Abstract
Aim
Non‐alcoholic fatty liver disease (NAFLD)‐related advanced liver fibrosis (Stage 3 or 4) was reported to be linked to worse prognosis in patients with heart failure with preserved ejection fraction (HFpEF). This study aims to assess the relationship between liver fibrosis scores and new‐onset atrial fibrillation (AF) incidence in patients with HFpEF in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial.
Methods and results
Baseline liver fibrosis levels, assessed by NAFLD fibrosis score (NFS) or Fibrosis‐4 index (FIB‐4), with AF incidence were expressed as hazard ratios (HRs) using the Cox proportional hazard model. The risk for advanced fibrosis was estimated to be 21.5% (447/2072) as assessed by FIB‐4 (>3.25) and 4.2% (88/2072) as assessed by NFS (>0.676) in HFpEF patients without baseline AF. After a median follow‐up of 3.11 years, 106 new‐onset AF cases occurred. In multivariate analysis, elevated NFS [NFS = −1.455–0.676: HR 2.44, 95% confidence interval (CI) 1.27–4.68; NFS > 0.676: HR 3.36, 95% CI 1.27–6.80; per 1 unit increase: HR 1.15, 95% CI 1.01–1.32], not FIB‐4 (FIB‐4 = 1.45–3.25: HR 1.02, 95% CI 0.67–1.55; FIB‐4 > 3.25: HR 1.69, 95% CI 0.76–3.79; per 1 unit increase: HR 1.13, 95% CI 0.93–1.37), was associated with increased AF incidence. The NFS (C‐index 0.662), not FIB‐4 (C‐index 0.531), had a moderate predictive ability in predicting incident AF.
Conclusions
Among patients with HFpEF, the risk of advanced liver fibrosis is associated with an increased incidence of new‐onset AF and may be a novel predictor for new‐onset AF. Additional studies are needed to confirm our results.
Keywords: Heart failure, Liver fibrosis, FIB‐4, NFS, Risk prediction, Atrial fibrillation
Introduction
Chronic liver diseases, such as non‐alcoholic fatty liver disease (NAFLD), are common in patients with heart failure with preserved ejection fraction (HFpEF) due to shared risk factors (alcohol abuse, drugs, inflammation, autoimmunity, infections), as well as complex cardiohepatic interactions. 1 Advanced liver fibrosis (Stage 3 or 4) is the primary pathology of many chronic liver diseases and was detected in 32% of patients with chronic heart failure via the gold standard of biopsies. 2 Regarding HFpEF, the prevalence of advanced liver fibrosis is in the range 37.5–53% based on several cohorts 3 , 4 , 5 , 6 assessed by the NAFLD fibrosis score (NFS) or Fibrosis‐4 index (FIB‐4) score, simple non‐invasive tools developed to distinguish the severity of liver fibrosis in liver diseases. 7 , 8 , 9 Furthermore, accumulating evidence suggests that liver fibrosis is independent of other established risk factors for heart failure 10 , 11 and significantly contributes to heart failure progression, cardiovascular events, and all‐cause death in patients with HFpEF. 1 , 4
Atrial fibrillation (AF) is one of the most common comorbidities in HFpEF, presenting in approximately one‐third of patients. There is a close link between HFpEF and AF, and each condition independently contributes to poor outcomes—the so‐called vicious twins. Therefore, identifying the potential risk factors for AF in HFpEF is clinically important for preventing AF and thus improving prognosis, especially in stroke. Evidence from epidemiological studies has shown a positive association between advanced liver fibrosis and the risk of AF in patients with NAFLD or Type 2 diabetes. 12 , 13
Additionally, advanced liver fibrosis is not uncommon in patients with HFpEF. Given this background, we are curious as to whether liver fibrosis contributes to the increased incidence of new‐onset AF in patients with HFpEF. Based on the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, we conducted a second analysis to investigate the relationship between liver fibrosis assessed by NFS and FIB‐4 scores and incident AF in patients with HFpEF.
Methods
The study population was from TOPCAT, an international, multicentre randomized, double‐blinded, placebo‐controlled trial that assessed the effect of spironolactone on clinical outcomes in patients with HFpEF compared with placebo. 14 TOPCAT complied with the Declaration of Helsinki and received ethical approval. All patients signed informed consent before randomization. All relevant data were obtained from the National Heart, Lung, and Blood Institute.
Liver fibrosis was assessed by NFS and FIB‐4 scores. NFS and FIB‐4 scores were computed using the following formulas:
AF post‐randomization was determined by a follow‐up electrocardiogram (ECG), event questionnaire, and review of relevant medical records every 4 months during the first year of the study and every 6 months thereafter. AF occurrence was adjudicated by the clinical endpoint committee. The Cox proportional hazard model with and without multivariable adjustment was used to examine the associations [expressed as hazard ratios (HRs) and 95% confidence intervals (CIs)] of NFS or FIB‐4 scores with the incidence of AF. The selected variables were from a backward stepwise method and additional clinically relevant factors. We evaluated the discriminatory ability of the NFS or FIB‐4 for predicting AF using the C‐indices. For internal model and score validation, we used the k‐fold in 10‐fold cross‐validation with 200 repetitions. Subgroup analysis stratified by prespecified variables in the main TOPCAT: sex (male vs. female), region (the Americas vs. non‐Americas), and treatment arm (spironolactone vs. placebo).
Sensitivity analysis was conducted by (i) recalculating the estimated effect by a competing risk model (death was defined as a competent event) and (ii) excluding patients with concomitant use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) and use of any beta‐blocker. All statistical analyses were performed with Stata software (version 16.0) and R software (version 4.1.2). A two‐sided P‐value <0.05 was considered statistically significant. Full details of the methods are presented in the Supporting Information.
Results
Baseline characteristics and prevalence of liver fibrosis score in heart failure with preserved ejection fraction
As shown in Figure 1 , after exclusion of 1217 HFpEF patients with baseline AF or missing AF status and missing individuals for calculating FIB‐4 or NFS scores [n = 6 for body mass index (BMI), n = 23 for platelet (PLT), n = 21 for alanine aminotransferase (ALT), n = 4 for aspartate aminotransferase (AST), n = 99 for albumin (ALB)], abnormal AST or ALT values were observed (n = 3). Finally, we included a studied population of 2072 without baseline AF, with 930 (44.9%) men and 1814 White participants (89.2%). The median ± interquartile range (IQR) values in all patients were 67 (60.0, 74.0) years for age, 30.6 (27.1, 35.4) kg/m2 for BMI, and 68 (61–75) bpm for heart rate. The median BNP (n = 744) and N‐terminal pro BNP (NT‐proBNP; n = 564) concentrations were 248 (IQR 144, 438) and 828 (IQR 456, 1668) pg/mL, respectively.
Figure 1.
