Abstract
Aims
To describe the effect of subsequent pregnancies (SSP) on left ventricular (LV) function and outcomes in patients with peripartum cardiomyopathy (PPCM).
Methods
Among146 women with PPCM who were prospectively followed at two medical centres in Israel (2007–2019), 75 SSPs (in 50 women) were identified: 8 miscarriages, 8 terminations, and 59 life birth.
Results
Forty‐five patients with 59 full‐term SSPs [mean age was 32.9 ± 4.1 years, LV ejection fraction (LVEF) 57.7 ± 5.1%] were analysed.
Data on LVEF at 1‐month post‐delivery were available in 46 and at 6 months in 36 SSPs. There was a small decrease in the mean LVEF, mostly at third trimester (57.2 ± 5.6 vs. 54.4. ± 7.3, P < 0.001); and at 1‐mont (57.9 ± 5.7% vs. 55.4 ± 6.1%, P = 0.001) and at 6‐month post‐delivery (57.4 ± 6.1 vs. 55.3 ± 7.9%, P = 0.03).
In patients with pre‐SSP LV LVEF ≥55%, a mild reduction in the mean group LVEF was seen at 1‐month post‐delivery (P = 0.009). One patient with pre‐SSP LVEF ≥55% developed severe relapse. In patients with pre‐SSP LVEF <55%, a mild reduction in LVEF was obtained mostly at third trimester (51.1 ± 5.6 vs 47.0 ± 7.4%, P < 0.001), which persisted at 6 months (P = 0.03). A relapse was observed in three (25%) women with LVEF <55%. There was no maternal mortality, 32 patients delivered by caesarean section, and there were no foetal complications.
Conclusions
Our study indicates a favourable outcome and low likelihood of maternal mortality associated with SSP in women with a history of PPCM and recovered LV systolic function. SSP was associated with a slight reduction in LVEF mostly during the third trimester, which persisted up to 6 months after delivery.
Keywords: Subsequent, Pregnancies, Peripartum, Cardiomyopathy, Outcome
Introduction
One of the main concerns for women who experienced peripartum cardiomyopathy (PPCM) is the safety of subsequent pregnancy (SSP). SSP in women with a history of PPCM is associated with a risk for recurrent cardiac dysfunction, clinical deterioration, and even mortality. 1 , 2 , 3 , 4 This risk is substantially higher in women with unrecovered left ventricular (LV) function before SSP. A number of publications reported lower mortality and better cardiac function at follow‐up in women with recovered LV ejection fraction (LVEF) before an SSP, 5 , 6 but still, recovered LV function does not guarantee an uncomplicated SSP. 5 , 7 Although impaired LV function assessed by speckle tracking and dobutamine stress echocardiography has been reported in women with a history of PPCM and recovered LVEF (≥50%), 8 , 9 it was not tested in women with SSP; therefore, the best predictor for deterioration of cardiac function remains a pre‐pregnancy LVEF. Based on limited available information, the 2018 European Society of Cardiology guidelines for the management of cardiovascular diseases during pregnancy discourage SSP in women in persistent LV dysfunction (LVEF <50–55%). 10
Studies evaluating risks of SSP in women with a history of PPCM have been limited by a small number of patients and heterogeneous population from different geographical regions with different rates of LV recovery and mortality, which may affect the outcome of SSPs. In addition, most studies were retrospective or observational with inconsistent data collection and inconsistent assessment of LV function. In this prospective study, we aimed to further define the effect of SSP on cardiac and obstetric outcomes of SSP in patients with a previous history of PPCM.
Methods
The protocol was approved by the Human Research Institutional Review Board of Kaplan and Sheba Medical Centers.
Patients
One‐hundred forty‐six women with a diagnosis of PPCM were prospectively followed at the Kaplan and Sheba Medical Center in Israel (2001–2019). PPCM was defined as an idiopathic cardiomyopathy diagnosed during pregnancy or within 5 months of delivery with echocardiographic LVEF 45%. 11 , 12 , 13 LV recovery was defined as LVEF ≥55% at any time during follow‐up. We identified 50 women with 75 SSP and prospectively analysed their clinical, echocardiographic, and obstetrical data. Patients were followed at a specialized cardio‐obstetrics clinics. Exercise stress echo was performed and showed normal contractile reserve, which was defined as increase in LVEF ≥15%, in all patients with LV recovery before SSP. The echocardiographic studies were performed in both institutional dedicated echo labs during the patient's regular follow‐up visits. The reading physicians were aware only of the patient's previous cardiac history and their current clinical condition. The echocardiograms were reviewed (blinded to patient's information) by specialized echocardiographer in both centres (RK and SG) with verification of LV function and size.
