Abstract
Aims
Anti‐mitochondrial antibody (AMA)‐positive myositis is frequently associated with various cardiac involvements, such as arrhythmia and left ventricular (LV) dysfunction. However, the efficacy of immunosuppressive therapy in these complications remains unknown. This study aimed to investigate the cardiac response to immunosuppressive therapy in patients with AMA‐positive myositis.
Methods and results
The clinical data of 15 AMA‐positive myositis patients with cardiac involvement were retrospectively collected at our centre. To evaluate the effects of immunosuppressive therapy, echocardiographic and laboratory data of patients who received glucocorticoid therapy with additional immunosuppressants (n = 6) and those who did not (n = 6) were compared. Also, the characteristics of patients with or without >5% LV ejection fraction (LVEF) decline during the follow‐up period (n = 5 vs. n = 7) were compared. Thirteen patients (87%) had arrhythmias, and eight patients (53%) had LV wall motion abnormalities. Although arrhythmias decreased after treatment, reduced LVEF and LV wall motion abnormalities persisted. Further investigation revealed an increased LV end‐systolic dimension and reduced LVEF in patients without additional immunosuppressive therapy, while those in patients with additional immunosuppressive therapy were maintained. Six of seven patients (86%) without LVEF decline received additional immunosuppressive therapy, whereas no patients with LVEF decline had additional immunosuppressive therapy.
Conclusions
Cardiac involvement in AMA‐positive myositis may worsen even with glucocorticoid monotherapy, and there might be some associations between the change of LV function and additional immunosuppressive therapy.
Keywords: Anti‐mitochondrial antibody, Myositis, Cardiac involvement, Immunosuppressive therapy
Introduction
Inflammation is a major cause of myocardial damage. 1 Although sarcoidosis is a representative myocardial disease caused by inflammation, few other diseases, including autoimmune diseases, have been widely and concisely investigated. 2 , 3 Among them, myocardial complications in the setting of anti‐mitochondrial antibody (AMA)‐positive myositis are important and may cause various cardiovascular complications, such as myocarditis and arrhythmia, as the main symptoms. 4 Indeed, there are some cases in which diagnosis may be achieved from cardiac complications. However, there are limited reports on concomitant cardiac involvement in patients with AMA‐positive myositis.
The typical treatment for AMA‐positive myositis includes glucocorticoids, with or without other immunosuppressive therapies, similar to other myositis. 5 , 6 However, there are no comprehensive reports on the effectiveness of these treatments for cardiovascular complications.
The purpose of this study was to summarize the myocardial complications associated with AMA‐positive myositis experienced at a single centre, focusing on left ventricular systolic function, and to analyse the responsiveness of cardiac complications to immunosuppressive treatments.
Methods
Patients
Clinical data were collected retrospectively from patients suffering from cardiac dysfunction and with AMA‐positive myositis diagnosed by the Department of Neurology at the University of Tokyo from January 2000 to July 2021. The diagnosis of inflammatory myositis was based on the criteria proposed by 2017 EULAR/ACR classification criteria (probable or definite). 7 Serum AMA‐M2 levels were measured by MESACUP‐2 Test Mitochondria M2 kit (Medical and Biological Laboratories) or Elia M2 Well (Thermo Fisher Scientific). Following previous reports, 4 , 8 , 9 , 10 we included patients who were positive for these tests in this study and called them ‘AMA‐positive myositis’. Cardiac involvement was defined as one or more of the following: arrhythmia, reduced left ventricular ejection fraction (LVEF) < 50%, and LV wall motion abnormality. Arrhythmias included supraventricular arrhythmia (premature atrial contraction, atrial fibrillation, atrial flutter, atrial tachycardia, and paroxysmal supraventricular tachycardia), ventricular arrhythmia (premature ventricular contraction and non‐sustained or sustained ventricular tachycardia), atrioventricular block (1st, 2nd, and complete atrioventricular block), and sick sinus syndrome. These arrhythmias were detected by 12‐lead or 24‐h Holter electrocardiogram (ECG). LVEF and LV wall motion abnormality were measured by echocardiography. All patients underwent systematic screening for cardiomyopathies. In particular, coronary angiography ruled out ischaemic cardiomyopathy in patients with reduced LVEF <50%. The study protocol conformed to the tenets of the Declaration of Helsinki and was reviewed and approved by the University of Tokyo Institutional Review Board (approval number: 2650).
Evaluation
The follow‐up of patients and their clinical data ended in September 2021. Symptoms of suspected myopathy, heart failure, arrhythmia, and myocarditis (muscle weakness, muscle pain, dyspnoea, oedema, palpitations, and chest pain) were defined as initial symptoms. Disease duration before the diagnosis of AMA‐positive myositis was defined as the time from the occurrence of initial symptoms.
