Abstract
Background
Post-viral respiratory symptoms are common among patients with asthma. Respiratory symptoms after acute COVID-19 are widely reported in the general population, but large-scale studies identifying symptom risk for patients with asthma are lacking.
Objective
To identify and compare risk for post–acute COVID-19 respiratory symptoms in patients with and without asthma.
Methods
This retrospective, observational cohort study included COVID-19–positive patients between March 4, 2020, and January 20, 2021, with up to 180 days of health care follow-up in a health care system in the Northeastern United States. Respiratory symptoms recorded in clinical notes from days 28 to 180 after COVID-19 diagnosis were extracted using natural language processing. Cohorts were stratified by hospitalization status during the acute COVID-19 period. Univariable and multivariable analyses were used to compare symptoms among patients with and without asthma adjusting for demographic and clinical confounders.
Results
Among 31,084 eligible patients with COVID-19, 2863 (9.2%) had hospitalization during the acute COVID-19 period; 4049 (13.0%) had a history of asthma, accounting for 13.8% of hospitalized and 12.9% of nonhospitalized patients. In the post–acute COVID-19 period, patients with asthma had significantly higher risk of shortness of breath, cough, bronchospasm, and wheezing than patients without an asthma history. Incident respiratory symptoms of bronchospasm and wheezing were also higher in patients with asthma. Patients with asthma who had not been hospitalized during acute COVID-19 had additionally higher risk of cough, abnormal breathing, sputum changes, and a wider range of incident respiratory symptoms.
Conclusion
Patients with asthma may have an under-recognized burden of respiratory symptoms after COVID-19 warranting increased awareness and monitoring in this population.
Key words: SARS-CoV-2, Coronavirus, Post-viral, Natural language processing, Electronic health records
Abbreviations used: aOR, Adjusted odds ratio; BMI, Body mass index; CCI, Charlson comorbidity index; CF, Cystic fibrosis; CI, Confidence interval; COPD, Chronic obstructive pulmonary disease; EHR, Electronic health record; ICD-9 and ICD-10-CM, International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification; ILD, Interstitial lung disease; NLP, Natural language processing; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VA, Veterans Affairs
What is already known about this topic? Post-viral respiratory symptoms are common in patients with asthma, and post–acute COVID-19 respiratory symptoms are widely experienced by the general population. However, post–acute COVID-19 symptom risk for patients with asthma is poorly understood.
What does this article add to our knowledge? In this large study of electronic clinical notes from >31,000 patients up to 180 days after acute COVID-19, patients with asthma have higher risk of respiratory symptoms—and of developing new symptoms—compared with patients without asthma.
How does this study impact current management guidelines? Our findings that patients with asthma are at greater risk for respiratory symptoms after COVID-19 compared with patients without asthma may shape patient and provider expectations of the post–COVID-19 period, inform care management, and justify resource allocation.
Prolonged symptoms after a viral respiratory tract infection are common and often more severe in patients with asthma.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is a multisystem infection that can involve the respiratory tract. Post–acute COVID-19 symptoms (≥28 days after infection) are increasingly reported among patients independent of acute disease severity.2, 3, 4 Patients with asthma are not at increased risk of COVID-19 infection or more severe acute outcomes than patients without asthma;5 , 6 however, post–acute symptom burden for these patients is not well described.
In the general population, COVID-19 survivors are at twice the risk for incident (conditions likely attributable to post–COVID-19) “respiratory signs and symptoms” than matched patients without COVID-19.7 Post–acute COVID-19 respiratory symptoms including cough, dyspnea, and chest pain are commonly identified in population-level prospective and retrospective studies.8, 9, 10 In studies of previously hospitalized and nonhospitalized patients, incident11 and persistent dyspnea is frequently reported.2 , 12, 13, 14 In a large Veterans Affairs (VA) study of incident post–COVID-19 sequelae, respiratory conditions were the most common excess burden at 6 months and reflected in high incident use of respiratory (ie, inhalers) medications.15 Hospitalization for acute COVID-19 may impact the scope of post–acute symptoms3 , 16, 17, 18 including lung function abnormalities,19 though persistent respiratory symptoms may not be directly associated with acute infection severity.20
Despite the widespread risk of post–acute COVID-19 respiratory symptoms, few studies have looked specifically at patients with pre-existing pulmonary comorbidities in the post–COVID-19 period or focused on study subpopulations with asthma or obstructive respiratory disease.12 , 21, 22, 23, 24 Early data suggest that patients with pre-existing pulmonary disease (including asthma) have higher relative risk of persistent post–COVID-19 symptoms at follow-up and impaired performance status.23 Survey data support that patients with asthma feel that their breathing is worse after COVID-19.25, 26, 27 However, small sample sizes and selection biases may limit generalizability.
Multiple prior studies have used structured data (eg, International Classification of Disease [ICD] diagnosis codes) for studying post–COVID-19 symptoms;10 , 28 , 29 however, these are limited to predefined coded diagnoses and may miss the extent and variety of symptoms documented in the patient-provider encounter clinical (free-text) notes. Electronic health record (EHR) notes provide a source of large volume, granular, longitudinal data for capturing post–acute COVID-19 symptoms. Free-text data can be processed automatically by natural language processing (NLP) tools to identify relevant symptoms.30, 31, 32 Although NLP approaches have been used in the context of acute COVID-19 symptom identification,33 , 34 few studies have reported on post–acute symptoms.
As SARS-CoV-2 variants increase the scale and spread of the pandemic, identifying post–COVID-19 symptoms and quantifying risk in specific populations clinically vulnerable to post-viral symptoms is imperative to inform risk stratification strategies and allocation of limited rehabilitation and population health resources. We hypothesized that patients with asthma would have greater risk of post–acute COVID-19 respiratory symptoms than patients without asthma.
Methods
Study population
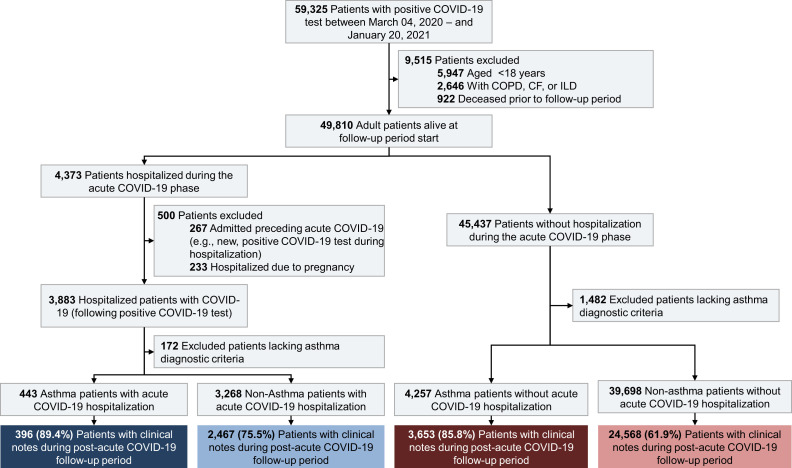
We conducted a retrospective, observational cohort study of adult patients after acute COVID-19 at Mass General Brigham, an academic health system in the Northeast United States. We identified patients who tested positive for COVID-19 by nasal or oropharyngeal polymerase chain reaction test between March 4, 2020, and January 20, 2021. We defined days 0 to 27 as the acute COVID-19 period and days 28 to 180 as the post–acute COVID-19 follow-up period. Patients were followed until the end of the follow-up period, death, or censored by study end (July 19, 2021). Patient cohort inclusion and exclusion criteria are shown in Figure 1 . First, we excluded patients who met the following criteria: (1) age <18 years at positive COVID-19 test; (2) presence of >1 ICD-coded chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), or interstitial lung disease (ILD); (3) death before the start of the post–acute COVID-19 follow-up period; (4) acute COVID-19 diagnosis during ongoing hospitalization (ie, current admission started before COVID-19 diagnosis); and (5) hospitalizations for pregnancy. Remaining patients with a hospitalization during the acute COVID-19 period were stratified into the “hospitalization” cohort. The remainder of patients were stratified into the “nonhospitalization” cohort.