LV dimensions and LVEF by biplane Simpson's rule were measured according to the American Society of Echocardiography recommendations as well as the assessment by conventional and tissue Doppler imaging 14 [peak velocities of early (E) and late (A) diastolic filling and deceleration time; and by TDI: early (E′) and late diastolic velocity (A′)]. RV function was assessed visually and defined as normal/mildly reduced/reduced RV function. TAPSE was not measured in all patients. Raw data of 14 women were stored digitally as DICOM cine loops and transferred for offline analysis to a workstation with the EchoPAC software (PC Dimension version 5.0.1, GE Vingmed Ultrasound, Horten, Norway). Longitudinal strain (LGS) was analysed in the three apical views (which were then averaged). Relapse of PPCM during an SSP was defined as a decrease of LVEF to 45% or less with or without symptoms or absolute decrease in LVEF of ≥10% in patients with persistent LV dysfunction before their SSP. 3
Statistical methods
Continuous variables are presented as mean ± SD. A repeated measures analysis of variance was used to determine any significant differences between variability over time. Friedman test was used to detect differences in LVEF across the trimesters and post‐delivery. Wilcoxon signed rank test was used to detect differences in LVEF between two time points. P value < 0.05 was considered significant. Significance values were adjusted by the Bonferroni correction for multiple tests. Statistical analysis was performed using IBM SPSS version 25.0 (Armonk, NY, USA).
Results
Among 146 women with PPCM who were prospectively followed at the Kaplan and Sheba Medical Centers between 2001 and 2019, 50 women had 75 SSPs.
Index pregnancy characteristics
The clinical characteristics of patients with SSP at the time their index event is presented in Table 1 . The mean age at the time of PPCM presentation was 29.3 ± 4.7 years; the mean parity was 1.7 ± 1.0. Twin pregnancies were reported in 20%. Gestational hypertension during pregnancy was present in 20% and pre‐eclampsia in 30%. Symptoms developed post‐partum in 78% of women. The LVEF at diagnosis was 35.9 ± 8.7% and 53.0 ± 9.6% at 6‐month follow up. Recovery of LV function (LVEF ≥50%) was reported in 44 (88%) and (LVEF ≥55%) in 42 (84%) women. All patients received ACEi/ARB and B blockers and 14% (7/50) received bromocriptine during their index pregnancy.
Table 1.
Clinical characteristics of women with SSP at their index pregnancy
| Mean ± SD; n (%) | |
|---|---|
| Total women | 50 |
| Age (years) | 29.3 ± 4.7 |
| Origin | |
| European | 13 (28%) |
| North Africa | 16 (34%) |
| East Africa | 14 (29%) |
| Middle East | 7 (9%) |
| Parity | 1.7 ± 1.0 |
| Multipara | 21 (42%) |
| Twin or triplet pregnancy | 10 (20%) |
| Gestational hypertension | 10 (20%) |
| Pre‐eclampsia/eclampsia | 15(30%) |
| Gestational diabetes mellitus | 0 (0%) |
| Occurrence of symptoms antepartum | 11 (22%) |
| Gestational age of delivery (weeks) | 37.4 ± 3.2 |
| Caesarean delivery | 37 (74%) |
| Birth weight (kg) | 2770.3 ± 786.4 |
| LVEF (%) at presentation | 35.9 ± 8.7 |
| LVEF (%) at ≥6 months | 53.0 ± 9.6 |
| LV recovery to LVEF ≥55% at any time | 42 (84%) |
| LV recovery to LVEF ≥50% at any time | 44(88%) |
| Major complications | 20 (40%) |
LV, left ventricle; LVEF, left ventricle ejection fraction.
Major complications included death, heart transplantation, temporary circulatory support, cardiopulmonary arrest, fulminant pulmonary oedema/ventilation, thromboembolic complications, ventricular tachycardia, defibrillator, or pacemaker implantation.
Major complications including death, heart transplantation, temporary circulatory support, cardiopulmonary arrest, fulminant pulmonary oedema/ventilation, thromboembolic complications, ventricular tachycardia, defibrillator, or pacemaker implantation were reported in 22 (40%) patients.
SSP clinical characteristics
Out of all 75 SSPs, termination of pregnancy was reported in 16 cases (13 women). Eight of these patients terminated per medical advice, five had reduced LVEF with a mean of 48.4 ± 4.7% before termination, and two patients had LVEF of 60%. In five patients with reduced LVEF, the mean LVEF before termination was 48.4 ± 4.7% and 45.6 ± 5.6% at 6‐month follow‐up (P = 0.2). Eight women with spontaneous abortion had LVEF ≥55% with no changes at 6‐month follow‐up (58.0 ± 2.7 vs. 58 ± 2.3, P = ns).
Fifty‐nine pregnancies resulted in live births and were included in the analysis (Figure 1 ). Thirty‐three women had one pregnancy, 10 had two, and two had three pregnancies. Their clinical characteristics during SSPs are presented in Table 2 . The time from the index event to SSP was 50.3 ± 28.6 (5–132) months. Beta‐blockers were used in 17 women (29%), and furosemide and hydralazine/nitrates were used in three patients with LV dysfunction. Bromocriptine was not used in our patients during subsequent pregnancy. The occurrence of symptoms (shortness of breath, fatigue, chest pain) was reported in 31%; hypertension during pregnancy was found in 5% and diabetes in 5% of the patients. Thirty‐two women (54%) delivered by caesarean section (CS) all for obstetric indications. No foetal complications were reported. Data on breastfeeding were available in 31 women, and 19 of them (61%) breastfed their babies.
Figure 1.
