Abstract
Aims
The present study aimed to evaluate the prognostic value of atrial strain and strain rate (SR) parameters derived from cardiac magnetic resonance (CMR) feature tracking (FT) in patients with ischaemic and non‐ischaemic dilated cardiomyopathy with heart failure with reduced ejection fraction (HFrEF) but without atrial fibrillation.
Methods and results
A total of 300 patients who underwent CMR with left ventricular ejection fraction (LVEF) ≤ 40% and ischaemic or non‐ischaemic dilated cardiomyopathy were analysed in this retrospective study. Major adverse cardiac events (MACEs) include cardiovascular death, heart transplantation, and rehospitalization for worsening HF. Ninety‐four patients had MACEs during median follow‐up of 3.84 years. Multivariate Cox regression models adjusted for common clinical and CMR risk factors detected a significant association between LA‐εs and MACE in ischaemic (HR = 0.94/%; P = 0.002), non‐ischaemic dilated cardiomyopathy (HR = 0.88/%; P = 0.001), or all included patients (HR = 0.87; P < 0.001). LA‐εs provided incremental prognostic value over conventional outcome predictors (Uno C statistical comparison model: from 0.776 to 0.801, P < 0.0001; net reclassification improvement: 0.075, 95% CI: 0.0262–0.1301). Kaplan–Meier analysis revealed that the risk of MACE occurrence increased significantly with lower tertiles of left atrial reservoir strain (LA‐εs) (log‐rank P < 0.0001). Patients in the worst LA‐εs tertile faced a significantly increased risk of MACEs irrespective of late gadolinium enhancement (LGE) (log‐rank P < 0.0001).
Conclusions
LA‐εs derived from CMR FT has a significant prognostic impact on patients with ischaemic or non‐ischaemic dilated cardiomyopathy, incremental to common clinical and CMR risk‐factors.
Keywords: Cardiac magnetic resonance, Prognosis, Cardiomyopathy, Left atrial strain, Feature tracking
Introduction
Heart failure (HF) remains a major challenge for patients and health care systems worldwide, especially with heart failure with reduced ejection fraction (HFrEF), who show rapid progression of HF, 1 despite best care, with approximately 50% dying within 5 years of diagnosis. 2 For patients with HF, prognostic evaluation not only performs risk stratification but is also significant in guiding clinical treatment decisions. 3 Cardiac magnetic resonance (CMR), echocardiography, and other imaging methods can comprehensively evaluate the structure and function of the heart and play a major role in the prognostic evaluation of patients with HF. CMR has the advantages of high spatial resolution, clearer visualization of atrial endocardial borders, and multiplanar imaging. It is increasingly used as a standard tool to assess the heart, 4 while late gadolinium enhancement (LGE) by CMR reflects the extent of tissue fibrosis, determines the underlying cause of left ventricular (LV) dysfunction, and is an independent predictor of adverse cardiovascular outcomes in patients with advanced HF. 5 For patients with advanced HF, the degree of impaired LV function loses its prognostic value. 6 Atrial remodelling and functional changes reflect ventricular systolic and diastolic dysfunction rather sensitively. 7 Atrial strain from echocardiography or CMR is an independent predictor of prognosis in patients with HF. 8 , 9 , 10 , 11 However, these studies either consider only non‐ischaemic dilated cardiomyopathy (NIDCM) or focus on a specific atrium. The predictive value of atrial strain in patients with ischaemic and non‐ischaemic dilated cardiomyopathy has not yet been studied. The present study aimed to investigate the long‐term predictive value of atrial strain derived from CMR in patients with ischaemic cardiomyopathy (ICM) and NIDCM with HFrEF but atrial fibrillation (AF).
