Abstract
Aims
To describe clinical characteristics and outcomes for those with STEMI and reduced left ventricular ejection fraction (LVEF) in low‐income and middle‐income countries (LMICs).
Methods and results
Adults presenting with STEMI to two government‐owned tertiary care centres in Delhi, India were prospectively enrolled in the North India ST‐elevation myocardial infarction (NORIN‐STEMI) registry. LVEF was evaluated at presentation and clinical characteristics were compared across LVEF categories. Overall, 3597 patients were included, of whom 468 (13%) had LVEF >50%, 1482 (41%) had mildly reduced LVEF (40–49%), 1357 (38%) had moderately reduced LVEF (30–39%), and 290 (8%) had severely reduced LVEF (<30%). Presentation delay >24 h, prior MI, and hyperlipidaemia were associated with decreasing LVEF category. Although most patients with reduced LVEF were discharged on appropriate guideline‐directed therapies, adherence at 1 year was low (ACE inhibitor/ARB 91% to 41%, beta blocker 98% to 78%, aldosterone receptor antagonist 69% to 6%). After multivariable adjustment, a Cox regression model showed moderately reduced LVEF (HR 1.77, 95% CI 1.20, 2.60) and severely reduced LVEF (HR 3.63, 95% CI 2.41, 5.48) were associated with increased risk of all‐cause mortality compared with LVEF ≥50%.
Conclusions
On presentation for STEMI, almost 90% of NORIN‐STEMI participants had at least mildly reduced LVEF and almost half had LVEF <40%. Patients with LVEF <40% had significantly higher risk of mortality at 1 year, and adherence to guideline‐directed therapies at 1 year was poor. Systematic initiatives to improve access to timely revascularization and guideline‐directed therapies are essential in advancing STEMI care in LMICs.
Keywords: ST‐elevation myocardial infarction, heart failure, NORIN‐STEMI, atherosclerotic cardiovascular disease, left ventricular dysfunction
Introduction
Ischaemic heart disease is the most common cause of heart failure (HF) worldwide. 1 , 2 , 3 ST‐segment elevation myocardial infarction (STEMI) is the most feared presentation of ischaemic heart disease and is associated with significant morbidity and mortality. Over the past two decades, prompt revascularization strategies and timely administration of medical therapies have improved outcomes in patients with STEMI in high income countries. 4 , 5 , 6 , 7 However, delays in revascularization largely stemming from delayed presentation in low‐income and middle‐income countries (LMICs) have blunted the magnitude of benefit from these treatments. 8 Consequently, these delays are associated with significant myocardial injury and hence, a large population at high risk for reduced left‐ventricular ejection fraction (LVEF) and HF. 9 , 10
STEMI remains an important pathway to reduced LVEF, HF, and poor outcomes in most of the world, including India. However, contemporary data quantifying reduced LVEF after STEMI and its association with subsequent clinical outcomes in LMICs are sparse. We use the prospective North India ST‐elevation Myocardial Infarction registry (NORIN‐STEMI) database to evaluate the distribution of LVEF among those presenting with STEMI in North India and the impact of reduced LVEF on clinical outcomes.
Methods
Study population
The design of NORIN‐STEMI has been previously described in detail. 11 Briefly, NORIN‐STEMI is a prospective, ongoing observational registry of all consenting adults (≥18 years of age) presenting with STEMI at two government‐owned, PCI‐capable tertiary care centres in New Delhi, India, from January 2019 to February 2020. A diagnosis of STEMI was based on the European Society of Cardiology/American College of Cardiology Foundation/American Heart Association/World Heart Federation Task Force for the fourth Universal Definition of Myocardial Infarction. 12
Determination of left ventricular ejection fraction and cardiovascular risk factors
All patients underwent transthoracic echocardiography on presentation. LVEF was determined visually by experienced cardiologists, per institutional standard, and was categorized into normal LVEF (>50%), mildly reduced LVEF (40–49%), moderately reduced LVEF (30–39%), and severely reduced LVEF (<30%). Patients with a pre‐existing diagnosis of HF were excluded from this analysis. Diabetes mellitus was defined as a chart or self‐reported history of diabetes mellitus or glycated haemoglobin (HbA1c) ≥ 6.5% at presentation. Hyperlipidaemia was defined as a chart or self‐reported history of hyperlipidaemia or by National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines (total cholesterol ≥240 mg/dL, calculated low‐density lipoprotein ≥160 mg/dL, or high‐density lipoprotein <40 mg/dL). Follow‐up data on outcomes during the hospital stay as well as at 30 days and 1 year following the acute event were obtained either from hospital records, outpatient clinic visit records, or through direct telephone contact with the patient.
Medication adherence
Historical medications were recorded for each patient based on oral and written patient history. Medications administered at the hospital or prescribed upon discharge were recorded as in the medical record. Adherence to medications at follow up time points were ascertained via direct telephone communication with patients or by medical record at follow‐up visits.
