Abstract
Caffeine is the most widely consumed psychostimulant drug which could affect learning and memory acting through central adenosine receptors. Although caffeine has been suggested to impair the acquisition and the expression of auditory fear conditioning, its effect on the extinction has not been elucidated. To address this issue, in the present study, we investigated whether caffeine affects the extinction of conditioned fear in an auditory fear conditioning paradigm. During conditioning, rats received pairings of auditory cues (conditioned stimulus, CS) and foot shocks (unconditioned stimulus). On the next day, the rats were intraperitoneally administrated saline or caffeine (5 or 10 mg/kg) and then subjected to the extinction training, in which CSs were repeatedly presented without the foot shocks. Twenty‐four hours later, the rats were re‐exposed to the presentations of CSs (retrieval test). We found an acute caffeine administration dose‐dependently decreased freezing rates during the presentations of CS in the extinction training. Furthermore, caffeine‐treated animals showed lower conditioned freezing responses in the retrieval test. These findings suggest that caffeine facilitates the extinction of conditioned fear.
Keywords: caffeine, extinction, fear conditioning, rats
1. INTRODUCTION
Caffeine is the most widely consumed psychoactive drug which promotes wakefulness and facilitates daily performances, especially in fatigued individuals. 1 In particular, previous research have shown that an intake of low‐dose caffeine reliably enhances fundamental cognitive processes such as attention, 2 and improves learning and memory in human subjects. 3 Animal studies have also revealed a promnesic effect of acute caffeine treatment in normal mice and rats using varieties of cognitive tasks including visual discrimination tasks, 4 object, spatial, and social recognition tests. 5 , 6 Once absorbed in the periphery, caffeine passes through blood‐brain barrier and acts as a non‐specific adenosine receptor antagonist which blocks both A1 and A2 receptors in the central nervous system. The facilitative effects of caffeine on learning and memory have been considered to be mediated by the blockade of Gi/o‐coupled adenosine A1 receptors that eventually promote neural plasticity such as long‐term potentiation. 7 , 8 , 9 , 10
Associative fear learning and its extinction has been assessed in the auditory or contextual fear conditioning paradigm. During the conditioning, animals learn the association between preceding auditory conditioned stimulus (CS) or surrounding contextual information and subsequent aversive unconditioned stimulus (US) such as mild electric shocks. 11 On the other hand, animals learn CS‐No US association during the extinction where only the CSs are repeatedly presented. Understanding the effects of drugs on these learning processes are important to find a potential molecular target for the pharmacological treatment of stress‐related mental disorders. Previous studies have shown that caffeine treatments impair both the acquisition and the expression of auditory 12 and contextual fear conditioning. 13 , 14 However, its effect on the extinction of conditioned fear is yet to be investigated. Given that the extinction of conditioned fear is executed through the distinct neural circuit mechanism from that of acquisition, 15 caffeine could have a different effect on the fear extinction. Furthermore, investigating its effect on the extinction is valuable as a preclinical study aiming at drug therapy for the patient already suffering from stress‐related mental disorders. Therefore, in the present study, we examined the effect of acute systemic treatment of caffeine on the fear extinction in the auditory fear conditioning paradigm in rats.
2. METHODS
Forty‐one 8‐week‐old male Sprague–Dawley rats (Institute for Animal Reproduction, Ibaraki, Japan) were used. Rats were group‐housed (2‐4 rats per cage) in plastic cages (W380 × D210 × H200 mm) with beddings (spruce wood shavings, Jackson Laboratory Japan, Kanagawa, Japan), and kept on a 12:12 hours light‐dark cycle (light on 0800‐2000) with free access to food pellets (MF, ORIENTAL YEAST CO., LTD., Tokyo, Japan) and tap water throughout the experiment. All efforts were made to minimize the number of animals used and their suffering. Experimental procedures were approved by the University of Tsukuba Committee on Animal Research.
