Abstract
Aims
The Guidelines for the Pharmacotherapy of Schizophrenia were established to improve the quality of medical care, and the EGUIDE project was conducted to train clinicians on guideline usage. A quality indicator (QI) was established to measure the prevalence of the guidelines, and a survey was conducted, which revealed a gap between the guidelines and actual clinical practice (evidence‐practice‐gap). The purpose of this study was to develop an individual fitness score (IFS) formula that expresses the degree to which prescribers adhere to the Guidelines for Pharmacological Therapy of Schizophrenia in a simple manner, and to determine the validity of this formula from a survey of the prescriptions of the EGUIDE project participants'.
Methods
To establish appropriate scores, members discussed the proposed formula and then voted on them. The IFS formula developed was set up so that antipsychotic monotherapy would be given 100 points, with points deducted if concomitant or adjunctive antipsychotic medications were used, and a minimum score of 0. To validate this formula, prescriptions of hospitalized schizophrenic patients at admission and at discharge were scored and compared.
Result
IFS points vary and ranged from 0 to100. The average pre‐admission score for all subjects was 45.6, and the average score at discharge was 54, those were significantly higher during discharge.
Conclusions
We developed an IFS formula, a tool to easily visualize the degree to which current prescriptions conform to the guidelines for the pharmacological treatment of schizophrenia.
Keywords: clinical practice guideline, individual fitness score (IFS), prescription, quality indicator, schizophrenia
1. INTRODUCTION
Clinical practice guidelines are developed to standardize and improve the quality of medical care, and several guidelines for schizophrenia have been published. 1 , 2 , 3 , 4 In Japan, the “Guidelines for Pharmacological Therapy of Schizophrenia” by the Japanese Society of Neuropsychopharmacology has been published. 1 However, it is unclear to what extent the guidelines are followed in clinical settings. Thus, we launched the “Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)” project to disseminate the guidelines and standardize medical practice. 5 , 6 , 7 , 8 The project aimed to ensure the social implementation of treatment guidelines for schizophrenia by conducting training sessions and evaluating whether trained psychiatrists practice following these guidelines. The training session lasted for 1 day and consisted of a lecture on treatment guidelines and a group discussion session. Approximately 1500 people attended the course from 2016 to 2021. The training course was designed to ensure that practice guidelines are implemented in the society using indicators, called quality indicators (QI), developed and used in a wide range of clinical medicine. For example, mortality rates have been set as a QI to verify the significance of the presence or the absence of tests or therapeutic interventions. 9
The field of psychiatry deals primarily with chronic illnesses, and there is a need for new indicators that can be repeatedly assessed over time rather than the use of short‐term events such as death. We set up our own QI and have surveyed and reported how schizophrenia pharmacological treatment is administered in Japan. 5 , 6 , 7 , 8 , 10 , 11 , 12 , 13 The survey results showed that those who took the course improved their understanding of the guidelines 5 and their guideline‐oriented practice. 10 Regarding the actual prescribing culture in clinical practice, analysis of pre‐course data showed that there was an evidence‐practice gap between the standard treatment proposed by the guidelines and clinical practice and there were significant differences between facilities, and standardization was lagging. For example, although schizophrenia pharmacological treatment guidelines recommend monotherapy with second‐generation antipsychotics (SGAs), 1 the monotherapy prescription rate in Japan is only 50%‐60%, and this rate varies widely by facility. 7
The QI we developed provided multiple indicators of compliance with the guidelines on prescribing. However, there is need for a simpler indicator that can visualize the degree of conformity to prescribing guidelines. It is, for example, an indicator that combines several indicators into one and shows them in one numerical value per prescription. This is because if a simple indicator can indicate the degree of conformity of individual patients, the evidence‐practice gap can be clarified, and improvement measures to fill the gap can be proposed and implemented. Furthermore, if improvements can be made, evidence‐based psychiatry can be provided, which will benefit patients. Such measures are needed in a wide range of clinical areas and their creation would be helpful in all areas of medicine.