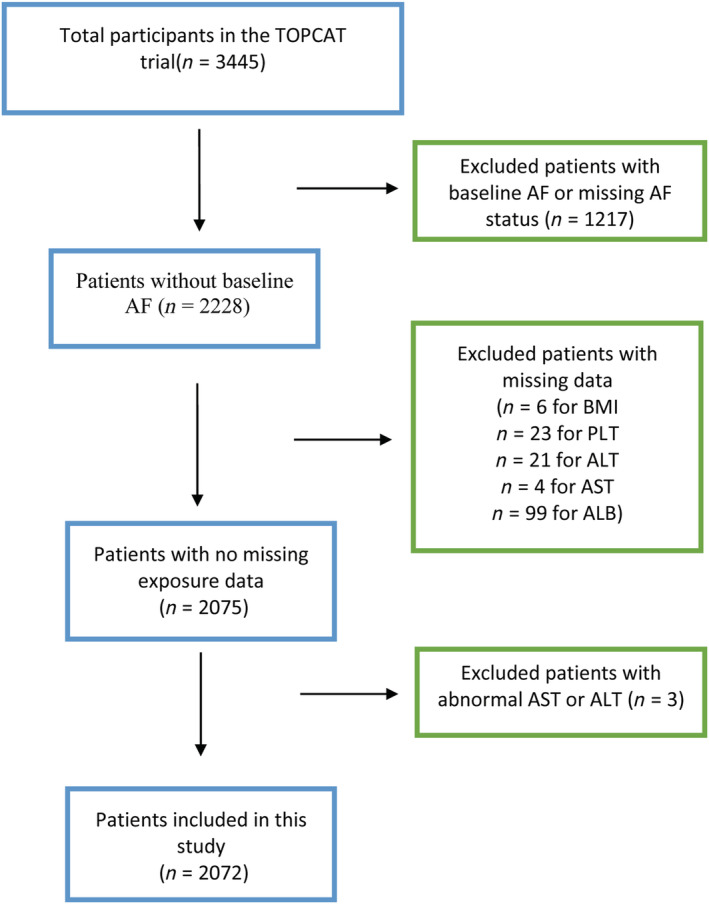
Flow chart of study selection of liver fibrosis scores from the TOPCAT trial. AF, atrial fibrillation; ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; PLT, platelet; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial.
The characteristics of participants across the NFS and FIB‐4 groups are shown in Table 1 . The median liver fibrosis scores in the overall cohort were −0.50 (IQR −1.48, 0.51) and 1.42 (IQR 1.07, 1.93) as assessed by NFS and FIB‐4, respectively. Overall, the prevalence of a high risk for advanced liver fibrosis was estimated to be higher by NFS than by FIB‐4. The low, moderate, and high risk rates for liver fibrosis scores were 25.7, 52.8, and 21.6%, respectively, as assessed by NFS and 51.5, 44.3, and 4.2%, respectively, as assessed by FIB‐4. There was a significant difference in the baseline characteristics across risk for liver fibrosis groups, such as age, heart function, BMI, smoking history, and ALT and ALB levels. Only a small proportion of patients provided echocardiographic data at baseline. The echocardiographic data at baseline across the NFS and FIB‐4 groups are presented in Supporting Information, Tables S1 and S2 .
Table 1.
Baseline characteristics stratified by Fibrosis‐4 index and non‐alcoholic fatty liver disease fibrosis score groups
| Overall | NFS < −1.455 | NFS = −1.455–0.676 | NFS > 0.676 | FIB‐4 < 1.45 | FIB‐4 = 1.45–3.25 | FIB‐4 > 3.25 | |
|---|---|---|---|---|---|---|---|
| (n = 2072) | (n = 532) | (n = 1,093) | (n = 447) | (n = 1,067) | (n = 917) | (n = 88) | |
| Demographic | |||||||
| Age (years) | 67.0 (60.0, 74.0) | 62.0 (56.0, 69.0) | 69.0 (61.0, 75.0) | 70.0 (62.0, 77.0)* | 63.0 (57.0, 70.0) | 70.0 (63.0, 77.0) | 73.0 (66.0, 80.0)* |
| Sex, male | 930 (44.9) | 229 (43.0) | 479 (43.8) | 222 (49.7) | 458 (42.9) | 424 (46.2) | 48 (54.5) |
| Race, White | 1814 (87.5) | 506 (95.1) | 961 (87.9) | 347 (77.6)* | 926 (86.8) | 812 (88.5) | 76 (86.4) |
| Current smoking | 261 (12.6) | 98 (18.4) | 128 (11.7) | 35 (7.8)* | 153 (14.3) | 95 (10.4) | 13 (14.8)* |
| Ever smoking | 695 (33.5) | 136 (25.6) | 363 (33.2) | 196 (43.8)* | 348 (32.6) | 316 (34.5) | 31 (35.2) |
| Never smoking | 1116 (53.9) | 298 (56.0) | 602 (55.1) | 216 (48.3)* | 566 (53.0) | 506 (55.2) | 44 (50.0) |
| BMI, kg/m2 | 30.6 (27.1, 35.4) | 27.8 (25.0, 31.1) | 30.4 (27.1, 34.5) | 37.0 (32.3, 43.0)* | 41.0 (27.4, 36.5) | 30.1 (26.7, 34.6) | 30.8 (27.6, 34.5)* |
| Heart rate, bpm | 68 (61, 75) | 68 (62, 69) | 69 (61, 75) | 70 (62, 77)* | 68 (62, 75) | 68 (60, 74) | 64.5 (60, 74) |
| SBP, mmHg | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (120, 140) | 130 (118, 136) |
| DBP, mmHg | 80 (70, 84) | 80.0 (75, 85) | 80 (70, 85) | 71 (62, 80)* | 80 (70, 85) | 80 (70, 83) | 70 (60, 80) |
| NYHA III or IV class | 604 (29.2) | 118 (22.2) | 296 (27.1) | 190 (42.5)* | 282 (26.5) | 299 (32.6) | 23 (26.1)* |
| Chronic health conditions, n% | |||||||
| Peripheral vascular disease | 193 (9.3) | 31 (5.8) | 92 (8.4) | 70 (15.7)* | 92 (8.6) | 91 (9.9) | 10 (11.4) |
| COPD | 219 (10.6) | 41 (7.7) | 109 (10.0) | 69 (15.4)* | 114 (10.7) | 99 (10.8) | 6 (6.8) |
| Hypertension | 1905 (91.9) | 486 (91.4) | 998 (91.3) | 421 (94.2) | 993 (93.1) | 833 (90.8) | 79 (89.8) |
| Diabetes mellitus | 698 (33.7) | 44 (8.3) | 322 (29.5) | 332 (74.3)* | 379 (35.5) | 290 (31.6) | 29 (33.0) |
| Previous MI | 579 (27.9) | 158 (29.7) | 312 (28.5) | 109 (24.4) | 298 (27.9) | 258 (28.1) | 23 (26.1) |
