Abstract
Aims
The role of sex in determining the profile and the outcomes of patients with myocarditis is largely unexplored. We evaluated the impact of sex as a modifier factor in the clinical characterization and natural history of patients with definite diagnosis of myocarditis.
Methods and results
We retrospectively analysed a single‐centre cohort of consecutive patients with definite diagnosis of myocarditis (i.e. endomyocardial biopsy or cardiac magnetic resonance proven). Specific sub‐analyses were performed in cohorts of patients with chest pain, ventricular arrhythmias, and heart failure as different main symptoms at presentation. The primary outcome measure was a composite of all‐cause mortality or heart transplantation (HTx). We included 312 patients, of which 211, 68% of the whole population, were males. Despite no clinically relevant differences found at baseline presentation, males had a higher indexed left ventricular end‐diastolic volume (62 ± 23 mL/m2 vs. 52 ± 20 mL/m2, P = 0.011 in males vs. females, respectively) at follow‐up evaluation. At a median follow‐up of 72 months, 36 (17%) males vs. 8 (8%) females experienced death or HTx (P = 0.033). Male sex emerged as predictors of all‐cause mortality or HTx in every combination of covariates (HR 2.600; 1.163–5.809; P = 0.020). Results were agreeable regardless of the main symptom of presentation.
Conclusions
In a large cohort of patients with definite diagnosis of myocarditis, females experienced a more favourable long‐term prognosis than males, despite a similar clinical profile at presentation.
Keywords: Sex differences, Myocarditis, Epidemiology, Prognosis
Introduction
The presence of sex‐related differences in the epidemiology and pathophysiology of cardiovascular diseases has become evident over time, with women experiencing more favourable natural history in several cardiovascular conditions compared with men. 1 The specific adverse cardiac remodelling and the observed unfavourable cardiac response to stress might be the putative mechanism underlying the greater susceptibility to the development of heart failure (HF) or dilated cardiomyopathy (DCM) in males, 2 but conclusive evidence is currently lacking.
Myocarditis is an inflammatory disease of the myocardium characterized by great heterogeneity of clinical presentation and evolution. 3 , 4 In this condition, a key role seems to be played by a maladaptive response of the immune system to specific triggers, 5 and the presence of specific individual genetic background has been postulated. 6 However, the role of sex in clinical presentation and evolution of myocarditis has been largely unexplored, except for data from animal studies showing more severe disease in males. 7 Recent studies report a female‐to‐male ratio between 1:1.5 and 1:1.7 in series of patients with myocarditis. 8 This finding was further confirmed in populations of non‐ischaemic DCM, where males were more susceptible to develop disease characterized by poor outcome, regardless of the response to therapy. 2 , 9 Conversely, women frequently exhibit stronger innate and adaptive immune responses than men, and this could theoretically contribute to their increased susceptibility to inflammatory and autoimmune diseases. 10
The aim of the present study, therefore, was to investigate the potential impact of sex in the clinical presentation and long‐term follow‐up in a large cohort of unselected patients with a definite diagnosis of myocarditis.
Methods
This was a single‐centre, retrospective, observational cohort study conducted at the Cardiothoracovascular Department of the Trieste University Hospital, Italy. Informed consent was obtained from all subjects, and the study was conducted under the local Regional Institutional Review Board approved the study (identifier 43_2009).
Study design, inclusion, and exclusion criteria
We retrospectively analysed data of consecutive patients who received a circumstantiated diagnosis of myocarditis between 2005 and 2019. 11
The diagnosis of myocarditis was confirmed by endomyocardial biopsy (EMB) or by cardiac magnetic resonance (CMR), according to the recommendation of the position statement on the diagnosis and management of myocarditis from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. 12 In detail, EMB was reserved to patients with high‐risk or intermediate‐risk syndromes, as previously reported. 3 The diagnosis was made in accordance with Dallas Criteria 13 and immunohistochemistry analysis 11 ; cases with borderline myocarditis were excluded from the present study. In low‐risk patients [i.e. patients with chest pain, normal left ventricular ejection fraction (LVEF), and electrically and haemodynamically stable], the diagnosis was confirmed by CMR according to Lake Louise Criteria. 3 , 14 , 15 Only CMR exams performed within 14 days from index admission were included in this analysis. In the acute phase, patients older than 35 years and/or ≥1 risk factor for coronary artery disease in the presence of troponin elevation consistent with myocardial infarction underwent coronary angiography (n = 162) or computed tomography (n = 9) to exclude coronary artery disease. Finally, patients with sarcoidosis and eosinophilic and giant cell myocarditis were excluded from this analysis.
Pre‐specified criteria for final inclusion were centrally revised by two cardiologists (M.C., M.M.).
Clinical and echocardiographic data were systematically evaluated at diagnosis and at last available clinical evaluation. All the echocardiographic and CMR images were independently revised by operators (M.C., G.G., C.B., and A.P.), blinded to the outcome of the patients.
According to the primary symptom at clinical presentation, patients were categorized in three groups: HF, arrhythmic (i.e. hypokinetic or hyperkinetic life‐threatening ventricular arrhythmias), and chest pain. Patient with fulminant myocarditis were mostly in the HF group. When two or more symptoms were present, the predominant clinical presentation was chosen in order to classify the patient.
