Abstract
Aims
This study aimed to investigate the association between trimethylamine N‐oxide (TMAO) and the prognosis and association between high‐sensitivity C‐reactive protein (hsCRP) and TMAO‐associated cardiovascular risk in patients with acute myocardial infarction (AMI) complicated by heart failure (HF).
Methods and results
A total of 985 patients presenting with AMI and HF were consecutively enrolled at the Fuwai Hospital between March 2017 and January 2020. Patients were stratified into groups according to tertiles of TMAO levels and the median hsCRP levels. The primary endpoint was major adverse cardiac events (MACE), including all‐cause death, recurrence of myocardial infarction, and rehospitalization due to HF. During a median follow‐up of 716 days, 138 (14.0%) patients experienced MACE. Cox regression analyses showed that the adjusted hazard ratio (HR) for MACE was higher in patients in tertile 3 [TMAO > 9.52 μmol/L, HR: 1.85, 95% confidence interval (CI): 1.18–2.89; P = 0.007] than in tertile 1 (TMAO < 4.74 μmol/L), whereas no significant differences were detected between the patients in tertiles 1 and 2 (TMAO = 4.74–9.52 μmol/L, HR: 0.96, 95% CI: 0.59–1.58; P = 0.874). Restricted cubic spline regression depicted an S‐shaped association between TMAO and MACE (P for nonlinearity = 0.012). In the setting of hsCRP above the median level (6.68 mg/L), per unit increase of TMAO was associated with a 20% increase of MACE risk (HR: 1.20, 95% CI: 1.05–1.37, P = 0.009); increasing tertiles of TMAO were significantly associated with a higher risk of MACE (adjusted P = 0.007 for interaction; P < 0.001 for trend across tertiles). The Kaplan–Meier analysis indicated that patients in tertile 3 had a significantly lower event‐free survival (P = 0.001) when the hsCRP level was above the median level. No similar association between TMAO and MACE was observed when the hsCRP level was below the median level.
Conclusions
High plasma TMAO levels were independently correlated with poor prognosis in patients with AMI complicated by HF, especially in those with higher hsCRP levels. There was an S‐shaped relationship between TMAO and HR for MACE.
Keywords: Trimethylamine N‐oxide, Inflammation, Acute myocardial infarction, Heart failure
Introduction
Plasma trimethylamine N‐oxide (TMAO) is one of the metabolites produced by gut microbiota. It has been proven to be involved in many pathophysiological processes and be related to the prognosis of patients with coronary artery disease and heart failure (HF). 1 , 2 A meta‐analysis demonstrated that plasma TMAO level was positively and dose‐dependently associated with an increased cardiovascular risk in the general population. 3 The TMAO level is recognized as an independent risk factor for major adverse cardiovascular and cerebrovascular events in patients with acute coronary syndrome. 4 Our previous study found that TMAO might serve as a potential indicator of the characteristics of coronary artery plaques. 5 Recent studies have displayed that elevated plasma levels of TMAO are associated with poor outcomes in a population with HF. 6 The combination of TMAO with B‐type natriuretic peptide (BNP) could improve the prognostic value of mortality in patients with HF with preserved ejection fraction (HFpEF). 7 However, most previous studies have focused on patients with chronic HF, 8 , 9 and it is uncertain whether TMAO levels have the same effects on patients with acute HF.
High‐sensitivity C‐reactive protein (hsCRP), a systemic inflammatory response marker, is extensively used to evaluate inflammatory risk in patients with myocardial infarction (MI). 10 , 11 , 12 Some studies have suggested that TMAO may activate the inflammation cascade. 13 , 14 However, it remains unclear whether TMAO is related to a poorer prognosis in the population with acute myocardial infarction (AMI) and HF and whether it is associated with increased cardiovascular risk determined by hsCRP. Therefore, we intended to explore the association between TMAO and the prognosis and the association between hsCRP levels and TMAO‐associated cardiovascular risk in patients with AMI complicated by HF.
Methods
Study population and design
The current study was conducted under the Declaration of Helsinki and obtained ethnic approval from the Ethics Committee of Fuwai Hospital. All patients signed a written informed consent form. Patients presenting with AMI and HF admitted to the emergency department of Fuwai Hospital between March 2017 and January 2020 were prospectively enrolled in this study cohort. The diagnostic criteria of AMI were based on the Fourth Universal Definition of Myocardial Infarction and guidelines, 15 , 16 , 17 including ST‐segment elevation MI and non‐ST‐segment elevation MI. HF was diagnosed and classified based on typical symptoms and signs, laboratory tests, echocardiogram, and X‐ray findings, following guidelines and statements by the European Society of Cardiology and Heart Failure Society of America. 18 , 19 We excluded patients who were in the acute phase of infectious or inflammatory diseases, missing hsCRP test results, or missing follow‐up records. A flowchart of patient selection process is shown in Supporting Information, Figure S1 . Relevant electronic medical records were retrieved using the hospital information system for subsequent analysis. Clinical data, including demographics, physical examination, medical history, blood test results, echocardiogram data, and medication at discharge, were obtained.
Laboratory testing
Blood samples were gathered into tubes with ethylenediaminetetraacetic acid through radial or femoral artery before percutaneous coronary intervention (PCI). These were processed at 4°C within 3 h, and then stored at −80°C until further analysis. As mentioned previously, the stable isotope dilution high‐performance liquid chromatography with online electrospray ionization tandem mass spectrometry was used to measure plasma TMAO levels, using an API 3200 triple quadrupole mass spectrometer (AB SCIEX, Framingham, Massachusetts) with a d9(trimethyl)‐labelled internal standard. 20 HsCRP testing was routinely collected via cubital or basilic veins in an overnight fasting state on the day after the PCI procedure and measured using an immunoturbidimetric assay (Beckmann Assay, Bera, California). The other blood test indicators are routinely detected in the hospital central laboratory. The N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and cardiac troponin I (cTnI) levels were measured several times during hospitalization, and the baseline and peak values were recorded.
Endpoints and follow‐up
The primary endpoint was a composite of all‐cause death, recurrence of MI, and rehospitalization due to HF, called major adverse cardiac events (MACE). Endpoint data were collected by professionals in charge of follow‐up via outpatient visits and telephone interviews. Routine follow‐up of patients was performed at 6 and 12 months after PCI and annually thereafter. The protocol for follow‐up was ratified by the Institutional Review Board of Fuwai Hospital.
