Abstract
Aims
The objective of the present study is to assess the bidirectional association between heart failure (HF) and atrial fibrillation (AF) using real‐world data.
Methods and results
From an electronic health recording with a population of 3 799 885 adult subjects, those with prevalent or incident HF were selected and followed throughout a study period of 5 years. Prevalence and incidence of AF, and their impact in the risk for acute HF hospitalization, worsening renal function, ischaemic and haemorrhagic stroke, and all‐cause mortality were identified. We analysed all incident and prevalent patients with HF and AF, 128 086 patients (S1), and subsequently analysed a subset of patients with incident HF and AF, 57 354 patients (S2). We analysed all incident and prevalent patients with HF and AF, 128 086 patients (S1), and subsequently a subset of patients with incident HF and AF, 57 354 patients (S2). The prevalence of AF was 59 906 (46.7%) of the HF patients, while incidence in the S2 was 231/1000 patients/year. In both cohorts, S1 and S2, AF significantly increases the risk of acute heart failure hospitalization [incidence 79.1/1000 and 97.5/1000 patients/year; HR 1.53 (1.48–1.59 95% CI) and HR 1.32 (1.24–1.41 95% CI), respectively], risk of decreased renal function (eGFR reduced by >20%) [66.2/1000 and 94.0/1000 patients/year; HR 1.13 (1.09–1.18 95% CI) and HR 1.22 (1.14–1.31 95% CI), respectively] and all‐cause mortality [203/1000 and 294/1000 patients/year; HR 1.62 (1.58–1.65 95% CI) and HR 1.65 (1.59–1.70 95% CI), respectively]. The number of episodes of hospitalization for acute heart failure was also significantly higher in the AF patients (27 623 vs. 10 036, P < 0.001). However, the risk for ischaemic stroke was reduced in the AF subjects [HR 0.66 (0.63–0.74 95% CI)], probably due to the anticoagulant treatment.
Conclusions
AF is associated with an increment in the risk of episodes of acute heart failure as well as decline of renal function and increment of all‐cause mortality.
Keywords: Heart failure, Atrial fibrillation, Hospitalization, Renal function, Stroke, All‐cause mortality
1. Introduction
Heart failure (HF) is a multifactorial disease with increasing prevalence 1 , 2 and with co‐morbidities that contribute to the progressive decline of heart function, increase hospitalization, and mortality. One of the most frequent co‐morbidities is atrial fibrillation, the most common sustained arrhythmia in the aged population. This condition develops as a consequence of multiple structural and haemodynamic conditions that affects heart function and contributes to the reduction of cardiac output. Moreover, AF increments risk for thromboembolic disease. Prevalence of both diseases, HF and AF, is expected to rise in the years to come as a result of higher life expectancy and the increment in the prevalence of cardiovascular risk factors, an alarming trend given that both AF and HF are accompanied by significant morbidity and mortality. Although the conditions may exist independently, they often go hand in hand as each of them is able to aggravate the other. 3 In addition, both HF and AF share a risk profile with coincident risk factors.
Several studies have analysed the interaction between AF and HF. Incident HF in AF patients is more frequent than stroke, 4 and it increases mortality twice as compared with AF control patients, 5 , 6 , 7 with the risk being even greater in younger patients. 7 Moreover, prevalence of AF in HF patients increases proportionally with the functional grade, achieving until the 50% in the functional class 4 of the NYHA. 8 The relevance of co‐morbidities in the cross link between HF and AF has not been considered until recently. 9 , 10 , 11
Randomized clinical trials and specific disease‐driven registers provided a high level of evidence on the natural history of this condition and on the impact of therapy. However, due to patient selection and/or data collected in cardiology centres, clinical relevant information may differ from the observed in daily clinical practice at primary care. 12 The risk for acute heart failure hospitalization, stroke, and mortality in HF patients with AF was biased due to the selection criteria of the registries. Real‐world data (RWD) obtained from electronic health records (EHR) is able to collect a large amount of information that can aid in evaluating treatment performance.
The objective of the present study is to assess the bidirectional association between HF and AF in HF patients using RWD. This study considers that EHR‐based studies from the general practice can be a representative setting to evaluate burden of disease associated with health conditions such as AF in HF.
2. Subjects and methods
2.1. Study population and baseline data collection
The study population was recruited from the universal health care system of the Valencian community with a population of 3 799 885 people older than 18 in 2012. One unique electronic centralized clinical record per patient exists. Total population data were extracted for the period of time between 1 January 2012 and 31 December 2016. Each patient is registered in the information systems, which collect information on outpatient and inpatient care, including diagnoses, medical and surgical procedures, imaging and laboratory tests, and pharmaceutical prescriptions, among others. This information was available for the research, anonymized in accordance with the Spanish Law of Data Protection, and with the approval for its study by the Committee for Ethics and Clinical Trials of the Hospital Clinico of Valencia. Spanish Law 3/2018 of Data Protection and Guarantee of Digital Rights and corresponding European norms (GDPR) 13 were followed.
Men and women were included in the study if they had a diagnosis of HF. The study population consisted of registered patients older than 24 years of age at baseline, with an active diagnosis of HF at some point during the study period, which amounted to 128 086 patients. Analysis of the total population (Study 1) and of those with incident HF (Study 2) during the study period was performed. During the follow‐up, incident AF was recorded. ICD codes used to select HF, AF as well as stroke, diabetes, and hypertension were in Supporting Information, Table S1 .