Echocardiography
Echocardiographic analysis was performed by expert operators, blinded to patients' data. Left ventricle (LV) quantitative analysis was performed based on 2015 and 2016 ASE/EACVI recommendations, and LVEF was calculated according to the modified Simpson rule in apical four‐chamber and two‐chamber views. 16 , 17
CMR assessment
CMR was performed using 1.5‐T scanners with dedicated cardiac software, a phased‐array surface receiver coil, and vectorcardiogram triggering. CMR images were acquired according to the protocols recommended by the Society for Cardiovascular Magnetic Resonance. 18 In detail, we acquired cine steady‐state free precession (cine‐SSFP) images during apnoea, T2‐weighted short‐tau inversion recovery (STIR) imaging and/or T2 mapping for myocardial oedema, and late gadolinium enhancement (LGE) in T1‐weighted inversion recovery sequences at 5–10 min after gadolinium injection (0.1 mmol/kg) in the short‐axis (9–14 images covering the entire LV), two‐chamber, three‐chamber, and four‐chamber planes.
Ventricular volumes and morphology were quantified from the cine images and the presence of LGE was visually assessed. Oedema was evaluated using the signal intensity ratio of the myocardium versus skeletal muscle on T2‐weighted images, 19 and regional enhancement was evaluated on an 18‐segment model of the LV.
Outcome measures
The primary study outcome was a composite of all‐cause death and heart transplantation (HTx). Information concerning the study endpoint and the causes of death were obtained directly from the patient during the follow‐up examinations or by telephone contact with patients, their relatives, or general practitioners. The end of follow‐up was established as 30 June 2021. These events were collected at scheduled follow‐up evaluations, from electronic health record system, and, if needed, through telephone contacts with patients' general practitioners and/or relatives. CV events were independently assessed by two cardiologists (M.C., A.P.) blinded to patients' baseline characteristics.
Statistical analysis
Categorical data are presented as percentages and numbers, normally distributed continuous data as mean ± standard deviation, and non‐normally distributed variables as median and interquartile range (IQR). Unpaired and paired Student's t‐test was used, when appropriate, for comparison of normally distributed data, whereas Mann–Whitney test was used for abnormally distributed data. The χ2 test or the Fisher test was used, when appropriate, to compare categorical variables expressed as proportion.
The Kaplan–Meier method was used to estimate the global survival and the composite end point curve, and the log‐rank test was used to compare the curves.
Univariable Cox proportional hazards models were applied to find predictors of the endpoint. Considering the limited number of the events, we evaluated the adjusted sex effect, estimating a set of different multivariable Cox models, each time changing the combination of the other covariates and considering only variables with P < 0.1 at the univariate analysis. We defined a P value < 0.05 as statistically significant.
All analyses were performed using IBM SPSS Statistics 24 package (New York, NY) package (New York, NY) statistical software and the software R (R Foundation for Statistical Computing, Vienna, Austria; https://www.r‐project.org), packages ‘rms’, ‘survminer’.
Results
Clinical characterization
The overall population included 406 patients. In 86 patients, CMR and EMB were negative and were excluded from the cohort. Of the 320 patients remaining, eight were excluded due to diagnosis of sarcoidosis and eosinophilic or giant cell myocarditis. Thus, the study population included 312 patients (68% males) with histologically proven [37.5%, n = 117, of which 78 patients (67%) were male and 39 patients (33%) female] or CMR‐confirmed myocarditis (62.5%, n = 195) (Figure S1 ). Of them, 60% (n = 187) presented with chest pain, 34% (n = 106) with HF, and 6% (n = 19) with life‐threatening ventricular arrhythmias. In each of these subgroups, the proportion of males was consistently higher than females: 73% in chest pain subgroup, 59% in HF subgroup, and, 63% in arrhythmia subgroup (global P value = 0.045).
Baseline, last clinical, and echocardiographic data characteristics of the study population comparing males and females were available for 255 patients and are summarized in Table 1 . The time from baseline to the last available clinical or imaging data was a median of 30 [IQR 10–77] months. At baseline, males and females had similar age, LVEF and LV dimensions. Compared to females, males showed a higher rate of LGE at CMR (40 vs. 23% in males and females, respectively, P < 0.001). This was particularly evident in the chest pain subgroup (49 vs. 29% in males and females, respectively; P < 0.001). LGE pattern was non‐ischaemic in all patients. Interestingly, at last available follow‐up, males had significantly dilated LV compared with females [left ventricle end‐diastolic volume indexed (LVEDVi) and left ventricle end‐systolic volume index (LVESVi) 62 ± 23 mL/m2 vs. 52 ± 20 mL/m2, P = 0.011, and 30 ± 21 mL/m2 vs. 25 ± 14 mL/m2, P = 0.046, in males and females, respectively].
Table 1.