Figure 1.
Cohort selection diagram for patients with electronic health record (EHR) clinical notes in the post–acute COVID-19 follow-up period. Asthma diagnosis defined as ≥2 separate encounters with an International Classification of Disease (ICD) asthma code (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org) as a primary or secondary diagnosis within the past 2 years before positive COVID-19 testing, or active asthma diagnosis on the patient EHR problem list. Patients with a single ICD code for asthma or inactive or resolved asthma diagnosis on the problem list were excluded. Acute COVID-19 period = days 0-27; post–acute COVID-19 follow-up period= days 28-180. CF, Cystic fibrosis; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease.
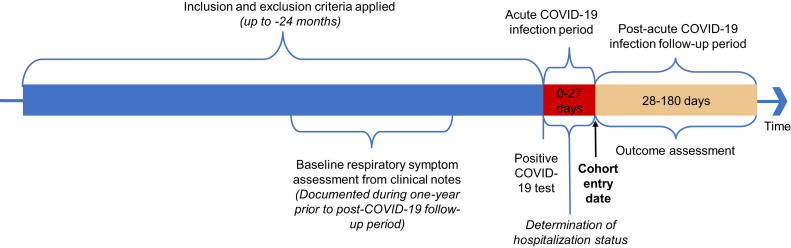
Next, we identified the population of patients with asthma within both cohorts. Asthma was defined based on an active asthma diagnosis on the patient problem list35 or ≥2 separate encounters with ICD-9 and/or ICD-10 codes (Table E1, available in this article’s Online Repository at www.jaci-inpractice.org) as a primary or secondary diagnosis within the past 2 years before the positive COVID-19 test. Patients with a single asthma code or an inactive or resolved problem of asthma on the problem list were excluded to minimize misclassification bias. Similar definitions have been used6 , 36 and internally validated with chart reviews at our institution.5 In the final step, we excluded patients without any clinical notes in the follow-up period. For the incident symptom subgroup analysis only (described in the Statistical analyses section, below), patients without any clinical notes in the year preceding COVID-19 diagnosis were excluded. We considered the first day of the follow-up period as the entry date for cohort analysis (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org).
Figure E1.
Study design.
Symptom analysis included symptoms documented by providers in notes from a patient-provider encounter, including in person, virtual video, and telephone visits. Note types were not classified by specialty due to missing data and providers’ multiple specialties. Symptoms were identified using a previously described post–acute COVID-19 lexicon.32 The Medical Text Extraction, Reasoning and Mapping System NLP tool was used to automatically extract symptoms.37 The complete symptom lexicon is available on GitHub.38 Our group’s recent validation of the post–acute COVID-19 symptom lexicon use by NLP achieved an averaged precision of 0.94 and an estimated recall of 0.84 in clinical notes.32
Patient demographic data, including insurance enrollment, education, marital status, and smoking history, were obtained from self-reported EHR data. Sex reflects the patient’s registered legal/administrative sex. Body mass index (BMI) was extracted from last measured BMI (at or before COVID-19 diagnosis encounter). Comorbid diseases were defined by at ≥2 ICD codes or the presence on the active problem list. The Charlson comorbidity index (CCI) was calculated using at least ≥1 ICD code in the year before COVID-19 diagnosis. Baseline respiratory symptoms were defined as the presence or absence of each respiratory symptom in clinical notes documented in the 1 year before the study cohort entry date.
Outcomes
The primary outcome was risk for post–acute COVID-19 respiratory symptoms. The secondary outcome was the risk of incident respiratory symptoms after acute COVID-19, defined as symptoms documented in post–COVID-19 notes that were previously absent from notes in the year before cohort entry.
Statistical analyses
Risks of post–acute COVID-19 respiratory symptoms were compared in patients with and without asthma using logistic regression models, stratified by acute COVID-19 hospitalization status. In multivariable-adjusted logistic regression models, we adjusted for age, sex, race, ethnicity, education attained, marital status, insurance type, smoking history, BMI, comorbid diseases previously shown to impact acute COVID-19 disease severity, and the CCI.39 Additional multivariable analyses were performed to account for the presence of the respiratory symptoms at baseline. Next, among all patients who did not have documented respiratory symptoms in the defined pre–COVID-19 period (Figure E1, available in this article’s Online Repository at www.jaci-inpractice.org), we measured the percentages of and compared the risk of incident post–acute COVID-19 respiratory symptoms (secondary outcome) in patients with and without asthma.
The Mann-Whitney-Wilcoxon test was used for the analysis of continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Statistical significance was defined by a 2-sided P value of ≤.05. Bonferroni correction was used to adjust for multiple testing in hospitalized and nonhospitalized cohorts. Full results for crude and multivariable adjusted odds ratios (aORs) are presented in the table detailing level of significance. Statistical analyses were performed in R software, version 3.5.3 (R Foundation for Statistical Computing).
The study was approved by the Mass General Brigham Institutional Review Board (2020P000816).
Results
A total of 59,325 patients had a positive COVID-19 test result during the study period (Figure 1). Of these, 31,084 met study inclusion criteria, accounting for a total of 687,683 patient notes included for post–acute COVID-19 symptom analysis. Of the study cohort, 4049 (13.0%) had a history of asthma; 2863 (9.2%) were hospitalized during their acute COVID-19 period and 28,221 (90.8%) without a documented hospitalization. In the hospitalization cohort, 396 (13.8%) patients had a prior diagnosis of asthma compared with 3653 (12.9%) of nonhospitalized patients with asthma (Table I ).
Table I.