2.2. Cardiovascular risk factors and events definition
Hypertension and diabetes are defined based on physician diagnosis through ICD codes. Serum total cholesterol and high density‐lipoprotein (HDL) cholesterol were measured enzymatically. High density‐lipoprotein (HDL cholesterol) was calculated by using the Friedwald formula. Dyslipidaemia was defined by a total cholesterol >200 mg/dL and/or treatment with lipid lowering drugs. Serum creatinine was measured and estimated glomerular filtration rate (eGFR) was calculated from creatinine, age, and sex using the CKD‐EPI formula. 14 If ICD codes or other diagnoses based on lab values were missing, co‐morbidity was classified as no present.
Hospitalization data due to acute heart failure, ischaemic stroke, haemorrhagic stroke, and all‐cause mortality until the 31 December 2016 were collected. Events were assigned from ICD codes recorded at discharge, from hospitalizations or from the emergency room. Death numbers were extracted from the death registry. Follow‐up was calculated as the difference between the inclusion date and the date of the event, death, or 31 December 2016, whichever occurred first.
2.3. Statistical analysis
Two different sets of information were collected and analyses were performed: (i) Substudy 1 (S1), included all of the subjects with HF present at the starting point of the study, plus the patients with newly developed HF during the study period; (ii) Substudy 2 (S2), included subjects with a new diagnosis of HF during the study period in the absence of previous AF. Differences between subjects with or without AF were assessed by one‐way ANOVA and χ 2 for continuous and categorical variables. Incidence rate of events was expressed as number of cases/1000 patients/year. The hazard ratio (HR) of events based on the two groups, HF with and without AF, was evaluated by means of cumulative survival rates and Cox proportional hazards regression models. Proportional hazard and nonlinearity have not systematically been tested, although the large data set does reduce the risk of biased estimates. Models were adjusted by clinically relevant factors, including age, sex, hypertension, diabetes, coronary heart disease, dyslipidaemia, and drugs used for HF treatment or anticoagulation if required. When data, mainly lab values, were not available at the time of inclusion, a 6 month window around the time of study inclusion were used to consider the value as baseline. The free statistical software R 3.6.1 was used for the analyses.
3. Results
3.1. General characteristics of the study population
The number and main characteristics of patients in the two groups, S1 and S2, are shown in Table 1 . The average age was 75.6 years old, with a small predominance in women, 54.6%. The highest co‐morbidity was hypertension in 88%, followed by diabetes in 44% and coronary heart disease in 34%. Among subjects in which creatinine values were available, an eGFR <60 mL/min/1.73 m2 was present in 27%. The treatments that the patients received are also shown in Table 1 , being the most frequent diuretics in 84.1%, beta‐blockers in 50.2%, ARBs in 45.7%, ACE inhibitors in 34.4%, and anti‐aldosterone drugs in 29%. Although the number of HF subjects largely differs among the two study groups, no significant differences exist for the main characteristics.
Table 1.
General characteristics of the heart failure study population with and without atrial fibrillation
| Variable | Total population (S1) | Incident population (S2) | ||||
|---|---|---|---|---|---|---|
| Patients | No AF | AF | Patients | No AF | AF | |
| Number | 128 086 | 68 180 | 59 906 (46.7) | 57 354 | 43 003 | 14 351 (25.0) |
| Age (years) | 75.6 (11.6) | 74.2 (12.8) | 77.3 (9.9) | 74.1 (12.3) | 73.4 (12.8) | 76.2 (10.7) |
| Sex (female) | 69 994 (54.6) | 37 660 (55.2) | 32 334 (54.0) | 30 635 (53.4) | 23 140 (53.8) | 7495 (52.2) |
| Hypertension | 112 418 (87.8) | 58 179 (85.3) | 54 239 (90.5) | 49 023 (85.5) | 36 432 (84.7) | 12 591 (87.7) |
| Dyslipidaemia | 87 620 (68.4) | 47 210 (69.2) | 40 410 (67.5) | 39 048 (68.1) | 29 606 (68.8) | 9442 (65.8) |
| Diabetes mellitus | 56 670 (44.2) | 29 568 (43.4) | 27 102 (45.2) | 24 367 (42.5) | 18 211 (42.3) | 6156 (42.9) |
| CHD | 43 305 (34.0) | 22 597 (33.1) | 20 708 (34.6) | 22 941 (40.0) | 13 537 (31.5) | 4580 (31.9) |
| CKD | ||||||
| eGFR <60 mL/min/1.73 m2 |
34 829 (27.2) [80 631] |
16 615 (24.4) [42 533] |
18 214 (30.4) [38 098] |
13 967 (24.4) [33 989] |
9865 (22.9) [25 087] |