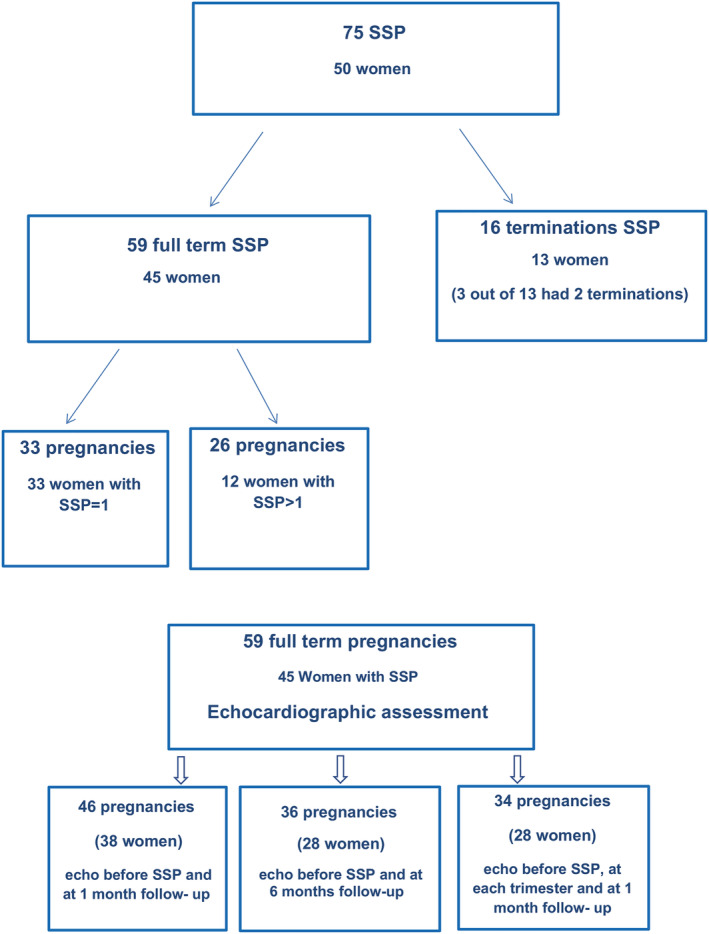
Derivation of the patient population of women with subsequent pregnancies and available echocardiographic follow‐up at different time points.
Table 2.
Maternal and obstetric outcomes of subsequent pregnancies
| Mean ± SD; n (%) | |
|---|---|
| Total subsequent pregnancies | 75 |
| SSP > 1 | 21 |
| Age (years at SSP) | 32.9± 4.1 |
| Time from PPCM to SSP (months) | 50.3 ± 28.6 (5–132) |
| Termination of pregnancy | 9 (12%) |
| Miscarriages | 7(9.33%) |
| Life birth | 59 (79%) |
| Gestation hypertension/pre‐eclampsia | 3 (5%) |
| Gestational diabetes mellitus | 3 (5%) |
| Symptoms (fatigue, chest pain, dyspnoea) | 18 (31%) |
| Medications during SSP | |
| Beta‐blockers | 17 (29%) |
| Furosemide | 3 (5%) |
| Baby aspirin | 6 (10%) |
| Hydralazine/nitrates | 3 (5%) |
| Gestational age at delivery (weeks) | 38 ± 1.6 |
| Vaginal delivery | 27 (46%) |
| Caesarean delivery | 32 (54%) |
| Birth weight (grams) | 2,969 ± 405 |
| Breastfeeding (n = 31) | 19 (61%) |
SSP, subsequent pregnancies.
LV function follow‐up during SSPs
Data on the LVEF before SSP and up to 1‐month post‐delivery were available in 46 pregnancies. There was a small but statistically significant decrease of the mean LVEF (57.9 ± 5.7% 55.4 ± 6.1%, P = 0.001) at 1 month after delivery. Parameters of diastolic function before SSP were in the normal ranges and remained unchanged at follow‐up: The mean E was 84.0 ± 17.4 vs. 88.0 ± 13.1 cm/s (P = ns), the mean A was 54.3 ± 11.6 vs. 62 ± 10 cm/s (P = ns), the E/A ratio 1.6 ± 0.4 vs. 1 E/A ratio 1.5 ± 0.3 (P = ns), the E′ was 10.7 ± 2.6 vs. 10.4 ± 3.1 cm/s (P = ns), and the E/E′ ratio was 6.8 ± 2.1 vs. E/E′ ratio was 7.3 ± 1.8 (P = ns). Echocardiographic measurements of the LVEF at 6‐month follow‐up were available in 36 SSPs (28 women). The mean group LVEF at 6 months remained somewhat lower than the pre‐SSP LVEF (57.4 ± 6.1 vs. 55.3 ± 7.9%, P = 0.025), mostly in women with pre‐pregnancy LVEF<55% (n = 10) (49.7 ± 5.8 vs. 46.6 ± 10.1, P = 0.03). RV function was normal before SSP and remained unchanged at follow‐up.