Demographic and clinical characteristics of the post–COVID-19 study cohort
| Characteristic | All patients (n = 31,084) | Hospitalization (n = 2863) |
No hospitalization (n = 28,221) |
||||
|---|---|---|---|---|---|---|---|
| Asthma (n = 396) | No asthma (n = 2467) | P value# | Asthma (n =3653) | No asthma (n = 24,568) | P value | ||
| Demographic | |||||||
| Age∗ (y), mean (SD) | 48.8 (17.8) | 57.6 (16.6) | 61.3 (17.1) | <.001 | 46.4 (16.7) | 47.7 (17.6) | <.001 |
| 18-29 | 5677 (18.3) | 23 (5.8) | 104 (4.2) | .006 | 755 (20.7) | 4795 (19.5) | <.001 |
| 30-39 | 4981 (16.0) | 39 (9.8) | 211 (8.6) | 606 (16.6) | 4125 (16.8) | ||
| 40-49 | 4957 (15.9) | 54 (13.6) | 273 (11.1) | 637 (17.4) | 3993 (16.3) | ||
| 50-59 | 6477 (20.8) | 94 (23.7) | 488 (19.8) | 812 (22.2) | 5083 (20.7) | ||
| 60-69 | 4912 (15.8) | 89 (22.5) | 549 (22.3) | 525 (14.4) | 3749 (15.3) | ||
| 70-80 | 2576 (8.3) | 59 (14.9) | 458 (18.6) | 242 (6.6) | 1817 (7.4) | ||
| ≥80 | 1504 (4.8) | 38 (9.6) | 384 (15.6) | 76 (2.1) | 1006 (4.1) | ||
| Sex†, male, N (%) | 12,509 (40.2) | 140 (35.4) | 1376 (55.8) | <.001 | 1053 (28.8) | 9940 (40.5) | <.001 |
| Race‡, N (%) | |||||||
| White | 20,383 (65.6) | 221 (55.8) | 1334 (54.1) | .162 | 2508 (68.7) | 16,320 (66.4) | <.001 |
| Black | 3230 (10.4) | 75 (18.9) | 389 (15.8) | 378 (10.3) | 2388 (9.7) | ||
| Asian | 914 (2.9) | 14 (3.5) | 98 (4.0) | 78 (2.1) | 724 (2.9) | ||
| Other/unknown | 6557 (21.1) | 86 (21.7) | 646 (26.2) | 689 (18.9) | 5136 (20.9) | ||
| Ethnicity, Hispanic‡ | 7139 (23.0) | 101 (25.5) | 727 (29.5) | .12 | 864 (23.7) | 5447 (22.2) | .047 |
| Education level‡, N (%) | |||||||
| College and above | 11,030 (35.5) | 141 (35.6) | 619 (25.1) | <.001 | 1326 (36.3) | 8944 (36.4) | <.001 |
| High school or equivalent | 9729 (31.3) | 147 (37.1) | 952 (38.6) | 1215 (33.3) | 7415 (30.2) | ||
| Did not complete high school | 3004 (9.7) | 55 (13.9) | 444 (18.0) | 334 (9.1) | 2171 (8.8) | ||
| Unknown | 7321 (23.6) | 53 (13.4) | 452 (18.3) | 778 (21.3) | 6038 (24.6) | ||
| Marital status‡, N (%) | |||||||
| Single | 11,499 (37.0) | 120 (31.9) | 556 (31.5) | .013 | 1545 (42.3) | 9041 (36.8) | <.001 |
| Married/partnered | 15,017 (48.3) | 176 (46.8) | 818 (46.3) | 1657 (45.4) | 12,050 (49.0) | ||
| Divorced | 2126 (6.8) | 47 (12.5) | 165 (9.3) | 253 (6.9) | 1578 (6.4) | ||
| Widowed | 1062 (3.4) | 29 (7.7) | 175 (9.9) | 98 (2.7) | 701 (2.9) | ||
| Unknown | 1380 (4.4) | 4 (1.1) | 53 (3.0) | 100 (2.7) | 1198 (4.9) | ||
| Insurance type, N (%) | |||||||
| Commercial | 21,441 (69.3) | 218 (55.2) | 1168 (47.4) | .035 | 2606 (71.4) | 17,449 (71.4) | <.001 |
| Medicare | 4526 (14.6) | 798 (32.4) | 108 (27.3) | 447 (12.2) | 3173 (13.0) | ||
| Medicaid | 4511 (14.6) | 66 (16.7) | 469 (19.0) | 577 (15.8) | 3399 (13.9) | ||
| Others | 1583 (5.1) | 3 (0.8) | 30 (1.2) | 406 (1.7) | 21 (0.6) | ||
| Smoking history‡, N (%) | |||||||
| Never smoker | 21,127 (68.0) | 273 (68.9) | 1491 (60.4) | <.001 | 2554 (69.9) | 16,809 (68.4) | <.001 |
| Current smoker | 1459 (4.7) | 21 (5.3) | 108 (4.4) | 195 (5.3) | 1135 (4.6) | ||
| Former smoker | 6915 (22.2) | 96 (24.2) | 729 (29.6) | 882 (24.1) | 5208 (21.2) | ||
| Unknown | 1583 (5.1) | 6 (1.5) | 139 (5.6) | 22 (0.6) | 1416 (5.8) | ||
| Body mass index, mean (SD) | 29.4 (6.6) | 32.6 (7.8) | 29.5 (6.6) | <.001 | 31.1 (7.5) | 29.1 (6.4) | <.001 |
| ≤24.9 | 7541 (24.3) | 59 (14.9) | 589 (23.9) | <.001 | 746 (20.4) | 6147 (25.0) | |
| 25-29.9 | 9654 (31.1) | 108 (27.3) | 863 (35.0) | 1061 (29.0) | 7620 (31.0) | ||
| ≥30 | 11,511 (37.0) | 229 (57.8) | 1015 (41.1) | 1793 (49.1) | 8474 (34.5) | ||
| Unknown | 2378 (7.7) | n/a | n/a | 53 (1.5) | 2327 (9.5) | ||
| Comorbidities§, N (%) | |||||||
| Diabetes mellitus | 4065 (13.1) | 127 (32.1) | 737 (29.9) | .409 | 547 (15.0) | 2654 (10.8) | <.001 |
| Hypertension | 9452 (30.4) | 246 (62.1) | 1326 (53.7) | .002 | 1176 (32.2) | 6704 (27.3) | <.001 |
| Chronic kidney disease | 1931 (6.2) | 65 (16.4) | 484 (19.6) | .151 | 172 (4.7) | 1210 (4.9) | .6 |
| Chronic liver disease | 2196 (7.1) | 60 (15.2) | 278 (11.3) | .032 | 348 (9.5) | 1510 (6.1) | <.001 |
| Cardiovascular disease | 4414 (14.2) | 156 (39.4) | 787 (31.9) | .004 | 560 (15.3) | 2911 (11.8) | <.001 |
| Anxiety | 5475 (17.6) | 114 (28.8) | 422 (17.1) | <.001 | 1007 (27.6) | 3932 (16.0) | <.001 |
| Depression | 4865 (15.7) | 132 (33.3) | 503 (20.4) | <.001 | 906 (24.8) | 3324 (13.5) | <.001 |
| Charlson comorbidity index | 0.7 (1.7) | 1.5 (2.5) | 2.0 (2.6) | <.001 | 1.2 (1.9) | 0.5 (1.5) | <.001 |
| Baseline respiratory symptoms | |||||||
| Shortness of breath | 4607 (14.8) | 158 (39.9) | 422 (17.1) | <.001 | 1105 (30.2) | 2922 (11.9) | <.001 |
| Cough | 4996 (16.1) | 152 (38.4) | 389 (15.8) | <.001 | 1119 (30.6) | 3336 (13.6) | <.001 |
| Bronchospasm | 624 (2.0) | 52 (13.1) | 15 (0.6) | <.001 | 452 (12.4) | 105 (0.4) | <.001 |
| Wheezing | 2339 (7.5) | 154 (38.9) | 151 (6.1) | <.001 | 1192 (32.6) | 842 (3.4) | <.001 |
| Abnormal breathing | 1408 (4.5) | 66 (16.7) | 174 (7.1) | <.001 | 296 (8.1) | 872 (3.5) | <.001 |
| Respiratory syndrome | 517 (1.7) | 11 (2.8) | 28 (1.1) | .017 | 142 (3.9) | 336 (1.4) | <.001 |
| Sputum color change | 294 (0.9) | 21 (5.3) | 27 (1.1) | <.001 | 82 (2.2) | 164 (0.7) | <.001 |
| Stridor | 155 (0.5) | 3 (0.8) | 19 (0.8) | 1.0 | 38 (1.0) | 95 (0.4) | <.001 |
Hospitalization status determined by treatment site during the acute COVID-19 period (days: 0-27).
n/a, Not applicable; SD, standard deviation.
Age at cohort entry (COVID-19–positive test result).
Legal sex. Gender identity not available.
Self-reported.
Defined as ≥1 ICD code in the 12 months before the positive COVID-19 test result.