Statistical analysis
Mean ± standard deviation or median with interquartile range (IQR) for continuous variables and number (percentage) for categorical variables were reported. For continuous variables with normal distribution, one‐way analysis of variance or the Kruskal–Wallis H test was used for comparison among groups, while for categorical variables, the chi‐square test or Fisher's exact test was conducted. To explore the impact of TMAO and hsCRP on cardiovascular risk in patients with AMI and HF, we divided the patients into three groups according to plasma TMAO levels and further grouped them into two categories based on the median hsCRP levels. The correlation of variables and TMAO were ln‐transformed and subsequently analysed using Spearman correlation tests with coefficients reported for 1000 bootstrapped samples. The event‐free survival distribution of groups was evaluated using Kaplan–Meier analysis and log‐rank test. The relationship between variables (including TMAO) and the primary endpoints was first assessed using univariate Cox proportional hazards regression. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were then computed using multivariate Cox regression analysis, adjusting for the following factors: age, sex, body mass index, estimated glomerular filtration rate (eGFR), current smoking, HF type, hypertension, diabetes mellitus (DM), peripheral arterial disease, and history of stroke and MI. A forest plot was graphically displayed to summarize the results of the various subgroup analyses of the HR of MACE comparing the first and third tertiles of plasma TMAO levels. Possible nonlinear associations were evaluated using restricted cubic spline (RCS) regression with TMAO as a continuous variable with four knots. Finally, we tested the effect of TMAO and its interaction with hsCRP on the HRs for MACE. All data were analysed using the SPSS software (version 26.0; IBM Corp., Armonk, New York, USA) and R (http://www.r‐project.org/) statistical packages. A P‐value <0.05 was considered as indicative of statistical significance.
Results
Baseline characteristics and the association between trimethylamine N‐oxide and laboratory variables
Between March 2017 and January 2020, 1006 patients were admitted for AMI complicated by HF, of which 985 patients with complete clinical data were ultimately included in this study (Supporting Information, Figure S1 ). Table 1 presents the detailed baseline characteristics of the included patients. The median age of total patients was 63 (IQR 54–70) years, and 766 (77.8%) patients were male. Overall, the median plasma levels of TMAO were 6.7 (IQR 4.0–11.7) μmol/L. When grouped by the Killip class, the plasma levels of TMAO in patients with Killip I–IV were 6.4 (IQR 3.8–11.3), 9.2 (IQR 4.6–13.9), 10.5 (IQR 5.5–18.1), and 13.4 (IQR 6.1–23.3) μmol/L, respectively, showing an increasing trend (P < 0.001). Besides, the plasma levels of TMAO for patients with HF with mildly reduced ejection fraction (HFmrEF), HF with preserved ejection fraction (HFpEF), and HF with reduced ejection fraction (HFrEF) were 6.4 (IQR 3.8–11.2), 7.0 (IQR 4.4–12.6), and 8.0 (IQR 4.6–17.9) μmol/L, respectively, also showing an increasing trend (P = 0.007). Patients in tertile 3 of TMAO levels were inclined to be older and presented with a higher prevalence of previous strokes, Killip II–IV, HF with reduced ejection fraction (HFrEF), and chronic kidney disease (CKD). Besides, TMAO levels were positively associated with peak NT‐proBNP levels (r = 0.087, P = 0.002), and inversely correlated with hsCRP levels (r = −0.062, P = 0.016), baseline and peak cTnI levels (r = −0.028 and −0.038, P = 0.013 and 0.019, respectively), eGFR (r = −0.495, P < 0.001), and low‐density lipoprotein‐cholesterol levels (r = −0.281, P < 0.001), as shown in Supporting Information, Table S1 .
Table 1.
Baseline characteristics according to plasma TMAO levels
| Variables | Total (n = 985) | Tertile 1 (n = 328) | Tertile 2 (n = 329) | Tertile 3 (n = 328) | P‐value |
|---|---|---|---|---|---|
| Male | 766 (77.8) | 249 (75.9) | 264 (80.2) | 253 (77.1) | 0.388 |
| Age (years) | 63.0 (54.0, 70.0) | 61.0 (52.0, 67.0) | 62.0 (54.0, 68.0) | 66.0 (57.0, 73.0) | <0.001 |
| BMI (kg/m2) | 25.7 (23.3, 27.8) | 25.0 (22.9, 27.7) | 25.7 (23.5, 27.8) | 25.9 (23.5, 27.8) | 0.053 |
| Killip (II–IV) | 142 (14.4) | 31 (9.5) | 39 (11.9) | 72 (22.0) | <0.001 |
| LVEF (%) | 55.0 (48.0, 58.0) | 55.0 (50.0, 58.0) | 55.0 (48.0, 58.0) | 55.0 (46.0, 59.0) | 0.132 |
| MI‐type | 0.746 | ||||
| STEMI | 917 (93.1) | 305 (93.0) | 304 (92.4) | 308 (93.9) | |
| NSTEMI | 68 (6.9) | 23 (7.0) | 25 (7.6) | 20 (6.1) | |
| HF type | 0.012 | ||||
| HFpEF | 713 (72.4) | 253 (77.1) | 239 (72.6) | 221 (67.4) | |
| HFmrEF | 207 (21.0) | 57 (17.4) | 75 (22.8) | 75 (22.9) | |
| HFrEF | 65 (6.6) | 18 (5.5) | 15 (4.6) | 32 (9.8) | |
| Medical history | |||||
| Current smoker | 697 (70.8) | 219 (66.8) | 246 (74.8) | 232 (70.7) | 0.079 |