Statistical analyses
Distributions of continuous variables and proportions of categorical variables were examined. Categorical variables were evaluated with χ 2 or Fisher's exact test and continuous variables were compared with Kruskal–Wallis or Mann–Whitney U test, as appropriate. Multivariable Cox proportional hazards models evaluated the association of LVEF categories with all‐cause mortality to 1 year. Cox regression models were adjusted for age, sex, diabetes mellitus, hyperlipidaemia, tobacco use, obesity, prior myocardial infarction, prior cerebrovascular accident, and revascularization. Finally, the association of baseline and presenting characteristics with decreasing EF category was evaluated with ordinal logistic regression. Adjusted odds ratios (OR) and 95% confidence intervals (95% CI) represent the log odds of decreasing the EF category by 1 (e.g. ≥50% to 40–49%, 40–49% to 30–39%, etc.). All analyses were performed using SAS 9.4 (SAS Inc, Cary, NC).
Results
A total of 3635 consecutive patients presenting with STEMI were enrolled in the registry; 38 (1%) had a history of HF and 3597 were included in the present study. Median age was 55 years (IQR 46–62) and 16% were women.
On presentation, 468 (13%) patients had LVEF >50%, 1482 (41%) had mildly reduced LVEF (40–49%), 1357 (38%) had moderately reduced LVEF (30–39%), and 290 (8%) had severely reduced LVEF (<30%). Coronary angiography was performed in 45% of patients with severely reduced LVEF compared with over 70% in all other EF categories (P < 0.001 for each). PCI was performed in 38% of patients with severely reduced LVEF compared with 65% or higher for each of the other EF categories (P < 0.001 for each), Figure 1 . Thrombolysis was utilized in 10% of those with severely reduced LVEF, 12% of those with moderately reduced LVEF, 15% with mildly reduced LVEF, and 14% with normal LVEF, Table 1 .
Figure 1.
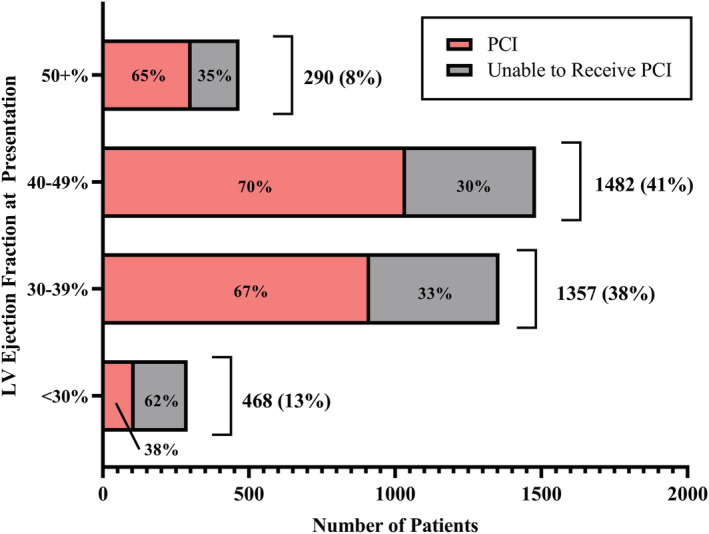
Left ventricular ejection fraction at presentation in NORIN‐STEMI participants.
Table 1.
Baseline and clinical characteristics of NORIN‐STEMI participants, stratified by left ventricular ejection fraction at presentation
| LVEF ≥50% (N = 468, 13%) | LVEF 40–49% (N = 1482, 41%) | LVEF 30–39% (N = 1357, 38%) | LVEF <30% (N = 290, 8%) | |
|---|---|---|---|---|
| Age, median (IQR) | 55 (46–61) | 54 (45–60) | 55 (45–62) | 58 (48–65) |
| Women, n (%) | 74 (16%) | 219 (15%) | 231 (17%) | 49 (17%) |
| Literacy | ||||
| Illiterate | 206 (44%) | 726 (49%) | 741 (55%) | 157 (54%) |
| High school graduate | 67 (14%) | 254 (17%) | 197 (15%) | 35 (12%) |
| Socioeconomic status | ||||
| Upper | 12 (3%) | 34 (2%) | 40 (3%) | 13 (4%) |
| Middle | 342 (73%) | 1088 (73%) | 900 (66%) | 203 (70%) |
| Lower | 114 (24%) | 360 (24%) | 417 (31%) | 74 (26%) |
| Insurance | ||||
| CGHS | 4 (1%) | 12 (1%) | 17 (1%) | 0 (0%) |
| Private | 5 (1%) | 9 (1%) | 7 (1%) | 3 (1%) |
| Self‐pay | 459 (98%) | 1461 (99%) | 1333 (98%) | 287 (99%) |
| Medical history, n (%) | ||||
| Diabetes mellitus (on history or presentation) | 152 (32%) | 450 (30%) | 460 (34%) | 113 (39%) |