| Previous stroke | 133 (6.4) | 24 (4.5) | 73 (6.7) | 36 (8.1) | 73 (6.8) | 56 (6.1) | 4 (4.5) |
| CABG | 259 (12.5) | 47 (8.8) | 127 (11.6) | 85 (19.0)* | 113 (10.6) | 132 (14.4) | 14 (15.9)* |
| PCI | 313 (15.1) | 57 (10.7) | 158 (14.5) | 98 (21.9)* | 151 (14.2) | 149 (16.2) | 13 (14.8) |
| Thyroid disease | 260 (12.5) | 56 (10.5) | 135 (12.4) | 69 (15.4) | 135 (12.7) | 115 (12.5) | 10 (11.4) |
| Dyslipidaemia | 1206 (58.2) | 264 (49.6) | 625 (57.2) | 317 (70.9)* | 613 (57.5) | 535 (58.3) | 58 (65.9) |
| C2HEST score | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0)* | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0)* |
| Measurements | |||||||
| eGFR, mL/min × 1.73 m2 | 67.0 (55.4, 80.6) | 71.5 (60.7, 84.3) | 66.0 (55.5, 79.5) | 63.1 (49.8, 77.2)* | 69.7 (57.0, 83.8) | 64.8 (53.9, 77.5) | 59.4 (64.8, 77.5)* |
| White blood cell count, 103/mL | 6.7 (5.6, 7.9) | 6.8 (5.6, 7.9) | 6.5 (5.5, 7.8) | 6.9 (5.8, 8.2)* | 7.0 (5.8, 8.4) | 6.4 (5.4, 7.5) | 6.3 (5.0, 7.2)* |
| Haemoglobin, g/dL | 13.1 (12.2, 14.3) | 13.5 (12.6, 14.6) | 13.2 (12.3, 14.3) | 12.7 (11.6, 13.9)* | 13.2 (12.2, 14.3) | 13.1 (12.2, 14.2) | 13.0 (11.5, 14.3) |
| Platelet (k/μL) | 228 (196, 265) | 268 (236, 310) | 220 (196, 254) | 195 (162, 229)* | 252 (221, 290) | 207 (180, 235) | 157.5 (128.3, 192)* |
| AST, U/L | 23.0 (18.0, 29.0) | 22.0 (17.43, 29.0) | 23.0 (18.0, 29.0) | 23.0 (18.0, 30.0) | 20.0 (16.0, 24.0) | 26.0 (21.0, 33.0) | 39.0 (30.0, 52.6)* |
| ALT, U/L | 21.9 (15.8, 31.0) | 25.0 (18.0, 34.0) | 21.0 (15.0, 30.0) | 19.0 (14.0, 28.0)* | 22.4 (16.0, 31.0) | 21.0 (15.0, 30.0) | 21.3 (14.0, 30.0)* |
| ALP, U/L | 90.0 (67.0, 126.0) | 95.0 (69.0, 135.7) | 89.0 (66.0, 125.5) | 87. 0 (67.0, 120.0)* | 90.0 (68.0, 127.0) | 88.0 (66.0, 124.5) | 103.0 (64.0 (135.7) |
| ALB, g/dL | 4.1 (3.8, 4.4) | 4.4 (4.1, 4.8) | 4.1 (3.8, 4.4) | 3.9 (3.5, 4.1)* | 4.1 (3.8, 4.5) | 4.1 (3.8, 4.4) | 4.1 (3.7, 4.4)* |
| BNP a | 227.0 (133.0, 427.5) | 214.0 (132.0, 426.0) | 203.0 (132.0, 424.0) | 244.5 (139.0, 432.8) | 198.0 (121.0, 410.0) | 236.0 (142.0, 433.5) | 242.5 (156.3, 428.8) |
| NT‐pro BNP a | 646.0 (386.0, 1462.0) | 571.5 (191.0, 1492.0) | 680.0 (406.75, 1327.8) | 660.0 (386.0, 1630.0) | 604.0 (284.0, 1354.0) | 715.5 (432.8, 1616.5) | 591.0 (232.0, 1585.0) |
M (IQR) for non‐normally distributed data, M ± SD for normally distributed data, and n (%) for categoric variables. Calculations of FIB‐4 and NFS are described in the Methods section. Calculation of C2HEST can be found in our previous article. 15
ALB, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FIB‐4, Fibrosis‐4 index; MI, myocardial infarction; NFS, non‐alcoholic fatty liver disease fibrosis score; NT‐pro BNP, N‐terminal proBNP; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SBP, systolic blood pressure.
BNP or NT‐pro BNP values were available in 442 or 351 patients, respectively.
P < 0.05.
New‐onset atrial fibrillation during follow‐up
During a median follow‐up of 3.11 years among 6893 person‐years, 106 new‐onset AF cases were detected. The baseline characteristics with and without AF incidence post‐randomization are shown in Table 2 . The patients with AF incidence had a larger mitral regurgitation (MR) jet area, higher left atrial area, higher MR jet area‐to‐left atrial area ratio, lower Peak A wave velocity, and lower left atrial volume index. There were no significant differences in the remaining echocardiographic parameters across the two studied groups (see Supporting Information, Table S3 ).
Table 2.