Methods
Study population and study design
A total of 326 patients with ICM and NIDCM with HFrEF but AF who visited Beijing Anzhen Hospital, Capital Medical University from January 2015 to April 2020 were included in this retrospective study. These patients underwent electrocardiography, biochemical analysis, standard echocardiography, and CMR examinations, including cine and LGE imaging. ICM was defined as the presence of systolic dysfunction accompanied by one of the following: (i) history of myocardial infarction or revascularization and (ii) presence of at least 75% stenosis in the left main or anterior descending artery or >75% stenosis in at least two coronary arteries. 12 DCM is defined as left or biventricular systolic dysfunction and dilatation that cannot be explained by abnormal loading conditions or coronary artery disease. 13 Chronic kidney disease (CKD) was defined as abnormalities in kidney function or structure present for more than 3 months, with implications for health. 14 The vean contracta(VC) was typically imaged in a view perpendicular to the commissural line. A VC of <3 mm indicated mild mitral regurgitation (MR), whereas a width of ≥7 mm suggested severe MR. 15 Pulmonary hypertension (PH) was considered if patients had a systolic pulmonary arterial pressure (sPAP) of 40 mmHg. 16 PH was categorized as ‘mild’ (40–54 mmHg), ‘moderate’ (55–64 mmHg), or ‘severe’ (>65 mmHg). 17 The inclusion criteria were impaired left ventricular ejection fraction (LVEF) ≤ 40% as determined by CMR. 1 Conversely, patients with congenital heart disease, infiltrative cardiomyopathy, severe valvular heart disease, history of cardiac resynchronization therapy, implantable cardioverter defibrillator, and images from CMR that could not be analysed were excluded. Subsequently, 26 patients were excluded because the image quality could not be assessed, and finally, 300 patients (ICM: n = 131, NIDCM: n = 169) were included in the study.
Cardiac magnetic resonance acquisition
All CMR images were electrocardiogram (ECG)‐gated. CMR was conducted on a 3.0 T scanner (Magnetom Verio; Siemens AG Healthcare, Erlangen, Germany, or MR750W, General Electric Healthcare, Waukesha, WI, USA). Standard scanning protocols, including steady‐state free precession (SSFP) breath‐hold cine images and LGE using gadolinium, conforming to current international guidelines, were utilized. 18 The cine images were obtained in multiple short‐axis and three long‐axis views, with the entire LV and right ventricle (RV) from the base to apex containing slices (8 mm thickness, no cross‐gap) at 25 stages per cardiac cycle in consecutive short‐axis. The long axis planes had 5‐mm slice thickness (2‐chamber, 4‐chamber, and 3‐chamber views) without spacing intersection gap. 6 Using the prospective ECG‐gated gradient echo sequence, LGE images were obtained 10 min after intravenous injection of 0.2 mmol/kg gadolinium chelating contrast agent. The sequence parameters were as follows: repetition time/echo time, 4.1/1.6 ms; flip angle, 20°; image matrix, 256 × 130.
Cardiac megnetic resonance analysis and assessment of left ventricular and atrial function
The function of the LV and biatrium was analysed using CVI42 commercial software (Circle Cardiovascular Imaging, Calgary, AB, Canada). Semi‐automatic analysis was performed using ventricular short‐axis and 4‐chamber or 2‐chamber or 3‐chamber cardiac sequences to identify and delineate the ventricular epicardial and epicardial boundaries at the end of the systole and diastole (papillary muscles were included in the blood pool); the inaccuracies were corrected by cardiovascular imaging professionals with >10 years of experience. LVEF and body surface area (BSA) functional indicators were obtained, including end‐diastolic volume (EDV), end‐systolic volume (ESV), stroke volume (SV), and ventricular mass (MASS). Epicardial and endocardial contours were manually placed on LGE images, which appeared in any area with signal intensity 5 SD above normal myocardium.