We compared the echocardiographic and laboratory data of the patients who did and did not receive additional immunosuppressive therapy with prednisolone (PSL) (Figure 1 ). Patients underwent two‐dimensional and Doppler echocardiography by experienced operators in accordance with the guidelines of the American Society of Echocardiography. 11 LVEF was calculated by Teichholz's formula. Baseline data (‘Baseline’ time point), was collected at the time of AMA‐positive myositis diagnosis and compared with the latest data before the end of follow‐up (‘End’ time point). The change in LVEF (delta LVEF) was calculated as follows: (LVEF at ‘End’) – (LVEF at ‘Baseline’). Additionally, we investigated the characteristics of patients whose LVEF declined >5% from the baseline data during the follow‐up period.
Figure 1.

Flowchart of study patients. AMA, anti‐mitochondrial antibody; PSL, prednisolone.
Treatments
The treatment is based on the protocol for polymyositis and dermatomyositis. Myositis was initially treated with glucocorticoids with the dose of 0.5–1.0 mg/kg/day. 12 Additional immunosuppressants, such as azathioprine (AZA), methotrexate (MTX), mycophenolate mofetil (MMF) and tacrolimus (TAC), were subsequently used to achieve clinical remission and glucocorticoid sparing based on the evidence of better efficacy in early combination with glucocorticoids. 5 , 6 , 13 , 14 , 15 Intravenous immunoglobulin (IVIG) was considered in patients who were refractory or contraindicated with glucocorticoids, with or without additional immunosuppressive therapy. 16 , 17
Cardiovascular complications were treated with medications following the general treatment guidelines for each arrhythmia and heart failure with reduced ejection fraction. Device therapies, such as pacemakers, were also performed following the appropriate guidelines for cardiac complications.
Statistics
Data are presented as median (interquartile range, IQR) or number (percentage). Statistical analyses were performed using GraphPad Prism software (version 9.2.0; GraphPad Software, San Diego, CA, USA). The Mann–Whitney U test for continuous variables and the Fisher's exact test for categorical variables were performed to compare the two groups. A Wilcoxon matched‐pair signed‐rank test was conducted to compare the ‘Baseline’ and the ‘End’ data to evaluate the treatments. The threshold for statistical significance was set at P < 0.05.
Results
Patient characteristics
Fifteen AMA‐positive myositis patients with cardiac involvement were identified for the study. The clinical characteristics of the patients are shown in Table 1 . Of the 15 patients, 12 (80%) were female, and the median age at diagnosis of AMA‐positive myositis was 59 years (IQR 51–71). The median disease duration before diagnosis was 61 months (IQR 32–92) and the median follow‐up duration after diagnosis was 51 months (IQR 9–96). Nine patients (60%) exhibited dyspnoea, which was the most common initial symptom. Thirteen patients (87%) visited the cardiovascular department after their initial symptoms regardless of the presence or absence of muscle weakness. In addition, 11 patients (73%) developed cardiovascular diseases (CVDs) preceding AMA‐positive myositis diagnosis. All patients had ECG abnormalities, and a significant proportion (13/15) had arrhythmias. Nine patients (60%) showed echocardiographic abnormalities. Among them, eight patients had left ventricular wall motion abnormalities. Seven patients (47%) underwent right ventricular endomyocardial biopsy (EMB). There was no sign of active myocarditis, but four patients showed interstitial fibrosis. Regarding laboratory results, the median creatine kinase (CK) was 628 U/L (IQR 256–1077), and the median CK‐MB level was 15 U/L (IQR 8–23). The median brain natriuretic peptide (BNP) level was 114 pg/mL (IQR 74–323).
Table 1.
Clinical characteristics of 15 AMA‐positive myositis associated with cardiac involvement
| Characteristics | n = 15 |
|---|---|
| Sex ratio, M/F | 3/12 |
| Age at diagnosis, years, median (IQR) | 59 (51–71) |
| Disease duration before diagnosis, months, median (IQR) | 61 (32–92) |
| CVD preceding myositis diagnosis, n (%) | 11 (73) |
| Initial visit to cardiovascular department, n (%) | 13 (87) |
| Follow‐up period, months, median (IQR) | 51 (9–96) |
| Cardiac involvement, n (%) | |
| Abnormal ECG | 15 (100) |
| Arrhythmia | 13 (87) |
| LV wall motion abnormality | 8 (53) |
| Initial symptoms, n (%) | |
| No symptom | 1 (7) |
| Dyspnoea | 9 (60) |
| Muscle weakness | 4 (27) |
| Muscle pain | 1 (7) |
| Palpitations | 5 (33) |
| Chest pain | 2 (13) |
| Oedema | 0 |
| Baseline laboratory data, median (IQR) | |
| CK, U/L | 628 (256–1077) |
| CK‐MB, U/L | 15 (8–23) |
| BNP, pg/mL | 114 (74–323) |
Abbreviations: AMA, anti‐mitochondrial antibody; BNP, brain natriuretic peptide; CK, creatine kinase; CVD, cardiovascular disease; ECG, electrocardiogram; IQR, interquartile range; LV, left ventricular.