The demographic and clinical characteristics of the post–acute COVID-19 study cohort are detailed in Table I. The mean age of the cohort was 48.8 (standard deviation, 17.9) years, with predominantly female (59.8%) and White (65.6%) patients; 23% of the cohort was Hispanic. Across cohorts, patients with asthma (compared with those without asthma) were younger, with a higher percentage of female patients, higher mean BMI, and fewer smokers.
The CCI was higher for patients without asthma in the hospitalization cohort, though patients with asthma were more likely to have comorbidities of hypertension, cardiovascular disease, and liver disease. In the nonhospitalization cohort, patients with asthma had a higher CCI consistent with higher rates of multiple chronic comorbidities except for chronic kidney disease than patients without asthma. As expected, the presence of respiratory symptoms in clinical notes at baseline (pre–COVID-19 infection) was broadly higher in patients with asthma than in patients without asthma.
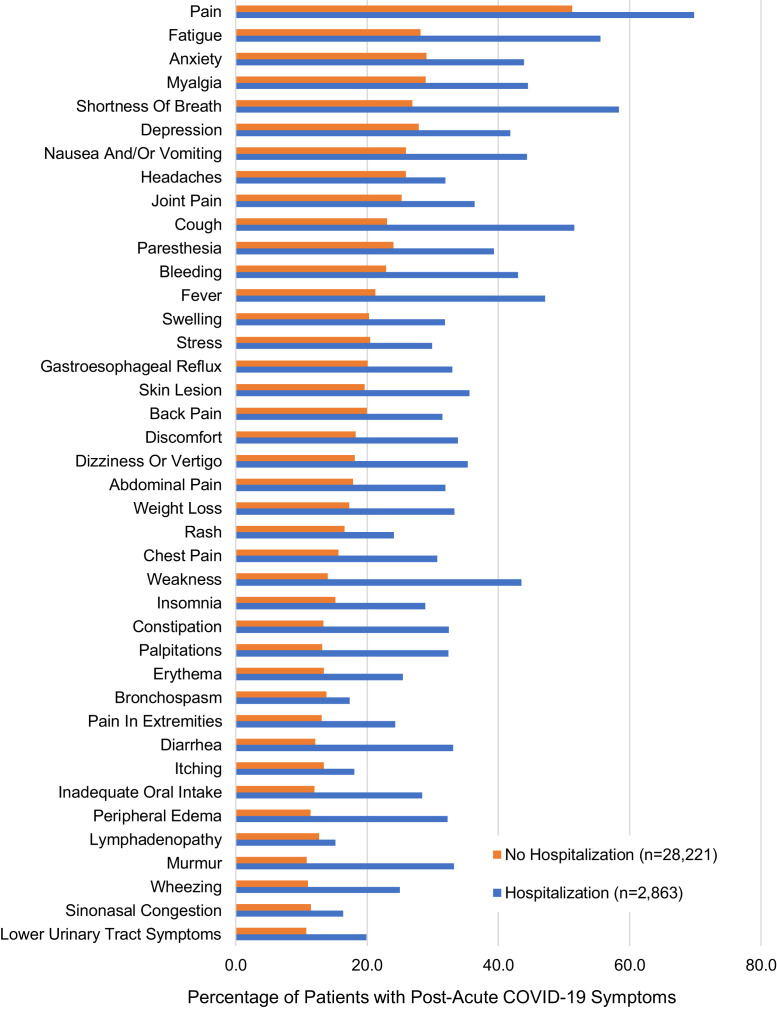
Respiratory symptoms (eg, shortness of breath and cough) were among the most common symptoms identified in the post–COVID-19 corpus of notes (Figure E2, available in this article’s Online Repository at www.jaci-inpractice.org). Greater than half of all patients in the hospitalization cohort, regardless of asthma status, had shortness of breath and approximately 50% or more had cough in the post–acute COVID-19 period (Table II ). These symptoms in patients without a hospitalization history were overall lower but approaching 1 in 5 or 1 in 4 patients even among those without an asthma history. In multivariable analysis 1, accounting for demographic and clinical covariates, the risk of shortness of breath and cough symptoms was higher in patients with asthma (aOR, 1.69 [95% confidence interval (CI), 1.33-2.16] and 1.53 [95% CI, 1.22-1.93] in the hospitalized group, and aOR [shortness of breath], 1.72 [95% CI, 1.59, 1.86] and aOR [cough], 1.63 [95% CI, 1.51-1.76] in the nonhospitalizedgroup). In multivariable analysis 2, which additionally accounted for the presence of each respiratory symptom at baseline, the risk estimate outcomes were similar.
Figure E2.
Comparison of patients with post–acute COVID-19 symptoms in electronic health record (EHR) notes by acute hospitalization status. Forty most common symptoms extracted from the free-text clinical notes in the EHR during the post–acute COVID-19 follow-up study period (days 28-180) for 31,084 patients. Symptoms stratified by patient hospitalization status during the acute COVID-19 period (days 0-27).
Table II.
Risk of respiratory symptoms after acute COVID-19 among patients with and without asthma
| Symptom† | Hospitalization (n = 2863)∗ |
Nonhospitalization (n = 28,221) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Asthma (n = 396) | No asthma (n = 2467) | Crude, OR (95% CI) | Multivariable adjusted 1‡, OR (95% CI) | Multivariable adjusted 2§, OR (95% CI) | Asthma (n = 3653) | No asthma (n = 24,568) | Crude, OR (95% CI) | Multivariable adjusted 1||, OR (95% CI) | Multivariable adjusted 2§, OR (95% CI) | |
| Shortness of breath | 282 (71.2) | 1390 (56.3) | 1.92 (1.52, 2.42)¶ | 1.69 (1.33, 2.16)¶ | 1.53 (1.20, 1.97)¶ | 1482 (40.6) | 6103 (24.8) | 2.07 (1.92, 2.22)¶ | 1.72 (1.59, 1.86)¶ | 1.57 (1.45, 1.70)¶ |
| Cough | 245 (61.9) | 1232 (49.94) | 1.63 (1.31, 2.03)¶ | 1.53 (1.22, 1.93)¶ | 1.39 (1.10, 1.76)# | 1250 (34.2) | 5256 (21.4) | 1.91 (1.77, 2.06)¶ | 1.63 (1.51, 1.76)¶ | 1.50 (1.39, 1.63)¶ |
| Bronchospasm | 307 (77.5) | 190 (7.7) | 41.34 (31.43, 54.88)¶ | 40.71 (30.31, 55.29)¶ | 36.99 (27.47, 50.36)¶ | 2413 (66.1) | 1482 (6.0) | 30.31 (27.82, 33.06)¶ | 27.42 (25.05, 30.03)¶ | 25.46 (23.20, 27.95)¶ |
| Wheezing | 219 (55.3) | 497 (20.1) | 4.9 (3.93, 6.12)¶ | 4.88 (3.86, 6.18)¶ | 3.71 (2.89, 4.76)¶ | 1489 (40.8) | 1611 (6.6) | 9.81 (9.02, 10.66)¶ | 8.09 (7.42, 8.82)¶ | 5.17(4.70, 5.69)¶ |
| Abnormal breathing | 113 (28.5) | 709 (28.7) | 0.99 (0.78, 1.25) | 1.03 (0.80, 1.32) | 0.99 (0.77, 1.27) | 375 (10.3) | 1774 (7.2) | 1.47 (1.31, 1.65)¶ | 1.20 (1.06, 1.35)# | 1.16 (1.03, 1.31)∗∗ |
| Sputum color change | 18 (4.5) | 144 (5.8) | 0.77 (0.45, 1.24) | 0.99 (0.57, 1.63) | 0.82 (0.46, 1.39) | 50 (1.4) | 155 (0.6) | 2.19 (1.57, 2.99)¶ | 1.87 (1.32, 2.61)¶ | 1.77 (1.25, 2.48)# |
| Stridor | 11 (2.8) | 79 (3.2) | 0.86 (0.43, 1.57) | 0.78 (0.38, 1.46) | 0.79 (0.38, 1.48) | 41 (1.1) | 175 (0.7) | 1.58 (1.11, 2.2)# | 1.19 (0.82, 1.68) | 1.11 (0.77, 1.58) |
Text in boldface indicates statistical significance after Bonferroni correction for multiple testing (for hospitalized patients: 23 tests in multivariable analysis 1 and 24 tests in multivariable analysis 2; for nonhospitalized patients, 27 tests in multivariable analysis 1 and 28 tests in multivariable analysis 2) with a significance level of <.05.