The purpose of this study was to develop an individual fitness score (IFS) formula that expresses the degree to which prescribers adhere to the Guidelines for Pharmacological Therapy of Schizophrenia, in a simple manner and to determine the validity of this formula from a survey of the prescriptions of the EGUIDE project participants'.
2. METHODS
2.1. Creation of IFS formula
We aimed to create a formula to visualize the compliance of prescriptions with the guidelines for pharmacotherapy of schizophrenia. The Guidelines for the Pharmacotherapy of Schizophrenia 1 apply to patients with a confirmed diagnosis of schizophrenia. Therefore, the formula was designed to evaluate whether the treatment conformed to the treatment guidelines for schizophrenia, with individual patient characteristics, such as the presence or the absence of comorbidities, excluded from the evaluation items. With reference to the level of recommendation, the formula assumed that 100 points were given for complete adherence to the guidelines, and points were deducted for any treatment that was not recommended. The number of points deducted for each treatment item was determined using the modified Delphi method by the EGUIDE project members. To establish appropriate scores, members discussed the proposed scores in an online meeting and then voted on them. The appropriateness of the scores for each item was evaluated using the two‐case method. The rounds were conducted four times between April 2021 and November 2021 and in each round, the voting rate was 100%. Twenty participants attended the final round, and consensus was reached on the setting of scores for each item, with the approval of at least 90% (18 participants) of the participants.
Table 1 shows each treatment item and the formula for calculating point reductions. According to the Guidelines for Pharmacological Therapy of Schizophrenia, 1 the treatment strategies differ between nontreatment‐resistant and treatment‐resistant schizophrenia. Therefore, the formulas were developed separately for nontreatment‐resistant and treatment‐resistant schizophrenia. For nontreatment‐resistant schizophrenia, monotherapy with SGAs at appropriate doses was given 100 points, deducting points for any concomitant use of nonrecommended medications. For the concomitant use of antipsychotics, points were deducted for excessive high‐dose prescriptions, with a 25‐point deduction for exceeding the appropriate dose and 50‐point deduction for exceeding the dose by more than 1.5 times. For multiple‐drug combinations, the points to be deducted were set at 25 points for two‐drug combinations and 50 for three or more drugs, with a larger point reduction for higher doses. As the guidelines recommend the use of SGAs rather than first‐generation antipsychotics (FGAs), the use of FGAs attracts a 5‐point deduction. The concomitant use of psychotropic drugs, such as antidepressants, is not recommended in the practice guidelines. Therefore, points were deducted for concomitant use of such drugs, including 15 points for one drug, 35 for two drugs, and 55 for three or more drugs. In particular, the use of dopaminergic agents attracts a deduction of 80 points because their effects are contrary to those of antipsychotic agents, and their concomitant use is not rational.
TABLE 1.
Formula for individual fitness score
| Nontreatment‐resistant schizophrenia | Treatment‐resistant schizophrenia | |||
|---|---|---|---|---|
| Clozapine or ECT | NA | CLZ(+) | CLZ(−) | |
| ECT(+) | ECT(−) | |||
| 0 | −20 | −60 | ||
| SGA monotherapy, less than MTD | 0‐5 | 0 | 0 | |
| FGA monotherapy, less than MTD | −5 | −5 | ||
| SGA monotherapy, <1.5 times of MTD | −25 | −25 | −25 | |
| FGA monotherapy, <1.5 times of MTD | −30 | −30 | −30 | |
| SGA monotherapy, more than 1.5 times of MTD | −50 | −50 | −50 | |
| FGA monotherapy, more than 1.5 times of MTD | −55 | −55 | −55 | |
| SGA + SGA, total CPZ equivalent <1000 | −25 | −30 | −25 | −25 |
| SGA + FGA, total CPZ equivalent <1000 | −30 | −30 | −30 | |
| FGA + FGA, total CPZ equivalent <1000 | −35 | −40 | −35 | −35 |
| SGA + SGA, total CPZ equivalent ≧1000, <2000 | −35 | −35 | −35 | |
| SGA + FGA, total CPZ equivalent ≧1000, <2000 | −40 | −40 | −40 | |
| FGA + FGA, total CPZ equivalent ≧1000, <2000 | −45 | −45 | −45 | |
| SGA + SGA, total CPZ equivalent ≧2000 | −50 | −50 | −50 | |