| Hypertension | 138 (30%) | 377 (25%) | 408 (30%) | 95 (33%) |
| Atrial fibrillation | 11 (2%) | 17 (1%) | 34 (3%) | 8 (3%) |
| Hyperlipidaemia (on history or presentation) | 239 (51%) | 815 (55%) | 745 (55%) | 179 (62%) |
| Prior cerebrovascular accident | 4 (1%) | 22 (2%) | 13 (1%) | 3 (1%) |
| Prior myocardial infarction | 43 (9%) | 140 (9%) | 197 (15%) | 52 (18%) |
| Tobacco use | 280 (60%) | 844 (57%) | 710 (52%) | 156 (54%) |
| Obesity | 52 (11%) | 164 (11%) | 168 (12%) | 28 (10%) |
| In‐hospital characteristics, n (%) | ||||
| MI type on ECG | ||||
| Anterior MI | 194 (42%) | 688 (46%) | 882 (65%) | 194 (67%) |
| Anteroinferior MI | 3 (1%) | 4 (<1%) | 9 (1%) | 2 (1%) |
| Anterolateral MI | 2 (<1%) | 8 (1%) | 9 (1%) | 0 (0%) |
| Inferior MI | 255 (55%) | 751 (51%) | 437 (32%) | 89 (31%) |
| Inferolateral MI | 1 (<1%) | 2 (<1%) | 4 (<1%) | 0 (0%) |
| Inferior and posterior MI | 3 (1%) | 2 (<1%) | 2 (<1%) | 1 (<1%) |
| Lateral MI | 6 (1%) | 22 (2%) | 8 (1%) | 3 (1%) |
| Posterior MI | 4 (1%) | 5 (<1%) | 6 (<1%) | 1 (<1%) |
| Ventricular fibrillation | 3 (1%) | 5 (<1%) | 9 (1%) | 7 (2%) |
| Regional wall motion abnormality | 190 (41%) | 1181 (80%) | 1239 (91%) | 243 (84%) |
| Mitral regurgitation | ||||
| None | 434 (93%) | 1112 (75%) | 823 (61%) | 145 (50%) |
| Mild | 27 (6%) | 295 (20%) | 477 (35%) | 90 (31%) |
| Moderate | 6 (1%) | 73 (5%) | 53 (4%) | 50 (17%) |
| Severe | 1 (<1%) | 2 (<1%) | 4 (<1%) | 5 (2%) |
| In‐hospital interventions, n (%) | ||||
| Symptom onset to presentation time | ||||
| <1 h | 298 (64%) | 971 (66%) | 848 (62%) | 169 (58%) |
| <3 h | 371 (79%) | 1199 (81%) | 1020 (75%) | 210 (72%) |
| <12 h | 421 (90%) | 1331 (90%) | 1170 (86%) | 237 (82%) |
| <24 h | 439 (94%) | 1394 (94%) | 1224 (90%) | 251 (87%) |
| <72 h | 455 (97%) | 1420 (96%) | 1265 (93%) | 258 (89%) |
| ≥72 h | 13 (3%) | 62 (4%) | 92 (7%) | 32 (11%) |
| Coronary angiography | 335 (72%) | 1104 (75%) | 1015 (75%) | 129 (45%) |
| Site of access (missing = 31) | ||||
| Radial | 13 (4%) | 15 (1%) | 26 (3%) | 7 (6%) |
| Femoral | 320 (96%) | 1084 (99%) | 967 (97%) | 120 (96%) |
| Percutaneous coronary intervention | 304 (65%) | 1038 (70%) | 915 (67%) | 109 (38%) |
| Thrombolysis | 67 (14%) | 216 (15%) | 159 (12%) | 28 (10%) |
Compared with those with normal or mildly reduced LVEF (≥40%), those with at least moderately reduced LVEF (<40%) were more likely to have diabetes mellitus, hypertension, and prior MI. Although most presented within 1 h, those with moderate/severely reduced LVEF had longer symptom onset to presentation time (75% within 3 h) compared with those with LVEF ≥40% (81%, P < 0.001), Figure 2 . Compared with the same population, patients with moderate/severely reduced EF were more likely to have an anterior wall MI (65% vs. 45%, P < 0.001), Table 1 .
Figure 2.
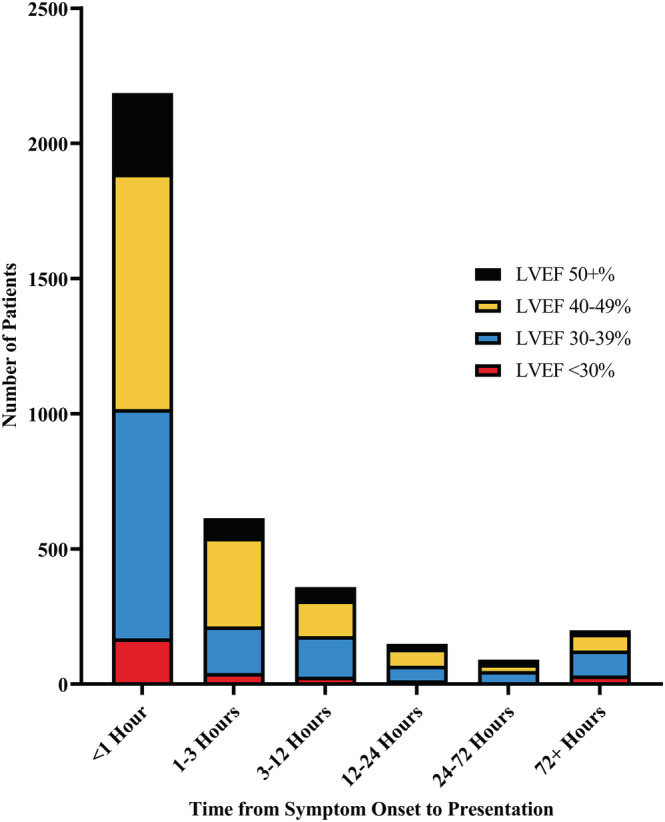
Time from symptom onset to healthcare presentation in NORIN‐STEMI participants, stratified by left ventricular ejection fraction at presentation.