Left atrial (LA) strain parameters were analysed using 2‐chamber and 4‐chamber cine images, while the right atrial (RA) strain parameters were analysed using 4‐chamber cine images. LA endocardial and epicardial borders (except pulmonary veins and LA appendages) were traced manually when the LA was the largest and smallest in 2‐chamber and 4‐chamber cine images, respectively, using a point‐matching method by cardiovascular imaging specialists with > 10 years of experience 19 (Figure 1 ). LA endocardial and epicardial borders then propagated automatically to all frames during the heart cycle (25 frames/heart cycle). LA global strain and strain rate were calculated as the average of 2‐chamber and 4‐chamber views. The endocardial and epicardial contours of the RA were manually drawn in end‐systolic images, followed by automatic software‐driven tracking of the endocardial and epicardial contours throughout the cardiac cycle 20 (Figure 1 ). In case of unsatisfactory feature tracking (FT), the endocardial border was readjusted manually, and the extension algorithm was reapplied.
Figure 1.
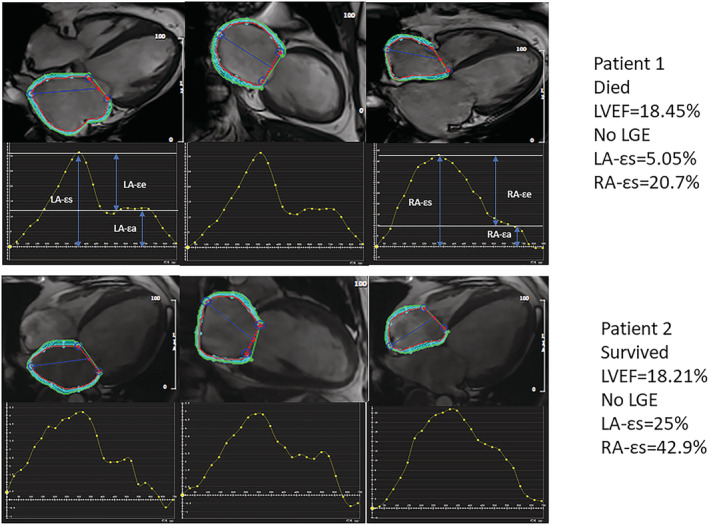
Measurement of atrial strain. The top panel shows a patient who died and the bottom panel shows a patient who survived.
A total of 30 cases were selected randomly to study the intra‐observer and inter‐observer variability regarding atrial strain parameters. For interobserver variability, measurements were repeated by a second independent observer, blinded to the results of the first observer. For the intraobserver variability study, these 30 patients were analysed repeatedly by the same observer after an interval of 3 months. Thus, the functional and strain parameters of the atria were obtained, including reservoir strain (εs), conduit strain (εe), booster strain (εa), peak positive strain rate (SRs), peak early negative strain rate (SRe) and peak late negative strain rate (SRa).
Follow‐up
Clinical follow‐up is the assessment of patients every 6 months by telephone and/or postal questionnaires. The primary outcome was a composite of major adverse cardiac events (MACEs), including cardiovascular death (sudden cardiac death (SCD), HF death, stroke, or thromboembolic event), heart transplantation, and rehospitalization for worsening HF. Patients were followed up until early March 2022. Also, the time point of lost patients was recorded as the last follow‐up.
