Abstract
Aims
To investigate the outcomes and associated costs of haemodynamic‐guided heart failure (HF) management with a pulmonary artery pressure (PAP) sensor in a multicentre European cohort.
Methods and results
Data from all consecutive patients receiving a PAP sensor in Ziekenhuis Oost‐Limburg, University Hospital Zurich and Sheffield Teaching Hospitals NHS Foundation Trust before January 2021 were collected. Medication changes, total number of HF hospitalizations and HF related health care costs (composed of HF hospitalizations, outpatient cardiology visits and monitoring costs) were compared between the pre‐implantation and post‐implantation period at 3, 6, and 12 months. PAP evolution post‐implantation were grouped according to baseline mPAP ≥25 mmHg versus <25 mmHg and changes from baseline were analyzed via an area under the curve (AUC) analysis. A total of 48 patients received a PAP sensor (29 CardioMEMS and 19 Cordella devices) with a median follow‐up of 19 (13–30) months. Mean age was 71 ± 10 years, 25.0% were female, 68.8% had a left ventricular ejection fraction < 50%, median NT‐proBNP was 1801 (827–4503) pg/mL, and 89.6% were in NYHA class III. The number of diuretic therapy changes were non‐significantly increased after 3 months (49 vs. 82; P = 0.284) and 6 months (82 vs. 127; P = 0.093) with a significant increase noted after 12 months (118 vs. 195; P = 0.005). The mPAP AUC decreased by −1418 mmHg‐days for patients with a baseline mean PAP ≥ 25 mmHg. The number of HF hospitalizations was reduced for all patients after 6 (34 vs. 17; P = 0.014) and 12 months (48 vs. 29; P = 0.032). HF related health care costs were reduced from € 6286 to € 3761 at 6 months (P = 0.012) and from € 8960 to € 6167 at 12 months (P = 0.032).
Conclusion
Haemodynamic‐guided HF management reduces HF hospitalizations and HF related health care costs in selected HF patients amongst different European health care systems.
Keywords: Heart failure, Pulmonary artery pressure monitoring, Telemonitoring, Diuretics, Health care costs
Introduction
Despite major advances in heart failure (HF) treatment in recent decades, a high number of patients continue to be admitted to hospital with decompensated HF. HF hospitalizations are associated with a 5% in‐hospital mortality and 25% mortality within 1 year of discharge. 1 The typical clinical signs of congestion in decompensated HF are often preceded by an increase in cardiac filling pressures in the weeks before symptoms develop. 2 Therefore, remote monitoring of cardiac filling pressures may allow physicians to detect decompensated HF in an earlier stage to intervene faster, potentially reducing the need for hospitalization. In the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in New York Heart Association Class III Heart Failure Patients) trial, remote monitoring of pulmonary artery pressures (PAP), using a CardioMEMS sensor (Abbott), reduced the total number of HF hospitalizations in NYHA III class HF patients already after 6 months. 3 Based upon these results, the Food and Drug Administration (FDA) approved its use in the USA since 2014. Since then, other observational studies have replicated the results of the trial 4 , 5 , 6 , 7 and the European Society of Cardiology guidelines continue to recommend a class IIb indication for its use in HF with reduced ejection fraction. 8 However, European experience with remote PAP monitoring devices is limited and only one prospective multicentre European study has been published so far, showing a 62% reduction in the number of HF events after 1 year. 9 It remains largely unknown whether the beneficial effects of remote PAP monitoring hold true outside clinical trial settings in Europe. The objective of this study was to assess the outcomes and associated costs of remote PAP monitoring in a multicentre ‘real world’ European cohort.
Methods
Data collection
Data from all consecutive HF patients with a successful remote PAP sensor implantation before January 2021 was retrospectively collected from three advanced HF tertiary care centres experienced (Ziekenhuis Oost‐Limburg, Genk, Belgium; University Hospital Zurich, Zurich, Switzerland; Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom). During this period both CardioMEMS devices (Abbott, Sylmar, CA, USA) and Cordella devices (Endotronix Inc., Lisle, IL, USA) 10 were implanted. The indication for implantation was symptomatic HF with a previous HF decompensation warranting intensification of diuretic therapy. Every patient was discussed in a multidisciplinary HF team to evaluate the indication before implantation. Follow‐up before and after the implantation was done by the same HF team. Baseline data at the time of PAP sensor implantation consisting of demographics, comorbidities, physical exam, left ventricular ejection fraction, right heart catheterization measures, and laboratory measurements were retrieved from patient records. Baseline risk was assessed using the MAGGIC (Meta‐Analysis Global Group in Chronic Heart Failure) risk score estimating 1 and 3 year mortality. 11 The full medication list at baseline and at last follow‐up were collected. In addition, all diuretic changes from 1 year before up to 1 year after the PAP sensor implantation were acquired. The study was approved by the Institutional Review Board (19/0058R and IRAS 269248/STH20422).