4102 (28.6) [8902] |
| eGFR and UAE |
21 966 (17.1) [37 426] |
10 375 (15.2) [19 366] |
11 591 (19.3) [18 060] |
8048 (14.0) [14 194] |
5670 (13.2) [10 372] |
2378 (16.6) [3822] |
| Drug treatment | ||||||
| Beta‐blockers | 64 316 (50.2) | 28 177 (41.3) | 36 139 (60.3) | 26 507 (46.2) | 17 656 (41.1) | 8851 (61.7) |
| ACEi | 44 124 (34.4) | 22 945 (33.7) | 21 179 (35.4) | 19 521 (34.0) | 14 108 (32.8) | 5413 (37.7) |
| ARB | 58 601 (45.7) | 30 747 (45.1) | 27 854 (46.5) | 25 461 (44.4) | 18 856 (43.8) | 6605 (46.0) |
| CCB | 41 787 (32.6) | 22 672 (33.3) | 19 115 (31.9) | 19 112 (33.3) | 14 131 (32.9) | 4981 (34.7) |
| Aliskiren | 1312 (1.0) | 686 (1.0) | 626 (1.0) | 238 (0.4) | 175 (0.4) | 63 (0.4) |
| Diuretics | 107 782 (84.1) | 53 982 (79.2) | 53 800 (89.8) | 46 927 (81.8) | 34 151 (79.4) | 12 776 (89.0) |
| Anti‐aldosterone | 37 142 (29.0) | 15 497 (22.7) | 21 645 (36.1) | 13 903 (24.2) | 9144 (21.3) | 4759 (33.1) |
| Alfa‐blockers | 13 659 (10.6) | 7165 (10.5) | 6494 (10.8) | 6165 (10.7) | 4529 (10.5) | 1636 (11.4) |
| Sacubitril | 312 (0.2) | 144 (0.2) | 168 (0.3) | 144 (0.3) | 93 (0.2) | 51 (0.4) |
| Anticoagulant | ||||||
| VKA | 42 607 (33.3) | 5066 (7.4) | 37 541 (62.7) | 10 258 (17.9) | 2910 (6.8) | 7348 (51.2) |
| NOAC | 922 (0.1) | 172 (0.3) | 9050 (15.1) | 2631 (4.5) | 97 (0.2) | 2534 (17.7) |
Abbreviations: ACEi, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; CCB, calcium channel blockers; CHD, coronary heart disease; CKD, chronic kidney disease; eGFR, estimate glomerular filtration rate; NACO, new oral anticoagulant drugs; VKA, vitamin K antagonists.
Note: Values in parentheses are percentage. Values in square brackets are the total number of subjects with renal function available for the analysis.
The number of patients with AF differed between group S1 and S2, the highest 59 906 (46.7%) and 14 351 (25%), respectively. In the S1, patients with AF were older, 77.3 year versus 74.2; have a slightly less frequency in women, 54% versus 55.2%; and more hypertension, 90.5% versus 85.3%; chronic kidney disease, 30.4% versus 24.4%; diabetes, 45.2% versus 43.4%; and coronary heart disease, 34.6% versus 33.1%. Concerning treatments, more diuretics, anti‐aldosterone drugs, and beta‐blockers were present in AF patients. The characteristics of patients in S2 were similar to those of S1, with a higher percentage of treatment with diuretics, ACE inhibitors, ARBs, and anti‐aldosterone drugs. Anticoagulant treatment predominated with VKA as compared with NOAC in both S1 and S2, but no anticoagulant treatment was present in 22.2% and 31.1% of the S1 and S2 groups, respectively.
The total number of events recorded are in Table 2 .
Table 2.
Incidence of events during the study period
| Variable | Total prevalence (S1) | Incident population (S2) | ||
|---|---|---|---|---|
| Number (%) | Incidence 1000/year | Number (%) | Incidence 1000/year | |
| Number | 128 086 | 57 354 | ||
| Atrial fibrillation | 30 278 (23.6) | 116 | 14 351 (25.0) | 116 |
| AHF hospitalization | 21 751 (17.0) | 79.1 | 7251 (12.6) | 79.1 |
| Ischaemic stroke | 17 088 (13.3) | 60.9 | 6573 (11.5) | 60.9 |
| Haemorrhagic stroke | 811 (0.6) | 2.7 | 288 (0.5) | 2.7 |
| >20% eGFR decrease | 18 513 (23.0) | 66.2 | 7067 (20.8) | 66.2 |
| All‐cause mortality | 61 420 (48.0) | 203 | 24 305 (42.4) | 203 |
Abbreviations: AHF, acute heart failure; eGFR, estimate glomerular filtration rate.
Note: Values in parentheses are percentage.
3.2. Prevalence and incidence of AF in HF
In the total population, prevalence of AF was present in 59 906 patients, 46.7%; among them 20 879 corresponded to patients/year, Figure 1A . The risk for incident AF increased slightly by age (HR 1.02, P95th CI 1.02–1.02) and was lower in women as compared with men (HR 0.78, P95th CI 0.75–0.80). Hypertension (HR 1.04, P95th CI 0.99–1.08) and diabetes (HR 1.03, P95th CI 1.0–1.06) marginally increased the risk. Treatment with diuretics (HR 0.55, P95th CI 0.53–0.57), anti‐aldosterone drugs (HR 0.90, P95th CI 0.87–0.94), beta‐blockers (HR 0.96, P95th CI 0.93–0.99) and RAS blockers, ACE inhibitors (HR 0.71, P95th CI 0.68–0.73), and ARBs (HR 0.87, P95th CI 0.84–0.90) reduced the risk of AF. Few patients received treatment with the renin inhibitor aliskiren and the combination of valsartan/neprilysin. A forest plot of risk is shown in Supporting Information, Figure S1A . In subjects in which eGFR values were available, the forest plot is in Supporting Information, Figure S1B .
Figure 1.