Detailed information on the treatments is summarized in Table 2 . Treatments revealed that 12 patients (80%) underwent PSL therapy and eight of them received additional immunosuppressive therapy. The initial median dose of PSL was 35 (IQR 30–53) mg/day, and 5/12 (42%) patients received concomitant immunosuppressive drugs for induction therapy. Also, six patients (40%) were treated with IVIG. On the other hand, 11 patients (73%) had already received cardiac drug therapy at the time of AMA‐positive myositis diagnosis, 10 patients continued their medication until their last visit, and one patient became drug‐free owing to no recurrence of arrhythmia.
Table 2.
Treatments and results of cardiac investigations at baseline and last visit
| Baseline | Last visit | |
|---|---|---|
| Treatment, n (%) | n = 15 | n = 15 |
| No cardiac drug therapy | 4 (27) | 5 (33) |
| Beta‐blockers | 8 (53) | 9 (60) |
| ACE inhibitors/ARBs | 7 (47) | 8 (53) |
| MRAs | 2 (13) | 3 (20) |
| Inodilators | 1 (7) | 2 (13) |
| No immunosuppressive therapy | 3 (20) | |
| Prednisolone ≤10 mg/day | 6 (40) | |
| Prednisolone >10 mg/day | 6 (40) | |
| Additional immunosuppressive therapy | 8 (53) | |
| IVIG | 6 (40) | |
| Electrocardiography, n (%) | n = 15 | n = 14 |
| Normal | 0 | 0 |
| Bundle branch block | 4 (27) | 4 (29) |
| ST‐T wave changes | 3 (20) | 2 (14) |
| Abnormal Q waves | 1 (7) | 1 (7) |
| Supraventricular arrhythmia | 9 (60) | 5 (36) |
| Ventricular arrhythmia | 10 (67) | 3 (21) |
| Sick sinus syndrome | 1 (7) | 0 |
| Atrioventricular block | 2 (13) | 0 |
| Pacemaker rhythm | 0 | 5 (36) |
| Echocardiography, n (%) | n = 14 | n = 12 |
| Normal | 5 (36) | 2 (17) |
| LVEF <50% | 7 (50) | 7 (58) |
| Global LV wall motion abnormality | 8 (57) | 7 (58) |
| Regional LV wall motion abnormality | 4 (29) | 7 (58) |
| Right ventricular endomyocardial biopsy, n (%) | n = 7 | |
| Non‐specific finding | 3 (43) | |
| Fibrosis | 4 (57) | |
| Cardiac MRI, n (%) | n = 6 | n = 2 |
| Normal | 2 (33) | 0 |
| T2 high‐intensity areas | 3 (50) | 1 (50) |
| Late gadolinium enhancement | 4 (67) | 2 (100) |
Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; IVIG, intravenous immunoglobulin; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; MRI, magnetic resonance imaging.
Cardiac complications and clinical events
Eight patients (53%) required hospitalization after initial treatment, in which half of the cause was attributed to cardiac involvement, such as heart failure and arrhythmia. Also, six patients (40%) underwent cardiac device implantation (cardiac resynchronization therapy, n = 1; implantable cardioverter defibrillator, n = 3; left ventricular assist device, n = 1; pacemaker, n = 1). Among patients who underwent ECG and echocardiography (n = 12–15), the complications of supraventricular and ventricular arrhythmias at the baseline visit decreased at the last visit (60% to 36%, 67% to 21%, respectively). However, reduced LVEF and global LV wall motion abnormality remained at a high rate (50% to 58%, 57% to 58%, respectively). Moreover, regional wall motion abnormalities observed in 4/14 (29%) patients increased to 7/12 (58%) patients (apex, 11%; inferior wall, 33%; intraventricular septum, 56%). Few patients underwent cardiac magnetic resonance imaging (cMRI); however, more than half of them had T2 high‐intensity areas and late gadolinium enhancement, which tended to correlate with regional wall motion abnormalities.