The experiment consisted of three phases, fear conditioning, extinction training, and retrieval test (Figure 1A, see below for details). The fear conditioning was performed in context A while the extinction training and the retrieval test were done in context B. In context A, animals were placed in a square transparent Plexiglas chamber with a grid floor (O'Hara & CO., LTD., Tokyo, Japan) which is in a sound‐isolating box (Muromachi Kikai CO., LTD., Tokyo, Japan). The interior was illuminated with 150 lx and background white noise (50 dB) was continuously given. In context B, a black‐colored square chamber with a smoothed floor was used in the same sound‐isolating box. The house light was turned off. The volume of background white noise was changed (60 dB), and the interior was fragranced by putting a small dish filled with peppermint soap (Magic soap, Dr. Bronner's, San Diego, CA, USA) outside of the black chamber.
FIGURE 1.
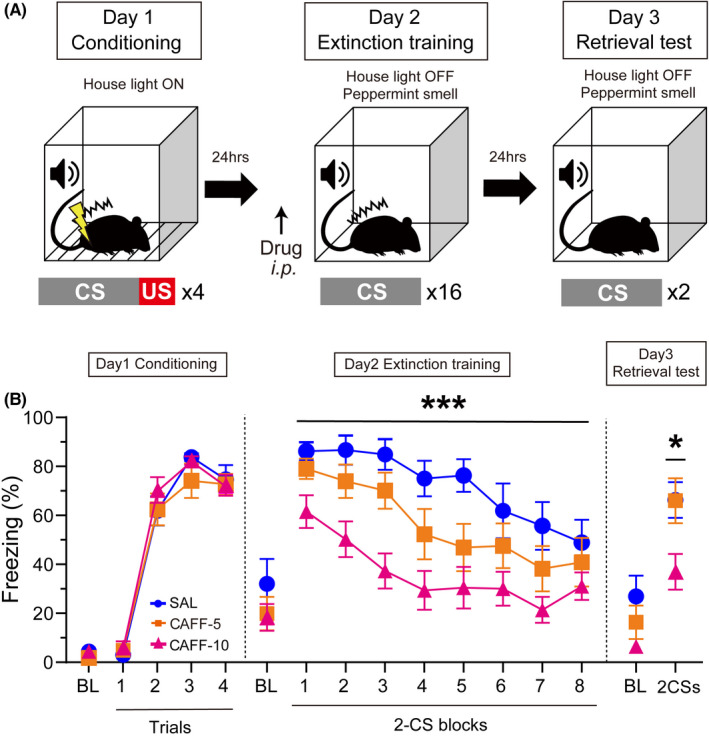
The effect of systemic injection of caffeine on the extinction learning in the auditory fear conditioning. A, Schematic illustration of the experiment. B, Freezing rate during CS periods on each experimental day. BL, baseline period; CAFF‐5, caffeine 5 mg/kg treated group; CAFF‐10, caffeine 10 mg/kg treated group; SAL, saline‐treated group. Data are presented as the mean ± SEM. *** Indicates a significant difference between SAL and CAFF‐10 (P < 0.001). * Indicates a significant difference between SAL/CAFF‐5 and CAFF‐10 (P < 0.05)
Caffeine (C0750, Sigma, MO, USA) was diluted in saline (SAL; Otsuka, Tokyo, Japan) to a final concentration of 5 and 10 mg/ml. In the experiment, the dose of 5 or 10 mg/kg was intraperitoneally injected. The dose of 10 mg/kg of caffeine was chosen based on a previous report showing that this dose of caffeine had no anxiolytic and anxiogenic effects in an open‐field test and an elevated plus maze test. 16 It has been also reported that this dose of caffeine did not have either rewarding or aversive effects in a conditioned place preference test. 17
During the fear conditioning, animals were placed into the chamber and received pairings of auditory CS (20 seconds, 65 dB, 10 kHz) and electric shock US after a baseline period (120 seconds). The foot shock US (2‐seconds scrambled foot shocks, 0.5 mA) was co‐terminated with the CS. The CS‐US pairings were repeated four times with variable intertrial intervals (ITIs) on average at 120 seconds. A 120 seconds after the last paring, the rats were removed from the apparatus and returned to the home cage. Twenty‐four hours later, the extinction training was performed (day 2). Fifteen minutes before the extinction training, 13 rats received an intraperitoneal injection of SAL (n = 13), 5 mg/kg (CAFF‐5, n = 14) or 10 mg/kg of caffeine (CAFF‐10, n = 14). During the extinction training, after a 5‐minutes baseline period, CS was repeatedly presented 16 times without the shock US delivery (120‐seconds ITI on average). The rats were returned to the home cage 120 seconds after the last CS presentation. Twenty‐four hours later (day 3), we tested whether the drug‐induced changes in the fear response observed in the extinction training remained without drug administration. During the retrieval test, after a 5‐minutes baseline period, CS was presented twice without the shock US (150‐seconds ITI). The rats were returned to the home cage 90 seconds after the last CS presentation. Rats' behavior was recorded by a head‐mount infrared camera, and the freezing behavior was analyzed using ANY‐maze software (Stoelting CO., LTD, Wood Dale, IL, USA). Freezing was defined as the cessation of all bodily movements except for breathing.