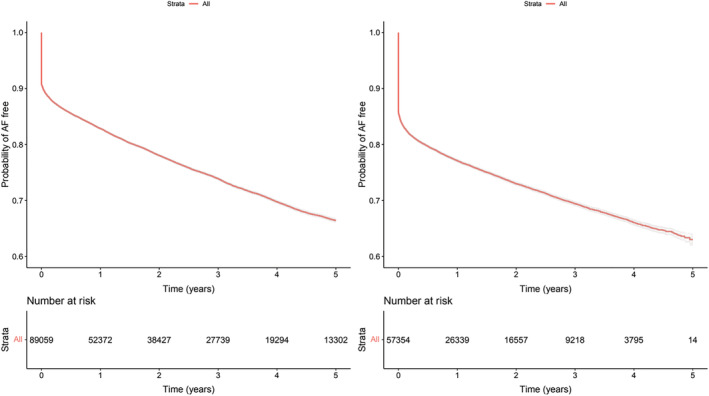
Survival curves with 95th confidence interval of being free of AF. Panel A, left side (S1) Total population; Panel B right side (S2) New diagnosis of HF.
In incident study, new diagnoses of HF in the absence of previous AF were seen in a total of 57 354 patients, of which 14 351 (25.0%) developed AF, incidence 231/1000 patients/year, Figure 1B . A large proportion of the incidents, 10 417 (72.6%) were diagnosed around 3 months of the HF diagnosis. The factors of risk for developing AF were similar to those observed in the total population, increasing with age, lower in women, and slightly increased in hypertension and diabetes. Treatment with diuretics, anti‐aldosterone drugs, beta‐blockers, and RAS blockers also reduced the risk; see the forest plot of risk in Supporting Information, Figure S1C , complemented in subjects with eGFR values available, the forest plot is in Supporting Information, Figure S1D .
3.3. AF and risk of hospitalization
AF is associated with increment in the number, incidence, and hospitalization due to acute heart failure in S1 and S2, Figure 2A,B . In S1, a total of 21 751 (17.0%) patients were hospitalized, incidence 79.1/1000 patients/year, while in S2, there were 7251 (12.6%) patients, incidence 97.5/1000 patients/year. Adjusted for potential confounders, AF increases the risk in S1 (HR 1.53, P95th CI 1.48–1.59) and in S2 (HR 1.32, P95th CI 1.24–1.41). The percentage of patients hospitalized with one, two, three, or more episodes among those with AF were 15%, 5%, and 5%, respectively, while in patients without AF, the percentages were 7%, 2%, and 1% (P < 0.001). The increased risk of hospitalization due to acute heart failure with AF was significant, accounting for a total of 27 623 hospitalizations versus the 10 036 from the non‐AF group (P < 0.001).
Figure 2.
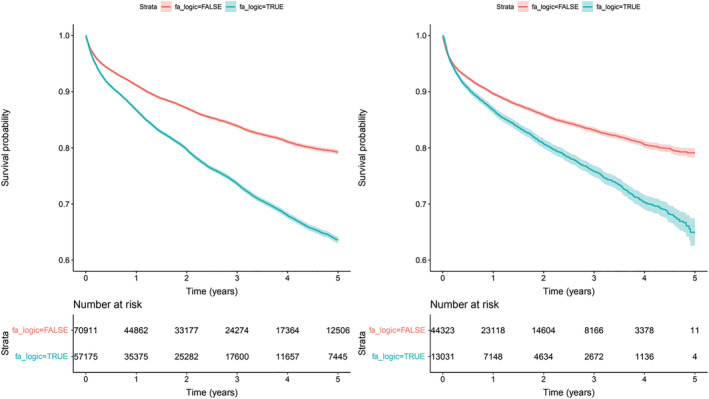
Risk of hospitalization by AF. Survival curves with 95th confidence interval. Panel A, left side (S1) Total population (p<0.0001); Panel, right side (S2) New diagnosis of HF (p<0.0001).
3.4. AF and neurologic events
An additional risk produced by AF in these patients is vascular embolism that mainly impacts the cerebrovascular territory. In S1, 17 088 (13.3%) ischaemic strokes were recorded with an incidence of 60.9/1000 patients/year with AF significantly increasing the risk (HR 1.17, P95th CI 1.12–1.21) (Figure 3A ). In S2, 6573 ischaemic strokes were recorded with an incidence of 87.7/1000 patients/year; however, in this case, AF presented a reduced risk (HR 0.66, P95th CI 0.63–0.74) (Figure 3B ). Concerning the impact of anticoagulant treatment, in S1, both NOAC and VKA significantly reduced the risk (HR 0.62 P95th CI 0.57–0.67; 0.88 P95th CI 0.84–0.92, respectively) (Figure 3C ), while in S2, only NOAC resulted in reducing risk (HR 0.61 P95th CI 0.54–0.68) as compared with VKA (HR 0.98 P95th CI 0.93–1.03) (Figure 3D ).
Figure 3.