Baseline and follow‐up characteristics of the whole population according to sex
| Data at baseline | |||
|---|---|---|---|
| Population N = 312 | F = 101 (32%) | M = 211 (68%) | P value |
| Age (years) | 42 ± 19 | 36 ± 17 | 0.678 |
| NYHA class, n (%) | 0.323 | ||
| I | 45 (55%) | 112 (64%) | |
| II | 13 (16%) | 24 (14%) | |
| III | 12 (15%) | 22 (13%) | |
| IV | 12 (15%) | 17 (10%) | |
| Diabetes mellitus, n (%) | 3 (3%) | 8 (4%) | 0.765 |
| Hypertension, n (%) | 16 (16%) | 26 (12%) | 0.403 |
| Family history for DCM, n (%) | 4(4) | 8(4) | 0.953 |
| QRS length (ms) | 93 ± 10 | 101 ± 16 | <0.001 |
| LVEF baseline (%) | 50 (30–62) | 56 (36–61) | 0.282 |
| LVEF baseline < 40%, n (%) | 33 (33) | 52 (25) | 0.244 |
| LVEDVi baseline (mL/m2) | 58 ± 30 | 60 ± 22 | 0.542 |
| MR moderate/severe, n (%) | 11 (11%) | 19 (9%) | 0.769 |
| RV systolic dysfunction, no. (%) | 10 (10%) | 16 (8%) | 0.868 |
| Restrictive pattern, n (%) | 23 (23) | 40 (27) | 0.447 |
| Non‐ischaemic LGE, n (%) | 23 (23%) | 83 (40%) | <0.001** |
| Beta‐blocker, n (%) | 48 (49%) | 73 (34%) | 0.031 |
| ACE‐i/ARB, n (%) | 26 (26%) | 36 (17%) | 0.067 |
| MRA, n (%) | 14 (14%) | 17 (10%) | 0.129 |
| Diuretics, no. (%) | 26 (26%) | 35 (16%) | 0.701 |
| Data at last available follow‐up | |||
|---|---|---|---|
| Population N = 255 | F = 78 (31%) | M = 177 (69%) | P value |
| Beta‐blocker, n (%) | 37 (61) | 56 (49) | 0.145 |
| ACE‐i/ARB, n (%) | 11 (18) | 19 (17) | 0.499 |
| MRA, n (%) | 29 (48) | 37 (32) | 0.130 |
| ICD at follow‐up, n (%) | 14 (18%) | 22 (13%) | 0.415 |
| LVEF at follow‐up (%) | 55 (49–64) | 57 (49–62) | 0.577 |
| LVEDVi at follow‐up, (mL/m 2 ) | 52 ± 20 | 62 ± 23 | 0.011 |
| LVESVi at follow‐up, (mL/m 2 ) | 25 ± 14 | 30 ± 21 | 0.046 |
ACE‐i, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BNP, brain natriuretic peptide; BMI, body mass index; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HR, heart rate; ICD, implantable cardiac defibrillator; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end‐diastolic volume indexed; LVESVi, left ventricular end‐systolic volume indexed; MRA mineral corticoid antagonists; MR, mitral regurgitation; NYHA, New York Heart Association; RV, right ventricle; SBP systolic blood pressure.
In bold P value < 0.05**. CMR was performed in 91, 58, and 51% of patients with chest pain, arrhythmias, and heart failure at the onset, respectively.
During follow‐up, ICD was implanted in 36 patients 11(%), 14 females and 22 males. The majority (i.e. 70%) was implanted in primary prevention for persistent severe left ventricular systolic dysfunction. The other patients (i.e. 30%) were implanted in secondary prevention after a life‐threatening ventricular arrhythmia at presentation.
Characteristics of sex‐specific patient profiles in the three subgroups according to the pattern of disease onset are described in Table S1 . In the subgroup of HF patients, despite no differences in LVEF, LVEDVi, and LVESVi at baseline, males showed higher LVEDVi and LVESVi values (77 ± 30 vs. 63 ± 25 mL/m2, P = 0.048 and 48 ± 28 vs. 33 ± 16 mL/m2, P = 0.034 in males and females, respectively) and significantly lower median LVEF values at last available follow‐up (42 vs. 50%, P = 0.004 in males and females, respectively). Consistently, in the chest pain and arrhythmic subgroups, males showed a more pronounced adverse LV remodelling in terms of LV volumes at last clinical available evaluation.
Outcomes
The outcomes data were available for all the patients. During a median follow‐up of 72 (IQR 43–122) months, 44 (14%) patients met the primary endpoint of all‐cause mortality or HTx. As shown in the Kaplan–Meier curves, male sex was associated with an increased risk of experiencing the primary endpoint compared to the female sex [36 (17%) vs. 8 (8%), P = 0.033] (Figure 1 ).
Figure 1.
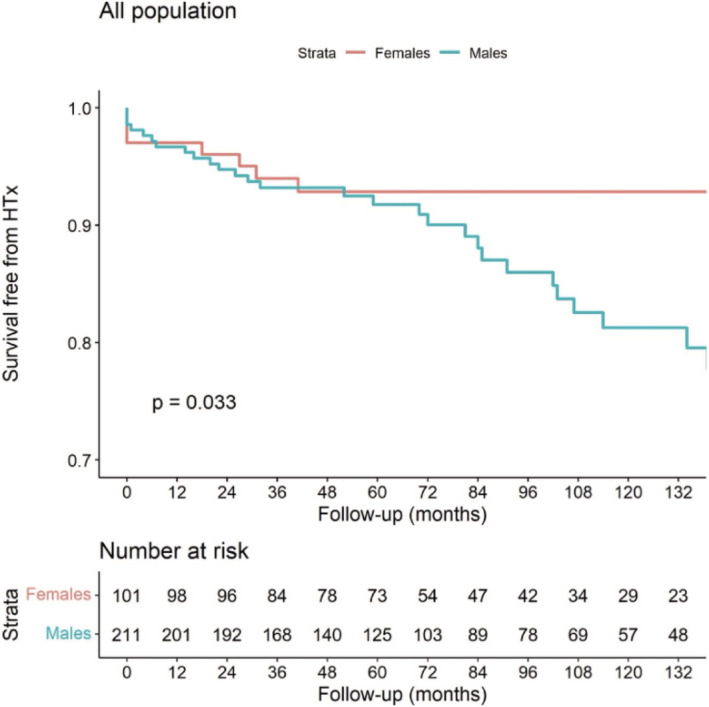
Long‐term HTx‐free survival according to the sex in the overall population.