Baseline characteristics of heart failure with preserved ejection fraction patients with and without incident atrial fibrillation during follow‐up
| Without incident AF | With incident AF | p | |
|---|---|---|---|
| (n = 1951) | (n = 121) | ||
| Demographic | |||
| Age (years) | 66.0 (59.0, 74.0) | 74.0 (65.5, 79.5) | <0.001 * |
| Sex, male | 866 (44.4) | 64 (52.9) | 0.068 |
| Race, White | 1714 (87.9) | 100 (82.6) | 0.092 |
| Current smoking | 254 (13.0) | 7 (5.8) | 0.020 * |
| Ever smoking | 637 (32.6) | 58 (47.9) | 0.001 * |
| Never smoking | 1060 (54.3) | 56 (46.3) | 0.085 |
| BMI (kg/m2) | 30.5 (27.0, 35.3) | 32.7 (27.9, 37.8) | 0.027 * |
| Heart rate (bpm) | 68 (62, 75) | 64 (60, 74) | 0.005 * |
| SBP (mmHg) | 130 (120, 140) | 131 (120, 140) | 0.748 |
| DBP (mmHg) | 80 (70, 85) | 73 (63, 80) | <0.001 * |
| NYHA class (III or IV) | 561 (28.8) | 43 (35.5) | 0.113 |
| Chronic health conditions | |||
| Peripheral vascular disease | 171 (8.8) | 22 (18.2) | 0.001 * |
| COPD | 202 (10.4) | 17 (14.0) | 0.199 |
| Hypertension | 1794 (92.0) | 111 (91.7) | 0.932 |
| Diabetes mellitus | 643 (33.0) | 55 (45.5) | 0.005 * |
| Congestive heart failure | 1451 (74.4) | 75 (62.0) | 0.003 * |
| Previous MI | 542 (27.8) | 3 7(30.6) | 0.506 |
| Previous stroke | 122 (6.3) | 11 (9.1) | 0.217 |
| CABG | 236 (12.1) | 23 (19.0) | 0.026 * |
| PCI | 285 (14.6) | 28 (23.1) | 0.011 * |
| Thyroid disease | 242 (12.4) | 18 (14.9) | 0.426 |
| Dyslipidaemia | 1122 (57.5) | 84 (69.4) | 0.010 * |
| C2HEST score | 2.0 (1.0, 2.0) | 3.0 (1.0,3.0) | <0.001 * |
| Measurements | |||
| eGFR (mL/min × 1.73 m2) | 67.3 (55.7, 80.9) | 62.5 (51.4, 75.7) | 0.013 * |
| White blood cell count (k/μL) | 6.7 (5.6, 7.9) | 6.9 (5.9, 8.5) | 0.053 |
| Haemoglobin (g/dL) | 13.2 (12.2, 14.3) | 13.1 (12.1, 14.1) | 0.601 |
| Platelet (k/μL) | 228.0 (197.0,265.0) | 215.0 (178.5,263.0) | 0.053 |
| AST (U/L) | 23.0 (18.0, 29.0) | 22.0 (17.0, 27.0) | 0.141 |
| ALT (U/L) | 22.0 (15.8, 31.0) | 20.0 (16.0, 27.0) | 0.263 |
| ALP (U/L) | 90.0 (67.0, 126.0) | 92.0 (71.0, 139.5) | 0.323 |
| ALB (g/dL) | 4.1 (3.8, 4.5) | 4.0 (3.7, 4.3) | 0.053 |
M (IQR) for non‐normally distributed data, M ± SD for normally distributed data, and n (%) for categoric variables.
AF, atrial fibrillation; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention; NYHA, New York Heart Association; SBP, systolic blood pressure.
P < 0.05.
The risk of AF incidence gradually increased across the risk of liver fibrosis score strata. According to NFS, AF incidence per 100 person‐years ranged from 0.58 at scores <−1.455 to 2.25 at scores >0.676. The rate of person‐years across the FIB‐4 group was higher, ranging from 1.33 per 100 person‐years at a FIB‐4 of <1.45 points to 2.55 per 100 person‐years at a score of >3.25.
Association between NFS and FIB‐4 levels and new‐onset atrial fibrillation in heart failure with preserved ejection fraction
Crude associations of the individual components of the NFS and FIB‐4 with new‐onset AF incidence are presented in Table 3 . Diabetes, age, ALT, and AST were positively associated with AF incidence, and platelets were inversely related to AF incidence, whereas no significant association was observed for ALB and BMI.
Table 3.
The univariate Cox regression analyses between the components of Fibrosis‐4 index and non‐alcoholic fatty liver disease fibrosis score and atrial fibrillation incidence
| HR (95% CI) | |
|---|---|
| Diabetes | 1.91 (1.28, 2.85)* |
| BMI, kg/m2 | 1.024 (0.997, 1.051) |
| Age, 10 years | 1.31 (1.26, 1.37)* |
| ALT, 10 U/L | 0.95 (0.92, 0.98)* |
| AST, 10 U/L | 0.90 (0.87, 0.93)* |
| ALB, g/dL | 1.05 (0.98, 1.11) |
| Platelet, 10/mm3 | 0.987 (0.981, 0.993)* |
ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CI, confidence interval; HR, hazard ratio.
P < 0.05.
The K‐M curve showed that AF incidence significantly increased with increasing NFS scores (P < 0.001); however, it was not significant according to FIB‐4 scores (P = 0.26) (Figure 2 ). In the Cox regression, the AF incidence was elevated with NFS groups (NFS = −1.455–0.676: HR 2.44, 95% CI 1.27–4.68; NFS > 0.676: HR 3.36, 95% CI 1.27–6.80) in all adjusted models, whereas it was not significant according to FIB‐4 (FIB‐4 = 1.45–3.25: HR 1.02, 95% CI 0.67–1.55; FIB‐4 > 3.25: HR 1.69, 95% CI 0.76–3.79) (Table 4 ).
Figure 2.
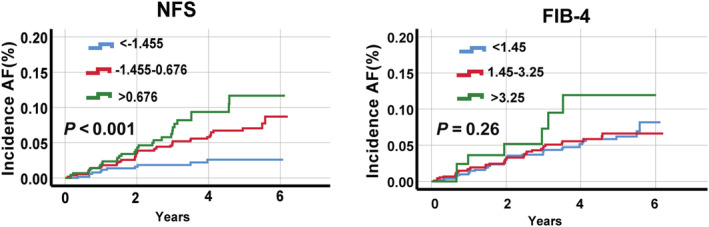
Kaplan–Meier curves for AF incidence across NFS and FIB‐4 groups in patients with heart failure with preserved ejection fraction. NFS, non‐alcoholic fatty liver disease fibrosis score; FIB‐4, Fibrosis‐4 index; AF, atrial fibrillation. Calculations of FIB‐4 and NFS are described in the Methods section.
Table 4.
Unadjusted and adjusted hazard ratio/95% confidence interval of association between liver fibrosis scores and atrial fibrillation incidence
| Fibrosis scores | Cases (%) | Person‐years | Incidence rate, per 100 person‐years | Crude | Model 1 | Model 2 |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| NFS b | ||||||
| Per 1 unit increase | 97 (4.7) | 6893 | 1.41 | 1.20 (1.05, 1.36)* | 1.19 (1.04, 1.36) | 1.15 (1.01, 1.32)* |
| NFS < −1.455 | 11 (2.1) | 1892 | 0.58 | Ref | Ref | Ref |
| NFS = −1.455–0.676 | 56 (5.1) | 3665 | 1.53 | 2.62 (1.37, 5.01)* | 2.61 (1.37, 4.99)* | 2.44 (1.27, 4.68)* |
| NFS > 0.676 | 30 (6.7) | 1336 | 2.25 | 3.80 (1.90, 7.59)* | 3.75 (1.88, 7.49)* | 3.36 (1.27, 6.80)* |
| P for trend | <0.001* | <0.001* | 0.001* | |||
| FIB‐4 a | ||||||
| Per 1 unit increase | 97 (4.7) | 6893 | 1.41 | 1.13 (0.94, 1.36) | 1.12 (0.94, 1.35) | 1.13 (0.93, 1.37) |
| FIB‐4 < 1.45 | 48 (4.5) | 3596 | 1.33 | Ref | Ref | Ref |
| FIB‐4 = 1.45–3.25 | 42 (4.6) | 3023 | 1.39 | 1.04 (0.69, 1.57) | 1.03 (0.68, 1.56) | 1.02 (0.67, 1.55) |
| FIB‐4 > 3.25 | 7 (8.0) | 274 | 2.55 | 1.91 (0.86, 4.21) | 1.86 (0.84, 4.12) | 1.69 (0.76, 3.79) |
| P for trend | 0.314 | 0.344 | 0.433 | |||
Model 1 was adjusted for sex.