| Hypertension | 654 (66.4) | 218 (66.5) | 213 (64.7) | 223 (68.0) | 0.678 |
| Hyperlipaemia | 914 (92.8) | 303 (92.4) | 301 (91.5) | 310 (94.5) | 0.306 |
| Diabetes mellitus | 358 (36.3) | 89 (27.1) | 111 (33.7) | 158 (48.2) | <0.001 |
| Previous stroke | 164 (16.6) | 38 (11.6) | 54 (16.4) | 72 (22.0) | 0.002 |
| CKD | 92 (9.3) | 21 (6.4) | 19 (5.8) | 52 (15.9) | <0.001 |
| PAD | 62 (6.3) | 16 (4.9) | 18 (5.5) | 28 (8.5) | 0.117 |
| Previous MI | 186 (18.9) | 59 (18.0) | 63 (19.1) | 64 (19.5) | 0.873 |
| Previous PCI | 175 (17.8) | 55 (16.8) | 62 (18.8) | 58 (17.7) | 0.784 |
| Previous CABG | 24 (2.4) | 8 (2.4) | 7 (2.1) | 9 (2.7) | 0.877 |
| Laboratory indexes | |||||
| TMAO (μmol/L) | 6.7 (4.0, 11.7) | 3.2 (2.2, 4.0) | 6.7 (5.6, 8.0) | 15.2 (11.7, 22.8) | <0.001 |
| eGFR (mL/min/1.732 m2 a ) | 77.0 (59.4, 97.2) | 81.7 (64.2, 103.2) | 78.9 (62.6, 98.3) | 68.8 (48.9, 89.1) | <0.001 |
| cTnI‐baseline (ng/mL) | 1.3 (0.2, 7.1) | 2.1 (0.3, 9.9) | 1.2 (0.2, 6.3) | 0.9 (0.1, 5.5) | 0.009 |
| cTnI‐peak (ng/mL) | 13.5 (3.8, 32.6) | 15.7 (5.7, 34.2) | 10.5 (2.8, 29.7) | 11.4 (3.0, 34.1) | 0.007 |
| NT‐proBNP‐baseline (ng/mL) | 439.3 (141.9, 1290.0) | 401.0 (147.2, 1093.0) | 420.6 (106.6, 1203.0) | 495.2 (168.1, 1531.0) | 0.090 |
| NT‐proBNP‐peak (ng/mL) | 1614.0 (783.9, 3174.0) | 1480.0 (762.6, 2890.0) | 1424.0 (686.1, 2890.0) | 1891.0 (878.8, 3806.0) | 0.001 |
| hsCRP (mg/L) | 6.7 (2.1, 11.3) | 7.9 (2.6, 11.4) | 6.2 (2.0, 11.3) | 5.8 (2.1, 11.2) | 0.241 |
| LDL‐C (mmol/L) | 2.6 (2.0, 3.2) | 2.8 (2.1, 3.4) | 2.6 (2.0, 3.2) | 2.4 (1.8, 3.0) | <0.001 |
| Angiography finding | |||||
| MVD, n (%) | 745 (75.6) | 239 (72.9) | 244 (74.2) | 262 (79.9) | 0.084 |
| Initial TIMI flow 0–1, n (%) | 561 (57.0) | 192 (58.5) | 197 (59.9) | 172 (52.4) | 0.122 |
| Aspiration, n (%) | 249 (25.3) | 88 (26.8) | 86 (26.1) | 75 (22.9) | 0.459 |
| PTCA, n (%) | 2 (0.2) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0.213 |
| Stenting, n (%) | 694 (70.5) | 231 (70.4) | 239 (72.6) | 224 (68.3) | 0.474 |
| Minimal diameter | 3.0 (2.8, 3.5) | 3.0 (2.8, 3.5) | 3.0 (2.8, 3.5) | 3.0 (2.8, 3.5) | 0.576 |
| Total length (mm) | 25.0 (18.0, 35.0) | 25.0 (18.0, 35.0) | 25.0 (18.0, 33.5) | 25.0 (18.0, 37.0) | 0.779 |
| Final TIMI Flow 3 | 945 (95.9) | 321 (97.9) | 315 (95.7) | 309 (94.2) | 0.058 |
| Medication | |||||
| Aspirin | 933 (94.7) | 315 (96.0) | 313 (95.1) | 305 (93.0) | 0.253 |
| Ticagrelor | 451 (45.8) | 169 (51.5) | 151 (45.9) | 131 (39.9) | 0.013 |
| Clopidogrel | 511 (51.9) | 153 (46.6) | 173 (52.6) | 185 (56.4) | 0.013 |
| ACEI/ARB | 685 (69.5) | 228 (69.5) | 238 (72.3) | 219 (66.8) | 0.145 |
| Βeta‐blocker | 840 (85.3) | 286 (87.2) | 287 (87.2) | 267 (81.4) | 0.075 |
| Statins | 933 (94.7) | 317 (96.6) | 309 (93.9) | 307 (93.6) | 0.174 |
Continuous variables are presented as medians (25 − 75th percentiles), and categorical variables are reported as counts (%). ACEIs/ARBs, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers; BMI, body mass index; CABG, Coronary artery bypass graft (ing); CKD, chronic kidney disease; cTnI, cardiac troponin I; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hsCRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MVD, multiple vessels disease; NSTMEI, Non‐ST‐segment elevation myocardial infarction; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; STMEI, ST‐segment elevation myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; TIMI flow, Thrombolysis In Myocardial Infarction flow; TMAO, trimethylamine‐N‐oxide.
Estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal Disease formula.
Trimethylamine N‐oxide and study endpoints
There were 138 (14.0%) patients who experienced MACE during the follow‐up, and the median follow‐up time was 716 days. The Kaplan–Meier analysis of TMAO stratified by tertiles is shown in Figure 1 . The results showed a significant increase in MACE risk in the tertile 3 (P < 0.001), similar to the risk for all‐cause death and recurrence of MI (P < 0.001), whereas the difference for rehospitalization due to HF was not statistically significant (P = 0.260). The forest plot in Figure 2 compares the HR for MACE between the patients in tertile 3 and tertile 1 according to different subgroups. The results showed that patients with higher TMAO levels had a higher risk of MACE regardless of age, sex, concomitant hypertension and DM, and history of stroke. In the tertile 3, women, younger patients (<65 years old), and patients with worse renal function had a relatively higher risk. However, when it comes to hsCRP level, only in the setting of hsCRP more than the median level, patients with higher TMAO levels had a significantly higher risk.
Figure 1.
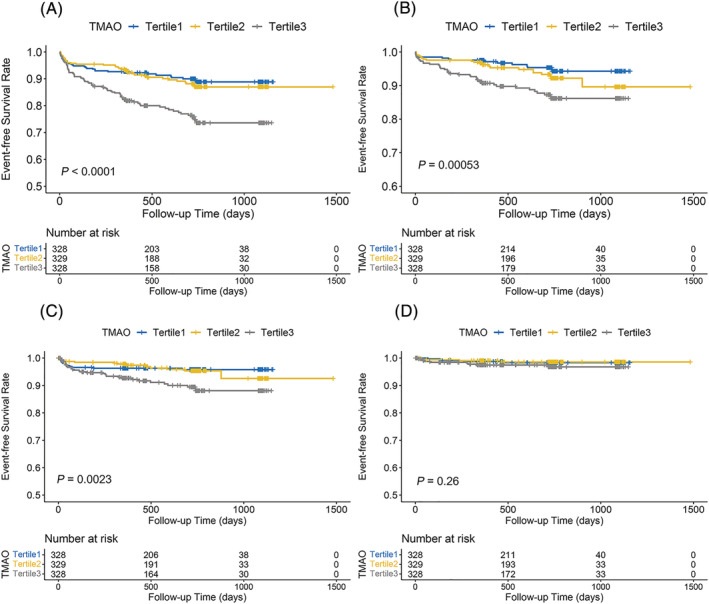
Kaplan–Meier curve for cumulative event‐free survival in different patient groups stratified by TMAO levels. (A) major adverse cardiac event, (B) all‐cause death, (C) myocardial infarction, (D) rehospitalization due to heart failure. TMAO, trimethylamine‐N‐oxide.
Figure 2.