Pharmacological effect on LV function
Furthermore, to explore the pharmacological effects on LV function, we analysed several clinical parameters in patients who underwent echocardiography pre‐ and post‐treatment (n = 12) during the follow‐up (Figure 2 A ). We focused on the effect of additional immunosuppressive therapy other than PSL and compared six patients who received additional immunosuppressive therapy with PSL (AZA, n = 2; MMF, n = 1; MTX, n = 2; TAC, n = 1) and those who did not (n = 6; including three patients without PSL treatment). No significant difference was observed in the baseline echocardiographic data and the follow‐up period between the two groups. In the no additional immunosuppressive therapy group, all patients exhibited an increase in left ventricular end‐systolic dimension (LVDs) and reduced LVEF at the end of the follow‐up compared with baseline data (P = 0.03, P = 0.03, respectively), whereas those in the additional immunosuppressive therapy group were maintained. Additionally, median LVEF at the end of the follow‐up was significantly lower in the no additional immunosuppressive therapy group than in the additional immunosuppressive therapy group (25.5% vs. 66.0%, P = 0.04), and there was a significant difference in delta LVEF between the two groups (Figure 2 B , P = 0.004). Regarding laboratory data, the median CK level was higher in the additional immunosuppressive therapy group at the baseline (977 U/L vs. 244 U/L, P = 0.002), but there was no difference between the two groups at the end (102 U/L vs. 217 U/L, P = 0.31). The median CK‐MB level was not different between the two groups at the baseline (23 U/L vs. 11 U/L, P = 0.09) or at the end (7 U/L vs. 5 U/L, P = 0.76). Although no difference was found in the high‐sensitivity troponin I and BNP levels between the two groups, nor were there any significant changes after the treatments; not all patients underwent these tests.
Figure 2.
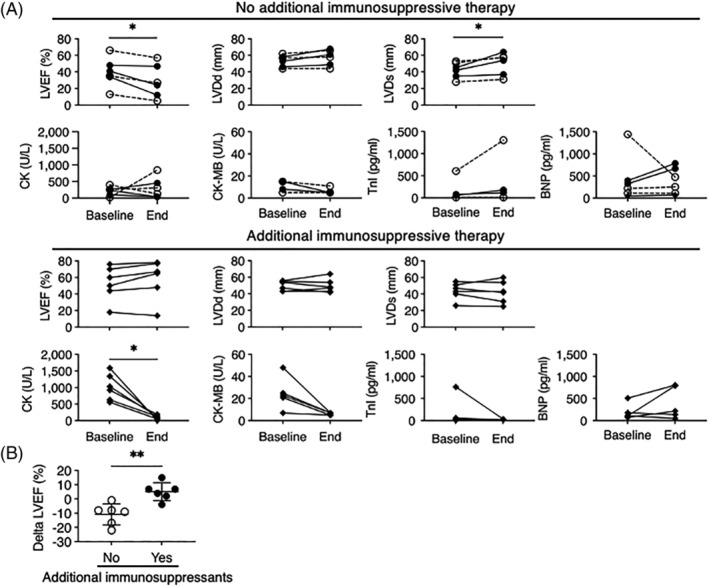
Changes of echocardiographic and laboratory data during the follow‐up period. (A) Echocardiographic and laboratory data changes in patients with and without additional immunosuppressive therapy. Patients who did not receive any immunosuppressive therapy are shown in dotted lines. (B) Delta LVEF between the two treatment groups. BNP, brain natriuretic peptide; CK, creatine kinase; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; TnI, troponin I. *P < 0.05, **P < 0.01.
Because LVEF decline was detected in the no additional immunosuppressive therapy group, we next compared the clinical characteristics of patients with and without >5% LVEF decline (n = 5 vs. n = 7) to reveal the factors associated with cardiac dysfunction (Table 3 ). Patients without LVEF decline showed higher median CK levels (924 U/L vs. 256 U/L, P = 0.03), and most of them received additional immunosuppressive therapy, whereas no patients with LVEF decline had additional immunosuppressive therapy (86% vs. 0%, P = 0.02). Additionally, 3/5 patients with LVEF decline had no PSL treatment (60% vs. 0%, P = 0.045). No differences were detected in age, sex, disease duration, baseline cardiac involvement, or echocardiographic data.
Table 3.