Similar results were seen in the subgroup of patients with HF at the onset. Of note, no female patients experienced the primary outcome measure in the chest pain group (Figure S2 ). In total, 32 patients (26 males and 6 females) died, and 31 patients (26 males and 5 females) underwent heart transplant. Of the 31 transplanted patients, 12 are still living, and 19 have passed away. Table S2 shows the incidences of the main components of endpoints during follow‐up in the overall population and in the three subgroups. Males had higher rates of major events both in HF and chest pain groups (Figure 2 ). Arrhythmic subgroup was too small in numbers to derive solid statistical conclusions. Table S3 shows the baseline and follow‐up characteristics dividing the population according to the primary outcome. The male sex was more prevalent in the event group showing that the prognostic role of sex was maintained among these subgroups.
Figure 2.
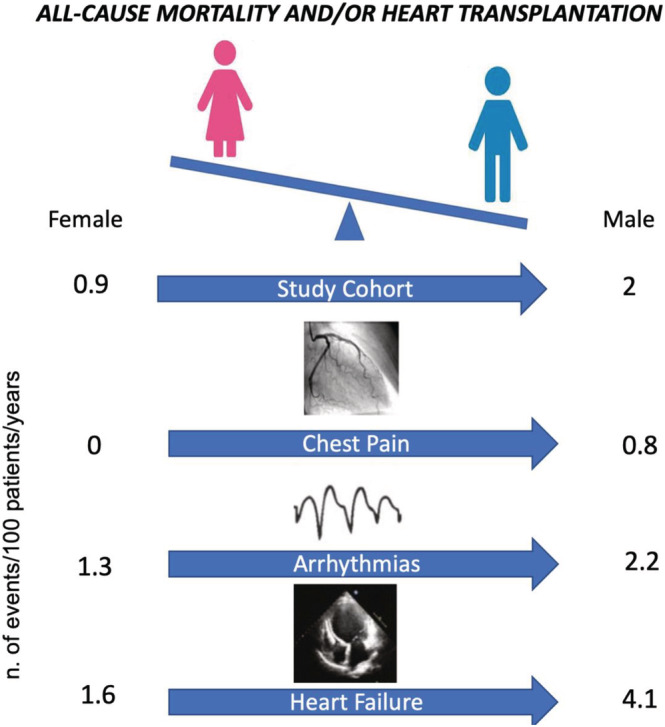
Incidence rates of all cause of mortality and/or HTX. Incidence rates are measured as number of events per 100 patients/years.
Additional analysis showing the role of sex in patients underwent only EMB or CMR was performed. For both groups, sex remained independently associated with outcome: (I) HR 4.129 (1.245–13.828; P = 0.021) among the 117 patients with biopsy‐proven myocarditis and (II) HR 8.229 (1.001–67.775; P = 0.045) among the 195 patients with CMR confirmed myocarditis.
Univariable and multivariable analyses showed male sex emerged as strongly associated to all‐cause mortality or HTx in every combination of covariates (Table S4 ). In the multivariable model with the highest chi‐square value, independent predictors of death‐HTx, other than sex (HR 2.600; 1.163–5.809; P = 0.020), were LVEF <50% (HR 11.496;1.964–67.277; P = 0.007), and restrictive filling pattern (HR 2.682; 1.243–5.788; P = 0.012) (Table 2 ).
Table 2.
Univariable analyses and main multivariable analysis
| Univariable | Multivariable a | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Male sex, n (%) | 3.319 (1.026–4.801) | 0.043 | 2.600 (1.163–5.809) | 0.020 |
| Age (years) | 0.999 (0.982–1.016) | 0.911 | ||
| BMI | 0.985 (0.914–1.062) | 0.698 | ||
| Diabetes mellitus | 0.909 (1.128–0.145) | 0.875 | ||
| Family history for DCM | 2.298 (0.298–17.709) | 0.425 | ||
| Onset with arrhythmia | 1.283 (0.455–3.618) | 0.637 | ||
| Onset with HF | 3.807 (1.965–7.373) | <0.001 | 0.970 (0.234–4.026) | 0.996 |
| Onset with chest pain | 0.197 (0.09–0.428) | <0.001 | ||
| Restrictive pattern | 3.103 (1.571–6.128) | 0.001 | 2.682 (1.243–5.788) | 0.012 |
| LVEF <50% at baseline | 14.004 (4.254–46.102) | <0.001 | 11.496 (1.964–67.277) | 0.007 |
| LVEDVi at baseline | 1.019 (1.010–1.027) | <0.001 | ||
| LBBB | 1.730 (0.607–4.928) | 0.305 | ||
| Beta‐blockers | 0.796 (0.311–2.041) | 0.635 |
BMI, body mass index; DBP, diastolic blood pressure; HF, heart failure; HR, heart rate; LBBB, left bundle branch block; LVEDVi, left ventricular end‐diastolic volume index; LVESVi, left ventricular end‐systolic volume index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SBP, systolic blood pressure.