| SGA + FGA, total CPZ equivalent ≧2000 | −55 | −55 | −55 | |
| FGA + FGA, total CPZ equivalent ≧2000 | −60 | −60 | −60 | |
| Combination therapy with 3 or more SGAs | −65 | −65 | −65 | |
| Combination therapy with 3 or more SGAs + FGAs | −70 | −70 | −70 | −70 |
| No antipsychotc prescription | −90 | −90 | −90 | −90 |
| No daily prescription (as needed prescrioption only) | −60 | −60 | −60 | −60 |
| Concomitant use of antidepressants, anxiolytics, hypnotics, mood stabilizers, antiepileptic drugs, and other psychotropic drugs (except dopaminergic and anticholinergic drugs) | 1 drug −15, 2 drugs −35, 3 drugs or more −55 a | |||
| Concomitant use of dopaminergic drugs (dopaminergic antiparkinsonian drugs, psychostimulants) | −80 for each | |||
| Concomitant use of anticholinergic drugs | <2 mg −5, <6 mg −10, >6 mg −30 | |||
Note: The individual fitness score was calculated by subtracting the number of points corresponding to each item from 100 points. 0 or less was set to zero.
Abbreviations: ECT, electroconvulsive therapy; FGA, first‐generation antipsychotic; MTD, maximum therapeutic dose; SGA, second‐generation antipsychotic.
In the case of clozapine, Li −5, Li + one drug −25, Li + two or more drugs −45.
Treatment with clozapine or electroconvulsive therapy (ECT) is recommended for treatment‐resistant schizophrenia. Therefore, a significant deduction of 60 points was made if none of these treatments were used.
If the total score was less than or equal to zero because of the point reduction, the score was uniformly set to zero.
The Guidelines for Pharmacological Therapy of Schizophrenia do not include “as needed prescriptions”, and therefore these were not included in the IFS formula.
2.2. Evaluation of IFS formula
To evaluate that the IFS formula adequately reflects the degree of compliance with the schizophrenia guidelines, we quantified and examined prescriptions for Japanese schizophrenia in patients using the IFS formula. Because, at the time of discharge from the hospital the patient's condition should be better than before admission, which is thought to be enhanced by the standard treatment according to the guidelines. Therefore, if the IFS is compared between admission and discharge, and if admission <discharge, then the IFS is considered valid.
A cross‐sectional, retrospective, observational study was conducted at the facilities participating in the EGUIDE project, referring to prescribing data prior to attending the training session. Prescribing data of schizophrenic patients who were discharged between April and September of each year from 2016 to 2020 were included.
The survey items included sex, age, diagnosis of treatment‐resistant, and nontreatment‐resistant schizophrenia, prescription details including clozapine, and administration of modified electroconvulsive therapy. Of these, those with duplicate admissions, those with a diagnosis of treatment‐resistant or nontreatment‐resistant schizophrenia were not listed, and those with incomplete data were excluded. Finally, 771 treatment‐resistant and 1840 nontreatment‐resistant schizophrenics were included in the study.
The study was approved by the Ethics Board of the National Center of Neuropsychiatry and the participating EGUIDE centers. This study was conducted in compliance with the principles of the Declaration of Helsinki. The study protocol was registered with the University Hospital Medical Information Network Registry (UMIN000022645).
2.3. Statistical analysis
Scores immediately before admission and at the time of discharge were calculated using the schizophrenia medication guideline compliance formula. Normality tests were conducted on the scores immediately before admission and at the time of discharge, and both were confirmed to be nonnormally distributed. The difference between the mean scores immediately before admission and at the time of discharge for each severity of illness was verified using Wilcoxon rank test. SPSS ver 25.0 (IBM, Armonk, NY, USA) was used for statistical analysis.
3. RESULTS
The background information of patient is presented in Table 2. Table 3 shows the preadmission and discharge IFS and the difference between discharge IFS and preadmission IFS. The positive difference for IFS on discharge‐admission (>0) was 36.2% for all subjects, 33.3% for nontreatment‐resistant schizophrenia, and 43.1% for treatment‐resistant schizophrenia, as shown in Table 3.