Clinical characteristics of patients with and without LVEF decline
| Characteristics | No decline | Decline | |
|---|---|---|---|
| n = 7 | n = 5 | P‐value | |
| Age at diagnosis, years, median (IQR) | 53 (53–60) | 59 (47–73) | 0.67 |
| Female, n (%) | 5 (71) | 5 (100) | 0.47 |
| Disease duration before diagnosis, months, median (IQR) | 61 (36–145) | 84 (18–96) | 0.64 |
| Follow‐up time period, months, median (IQR) | 79 (27–112) | 9 (4–102) | 0.26 |
| Cardiac involvement, n (%) | |||
| Arrhythmia | 5 (71) | 5 (100) | 0.47 |
| LV wall motion abnormality | 5 (71) | 4 (80) | >0.99 |
| Baseline laboratory data, median (IQR) | |||
| CK, U/L | 924 (556–1343) | 256 (141–326) | 0.03 |
| CK‐MB, U/L | 21 (8–25) | 9 (5–15) | 0.13 |
| BNP, pg/mL | 110 (63–181) | 323 (165–919) | 0.07 |
| Baseline echocardiographic data, median (IQR) | |||
| LVEF, % | 50 (44–70) | 35 (24–54) | 0.15 |
| LVDd, mm | 47 (43–55) | 57 (49–60) | 0.14 |
| LVDs, mm | 35 (26–43) | 45 (35–52) | 0.16 |
| Additional immunosuppressive therapy, n (%) | 6 (86) | 0 | 0.02 |
| No PSL therapy, n (%) | 0 | 3 (60) | 0.045 |
Abbreviations: BNP, brain natriuretic peptide; CK, creatine kinase; IQR, interquartile range; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; PSL, prednisolone.
Discussion
Here, we summarized the cases of AMA‐positive myositis‐complicated cardiac involvement at a single centre and analysed them with particular attention to the treatment responsiveness of cardiovascular dysfunction.
Cardiac involvement is the leading cause of death in inflammatory myositis. 6 , 18 Recently, the AMA‐M2 subtype was identified as an associated factor for cardiac involvement in a multicentre cross‐sectional study. 19 AMAs were detected in about 5%–10% of inflammatory myositis and the cardiac involvement was more frequent in the AMA‐positive myositis than other inflammatory myositis with a 30%–70% incidence rate. 4 , 8 , 9 , 20 , 21 , 22 The first symptoms were cardiovascular in about 50% of cases with cardiac involvement. 22 In our study, the most frequent initial symptom was dyspnoea (60%), and 87% of the patients first visited the cardiovascular department. Moreover, 73% of the patients developed CVDs prior to myositis diagnosis, suggesting the importance of early cardiac manifestations in AMA‐positive myositis. In particular, the frequency of arrhythmia was as high as 8%, followed by 53% of LV wall motion abnormalities. In a review by Maeda et al., cardiac complications resulted in arrhythmia in 8 of 24 cases, and about 20% cases had decreased cardiac contractility. 8 Various arrhythmias, such as atrioventricular block, supraventricular arrhythmia, and ventricular arrhythmia, were detected in the patients, similar to the manifestations of cardiac sarcoidosis (CS). Furthermore, regional LV wall motion abnormality, especially in the intraventricular septum, appeared on echocardiography during the follow‐up period, mimicking CS. Although no sign of active myocarditis was found by EMB, the sensitivity of EMB in patients with inflammatory myopathy with suspected myocarditis is not well known. 23 , 24 Also, patchy distribution of the inflammation may lead to false‐negative results. 1 On the other hand, cMRI detected 3/6 (50%) of patients with T2 high‐intensity areas and 4/6 (67%) of patients with late gadolinium enhancement that suggest underlying inflammation. To verify a more definite association between myocardial inflammation and cardiac involvement of AMA‐positive myositis, we should perform prospective research including a thorough examination of cMRI. Taken all together, these CS‐like manifestations indicate the critical role of cardiac muscle inflammation in AMA‐positive myositis‐complicated cardiac involvement and the importance of anti‐inflammatory treatment.
AMA‐positive myositis is treated with inflammatory myositis therapy. Glucocorticoids are the initial treatment, and traditional immunosuppressants, such as AZA and MTX, are combined to avoid long‐term glucocorticoid exposure or prevent refractory myositis. 5 , 6 IVIG is also used together with glucocorticoids in severe or refractory myositis. Previous studies have shown improved muscular strength in patients following these treatments. 8 However, the response of cardiac involvement to immunosuppressive treatment is unknown, and the cardiac outcomes in case reports are contradictory. Interestingly, some cases showed that cardiac involvement worsened or remained unchanged, even after glucocorticoid therapy improved muscle weakness and CK levels. 25 , 26 , 27 , 28 This outcome could be explained by the finding that cMRI detected myocarditis manifestations after glucocorticoid treatment in inflammatory myositis, which is clinically considered to be in remission. 2 Although glucocorticoid therapy might not be sufficient to suppress cardiac inflammation, there is no clear evidence for the use of additional immunosuppressants.