In bold P value < 0.05.
We report the multivariable model showing the highest chi‐square value among the ones estimated.
Discussion
The present study provides deeper insight into the sex‐related impact on clinical presentation and long‐term cardiovascular outcomes of myocarditis. To the best of our knowledge, this population represents a large cohort of well‐characterized patients with a substantial available follow‐up (median of 6 years) in which the possible prognostic role of sex was investigated across a wide clinical spectrum of myocarditis.
The main findings can be summarized as follows: (I) In the overall population and in each subgroup of clinical presentation, male sex was predominant. (II) Male and female patients with myocarditis have similar clinical and echocardiographic characteristics at baseline, except for a higher presence of LGE at CMR in males. (III) Male sex, systolic and diastolic dysfunction at baseline were associated with an increased risk of all‐cause mortality or HTx during follow‐up. This may have been driven by particularly adverse cardiac remodelling in males. (IV) The sex‐related difference in prognosis was particularly evident in the subgroup of HF patients, but was consistent in the chest pain subgroup, which are traditionally considered at low risk in light of a normal LVEF at presentation. Finally, the awareness of sex differences and the awareness of the ‘protective’ effect of female sex on the natural history of the disease might have major implications for clinical evaluation and modern management of patients with myocarditis.
The influence of sex in the pathophysiology of myocarditis
Women constitute a largely under‐represented and under‐investigated subgroup of patients, accounting for only 30% of participants in clinical trials. 20 Therefore, data from retrospective analyses on large cohorts with long‐term follow‐up are fundamental to understand the role of sex differences in specific settings of cardiovascular disease. From this perspective, the present study provides important information on sex‐related differences for the clinical management of patients with myocarditis. In fact, we found an association between sex and long‐term cardiovascular outcome. Our results are relevant considering that female sex is associated with a higher susceptibility to autoimmune diseases and are expected to have more severe consequences due to the stronger innate and adaptive immune responses. 10
Available studies exploring the mechanisms explaining our results are not conclusive and the ‘sex‐tailored approach’ is still a grey area under investigation. In the experimental myocarditis murine model, development of DCM was associated with sex‐related differences in the inflammatory response, partially influenced by differences in sex hormones. 21
In human myocarditis, male sex has been reported as a potential risk factor associated with a higher incidence of cardiac fibrosis and more intense cardiac inflammation, leading to the development of DCM. 22 , 23 Some initial insights are derived from the Intervention in Myocarditis and Acute Cardiomyopathy (IMAC)‐2 Study, which only included patients with HF and LVEF <40% due to idiopathic DCM, myocarditis, or peripartum cardiomyopathy and reported greater rates of myocardial recovery and longer HTx‐free survival in women compared with men. 22 In a recent study, the long‐term outcome of women affected by DCM was more favourable compared with those of men, and sex emerged as an important independent factor, particularly for cardiovascular outcome. 24 Our study confirms and expands this knowledge to a population including all spectrums of clinical onset of myocarditis.
Our knowledge on the role of sex in outcome prediction compared to other parameters remains incomplete. Although some studies investigated the role of sex in patients with myocarditis, the vast majority included selected cohorts, such as fulminant or arrhythmic myocarditis, or did not include the sex in univariable or multivariable analyses. 25 , 26 , 27 , 28 , 29
Current understanding of how sex‐related differences influence the prevalence and evolution of cardiovascular diseases is still poor and further research on large populations is needed.
The prognostic role of sex
In the present study, clinical and echocardiographic characteristics of patients at presentation were similar between men and women, but men showed worse outcomes. This data adds an important piece of information to the understanding of sex differences in this clinical context. Men are known to have larger hearts than women, 30 even after adjusting for body surface area, whereas women have increased LVEF values compared with men. 31 In our cohort, ventricular dimensions (even if indexed for body surface area) were similar between males and female at clinical presentation, but men had more dilated LV at follow‐up assessment, suggesting a possible sex difference in the evolution of the disease. Specifically, this finding suggests the intriguing possibility that males were less likely to develop LV reverse remodelling compared with females. However, focused studies on this topic are needed in the future. Interestingly, our results further highlight the role of CMR as important tool for prognostic stratification in myocarditis: Men did show higher rates of LGE, which has been previously demonstrated to predict a low probability of LV reverse remodelling. 32
Moreover, it might be possible to speculate how the association between sex and outcomes may be due to the influence of sex in determining not only the presence of LGE but also its extent. In fact, the extent of LGE might be the best variable to consider for a multivariable Cox analysis in future investigations. So far, standardization of LGE quantification is lacking and represents an important gap of knowledge, mostly in non‐ischaemic cardiomyopathies. According to these findings, female patients may be less prone to produce a fibrotic scar in the myocardium in response to an inflammatory cardiac injury, but more research is required in this field. Moreover, our results underline the need to assess myocardial derangement through evaluation of systolic and diastolic dysfunction, which were confirmed to be associated with an adverse outcome, as previously demonstrated in DCM. 33 Further studies are needed to explore the best techniques and parameters to detect and quantify the magnitude of ventricular derangement. Furthermore, a study evaluating this topic in the era of Lake Louise Criteria is not present yet and definitely needed.