Model 2 was adjusted for Model 1 + treatment arm, diabetes mellitus, smoking or ever smoking, body mass index, heart rate, estimated glomerular filtration rate, hypertension, thyroid, coronary artery bypass graft surgery, and percutaneous coronary intervention.
Model 2 was adjusted for Model 1 + treatment arm, smoking or ever smoking, heart rate, estimated glomerular filtration rate, hypertension, thyroid, coronary artery bypass graft surgery, and percutaneous coronary intervention.
P < 0.05.
When NFS and FIB‐4 were analysed as continuous variables, each point increase in the NFS was associated with a 15% increase in AF incidence (HR 1.15, 95% CI 1.01–1.32) after full adjustments; nevertheless, the association was not significant according to FIB‐4 (HR 1.13, 95% CI 0.93–1.37) (Table 4 ).
Discriminatory capacity of non‐alcoholic fatty liver disease fibrosis score and Fibrosis‐4 index
The discriminatory performances of the liver fibrosis scores for predicting incident AF in patients with HFpEF were expressed as the C‐indices. As shown in Figure 3 , NFS (C‐index 0.662, 95% CI 0.608–0.716, sensitivity 81.8%, specificity 48.1%), not FIB‐4 (C‐index 0.531, 95% CI 0.471–0.593, sensitivity 27.1%, specificity 78.2%), had moderate performance in predicting incident AF. The performance of NFS and FIB‐4 after internal validation showed a mean C‐index of 0.660 (IQR 0.604, 0.721) or 0.528 (IQR 0.429, 0.590).
Figure 3.
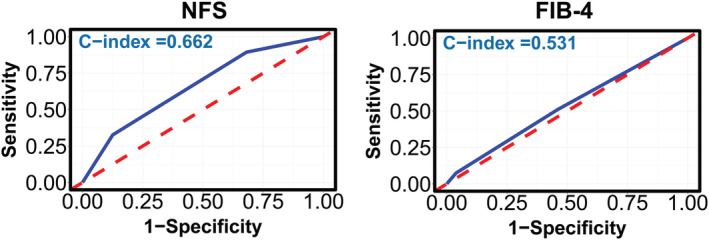
The receiver operating characteristic curves for predicting atrial fibrillation incidence in patients with heart failure with preserved ejection fraction, assessed by NFS and FIB‐4 scores. NFS, non‐alcoholic fatty liver disease fibrosis score; FIB‐4, Fibrosis‐4 index. The C‐statistic of the NFS and FIB‐4 score was calculated as category variables using a crude Cox regression model.
Sensitivity and subgroup analyses
As presented in the Supporting Information, Tables S4 and S5 , the sensitivity analyses confirmed the results by using a competing risk regression model, excluding the Russian/Georgian population and patients who were treated with ACEIs/ARBs and beta‐blockers.
The subgroup analyses for incident AF are described in Supporting Information, Tables S6–S8 . There were no significant interactions between NFS and subgroups based on gender (females vs. males) and region (Americas vs. Russia/Georgia), but an expected interaction for the treatment arm (spironolactone vs. placebo) was found (P = 0.01). According to FIB‐4, there were no interactions in all subgroups (all P > 0.05).
Discussion
Major findings
This post hoc analysis from TOPCAT demonstrated that in patients with HFpEF, an elevated NFS score rather than FIB‐4 was significantly associated with incident AF. The subgroup and sensitivity analyses confirmed results. Moreover, NFS has a moderate predictive ability for predicting AF occurrence. Overall, these findings suggested that among patients with HFpEF, advanced liver fibrosis was associated with increased new‐onset AF incidence and may be a novel predictor of new‐onset AF. To the best of our knowledge, this is the first study to assess the relationship between FIB‐4 or NFS and the incidence of AF in patients with HFpEF.
Heart failure and liver diseases often coexist and independently contribute to poor outcomes. 16 Advanced liver fibrosis is the primary determinant of progression to cirrhosis and liver failure. In a small‐cohort (N = 183) study by Miller et al. 5 including baseline AF, the prevalence of advanced liver fibrosis reached 48.6% as assessed by NFS (>0.676). As previously reported, the prevalence of advanced liver fibrosis was 37.65% in TOPCAT Americans, including baseline AF. In our study, advanced liver fibrosis was 21.5% in the overall population and increased to 29.3% in Americans. The results from Peters et al. 4 also showed that patients with higher NFS or FIB‐4 scores were more likely to have AF at baseline. These results suggest that HFpEF patients with AF probably have a greater incidence of hepatic fibrosis.
Recent evidence showed that liver fibrosis scores were significant predictors of cardiovascular events in HFpEF. 17 , 18 , 19 However, in the context of FIB‐4, our results seemed inconsistent with previous studies. Our results showed that the association between FIB‐4 and incident AF among patients with HFpEF was not significant. Notably, the diagnostic performance of NFS and FIB‐4 for liver fibrosis was comparable in patients with NAFLD. 20 , 21 However, the presence of advanced liver fibrosis assessed by FIB‐4 (4.2%) was much lower than that assessed by NFS (21.5%) in the present study. This inconsistency was interesting. To date, neither NFS nor FIB‐4 has been validated in HFpEF patients. However, a recent study by Miller et al. 5 suggested that compared with FIB‐4, NFS might be a more sensitive indicator in liver fibrosis in HFpEF, which might explain this inconsistency. Another important point to discuss here is the ability of liver fibrosis scores to exclude advanced fibrosis rather than detect significant/advanced fibrosis. 22 , 23 That is, FIB‐4 or NFS scores have a higher negative predictive value but lower positive predictive value. In light of this evidence, the presence of advanced fibrosis in many patients might be underestimated, including HFpEF. More data with other measures of imaging or biopsy are needed to assess the prevalence and prognosis of liver fibrosis in HFpEF.
NFS or FIB‐4 has been shown to predict death in the general population and clinical outcomes in patients without liver‐related diseases, including heart failure. 4 , 19 , 24 , 25 We showed that NFS has a moderate predictive ability for AF incidence in HFpEF. However, it should be emphasized that the aim of this study was not to elaborate a predictive model for new‐onset AF but to point out the role of advanced liver fibrosis on AF in HFpEF.