Almost all patients with LVEF <40% were discharged on an ACE inhibitor/angiotensin receptor blocker (ACE/ARB; 91%) and beta blocker (98%). Most (69%) also received an aldosterone receptor antagonist upon discharge. Of those surviving to 1 year (missing = 310, 10%), 78% remained adherent to beta‐blocker therapy, 43% were adherent to ACE/ARB, 6% reported taking aldosterone receptor antagonists, and 3% reported taking sacubitril/valsartan, Table 2 , Figures S1–S3 .
Table 2.
Medication history, administration during index hospitalization, and adherence at 1 year among NORIN‐STEMI participants by left ventricular ejection fraction at presentation
| LVEF ≥50% (N = 468, 13%) | LVEF 40–49% (N = 1482, 41%) | LVEF 30–39% (N = 1357, 38%) | LVEF <30% (N = 290, 8%) | |
|---|---|---|---|---|
| Historical medications | ||||
| Aspirin | 46 (10%) | 127 (9%) | 156 (12%) | 42 (15%) |
| Statin | 45 (10%) | 126 (9%) | 153 (11%) | 44 (15%) |
| Beta‐blocker | 48 (10%) | 159 (11%) | 193 (14%) | 51 (18%) |
| ACE inhibitor/ARB | 45 (10%) | 147 (9%) | 188 (14%) | 54 (19%) |
| Aldosterone receptor antagonist | 1 (<1%) | 9 (1%) | 5 (<1%) | 3 (1%) |
| Medications on arrival | ||||
| Aspirin | 466 (>99%) | 1476 (>99%) | 1353 (>99%) | 288 (99.3%) |
| Statin | 466 (>99%) | 1476 (>99%) | 1352 (>99%) | 287 (99%) |
| P2Y12 inhibitor | 468 (100%) | 1474 (>99%) | 1352 (>99%) | 288 (99%) |
| Beta‐blocker | 115 (25%) | 294 (20%) | 235 (17%) | 25 (9%) |
| Anticoagulant | 305 (65%) | 1038 (70%) | 914 (67%) | 110 (38%) |
| Discharge medications, n (%) 1 | ||||
| Aspirin | 455 (100%) | 1432 (>99%) | 1275 (>99%) | 221 (100%) |
| Statin | 453 (>99%) | 1429 (>99%) | 1266 (99%) | 221 (100%) |
| P2Y12 inhibitor | 452 (99%) | 1426 (>99%) | 1273 (>99%) | 217 (98%) |
| Beta‐blocker | 444 (98%) | 1417 (99%) | 1252 (98%) | 216 (98%) |
| ACE inhibitor/ARB | 363 (80%) | 1164 (81%) | 1250 (98%) | 215 (97%) |
| Aldosterone receptor antagonist | 97 (21%) | 359 (25%) | 883 (69%) | 146 (68%) |
| Guideline‐directed medical therapies at 1 year (missing = 310, 10%), n (%) 2 | ||||
| Aspirin | 334 (85%) | 1076 (87%) | 917 (85%) | 156 (87%) |
| P2Y12 inhibitor | 282 (72%) | 805 (65%) | 774 (72%) | 118 (66%) |
| Statin | 308 (79%) | 991 (80%) | 868 (80%) | 149 (83%) |
| Beta‐blocker | 309 (79%) | 989 (80%) | 843 (78%) | 142 (79%) |
| ACE inhibitor/ARB | 183 (47%) | 570 (46%) | 451 (42%) | 91 (51%) |
| Aldosterone receptor antagonist | 19 (5%) | 65 (5%) | 61 (6%) | 10 (6%) |
| Sacubitril/valsartan | 15 (4%) | 42 (3%) | 35 (3%) | 6 (3%) |
Of those surviving to discharge.
Of those surviving to 1 year.
Of those surviving to discharge, HF readmission within 30 days occurred in 6 (1%) patients with LVEF >50%, 23 (2%) with mildly reduced LVEF, 92 (7%) with moderately reduced LVEF, and 83 (38%) with severely reduced LVEF, Table 3 , Figure S4 . One year mortality occurred in 31 (7%) patients with normal LVEF at presentation, 120 (8%) with mildly reduced LVEF, 154 (11%) with moderately reduced LVEF, and 95 (33%) with severely reduced LVEF. After multivariable adjustment, mildly reduced LVEF had similar mortality to that of LVEF ≥50% (HR 1.27, 95% CI 0.85, 1.88); moderately reduced LVEF (HR 1.77, 95% CI 1.20, 2.60) and severely reduced LVEF (HR 3.62, 95% CI 2.41, 5.48) were associated with significantly increased risk of all‐cause mortality compared with LVEF ≥50%, Figure 3 .
Table 3.
Clinical outcomes among NORIN‐STEMI participants, stratified by left ventricular ejection fraction at presentation
| LVEF ≥50% (N = 468, 13%) | LVEF 40–49% (N = 1482, 41%) | LVEF 30–39% (N = 1357, 38%) | LVEF <30% (N = 290, 8%) | |
|---|---|---|---|---|
| In‐hospital mortality | 13 (3%) | 49 (3%) | 81 (6%) | 69 (24%) |
| 30 day all‐cause mortality | 18 (4%) | 80 (6%) | 109 (9%) | 80 (29%) |
| 30 day all‐cause readmission | 8 (2%) | 34 (3%) | 102 (9%) | 93 (49%) |
| 30 day heart failure readmission | 3 (1%) | 23 (2%) | 92 (7%) | 83 (38%) |
| 1 year all‐cause mortality | 31 (7%) | 120 (8%) | 154 (11%) | 95 (33%) |
30 day and 1 year outcomes are calculated as days from discharge.