Pulmonary artery pressure measurements and follow‐up
Before discharge, patients were trained in performing self‐measurements of PAP at home. They were instructed to perform daily PAP measurements that were sent to a secure database (Merlin.net™ for the CardioMEMS device and myCordella™ Patient Management Portal for the Cordella device). The PAP recordings of every patient were evaluated each working day by an allied healthcare provider trained in HF. Following a change in PAP, the patient was contacted by telephone to evaluate signs and symptoms of congestion. Treatment interventions based on changes in PAP pressures and decisions regarding an earlier ambulatory visit were non‐protocolized and left at the treating team's discretion. Treatment changes were mainly communicated to the patients over the telephone.
Endpoints
The number of diuretic changes were assessed at 3, 6, and 12 months prior to the PAP sensor implantation (antecedent hospitalizations) and at 3, 6, and 12 months after the PAP sensor implantation (incident hospitalizations). Other medications were assessed at the time of implantation and last follow‐up. In addition, the number of antecedent and incident HF hospitalizations, all‐cause hospitalizations, cardiology outpatient visits and all outpatient visits to the hospital (all specialties) were assessed at the same time intervals. HF hospitalization was defined as an event in which the patient was admitted to the hospital with a primary diagnosis of HF, the length of stay was at least 24 h (or extending over a calendar date), the patient exhibited new or worsening symptoms of HF on presentation, had objective evidence of new or worsening HF, and received initiation or intensification of treatment specifically for HF. 12 The occurrence of device‐related or system‐related complications was used as a safety endpoint and defined as any adverse event definitely or possibly related to the device or the implantation procedure requiring specific treatment and/or hospitalization. Both daily compliance with transmissions and weekly compliance were calculated for the first month and the first year.
Cost analysis
Health care related costs were calculated as the sum of the costs of HF hospitalizations, outpatient cardiology visits and device monitoring for antecedent and incident costs at 6 and 12 months. The cost of a HF hospitalization was estimated at an average of € 7608 for the participating countries (Belgium: € 7630; UK: € 2668; Switzerland € 12 528). The cost of a cardiology outpatient visit was similarly estimated at € 324 per visit (Belgium: € 208; UK: € 252; Switzerland: € 518). Monitoring cost was estimated at € 25 per patient per month, based upon staffing costs for daily monitoring (1 min/patient/day) and contacting patients (2 min/patient/week) at a rate of € 50/h. The time management estimates were based upon 3 months of daily time registration by monitoring nurses at Ziekenhuis Oost‐Limburg.
Statistical analysis
Continuous variables are displayed as mean ± standard deviation if normally distributed or otherwise as median (25th–75th percentile). Normality was checked by integrating visual inspection of the histogram, skewness, kurtosis, PP‐plots, QQ‐plots and Shapiro–Wilk testing. Categorical data are expressed as number (percentage). The incident and antecedent number of patients with a first HF hospitalization were compared using the McNemar's test at the predefined 3, 6, and 12 month time intervals. The number of diuretic changes and the number of HF hospitalizations were expressed as total number and compared using a Wilcoxon signed rank test. Patients who died or received a left ventricular assist device (LVAD) or transplant before this time interval were censored for both antecedent and incident analysis to allow for paired comparisons with patients serving as their own control. Cumulative changes in mean PAP (mPAP) were evaluated using an area under the curve (AUC) analysis, which quantifies frequency and duration of mPAP values below baseline (first week of home readings) using numeric integration. AUCs were analysed for all patients and for subgroups according to baseline mPAP (<25 and ≥25 mmHg). Changes in AUC and PAP were analysed using a paired t‐test, as were HF related health care costs.
Results
Study population
In 48 consecutive HF patients, 29 CardioMEMS and 19 Cordella devices were successfully implanted between April 2015 and January 2021 with a median follow‐up of 19 (13–30) months. Their baseline characteristics are displayed in Table 1 . Of note, patients were mostly male with a high comorbidity burden, a long‐standing diagnosis of HF and a high estimated 1 year mortality risk. The vast majority received loop diuretics and there was a high use of guideline directed medical and device therapy.
Table 1.