Our results supported the emerging concept of phenogroups of patients with HFpEF with distinct clinical characteristics and long‐term outcomes. A phenomapping analysis of the TOPCAT study identified a phenogroup that demonstrated a high prevalence of obesity, diabetes, chronic kidney disease, elevated renin levels, high pro‐inflammatory biomarkers, concentric left ventricular (LV) hypertrophy, and liver fibrosis. 26 Consistently, Salah et al. also proposed the novel term ‘advanced liver disease/cirrhosis HFpEF’, 16 representing the disease trajectory among HFpEF. Heart failure and AF commonly coexist, and patients with AF have worse outcomes. 27 Based on our results, we also supposed an increased incidence of AF with advanced liver fibrosis, which reinforced the concept of ‘advanced liver disease/cirrhosis HFpEF’. 16 The expected interaction of NFS for the response to spironolactone randomized therapy can also be mirrored by the heterogeneity in the clinical profiles among HFpEF patients.
Potential mechanisms
The mechanisms underlying the relationship between advanced liver fibrosis and incident AF in patients with HFpEF remain incompletely elucidated. Cardiac autonomic dysfunction has been suggested as a possible mechanism. Several studies have demonstrated that a dysfunctional autonomic nervous system could play a significant role in the initiation and perpetuation of AF. 28 , 29 NAFLD and advanced liver disease have proven to be independent risk factors for autonomic dysfunction. 30 In addition, advanced liver fibrosis can also lead to increased levels of neuropeptides that have been shown to be associated with the development of AF. 31 Moreover, vagal‐mediated AF is thought to be caused by vasoactive intestinal peptide. 32 , 33 Another potential mechanism behind the elevated risk of AF in the setting of advanced liver fibrosis is the abnormal pathway of fibrosis. Systemic fibrosis, fibrogenesis, and collagen conversion may be a possible link between HFpEF and NAFLD. 34 , 35 , 36 Elevated levels of galectin‐3 were observed in patients with liver disease. 37 , 38 Galectin‐3 is implicated in fibrosis of both the liver and heart and is associated with an increased incidence of AF. 39 , 40 Transforming growth factor beta 41 and connective tissue growth factor 42 may also be potential mediators that share the common pathway of fibrosis. Therefore, we assumed that in HFpEF patients, higher liver fibrosis scores may be indicators of higher central venous pressure with resultant liver fibrosis and impaired liver functional reserve and partly reflected by cardiac fibrosis, resulting in a higher incidence of AF.
Comparison with previous studies
Several studies showed that liver fibrosis scores were associated with worse outcomes in HFpEF. 17 , 18 , 19 In addition, a prospective study of 492 patients with HFpEF reported that NFS was associated with atrial pressure and left atrial volume and the risk of all‐cause mortality. 3 Our study reinforced these results, further showing that elevated NFS was significantly associated with incident AF and had a moderate predictive ability in predicting incident AF among patients with HFpEF.
Clinical implications
HFpEF is a heterogeneous disease that contains considerable phenotypic diversity and has no obvious effective treatment. 43 , 44 Considering that AF is very common in HFpEF and contributes to a poor prognosis, it is crucial for clinicians to develop strategies to reduce the incidence of AF in HFpEF. Several risk scoring models for predicting incident AF have been established 15 , 45 ; however, they lack excellent predictive performance in HFpEF. 15 Our findings provide important information, and liver fibrosis might be a new component in risk scores for AF in HFpEF.
Our findings suggest that advanced liver fibrosis was associated with increased new‐onset AF incidence and may be a predictor for new‐onset AF in patients with HFpEF. However, our study should not be interpreted as emphasizing the importance of liver biopsy in predicting the outcomes in HFpEF. Clinicians could incorporate liver fibrosis score assessment into their AF risk scoring models to prevent incident AF and predict the progression of HFpEF. On the other hand, physicians should be vigilant of the changes in transaminase and platelets, which might indicate changes in liver fibrosis, especially when using medications that affect liver function, such as statins, amiodarone, and warfarin.
Strength and limitations
Previous reports demonstrated that advanced liver fibrosis was associated with poor prognosis in patients with heart failure. 3 , 18 , 19 , 46 Our analysis extended these studies and has several important strengths. This is the first study to explore the association between liver fibrosis scores and incident AF in patients with HFpEF.
The present study has several limitations. First, it should be emphasized that the liver fibrosis level assessed in the present study is based on scoring systems. Although the diagnostic accuracy of FIB‐4 and NFS has been tested in patients with certain liver diseases, such as NAFLD or hepatitis B virus infection, our results might be explained as exploratory before the diagnostic performance of NFS or FIB‐4 in liver fibrosis in heart failure is well validated. Second, this is a retrospective study, and we cannot infer direct causality. Measured and unmeasured confounding may influence our results. Third, our population was based on a clinical trial, and most patients were White, which may not be completely representative of real‐world patients; thus, it may limit the generalizability of our findings. Fourth, we evaluated the NFS and FIB‐4 at baseline; changes in the NFS and FIB‐4 during hospitalization or after discharge remain unclear. Lastly, the current evidence suggests that FIB‐4 and NFS do not perform accurately in some populations (e.g. age<35 years, morbidly obese adults), and further studies are needed to clarify the role of these scores in HFpEF.
Conclusion
Among patients with HFpEF, advanced liver fibrosis is associated with increased new‐onset AF incidence and may be a novel predictor of new‐onset AF. Additional studies are needed to confirm our results.
Conflict of interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (J.F.‐W., 82070237; Y.L.‐Z., 81870170; Y.X.‐C., 81970200 and 81770229; and X.L., 82100347), Natural Science Foundation of Guangdong Province (Y.L.‐Z., 2019A1515011682), and National High Technology Research and Development Program of Guangzhou (J.F.‐W., 20180304001 and 2019GZR110406004; and Y.L‐Z, 201704020044). All of the funding agencies had no role in the design, methods, subject recruitment, data collections, analysis, and preparation of the paper.
Supporting information
Table S1. Baseline echocardiographic characteristics stratified by NFS groups.
Table S2. Baseline echocardiographic characteristics stratified by Fibrosis‐4.
Table S3. Baseline echocardiographic characteristics stratified by AF incident post‐randomization.
Table S4. The Fibrosis scores and the risk of incident AF in the competing risk regression model.
Table S5. Association between Fibrosis scores and Atrial fibrillation in patients without concomitant use of ACE inhibitors or ARBs, or without concomitant use of any β‐blocker, NFS and FIB‐4 were analyzed as continuous variables.
Table S6. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in Americas cohort and non‐ Americas cohort, analyzed as continuous variables.
Table S7. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in male and female, analyzed as continuous variables.
Table S8. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in patients using spironolactone or placebo, analyzed as continuous variables.