TABLE 2.
Demographic data
| Total | Nontreatment‐resistant schizophrenia | Treatment‐resistant schizphrenia | |
|---|---|---|---|
| Number of patients | 2611 | 1840 | 771 |
| Age (mean ± SD) | 45.6 (±15.7) | 46.3 (±16.3) | 44.0 (±13.10) |
| Male (%) | 1118 (42.8) | 785 (42.7) | 333 (43.2) |
| Discharge | |||
| No prescription of antipsychotics (%) | 0.7 | 0.7 | 0.5 |
| Mono prescription of antipsychotic (%) | 59.8 | 58.3 | 63.3 |
| Poly prescription of antipsychotics (%) | 39.6 | 41.0 | 36.2 |
| No prescription of anxiplitic and hypnotics (%) | 35.5 | 34.8 | 37.4 |
| No prescription of antidepressants (%) | 91.5 | 90.9 | 92.2 |
| No prescription of mood stabilizers/antiepileptics (%) | 74.5 | 77.3 | 67.7 |
| No prescription of anticholinergic drugs (%) | 74.3 | 74.5 | 74.1 |
| Implementation of m‐ECT (%) | 8.1 | 3.2 | 19.8 |
| Prescription of clozapine (%) | 12.4 | 0.1 | 41.8 |
| Chlorpromazine equivalent for antipsychotics (SD) (mg/day) | 690.1 ± 427 | 642.1 ± 396.0 | 806.6 ± 472.4 |
| Biperiden equivalent for anticholinergics (SD) (mg/day) | 0.7 ± 1.5 | 0.7 ± 1.4 | 0.7 ± 1.5 |
| Imipramine equivalent for antidepressants (SD) (mg/day) | 7.5 ± 33.9 | 8.3 ± 36.2 | 5.6 ± 27.4 |
| Diazepam/nitrazepam equivalent for anxiolytics and hypnotics (SD) (mg/day) | 7.2 ± 11.8 | 7.0 ± 11.5 | 7.4 ± 12.5 |
Abbreviation: ECT: electro convulsive therapy.
TABLE 3.
Average individual fitness score (IFS) on admission and discharge for total, nontreatment‐resistant, and treatment‐resistant schizophrenia
| Total | Nontreatment‐resistant schizophrenia | Treatment‐resistant schizophrenia | |
|---|---|---|---|
| Number of patients, n | 2611 | 1840 | 771 |
| IFS on admission, average ± SD | 45.6 ± 0.7 | 55.1 ± 0.8 | 22.9 ± 1.2 |
| IFS on discharge, average ± SD | 54.0 ± 0.7 | 59.3 ± 0.7 | 41.4 ± 1.4 |
| P value a | <0.0001 | <0.001 | <0.0001 |
| IFS on discharge ‐ admission n (%) | |||
| <0 | 618 (23.7) | 522 (28.4) | 96 (12.5) |
| =0 | 1049 (40.2) | 706 (38.4) | 343 (44.5) |
| >0 | 944 (36.2) | 612 (33.3) | 332 (43.1) |
Abbreviation: SD, standard deviation.
Wilcoxson signed‐rank test admission vs discharge.
The average preadmission score for all subjects was 45.6, and the average score at discharge was 54. The average preadmission scores for nontreatment‐resistant and treatment‐resistant schizophrenia were 55.1 and 59.3, respectively, while those for discharge were 22.9 and 41.4, respectively. Comparing these scores between preadmission and discharge, a significant increase was observed in the latter.
The relationship between the scores before and after hospitalization is shown using correlation and bubble plots in Figure 1. Scores at admission are plotted on the horizontal axis and the number of admission points on the vertical axis, with the circle sizes varying according to the number of cases. The IFS at admission and discharge ranged from 0 to 100.
FIGURE 1.