CI, Confidence interval; OR, odd ratio.
Hospitalization status determined by the treatment site during the acute COVID-19 period (days: 0-27).
Symptoms were ordered by prevalence in the entire study population (n = 31,084).
Multivariable analysis where the variables were chosen based on the P value ≤.1 calculated using the Wilcoxon test, χ2 test, or Fisher’s exact test. The variables included age; sex; education level; marital status; insurance type; smoking history; body mass index; comorbidities including hypertension, chronic liver disease, cardiovascular disease, anxiety, and depression; and Charlson comorbidity index.
Multivariable analysis 2 included all the variables in multivariable analysis 1 plus the corresponding baseline respiratory symptom.
Multivariable analysis where the variables were chosen based on the P value ≤.1 calculated using the Wilcoxon test, the χ2 test, or Fisher’s exact test. The variables included age; sex; race; education level; marital status; insurance type; smoking history; body mass index; comorbidities including diabetes, hypertension, chronic liver disease, cardiovascular disease, anxiety, and depression; and Charlson comorbidity index.
Indicates a statistical significance level of the unadjusted P value <.001.
Indicates a statistical significance level of the unadjusted P value <.01.
Indicates a statistical significance level of the unadjusted P value <.05.
Respiratory symptoms of bronchospasm and wheezing were markedly elevated in patients with asthma across cohorts, with an aOR of 40.71 (95% CI, 30.31-55.29) and 4.88 (95% CI, 3.86-6.18) in hospitalized patients and an aOR of 27.42 (95% CI, 25.05-30.03) and 8.09 (95% CI, 7.42-8.82) in nonhospitalized patients, respectively. These symptom risks remained significantly elevated even after accounting for the baseline presence of these symptoms.
In the hospitalization cohort, risk of abnormal breathing and sputum color change did not differ significantly by asthma history; however, risks were increased for patients with asthma without hospitalization exposure (aOR, 1.20 [95% CI, 1.06-1.35] and aOR, 1.87 [95% CI, 1.32-2.61], respectively). The presence of stridor did not differ by asthma history in either cohort.
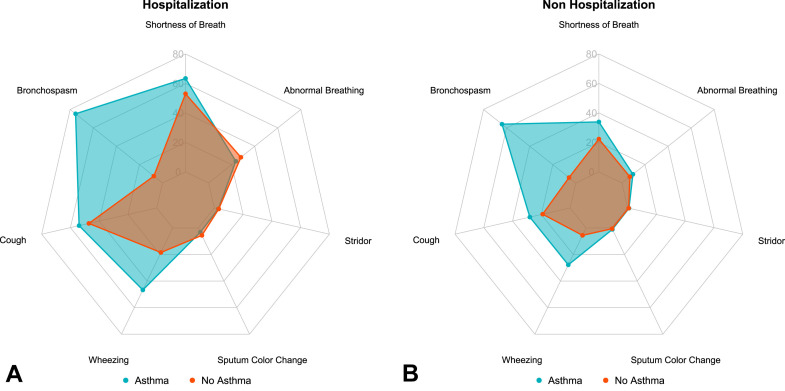
Figure 2 shows the incident risk of respiratory symptoms after acute COVID-19 among hospitalized and nonhospitalized cohorts. Among patients with asthma, 75.3% in the hospitalized cohort and 64.0% in the nonhospitalized cohort had a documented symptom of bronchospasm, having had no prior mention of bronchospasm in the pre–COVID-19 period. Among the nonhospitalized patients, risk of multiple respiratory symptoms, including shortness of breath (aOR, 1.57 [95% CI, 1.43-1.72]), cough (aOR, 1.44 [95% CI, 1.31-1.59]), bronchospasm (aOR, 26.22 [95% CI, 23.87-28.82]), wheeze (aOR, 5.54 [95% CI, 4.97-6.17]), abnormal breathing (aOR, 1.19 [95% CI, 1.04-1.36]), and sputum color change (aOR, 1.60 [95% CI, 1.09-2.29]), was newly documented among patients with asthma compared with those without asthma (Table III ). In the hospitalized cohort, only shortness of breath (aOR, 1.48 [95% CI, 1.11-1.98]), bronchospasm (aOR, 37.75 [95% CI, 27.88-51.70]), and wheeze (aOR, 4.01 [95% CI, 3.01-5.34]) were increased in patients with asthma compared with those without asthma. These findings remained statistically significant after Bonferroni correction in the nonhospitalization cohort. Bronchospasm and wheeze remained significant in the hospitalization cohort (Table III).
Figure 2.
Incident risk of respiratory symptoms after acute COVID-19. Radar charts display percentages of patients with documented incident respiratory symptoms after COVID-19, comparing patients with and without asthma history and stratified by hospitalization status during acute COVID-19 infection: (A) Hospitalization cohort; (B) nonhospitalization cohort.
Table III.
Association between asthma and risk of incident respiratory symptoms after acute COVID-19
| Outcome | Hospitalization |
Nonhospitalization |
||||||
|---|---|---|---|---|---|---|---|---|
| Asthma, n′/n (%)∗ | No asthma, n′/n (%)† | Crude, OR (95% CI) | Multivariable adjusted‡, OR (95% CI) | Asthma, n′/n (%)∗ | No asthma, n′/n (%)† | Crude, OR (95% CI) | Multivariable adjusted§, OR (95% CI) | |
| Shortness of breath | 151/238 (63.4) | 1084/2045 (53.0) | 1.54 (1.17, 2.04)|| | 1.48 (1.11, 1.98)|| | 865/2548 (33.9) | 4817/21,646 (22.3) | 1.80 (1.64, 1.96)¶ | 1.57 (1.43, 1.72)¶ |
| Cough | 132/244 (54.0) | 980/2078 (47.2) | 1.32 (1.01, 1.72)# | 1.29 (0.97, 1.70) | 709/2534 (28.0) | 4056/21,232 (19.1) | 1.65 (1.50, 1.81)¶ | 1.44 (1.31, 1.59)¶ |
| Bronchospasm | 259/344 (75.3) | 184/2452 (7.5) | 37.56 (28.30, 50.30)¶ | 37.75 (27.88, 51.70)¶ | 2049/3201 (64.0) | 1437/24,463 (5.9) | 28.50 (26.06, 31.19)¶ | 26.22 (23.87, 28.82)¶ |
| Wheezing | 113/242 (46.7) | 428/2316 (18.5) | 3.86 (2.94, 5.08)¶ | 4.01 (3.01, 5.34)¶ | 683/2461 (27.8) | 1326/23,726 (5.6) | 6.49 (5.85, 7.20)¶ | 5.54 (4.97, 6.17)¶ |
| Abnormal breathing | 78/330 (23.6) | 642/2293 (28.0) | 0.80 (0.60, 1.04) | 0.84 (0.63, 1.12) | 318/3357 (9.5) | 1608/23,696 (6.8) | 1.44 (1.27, 1.63)¶ | 1.19 (1.04, 1.36)|| |
| Sputum color change | 12/375 (3.2) | 137/2440 (5.6) | 0.56 (0.29, 0.97) | 0.73 (0.37, 1.29) | 39/3571 (1.1) | 149/24,404 (0.6) | 1.80 (1.24, 2.53)|| | 1.60 (1.09, 2.29)# |
| Stridor | 11/393 (2.8) | 75/2448 (3.1) | 0.90 (0.45, 1.64)) | 0.82 (0.40, 1.55) | 34/3615 (0.9) | 162/24,473 (0.7) | 1.42 (0.97, 2.04)) | 1.07 (0.72, 1.55) |
Text in boldface indicates statistical significance after Bonferroni correction for multiple testing (23 tests for hospitalized patients and 27 tests for nonhospitalized patients) with a significance level of .05.
