inclusion criteria between the two cohorts differed slightly. The index cohort had more strict inclusion criteria for subjects with HFpEF, leading to a lower number of subjects and possibly different clinical statuses of the patients with HFpEF.
Funding information
This work was supported by a grant from the European Research Council (ERC CoG 818715, SECRETE‐HF). Furthermore, support was received from grants from the Netherlands Heart Foundation (CVON SHE‐PREDICTS‐HF, grant 2017‐21; CVON RED‐CVD, grant 2017‐11; CVON PREDICT2, grant 2018‐30; and CVON DOUBLE DOSE, grant 2020B005), and by a grant from the leDucq Foundation [Cure PhosphoLambaN induced Cardiomyopathy (Cure‐PLaN)].
Conflicts of interest
The UMCG, which employs several of the authors, has received research grants and/or fees from AstraZeneca, Abbott, Bristol‐Myers Squibb, Novartis, Novo Nordisk, and Roche.
Supporting information
Table S1. Spearman correlation of IGFBP7 and clinical parameters.
Table S2. Hospitalization and mortality incidence by IGFBP7 tertiles, during the study follow‐up.
Table S3. Cox regression models for the prediction of hospitalization, all‐cause mortality and combined outcome in BIOSTAT‐CHF Validation cohort, by each doubling of IGFBP7.
Table S4. Baseline characteristics of subjects with LVEF <40%, by IGFBP7 tertiles.c
Table S5. Baseline characteristics of subjects with LVEF > 40%, by IGFBP7 tertiles.
Table S6. Biomarkers correlated with IGFBP7 in HF population.
Acknowledgements
Authors would like to thank Sylwia Figarska and Karla Arevalo Gomes for their insights over statistical analyses.
Bracun, V. , van Essen, B. , Voors, A. A. , van Veldhuisen, D. J. , Dickstein, K. , Zannad, F. , Metra, M. , Anker, S. , Samani, N. J. , Ponikowski, P. , Filippatos, G. , Cleland, J. G. F. , Lang, C. C. , Ng, L. L. , Shi, C. , de Wit, S. , Aboumsallem, J. P. , Meijers, W. C. , Klip, I. J. T. , van der Meer, P. , and de Boer, R. A. (2022) Insulin‐like growth factor binding protein 7 (IGFBP7), a link between heart failure and senescence. ESC Heart Failure, 9: 4167–4176. 10.1002/ehf2.14120.
References
- 1. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr, on behalf of the Heart Failure Association of the European Society of Cardiology . Heart failure Association of the European Society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019; 21: 715–731. [DOI] [PubMed] [Google Scholar]
- 2. de Boer RA, Daniels LB, Maisel AS, Januzzi JL. State of the art: Newer biomarkers in heart failure. Eur J Heart Fail. 2015; 17: 559–569. [DOI] [PubMed] [Google Scholar]
- 3. He S, Sharpless NE. Senescence in health and disease. Cell. 2017; 169: 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez‐Segura A, Nehme J, Demaria M. Hallmarks of cellular senescence. Trends Cell Biol. 2018; 28: 436–453. [DOI] [PubMed] [Google Scholar]
- 5. van den Bosch E, Bossers SSM, Kamphuis VP, Boersma E, Roos‐Hesselink JW, Breur JMPJ, ten Harkel ADJ, Kapusta L, Bartelds B, Roest AAW, Kuipers IM, Blom NA, Koopman LP, Helbing WA. Associations between blood biomarkers, cardiac function, and adverse outcome in a young fontan cohort. J Am Heart Assoc. 2021; 10: e015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen Z, Li L, Wu W, Liu Z, Huang Y, Yang L, Luo Q, Chen J, Hou Y, Song G. Exercise protects proliferative muscle satellite cells against exhaustion via the Igfbp7‐Akt‐mTOR axis. Theranostics. 2020; 10: 6448–6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jin L, Shen F, Weinfeld M, Sergi C. Insulin growth factor binding protein 7 (IGFBP7)‐related cancer and IGFBP3 and IGFBP7 crosstalk. Front Oncol. 2020; 10: 727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruan W, Kang Z, Li Y, Sun T, Wang L, Liang L, Lai M, Wu T. Interaction between IGFBP7 and insulin: A theoretical and experimental study. Sci Rep. 2016; 6: 19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin‐like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013; 4: e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chugh S, Ouzounian M, Lu Z, Mohamed S, Li W, Bousette N, Liu PP, Gramolini AO. Pilot study identifying myosin heavy chain 7, desmin, insulin‐like growth factor 7, and annexin A2 as circulating biomarkers of human heart failure. Proteomics. 2013; 13: 2324–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meessen JMTA, Cesaroni G, Mureddu GF, Boccanelli A, Wienhues‐Thelen UH, Kastner P, Ojeda‐Fernandez L, Novelli D, Bazzoni G, Mangiavacchi M, Agabiti N, Masson S, Staszewsky L, Latini R, on behalf of the PREDICTOR Investigators . IGFBP7 and GDF‐15, but not P1NP, are associated with cardiac alterations and 10‐year outcome in an elderly community‐based study. BMC Cardiovasc Disord. 2021; 21: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ibrahim NE, Afilalo M, Chen‐Tournoux A, Christenson RH, Gaggin HK, Hollander JE, Kastner P, Levy PD, Mang A, Masson S, Nagurney JT, Nowak RM, Pang PS, Peacock WF, Dipl‐Stat VR, Walters EL, Januzzi JL Jr. Diagnostic and prognostic Utilities of Insulin‐like Growth Factor Binding Protein‐7 in patients with dyspnea. JACC Heart Fail. 2020; 8: 415–422. [DOI] [PubMed] [Google Scholar]