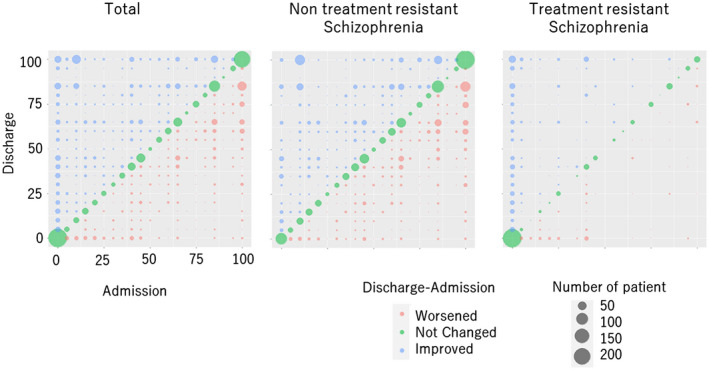
Correlation between individual fitness score at admission and discharge. The individual fitness score at admission was plotted on the x‐axis, and the individual fitness score at discharge was plotted on the y‐axis, with the number of cases indicated by the size of the circle.
4. DISCUSSION
We developed the individual fitness score (IFS) formula to measure compliance with the schizophrenia medication treatment guidelines. The compliance with prescription guidelines before and after admission to the EGUIDE project was examined before the guideline training began. In addition, the conformity of prescriptions to guidelines before and after hospitalization was examined. This is the first study that attempted to score the conformity of treatment to the Guidelines for Pharmacological Therapy of Schizophrenia.
As calculated using the IFS formula, the IFS at admission and discharge were generally low, with wide variations ranging from 0 to 100 points. The low scores could be attributed to several factors. First, the patients' treatment may have been inconsistent with the guidelines. The guidelines recommend monotherapy with antipsychotics and no concomitant use of anxiolytics, sleeping pills, or other medications. However, the treatment of schizophrenia in Japan has been shown to involve a low rate of monotherapy 7 , 12 and a high rate of concomitant use of sleeping pills. 13 In the IFS formulas developed in this study, points were deducted for the use of multiple antipsychotic medications and concomitant use of sleeping pills. Thus, in Japan, where the rate of monotherapy is low and the rate of concomitant use of sleeping pills is high, the score is expected to be low.
Second, ECT and clozapine are recommended in the practice guidelines for treatment‐resistant schizophrenia. However, only about 10 000 patients have been registered for clozapine treatment in Japan, 14 which is reflected in the low score. In other words, the low IFS reflected the state of medical practice in Japan. Furthermore, the conformance scores varied widely. The IFS for each case varied from 0 to 100 points, showing no consistent trend (Figure 1). The IFS were used to visualize a summary of the QI for each case. In previous studies, QI for individual items such as the monotherapy rate of antipsychotic medications was examined; however, the variation was large. 7 As the results of the present study were similar to those of previous studies, we believe that the large variation gives evidence of appropriate visualization.
Finally, in the present study, the IFS were significantly higher at the time of discharge than at the time of admission (Figure 1, Table 3). We believe that the IFS formula presented in this study reflects the actual clinical situation because the diagnosis is reviewed and appropriate treatment is given after the patient is hospitalized, and the patient is discharged after the treatment that is more compliant with the guidelines, even if the therapist is not aware of it.
The low individual fitness scores and high variability observed in this study indicated that the conformity formulas were valid.
This study has some limitations. First, the IFS was based on a clear diagnosis of treatment‐resistant or nontreatment‐resistant disease. Therefore, scores could not be calculated for patients with schizophrenia who do not have a stated treatment‐resistant diagnosis. However, Yasui‐Furukori et al 15 found that the higher the rate of treatment‐resistant schizophrenia diagnosis, the higher the rate of clozapine use, and that this diagnosis makes the difference between guideline‐based treatment or not. Hence, a diagnosis of treatment‐resistant schizophrenia needs to be popularized in psychiatric care in Japan. Second, the scores immediately before admission and at the time of discharge were calculated based on inpatient treatment data from facilities participating in the EGUIDE project, and outpatients with schizophrenia were not included in the evaluation. These limitations indicate that the results do not reflect the actual treatment conditions of all psychiatric institutions in Japan. Third, while most of the psychiatric hospitals in Japan are private psychiatric hospitals, most of the facilities participating in this study are university hospitals or public hospitals. Therefore, the IFS obtained in this study may be subjected to selection bias that does not accurately reflect the actual situation in Japan. In order to eliminate this bias, a study involving a larger number of facilities is needed. Fourth, this study did not collect data on patient status. Therefore, it is unclear what outcomes are associated with adherence to guidelines, i.e., with high IFS scores. It will be necessary to analyze the relationship between IFS and patients' condition profile to clarify whether following the guidelines is beneficial to patients. Finally, the IFS formula did not consider the presence or the absence of comorbidities. In cases of comorbidities, treatment that conform to the guidelines is not necessarily the best treatment.