Although the influence of sex on outcome was evident in the overall study population, subgroup analyses showed its prominent role in patients presenting with HF, highlighting the suggested genetic overlap between myocarditis and DCM, 34 in which it is known the detrimental role of male sex. 24 However, our results question the overall benign natural history of myocarditis presenting with chest pain and normal LVEF, suggesting that sex‐related differences are important in this setting. In fact, only males experienced the primary endpoint, although we did not observe hard events in the females in this specific subgroup.
Taking all things into consideration, these findings could indicate that men with myocarditis might require closer cardiological follow‐up, particularly if they present without LV systolic dysfunction, in light of a higher tendency towards unfavourable ventricular remodelling in the long‐term follow‐up, thereby affecting their overall survival. According to our results, a careful characterization of the presence and extent of ventricular derangement by means of a multiparametric evaluation including EKG, echocardiography, and CMR (even in myocarditis with low‐risk presentation 11 ) emerges as a critical tool in the contemporary management of myocarditis.
Finally, our study findings might suggest an important intimate connection between myocarditis and DCM. This connection is tangible and could be attributed to the combination of genetic and immunologic factors, with myocarditis being the hidden bridge, between these two uncharted territories. Therefore, in clinical practice, genetics and family history should be systematically evaluated, not only in cardiomyopathies but also in myocarditis. Conversely, the immunologic point of view might be the missing key in future diagnostic approach and management, not only of myocarditis but also of cardiomyopathies and HF, as suggested by recent clinical trials. 35
Limitations
There are several limitations that should be acknowledged. This is a single‐centre, retrospective analysis that was conducted on patients with myocarditis followed in a tertiary referral centre for the diagnosis and treatment of cardiomyopathies. Therefore, despite consistency in the entire clinical spectrum of myocarditis, these results may not be generalized to all patients presenting with the disease. Furthermore, the number of women in our cohort was relatively small, possibly reflecting underdiagnosis of women with myocarditis in clinical practice, as known for many other cardiovascular diseases. Additionally, the majority of the overall population was Caucasian, so we were not able to explore the presence of ethnicity differences in our study.
The long enrolment period, although providing the possibility for a reliable statistical analysis, can be considered a known limit of studies analysing rare diseases with low event rate. Evaluation of LGE presence and extent, as well as its prognostic value, could not be investigated, as CMR data were not available for all patients. Given its widely recognized prognostic role in myocarditis, it is reasonable to hypothesize that LGE, and especially the extent of LGE, would have been associated with outcome. Finally, it is unclear how pharmacological therapies, such as beta‐blockers and angiotensin‐converting enzyme inhibitors, have influenced the occurrence of the study outcome in our cohort. Further research is necessary to confirm findings from this study in larger multicentric populations.
Conclusion
In a large cohort of well‐characterized patients with confirmed myocarditis, our results suggest that male sex is associated with a higher risk of all‐cause mortality and HTx in long‐term follow‐up. Sex emerged as a factor affecting the natural history of myocarditis in terms of adverse ventricular remodelling and poor global outcome, particularly in male patients presenting with systolic and diastolic dysfunction.
Conflict of interest
All authors declare no conflict of interest.
Funding
Study not funded.
Supporting information
Table S1. Baseline and follow‐up characteristics according to sex in the subgroups of patients presenting with heart failure, chest pain and arrhythmias at the onset.
Table S2. Incidence rates of events as number of events per 100 patients/years.
Table S3. Baseline and follow‐up characteristics according to the primary outcome of death for all cause/heart transplant.
Table S4. Different multivariable Cox models, each time maintaining male sex and changing the combination of the other covariates.
Figure S1. Consort flow diagram of the population.
Figure S2. Long‐term HTx‐free survival according to the sex in the subgroups of patients presenting with chest pain (panel A). arrhythmias (panel B). heart failure (panel C). HTx indicates heart transplantation. Interestingly, no female experienced the outcome measure in chest pain group vs. 9 (6%) males. P = 0.1. The subgroup of patients with HF at the onset experienced more adverse events than the other groups. P = 0.019.
Acknowledgements
We would like to thank all the nuclear medicine doctors, haematologists, neurologists, pathologists, and nephrologists of the participating centres for providing their essential contribution in multidisciplinary teams for the care of patients with amyloidosis. We would like to thank Fondazione CRTrieste, Fondazione CariGO, Fincantieri, and all the healthcare professionals for the continuous support to the clinical management of patients affected by cardiomyopathies, followed in Heart Failure Outpatient Clinic of Trieste, and their families. Finally, a special thank is for the cardiac nurses of outpatient clinics involved in the study for their daily, professional management of patients and their relatives.
Castrichini, M. , Porcari, A. , Baggio, C. , Gagno, G. , Maione, D. , Barbati, G. , Medo, K. , Mestroni, L. , Merlo, M. , and Sinagra, G. (2022) Sex differences in natural history of cardiovascular magnetic resonance‐ and biopsy‐proven lymphocytic myocarditis. ESC Heart Failure, 9: 4010–4019. 10.1002/ehf2.14102.
Matteo Castrichini and Aldostefano Porcari contributed equally as first author.