In this study, the percentages of freezing response during CS were compared using either a one‐way ANOVA or a two‐way repeated measures ANOVA (drug × trial) followed by Tukey's multiple comparisons (P < 0.05). All statistical analyses were performed using Prism 9.3.1 (GraphPad Software, San Diego, CA, USA).
3. RESULTS
Freezing responses during the fear conditioning, the extinction training, and the retrieval test are shown in Figure 1B and Table S1. In the conditioning on day 1, animals successively learned auditory fear conditioning. A one‐way ANOVA revealed that there was no difference in freezing among groups during the 2‐minutes baseline period [F(2,38) = 2.027, P = 0.1457]. A two‐way repeated measures ANOVA revealed that CS‐evoked freezing was significantly increased during conditioning [F(3,114) = 205.6, P < 0.0001].
In the extinction training on day 2, the systemic administration of caffeine decreased freezing behavior. A one‐way ANOVA revealed that there was no difference in freezing among groups during the 5‐minutes baseline period [F(2,38) = 0.9413, P = 0.3990]. A two‐way repeated measures ANOVA revealed significant main effects of extinction training [F(7,266) = 18.90, P < 0.0001] and drug [F(2,38) = 8.975, P = 0.0006] in CS‐evoked freezing. Regarding the effect of the drug, freezing responses under the CAFF‐10 condition decreased significantly compared to those under the SAL control condition (P = 0.0004).
In the retrieval test on day 3, rats in the caffeine‐treated group still showed less freezing. A one‐way ANOVA revealed that there was no difference in freezing among groups during the 5‐minutes baseline period [F(2,38) = 2.634, P = 0.0849]. On the other hand, a one‐way ANOVA revealed a significant main effect of the drug [F(2,38) = 4.497, P = 0.0177], and subsequent post‐hoc analysis showed that CS‐evoked freezing responses in the CAFF‐10 group were significantly decreased compared to those in SAL group (P = 0.0365) and CAFF‐5 group (P = 0.0343).
4. DISCUSSION
In the present study, we investigated the effect of acute systemic treatment of caffeine on fear extinction in the auditory fear conditioning paradigm. We found that the caffeine treatment generally decreased conditioned fear response during the extinction training. Importantly, the decreased conditioned response to caffeine was also observed in the next day's retrieval test. Our results suggest the facilitative effect of caffeine on the fear extinction.
The fact that caffeine decreased freezing responses within the extinction training suggests not only its facilitative effects on fear extinction 18 but also its disruptive effects on fear expression. Indeed, previous studies have shown that caffeine treatment impairs the expression of conditioned fear in a retrieval test. 12 , 13 On the other hand, caffeine has an effect to enhance general locomotor activity in rodents. 19 Given this psychostimulative effect, we cannot rule out the possibility that the reduced conditioned fear response during the extinction training on day 2 could be attributed to increased general activity caused by the caffeine treatment.