Baseline characteristics
| Baseline characteristics | N = 48 |
|---|---|
| Age (years) | 71 ± 10 |
| Female sex | 12 (25.0%) |
| Co‐morbidities | |
| Arterial hypertension | 30 (62.5%) |
| Diabetes | 21 (43.8%) |
| Dyslipidaemia | 21 (43.8%) |
| Coronary artery disease | 29 (60.4%) |
| Atrial fibrillation | 36 (75.0%) |
| Stroke or TIA | 7 (14.6%) |
| Peripheral artery disease | 8 (16.7%) |
| COPD | 11 (22.9%) |
| Ischaemic aetiology of heart failure | 28 (58.3%) |
| Duration of heart failure (years) | 5.8 (3.4–9.6) |
| LVEF categories | |
| LVEF <50% | 33 (68.8%) |
| LVEF ≥50% | 15 (31.2%) |
| NYHA class | |
| II | 3 (6.3%) |
| III | 43 (89.6%) |
| IV | 2 (4.2%) |
| Physical exam | |
| Systolic blood pressure (mmHg) | 117 ± 25 |
| Diastolic blood pressure (mmHg) | 67 ± 11 |
| Heart rate (b.p.m.) | 72 ± 12 |
| Body mass index (kg/m2) | 28.2 (24.5–32.2) |
| Laboratory analysis | |
| Creatinine (mg/dL) | 1.50 (1.12–1.81) |
| Urea (mg/dL) | 71 (52–106) |
| eGFR (mL/min/1.73 m2) | 44 (34–64) |
| eGFR > 60 | 14 (29.2%) |
| eGFR 30–59 | 29 (60.4%) |
| eGFR < 30 | 5 (10.4%) |
| NT‐proBNP (pg/mL) | 1801 (827–4503) |
| Haemodynamics on right heart catheterization | |
| Systolic pulmonary artery pressure (mmHg) | 42 ± 15 |
| Diastolic pulmonary artery pressure (mmHg) | 16 ± 6 |
| Mean pulmonary artery pressure (mmHg) | 26 ± 9 |
| Pulmonary capillary wedge pressure (mmHg) | 16 ± 6 |
| Right atrial pressure (mmHg) | 7 ± 5 |
| Cardiac index (L/min/m2) | 2.4 ± 0.6 |
| Pulmonary vascular resistance (Wood Units) | 2.4 ± 1.4 |
| Pulmonary artery pressure sensor type | |
| CardioMEMS | 29 (60.4%) |
| Cordella | 19 (39.6%) |
| Therapy | |
| ACEi/ARB/ARNI | 41 (85.4%) |
| Beta‐blocker | 43 (89.6%) |
| MRA | 39 (81.3%) |
| SGLT2 inhibitor | 1 (2.1%) |
| Digoxin | 8 (16.7%) |
| Ivabradine | 2 (4.2%) |
| Loop diuretic | 47 (97.9%) |
| Thiazide | 9 (18.8%) |
| Antiplatelet | 13 (27.1%) |
| Anticoagulation | 35 (72.9%) |
| Statin | 30 (64.6%) |
| CRT | 17 (35.4%) |
| ICD | 25 (52.1%) |
| Risk assessment | |
| MAGGIC score | 29 (23–33) |
| Estimated 1 year mortality (%) | 22.7 (13.4–31.6) |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprylisin inhibitor; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SGLT2, sodium glucose cotransporter 2; TIA, transient ischaemic attack.
Medication changes
The number of diuretic changes pre‐implantation and post‐implantation are illustrated in Figure 1 . Although there were more diuretic changes both at 3 months (49 vs. 82; P = 0.284) and 6 months (82 vs. 127; P = 0.093) post‐implantation, statistical significance was only reached after 12 months (118 vs. 195; P = 0.005). A total of 43 (89.6%) patients had at least one diuretic change within the first 12 months after implantation after a median of 3 (1–6) months (Figure 2 A ).
Figure 1.
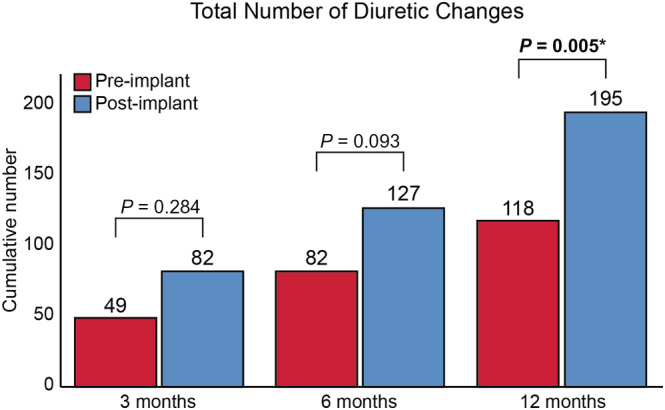
Total number of diuretic changes pre‐implantation and post‐implantation. *P < 0.05.
Figure 2.