Marco Merlo and Gianfranco Sinagra equally contributed as last authors.
References
- 1. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, Ky B, Santema BT, Sliwa K, Voors AA. Sex differences in heart failure. Eur Heart J 2019; 40: 3859–68c. [DOI] [PubMed] [Google Scholar]
- 2. Cleland JG, Swedberg K, Follath F, Komajda M, Cohen‐Solal A, Aguilar JC, Dietz R, Gavazzi A, Hobbs R, Korewicki J, Madeira HC, Moiseyev VS, Preda I, van Gilst W, Widimsky J, Freemantle N, Eastaugh J, Mason J, Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology . The EuroHeart failure survey programme—A survey on the quality of care among patients with heart failure in Europe part 1: Patient characteristics and diagnosis. Eur Heart J 2003; 24: 442–463. [DOI] [PubMed] [Google Scholar]
- 3. Sinagra G, Anzini M, Pereira NL, Bussani R, Finocchiaro G, Bartunek J, Merlo M. Myocarditis in clinical practice. Mayo Clin Proc 2016; 91: 1256–1266. [DOI] [PubMed] [Google Scholar]
- 4. Baggio C, Gagno G, Porcari A, Paldino A, Artico J, Castrichini M, Dal Ferro M, Bussani R, Merlo M. Myocarditis: Which role for genetics? Curr Cardiol Rep 2021; 23: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sinagra G, Porcari A, Gentile P, Artico J, Fabris E, Bussani R, Merlo M. Viral presence‐guided immunomodulation in lymphocytic myocarditis: An update. Eur J Heart Fail 2021; 23: 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campuzano O, Fernández‐Falgueras A, Sarquella‐Brugada G, Sanchez O, Cesar S, Mademont I, Allegue C, Mates J, Pérez‐Serra A, Coll M, Alcalde M, Iglesias A, Tiron C, Gallego MÁ, Ferrer‐Costa C, Hospital A, Escribano C, Dasí C, Borondo JC, Castellà J, Arbelo E, Medallo J, Brugada J, Brugada R. A genetically vulnerable myocardium may predispose to myocarditis. J Am Coll Cardiol 2015; 66: 2913–2914. [DOI] [PubMed] [Google Scholar]
- 7. Huber SA, Job LP, Auld KR. Influence of sex hormones on coxsackie B‐3 virus infection in Balb/c mice. Cell Immunol 1982; 67: 173–179. [DOI] [PubMed] [Google Scholar]
- 8. Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The myocarditis treatment trial investigators. N Engl J Med 1995; 333: 269–275. [DOI] [PubMed] [Google Scholar]
- 9. Cannata A, Manca P, Nuzzi V, Gregorio C, Artico J, Gentile P, Pio Loco C, Ramani F, Barbati G, Merlo M, Sinagra G. Sex‐specific prognostic implications in dilated cardiomyopathy after left ventricular reverse remodeling. J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16: 626–638. [DOI] [PubMed] [Google Scholar]
- 11. Anzini M, Merlo M, Sabbadini G, Barbati G, Finocchiaro G, Pinamonti B, Salvi A, Perkan A, di Lenarda A, Bussani R, Bartunek J, Sinagra G. Long‐term evolution and prognostic stratification of biopsy‐proven active myocarditis. Circulation 2013; 128: 2384–2394. [DOI] [PubMed] [Google Scholar]
- 12. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J 2013; 34: 2636–2648. [DOI] [PubMed] [Google Scholar]
- 13. Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, Olsen EG, Schoen FJ. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987; 1: 3–14. [PubMed] [Google Scholar]
- 14. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: A JACC White paper. J Am Coll Cardiol 2009; 53: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferreira VM, Schulz‐Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: Expert recommendations. J Am Coll Cardiol 2018; 72: 3158–3176. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16: 233–270. [DOI] [PubMed] [Google Scholar]
- 17. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29: 277–314. [DOI] [PubMed] [Google Scholar]
- 18. Kramer CM, Barkhausen J, Bucciarelli‐Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020; 22: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gräni C, Eichhorn C, Bière L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch‐Herold M, Kwong RY. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017; 70: 1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gong IY, Tan NS, Ali SH, Lebovic G, Mamdani M, Goodman SG, Ko DT, Laupacis A, Yan AT. Temporal trends of women enrollment in major cardiovascular randomized clinical trials. Can J Cardiol 2019; 35: 653–660. [DOI] [PubMed] [Google Scholar]
- 21. Fairweather D. Sex differences in inflammation during atherosclerosis. Clin Med Insights Cardiol 2014; 8: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J 3rd, Kip KE, Dec GW, IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: Results of the IMAC (intervention in myocarditis and acute cardiomyopathy)‐2 study. J Am Coll Cardiol 2011; 58: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cocker MS, Abdel‐Aty H, Strohm O, Friedrich MG. Age and gender effects on the extent of myocardial involvement in acute myocarditis: A cardiovascular magnetic resonance study. Heart 2009; 95: 1925–1930. [DOI] [PubMed] [Google Scholar]