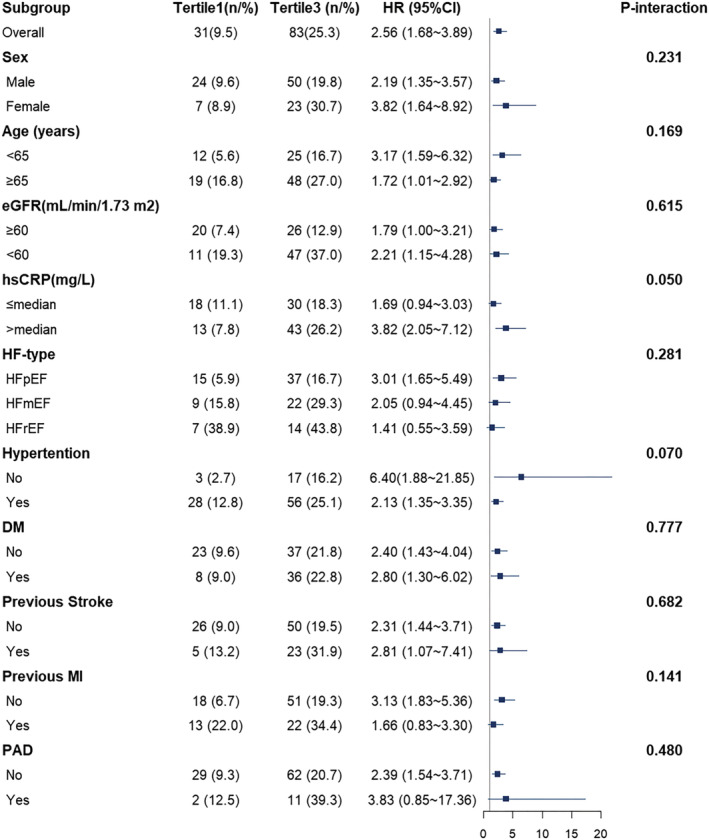
Forest plot of hazard ratios for major adverse cardiac events comparing first and third tertiles of plasma TMAO levels and stratified by baseline characteristics. DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; hsCRP, high‐sensitivity C‐reactive protein; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MI, myocardial infarction; PAD, peripheral artery disease; TMAO, trimethylamine‐N‐oxide.
Table 2 describes the univariable and multivariable relationships between all endpoints and TMAO levels. The results revealed that TMAO was an independent risk factor for MACE (P = 0.003) and recurrent MI (P = 0.011). Meanwhile, there were no statistical significances for the all‐cause death and rehospitalization due to HF (P = 0.125 and P = 0.607, respectively). The adjusted HR for MACE was higher in tertile 3 (>9.52 μmol/L, HR: 1.85, 95% CI: 1.18–2.89; P = 0.007) than in tertile 1 (<4.74 μmol/L), while no significant difference was detected between tertile 2 and tertile 1 (4.74–9.52 μmol/L, HR: 0.96, 95% CI: 0.59–1.58; P = 0.874). Similarly, patients in tertile 3 had a higher HR for recurrent MI than patients in tertile 1 (HR: 2.19, 95% CI: 1.13–4.26; P = 0.020).
Table 2.
Association between TMAO levels and all endpoints
| Endpoint | Group | Event (n, %) | Crude HR (95% CI) | P‐value | Adjusted HR (95% CI) a | P‐value |
|---|---|---|---|---|---|---|
| MACE | ||||||
| Tertile 1 | 31 (9.5) | 1 (Ref) | 1 (Ref) | |||
| Tertile 2 | 34 (10.3) | 1.11 (0.68–1.81) | 0.673 | 0.96 (0.59–1.58) | 0.874 | |
| Tertile 3 | 73 (22.3) | 2.57 (1.69–3.92) | <0.001 | 1.85 (1.18–2.89) | 0.007 | |
| Trend test | 138 (14.0) | 1.69 (1.36–2.10) | <0.001 | 1.42 (1.12–1.79) | 0.003 | |
| All‐cause death | ||||||
| Tertile 1 | 14 (4.3) | 1 (Ref) | 1 (Ref) | |||
| Tertile 2 | 20 (6.1) | 1.48 (0.75–2.92) | 0.264 | 1.14 (0.57–2.31) | 0.709 | |
| Tertile 3 | 38 (11.6) | 2.92 (1.58–5.40) | 0.001 | 1.63 (0.84–3.18) | 0.151 | |
| Trend test | 72 (7.3) | 1.75 (1.29–2.37) | <0.001 | 1.30 (0.93–1.80) | 0.125 | |
| reMI | ||||||
| Tertile 1 | 13 (4.0) | 1 (Ref) | 1 (Ref) | |||
| Tertile 2 | 13 (4.0) | 1.01 (0.47–2.18) | 0.975 | 0.92 (0.42–1.99) | 0.831 | |
| Tertile 3 | 30 (9.1) | 2.48 (1.29–4.76) | 0.006 | 2.19 (1.13–4.26) | 0.020 | |
| Trend test | 56 (5.7) | 1.67 (1.19–2.35) | 0.003 | 1.58 (1.11–2.23) | 0.011 | |
| reHF | ||||||
| Tertile 1 | 5 (1.5) | 1 (Ref) | 1 (Ref) | |||
| Tertile 2 | 4 (1.2) | 0.82 (0.22–3.04) | 0.762 | 0.69 (0.18–2.61) | 0.584 | |
| Tertile 3 | 9 (2.7) | 1.92 (0.64–5.73) | 0.242 | 1.28 (0.40–4.05) | 0.679 | |
| Trend test | 18 (1.8) | 1.45 (0.81–2.60) | 0.210 | 1.17 (0.64–2.16) | 0.607 | |
HR, hazard ratio; MACE, major adverse cardiac event; reMI, recurrent myocardial infarction; reHF, rehospitalization due to heart failure; TMAO, trimethylamine‐N‐oxide.
Adjusted for age, sex, body mass index, estimated glomerular filtration rate, current smoking, HF‐type, hypertension, diabetes mellitus, peripheral artery disease, and history of stroke and MI.
As a continuous variable, the RCS regression analysis displayed an S‐shaped relationship between TMAO levels and HR for MACE (P for nonlinearity = 0.012) after adjusting for the confounding factors (Figure 3 ). The cutoff level of TMAO for the predicted HR was 10.0 μmol/L. However, there was no statistical evidence to support a nonlinear association between TMAO and all‐cause death (P for nonlinearity = 0.237), recurrent MI (P for nonlinearity = 0.088), or rehospitalization due to HF (P for nonlinearity = 0.372).
Figure 3.
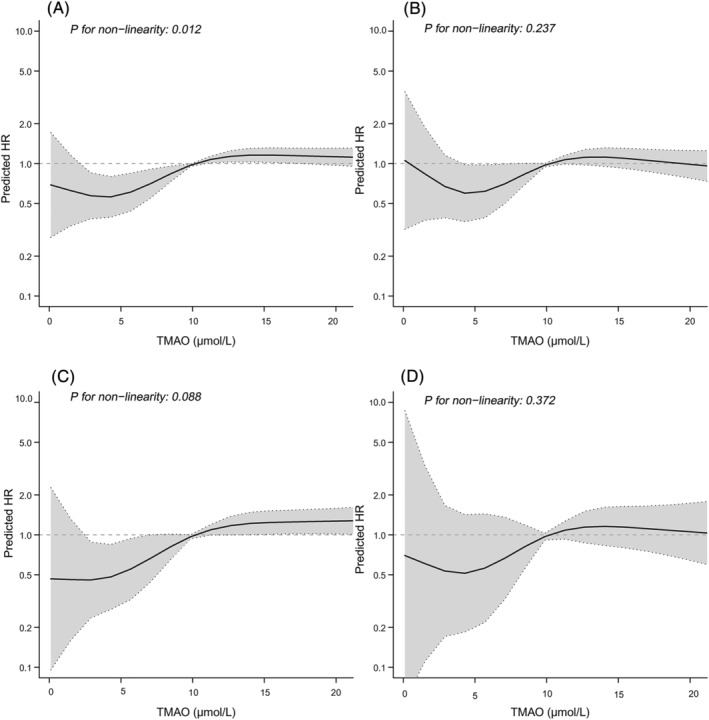
Continuous hazard ratios across TMAO for major adverse cardiac events (A), all‐cause death (B), myocardial infarction (C), and rehospitalization due to heart failure (D). HR, hazard ratio; TMAO, trimethylamine‐N‐oxide.