Figure 3.
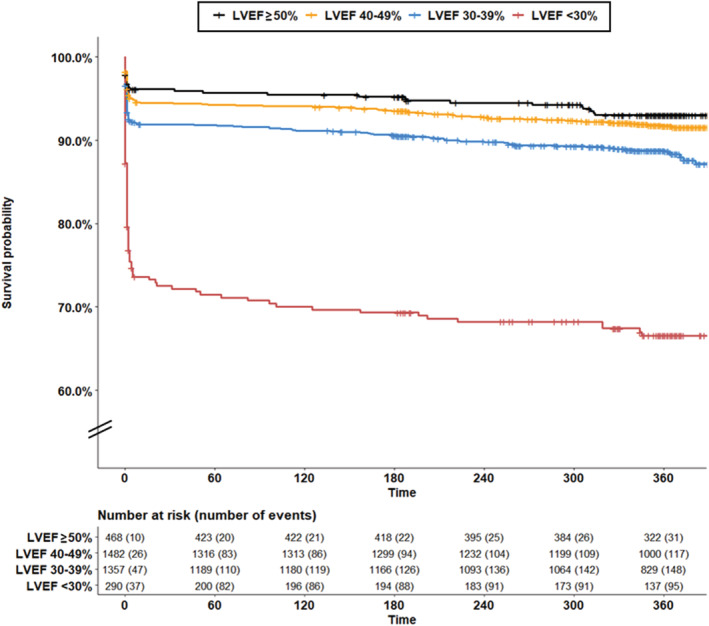
Survival of adults by left ventricular ejection fraction at presentation in NORIN‐STEMI participants.
In a multivariable ordinal logistic regression model, delay in presentation longer than 24 h (OR 1.69, 95% CI 1.34, 2.13), prior MI (OR 1.57, 95% CI 1.30, 1.90), hyperlipidaemia (OR 1.15, 95% CI 1.02, 1.30), and increasing age decile (OR 1.06, 95% CI 1.00, 1.11) were associated with significant odds of decreasing EF category; current tobacco use was associated with decreased odds (OR 0.87, 95% CI 0.76, 0.99), Figure 4 .
Figure 4.
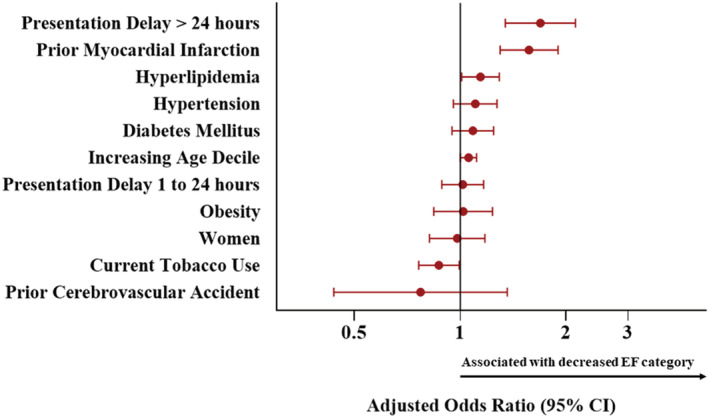
Adjusted odds ratios and 95% confidence intervals of decreasing left ventricular ejection fraction category for baseline characteristics in NORIN‐STEMI participants.
Discussion
In this analysis of the prospective NORIN‐STEMI registry, we found that almost 90% of patients presenting with STEMI to participating hospitals in North India had at least mildly reduced LVEF (<50%), and about half had at least moderately reduced LVEF (<40%). Consistent with studies from different populations, 3 , 6 moderate and severely reduced LVEF were associated with significantly increased risk of mortality to 1 year. Significant delay in presentation >24 h, prior MI, and hyperlipidaemia were the most important risk factors for progressive reduction in LVEF. Almost all patients with reduced LVEF were discharged on a beta blocker plus ACE/ARB and a majority also received an aldosterone receptor antagonist; however, adherence at 1 year was poor. Overall, these data highlight that a large proportion of those having STEMI in India have reduced LVEF, likely related to delays in presentation and subsequently, longer symptom to balloon time. Further quality initiatives and studies are warranted as this population is at significant risk of progression to heart failure with reduced ejection fraction (HFrEF) and higher morbidity/mortality.