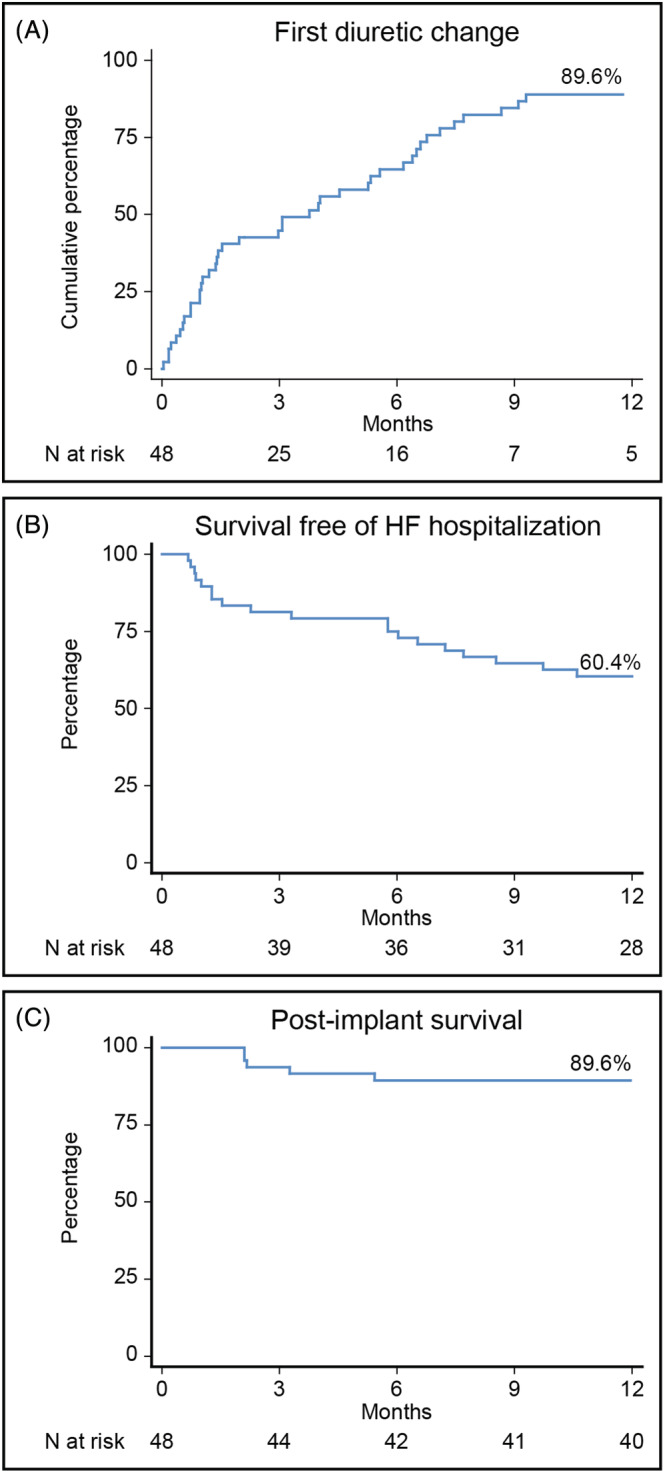
Post‐implantation timing of first events. HF, heart failure.
Of note, there were few changes in other HF drugs (Table 2 ) from the time of implantation to last follow‐up. In addition to an increase in the loop diuretic dose, a reduction in the number of patients taking an angiotensin converting enzyme inhibitor (ACEi), angiotensin receptor blocker (ARB) or angiotensin receptor‐neprilysin inhibitor (ARNI) was noted (41 to 33, P = 0.021). However, the dose in patients on ACEi, ARB or ARNI did not change. Reasons for stopping the ACEi, ARB or ARNI was renal failure in three (6.3%) patients and progressive HF with hypotension in six (12.5%) patients. Furthermore, there was an increase in the number of patients taking sodium glucose cotransporter 2 (SGLT2) inhibitors.
Table 2.
Dosing of HF drugs at time of implantation and last follow‐up
| Implant | Last follow‐up | P‐value | |
|---|---|---|---|
| Beta‐blocker | |||
| Yes | 43 (89.6%) | 40 (83.3%) | 0.375 |
| % of target dose | 54 ± 30 | 55 ± 34 | 0.530 |
| ACEi/ARB/ARNI | |||
| Yes | 41 (85.4%) | 33 (68.8%) | 0.021 |
| % of target dose | 42 ± 14 | 38 ± 24 | 0.184 |
| MRA | |||
| Yes | 39 (81.3%) | 38 (79.2%) | 1.000 |
| Spironolactone equivalent (mg) | 24 ± 8 | 24 ± 12 | 0.812 |
| Loop diuretic | |||
| Yes | 47 (97.9%) | 47 (97.9%) | 1.000 |
| Furosemide equivalent dose (mg) | 97 ± 89 | 154 ± 195 | 0.005 |
| Thiazide diuretic | |||
| Yes | 9 (18.8%) | 10 (20.8%) | 1.000 |
| HCT equivalent dose (mg) | 29 ± 13 | 30 ± 11 | 1.000 |
| SGLT2 inhibitor | 1 (2.1%) | 8 (16.7%) | 0.016 |
| Ivabradine | 2 (4.2%) | 2 (4.2%) | 1.000 |
| Digoxin | 8 (16.7%) | 8 (16.7%) | 1.000 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; HCT, hydrochlorothiazide; MRA, mineralocorticoid receptor antagonist; SGLT2, sodium glucose cotransporter 2.
% of target dose ranges from 0 to 100 and indicates how much of the recommended target dose in heart failure is achieved. For furosemide equivalent dose: 40 mg furosemide = 1 mg bumetanide = 20 mg torsemide. For HCT equivalent dose: 25 mg hydrochlorothiazide = 25 mg chlortalidone = 2.5 mg metolazone = 1.25 mg indapamide.
P‐value in bold indicates P < 0.05.