Associations between major adverse cardiac events stratified by high‐sensitivity C‐reactive protein and trimethylamine‐N‐oxide
The Kaplan–Meier curve of cumulative event‐free probability for the tertiles stratified by median hsCRP levels is shown in Figure 4 . The results demonstrated a significant difference among tertiles (P < 0.0001) in the setting of hsCRP above the median level (6.68 mg/L). However, when hsCRP level was below the median level, the difference among the tertiles was not statistically significant (P = 0.056).
Figure 4.
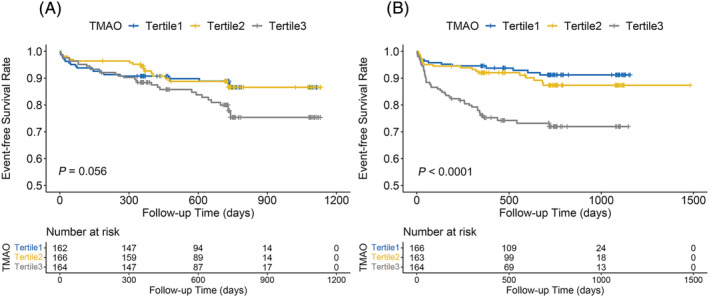
Kaplan–Meier curve for cumulative MACE‐free survival rate among TMAO tertiles stratified by hsCRP below median levels (A) and above median levels (B). hsCRP, high‐sensitivity C‐reactive protein; MACE, major adverse cardiac event; TMAO, trimethylamine‐N‐oxide.
As presented in Table 3 , in the setting of hsCRP above the median level, per unit increase of TMAO was associated with a 20% increase of MACE risk (HR: 1.20, 95% CI: 1.05–1.37, P = 0.009); patients in tertile 3 had a significantly higher risk for MACE than those in tertile 1 (HR: 2.91, 95% CI: 1.52–5.56; P = 0.001), whereas no significant differences were obtained between the patients in tertile 2 and tertile 1 (HR: 1.27, 95% CI: 0.62–2.61; P = 0.520). Additionally, there was a significant interaction for MACE between TMAO tertiles and hsCRP dichotomy (P for interaction = 0.007). Meanwhile, there were no similar findings when hsCRP levels were below the median level.
Table 3.
Association between MACE and TMAO levels stratified by hsCRP median levels
| Variable | n, total | n, event (%) | Crude HR (95% CI) | P‐value | Adjusted HR (95% CI) a | P‐value |
|---|---|---|---|---|---|---|
| hsCRP b | ||||||
| >median vs. ≤median | 1.17 (0.84–1.64) | 0.356 | 1.20 (0.85–1.69) | 0.292 | ||
| TMAO | ||||||
| >median vs. ≤median | 2.14 (1.50–3.05) | <0.001 | 1.56 (1.08–2.26) | 0.019 | ||
| hsCRP ≤ 6.68 mg/L | ||||||
| TMAO per SD c | ‐ | ‐ | 1.08 (0.88–1.32) | 0.482 | 0.89 (0.71–1.11) | 0.297 |
| TMAO by median | ||||||
| ≤median | 249 | 27 (10.8) | 1 (Ref) | 1 (Ref) | ||
| >median | 243 | 37 (15.2) | 1.46 (0.89–2.40) | 0.134 | 1.14 (0.66–1.95) | 0.646 |
| TMAO by tertile | ||||||
| Tertile 1 | 162 | 5 (6.6) | 1 (Ref) | 1 (Ref) | ||
| Tertile 2 | 82 | 10 (12.2) | 1.91 (0.65–5.59) | 0.238 | 1.78 (0.57–5.54) | 0.317 |
| Tertile 3 | 79 | 11 (13.9) | 2.14 (0.74–6.16) | 0.159 | 2.27 (0.73–7.03) | 0.155 |
| hsCRP > 6.68 mg/L | ||||||
| TMAO per SD c | ‐ | ‐ | 1.27 (1.14–1.41) | <0.001 | 1.20 (1.05–1.37) | 0.009 |
| TMAO by median | ||||||
| ≤median | 243 | 19 (7.8) | 1 (Ref) | 1 (Ref) | ||
| >median | 250 | 55 (22.0) | 3.12 (1.85–5.25) | <0.001 | 2.35 (1.36–4.07) | 0.002 |
| TMAO by tertile | ||||||
| Tertile 1 | 166 | 13 (7.8) | 1 (Ref) | 1 (Ref) | ||
| Tertile 2 | 163 | 18 (11.0) | 1.44 (0.71–2.94) | 0.315 | 1.27 (0.62–2.61) | 0.520 |
| Tertile 3 | 164 | 43 (26.2) | 3.86 (2.07–7.19) | <0.001 | 2.91 (1.52–5.56) | 0.001 |
HR, hazard ratio; hsCRP, high‐sensitivity C‐reactive protein; MACE, major adverse cardiac event; TMAO, trimethylamine‐N‐oxide.
Adjusted for age, sex, body mass index, estimated glomerular filtration rate, current smoking, HF‐type, hypertension, diabetes mellitus, peripheral artery disease, and history of stroke and MI.
The median level of hsCRP was 6.68 mg/L.
P = 0.041 for interaction between TMAO tertiles and hsCRP dichotomy for MACE, and adjusted P = 0.007.
Discussion
This prospective cohort study explored the association between plasma TMAO and hsCRP levels and the prognosis of patients with AMI and HF. The main finding was that high plasma TMAO levels were independently associated with poor outcomes, particularly in patients with higher hsCRP levels. There may be a potential congenerous effect of TMAO and inflammation on cardiovascular risk. Besides, an S‐shaped curve relationship was recorded between TMAO and HR for MACE.
TMAO is a small organic compound that is formed by the oxidation of trimethylamine in the host liver by flavin monooxygenases. 21 Alkaloids such as choline, carnitine, and betaine are converted to trimethylamine by intestinal flora. 22 , 23 Robust evidence suggests that TMAO is involved in immunity, inflammation, cholesterol metabolism, and atherothrombosis. The median (IQR) plasma levels of TMAO were 6.7 (4.0–11.7) μmol/L in this study, which is similar to the range reported in previous studies related to HF. 8 The concentration of TMAO may vary according to the disease state, course, and severity. In a systematic review, the median levels of TMAO ranged from 2.87 to 88 μmol/L in the general population, whereas patients with CKD had higher TMAO levels. 3 The plasma TMAO levels in the groups classified as New York Heart Association II, III, and IV groups were 3.5 ± 0.9, 6.0 ± 0.8, and 8.1 ± 1.0 μmol/L, respectively, showing a significantly increasing trend (P < 0.01) in the chronic HF population. 24 Similarly, levels of TMAO also showed an increasing trend according to the Killip class in this study. Thus, different levels of TMAO reflect various states of pathophysiological processes.