Statistical analysis
All analyses were carried out using SPSS (version 17.0, International Business Machines, Armonk, NY, USA) and R (http://www.R‐project.org). For continuous variables, data are presented as mean ± standard deviation (SD) for normal distribution and as median and interquartile range (IQR) for skewed distribution. Discrete data are reported as percentages and frequencies. Herein, we used a five‐repetition based multiple interpolation approach to address the missing data issue for the most important variable, type B natriuretic peptide (BNP). The reproducibility of atrial strain parameter measurements was analysed using the intraclass correlation coefficient (ICC), which was <0.4 with poor agreement and >0.75 with good agreement. The differences in baseline characteristics were compared using analysis of variance (ANOVA) or its nonparametric equivalents (Kruskal–Wallis test) for continuous variables and the χ 2 test for dichotomous variables. Cox regression was used to assess the prognostic value of various parameters to obtain hazard ratios (HRs) and 95% confidence intervals (CIs). Univariate Cox regression analysis was used to assess the association between each variable and MACEs over 5 years of patient follow‐up. The correlations between covariates were evaluated using Pearson's or Spearman's correlation coefficient. A correlation r > 0.7 was interpreted as a strong link between the two variables, and r < 0.3 was considered as a weak correlation. The association of LA parameters with outcomes was compared using area under curve (AUC) analysis. Clinical and radiographic risk factors for univariate predictors (P ≤ 0.05) were included as covariates in the multivariate Cox regression model. For variables with correlation >0.7, one variable was selected for inclusion in the multivariate Cox regression model based on clinical experience and previous literature. Because 10% of the patients lacked BNP, we applied the average of the five interpolated data points to the model with BNP and judged whether the interpolation was reliable by estimating the difference in the statistical results before and after interpolation. Survival curves were constructed using the Kaplan–Meier method and compared using the log‐rank test. The final model was compared with a model that did not include LA‐εs (LA reservoir strain), and model discrimination was compared by calculating the C‐statistic and the integrated discrimination improvement. 21 The incremental prognostic value of LA‐εs for the prediction of the primary endpoint was assessed using net reclassification improvement (NRI). A p‐value < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 300 patients (131 with ICM and 169 with NIDCM) with HFrEF without AF were included in this study. Tables 1 and 2 summarize the clinical baseline and CMR characteristics of patients stratified by LA‐εs tertiles, respectively. Supporting Information, Table S1 compares the baseline characteristics of ICM with NIDCM. The median age at baseline was 56 (IQR: 46–64) years, and 81% were men. The LA and RA strain and strain rate (SR) were reduced compared with the corresponding values reported previously in normal subjects. 11 LGE was present in 67.7% of patients. Statistically significant differences were observed in the functional parameters of LA and RA among the three groups. In addition, we counted the echocardiographic findings in 288 patients. There was a weak correlation between the degree of MR and PH and LA‐εs (r < 0.3).
Table 1.
Clinical baseline characteristics stratified by tertiles of LA‐εs