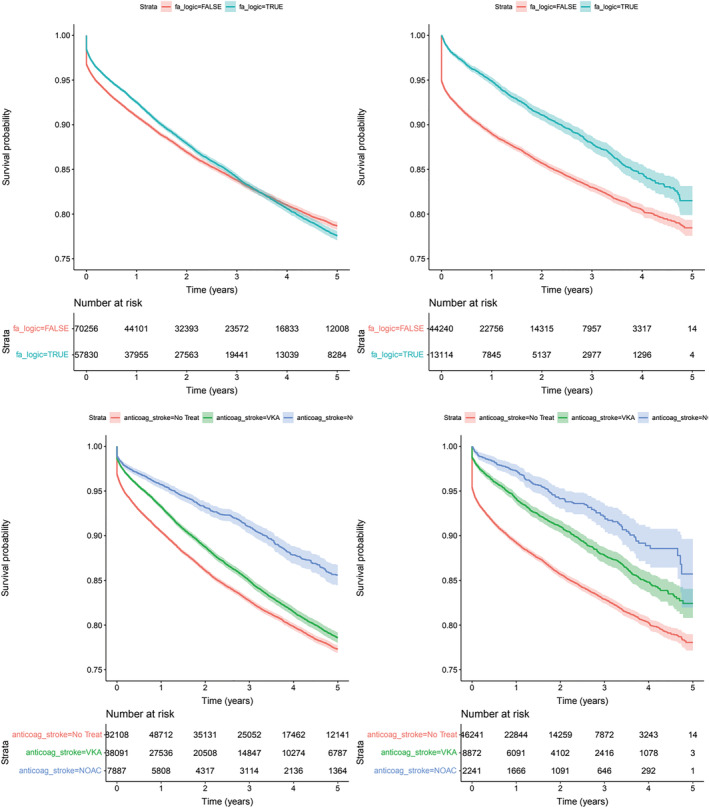
Risk of ischemic stroke by AF. Survival curves with 95th confidence interval and impact of anticoagulant treatment (blue NOAC, green VKA, red untreated). Panel A upper panel left side (S1) Total population (p=0.0035); Panel B upper panel right side (S2) New diagnosis of HF (p<0.0001); Panel C bottom panel left side (S1) Total population (p<0.0001); Panel D bottom panel right side (S2) New diagnosis of HF (p<0.0001).
The number of haemorrhagic strokes was 811 and 288 in S1 and S2, respectively, incidence 2.7/1000 patients/year and 3.5/1000 patients/year. AF increased the risk in S1 (HR 1.23, P95th CI 1.01–1.50) but not in S2 (HR 0.88, P95th CI 0.63–1.22).
3.5. AF and progression in renal dysfunction
The association of AF on a reduction of eGFR by >20% was analysed. In S1, 18 513 patients had significantly reduced eGFR values, incidence 66.2/1000 patients/year (Figure 4A ) with AF increasing the risk (HR 1.13, P95th CI 1.09–1.18, P < 0.001) more so than the presence of diabetes, hypertension, dyslipidaemia, and coronary heart disease. The association with an increment of risk was also demonstrated in S2 (Figure 4B ). The number of subjects with decreased eGFR values was 7067, incidence 94.0/1000 patients/year with AF increasing the risk (HR 1.22, P95th CI 1.14–1.31, P < 0.001) independent of the other potential factors. In the subgroup of patients with KDIGO risk classification, 10 425 (27.8%) and 3297 (23.2%) increased by at least one category in S1 and S2, respectively.
Figure 4.
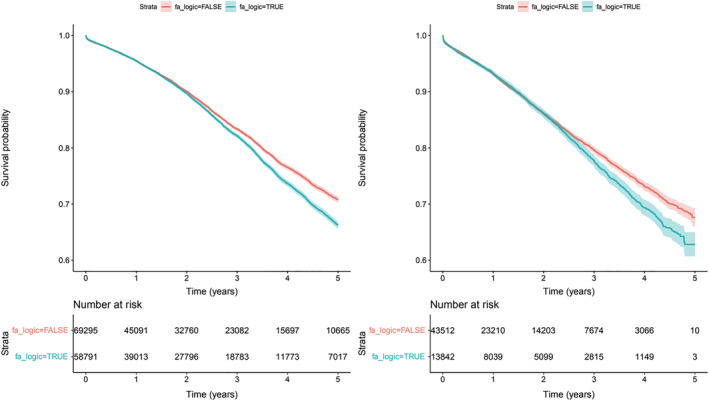
Risk of eGFR reduction >20%. Survival curves with 95th confidence interval. Panel A, left side (S1) Total population (p<0.0001); Panel B right side (S2) New diagnosis of HF (p=0.0006).
3.6. AF and mortality
All‐cause mortality in the total population was observed in a total of 61 420 patients, 32 450 (52.8%) of them with AF (Figure 5A ). Incidence was 203/1000 patients/year and AF significantly increased the risk (HR 1.62; 95th CI 1.58–1.65). In those with incident HF (Figure 5B ), S2, mortality was observed in 24 305, incidence 294/1000 patients/year. The risk of all‐cause mortality was significantly higher in subjects with AF (HR 1.65; 95th CI 1.59–1.70).
Figure 5.