Moreover, increasing numbers of studies have exhibited that TMAO may be a promising cardiovascular risk marker. A prospective multicentre cohort study by Lee et al. reported that for persistently higher levels of plasma TMAO in the patients aged >65 years were associated with more incident atherosclerotic cardiovascular disease (the extreme quintile vs. the lowest quintile, HR: 1.21, 95% CI, 1.02–1.42; P‐trend = 0.029). 25 Higher TMAO levels were an independent risk factor for short‐ and long‐term composite outcome of MI, stroke, need for revascularization, or all‐cause death in patients with acute coronary syndrome. 4 A nested case–control study indicated that the risk for cardiovascular death, MI, or stroke in patients with higher TMAO levels was elevated by approximately 50% (quartile 4 vs. quartile 1, odds ratio = 1.43, 95% CI, 1.06–1.93, P‐trend = 0.015) in patients with stable coronary artery diseases. 26 For patients with HF, a meta‐analysis reported that elevated TMAO levels predicted a higher risk of composite outcomes (including all‐cause death, hospitalization with HF, and heart transplantation) with a 1.68‐fold (95% CI: 1.44–1.96) increase in the HR of the highest tertile compared with that of the lowest tertile. 8 Our current study observed that, the relative risk for MACE was increased by 1.85 (95% CI: 1.18–2.89) times in patients with AMI and HF who had the highest levels of TMAO than that in the lowest tertile. In addition, this is the first study to show the S‐shaped association between TMAO and HR for MACE (P for nonlinearity = 0.012), implying that the HR of MACE may no longer increase after TMAO exceeds a certain level. However, the specific mechanism of this association requires further investigation.
Remarkably, our study reported that the higher risk for MACE was mainly driven by all‐cause death and recurrence of MI, and not rehospitalization due to HF. As reported in previous studies, the results for rehospitalization due to HF were inconsistent in different HF subtypes, especially in patients with acute HF or HFpEF. 27 , 28 For patients with acute HF, a study by Suzuki et al. reported that TMAO was not associated with the risk of death or rehospitalization due to HF, after adjusting for cofounders especially eGFR. 29 In contrast, Israr et al. indicated that TMAO was independently related to the short‐ and long‐term outcomes of a composite of all‐cause mortality or rehospitalization caused by HF. 30 Schuett et al. revealed that elevated TMAO levels could not predict all‐cause mortality and cardiovascular mortality in patients with HFpEF. 28 However, Kinugasa et al. reported that elevated TMAO levels at discharge were associated with an increased risk of post‐discharge cardiac events in patients with HFpEF. 31 Given the relatively mild symptoms and signs, and different pathophysiological processes of HFpEF, the results might be inverse. However, clinical data on acute HF or HFpEF are limited, and further studies are necessary to examine the association between TMAO levels and the prognosis in patients with acute HF or HFpEF. In addition, the precursors of trimethylamine, such as choline and carnitine, were proved to be associated with poor outcomes in patients with HF. A study by Israr et al. extensively suggested that circulating levels of multiple metabolites of the choline/carnitine‐TMAO pathway were graded associations with the severity and adverse prognosis of chronic HF. 32 It is worth expecting that combining TMAO with these biomarkers might play a better role in the risk stratification of different types of HF.
Currently, substantial evidence reveals that systemic inflammation is the predominant driver of atherosclerosis and the underlying pathology of cardiovascular diseases. 33 HsCRP is regarded as a marker for measuring the intensity of systemic inflammation. 34 In this study, we found that hsCRP levels were not associated with MACE risk (P = 0.077), which may be because we selected the median level of hsCRP as the cutoff while the cutoff was 2 or 3 mg/L to assess residual cardiovascular risk in most previous studies. 11 , 34 Besides, our previous study found that both low and high hsCRP levels were associated with increased risk of death in AMI patients, 12 which may partially explain that no significance in the median levels of hsCRP and MACE risk in this study. In addition, we observed that TMAO levels were negatively correlated with hsCRP levels (P = 0.009), which is consistent with the result of the previous study. 35 , 36 Whereas a meta‐analysis 37 reported that various sample sources and diseases would cause different results, and there was a non‐linear association between TMAO and CRP levels. More importantly, we observed that the difference in the risk for MACE between tertiles 3 and 1 was only statistically significant if the hsCRP level was above the median level. Furthermore, increasing hsCRP might enhance the effect of TMAO levels on the HR of MACE. These may be because TMAO and inflammation may have potential synergistic effects on cardiovascular risk. In the past few years, some experimental studies have suggested that TMAO activates different signalling pathways, resulting in the release of inflammatory cytokines, such as interleukin‐1β, which induces systemic inflammation. 13 , 14 , 38 Seldin et al. reported that TMAO could activate the nuclear factor‐κB signalling pathway, which is essential for the inflammatory effects of TMAO. Another study by Chen et al. revealed that TMAO caused vascular inflammation through activating the nucleotide‐binding oligomerization domain‐like receptor family pyrin domain‐containing 3 (NLRP3) inflammasome. Notably, NLRP3 inflammasome is required for TMAO to induce inflammatory cascades and can cause the release of interleukin‐1β and interleukin‐18, which extensively triggers systemic inflammation. These results may explain why the TMAO‐associated HR for MACE was significantly elevated only when hsCRP was elevated. However, it is unclear why there is a negative correlation between TMAO and hsCRP levels. Even so, it is worth noting that elevated hsCRP levels would strengthen the impact of TMAO on adverse outcomes. The potential mechanism of interaction between body inflammatory status and TMAO levels deserves further attention and research.
The present study had a few limitations that should be acknowledged. First, the study measured the TMAO concentrations before PCI, and changes in TMAO levels during the course were unavailable. Besides, dietary changes may influence the TMAO levels. Second, given that this study included patients with AMI and HF in the emergency department, there may have been bias of patient selection, resulting in a sample with relatively severe condition. Third, although this is a prospective cohort study, the causality between TMAO levels and its interaction with hsCRP and cardiovascular outcomes in patients with AMI and HF remains elusive. Furthermore, race and region may also affect TMAO levels. 27 , 39 Therefore, large‐scale, multicentre studies are needed to verify these findings.
Conclusions
In summary, increased TMAO levels before PCI were independently related to an increased risk of MACE in patients with AMI and HF, especially in those with elevated hsCRP levels. It is worth paying attention to the potential synergistic effects of TMAO and inflammation in the future. Notably, there is no linear relationship between the risk of MACE and increasing TMAO levels.
Funding
This study was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2016‐I2M‐1‐009), Fund of “Sanming” Project of Medicine in Shenzhen (No. SZSM201911017), and Shenzhen Key Medical Discipline Construction Fund (No. SZXK001).