Liu, X. , Chen, W. , Shao, W. , Jiang, Y. , Cao, Z. , He, W. , Wu, M. , Chen, Z. , Ma, J. , Chen, Y. , Yu, P. , Zhang, Y. , and Wang, J. (2022) Liver fibrosis scores and atrial fibrillation incidence in heart failure with preserved ejection fraction. ESC Heart Failure, 9: 3985–3994. 10.1002/ehf2.14087.
Xiao Liu, Wenya Chen, and Wen Shao are co‐first authors.
Contributor Information
Yuling Zhang, Email: zzhangyuling@126.com.
Jingfeng Wang, Email: wjingf@mail.sysu.edu.cn.
References
- 1. Itier R, Guillaume M, Ricci JE, Roubille F, Delarche N, Picard F, Galinier M, Roncalli J. Non‐alcoholic fatty liver disease and heart failure with preserved ejection fraction: from pathophysiology to practical issues. ESC Heart Fail. 2021; 8: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dhall D, Kim SA, Mc Phaul C, Kransdorf EP, Kobashigawa JA, Sundaram V, Mirocha J, Guindi M. Heterogeneity of fibrosis in liver biopsies of patients with heart failure undergoing heart transplant evaluation. Am J Surg Pathol. 2018; 42: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 3. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail. 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peters AE, Pandey A, Ayers C, Wegermann K, McGarrah RW, Grodin JL, Abdelmalek MF, Bekfani T, Blumer V, Diehl AM, Moylan CA, Fudim M. Association of liver fibrosis risk scores with clinical outcomes in patients with heart failure with preserved ejection fraction: findings from TOPCAT. ESC Heart Fail. 2021; 8: 842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miller A, McNamara J, Hummel SL, Konerman MC, Tincopa MA. Prevalence and staging of non‐alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. Sci. Rep. 2020; 10: 12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konerman MA, McNamara J, Hummel SL, Konerman MC. Prevalence of and characteristics associated with non‐alcoholic fatty liver disease among patients with heart failure with preserved ejection fraction. J Card Fail. 2018; 24: S51. [Google Scholar]
- 7. Ucar F, Sezer S, Ginis Z, Ozturk G, Albayrak A, Basar O, Ekiz F, Coban S, Yuksel O, Armutcu F, Akbal E. APRI, the FIB‐4 score, and Forn's index have noninvasive diagnostic value for liver fibrosis in patients with chronic hepatitis B. Eur. J. Gastroenterol. Hepatol. 2013; 25: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 8. Petta S, Vanni E, Bugianesi E, di Marco V, Cammà C, Cabibi D, Mezzabotta L, Craxì A. The combination of liver stiffness measurement and NAFLD fibrosis score improves the noninvasive diagnostic accuracy for severe liver fibrosis in patients with nonalcoholic fatty liver disease. Liver Int. 2015; 35: 1566–1573. [DOI] [PubMed] [Google Scholar]
- 9. Anstee QM, Lawitz EJ, Alkhouri N, Wong VWS, Romero‐Gomez M, Okanoue T, Trauner M, Kersey K, Li G, Han L, Jia C, Wang L, Chen G, Subramanian GM, Myers RP, Djedjos CS, Kohli A, Bzowej N, Younes Z, Sarin S, Shiffman ML, Harrison SA, Afdhal NH, Goodman Z, Younossi ZM. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019; 70: 1521–1530. [DOI] [PubMed] [Google Scholar]
- 10. Ostovaneh MR, Ambale‐Venkatesh B, Fuji T, Bakhshi H, Shah R, Murthy VL, Tracy RP, Guallar E, Wu CO, Bluemke DA, Lima JAC. Association of liver fibrosis with cardiovascular diseases in the general population: the Multi‐Ethnic Study of Atherosclerosis (MESA). Circ. Cardiovasc. Imaging. 2018; 11: e007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. So‐Armah KA, Lim JK, Lo Re V, Tate JP, Chang CCH, Butt AA, Gibert CL, Rimland D, Marconi VC, Goetz MB, Rodriguez‐Barradas MC, Budoff MJ, Tindle HA, Samet JH, Justice AC, Freiberg MS, for the Veterans Aging Cohort Study Project Team . FIB‐4 stage of liver fibrosis predicts incident heart failure among HIV‐infected and uninfected patients. Hepatology. 2017; 66: 1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cai X, Zheng S, Liu Y, Zhang Y, Lu J, Huang Y. Nonalcoholic fatty liver disease is associated with increased risk of atrial fibrillation. Liver Int. 2020; 40: 1594–1600. [DOI] [PubMed] [Google Scholar]
- 13. Park HE, Lee H, Choi SY, Kim HS, Chung GE. The risk of atrial fibrillation in patients with non‐alcoholic fatty liver disease and a high hepatic fibrosis index. Sci Rep. 2020; 10: 5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 15. Liang W, Wu Y, Xue R, Wu Z, Wu D, He J, Dong Y, Lip GYH, Zhu W, Liu C. C(2)HEST score predicts clinical outcomes in heart failure with preserved ejection fraction: a secondary analysis of the TOPCAT trial. BMC Med. 2021; 19: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salah HM, Pandey A, Soloveva A, Abdelmalek MF, Diehl AM, Moylan CA, Wegermann K, Rao VN, Hernandez AF, Tedford RJ, Parikh KS, Mentz RJ, McGarrah RW, Fudim M. Relationship of nonalcoholic fatty liver disease and heart failure with preserved ejection fraction. JACC Basic Transl Sci. 2021; 6: 918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. So‐Armah KA, Lim JK, Lo Re V 3rd, Tate JP, Chang CCH, Butt AA, Gibert CL, Rimland D, Marconi VC, Bidwell Goetz M, Ramachandran V, Brittain E, Long M, Nguyen KL, Rodriguez‐Barradas MC, Budoff MJ, Tindle HA, Samet JH, Justice AC, Freibergk MS, VACS Project Team . FIB‐4 stage of liver fibrosis is associated with incident heart failure with preserved, but not reduced, ejection fraction among people with and without HIV or hepatitis C. Prog Cardiovasc Dis. 2020; 63: 184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takae M, Fujisue K, Yamamoto E, Egashira K, Komorita T, Oike F, Nishihara T, Yamamoto M, Hirakawa K, Tabata N, Tokitsu T, Yamanaga K, Sueta D, Hanatani S, Nakamura T, Usuku H, Araki S, Arima Y, Takashio S, Suzuki S, Kaikita K, Matsushita K, Tsujita K. Prognostic significance of liver stiffness assessed by fibrosis‐4 index in patients with heart failure. ESC Heart Fail. 2021; 8: 3809–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato Y, Yoshihisa A, Kanno Y, Watanabe S, Yokokawa T, Abe S, Misaka T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver stiffness assessed by Fibrosis‐4 index predicts mortality in patients with heart failure. Open Heart. 2017; 4: e000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park H, Yoon EL, Kim M, Lee J, Kim JH, Cho S, Won Jun D, Nah EH. Comparison of diagnostic performance between FIB‐4 and NFS in metabolic‐associated fatty liver disease era. Hepatol. Res. 2021; 75: 725–726. [DOI] [PubMed] [Google Scholar]