In our study, 80% of patients received PSL therapy, and 53% of patients had additional immunosuppressants. There was no remarkable change in the optimal medical care for CVDs during the follow‐up period, probably because most cardiac involvements developed before the diagnosis of AMA‐positive myositis and started treatment in advance. At the last visit, LV dysfunction persisted, whereas arrhythmias drastically decreased. Furthermore, regional LV wall motion abnormality increased from 29% to 58%, suggesting residual cardiac inflammation. LVEF reduction and LVDs enlargement progressed in patients who did not receive additional immunosuppressive therapy. In contrast, most patients without LVEF decline received additional immunosuppressive therapy and had high CK levels, reflecting severe myositis. Thus, additional immunosuppressants may be required for the complete inhibition of inflammation to maintain LV function in the same way as to suppress severe myositis. Considering that no patients with LVEF decline received additional immunosuppressive therapy, careful attention to cardiac function is required in patients receiving no treatment or PSL treatment alone. It is notable that 27% of patients were hospitalized after their initial treatment due to cardiac involvement, indicating different reactions to treatment between cardiac involvement and myositis. Moreover, various immunosuppressive treatment responses among cardiac involvements are similar to those of CS, an inflammatory cardiomyopathy. 29 , 30 Although it is unclear whether arrhythmias were improved by immunosuppressive therapy rather than medication and device implantation, the response to immunosuppressive therapy might depend on the type of cardiac involvement or the phase of inflammation, as we previously reported. 31
In summary, there is a growing evidence that AMA‐positive myositis is frequently associated with cardiac involvements. However, no standardized immunosuppressive treatment is available. We demonstrated a series of AMA‐positive myositis‐complicated cardiac involvement cases that showed various cardiac responses to immunosuppressive therapy.
Limitations
This study has some limitations. First, this was a retrospective study conducted at a single centre. The number of patients was quite low, thereby limiting the statistical power. Ideally, these hypotheses should be tested in future prospective studies. In addition, it contains a variety of stages and a follow‐up period, which might distort the case distribution. In this study, the initial symptoms included those that might be derived from cardiac dysfunction; however, it might be quite difficult to determine whether the symptoms were accurately associated with AMA‐positive myositis‐complicated abnormalities. Third, because the time span of our cohort was from 2000 to 2021, the antigen specificities for detection of autoantibodies in the detection kit might be changed. Fourth, we might have dismissed arrhythmias that were undetectable on the ECG. Fifth, it is difficult to completely tell the difference between cardiac involvement derived from AMA‐positive myositis and primary cardiomyopathies, such as dilated cardiomyopathy, which could cause similar cardiac manifestation to AMA‐positive myositis.
Conflicts of interest
EA belongs to the Department, endowed by NIPRO‐Corp, Terumo‐Corp., Senko Medical‐Instrument‐Mfg., Century‐Medical, Inc., ONO‐pharmaceutical‐Co., Ltd. Medtronic‐JAPAN Co., Ltd, Nippon‐Shinyaku Co., Ltd, Mochida Pharmaceutical Co.; Boehringer Ingelheim Pharmaceuticals Inc., Abiomed‐Inc, AQuA‐Inc, Fukuda‐Denshi Co., Ltd, and Sun‐Medical‐Technology‐Research Corp.
Bujo, S. , Amiya, E. , Maeda, M. H. , Ishida, J. , Hatano, M. , Ishizuka, M. , Uehara, M. , Oshima, T. , Kojima, T. , Nakanishi, K. , Daimon, M. , Shimizu, J. , Toda, T. , and Komuro, I. (2022) The effect of immunosuppressive therapy on cardiac involvements in anti‐mitochondrial antibody‐positive myositis. ESC Heart Failure, 9: 4112–4119. 10.1002/ehf2.14138.
Funding information: This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan through Grant‐in‐Aid 21K08047 (to Amiya E).