The most important result in the present study is that the caffeine treatment immediately before the extinction training reduced conditioned freezing even in the next day's retrieval test, strongly suggesting the facilitating effect of caffeine on the fear extinction. The possibility that the psychostimulative effect of caffeine could remain until the retrieval test could be excluded because the half‐life of caffeine in the circulation is normally 3‐5 hours, 20 and the facilitative effect of caffeine on the locomotor activity does not last more than 5 hours even at a higher dose (20‐25 mg/kg) in mice. 21 , 22 In addition, another possibility that counter conditioning by the rewarding effect of caffeine weakened conditioned fear response could be also ruled out because our dose of caffeine (10 mg/kg) has no rewarding effects in a conditioned place preference test. 17
The extinction of conditioned fear is controlled by brain‐wide neural circuits, 15 , 23 and within this circuit, the role of the cortico‐amygdala pathway has been considered to be particularly important. 24 , 25 Notably, moderate to high levels of adenosine and adenosine A1 and A2A receptors have been identified in the amygdala and medial prefrontal cortex (mPFC). 20 Given that the blockade of A1 receptors facilitates synaptic plasticity in the hippocampus, 7 , 8 , 9 , 10 acute caffeine might block A1 receptors in the mPFC or amygdala and then enhance neural plasticity underlying the fear extinction. Furthermore, it has also been reported that caffeine treatment induces neurotransmitter releases such as dopamine 26 and noradrenaline. 27 Previous studies have suggested that dopamine release in the nucleus accumbens 28 and noradrenaline release in the mPFC 29 facilitate the extinction learning in the auditory fear conditioning paradigm. Therefore, it is also possible that the caffeine facilitated the fear extinction by adaptively changing dopamine and/or noradrenaline release in the fear extinction circuit.
AUTHOR CONTRIBUTIONS
TO conceptualized and designed experiments. KK performed the behavioral tests. TO and KK analyzed the data and wrote the manuscript. KY and YI helped to draft the manuscript. TO, KY, and YI supervised all aspects of the present study. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ANIMAL STUDIES
Animal experiments were approved by the University of Tsukuba Committee on Animal Research.
Supporting information
Table S1
ACKNOWLEDGMENT
This study was supported by grants from Japan Society for the Promotion of Science (JP21H00812 to K.Y.; JP19K03365 and JP20K03488 to K.Y. and Y.I.; JP19K21806 to K.Y., Y.I., and T.O.; JP19H01769, JP19K03385, JP19K21806, JP21K18557, JP21H00311, and JP22H01105 to T.O.).
Ozawa T, Kaseda K, Ichitani Y & Yamada K Caffeine facilitates extinction of auditory fear conditioning in rats. Neuropsychopharmacol Rep. 2022;42:521–525. 10.1002/npr2.12287
Takaaki Ozawa and Kodai Kaseda contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Fulgoni VL, Keast DR, Lieberman HR. Trends in intake and sources of caffeine in the diets of US adults: 2001–2010. Am J Clin Nutr. 2015;101:1081–7. 10.3945/ajcn.113.080077 [DOI] [PubMed] [Google Scholar]
- 2. McLellan TM, Caldwell JA, Lieberman HR. A review of caffeine's effects on cognitive, physical and occupational performance. Neurosci Biobehav Rev. 2016;71:294–312. 10.1016/j.neubiorev.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 3. Borota D, Murray E, Keceli G, Chang A, Watabe JM, Ly M, et al. Post‐study caffeine administration enhances memory consolidation in humans. Nat Neurosci. 2014;17:201–3. 10.1038/nn.3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paré W. The effect of caffine and seconal on a visual discrimination task. J Comp Physiol Psychol. 1961;54:506–9. 10.1037/H0040683 [DOI] [PubMed] [Google Scholar]
- 5. Onaolapo AY, Onaolapo OJ. Caffeine's influence on object recognition and working‐memory in prepubertal mice and its modulation by gender. Pathophysiology. 2015;22:223–30. 10.1016/J.PATHOPHYS.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 6. Prediger RDS, Takahashi RN. Modulation of short‐term social memory in rats by adenosine A1 and A2A receptors. Neurosci Lett. 2005;376:160–5. 10.1016/j.neulet.2004.11.049 [DOI] [PubMed] [Google Scholar]