In 34 SSPs, LVEF measurements were available at each trimester and 1–4 weeks post‐delivery. There was a small but statistically significant decrease in LVEF at the third trimester (P = 0.01) and early post‐partum (P = 0.03) (Figure 2 ). When dividing these 34 SSPs into two groups, the first with LVEF <55% (n = 11) and the second with LVEF ≥55% (n = 23), decline in LVEF was observed in both groups (Figure 3 ). Contrary to women with LVEF <55% before SSP, those with normal LVEF (≥55%) did not drop the LVEF below normal range. Patients with LVEF<50% showed a significant decrease in LVEF especially in the third trimester (mean LVEF 51.1 ± 5.7% vs. 47.0 ± 7.4%, P = 0.001), which remained >50% early post‐delivery. A relapse was observed in three (25%) women with LVEF <55% and only in one (3%) with pre‐SSP LVEF ≥55%. This woman who had LVEF of 60% by echo and 65% by MRI and had a completely normal stress echo test before her SSP developed severe relapse of PPCM. Her SSP course was unremarkable on bisoprolol until the last month when her LVEF dropped to 45% with no symptoms. An elective CS was planned by obstetric indication at 39 weeks, but she refused. At week 40 + 2, she was admitted with uterine rupture and underwent an urgent CS. The LVEF at discharge remained unchanged (45%) with no symptoms of heart failure (HF). Two weeks after discharge, she developed superficial thrombophlebitis and was treated with LMWH and antibiotics. Four days later, she presented with cough and shortness of breath with no fever and was treated as bronchitis by her family physician, with no improvement. A few days later, she was admitted to the CCU with signs of severe HF, low blood pressures (90/60 mmHg), tachycardia of 130 bpm, pulmonary congestion, pleural effusion, and leg oedema. Her echo revealed LVEF 15%, severe MR, moderate TR, pulmonary hypertension, and a small pericardial effusion. The NT‐proBNP level was 12.000 pg/mL. She was treated aggressively with IV diuretics and vasodilators. With maximal HF treatment (bisoprolol, spironolactone, enalapril, Lasix, LMWH), her LVEF gradually improved to 35–38% (mild MR and TR) on discharge (NT‐proBNP level was 320 pg/mL) and 45% at 5‐month follow‐up. She is still on bisoprolol and enalapril.
Figure 2.
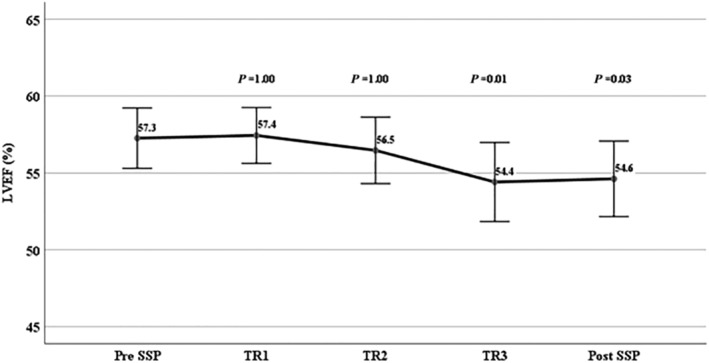
Changes in LVEF during each trimester and after (1 week to 1 month). SSP, subsequent pregnancy; TR, trimester.
Figure 3.
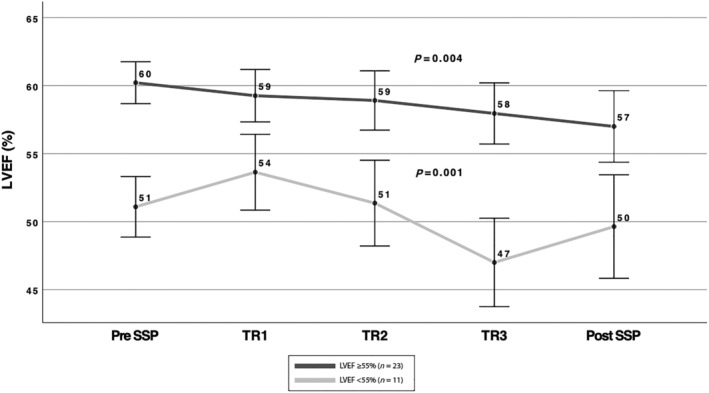
Changes in LVEF during each trimester and after (1 week to 1 month) delivery in patients with LVEF ≥55% and LVEF<55%. The black line represents changes in LVEF during pregnancies in patients with ≥55% (n = 23), and the grey line in patients LVEF <55% (n = 11). SSP, subsequent pregnancy; TR, trimester.
Ten women with more than one SSPs had a complete follow‐up (eight had two and two patients had three SSPs). No relapse occurred during the second or third SSP. No significant difference in changes of LVEF was seen between each SSP. The group average LVEF prior and 1‐month post‐partum at first SSP was 57.5 ± 3.5 vs. 55.0 ± 1.0%; at second, 56.5 ± 2.1 vs. 55.1 ± 1.2%; and at third, 58.0 ± 2.0 vs. 57.2 ± 0.8% (P = ns between each SSP). Based on these results, we did not find a clear cumulative effect of decrease in LVEF in women with >1 SSP.
In 14 women, two‐dimensional speckle tracking strain analysis was performed at each trimester. Figure 4 shows significant changes in LVEF (P < 0.001) in these women during pregnancy and post‐delivery, mostly at the third trimester (58 ± 6.5 vs. 53.0 ± .0.7, P = 0.04). Similarly, a significantly impaired longitudinal strain was obtained during SSPs (P < 0.001) in all patients, especially at the third trimester (−19.9 ± 2.0 vs. −17.0 ± 2.6, P < 0.0001). Analysis of individual data indicated impaired 2DS even in four (29%) patients who did not show a significant change in the LVEF.