- 13. Kalayci A, Peacock WF, Nagurney JT, Hollander JE, Levy PD, Singer AJ, Shapiro NI, Cheng RK, Cannon CM, Blomkalns AL, Walters EL, Christenson RH, Chen‐Tournoux A, Nowak RM, Lurie MD, Pang PS, Kastner P, Masson S, Gibson CM, Gaggin HK, Januzzi JL Jr. Echocardiographic assessment of insulin‐like growth factor binding protein‐7 and early identification of acute heart failure. ESC Heart Fail. 2020; 7: 1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gandhi PU, Gaggin HK, Sheftel AD, Belcher AM, Weiner RB, Baggish AL, Motiwala SR, Liu PP, Januzzi JL Jr. Prognostic usefulness of insulin‐like growth factor‐binding protein 7 in heart failure with reduced ejection fraction: A novel biomarker of myocardial diastolic function? Am J Cardiol. 2014; 114: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 15. Gandhi PU, Gaggin HK, Redfield MM, Chen HH, Stevens SR, Anstrom KJ, Semigran MJ, Liu P, Januzzi JL Jr. Insulin‐like growth factor–binding Protein‐7 as a biomarker of diastolic dysfunction and functional capacity in heart failure with preserved ejection fraction. JACC Hear Fail. 2016; 4: 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Januzzi JL, Packer M, Claggett B, Liu J, Shah AM, Zile MR, Pieske B, Voors A, Gandhi PU, Prescott MF, Shi V, Lefkowitz MP, McMurray JJV, Solomon SD. IGFBP7 (insulin‐like growth factor–binding Protein‐7) and Neprilysin inhibition in patients with heart failure. Circ Heart Fail. 2018; 11: e005133. [DOI] [PubMed] [Google Scholar]
- 17. Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, ter Maaten JM, Ng L, Ponikowski P, Samani NJ, Veldhuisen DJ, Zannad F, Zwinderman AH, Metra M. A systems BIOlogy study to TAilored treatment in chronic heart failure: Rationale, design, and baseline characteristics of BIOSTAT‐CHF. Eur J Heart Fail. 2016; 18: 716–726. [DOI] [PubMed] [Google Scholar]
- 18. Markousis‐Mavrogenis G, Tromp J, Ouwerkerk W, Ferreira JP, Anker SD, Cleland JG, Dickstein K, Filippatos G, Lang CC, Metra M, Samani NJ, The BIOSTAT‐CHF Consortium , de Boer RA, van Veldhuisen DJ, Voors AA, van der Meer P. Multimarker profiling identifies protective and harmful immune processes in heart failure: Findings from BIOSTAT‐CHF. Cardiovasc Res. 2022; 118: 1964–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, Ng LL, Metra M, ter Maaten JM, Lang CC, Hillege HL, van der Harst P, Filippatos G, Dickstein K, Cleland JG, Anker SD, Zwinderman AH. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. 2017; 19: 627–634. [DOI] [PubMed] [Google Scholar]
- 20. Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Lang CC, Ng LL, Zannad F, Zwinderman AH, Hillege HL, van der Meer P, Voors AA. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018; 72: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 21. Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003; 13: 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mlecnik B, Galon J, Bindea G. Comprehensive functional analysis of large lists of genes and proteins. J Proteomics. 2018; 171: 2–10. [DOI] [PubMed] [Google Scholar]
- 23. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 24. Del Pinto R, Ferri C. Inflammation‐accelerated senescence and the cardiovascular system: Mechanisms and perspectives. Int J Mol Sci. 2018; 19: 3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017; 120: 713–735. [DOI] [PubMed] [Google Scholar]
- 26. Neuman RB, Bloom HL, Shukrullah I, Darrow LA, Kleinbaum D, Jones DP, Dudley SC Jr. Oxidative stress markers are associated with persistent atrial fibrillation. Clin Chem. 2007; 53: 1652–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011; 301: H2181–H2190. [DOI] [PubMed] [Google Scholar]
- 28. Simmonds SJ, Cuijpers I, Heymans S. Cellular and molecular Di ff erences between HFpEF and HFrEF: A step ahead in an improved. Cell. 2020; 9: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gerety SS, Anderson DJ. Cardiovascular ephrinB2 function is essential for embryonic angiogenesis. Development. 2002; 129: 1397–1410. [DOI] [PubMed] [Google Scholar]
- 30. Cowan CA, Yokoyama N, Saxena A, Chumley MJ, Silvany RE, Baker LA, Srivastava D, Henkemeyer M. Ephrin‐B2 reverse signaling is required for axon pathfinding and cardiac valve formation but not early vascular development. Dev Biol. 2004; 271: 263–271. [DOI] [PubMed] [Google Scholar]
- 31. Su SA, Yang D, Wu Y, Xie Y, Zhu W, Cai Z, Shen J, Fu Z, Wang Y, Jia L, Wang Y, Wang JA, Xiang M. EphrinB2 regulates cardiac fibrosis through modulating the interaction of Stat3 and TGF‐β/Smad3 signaling. Circ Res. 2017; 121: 617–627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman correlation of IGFBP7 and clinical parameters.
Table S2. Hospitalization and mortality incidence by IGFBP7 tertiles, during the study follow‐up.
Table S3. Cox regression models for the prediction of hospitalization, all‐cause mortality and combined outcome in BIOSTAT‐CHF Validation cohort, by each doubling of IGFBP7.
Table S4. Baseline characteristics of subjects with LVEF <40%, by IGFBP7 tertiles.c
Table S5. Baseline characteristics of subjects with LVEF > 40%, by IGFBP7 tertiles.
Table S6. Biomarkers correlated with IGFBP7 in HF population.