CI, Confidence interval; OR, odd ratio.
The n denominator is the number of patients who had asthma but without the specific symptom (eg, shortness of breath) before COVID-19; the n′ numerator is the number of patients who did not have the specific symptom before COVID-19 infection but develop that symptom (eg, shortness of breath) after COVID infection; the % is the percentage of patients who developed the specific symptom (eg, shortness of breath).
The n denominator is the number of patients who did not have asthma and the specific symptom (eg, shortness of breath) before COVID-19; the n′ numerator is the number of patients who developed that symptom after COVID infection; the % is the percentage of patients who developed the specific symptom (eg, shortness of breath).
The variables included in the multivariable analysis were age; sex; education level; marital status; insurance type; smoking history; body mass index; comorbidities including hypertension, chronic liver disease, cardiovascular disease, anxiety, and depression; and Charlson comorbidity index.
The variables included in the multivariable analysis were age; sex; race; education level; marital status; insurance type; smoking history; body mass index; comorbidities including diabetes, hypertension, chronic liver disease, cardiovascular disease, anxiety, and depression; and Charlson comorbidity index.
Indicates a statistical significance level of the unadjusted P value <.01.
Indicates a statistical significance level of the unadjusted P value <.001.
Indicates a statistical significance level of the unadjusted P value <.05.
Discussion
Patients with asthma have well-established risk for post-viral respiratory symptoms generally, yet research on symptom identification, prevalence, and risk in the post–acute COVID-19 period for this high-risk population is limited.22 We leveraged clinical notes to identify provider-documented respiratory symptoms in over 30,000 patients after acute COVID-19 infection and found that patients with asthma are at increased risk for a range of respiratory symptoms after acute COVID-19, and that symptom type and degree of risk may vary by hospitalization exposure during the acute COVID-19 period.
We found high rates of cough and dyspnea in the patient cohort, consistent with prior studies10 , 12 , 21 , 22 , 40 and with similar ORs.7 Risk of shortness of breath and cough symptoms was increased by ≥1.4-fold among patients with asthma in both the hospitalization and nonhospitalized cohorts even after accounting for the prevalence of respiratory symptoms at baseline. Our study significantly adds to prior literature by comparing the risk of these symptoms in the general population to patients with asthma. Our study also accounts for important confounders missing from prior studies and strengthening our findings.10 Recent studies identifying asthma as an incident (ie, post-COVID-related) condition7 in the post–COVID-19 period among patients without preceding asthma are supported by our findings of bronchospasm and wheeze—hallmarks of asthma—in approximately 6% of the patients without asthma.10 , 15 Higher risk of bronchospasm and wheeze would be expected among patients with asthma compared with those without; however, this risk remained markedly elevated even in the subgroups of patients without these symptoms mentioned in the notes before COVID-19, suggesting a significant symptom burden in patients with asthma in the post–COVID-19 period. In contrast, studies on acute COVID-19 infection severity in patients with asthma have not identified increased severity or infection risk, despite the early presumption that patients with asthma would be at higher risk.5 , 6
Stratifying patients into hospitalization and nonhospitalization cohorts based on acute COVID-19 treatment site may additionally minimize post–acute infection symptom differences attributable to initial COVID-19 disease severity as well as to the hospitalization exposure itself, which may impact patients’ physical, mental, or emotional health.14 , 18 , 41, 42, 43 We did not test whether the risk of post–acute respiratory symptoms differed significantly between the hospitalized and nonhospitalized cohorts. However, our finding that patients with asthma in the nonhospitalization cohort had increased risk for a broader range of symptoms—and particularly for incident respiratory symptoms—than those in the hospitalization cohorts supports that the disease severity or hospitalization exposure may mediate symptom risk. Studies by other groups also suggest a potential disconnect between initial COVID-19 severity and post–acute COVID-19 symptom burden, as assessed by radiographic, biomarker, or functional outcomes.20 This complexity may be due to the increased heterogeneity of acute COVID-19 disease severity in the outpatient setting and limitations of accounting for this variability without hospital-level monitoring. Alternatively, the larger size of the nonhospitalized cohort, compared with the hospitalized cohort, may have increased our power to detect a broader range of symptoms. However, these findings suggest an unmet supportive care need for patients with initially mild-to-moderate disease or patient underutilization of rehabilitation services despite a high burden of post–acute COVID-19 symptoms.44 Our findings suggest that patients with asthma may be a high-priority group for these services, and future studies are needed to identify specific risk factors for respiratory symptoms within this population. Our findings also support interventions aimed at early detection and prevention/mitigation of post–acute COVID-19 symptoms or symptom duration. For example, clinical decision support tools could be used to flag patients with asthma and with a history of COVID. These patients could then be prioritized for referral pathways for rehabilitation services or for the use of patient-reported outcome screening tools in follow-up visits. These needs may also change with vaccination trends; future studies could assess the impact of vaccination on post-acute sequelae of COVID-19 symptoms in patients with asthma.
Strengths of this study include the application of NLP to identify symptom-level data in a clinically vulnerable population. Although prior studies have used NLP to study acute COVID-19, this is the first to apply NLP methodology to the post–acute COVID-19 domain.45 NLP enables symptom capture from free-text descriptions of patient clinical history, circumventing limitations in other EHR-based post–COVID-19 studies that used billing and diagnosis codes that may not capture symptom-level detail.15 , 29 , 30 Limitations of NLP include errors in symptom detection, due to, for example, unrecognized abbreviations, negations, and ambiguity of words. Despite these limitations, the NLP and symptom lexicon achieved high precision and recall scores as previously described,32 supporting its use for this work as well as its application to other NLP tools and machine learning models and for validation in other populations. The use of a real-world symptom-based lexicon also complements smaller, prospective studies of patient-reported symptoms and facilitates future longitudinal analysis through the use of the EHR in routine care.
There are several limitations to this study related to the observational, EHR-based study design including missing notes or incomplete data, surveillance bias, and unmeasured confounders. EHR data can be incomplete particularly for complex covariates like smoking, education, and BMI.46 , 47 Our analysis was limited to patients seeking medical care in our system, and therefore patients with unreported symptoms, or with care outside of our system, would be missed in our analysis. The use of an institutional EHR data source may also limit generalizability based on regional demographic differences or neighborhood-level socioeconomic status.48 Paradoxically, travel restrictions during the study period and telemedicine technology49 may have improved in-network data capture. Clinical note text from virtual video and telephone encounters was included in this study, maximizing data capture particularly among patients who might be precluded from studies requiring in-person visits for post–acute COVID-19 evaluation (ie, imaging or laboratory draws). In contrast, new patients to the system without any prior clinical notes would have been missed by this study. Character and tempo of post–COVID-19 symptoms may also depend in part on the specific variant involved, which we could not assess, and may be best investigated prospectively.