Conflict of interest
All authors declare no conflicts of interest.
Supporting information
Table S1. Spearman's correlation coefficients between variables and ln‐transformed TMAO levels.
Table S2. Association between variables and major adverse cardiac event.
Figure S1. Subject selection flow chart. AMI, acute myocardial infarction; HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein.
Figure S2. Kaplan–Meier curve for cumulative event‐free survival rate among groups stratified by TMAO and hsCRP median levels. A: major adverse cardiac event, B: all‐cause death, C: myocardial infarction, D: rehospitalization due to heart failure; hsCRP, high‐sensitivity C‐reactive protein; TMAO, trimethylamine‐N‐oxide.
Figure S3. Kaplan–Meier curve for cumulative event‐free survival rate among TMAO tertiles in patients with STEMI. A: major adverse cardiac event, B: all‐cause death, C: myocardial infarction, D: rehospitalization due to heart failure; STEMI, ST‐segment elevation myocardial infarction; TMAO, trimethylamine‐N‐oxide.
Li, N. , Zhou, J. , Wang, Y. , Chen, R. , Li, J. , Zhao, X. , Zhou, P. , Liu, C. , Song, L. , Liao, Z. , Wang, X. , Yan, S. , Zhao, H. , and Yan, H. (2022) Association between trimethylamine N‐oxide and prognosis of patients with acute myocardial infarction and heart failure. ESC Heart Failure, 9: 3846–3857. 10.1002/ehf2.14009.
Contributor Information
Hanjun Zhao, Email: 15210020808@163.com.
Hongbing Yan, Email: hbyanfuwai2018@163.com.
References
- 1. Cretoiu D, Ionescu RF, Enache RM, Cretoiu SM, Voinea SC. Gut microbiome, functional food, atherosclerosis, and vascular calcifications‐is there a missing link? Microorganisms 2021; 9: 1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Troseid M, Andersen GO, Broch K, Hov JR. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020; 52: 102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, Trimarco B, Esposito G, Perrino C. Gut microbe‐generated metabolite trimethylamine‐N‐oxide as cardiovascular risk biomarker: A systematic review and dose‐response meta‐analysis. Eur Heart J 2017; 38: 2948–2956. [DOI] [PubMed] [Google Scholar]
- 4. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Räber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Lüscher TF. Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017; 38: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan Y, Zhou J, Yang S, Li J, Zhao H, Song L, Yan H. Addition of plasma myeloperoxidase and trimethylamine N‐oxide to the GRACE score improves prediction of near‐term major adverse cardiovascular events in patients with ST‐segment elevation myocardial infarction. Front Pharmacol 2021; 12: 632075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou X, Jin M, Liu L, Yu Z, Lu X, Zhang H. Trimethylamine N‐oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail 2020; 7: 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salzano A, Israr MZ, Yazaki Y, Heaney LM, Kanagala P, Singh A, Arnold JR, Gulsin GS, Squire IB, McCann GP, Ng LL, Suzuki T. Combined use of trimethylamine N‐oxide with BNP for risk stratification in heart failure with preserved ejection fraction: Findings from the DIAMONDHFpEF study. Eur J Prev Cardiol 2020; 27: 2159–2162. [DOI] [PubMed] [Google Scholar]
- 8. Li W, Huang A, Zhu H, Liu X, Huang X, Huang Y, Cai X, Lu J, Huang Y. Gut microbiota‐derived trimethylamine N‐oxide is associated with poor prognosis in patients with heart failure. Med J Aust 2020; 213: 374–379. [DOI] [PubMed] [Google Scholar]
- 9. Salzano A, Cassambai S, Yazaki Y, Israr MZ, Bernieh D, Wong M, Suzuki T. The gut Axis involvement in heart failure: Focus on trimethylamine N‐oxide. Heart Fail Clin 2020; 16: 23–31. [DOI] [PubMed] [Google Scholar]
- 10. Ridker PM. Clinician's guide to reducing inflammation to reduce Atherothrombotic risk: JACC review topic of the week. J Am Coll Cardiol 2018; 72: 3320–3331. [DOI] [PubMed] [Google Scholar]
- 11. Carrero JJ, Andersson Franko M, Obergfell A, Gabrielsen A, Jernberg T. hsCRP level and the risk of death or recurrent cardiovascular events in patients with myocardial infarction: A healthcare‐based study. J Am Heart Assoc 2019; 8: e012638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen R, Liu C, Zhou P, Tan Y, Sheng Z, Li J, Zhou J, Chen Y, Song L, Zhao H, Yan H. Both low and high Postprocedural hsCRP associate with increased risk of death in acute coronary syndrome patients treated by percutaneous coronary intervention. Mediators Inflamm 2020; 2020: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seldin MM, Meng Y, Qi H, Zhu WF, Wang Z, Hazen SL, Lusis AJ, Shih DM. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐kappaB. J Am Heart Assoc 2016; 5: e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen M, Zhu X, Ran L, Lang H, Yi L, Mi M. Trimethylamine‐N‐oxide induces vascular inflammation by activating the NLRP3 Inflammasome through the SIRT3‐SOD2‐mtROS signaling pathway. J Am Heart Assoc 2017; 6: e006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264. [DOI] [PubMed] [Google Scholar]
- 16. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The task force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 17. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, ESC Scientific Document Group , Kastrati A, Mamas MA, Aboyans V, Angiolillo DJ, Bueno H, Bugiardini R, Byrne RA, Castelletti S, Chieffo A, Cornelissen V, Crea F, Delgado V, Drexel H, Gierlotka M, Halvorsen S, Haugaa KH, Jankowska EA, Katus HA, Kinnaird T, Kluin J, Kunadian V, Landmesser U, Leclercq C, Lettino M, Meinila L, Mylotte D, Ndrepepa G, Omerovic E, Pedretti RFE, Petersen SE, Petronio AS, Pontone G, Popescu BA, Potpara T, Ray KK, Luciano F, Richter DJ, Shlyakhto E, Simpson IA, Sousa‐Uva M, Storey RF, Touyz RM, Valgimigli M, Vranckx P, Yeh RW, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Jüni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2021; 42: 1289–1367. [DOI] [PubMed] [Google Scholar]
- 18. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 19. Bozkurt B, Coats A, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, Anker SD, Atherton J, Böhm M, Butler J, Drazner MH, Felker GM, Filippatos G, Fonarow GC, Fiuzat M, Gomez‐Mesa J–E, Heidenreich P, Imamura T, Januzzi J, Jankowska EA, Khazanie P, Kinugawa K, Lam CSP, Matsue Y, Metra M, Ohtani T, Francesco Piepoli M, Ponikowski P, Rosano GMC, Sakata Y, SeferoviĆ P, Starling RC, Teerlink JR, Vardeny O, Yamamoto K, Yancy C, Zhang J, Zieroth S. Universal definition and classification of heart failure. J Card Fail 2021; 27: 387–413. [DOI] [PubMed] [Google Scholar]