- 24. Cannatà A, Fabris E, Merlo M, Artico J, Gentile P, Pio Loco C, Ballaben A, Ramani F, Barbati G, Sinagra G. Sex differences in the long‐term prognosis of dilated cardiomyopathy. Can J Cardiol 2020; 36: 37–44. [DOI] [PubMed] [Google Scholar]
- 25. Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, Mantovani R, Varrenti M, Pedrotti P, Conca C, Mafrici A, Grosu A, Briguglia D, Guglielmetto S, Perego GB, Colombo S, Caico SI, Giannattasio C, Maestroni A, Carubelli V, Metra M, Lombardi C, Campodonico J, Agostoni P, Peretto G, Scelsi L, Turco A, di Tano G, Campana C, Belloni A, Morandi F, Mortara A, Cirò A, Senni M, Gavazzi A, Frigerio M, Oliva F, Camici PG, On behalf of the Registro Lombardo delle Miocarditi . Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter Lombardy registry. Circulation 2018; 138: 1088–1099. [DOI] [PubMed] [Google Scholar]
- 26. Peretto G, Sala S, Luca GD, Marcolongo R, Campochiaro C, Sartorelli S, Tresoldi M, Foppoli L, Palmisano A, Esposito A, De Cobelli F, Rizzo S, Thiene G, Basso C, Dagna L, Caforio ALP, Della Bella P. Immunosuppressive therapy and risk stratification of patients with myocarditis presenting with ventricular arrhythmias. JACC Clin Electrophysiol 2020; 6: 1221–1234. [DOI] [PubMed] [Google Scholar]
- 27. Ammirati E, Veronese G, Brambatti M, Merlo M, Cipriani M, Potena L, Sormani P, Aoki T, Sugimura K, Sawamura A, Okumura T, Pinney S, Hong K, Shah P, Braun Ö, van de Heyning CM, Montero S, Petrella D, Huang F, Schmidt M, Raineri C, Lala A, Varrenti M, Foà A, Leone O, Gentile P, Artico J, Agostini V, Patel R, Garascia A, van Craenenbroeck EM, Hirose K, Isotani A, Murohara T, Arita Y, Sionis A, Fabris E, Hashem S, Garcia‐Hernando V, Oliva F, Greenberg B, Shimokawa H, Sinagra G, Adler ED, Frigerio M, Camici PG. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol 2019; 74: 299–311. [DOI] [PubMed] [Google Scholar]
- 28. Caforio ALP, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy‐proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J 2007; 28: 1326–1333. [DOI] [PubMed] [Google Scholar]
- 29. Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H. Long‐term follow‐up of biopsy‐proven viral myocarditis: Predictors of mortality and incomplete recovery. J Am Coll Cardiol 2012; 59: 1604–1615. [DOI] [PubMed] [Google Scholar]
- 30. Salton CJ, Chuang ML, O'Donnell CJ, Kupka MJ, Larson MG, Kissinger KV, Edelman RR, Levy D, Manning WJ. Gender differences and normal left ventricular anatomy in an adult population free of hypertension. A cardiovascular magnetic resonance study of the Framingham heart study offspring cohort. J Am Coll Cardiol 2002; 39: 1055–1060. [DOI] [PubMed] [Google Scholar]
- 31. Chung AK, Das SR, Leonard D, Peshock RM, Kazi F, Abdullah SM, Canham RM, Levine BD, Drazner MH. Women have higher left ventricular ejection fractions than men independent of differences in left ventricular volume: The Dallas heart study. Circulation 2006; 113: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 32. Masci PG, Schuurman R, Andrea B, Ripoli A, Coceani M, Chiappino S, Todiere G, Srebot V, Passino C, Aquaro GD, Emdin M, Lombardi M. Myocardial fibrosis as a key determinant of left ventricular remodeling in idiopathic dilated cardiomyopathy. Circ Cardiovasc Imaging 2013; 6: 790–799. [DOI] [PubMed] [Google Scholar]
- 33. Pinamonti B, Di Lenarda A, Sinagra G, Camerini F. Restrictive left ventricular filling pattern in dilated cardiomyopathy assessed by Doppler echocardiography: Clinical, echocardiographic and hemodynamic correlations and prognostic implications. Heart muscle disease study group. J Am Coll Cardiol 1993; 22: 808–815. [DOI] [PubMed] [Google Scholar]
- 34. Artico J, Merlo M, Delcaro G, Cannatà A, Gentile P, De Angelis G, Paldino A, Bussani R, Ferro MD, Sinagra G. Lymphocytic myocarditis: A genetically predisposed disease? J Am Coll Cardiol 2020; 75: 3098–3100. [DOI] [PubMed] [Google Scholar]
- 35. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group . Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline and follow‐up characteristics according to sex in the subgroups of patients presenting with heart failure, chest pain and arrhythmias at the onset.
Table S2. Incidence rates of events as number of events per 100 patients/years.
Table S3. Baseline and follow‐up characteristics according to the primary outcome of death for all cause/heart transplant.
Table S4. Different multivariable Cox models, each time maintaining male sex and changing the combination of the other covariates.
Figure S1. Consort flow diagram of the population.
Figure S2. Long‐term HTx‐free survival according to the sex in the subgroups of patients presenting with chest pain (panel A). arrhythmias (panel B). heart failure (panel C). HTx indicates heart transplantation. Interestingly, no female experienced the outcome measure in chest pain group vs. 9 (6%) males. P = 0.1. The subgroup of patients with HF at the onset experienced more adverse events than the other groups. P = 0.019.