- 21. Lee J, Vali Y, Boursier J, Spijker R, Anstee QM, Bossuyt PM, Zafarmand MH. Prognostic accuracy of FIB‐4, NAFLD fibrosis score and APRI for NAFLD‐related events: a systematic review. Liver Int. 2021; 41: 261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaya E, Bakir A, Kani HT, Demirtas CO, Keklikkiran C, Yilmaz Y. Simple noninvasive scores are clinically useful to exclude, not predict, advanced fibrosis: a study in Turkish patients with biopsy‐proven nonalcoholic fatty liver disease. Gut Liver. 2020; 14: 486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non‐invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J. Hepatol. 2007; 46: 775–782. [DOI] [PubMed] [Google Scholar]
- 24. Unalp‐Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatology. 2017; 66: 84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parikh NS, Kamel H, Navi BB, Iadecola C, Merkler AE, Jesudian A, Dawson J, Falcone GJ, Sheth KN, Roh DJ, Elkind MSV, Hanley DF, Ziai WC, Murthy SB, Hanley DF, Butcher K, Davis SM, Gregson B, Lees KR, Lyden PD, Mayer SA, Muir K, Steiner T. Liver fibrosis indices and outcomes after primary intracerebral hemorrhage. Stroke. 2020; 51: 830–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail. 2020; 8: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016; 68: 2217–2228. [DOI] [PubMed] [Google Scholar]
- 28. Huang WA, Dunipace EA, Sorg JM, Vaseghi M. Liver disease as a predictor of new‐onset atrial fibrillation. J Am Heart Assoc. 2018; 7: e008703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014; 114: 1004–1021. [DOI] [PubMed] [Google Scholar]
- 30. Liu YC, Hung CS, Wu YW, Lee YC, Lin YH, Lin C, Lo MT, Chan CC, Ma HP, Ho YL, Chen CH. Influence of non‐alcoholic fatty liver disease on autonomic changes evaluated by the time domain, frequency domain, and symbolic dynamics of heart rate variability. PLoS ONE. 2013; 8: e61803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Y, Scherlag BJ, Fan Y, Varma V, Male S, Chaudhry MA, Huang C, Po SS. Inducibility of atrial fibrillation after GP ablations and “autonomic blockade”: evidence for the pathophysiological role of the nonadrenergic and noncholinergic neurotransmitters. J Cardiovasc Electrophysiol. 2013; 24: 188–195. [DOI] [PubMed] [Google Scholar]
- 32. Xi Y, James Chao ZY, Yan W, Abbasi S, Yin X, Mathuria N, Patel M, Fan C, Sun J, Wu G, Wang S, Elayda MA, Gao L, Wehrens XHT, Lin SF, Cheng J. Neuronally released vasoactive intestinal polypeptide alters atrial electrophysiological properties and may promote atrial fibrillation. Heart Rhythm. 2015; 12: 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xi Y, Wu G, Ai T, Cheng N, Kalisnik JM, Sun J, Abbasi S, Yang D, Fan C, Yuan X, Wang S, Elayda MA, Gregoric ID, Kantharia BK, Lin SF, Cheng J. Ionic mechanisms underlying the effects of vasoactive intestinal polypeptide on canine atrial myocardium. Circ Arrhythm Electrophysiol. 2013; 6: 976–983. [DOI] [PubMed] [Google Scholar]
- 34. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med. 2011; 43: 617–649. [DOI] [PubMed] [Google Scholar]
- 35. Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123: 1705–1725. [DOI] [PubMed] [Google Scholar]
- 36. Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast‐mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013; 10: 15–26. [DOI] [PubMed] [Google Scholar]
- 37. Gudowska M, Gruszewska E, Cylwik B, Panasiuk A, Rogalska M, Flisiak R, Szmitkowski M, Chrostek L. Galectin‐3 concentration in liver diseases. Ann Clin Lab Sci. 2015; 45: 669–673. [PubMed] [Google Scholar]
- 38. Hsu DK, Dowling CA, Jeng KC, Chen JT, Yang RY, Liu FT. Galectin‐3 expression is induced in cirrhotic liver and hepatocellular carcinoma. Int J Cancer. 1999; 81: 519–526. [DOI] [PubMed] [Google Scholar]
- 39. Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, McManus DD, Lubitz SA, Larson MG, Benjamin EJ. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014; 167: 729–34.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lippi G, Cervellin G, Sanchis‐Gomar F. Galectin‐3 in atrial fibrillation: Simple bystander, player or both? Clin Biochem. 2015; 48: 818–822. [DOI] [PubMed] [Google Scholar]
- 41. Xu F, Liu C, Zhou D, Zhang L. TGF‐β/SMAD pathway and its regulation in hepatic fibrosis. J Histochem Cytochem. 2016; 64: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008; 28: 1065–1079. [DOI] [PubMed] [Google Scholar]
- 43. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O’Connor CM, Lam CSP. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015; 65: 1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Himmelreich JCL, Veelers L, Lucassen WAM, Schnabel RB, Rienstra M, van Weert HCPM, Harskamp RE. Prediction models for atrial fibrillation applicable in the community: a systematic review and meta‐analysis. Europace. 2020; 22: 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi T, Watanabe T, Shishido T, Watanabe K, Sugai T, Toshima T, Kinoshita D, Yokoyama M, Tamura H, Nishiyama S, Arimoto T, Takahashi H, Yamanaka T, Miyamoto T, Kubota I. The impact of non‐alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels. 2018; 33: 733–739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline echocardiographic characteristics stratified by NFS groups.
Table S2. Baseline echocardiographic characteristics stratified by Fibrosis‐4.
Table S3. Baseline echocardiographic characteristics stratified by AF incident post‐randomization.
Table S4. The Fibrosis scores and the risk of incident AF in the competing risk regression model.
Table S5. Association between Fibrosis scores and Atrial fibrillation in patients without concomitant use of ACE inhibitors or ARBs, or without concomitant use of any β‐blocker, NFS and FIB‐4 were analyzed as continuous variables.
Table S6. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in Americas cohort and non‐ Americas cohort, analyzed as continuous variables.
Table S7. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in male and female, analyzed as continuous variables.
Table S8. Unadjusted and adjusted hazard ratios/95% confidence intervals describing association between Fibrosis scores and Atrial fibrillation in patients using spironolactone or placebo, analyzed as continuous variables.