References
- 1. Tschöpe C, Ammirati E, Bozkurt B, Caforio ALP, Cooper LT, Felix SB, Hare JM, Heidecker B, Heymans S, Hübner N, Kelle S, Klingel K, Maatz H, Parwani AS, Spillmann F, Starling RC, Tsutsui H, Seferovic P, Van Linthout S. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat Rev Cardiol. 2021; 18: 169–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mavrogeni S, Bratis K, Karabela G, Stavropoulos E, Sfendouraki E, Kolovou G. Myocarditis during acute inflammatory myopathies: Evaluation using clinical criteria and cardiac magnetic resonance imaging. Int J Cardiol. 2013; 164: e3–e4. [DOI] [PubMed] [Google Scholar]
- 3. Mavrogeni S, Markousis‐Mavrogenis G, Kolovou G. The Sphinx's riddle: Cardiovascular involvement in autoimmune rheumatic disease. BMC Cardiovasc Disord. 2016; 16: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albayda J, Khan A, Casciola‐Rosen L, Corse AM, Paik JJ, Christopher‐Stine L. Inflammatory myopathy associated with anti‐mitochondrial antibodies: A distinct phenotype with cardiac involvement. Semin Arthritis Rheum. 2018; 47: 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glaubitz S, Zeng R, Schmidt J. New insights into the treatment of myositis. Ther Adv Musculoskelet Dis. 2020; 12: 1759720X19886494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher‐Stine L, Mammen AL, Miller FW. Idiopathic inflammatory myopathies. Nat Rev Dis Primers. 2021; 7: 86. [DOI] [PubMed] [Google Scholar]
- 7. Lundberg IE, Tjärnlund A, Bottai M, Werth VP, Pilkington C, de Visser M, Alfredsson L, Amato AA, Barohn RJ, Liang MH, Singh JA, Aggarwal R, Arnardottir S, Chinoy H, Cooper RG, Dankó K, Dimachkie MM, Feldman BM, Torre IG‐DL, Gordon P, Hayashi T, Katz JD, Kohsaka H, Lachenbruch PA, Lang BA, Li Y, Oddis CV, Olesinska M, Reed AM, Rutkowska‐Sak L, Sanner H, Selva‐O'Callaghan A, Song Y‐W, Vencovsky J, Ytterberg SR, Miller FW, Rider LG, International Myositis Classification Criteria Project consortium, The Euromyositis register and The Juvenile Dermatomyositis Cohort Biomarker Study and Repository (JDRG) (UK and Ireland) . 2017 European league against rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann Rheum Dis. 2017; 76: 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maeda MH, Tsuji S, Shimizu J. Inflammatory myopathies associated with anti‐mitochondrial antibodies. Brain. 2012; 135: 1767–1777. [DOI] [PubMed] [Google Scholar]
- 9. Zhang L, Yang H, Lei J, Peng Q, Yang H, Wang G, Lu X. Muscle pathological features and extra‐muscle involvement in idiopathic inflammatory myopathies with anti‐mitochondrial antibody. Semin Arthritis Rheum. 2021; 51: 741–748. [DOI] [PubMed] [Google Scholar]
- 10. Minamiyama S, Ueda S, Nakashima R, Yamakado H, Sakato Y, Yamashita H, Sawamoto N, Fujimoto R, Nishino I, Urushitani M, Mimori T, Takahashi R. Thigh muscle MRI findings in myopathy associated with anti‐mitochondrial antibody. Muscle Nerve. 2020; 61: 81–87. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt J‐U. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 12. Nzeusseu A, Brion F, Lefèbvre C, Knoops P, Devogelaer JP, Houssiau FA. Functional outcome of myositis patients: Can a low‐dose glucocorticoid regimen achieve good functional results? Clin Exp Rheumatol. 1999; 17: 441–446. [PubMed] [Google Scholar]
- 13. Oddis CV, Aggarwal R. Treatment in myositis. Nat Rev Rheumatol. 2018; 14: 279–289. [DOI] [PubMed] [Google Scholar]
- 14. Gordon PA, Winer JB, Hoogendijk JE, Choy EHS, Cochrane Neuromuscular Group . Immunosuppressant and immunomodulatory treatment for dermatomyositis and polymyositis. Cochrane Database Syst Rev. 2012; 2012: CD003643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller JAL, Walsh Y, Saminaden S, Lecky BRF. Randomised double blind trial of methotrexate and steroids compared with azathioprine and steroids in the treatment of idiopathic inflammatory myopathy. (ABN abstracts). J Neurol Neurosurg Psychiatry. 2002; 73: 222–223. [Google Scholar]