Cohort eligibility criteria excluded patients with COPD, ILD, or CF based on published literature on asthma phenotypes36 and manual chart review for the internal validation of the asthma phenotype. Of the 2646 patients excluded with these 3 diagnoses, 1138 (43.0%) had at least 1 code for asthma, supporting the exclusion criteria to minimize misclassification as asthma/nonasthma history. However, this may have excluded some patients with more severe asthma on the asthma-COPD overlap spectrum. Patients with asthma-COPD overlap may be at higher risk of acute COVID-19,6 though the implications for post–COVID-19 symptoms among patients with COPD is an active area of study.21
Relying on provider-documented, patient-centered symptom reporting may introduce subjectivity.20 Providers may translate patient-reported symptoms into clinical terminology such as “stridor,” “abnormal breathing,” or “bronchospasm.” We did not correlate symptoms with service or procedure codes (ie, orders for pain medications), radiographic data, or asthma control measures.19 , 20 , 23 , 50 As in routine clinical care, it is difficult to confirm whether symptoms detected in follow-up can be directly attributed to COVID-19 infection. Few studies have addressed this question methodologically: one study of a large VA population used negative-outcome and negative-exposure controls, finding that incident respiratory conditions were still the most common excess burden at 6 months after COVID-19.15 A United States EHR-based study of patients who did not require hospitalization for acute COVID-19 examined symptoms “potentially related to COVID-19” using incident ICD codes, precluding those from patients who had matching codes in the preceding 12 months.10 Although this may have undercaptured symptoms in patients with symptomatic, pre-existing chronic diseases, asthma was associated with a higher count of new post–COVID-19 visits/diagnoses, supporting our findings of both higher follow-up rates and symptom risk. Future research adopting these approaches will be critical to strengthen associations between COVID-19 infection and post–COVID-19 symptoms, investigate underlying mechanisms, and justify targeted post–acute COVID-19 care.
Conclusion
To date, asthma does not appear to be a risk factor for acute COVID-19, which should be reassuring for patients with asthma and the providers who care for them. However, in this study of symptoms extracted with NLP from free-text clinical EHR notes, we found that patients with asthma have significantly higher risk for multiple respiratory symptoms, and incident symptom development, up to 6 months after acute COVID-19 diagnosis compared with patients without an asthma history. These findings flag an important, emerging clinical concern and highlight the need for longitudinal evaluation and intervention for patients with asthma within the increasingly large population of COVID-19 survivors.
Acknowledgments
We thank Mass General Brigham Enterprise Data Warehouse for providing data and technical support.
L. Wang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: L. Wang, D. Foer; acquisition, analysis, or interpretation of data: all authors; draft of the manuscript: L. Wang, D. Foer; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: L. Wang, D. Foer, Y. Zhang; administrative, technical, or material support: L. Zhou; supervision: E. W. Karlson, D. W. Bates, L. Zhou.
Footnotes
The authors were supported by the National Institutes of Health (grant numbers R01AI150295, R01HS025375, K23HL161332, K99AG075190, VERITY P30 AR070253). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Portions of this work were presented in abstract form at the American Thoracic Society annual conference (May 2022).
Conflicts of interest: D. Foer receives research funding from CRICO and IBM Watson Health unrelated to this work. L. Zhou receives research funding from IBM Watson Health unrelated to this work. D. W. Bates consults for EarlySense, which makes patient safety monitoring systems. He receives cash compensation from CDI (Negev), Ltd, which is a not-for-profit incubator for health IT startups. He receives equity from ValeraHealth, which makes software to help patients with chronic diseases; equity from Clew, which makes software to support clinical decision-making in intensive care; equity from MDClone, which takes clinical data and produces deidentified versions of it; equity from AESOP, which makes software to reduce medication error rates. He receives cash compensation and equity from FeelBetter. He receives research funding from IBM Watson Health. These disclosures are unrelated to this work. The rest of the authors declare that they have no relevant conflicts of interest.
Online Repository
Table E1.
International Classification of Disease (ICD) codes used for asthma inclusion criteria
| Code | Description |
|---|---|
| ICD-10 codes | |
| J45 | Asthma |
| J45.2 | Mild intermittent asthma |
| J45.20 | Mild intermittent asthma uncomplicated |
| J45.21 | Mild intermittent asthma with (acute) exacerbation |
| J45.22 | Mild intermittent asthma with status asthmaticus |
| J45.3 | Mild persistent asthma |
| J45.30 | Mild persistent asthma uncomplicated |
| J45.31 | Mild persistent asthma with (acute) exacerbation |
| J45.32 | Mild persistent asthma with status asthmaticus |
| J45.4 | Moderate persistent asthma |
| J45.40 | Moderate persistent asthma uncomplicated |
| J45.41 | Moderate persistent asthma with (acute) exacerbation |
| J45.42 | Moderate persistent asthma with status asthmaticus |
| J45.5 | Severe persistent asthma |
| J45.50 | Severe persistent asthma uncomplicated |
| J45.51 | Severe persistent asthma with (acute) exacerbation |
| J45.52 | Severe persistent asthma with status asthmaticus |
| J45.9 | Other and unspecified asthma |
| J45.90 | Unspecified asthma |
| J45.901 | Unspecified asthma with (acute) exacerbation |
| J45.902 | Unspecified asthma with status asthmaticus |
| J45.909 | Unspecified asthma uncomplicated |
| J45.99 | Other asthma |
| J45.990 | Exercise-induced bronchospasm |
| J45.991 | Cough variant asthma |
| J45.998 | Other asthma |
| ICD-9 codes | |
| 493 | Asthma |
| 493.0 | Extrinsic asthma |
| 493.00 | Extrinsic asthma, unspecified |
| 493.01 | Extrinsic asthma with status asthmaticus |
| 493.02 | Extrinsic asthma with (acute) exacerbation |
| 493.1 | Intrinsic asthma |
| 493.10 | Intrinsic asthma, unspecified |
| 493.11 | Intrinsic asthma with status asthmaticus |
| 493.12 | Intrinsic asthma with (acute) exacerbation |
| 493.2 | Chronic obstructive asthma |
| 493.20 | Chronic obstructive asthma, unspecified |
| 493.21 | Chronic obstructive asthma with status asthmaticus |
| 493.22 | Chronic obstructive asthma with (acute) exacerbation |
| 493.8 | Other forms of asthma |
| 493.81 | Exercise-induced bronchospasm |
| 493.82 | Cough variant asthma |
| 493.9 | Asthma unspecified |
| 493.90 | Asthma, unspecified type, unspecified |
| 493.91 | Asthma, unspecified type, with status asthmaticus |
| 493.92 | Asthma, unspecified type, with (acute) exacerbation |
References
- 1.Busse W.W., Lemanske R.F., Jr., Gern J.E. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2010;376:826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandal S., Barnett J., Brill S.E., Brown J.S., Denneny E.K., Hare S.S., et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76:396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P., Cuapio A., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11 doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson L.B., Wang L., Fu X., Wallace Z.S., Long A.A., Zhang Y., et al. COVID-19 severity in asthma patients: a multi-center matched cohort study. J Asthma. 2022;59:442–450. doi: 10.1080/02770903.2020.1857396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L., Foer D., Bates D.W., Boyce J.A., Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. 2020;146:808–812. doi: 10.1016/j.jaci.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bull-Otterson L. Post-COVID conditions among adult COVID-19 survivors aged 18–64 and ≥65 years—United States, March 2020–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:713–717. [Google Scholar]