Pulmonary artery pressures
Baseline device measured pressures were dPAP 18 ± 11 mmHg, mPAP 27 ± 14 mmHg and sPAP 41 ± 20 mmHg. Twenty‐eight (58.3%) patients had a baseline mPAP <25 mmHg and 20 (41.7%) had a baseline mPAP ≥25 mmHg. The changes in AUC according to baseline mPAP are shown in Figure 3 . For patients with a baseline mPAP <25 mmHg the AUC was positive (1370 ± 2248 mmHg‐days) and mPAP was similar between baseline 16 ± 6 mmHg and after 1 year 19 ± 13 mmHg (P = 0.127). For patients with a baseline mPAP ≥25 mmHg the AUC was negative (−1418 ± 2541 mmHg‐days) and mPAP decreased non‐significantly from 40 ± 9 mmHg baseline to 34 ± 11 (P = 0.085) after 1 year. Compliance with daily transmissions was 87 ± 20% within the first month and 66 ± 32% within the first year. Weekly compliance was 99 ± 6% within the first month and 94 ± 11% within the first year.
Figure 3.
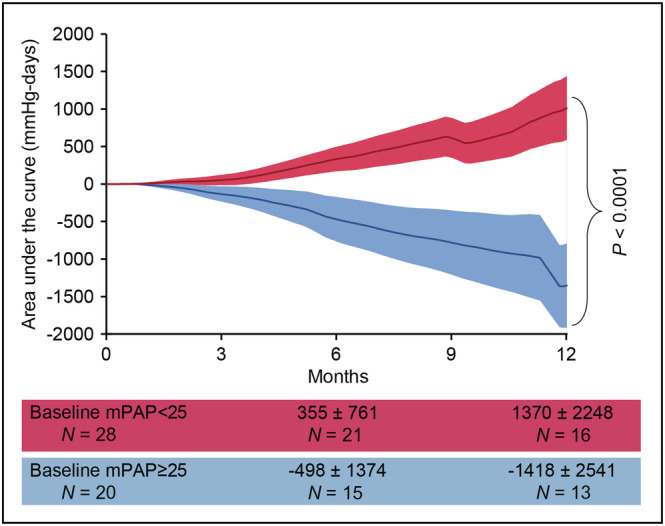
Area under the curve changes in pulmonary artery pressures post‐implantation. mPAP: mean pulmonary artery pressure.
Heart failure hospitalization and mortality
Within the first 3 months post‐implantation, there was no statistically significant difference in the number of patients with a first HF hospitalization compared with the 3 months pre‐implantation (17 [35.4%] vs. 9 [18.8%]; P = 0.115). The number of first HF hospitalizations was reduced after 6 months (13 [27.1%] vs. 21 [51.2%]; P = 0.017) and 12 months (33 [68.8%] vs. 19 [39.8%]; P = 0.001). The median time to first HF hospitalization after implantation was 6 (1–8) months (Figure 2 B ). Consistent with this finding, the total number of HF hospitalizations were a reduced at 6 months (34 vs. 17; P = 0.014) and 12 months (48 vs. 29; P = 0.032) post‐implant (Figure 4 A ). There was no statistical significant difference in the number of all‐cause hospitalizations, outpatient cardiology visits, and total outpatient visits (Figure 4 D B– 4 ).
Figure 4.
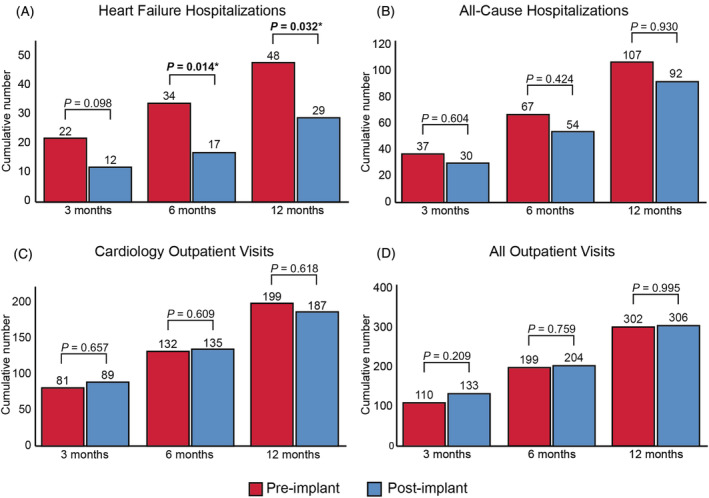
Total number of events pre‐implantation and post‐implantation. *P < 0.05.
Within the first 12 months after implantation, three (6.3%) patients received an LVAD or heart transplantation, and five (10.4%) patients died: three died because of HF, one because of endocarditis, and one because of thromboembolism. Post‐implantation 12 month survival is depicted in Figure 2 C .
Device complications and system performance
There were no serious device‐related or system‐related complications. There were two patients (one Cordella and one CardioMEMS) that had temporary transmission problems that were resolved.
Health care costs
The HF related health care costs per patient dropped from € 6286 ± 6357 to € 3761 ± 5185 at 6 months (P = 0.012) and from € 8960 ± 7892 to € 6167 ± 7404 at 12 months (P = 0.032) (Figure 5 ), mainly driven by the reduction in HF hospitalizations.
Figure 5.