Health infrastructure innovations that have dramatically improved availability and timing of revascularization in high‐income countries have not yet materialized in LMICs. Ischaemic heart disease and STEMI remain the most important pathway to HF in the Indian population, and we found a staggering proportion of those with STEMI presented with reduced LVEF. Readmission for HF at 30 days (indicating incident HF) occurred in 7% and 39% of patients with moderate and severely reduced LVEF, respectively. LV dysfunction may develop at the onset of STEMI due to extensive myocardial necrosis or later in the course of disease process due to ventricular remodelling. 3 Delay in presentation is well documented in India and may contribute to either of these mechanisms. While median symptom to door time in our registry was shorter than 4.5 h reported from a prospective registry of acute coronary syndrome admissions in Kerala, 13 one‐fourth of our population presented after a delay of over 3 h, and almost 10% after over 24 h. Those presenting with such a delay are more likely to have substantial myocardial necrosis and LVSD in the setting of completed STEMI, and only 38% of those with LVEF<30% were able to undergo PCI. Low rates of revascularization observed in the present study are concerning and likely related to a variety of factors. First, over 10% of patients with severely reduced LVEF presented beyond the time window for primary PCI. Second, access to mechanical circulatory support in participating hospitals is limited to a finite number of intra‐aortic balloon pumps. Thus, operators may be hesitant to offer revascularization in patients who present in clinical shock (which we were limited in discerning in the present study). Third, 98% of patients were self‐pay; while medical therapies are generally cost‐free, there is a nominal cost incurred for procedures at participating centres and patients often refuse intervention due to the financial burden. Finally, patients may refuse the procedure for other personal reasons. Minimizing barriers to presentation to PCI‐capable hospitals for emergency care is of particular importance in improving future outcomes in this population.
India and other LMICs have demonstrated higher rates of acute coronary syndrome and cardiovascular mortality despite lower baseline cardiovascular risk profiles compared with high‐income countries. 14 This is likely attributable to poor access to outpatient care, missed screening opportunities, and presentation in later stages of disease. A prior population study demonstrated 80% of those with prior MI or stroke in India did not take any form of secondary prevention, and therefore these patients are at exceedingly high risk for advanced atherosclerotic disease and extensive myocardial necrosis during subsequent events. 15 Indeed, prior MI was the most important risk factor for progressive reduced LVEF in our registry. Hyperlipidaemia was another risk factor for reduced LVEF and, despite over half of patients in our study presenting with lipids diagnostic for hyperlipidaemia, only 10% reported statin use at baseline. 16 Almost all patients with reduced LVEF in our registry were appropriately discharged on a beta blocker and ACE/ARB; however, less than half were adherent at 1 year. Aldosterone receptor antagonists, another important component of guideline‐directed medical therapy (GDMT) in patients with post‐MI LV dysfunction, 17 had extremely poor adherence at 1 year (6%). This is consistent with a study from Kerala demonstrating consistently low adherence to GDMT in those with existing HF. 18 Access to post‐MI GDMT remains an important barrier to optimal post‐MI care in North India. Further studies and initiatives are warranted to improve access and adherence to both primary and secondary prevention as well as GDMT in this population.
Moderate to severely reduced LVEF during index STEMI hospitalization incurs worsening disability life years, increases longitudinal healthcare costs, and is associated with at least double the risk of 1 year mortality in the North Indian population. Collectively, our data call for sustainable initiatives that target timely presentation and revascularization, risk factor screening and management, and access to primary/secondary prevention and GDMT to ameliorate risk of LV dysfunction post‐STEMI and thus improve long‐term health outcomes.
Our study has some limitations. First, LVEF was determined visually by cardiologists and not by bi‐plane Simpson's method, as is the institutional standard at participating centres. History of clinical HF was able to be ascertained via medical record review and questionnaire; however, history of reduced LVEF without HF was unable to be reliably obtained; thus, it is probable some patients had baseline reduced LVEF without clinical HF and estimates of reduced LVEF due to STEMI are likely overestimated. Second, advanced laboratory data and clinical signs of HF such as volume overload, need for diuretics or inotropes, and follow‐up LVEF were unavailable. While we can presume incident HF in those readmitted for HF at 30 days, we were unable to differentiate transient reduced LVEF after STEMI from incipient HF after STEMI in those who were not readmitted. Third, given previously documented lack of screening and access to healthcare for many adults in North India, it is possible that some patients had reduced LVEF at baseline. Similarly, many baseline characteristics, including HF, and medications were collected via self‐report and thus are subject to recall bias. Fourth, medication data at discharge were recorded via the medical record and at follow‐up were recorded primarily by telephonic communication, which may partially explain some differences. Also, follow‐up medication data at interim time intervals prior to 1 year were not collected. Lastly, enrolment in NORIN‐STEMI is restricted to two government‐owned hospitals in Delhi, and therefore results may not be generalizable to larger populations in question.
Conclusions
On presentation for STEMI, almost 90% of NORIN‐STEMI participants had at least mildly reduced LVEF <50%, and almost half had LVEF <40%. Patients with LVEF <40% had poor adherence to GDMT and significantly increased risk of mortality at 1 year. Prior MI and hyperlipidaemia were the most important risk factors for reduced LVEF at presentation. Ischaemic heart disease remains an important pathway to HFrEF and poor outcomes in adults in North India. Stepwise initiatives that emphasize access to timely revascularization, risk factor screening, primary/secondary prevention, and GDMT are essential to improve outcomes in this population Figure 5 .
Figure 5.