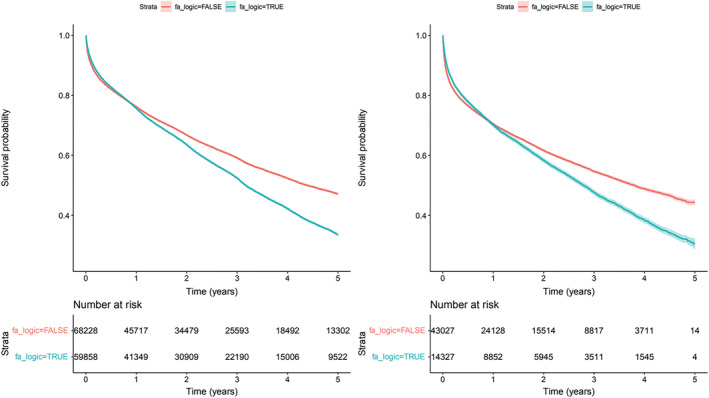
Risk of all‐cause mortality by AF. Survival curves with 95th confidence interval. Panel A left side (S1) Total population (p<0.0001); Panel B right side (S2) New diagnosis of HF (p<0.0001).
4. Discussion
In a large cohort of patients with HF, the bidirectional association with AF was assessed using RWD, allowing to observe an unselected groups of patients encountered in the clinical practice setting. In HF patients, the risk of developing AF increased with age and was lower for women as compared with men. A diagnosis of previous hypertension or diabetes did not increase the risk of HF when AF is present. Treatment with diuretics, anti‐aldosterone drugs, beta‐blockers and RAS blockers, ACE inhibitors, and ARBs seems to be that reduced the risk of developing AF. Patients with HF who develop AF have a higher risk of acute heart failure hospitalization and haemorrhagic stroke, faster renal function reduction, and all‐cause mortality. The decreased risk of stroke and TIA observed can be explained by the anticoagulant treatment received in the AF patients.
AF is a frequent condition that requires an integrated management, the so‐called Atrial Fibrillation Better Care (ABC) pathway with holistic approach due to the high frequency of co‐morbidities, one of the most relevant due to the clinical consequences is HF. 15 The Guidelines of the ESC developed with European Heart Rhythm Association (EHRA) of the ESC recognized that the management of patients with AF and HF is often challenging and that all patients with HF and AF should receive guideline‐adherent HF therapy. 16 However, in a recent meta‐analysis, the adherence to the ABC is low, around 21%, and heterogeneous in the application, 17 the same that in the ESC‐EHRA EURObservational Research Programme in AF General Long‐Term Registry. 18
The present study included one of the largest numbers of patients with HF published, with a 5 year follow‐up. In the design, we divided the study population into two blocks: one including all patients available and the other including patients without HF or AF before the starting point of the study. In the prevalence, S1, analysis was unable to provide the natural history of AF in HF because many of the patients had AF before the study period. It was possible, however, to assess the clinical load of having AF in HF patients. In the incident, S2, it was possible to assess a precise time of risk for the development of AF in new incident HF.
Heart failure is one of the most influential factors in increasing the risk of AF. In fact, HF and age‐per‐HF are components of the all score risk for AF. 19 , 20 , 21 The increased risk of HF patients to develop AF can primarily be explained by structural atrial remodelling, mitral valve regurgitation, and altered neurohumoral balances. A large number of studies reported that the prevalence of AF in HF patients reportedly differ due to the particularities of the different registers, from 23% to 33% in HF with reduced ejection fraction, to 33% to 65% in HF with preserved ejection fraction. 22 , 23 , 24 , 25 In the present study, the prevalence of AF in HF patients was 47% without considering the type of ejection fraction.
Studies of incidence, however, are scarce because the initiation of HF or AF is not easily identified in previous registers. In the present study, the incidence design allowed for the obtention of reference points for the calculation because subjects were selected without previous diagnosis of HF and in the absence of AF. Overall, the incidence of AF and events was higher in the recently diagnosed patients (Table 2 ). The incidence of AF in these patients was 236/1000 patients/year, figure that was twice the incidence observed for AF in the total population, 116/1000 patients/year. Differences between the two incidences of AF can be explained because a large proportion of patients, both HF and AF, are diagnosed at the same time. Concerning the differences in events, patients with long‐term AF are clinically more stable and consequently have less risk.
It is also relevant to note that AF increments the incidence and the prognosis of HF, driven by haemodynamic alterations and over‐active regulatory mechanisms. Loss of atrial contraction, irregularity of ventricular contractions, tachycardia, neurohormonal activation, and structural myocardial changes are among the factors related to the incidences of AF. 3 In the present study, AF was associated with the risk and number of hospitalizations due to acute heart failure and all‐cause mortality.
Mortality in the incidence group was significantly higher than in the prevalence group, in agreement with previous observations in which developing incident AF after a diagnosis of HF appears to confer a much greater risk of long‐term mortality than prevalent AF. 26 A recent diagnosis of AF could potentially introduce elements that could increase the risk such as a sudden change from sinus rhythm and/or additional added medications, antiarrhythmics, and anticoagulants.
Two more aspects observed are worth commenting. The first is the association of AF on renal function in HF. 27 The importance of renal function on the natural history of HF has been reported as being one of the most relevant prognostic markers. Chronic renal disease, defined by reduced eGFR or urinary albumin excretion, is associated with increased risk of all‐cause mortality, cardiovascular mortality, and hospitalization. 28 The impact of CKD is independent of age, functional class, duration of HF, haemoglobin, or diabetes mellitus. 29 Moreover, the lower the renal function increases mortality. In the present cohort, AF is associated with reduction of >20% of eGFR in the relative short observational period. The mechanism in reducing renal function may be related due the haemodynamic changes associated to AF that will impact by reducing the renal blood flow or by congestion in the venous territory.