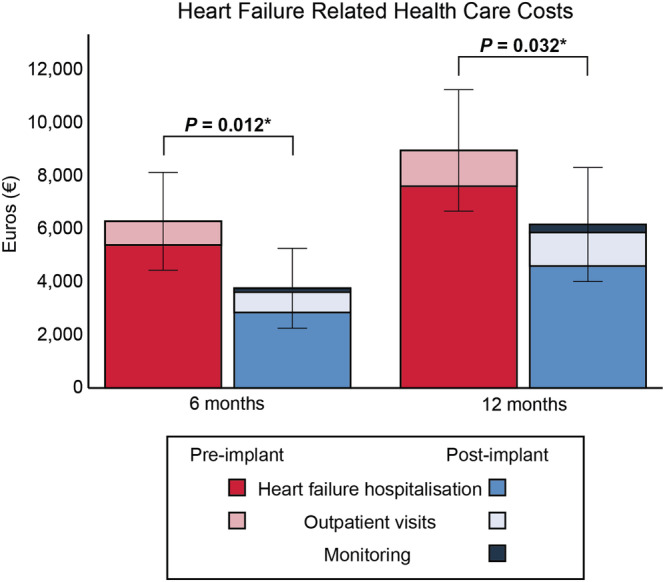
Heart failure related health care costs per patient pre‐implantation and post‐implantation. *P < 0.05.
Discussion
In this multinational European observational study, 1 year of ambulatory PAP‐guided therapy in HF patients led to (i) an increase in diuretic therapy changes; (ii) a decrease in mPAP in patients with a baseline mPAP ≥25 mmHg; (iii) a decrease in the number of HF hospitalizations; and (iv) decreased HF related health care costs.
This is the first study to report on outcomes in patients with different PAP sensor systems including the Cordella and the CardioMEMS device. Adherence to guideline directed medical therapy was high and, consistent with prior studies, the number of therapeutic changes made following PAP monitoring was increased. 9 , 13 , 14 The majority of treatment changes were to diuretic dose and class, which are the preferred means to alleviate congestion and decrease filling pressures. 8 There were few changes in other HF drugs between the time of implantation and last follow‐up. Of note, there were fewer patients receiving ACEi, ARB, or ARNI at last follow‐up due to progression to either end‐stage renal failure or end‐stage HF. This is in concordance with the high risk status of this population with an estimated median 1 year mortality of 22.7% at the time of implantation.
The therapeutic interventions translated in a trend to 6 mmHg decrease in mPAP in patients with baseline mPAP ≥25 mmHg after 12 months. Keeping PAP within a predefined range is the main goal of haemodynamic monitoring and even small changes over time can have a significant effect on outcome. Our results are in line with previous studies that have reported an absolute decrease of 1–5 mmHg after 12 months. 3 , 6 , 9 , 14 , 15
PAP‐guided therapy resulted in a reduction in total HF hospitalizations of 50% after 6 months and 40% after 12 months. In the CHAMPION trial remote PAP monitoring reduced HF hospitalizations by 33% after a mean follow‐up of 18 months in NYHA class III patients with a previous HF hospitalization 16 and these results were reproduced in several post‐approval observational studies. 4 , 5 , 6 , 9 , 14 In contrast, the very recent GUIDE‐HF (Haemodynamic‐Guided Management of Heart Failure) trial in NYHA class II–IV patients with a previous HF hospitalization or elevated N‐terminal pro‐BNP failed to show a benefit of remote PAP monitoring on all‐cause mortality and total HF events. 17 However, a prespecified COVID‐19 sensitivity analysis suggested a significant impact of COVID‐19 on the results. In the pre‐COVID‐19, remote PAP monitoring resulted in a 19% reduction in the primary endpoint. Of note, both randomized trials were exclusively conducted in North‐America and only with the CardioMEMS device. To date, only two European observational studies have been published. In the MEMS‐HF (CardioMEMS European Monitoring Study for Heart Failure) study, remote PAP monitoring with CardioMEMS in 234 NYHA class III patients from Germany, the Netherlands and Ireland, with a previous HF hospitalization, led to a 62% reduction in HF hospitalizations after 1 year. 9 In addition, the COAST (CardioMEMS HF System Post‐Market Study) study showed an 82% reduction after 1 year in 100 NYHA Class III patients from the UK with a previous HF hospitalization. Importantly, our study corroborates that remote PAP monitoring is safe and reduces HF hospitalization in diverse European health care systems as well as in routine care outside of the strict conduct of a trial. Despite the relative small sample size, statistical significance was already met by 6 months. Indeed, in the CHAMPION trial, the number needed to treat for 6 months to prevent one HF hospitalization was only 8. 3 Currently, two randomized trials are investigating remote PAP monitoring with the CardioMEMS device in NYHA class III patients with a previous HF hospitalization in Europe. The primary endpoint of PASSPORT‐HF (Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes) study is the composite of the number of unplanned HF‐related rehospitalizations or all‐cause mortality after 12 months, 18 while the primary endpoint of MONITOR‐HF (a randomized comparison of the effect of haemodynamic monitoring with CardioMEMS in addition to standard care on quality of life and hospitalizations in patients with chronic heart failure) study is quality of life. 19 These studies will provide more data on the effect of remote PAP monitoring in Europe.