The IFS formula developed in this study visualizes prescribing practice and enables us to understand the evidence‐practice gap between current treatment and standard of care. In the future, it will be possible to examine the reasons for this gap, review current treatment, and promote equalization. We hope that this will bring drug treatment for schizophrenia closer to the standard treatment proposed by the guidelines. In practice, it will be necessary to encourage healthcare practitioners to ensure the diagnosis of treatment‐resistant schizophrenia and simplification of prescribing are key points. If it is possible to review treatment outcomes with standard treatment, it will be possible to evaluate and revise the guidelines. Repeated evaluation and revision of the guidelines will lead to the social implementation of evidence‐based practice. Ultimately, evidence‐based psychiatry is expected to improve patients' quality of life.
5. CONCLUSIONS
We developed an IFS formula, a tool to easily visualize the degree to which current prescriptions conform to the guidelines for the pharmacological treatment of schizophrenia. The use of this formula is expected to promote dissemination of treatment guidelines and improve the quality of medical care.
AUTHOR CONTRIBUTIONS
KI and KF were involved in data collection and data analysis and wrote the first draft of the manuscript. NH, YY, HY, HH, KY, HI, KO, HM, FK, KI, SO and NY‐F were involved in the data analysis and contributed to the interpretation of the data and writing of the manuscript. NH, JI, KO, KA, YT, TN, HK, TO, RF, MK, SK, CK, MM, MS, HY, JM, KM, MU, TK, TO, EK, AH and SN contributed to the interpretation of the data and data collection. KW were involved in the study design and contributed to the interpretation of the data. RH supervised the entire project, collected the data and was involved in the design, analysis, and interpretation of the data. All authors contributed to and approved the final article.
FUNDING INFORMATION
This project was funded by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP16dk0307060, JP19dk0307083, and JP22dk0307112, JSPS KAKENHI Grant Number JP21K17261, the Health and Labor Sciences Research Grants (H29‐Seishin‐Ippan‐001, 19GC1201), the Japanese Society of Neuropsychopharmacology, the Japanese Society of Mood Disorders, the Japanese Society of Clinical Neuropsychopharmacology, and the Japanese Society of Psychiatry and Neurology. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
INSTITUTIONAL REVIEWER BOARD APPROVAL
This study was approved by the ethics committees of the National Center of Neurology and Psychiatry (B2022‐044) and each participating university, hospital, and clinic.
INFORMED CONSENT
All participants provided their written informed consent.
TRIAL REGISTRATION NUMBER
The University Hospital Medical Information Network Registry (UMIN000022645).
ACKNOWLEDGMENTS
We thank all the individuals who participated in this study for their cooperation.
Inada K, Fukumoto K, Hasegawa N, Yasuda Y, Yamada H, Hori H, et al. Development of individual fitness score for conformity of prescriptions to the “Guidelines For Pharmacological Therapy of Schizophrenia”. Neuropsychopharmacol Rep. 2022;42:502–509. 10.1002/npr2.12293
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy and ethical restrictions (ie, we did not obtain informed consent on the public availability of raw data).