Figure 4.
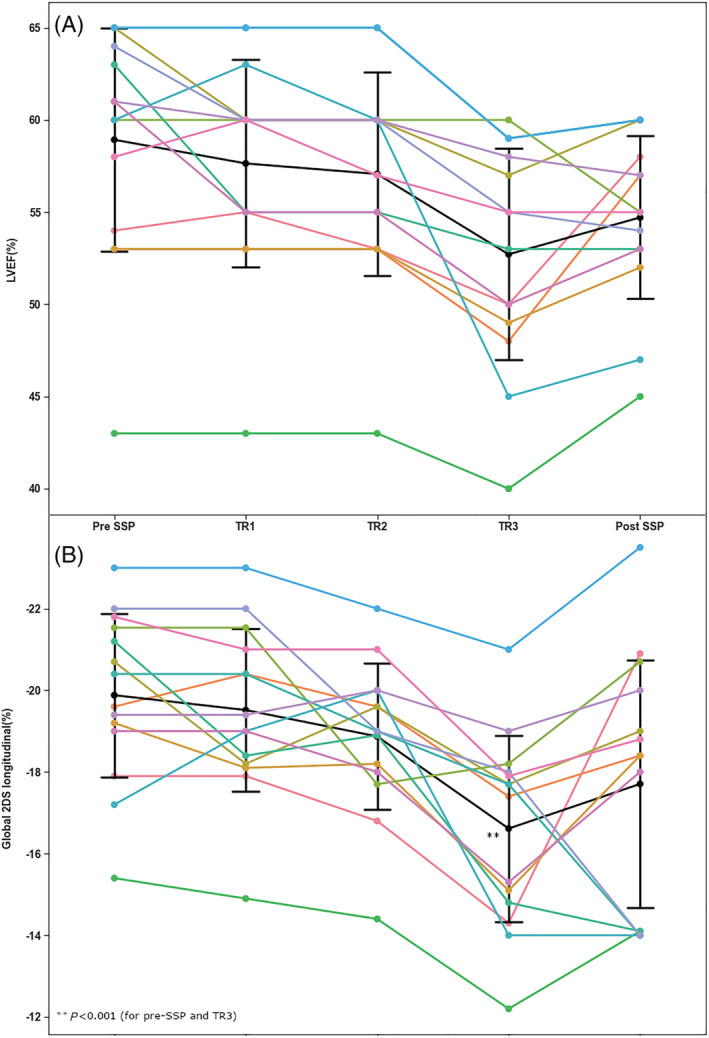
Individual changes in LVEF and 2D global longitudinal strain during each trimester and after (1 week to 1 month) delivery. (A) Individual changes in LVEF during each trimester and after (1 week to 1 month) delivery. The black line represents changes in the mean LVEF. P < 0.001 between all time points. LVEF, left ventricular ejection fraction. (B) Changes in global longitudinal strain in LVEF during each trimester and after (1 week to 1 month) delivery. The black line represents changes in the mean global longitudinal strain. P < 0.001 between all time points. 2DS, two‐dimensional strain; LVEF, left ventricular ejection fraction; SSP, subsequent pregnancy; TR, trimester.
Discussion
In this prospective observational study, we provided detailed information on outcomes of SSP and changes in LV function in women with a history of PPCM. Most of our patients experienced LV recovery (LVEF ≥55%) after their index event and according to the recent European Society of Cardiology guidelines for the management of cardiovascular diseases would not be advised against additional pregnancy. The main findings of this study are the following: (i) Subsequent pregnancies even in women with recovered LVEF were associated with a slight reduction in LVEF, which persisted up to 6 months after delivery. (ii) This decrease in LVEF was observed mostly in the third trimester and was more prominent in patients with LVEF <55% prior to SSP. In patients with LVEF ≥55%, mean LVEF was mildly reduced but remained within normal ranges. (iii) A relapse was observed only one patient among patients with LV recovery to LV ≥55% and in three patients (25%) in women with LV LVEF <55% and was not associated with mortality.
The risk of SSP is the most common concern raised by women with a history of PPCM. 15 Several studies have shown a substantial risk of SSP related deterioration of LV function. Despite considerable geographical and ethnic differences, different data collection methods and variability of LV pre‐SSP and post‐SSP assessment information, the majority of these studies have identified pre‐SSP LVEF to be a strong predictor of deterioration of cardiac function during SSP. 5 , 16
The PPCM relapse was low in our cohort of patients with LV recovery (LV ≥55%) and significantly lower in patients with persistent LV dysfunction than previously reported. 3 , 4 , 5 , 6 , 17 , 18 In our study, we used the definition of relapse similar to the study by Fett et al. 3 who reported on 61 post‐PPCM pregnancies and described relapses of PPCM in 29% of the entire group, with a significantly higher rate (46%) in women with LVEFs <55% and 17% in women with LVEFs ≥55%. Using the same definition of relapse, Codsi et al. 6 reported on SSP in 25 patients in the USA with a history of PPCM with LV recovery defined as LVEF ≥50%. The relapse rate of PPCM among patients with LVEF ≥50% was 21%. Lower rates of relapse in our cohort from Israel may be explained by differences in patient population including ethnicity and higher level of LVEF used for definition of recovery (≥55%).