- 16. Miyasaka N, Hara M, Koike T, Saito E, Yamada M, Tanaka Y. GB‐0998 study group. Effects of intravenous immunoglobulin therapy in Japanese patients with polymyositis and dermatomyositis resistant to corticosteroids: A randomized double‐blind placebo‐controlled trial. Mod Rheumatol. 2012; 22: 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patwa HS, Chaudhry V, Katzberg H, Rae‐Grant AD, So YT. Evidence‐based guideline: Intravenous immunoglobulin in the treatment of neuromuscular disorders: Report of the therapeutics and technology assessment Subcommittee of the American Academy of neurology. Neurology. 2012; 78: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 18. Lundberg IE. The heart in dermatomyositis and polymyositis. Rheumatology. 2006; 45: iv18–iv21. [DOI] [PubMed] [Google Scholar]
- 19. Zhang L, Zhu H, Yang P, Duan X, Wei W, Wu Z, Fang Y, Li Q, Liu S, Shi X, Li H, Wu C, Zhou S, Leng X, Zhao J, Xu D, Wu Q, Tian X, Li M, Zhao Y, Wang Q, Zeng X, Chinese Rheumatism Data Center‐Myositis Registry (CRDC‐MYO) . Myocardial involvement in idiopathic inflammatory myopathies: A multi‐center cross‐sectional study in the CRDC‐MYO registry. Clin Rheumatol. 2021; 40: 4597–4608. [DOI] [PubMed] [Google Scholar]
- 20. Mauhin W, Mariampillai K, Allenbach Y, Charuel JL, Musset L, Benveniste O. Anti‐mitochondrial antibodies are not a hallmark of severity in idiopathic inflammatory myopathies. Joint Bone Spine. 2018; 85: 375–376. [DOI] [PubMed] [Google Scholar]
- 21. Hou Y, Liu M, Luo YB, Sun Y, Shao K, Dai T, Li W, Zhao Y, Yan C. Idiopathic inflammatory myopathies with anti‐mitochondrial antibodies: Clinical features and treatment outcomes in a Chinese cohort. Neuromuscul Disord. 2019; 29: 5–13. [DOI] [PubMed] [Google Scholar]
- 22. Uenaka T, Kowa H, Ohtsuka Y, Seki T. Less limb muscle involvement in myositis patients with anti‐mitochondrial antibodies. Eur Neurol. 2017; 78: 290–295. [DOI] [PubMed] [Google Scholar]
- 23. Myhr KA, Pecini R. Management of Myocarditis in myositis: Diagnosis and treatment. Curr Rheumatol Rep. 2020; 22: 49. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Fang L, Chen W, Zhu Y, Lin X, Wang Y, Li X, Wang Q, Liu Z. Identification of characteristics of overt myocarditis in adult patients with idiopathic inflammatory myopathies. Cardiovasc Diagn Ther. 2020; 10: 405–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kadosaka T, Tsujinaga S, Iwano H, Kamiya K, Nagai A, Mizuguchi Y, Motoi K, Omote K, Nagai T, Yabe I, Anzai T. Cardiac involvement with anti‐mitochondrial antibody‐positive myositis mimicking cardiac sarcoidosis. ESC Heart Fail. 2020; 7: 4315–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamanaka T, Fukatsu T, Ichinohe Y, Hirata Y. Antimitochondrial antibodies‐positive myositis accompanied by cardiac involvement. BMJ Case Rep. 2017; 2017: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takahashi F, Sawada J, Minoshima A, Sakamoto N, Ono T, Akasaka K, Takei H, Nishino I, Hasebe N. Antimitochondrial antibody‐associated myopathy with slowly progressive cardiac dysfunction. Intern Med. 2021; 60: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hasegawa Y, Izumi D, Kashimura T, Minamino T. Life‐threatening ventricular arrhythmia and left ventricular dysfunction associated with anti‐mitochondrial antibody‐positive myositis: A case report. Eur Heart J Case Rep. 2021; 5: ytab469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilotra NA, Griffin JM, Pavlovic N, Houston BA, Chasler J, Goetz C, Chrispin J, Sharp M, Kasper EK, Chen ES, Blankstein R, Cooper LT, Joyce E, Sheikh FH. Sarcoidosis‐related cardiomyopathy: Current knowledge, challenges, and future perspectives state‐of‐the‐art review. J Card Fail. 2022; 28: 113–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Giblin GT, Murphy L, Stewart GC, Desai AS, Di Carli MF, Blankstein R, Givertz MM, Tedrow UB, Sauer WH, Hunninghake GM, Dellaripa PF, Divakaran S, Lakdawala NK. Cardiac sarcoidosis: When and how to treat inflammation. Card Fail Rev. 2021; 7: e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bujo S, Amiya E, Kojima T, Yamada S, Maki H, Ishizuka M, Uehara M, Hosoya Y, Hatano M, Kubota A, Toda T, Komuro I. Variable cardiac responses to immunosuppressive therapy in anti‐mitochondrial antibody‐positive myositis. Can J Cardiol. 2019; 35: 1604.e9–1604.e12. [DOI] [PubMed] [Google Scholar]