- 20. Wang Z, Levison BS, Hazen JE, Donahue L, Li XM, Hazen SL. Measurement of trimethylamine‐N‐oxide by stable isotope dilution liquid chromatography tandem mass spectrometry. Anal Biochem 2014; 455: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Warrier M, Shih DM, Burrows AC, Ferguson D, Gromovsky AD, Brown AL, Marshall S, McDaniel A, Schugar RC, Wang Z, Sacks J, Rong X, Vallim TA, Chou J, Ivanova PT, Myers DS, Brown HA, Lee RG, Crooke RM, Graham MJ, Liu X, Parini P, Tontonoz P, Lusis AJ, Hazen SL, Temel RE, Brown JM. The TMAO‐generating enzyme Flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep 2015; 10: 326–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368: 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WHW, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dong Z, Liang Z, Wang X, Liu W, Zhao L, Wang S, Hai X, Yu K. The correlation between plasma trimethylamine N‐oxide level and heart failure classification in northern Chinese patients. Ann Palliat Med 2020; 9: 2862–2871. [DOI] [PubMed] [Google Scholar]
- 25. Lee Y, Nemet I, Wang Z, Lai HTM, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Sotoodehnia N, Budoff M, DiDonato JA, McKnight B, Tang WHW, Psaty BM, Siscovick DS, Hazen SL, Mozaffarian D. Longitudinal plasma measures of trimethylamine N‐oxide and risk of atherosclerotic cardiovascular disease events in community‐based older adults. J Am Heart Assoc 2021; 10: e020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gencer B, Li XS, Gurmu Y, Bonaca MP, Morrow DA, Cohen M, Bhatt DL, Steg PG, Storey RF, Johanson P, Wang Z, Hazen SL, Sabatine MS. Gut microbiota‐dependent trimethylamine N‐oxide and cardiovascular outcomes in patients with prior myocardial infarction: A nested case control study from the PEGASUS‐TIMI 54 trial. J Am Heart Assoc 2020; 9: e015331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yazaki Y, Aizawa K, Israr MZ, Negishi K, Salzano A, Saitoh Y, Kimura N, Kono K, Heaney L, Cassambai S, Bernieh D, Lai F, Imai Y, Kario K, Nagai R, Ng LL, Suzuki T. Ethnic differences in association of outcomes with trimethylamine N‐oxide in acute heart failure patients. ESC Heart Fail 2020; 7: 2373–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schuett K, Kleber ME, Scharnagl H, Lorkowski S, März W, Niessner A, Marx N, Meinitzer A. Trimethylamine‐N‐oxide and heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol 2017; 70: 3202–3204. [DOI] [PubMed] [Google Scholar]
- 29. Suzuki T, Heaney LM, Bhandari SS, Jones DJ, Ng LL. Trimethylamine N‐oxide and prognosis in acute heart failure. Heart 2016; 102: 841–848. [DOI] [PubMed] [Google Scholar]
- 30. Israr MZ, Bernieh D, Salzano A, Cassambai S, Yazaki Y, Heaney LM, Jones DJL, Ng LL, Suzuki T. Association of gut‐related metabolites with outcome in acute heart failure. Am Heart J 2021; 234: 71–80. [DOI] [PubMed] [Google Scholar]
- 31. Kinugasa Y, Nakamura K, Kamitani H, Hirai M, Yanagihara K, Kato M, Yamamoto K. Trimethylamine N‐oxide and outcomes in patients hospitalized with acute heart failure and preserved ejection fraction. ESC Heart Fail 2021; 8: 2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Israr MZ, Zhan H, Salzano A, Voors AA, Cleland JG, Anker SD, Metra M, van Veldhuisen D, Lang CC, Zannad F, Samani NJ, Ng LL, Suzuki T, BIOSTAT‐CHF investigators (full author list as appendix) . Surrogate markers of gut dysfunction are related to heart failure severity and outcome‐from the BIOSTAT‐CHF consortium. Am Heart J 2022; 248: 108–119. [DOI] [PubMed] [Google Scholar]
- 33. Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL‐1 pathway in atherosclerosis. Circ Res 2018; 122: 1722–1740. [DOI] [PubMed] [Google Scholar]
- 34. Ridker PM. A test in context: High‐sensitivity C‐reactive protein. J Am Coll Cardiol 2016; 67: 712–723. [DOI] [PubMed] [Google Scholar]
- 35. Kaysen GA, Johansen KL, Chertow GM, Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW, Fiehn O. Associations of trimethylamine N‐oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to Dialysis. J Ren Nutr 2015; 25: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Trøseid M, Ueland T, Hov JR, Svardal A, Gregersen I, Dahl CP, Aakhus S, Gude E, Bjørndal B, Halvorsen B, Karlsen TH, Aukrust P, Gullestad L, Berge RK, Yndestad A. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J Intern Med 2015; 277: 717–726. [DOI] [PubMed] [Google Scholar]
- 37. Farhangi MA, Vajdi M. Novel findings of the association between gut microbiota‐derived metabolite trimethylamine N‐oxide and inflammation: Results from a systematic review and dose‐response meta‐analysis. Crit Rev Food Sci Nutr 2020; 60: 2801–2823. [DOI] [PubMed] [Google Scholar]
- 38. Zhang X, Li Y, Yang P, Liu X, Lu L, Chen Y, Zhong X, Li Z, Liu H, Ou C, Yan J, Chen M. Trimethylamine‐N‐oxide promotes vascular calcification through activation of NLRP3 (nucleotide‐binding domain, leucine‐rich‐containing family, pyrin domain‐Containing‐3) Inflammasome and NF‐kappaB (nuclear factor kappaB) signals. Arterioscler Thromb Vasc Biol 2020; 40: 751–765. [DOI] [PubMed] [Google Scholar]
- 39. Yazaki Y, Salzano A, Nelson CP, Voors AA, Anker SD, Cleland JG, Lang CC, Metra M, Samani NJ, Ng LL, Suzuki T. Geographical location affects the levels and association of trimethylamine N‐oxide with heart failure mortality in BIOSTAT‐CHF: A post‐hoc analysis. Eur J Heart Fail 2019; 21: 1291–1294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Spearman's correlation coefficients between variables and ln‐transformed TMAO levels.
Table S2. Association between variables and major adverse cardiac event.
Figure S1. Subject selection flow chart. AMI, acute myocardial infarction; HF, heart failure; hsCRP, high‐sensitivity C‐reactive protein.
Figure S2. Kaplan–Meier curve for cumulative event‐free survival rate among groups stratified by TMAO and hsCRP median levels. A: major adverse cardiac event, B: all‐cause death, C: myocardial infarction, D: rehospitalization due to heart failure; hsCRP, high‐sensitivity C‐reactive protein; TMAO, trimethylamine‐N‐oxide.
Figure S3. Kaplan–Meier curve for cumulative event‐free survival rate among TMAO tertiles in patients with STEMI. A: major adverse cardiac event, B: all‐cause death, C: myocardial infarction, D: rehospitalization due to heart failure; STEMI, ST‐segment elevation myocardial infarction; TMAO, trimethylamine‐N‐oxide.