Three retrospective reports from the USA were published on outcomes of SSP. Elkayam et al. 4 described reductions of >20% in the LVEF in 21% of the group with LV recovery with no mortality cases and in 44% of pregnancies with persistent LV dysfunction with high mortality rate of 19%. Clinical worsening of HF was observed in almost third of the women with PPCM during their SSPs in another two studies. 19 , 20 In contrast to the current study, these three retrospective reports described a higher percentage of patient with persistent LV dysfunction and significantly lower mean LVEF before SSP. In addition, more than half of the patients included in these studies were of African American descent, a population that has been previously shown to have lower rates of LV function recovery and worse survival. In the recently published comprehensive review of literature, Elkayam 5 summarized the data on 191 SSPs and showed that the risk of deterioration of LV function and mortality in patients with persistent LV dysfunction before SSP was much higher compared to those who normalized LV function (48% vs. 27% and 16% vs. 0%, respectively). Although mortality rate was low, three patients with recovered LV function before SSP developed severe complications including cardiac arrest, cardiogenic shock, ventricular arrhythmias, and even need for temporary LV assist devices. In line with our report, normalized LV function was usually associated with good outcome but did not guarantee an uncomplicated subsequent pregnancy. 5 , 7
Two prospective studies on SSP were published recently. The study from Germany, Scotland, and South Africa by Hilfiker‐Kleiner et al. included 34 women with SSP; 75% of the patients with persistent LV dysfunction (LVEF <50%) were of African origin. Once again, this study demonstrated a high risk of relapse defined as LVEF <50% in women with persistent LV dysfunction (compared with 25% in the current study). Like the results of our study, there was a 12% decrease in LVEF in women with recovered cardiac function prior to SSP, which persisted at 6 months in half of the patients, while no mortality was reported. While this study described a better outcome in women who received bromocriptine in addition to standard therapy, none of our patients received it. Recently, Yameogo et al. reported mortality rate as high as 48% in 29 African women with SSPs. Notably, that 2/3 of the patients included in this study had persistent LV dysfunction before the SSP, 18 suggesting that their clinical course and changes in LV function during SSP may be worse than those observed in Caucasians. Regional differences in myocardial recovery were recently reported by the PPCM ESC EORP registry of higher recovery rates in Asia‐Pacific (62%) and Europe (57%), lower in Africa, and the lowest in the Middle East (25%). 21 In addition, the definition of relapse and cut‐off of LVEF for recovery used in this study differed from ours; therefore, direct comparison between these studies is difficult.
Regarding changes in LV function during SSP and after delivery, in women with pre‐SSP LV recovery (LVEF ≥55%), we found a mild and not clinically significant reduction in the mean group LVEF; in addition, all patients but one remained with LVEF ≥55% at 6 months after delivery. This finding was supported by the study from Codsi et al. that described normalization of the LVEF during 0–24 months after delivery in all patients. 6 Importantly, the largest reduction in LVEF in our study was obtained mostly at the third trimester and 1‐month post‐partum in patients with LVEF <55% and persisted up to 6 months. Similar findings on timing of the reduction of LVEFs during SSP were reported previously. 5 , 22
Other indicators of full cardiac function recovery in addition to LVEF were suggested to predict risk for relapse of PPCM during SSP. Fett et al. 3 reported on nine patients with recovered LV function before SSP who underwent stress echocardiography demonstrating normal contractile reserve and did not experience a relapse during their SSP. Although this finding may suggest better outcome of SSP in women with LV recovery and normal contractile reserve, it has not been tested in a larger number of patients and in patients with evidence for a decreased contractile reserve. In our study, a severe relapse occurred in a woman with normal contractile reserve and a normal cardiac MRI. A previous study by our group demonstrated the presence of residual impairment of cardiac function as indicated by reduced myocardial strain after recovery of LVEF in women with PPCM at least 12 months after the diagnosis. 8 In the current study, we found an altered longitudinal 2DS during SSP even in patients with unchanged LVEF, suggesting the presence of some impairment of LV systolic function. The implications of this finding should be tested in a prospective study including a large number of women with SSP.
Regarding obstetric complications, in line with our data, high rates of caesarean delivery for obstetrical reasons were reported in previous studies, 6 , 22 which were mostly due to obstetrical rather than cardiac reasons. Preterm labour and induction of labour for hypertensive disorders were described in patients with PPCM during SSP. 6 Due to very low incidence of hypertension and pre‐eclampsia in our cohort, these complications were not reported.
As mentioned above, two prospective studies published by Hilfiker‐Kleiner et al. (n = 34) and Yameogo et al. (n = 29) described the outcomes of SSP in patients with a history of PPCM in a population where the majority were women of African descent with a predominance of low socio‐economic status and low recovery rates and high mortality. Our prospective observational study included a substantially larger number of pregnancies compared to the previous studies. It is important to note that the present and our previously published studies 8 represent a different, lower‐risk patient population with a significantly better prognosis at the index PPCM event and was managed during SSP in a dedicated cardio‐obstetric clinic at two tertiary care centres, which could have had an effect on the outcome. All this can provide important additional information for the challenging counselling of different patient populations of women with a history of PPCM. In addition, in contrast to other studies examining the outcome of SSP in women with PPCM, echocardiographic information of LV function during SSP and at follow‐up was not obtained from medical records but was prospectively assessed and reviewed in both institutional echo labs providing special attention to accurate measurements of LVEF. In addition, changes in 2DS may add further prognostic information over LVEF for future risk stratification. More information is necessary in order to assess the long‐term clinical implications of this finding.