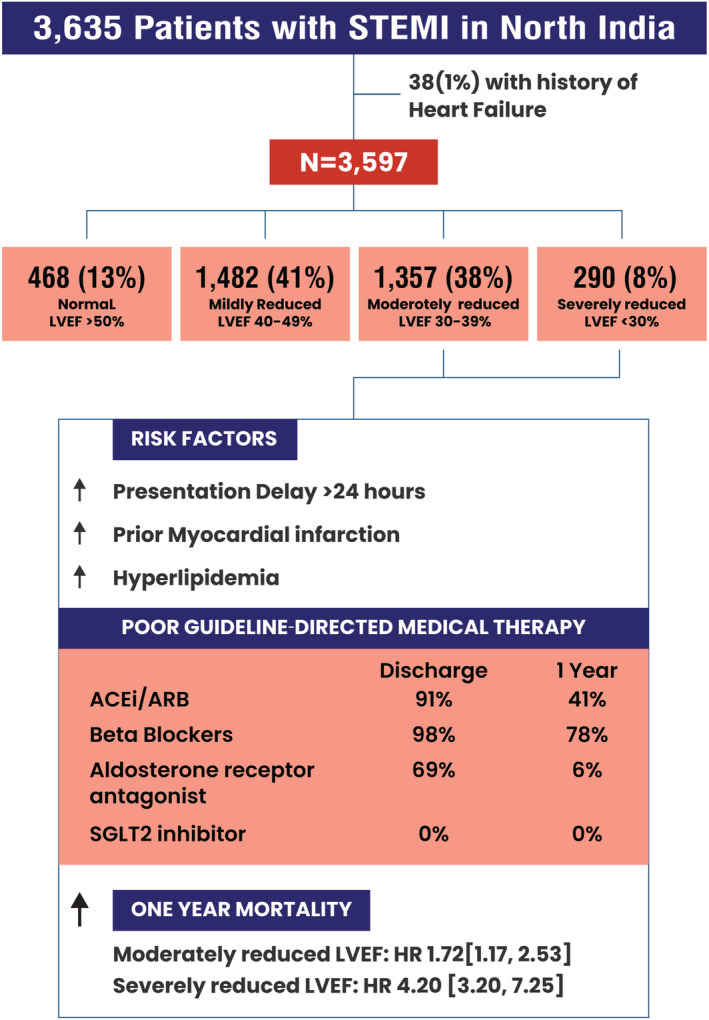
Central Illustration: Prevalence and prognostic significance of reduced left ventricular ejection fraction among patients with STEMI in North India.
Conflict of interest
Dr. Vaduganathan has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with Novartis, AstraZeneca, and Roche Diagnostics, and participates on clinical trial committees for studies sponsored by Galmed, Impulse Dynamics, Bayer AG, Novartis, and Occlutech. Dr. Bhatt discloses the following relationships ‐ Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol‐Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co‐Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Bristol‐Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. Dr Fonarow reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Janssen, Medtronic, Merck, and Novartis. The remaining authors report no disclosures, financial or otherwise.
Supporting information
Figure S1. Historical medications stratified by left ventricular ejection fraction category.
Figure S2. Discharge medications stratified by left ventricular ejection fraction category.
Figure S3. One‐year medications stratified by left ventricular ejection fraction category.
Figure S4. Outcomes stratified by left ventricular ejection fraction category.
Hendrickson, M. J. , Arora, S. , Vaduganathan, M. , Fonarow, G. C. , MP, G. , Bansal, A. , Batra, V. , Kunal, S. , Bhatt, D. L. , Gupta, M. , and Qamar, A. (2022) Prevalence and prognostic implications of reduced left ventricular ejection fraction among patients with STEMI in India. ESC Heart Failure, 9: 3836–3845. 10.1002/ehf2.14055.
References
- 1. Gheorghiade M, Bonow RO. Chronic heart failure in the United States: A manifestation of coronary artery disease. Circulation. 1998; 97: 282–289. [DOI] [PubMed] [Google Scholar]
- 2. Lund LH, Mancini D. Heart failure in women. Med Clin North Am. 2004; 88: 1321–1345. [DOI] [PubMed] [Google Scholar]
- 3. White HD, Cross DB, Elliott JM, Norris RM, Yee TW. Long‐term prognostic importance of patency of the infarct‐related coronary artery after thrombolytic therapy for acute myocardial infarction. Circulation. 1994; 89: 61–67. [DOI] [PubMed] [Google Scholar]
- 4. Townsend N, Wilson L, Bhatnagar P, Wickramasinghe K, Rayner M, Nichols M. Cardiovascular disease in Europe: Epidemiological update 2016. Eur Heart J. 2016; 37: 3232–3245. [DOI] [PubMed] [Google Scholar]
- 5. Jernberg T, Omerovic E, Hamilton E, Lindmark K, Desta L, Alfredsson J, Lundberg A, Kellerth T, Erlinge D. Prevalence and prognostic impact of left ventricular systolic dysfunction after acute myocardial infarction. Eur Heart J [Internet]. 2020. [cited 2021 Nov 4];41. Available from: https://academic.oup.com/eurheartj/article/doi/10.1093/ehjci/ehaa946.1796/6004608 (Accessed 11/22/21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population Trends in the Incidence and Outcomes of Acute Myocardial Infarction. N Engl J Med. 2010; 362: 2155–2165. [DOI] [PubMed] [Google Scholar]
- 7. Gerber Y, Weston SA, Berardi C, McNallan SM, Jiang R, Redfield MM, Roger VL. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: A community study. Am J Epidemiol. 2013; 178: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandrashekhar Y, Alexander T, Mullasari A, Kumbhani DJ, Alam S, Alexanderson E, Bachani D, Wilhelmus Badenhorst JC, Baliga R, Bax JJ, Bhatt DL, Bossone E, Botelho R, Chakraborthy RN, Chazal RA, Dhaliwal RS, Gamra H, Harikrishnan SP, Jeilan M, Kettles DI, Mehta S, Mohanan PP, Kurt Naber C, Naik N, Ntsekhe M, Otieno HA, Pais P, Piñeiro DJ, Prabhakaran D, Reddy KS, Redha M, Roy A, Sharma M, Shor R, Adriaan Snyders F, Weii Chieh Tan J, Valentine CM, Wilson BH, Yusuf S, Narula J. Resource and Infrastructure‐Appropriate Management of ST‐Segment Elevation Myocardial Infarction in Low‐ and Middle‐Income Countries. Circulation. 2020. [cited 2022 Jan 14];141:2004–2025. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.119.041297 (Accessed 11/22/21). [DOI] [PubMed] [Google Scholar]