REFERENCES
- 1. Japanese Society of Neuropsychopharmacology . Guideline for pharmacological therapy of schizophrenia. Neuropsychopharmacol Rep. 2021;41(3):266–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crockford D, Addington D. Canadian schizophrenia guidelines: schizophrenia and other psychotic disorders with coexisting substance use disorders. Can J Psychiatry. 2017;62(9):624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castle DJ, Galletly CA, Dark F, Humberstone V, Morgan VA, Killackey E, et al. The 2016 Royal Australian and New Zealand College of Psychiatrists guidelines for the management of schizophrenia and related disorders. Med J Aust. 2017;206(11):501–5. [DOI] [PubMed] [Google Scholar]
- 4. Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13(5):318–78. [DOI] [PubMed] [Google Scholar]
- 5. Numata S, Nakataki M, Hasegawa N, Takaesu Y, Takeshima M, Onitsuka T, et al. Improvements in the degree of understanding the treatment guidelines for schizophrenia and major depressive disorder in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. 2021;41(2):199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iida H, Iga J, Hasegawa N, Yasuda Y, Yamamoto T, Miura K, et al. Unmet needs of patients with major depressive disorder ‐ findings from the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)’ project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci. 2020;74(12):667–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ichihashi K, Hori H, Hasegawa N, Yasuda Y, Yamamoto T, Tsuboi T, et al. Prescription patterns in patients with schizophrenia in Japan: first‐quality indicator data from the survey of “Effectiveness of Guidelines for Dissemination and Education in psychiatric treatment (EGUIDE)” project. Neuropsychopharmacol Rep. 2020;40(3):281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takaesu Y, Watanabe K, Numata S, Iwata M, Kudo N, Oishi S, et al. Improvement of psychiatrists' clinical knowledge of the treatment guidelines for schizophrenia and major depressive disorders using the ‘Effectiveness of Guidelines for Dissemination and Education in Psychiatric Treatment (EGUIDE)’ project: a nationwide dissemination, education, and evaluation study. Psychiatry Clin Neurosci. 2019;73(10):642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takada T, Isaji S, Mayumi T, Yoshida M, Takeyama Y, Itoi T, et al. JPN clinical practice guidelines 2021 with easy‐to‐understand explanations for the management of acute pancreatitis. J Hepato Bil Pancreat Sci. 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10. Yamada H, Motoyama M, Hasegawa N, Miura K, Matsumoto J, Ohi K, et al. A dissemination and education programme to improve the clinical behaviours of psychiatrists in accordance with treatment guidelines for schizophrenia and major depressive disorders: the effectiveness of guidelines for dissemination and education in psychiatric treatment (EGUIDE) project. BJPsych Open. 2022;8(3):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ichihashi K, Kyou Y, Hasegawa N, Yasui‐Furukori N, Shimizu Y, Hori H, et al. The characteristics of patients receiving psychotropic pro re nata medication at discharge for the treatment of schizophrenia and major depressive disorder: a nationwide survey from the EGUIDE project. Asian J Psychiatr. 2022;69:103007. [DOI] [PubMed] [Google Scholar]
- 12. Hashimoto N, Yasui‐Furukori N, Hasegawa N, Ishikawa S, Numata S, Hori H, et al. Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real‐world evidence from the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) psychiatric treatment project. Asian J Psychiatr. 2021;63:102744. [DOI] [PubMed] [Google Scholar]
- 13. Furihata R, Otsuki R, Hasegawa N, Tsuboi T, Numata S, Yasui‐Furukori N, et al. Hypnotic medication use among inpatients with schizophrenia and major depressive disorder: results of a nationwide study. Sleep Med. 2022;89:23–30. [DOI] [PubMed] [Google Scholar]
- 14. Toyoda K, Hata T, Yamauchi S, Kinoshita S, Nishihara M, Uchiyama K, et al. A descriptive study of 10‐year clozapine use from the nationwide database in Japan. Psychiatry Res. 2021;297:113764. [DOI] [PubMed] [Google Scholar]
- 15. Yasui‐Furukori N, Muraoka H, Hasegawa N, Ochi S, Numata S, Hori H, et al. Association between the examination rate of treatment‐resistant schizophrenia and the clozapine prescription rate in a nationwide dissemination and implementation study. Neuropsychopharmacol Rep. 2022;42(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy and ethical restrictions (ie, we did not obtain informed consent on the public availability of raw data).