This study demonstrated that PAP monitoring reduced HF related health care costs by ~40% after 6 and 12 months, which was mainly driven by a reduction in HF hospitalization cost. Of note, the estimated costs did not take the cost of the device and implantation into account. A recent cost‐effectiveness analysis including the device cost, using the event rates of the CHAMPION trial and cost estimates from different European countries, estimated that PAP‐guided HF management increased costs by € 14 030 over 10 years with an incremental cost‐effectiveness ratio (ICER) of € 24 772 per quality‐adjusted life year (QALY) gained. 20 Besides the cost of the device, cost‐effectiveness is influenced by the rate reduction in HF hospitalization and mortality and the cost of a HF hospitalization. More data on cost‐effectiveness in a European setting will be provided by the aforementioned MONITOR‐HF study. 19
Limitations
This study is subject to certain limitations. Firstly, the sample size was small, representing the limited experience with PAP‐guided HF management in Europe. Small sample sizes could be less representative for the entire population and hamper generalizability of the results. This was partially overcome by the multicentre nature of the study, including three different countries and the consecutive inclusion of patients. In addition, the small sample size could lead to type II error. Therefore, non‐significant results should be interpreted with caution Secondly, this was a retrospective study, which makes it vulnerable to selection bias and confounders. Thirdly, the progressive nature of HF might also influence the event rate during follow‐up. Fourthly, due to the unblinded nature of the study, there is a potential performance bias as the treating physicians might have acted differently after the PAP sensor was implanted. Finally, costs were estimated based upon generalized assumptions. In addition, quality of life was not collected so that no formal cost‐effectiveness could be calculated.
Conclusion
Remote PAP monitoring is safe and led to a reduction in HF hospitalizations already after 6 months in a multicentre, high‐risk European HF population and was associated with a reduction in HF related health care costs.
Conflict of interest
A.R. received grant funding from Medical Research Council and Wellcome Trust; research support from Novarits, Johnson and Johnson, Medtronic, Abbott, SoniVie, Endotronix; consulting fees from SoniVie, Endotronix. A.F. received grants/research support form Novartis, AstraZeneca and Berlin Heart, as well as honoraria for consulting, lecturing or advisory boards from Abbott, Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius, Medtronic, MSD, Mundipharma, Novartis, Pfizer, Pierre Fabre, Roche, Vifor, and Zoll. F.R. has not received personal payments by pharmaceutical companies or device manufacturers in the last 3 years (remuneration for the time spent in activities, such as participation as steering committee member of clinical trials and member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research, educational and/or travel grants from Abbott, Amgen, AstraZeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Trama Solutions, V‐Wave, Vascular Medical, Vifor, Wissens Plus, ZOLL. The research and educational grants do not impact on F.R.'s personal remuneration. O.F. receives financial support from Endotronix. M.D. and P.N. report no conflicts of interest. W.M. received research grants/consultancy fees from Novartis, Vifor, Medtronic, Abbott, AstraZenica, Boehringer Ingelheim. J.D., M.S., and J.M. have no conflicts of interest.
Acknowledgements
We thank our HF nurses Jan Vercammen, Evert Luwel, Veerle Kockaerts, and Wendy Ceyssens for their help to estimate time consumption of the remote monitoring. Joren Schouteden also deserves our gratitude for his help with data collection.
Dauw, J. , Sokolski, M. , Middleton, J. T. , Nijst, P. , Dupont, M. , Forouzan, O. , Rothman, A. M. K. , Ruschitzka, F. , Flammer, A. J. , and Mullens, W. (2022) Ambulatory haemodynamic‐guided management reduces heart failure hospitalizations in a multicentre European heart failure cohort. ESC Heart Failure, 9: 3858–3867. 10.1002/ehf2.14056.