- 7. Arai A, Lynch G. Factors regulating the magnitude of long‐term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–84. 10.1016/0006-8993(92)90181-8 [DOI] [PubMed] [Google Scholar]
- 8. Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. 10.1146/annurev.neuro.24.1.31 [DOI] [PubMed] [Google Scholar]
- 9. Simons SB, Caruana DA, Zhao M, Dudek SM. Caffeine‐induced synaptic potentiation in hippocampal CA2 neurons. Nat Neurosci. 2012;15:23–5. 10.1038/nn.2962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costenla AR, Diógenes MJ, Canas PM, Rodrigues RJ, Nogueira C, Maroco J, et al. Enhanced role of adenosine A 2A receptors in the modulation of LTP in the rat hippocampus upon ageing. Eur J Neurosci. 2011;34:12–21. 10.1111/j.1460-9568.2011.07719.x [DOI] [PubMed] [Google Scholar]
- 11. Ozawa T, Johansen JP. Learning rules for aversive associative memory formation. Curr Opin Neurobiol. 2018;49:148–57. 10.1016/J.CONB.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 12. Dubroqua S, Low SRL, Yee BK, Singer P. Caffeine impairs the acquisition and retention, but not the consolidation of Pavlovian conditioned freezing in mice. Psychopharmacology (Berl). 2015;232:721–31. 10.1007/s00213-014-3703-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Corodimas KP, Pruitt JC, Stieg JM. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology (Berl). 2000;152:376–82. 10.1007/s002130000557 [DOI] [PubMed] [Google Scholar]
- 14. Simões AP, MacHado NJ, Gonçalves N, Kaster MP, Simões AT, Nunes A, et al. Adenosine A2Areceptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology. 2016;41:2862–71. 10.1038/npp.2016.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. 10.1111/j.1460-9568.2010.07101.x [DOI] [PubMed] [Google Scholar]
- 16. Bhattacharya SK, Satyan KS, Chakrabarti A. Anxiogenic action of caffeine: an experimental study in rats. J Psychopharmacol. 1997;11:219–24. 10.1177/026988119701100304 [DOI] [PubMed] [Google Scholar]
- 17. Brockwell NT, Eikelboom R, Beninger RJ. Caffeine‐induced place and taste conditioning: production of dose‐dependent preference and aversion. Pharmacol Biochem Behav. 1991;38:513–7. 10.1016/0091-3057(91)90006-N [DOI] [PubMed] [Google Scholar]
- 18. Plendl W, Wotjak CT. Dissociation of within‐and between‐session extinction of conditioned fear. J Neurosci. 2010;30:4990–8. 10.1523/JNEUROSCI.6038-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yacoub MEl, Ledent C, Ménard JF, Parmentier M, Costentin J, Vaugeois JM. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A1and A2A receptors. Br J Pharmacol. 2000;129:1465–73. 10.1038/sj.bjp.0703170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fredholm BB. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. 10.1111/j.1600-0773.1995.tb00111.x [DOI] [PubMed] [Google Scholar]
- 21. Flores AE, Flores JE, Deshpande H, Picazo JA, Xie X, Franken P, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54:225–33. 10.1109/TBME.2006.886938 [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi K, Shimizu N, Matsushita S, Murata T. The assessment of mouse spontaneous locomotor activity using motion picture. J Pharmacol Sci. 2020;143:83–8. 10.1016/j.jphs.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 23. Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. 10.1146/annurev.psych.121208.131631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–94. 10.1038/nn.2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hagihara KM, Bukalo O, Zeller M, Aksoy‐Aksel A, Karalis N, Limoges A, et al. Intercalated amygdala clusters orchestrate a switch in fear state. Nature. 2021;594:403–7. 10.1038/s41586-021-03593-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Solinas M, Ferre S, You Z‐B, Karcz‐Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22:6321–4. 10.1016/j.jlumin.2017.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berkowitz BA, Tarver JH, Spector S. Release of norepinephrine in the central nervous system by theophylline and caffeine. Eur J Pharmacol. 1970;10:64–71. 10.1016/0014-2999(70)90158-5 [DOI] [PubMed] [Google Scholar]
- 28. Luo R, Weitemier A, Aquili L, Koivumaa J, McHugh TJ, Johansen P. A dopaminergic switch for fear to safety transitions. Nat Commun. 2018;9:2483. 10.1038/s41467-018-04784-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, et al. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci. 2017;20:1602–11. 10.1038/nn.4642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.