The second is the impact of AF on potentially suffering a stroke in patients with HF. While in the entire population AF increases the risk of stroke, in the relative short‐term follow‐up of incident HF, the development of AF not only did not increase the risk of stroke even it is reduced. One possible explanation is the fact that AF patients receive anticoagulant treatment, and this protects not only against embolic stroke but also from those due to atherosclerosis. Anticoagulant treatment with NOAC provided significant protection greater than VKA treatment.
Considering the above‐mentioned data of the impact of AF in the presence of HF, efforts should be apply to follow the recommendations of the guidelines under the concept that multimorbidity requires an integrate care approach. A step‐based approach in the HF treatment will reduce decompensations and reduce the risk to develop AF. Prompt recognition and anticoagulant treatment, mainly NOACs, will reduce the risk of decompensation and thrombo‐embolic disease.
Strengths and limitations of the study should be contemplated. A large number of patients with AF have been analysed with a total high patient‐year accounting for potential confounders such age, sex, and major cardiovascular and metabolic diseases; however, the origin of each of the patients was not clearly established. Limitations include all that are inherent to the EHR, even though efforts were made to minimize these limitations and only patients with the required variables for the analysis were included. Likewise, no stratification according to the HF phenotype was available, even though co‐morbidities were taken into account in the multivariate analysis of risk. Particularly, a limitation was the lack of renal function assessment in a large proportion of subjects. The reasons include the lack of enough eGFR available for adequately assess of changes in renal function or the reduced number in which UAE was measured. Reasons for no anticoagulant treatment, quality of VKA control and dosage, and differences among the NOAC molecules were not contemplated. The fact that the study period finished in 2016 reduced the number of patients treated with NOACs. Concerning the impact of other drugs in the risk to develop AF is biased due to a confounding by indication. Finally, survival analysis in cardiovascular research assume competing risk, that if is not considered, like in the present study, an overestimation of risk is present.
In summary, AF is a highly prevalent co‐morbidity in patients with HF, which occurs more in older patients and male patients who share hypertension, diabetes, or CKD, although HF treatment reduces the risk. Development of AF is associated with the incidence of acute heart failure hospitalization and mortality and with a faster decline in the eGFR. Efforts to reduce the incidence of AF in HF patients will result in an increased quality of life and survival.
Conflicts of interest
None declared.
Supporting information
Figure S1. Forest plot of factors related with the new development of AF. Panel A (S1) Total population; Panel B (S1) Total population with eGFR available. Panel C (S2) New diagnosis of HF; Panel D (S2) New diagnosis of HF with eGFR available.
Table S1. ICD‐Codes used in the manuscript.
Acknowledgement
We acknowledge the contribution of the Health Authorities of the “Conselleria de Salud Universal” Generalitat Valenciana for allowing to use the data.
Diaz, J. , Martinez, F. , Calderon, J. M. , Fernandez, A. , Sauri, I. , Uso, R. , Trillo, J. L. , Redon, J. , and Forner, M. J. (2022) Incidence and impact of atrial fibrillation in heart failure patients: real‐world data in a large community. ESC Heart Failure, 9: 4230–4239. 10.1002/ehf2.14124.
Josep Redon and Maria Jose Forner contributed equally to this work.
This work was supported by the Innovative Medicines Initiative (IMI2‐FPP116074‐2), BIGMEDILYTICS (ICT‐15‐780495), and CIBERObn Institute of Health Carlos III (PI16/01402).
References
- 1. Michaud GF, Stevenson WG. Atrial Fibrillation. N Engl J Med. 2021; 384: 353–361. [DOI] [PubMed] [Google Scholar]
- 2. John RM, Michaud GF, Stevenson WG. Atrial fibrillation hospitalization, mortality, and therapy. Eur Heart J. 2018; 39: 3958–3960. [DOI] [PubMed] [Google Scholar]
- 3. Verhaert DVM, Brunner‐La Rocca HP, van Veldhuisen DJ, Vernooy K. The bidirectional interaction between atrial fibrillation and heart failure: Consequences for the management of both diseases. Europace. 2021; 23: ii40–ii45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nedkoff L, Kelty EA, Hung J, Thompson SC, Katzenellenbogen JM. Differences in stroke risk and cardiovascular mortality for aboriginal and other Australian patients with atrial fibrillation. Med J Aust. 2020; 212: 215–221. [DOI] [PubMed] [Google Scholar]
- 5. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, Piccini JP, Fonarow GC. ORBIT‐AF investigators and patients. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017; 5: 44–52. [DOI] [PubMed] [Google Scholar]