References
- 1. Cowie MR, Anker SD, Cleland JGF, Felker GM, Filippatos G, Jaarsma T, Jourdain P, Knight E, Massie B, Ponikowski P, López‐Sendón J. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Failure. 2014; 1: 110–145. [DOI] [PubMed] [Google Scholar]
- 2. Zile MR, Bennett TD, Sutton SJM, Cho YK, Adamson PB, Aaron MF, Aranda JM Jr, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ. Transition from chronic compensated to acute d compensated heart failure: Pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008; 118: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 3. Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S, Weiner S. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: A randomised controlled trial. Lancet. 2011; 377: 658–666. [DOI] [PubMed] [Google Scholar]
- 4. Desai AS, Bhimaraj A, Bharmi R, Jermyn R, Bhatt K, Shavelle D, Redfield MM, Hull R, Pelzel J, Davis K, Dalal N. Ambulatory Hemodynamic Monitoring Reduces Heart Failure Hospitalizations in “Real‐World” Clinical Practice. J Am Coll Cardiol. 2017; 69: 2357–2365. [DOI] [PubMed] [Google Scholar]
- 5. Abraham J, Bharmi R, Jonsson O, Oliveira GH, Artis A, Valika A, Capodilupo R, Adamson PB, Roberts G, Dalal N, Desai AS. Association of Ambulatory Hemodynamic Monitoring of Heart Failure with Clinical Outcomes in a Concurrent Matched Cohort Analysis. JAMA Cardiol. 2019; 4: 556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shavelle DM, Desai AS, Abraham WT, Bourge RC, Raval N, Rathman LD, Heywood JT, Jermyn RA, Pelzel J, Jonsson OT, Costanzo MR. Lower Rates of Heart Failure and All‐Cause Hospitalizations during Pulmonary Artery Pressure‐Guided Therapy for Ambulatory Heart Failure: One‐Year Outcomes from the CardioMEMS Post‐Approval Study. Circ Heart Fail. 2020; 13: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mullens W, Martens P, Forouzan O, Dauw J, Vercammen J, Luwel E, Ceyssens W, Kockaerts V, Ameloot K, Dupont M. Effects of dapagliflozin on congestion assessed by remote pulmonary artery pressure monitoring. ESC Hear Fail. 2020; 7: 2071–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 9. Angermann CE, Assmus B, Anker SD, Asselbergs FW, Brachmann J, Brett ME, Brugts JJ, Ertl G, Ginn G, Hilker L, Koehler F, Rosenkranz S, Zhou Q, Adamson PB, Böhm M, MEMS‐HF Investigators . Pulmonary artery pressure‐guided therapy in ambulatory patients with symptomatic heart failure: the CardioMEMS European Monitoring Study for Heart Failure (MEMS‐HF). Eur J Heart Fail. 2020; 1: 1–11. [DOI] [PubMed] [Google Scholar]
- 10. Mullens W, Sharif F, Dupont M, Rothman AMK, Wijns W. Digital health care solution for proactive heart failure management with the Cordella Heart Failure System: results of the SIRONA first‐in‐human study. Eur J Heart Fail. 2020; 22: 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 12. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascu). J Am Coll Cardiol. 2015; 66: 403–469. [DOI] [PubMed] [Google Scholar]
- 13. Costanzo MR, Stevenson LW, Adamson PB, Desai AS, Heywood JT, Bourge RC, Bauman J, Abraham WT. Interventions Linked to Decreased. JACC Hear Fail. 2016; 4: 333–344. [DOI] [PubMed] [Google Scholar]
- 14. Cowie MR, Flett A, Cowburn P, Foley P, Chandrasekaran B, Loke I, Critoph C, Gardner RS, Guha K, Betts TR, Carr‐White G, Zaidi A, Lim HS, Hayward C, Patwala A, Rogers D, Pettit S, Gazzola C, Henderson J, Adamson PB. Real‐world evidence in a national health service: results of the UK CardioMEMS HF System Post‐Market Study. ESC Hear Fail. 2022; 9: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heywood JT, Jermyn R, Shavelle D, Abraham WT, Bhimaraj A, Bhatt K, Sheikh F, Eichorn E, Lamba S, Bharmi R, Agarwal R, Kumar C, Stevenson LW. Impact of Practice‐Based Management of Pulmonary Artery Pressures in 2000 Patients Implanted with the CardioMEMS Sensor. Circulation. 2017; 135: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 16. Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: Complete follow‐up results from the CHAMPION randomised trial. Lancet. 2016; 387: 453–461. [DOI] [PubMed] [Google Scholar]
- 17. Lindenfeld J, Zile MR, Desai AS, Bhatt K, Ducharme A, Horstmanshof D, Krim SR, Maisel A, Mehra MR, Paul S, Sears SF, Sauer AJ, Smart F, Zughaib M, Castaneda P, Kelly J, Johnson N, Sood P, Ginn G, Henderson J, Adamson PB, Costanzo MR. Haemodynamic‐guided management of heart failure (GUIDE‐HF): a randomised controlled trial. Lancet. 2021; 6736: 1–11. [DOI] [PubMed] [Google Scholar]
- 18. Störk S, Bernhardt A, Böhm M, Brachmann J, Dagres N, Frantz S, Hindricks G, Köhler F, Zeymer U, Rosenkranz S, Angermann C, Aßmus B. Pulmonary artery sensor system pressure monitoring to improve heart failure outcomes (PASSPORT‐HF): rationale and design of the PASSPORT‐HF multicenter randomized clinical trial. Clin Res Cardiol. 2022: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brugts JJ, Veenis JF, Radhoe SP, Linssen GCM, van Gent M, Borleffs CJW, van Ramshorst J, van Pol P, Tukkie R, Spee RF, Emans ME, Kok W, van Halm V, Handoko L, Beeres SLMA, Post MC, Boersma E, Lenzen MJ, Manintveld OC, Koffijberg H, van Baal P, Versteegh M, Smilde TD, van Heerebeek L, Rienstra M, Mosterd A, Delnoy PPH, Asselbergs FW, Brunner‐La Rocca HP, de Boer RA. A randomised comparison of the effect of haemodynamic monitoring with CardioMEMS in addition to standard care on quality of life and hospitalisations in patients with chronic heart failure: Design and rationale of the MONITOR HF multicentre randomised clinical trial. Neth Hear J. 2020; 28: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cowie MR, Simon M, Klein L, Thokala P. The cost‐effectiveness of real‐time pulmonary artery pressure monitoring in heart failure patients: a European perspective. Eur J Heart Fail. 2017; 19: 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]