- 8.Song W.J., Hui C.K.M., Hull J.H., Birring S.S., McGarvey L., Mazzone S.B., et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med. 2021;9:533–544. doi: 10.1016/S2213-2600(21)00125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez-Romieu A.C., Leung S., Mbanya A., Jackson B.R., Cope J.R., Bushman D., et al. Health care utilization and clinical characteristics of nonhospitalized adults in an integrated health care system 28-180 days after COVID-19 diagnosis—Georgia, May 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:644–650. doi: 10.15585/mmwr.mm7017e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weerahandi H., Hochman K.A., Simon E., Blaum C., Chodosh J., Duan E., et al. Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med. 2021;36:738–745. doi: 10.1007/s11606-020-06338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdallah S.J., Voduc N., Corrales-Medina V.F., McGuinty M., Pratt A., Chopra A., et al. Symptoms, pulmonary function and functional capacity four months after COVID-19. Ann Am Thorac Soc. 2021;18:1912–1917. doi: 10.1513/AnnalsATS.202012-1489RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellan M., Soddu D., Balbo P.E., Baricich A., Zeppegno P., Avanzi G.C., et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aly Z., Xie Y., Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chopra V., Flanders S.A., O’Malley M., Malani A.N., Prescott H.C. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend L., Dowds J., O’Brien K., Sheill G., Dyer A.H., O’Kelly B., et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann Am Thorac Soc. 2021;18:997–1003. doi: 10.1513/AnnalsATS.202009-1175OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpin S.J., McIvor C., Whyatt G., Adams A., Harvey O., McLean L., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 22.Eggert L.E., He Z., Collins W., Lee A.S., Dhondalay G., Jiang S.Y., et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy. 2022;77:173–185. doi: 10.1111/all.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sonnweber T., Boehm A., Sahanic S., Pizzini A., Aichner M., Sonnweber B., et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res. 2020;21:276. doi: 10.1186/s12931-020-01546-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tenforde M.W., Kim S.S., Lindsell C.J., Rose E.B., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:993. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philip K.E.J., Buttery S., Williams P., Vijayakumar B., Tonkin J., Cumella A., et al. Impact of COVID-19 on people with asthma: a mixed methods analysis from a UK wide survey. BMJ Open Respir Res. 2022;9 doi: 10.1136/bmjresp-2021-001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-de-Las-Penas C., Torres-Macho J., Velasco-Arribas M., Arias-Navalon J.A., Guijarro C., Hernandez-Barrera V., et al. Similar prevalence of long-term post-COVID symptoms in patients with asthma: a case-control study. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Pachon E., Grau-Delgado J., Soler-Sempere M.J., Zamora-Molina L., Baeza-Martinez C., Ruiz-Alcaraz S., et al. Low prevalence of post-COVID-19 syndrome in patients with asthma. J Infect. 2021;82:276–316. doi: 10.1016/j.jinf.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taquet M., Luciano S., Geddes J.R., Harrison P.J. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2021;8:130–140. doi: 10.1016/S2215-0366(20)30462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estiri H., Strasser Z.H., Brat G.A., Semenov Y.R., Patel C.J. Consortium for Characterization of COVID-19 by EHR (4CE), Patel CJ, et al. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med. 2021;19:249. doi: 10.1186/s12916-021-02115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koleck T.A., Dreisbach C., Bourne P.E., Bakken S. Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review. J Am Med Inform Assoc. 2019;26:364–379. doi: 10.1093/jamia/ocy173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.South B.R., Shen S., Jones M., Garvin J., Samore M.H., Chapman W.W., et al. Developing a manually annotated clinical document corpus to identify phenotypic information for inflammatory bowel disease. BMC Bioinformatics. 2009;10(Suppl 9):S12. doi: 10.1186/1471-2105-10-S9-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L., Foer D., MacPhaul E., Lo Y.C., Bates D.W., Zhou L. PASCLex: a comprehensive post-acute sequelae of COVID-19 (PASC) symptom lexicon derived from electronic health record clinical notes. J Biomed Inform. 2022;125 doi: 10.1016/j.jbi.2021.103951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lybarger K., Ostendorf M., Thompson M., Yetisgen M. Extracting COVID-19 diagnoses and symptoms from clinical text: a new annotated corpus and neural event extraction framework. J Biomed Inform. 2021;117 doi: 10.1016/j.jbi.2021.103761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J., Abu-El-Rub N., Gray J., Pham H.A., Zhou Y., Manion F.J., et al. COVID-19 SignSym: a fast adaptation of a general clinical NLP tool to identify and normalize COVID-19 signs and symptoms to OMOP common data model. J Am Med Inform Assoc. 2021;28:1275–1283. doi: 10.1093/jamia/ocab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang E.C.-H., Wright A. Characterizing outpatient problem list completeness and duplications in the electronic health record. J Am Med Inform Assoc. 2020;27:1190–1197. doi: 10.1093/jamia/ocaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivade C., Raghavan P., Fosler-Lussier E., Embi P.J., Elhadad N., Johnson S.B., et al. A review of approaches to identifying patient phenotype cohorts using electronic health records. J Am Med Inform Assoc. 2014;21:221–230. doi: 10.1136/amiajnl-2013-001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L., Plasek J.M., Mahoney L.M., Karipineni N., Chang F., Yan X., et al. Using Medical Text Extraction, Reasoning and Mapping System (MTERMS) to process medication information in outpatient clinical notes. AMIA Annu Symp Proc. 2011;2011:1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 38.GitHub Post-COVID-19 symptoms. https://github.com/bylinn/post_covid19_symptoms
- 39.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 40.Sonnweber T., Sahanic S., Pizzini A., Luger A., Schwabl C., Sonnweber B., et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021;57 doi: 10.1183/13993003.03481-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kingery J.R., Bf Martin P., Baer B.R., Pinheiro L.C., Rajan M., Clermont A., et al. Thirty-day post-discharge outcomes following COVID-19 infection. J Gen Intern Med. 2021;36:2378–2385. doi: 10.1007/s11606-021-06924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somani S.S., Richter F., Fuster V., De Freitas J.K., Naik N., Sigel K., et al. Characterization of patients who return to hospital following discharge from hospitalization for COVID-19. J Gen Intern Med. 2020;35:2838–2844. doi: 10.1007/s11606-020-06120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X., Liu X., Zhou Y., Yu H., Li R., Zhan Q., et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foer D, Wang L, Karlson E, Bates D, Zhou L. Post-acute COVID-19 symptoms in patients with asthma: a natural language processing-based study. B34: Global Impact of the COVID-19 Pandemic. American Thoracic Society; 2022:A2694.
- 46.Polubriaginof F., Salmasian H., Albert D.A., Vawdrey D.K. Challenges with collecting smoking status in electronic health records. AMIA Annu Symp Proc. 2017;2017:1392–1400. [PMC free article] [PubMed] [Google Scholar]
- 47.Wells B.J., Chagin K.M., Nowacki A.S., Kattan M.W. Strategies for handling missing data in electronic health record derived data. EGEMS (Wash DC) 2013;1:1035. doi: 10.13063/2327-9214.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez J.A., Betancourt J.R., Sequist T.D., Ganguli I. Differences in the use of telephone and video telemedicine visits during the COVID-19 pandemic. Am J Manag Care. 2021;27:21–26. doi: 10.37765/ajmc.2021.88573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Castro R., Vasconcello-Castillo L., Alsina-Restoy X., Solis-Navarro L., Burgos F., Puppo H., et al. Respiratory function in patients post-infection by COVID-19: a systematic review and meta-analysis. Pulmonology. 2021;27:328–337. doi: 10.1016/j.pulmoe.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]