Due to the observational nature of the study, most patients but not all of them arrived at all the scheduled echocardiographic examinations.
In summary, our study supports previous reports indicating a favourable outcome and low likelihood of maternal mortality associated with SSP in women with a history of PPCM and recovered LV systolic function. The study, however, also confirms that SSP in women with a history of PPCM is associated with a mild and partially persistent reduction in LV function especially in women with LVEF <55%. The long‐term clinical significance of this change in LVEF requires further investigation. Similar to the typical presentation of PPCM, fall in LVEF associated with SSP occurs during the last trimester or the first month post‐partum and should be anticipated. Altered 2DS was observed even in women with unchanged normal LVEF. More information is necessary in order to assess the long‐term clinical implications of this finding.
Even though the prognosis of SSP in patients with normalized LV function seems to be good, the life‐threatening deterioration in cardiac function in a single patient with complete recovery of LVEF prior to SSP in this study and similar anecdotal reports elsewhere 5 emphasize the need of pre‐conceptual evaluation for women with a history of PPCM who contemplate an additional pregnancy. Women should be counselled about the risk of recurrence during subsequent pregnancy and need to be closely followed by a multidisciplinary team of obstetricians and cardiologists during pregnancy and the post‐partum period. 1 , 5 , 23
Conflict of interest
None declared.
Acknowledgements
We thank Ms Metal Biner for her contributions in clinical data collection and Mrs Orly Edri and Anat Mahadi for their assistance in echo data acquisition and collection.
Goland, S. , George, J. , Elkayam, U. , Shimoni, S. , Fugenfirov, I. , Vaisbuch, E. , Arad, M. , Freimark, D. , Simchen, M. , and Kuperstein, R. (2022) Contemporary outcome of subsequent pregnancies in patients with previous peripartum cardiomyopathy. ESC Heart Failure, 9: 4262–4270. 10.1002/ehf2.14141.
References
- 1. Sliwa K, Petrie MC, Hilfiker‐Kleiner D, Mebazaa A, Jackson A, Johnson MR, van der Meer P, Mbakwem A, Bauersachs J. Long‐term prognosis, subsequent pregnancy, contraception and overall management of peripartum cardiomyopathy: Practical guidance paper from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2018; 20: 951–962. [DOI] [PubMed] [Google Scholar]
- 2. Hilfiker‐Kleiner D, Haghikia A, Nonhoff J, Bauersachs J. Peripartum cardiomyopathy: Current management and future perspectives. Eur Heart J. 2015; 36: 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet. 2010; 109: 34–36. [DOI] [PubMed] [Google Scholar]
- 4. Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, Wani OR, Hameed A, Gviazda I, Shotan A. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001; 344: 1567–1571. [DOI] [PubMed] [Google Scholar]
- 5. Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol. 2014; 64: 1629–1636. [DOI] [PubMed] [Google Scholar]
- 6. Codsi E, Rose CH, Blauwet LA. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol. 2018; 131: 322–327. [DOI] [PubMed] [Google Scholar]
- 7. Fett JD, Shah TP, McNamara DM. Why do some recovered peripartum cardiomyopathy mothers experience heart failure with a subsequent pregnancy? Curr Treat Options Cardiovasc Med. 2015; 17: 354. [DOI] [PubMed] [Google Scholar]
- 8. Goland S, Weinstein JM, Zalik A, Kuperstein R, Zilberman L, Shimoni S, Arad M, Ben Gal T, George J. Angiogenic imbalance and residual myocardial injury in recovered peripartum cardiomyopathy patients. Circ Heart Fail. 2016; 9: e003349. [DOI] [PubMed] [Google Scholar]
- 9. Lampert MB, Weinert L, Hibbard J, Korcarz C, Lindheimer M, Lang RM. Contractile reserve in patients with peripartum cardiomyopathy and recovered left ventricular function. Am J Obstet Gynecol. 1997; 176: 189–195. [DOI] [PubMed] [Google Scholar]