- 9. Tromp J, Ouwerkerk W, Cleland JGF, Angermann CE, Dahlstrom U, Tiew‐Hwa Teng K, Bamadhaj S, Ertl G, Hassanein M, Perrone SV, Ghadanfar M, Schweizer A, Obergfell A, Filippatos G, Collins SP, Lam CSP, Dickstein K. Global Differences in Burden and Treatment of Ischemic Heart Disease in Acute Heart Failure: REPORT‐HF. JACC Hear Fail. 2021; 9: 349–359. [DOI] [PubMed] [Google Scholar]
- 10. Weir RAP, McMurray JJV, Velazquez EJ. Epidemiology of Heart Failure and Left Ventricular Systolic Dysfunction after Acute Myocardial Infarction: Prevalence, Clinical Characteristics, and Prognostic Importance. Am J Cardiol. 2006; 97: 13–25. [DOI] [PubMed] [Google Scholar]
- 11. Arora S, Qamar A, Gupta P, Vaduganathan M, Chauhan I, Tripathi AK, Sharma VY, Bansal A, Fatima A, Jain G, Batra V, Tyagi S, Khandelwal L, Kaul P, Rao SV, Girish MP, Bhatt DL, Gupta MD. Design and rationale of the North Indian ST‐Segment Elevation Myocardial Infarction Registry: A prospective cohort study. Clin Cardiol. 2019; 42: 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth Universal Definition of Myocardial Infarction (2018). Glob Heart. 2018; 13: 305–338. [DOI] [PubMed] [Google Scholar]
- 13. Mohanan PP, Mathew R, Harikrishnan S, Krishnan MN, Zachariah G, Joseph J, Eapen K, Abraham M, Menon J, Thomas M, Jacob S, Huffman MD, Prabhakaran D. Presentation, management, and outcomes of 25 748 acute coronary syndrome admissions in Kerala, India: Results from the Kerala ACS Registry. Eur Heart J. 2013; 34: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, Bo J, Lou Q, Lu F, Liu T, Yu L, Zhang S, Mony P, Swaminathan S, Mohan V, Gupta R, Kumar R, Vijayakumar K, Lear S, Anand S, Wielgosz A, Diaz R, Avezum A, Lopez‐Jaramillo P, Lanas F, Yusoff K, Ismail N, Iqbal R, Rahman O, Rosengren A, Yusufali A, Kelishadi R, Kruger A, Puoane T, Szuba A, Chifamba J, Oguz A, McQueen M, McKee M, Dagenais G. Cardiovascular Risk and Events in 17 Low‐, Middle‐, and High‐Income Countries. N Engl J Med. 2014; 371: 818–827. [DOI] [PubMed] [Google Scholar]
- 15. Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, Gupta R, Kelishadi R, Iqbal R, Avezum A, Kruger A, Kutty R, Lanas F, Liu L, Wei L, Lopez‐Jaramillo P, Oguz A, Rahman O, Swidan H, Yusoff K, Zatonski W, Rosengren A, Teo KK. Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE Study): A prospective epidemiological survey. Lancet. 2011; 378: 1231–1243. [DOI] [PubMed] [Google Scholar]
- 16. Arora S, Qamar A, Gupta P, Hendrickson M, Singh A, Vaduganathan M, Pandey A, Bansal A, Batra V, Mukhopadhyay S, Yusuf J, Tyagi S, Girish M, Kaul P, Bhatt DL, Gupta M. Guideline based eligibility for primary prevention statin therapy – Insights from the North India ST‐elevation myocardial infarction registry (NORIN‐STEMI). J Clin Lipidol. 2021. [cited 2021 Dec 28];0. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1933287421003494 (Accessed 11/22/21). [DOI] [PubMed] [Google Scholar]
- 17. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction. N Engl J Med. 2003; 348: 1309–1321. [DOI] [PubMed] [Google Scholar]
- 18. Joseph S, Panniyammakal J, Abdullakutty J, S S, Vaikathuseril LJ, Joseph J, Mattummal S, Punnose E, Unni G, Natesan S, Sivadasanpillai H. The Cardiology Society of India‐Kerala Acute Heart Failure Registry: poor adherence to guideline‐directed medical therapy. Eur Heart J. 2021. [cited 2021 Dec 28];Available from: https://academic.oup.com/eurheartj/advance‐article/doi/10.1093/eurheartj/ehab793/6471440 (Accessed 12/05/21). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Historical medications stratified by left ventricular ejection fraction category.
Figure S2. Discharge medications stratified by left ventricular ejection fraction category.
Figure S3. One‐year medications stratified by left ventricular ejection fraction category.
Figure S4. Outcomes stratified by left ventricular ejection fraction category.