- 6. Gawałko M, Budnik M, Gorczyca I, Jelonek O, Uziębło‐Życzkowska B, Maciorowska M, Wójcik M, Błaszczyk R, Tokarek T, Rajtar‐Salwa R, Bil J, Wojewódzki M, Szpotowicz A, Krzciuk M, Bednarski J, Bakuła‐Ostalska E, Tomaszuk‐Kazberuk A, Szyszkowska A, Wełnicki M, Mamcarz A, Kapłon‐Cieślicka A. Characteristics and treatment of atrial fibrillation with respect to the presence or absence of heart failure. Insights from the multicenter polish atrial fibrillation (POL‐AF) registry. J Clin Med. 2021; 10: 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber C, Hung J, Hickling S, Nedkoff L, Murray K, Li I, Briffa TG. Incidence, predictors and mortality risk of new heart failure in patients hospitalised with atrial fibrillation. Heart. 2021; 107: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 8. Kane PM, Murtagh FE, Ryan K, Mahon NG, McAdam B, McQuillan R, Ellis‐Smith C, Tracey C, Howley C, Raleigh C, O'Gara G, Higginson IJ, Daveson BA. The gap between policy and practice: A systematic review of patient‐centred care interventions in chronic heart failure. Heart Fail Rev. 2015; 20: 673–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagamine T, Gillette B, Pakhomov A, Kahoun J, Mayer H, Burghaus R, Lippert J, Saxena M. Multiscale classification of heart failure phenotypes by unsupervised clustering of unstructured electronic medical record data. Sci Rep. 2020; 10: 21340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kozieł M, Teutsch C, Bayer V, Lu S, Gurusamy VK, Halperin JL, Rothman KJ, Diener HC, Ma CS, Huisman MV, Lip GYH, GLORIA‐AF Investigators . Changes in anticoagulant prescription patterns over time for patients with atrial fibrillation around the world. J Arrhythm. 2021; 37: 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gevaert AB, Tibebu S, Mamas MA, Ravindra NG, Lee SF, Ahmad T, Ko DT, Januzzi JL Jr, Van Spall HGC. Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Fail. 2021; 8: 2741–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirose J, Wakata Y, Tagi M, Tamaki Y. The role of medical informatics in the management of medical information. J Med Invest. 2020; 67: 27–29. [DOI] [PubMed] [Google Scholar]
- 13. The EU General Data Protection Regulation GDPR (accesed February 15th, 2021). https://gdpr‐info.eu/
- 14. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD‐EPI (chronic kidney disease epidemiology collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lip GYH. The ABC pathway: An integrated approach to improve AF management. Nat Rev Cardiol. 2017; 14: 627–628. [DOI] [PubMed] [Google Scholar]
- 16. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2020; 2021: 373–498. [DOI] [PubMed] [Google Scholar]
- 17. Romiti GF, Pastori D, Rivera‐Caravaca JM, Ding WY, Gue YX, Menichelli D, Gumprecht J, Kozieł M, Yang PS, Guo Y, Lip GYH, Proietti M. Adherence to the ‘Atrial fibrillation better Care’ pathway in patients with atrial fibrillation: Impact on clinical outcomes‐a systematic review and meta‐analysis of 285,000 patients. Thromb Haemost. 2022; 122: 406–414. [DOI] [PubMed] [Google Scholar]
- 18. Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer M, Potpara T, Dan GA, Kalarus Z, Tavazzi L, Maggioni AP, Boriani G; ESC‐ EORP Atrial Fibrillation General Long‐Term Registry Investigators Group . Relation of outcomes to ABC (atrial fibrillation better care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC‐EHRA EORP atrial fibrillation general long‐term (AFGen LT) registry. Europace 2021;23:174–183. [DOI] [PubMed] [Google Scholar]
- 19. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham heart study): A community‐based cohort study. Lancet. 2009; 373: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shulman E, Kargoli F, Aagaard P, Hoch E, Di Biase L, Fisher J, Gross J, Kim S, Krumerman A, Ferrick KJ. Validation of the Framingham heart study and CHARGE‐AF risk scores for atrial fibrillation in Hispanics, African‐American, and non‐Hispanic whites. Am J Cardiol. 2016; 117: 76–83. [DOI] [PubMed] [Google Scholar]
- 21. Aronson D, Shalev V, Katz R, Chodick G, Mutlak D. Risk score for prediction of 10‐year atrial fibrillation: A community‐based study. Thromb Haemost. 2018; 118: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 22. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J. 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 23. Ling LH, Kistler PM, Kalman JM, Schilling RJ, Hunter RJ. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol. 2016; 13: 131–147. [DOI] [PubMed] [Google Scholar]
- 24. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial fibrillation begets heart failure and vice versa: Temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016; 133: 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ariyaratnam JP, Lau DH, Sanders P, Kalman JM. Atrial fibrillation and heart failure: Epidemiology, pathophysiology, prognosis, and management. Card Electrophysiol Clin. 2021; 13: 47–62. [DOI] [PubMed] [Google Scholar]
- 26. Odutayo A, Wong CX, Williams R, Hunn B, Emdin CA. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: A systematic review and meta‐analysis. J Card Fail. 2017; 23: 56–62. [DOI] [PubMed] [Google Scholar]
- 27. Lai AC, Bienstock SW, Sharma R, Skorecki K, Beerkens F, Samtani R, Coyle A, Kim T, Baber U, Camaj A, Power D, Fuster V, Goldman ME. A personalized approach to chronic kidney disease and cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2021; 77: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 28. Murton M, Goff‐Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, Nolan S, Sörstadius E, Pecoits‐Filho R, Tuttle K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: A systematic review. Adv Ther. 2021; 38: 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Löfman I, Szummer K, Dahlström U, Jernberg T, Lund LH. Associations with and prognostic impact of chronic kidney disease in heart failure with preserved, mid‐range, and reduced ejection fraction. Eur J Heart Fail. 2017; 19: 1606–1614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Forest plot of factors related with the new development of AF. Panel A (S1) Total population; Panel B (S1) Total population with eGFR available. Panel C (S2) New diagnosis of HF; Panel D (S2) New diagnosis of HF with eGFR available.
Table S1. ICD‐Codes used in the manuscript.