- 10. Regitz‐Zagrosek V, Roos‐Hesselink JW, Bauersachs J, Blomström‐Lundqvist C, Cífková R, de Bonis M, Iung B, Johnson MR, Kintscher U, Kranke P, Lang IM, Morais J, Pieper PG, Presbitero P, Price S, Rosano GMC, Seeland U, Simoncini T, Swan L, Warnes CA, ESC Scientific Document Group , Deaton C, Simpson IA, Aboyans V, Agewall S, Barbato E, Calda P, Coca A, Coman IM, de Backer J, Delgado V, di Salvo G, Fitzsimmons S, Fitzsimons D, Garbi M, Gevaert S, Hindricks G, Jondeau G, Kluin J, Lionis C, McDonagh TA, Meier P, Moons P, Pantazis A, Piepoli MF, Rocca B, Roffi M, Rosenkranz S, Sarkozy A, Shlyakhto E, Silversides CK, Sliwa K, Sousa‐Uva M, Tamargo J, Thorne S, van de Velde M, Williams B, Zamorano JL, Windecker S, Aboyans V, Agewall S, Barbato E, Bueno H, Coca A, Collet JP, Coman IM, Dean V, Delgado V, Fitzsimons D, Gaemperli O, Hindricks G, Iung B, Jüni P, Katus HA, Knuuti J, Lancellotti P, Leclercq C, McDonagh TA, Piepoli MF, Ponikowski P, Richter DJ, Roffi M, Shlyakhto E, Simpson IA, Sousa‐Uva M, Zamorano JL, Hammoudi N, Piruzyan A, Mascherbauer J, Samadov F, Prystrom A, Pasquet A, Caluk J, Gotcheva N, Skoric B, Heracleous H, Vejlstrup N, Maser M, Kaaja RJ, Srbinovska‐Kostovska E, Mounier‐Vehier C, Vakhtangadze T, Rybak K, Giannakoulas G, Kiss RG, Thrainsdottir IS, Erwin RJ, Porter A, Geraci G, Ibrahimi P, Lunegova O, Mintale I, Kadri Z, Benlamin H, Barysiene J, Banu CA, Caruana M, Gratii C, Haddour L, Bouma BJ, Estensen ME, Hoffman P, Petris AO, Moiseeva O, Bertelli L, Tesic BV, Dubrava J, Koželj M, Prieto‐Arévalo R, Furenäs E, Schwerzmann M, Mourali MS, Ozer N, Mitchenko O, Nelson‐Piercy C. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018; 39: 3165–3241. [DOI] [PubMed] [Google Scholar]
- 11. Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, Tobin JR, Gunnar RM. Natural course of peripartum cardiomyopathy. Circulation. 1971; 44: 1053–1061. [DOI] [PubMed] [Google Scholar]
- 12. Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, Hosenpud JD, Ansari A, Baughman KL. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000; 283: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 13. Bauersachs J, König T, van der Meer P, Petrie MC, Hilfiker‐Kleiner D, Mbakwem A, Hamdan R, Jackson AM, Forsyth P, Boer RA, Mueller C, Lyon AR, Lund LH, Piepoli MF, Heymans S, Chioncel O, Anker SD, Ponikowski P, Seferovic PM, Johnson MR, Mebazaa A, Sliwa K. Pathophysiology, diagnosis and management of peripartum cardiomyopathy: A position statement from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail. 2019; 21: 827–843. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 15. Hess RF, Weinland JA. The life‐changing impact of peripartum cardiomyopathy: An analysis of online postings. MCN: Am J Matern Child Nurs. 2012; 37: 241–246. [DOI] [PubMed] [Google Scholar]
- 16. Hilfiker‐Kleiner D, Haghikia A, Masuko D, Nonhoff J, Held D, Libhaber E, Petrie MC, Walker NL, Podewski E, Berliner D, Bauersachs J, Sliwa K. Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail. 2017; 19: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 17. Fett JD. Reducing the risks for relapse of heart failure in a subsequent pregnancy after peripartum cardiomyopathy? Future Cardiol. 2017; 13: 305–310. [DOI] [PubMed] [Google Scholar]
- 18. Yaméogo NV, Samadoulougou AK, Kagambèga LJ, Kologo KJ, Millogo GRC, Thiam A, Guenancia C, Zansonré P. Maternal and fetal prognosis of subsequent pregnancy in black African women with peripartum cardiomyopathy. BMC Cardiovasc Disord. 2018; 18: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Habli M, O'Brien T, Nowack E, Khoury S, Barton JR, Sibai B. Peripartum cardiomyopathy: Prognostic factors for long‐term maternal outcome. Am J Obstet Gynecol. 2008; 199: 415.e1–415.e5. [DOI] [PubMed] [Google Scholar]
- 20. Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC. Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol. 2009; 201: 171.e1–171.e5. [DOI] [PubMed] [Google Scholar]
- 21. Sliwa K, Petrie MC, van der Meer P, Mebazaa A, Hilfiker‐Kleiner D, Jackson AM, Maggioni AP, Laroche C, Regitz‐Zagrosek V, Schaufelberger M, Tavazzi L, Roos‐Hesselink JW, Seferovic P, van Spaendonck‐Zwarts K, Mbakwem A, Böhm M, Mouquet F, Pieske B, Johnson MR, Hamdan R, Ponikowski P, van Veldhuisen DJ, McMurray JJV, Bauersachs J. Clinical presentation, management, and 6‐month outcomes in women with peripartum cardiomyopathy: An ESC EORP registry. Eur Heart J. 2020; 41: 3787–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sliwa K, Forster O, Zhanje F, Candy G, Kachope J, Essop R. Outcome of subsequent pregnancy in patients with documented peripartum cardiomyopathy. Am J Cardiol. 2004; 93: 1441–1443. [DOI] [PubMed] [Google Scholar]
- 23. Davis MB, Arany Z, McNamara DM, Goland S, Elkayam U. Peripartum cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020; 75: 207–221. [DOI] [PubMed] [Google Scholar]