| Characteristics | Total | LA εs<8.0% | LA εs8% to 15.9% | LA εs > 15.9% | P‐value |
|---|---|---|---|---|---|
| Demographic and clinical data | |||||
| Age, median (IQR), years | 56 (46, 64) | 55 (45, 63.75) | 59 (48, 65) | 57 (43.5, 63.50) | 0.352 |
| Male, % | 81.00 | 84.20 | 83.00 | 75.80 | 0.616 |
| BMI, median (IQR), kg/m2 | 25.19 (22.28, 27.68) | 25.43 (21.92, 28.26) | 24.77 (22.89, 26.73) | 23.67 (19.76, 27.52) | 0.318 |
| Diabetes mellitus, % | 31.30 | 27.80 | 37.90 | 29.40 | 0.209 |
| Hypertension, % | 47.70 | 47.20 | 49.50 | 41.20 | 0.802 |
| CKD, % | 6.00 | 7.20 | 4.90 | 0.00 | 0.406 |
| SBP, median (IQR), mmHg | 120 (108, 130) | 120 (106, 130) | 120 (108, 130) | 119 (107.5, 120) | 0.018 |
| DBP, median (IQR), mmHg | 74 (69, 80) | 75 (69, 80) | 70 (68, 80) | 80 (71, 83.5) | 0.690 |
| Heart rate, median (IQR), b.p.m. | 80 (70, 90) | 82 (72, 96) | 75 (67, 88) | 71 (65.5, 94) | 0.248 |
| QRS duration, median (IQR), ms | 110 (99, 129) | 110 (100, 129.5) | 114 (98, 136) | 107 (97.5, 117.5) | 0.000 |
| LBBB on EKG, % | 12.30 | 12.20 | 14.60 | 0.00 | 0.238 |
| RBBB on EKG, % | 6.00 | 7.20 | 4.90 | 0.00 | 0.406 |
| Intraventricular block on EKG, % | 8.30 | 8.90 | 7.80 | 5.60 | 0.883 |
| Smoking, % | 68.00 | 68.30 | 67.00 | 70.60 | 0.947 |
| Drinking, % | 50.00 | 53.30 | 47.70 | 41.10 | 0.362 |
| Medication | |||||
| ACEI or ARB, % | 85.50 | 86.40 | 84.30 | 85.70 | 0.929 |
| Beta‐blocker, % | 84.70 | 85.90 | 83.30 | 84.90 | 0.895 |
| Diuretic, % | 77.30 | 76.70 | 78.30 | 77.00 | 0.965 |
| Spironolactone, % | 78.40 | 81.20 | 77.10 | 76.70 | 0.744 |
| Blood results | |||||
| BNP, median (IQR), pg/mL (n = 270, 90%) | 359.50 (142.50, 942.00) | 388 (152.05, 915.00) | 460.00 (159.00, 1147.00) | 178.00 (68.00, 392.50) | 0.024 |
| ALT, median (IQR), U/L | 27.5 (18, 44) | 29 (18, 44) | 25 (17, 43) | 27 (21, 38) | 0.502 |
| AST, median (IQR), U/L | 25 (20, 37.75) | 26 (20, 33) | 24 (19, 31) | 24 (21, 31) | 0.245 |
| Creatinine, median (IQR), μmol/L | 82.85 (69.73, 95.98) | 84.65 (71.23, 97.30) | 80.70 (69.20, 92.90) | 80.80 (65.10, 90.90) | 0.154 |
| Glucose, median (IQR), mmol/L | 5.67 (5.14, 7.38) | 5.63 (5.12, 6.79) | 5.88 (5.20, 7.77) | 5.73 (5.22, 7.64) | 0.248 |
| TC, mean (SD), mmol/L | 4.14 ± 1.15 | 4.14 ± 1.10 | 4.06 ± 1.25 | 4.48 ± 1.09 | 0.373 |
| TG, median (IQR), mmol/L | 1.23 (0.93, 1.83) | 1.21 (0.93, 1.70) | 1.41 (1.20, 1.70) | 0.94 (0.69, 2.32) | 0.128 |
| HDL‐C, median (IQR), mmol/L | 0.96 (0.81, 1.17) | 0.94 (0.80, 1.13) | 0.98 (0.83, 1.22) | 1.04 (0.91, 1.27) | 0.145 |
| LDL‐C, median (IQR), mmol/L | 2.50 (2.01, 3.17) | 2.51 (2.02, 3.20) | 2.46 (1.95, 3.05) | 2.79 (2.25, 3.43) | 0.334 |
| Na+, median (IQR), mmol/L | 139.30 (137.70, 141.40) | 139.45 (137.80, 141.86) | 139.30 (137.60, 141.20) | 139.10 (137.10, 140.70) | 0.314 |
| Cl−, median (IQR), mmol/L | 101.95 (99.40, 104.18) | 101.90 (99.32, 104.10) | 102.10 (99.60, 104.20) | 101.60 (98.85, 104.55) | 0.994 |
| hs‐CRP, median (IQR), mg/L | 2.13 (0.86, 5.86) | 2.42 (1.00, 5.65) | 2.12 (0.63, 7.21) | 1.43 (0.71, 2.23) | 0.277 |
| White blood cell, median (IQR), g/L | 6.90 (5.67, 8.35) | 7.03 (5.73, 8.28) | 6.78 (5.73, 8.47) | 6.49 (4.38, 8.70) | 0.620 |
| Red blood cell, median (IQR), g/L | 4.84 (4.42, 5.21) | 4.92 (4.45, 5.25) | 4.73 (4.38, 5.10) | 4.75 (4.52, 5.15) | 0.165 |
| Platelets, median (IQR), g/L | 203.00 (167.00, 253.30) | 203.00 (168.25, 256.00) | 210.00 (164.00, 256.00) | 191.00 (147.50, 231.50) | 0.348 |
| Echocardiographic parameters | |||||
| MR severity (n = 288, 96%) | |||||
| No, % | 8.00 | 5.10 | 7.40 | 11.60 | 0.246 |
| Mild, % | 45.30 | 37.80 | 40.40 | 57.90 | 0.010 |
| Moderate, % | 23.00 | 20.40 | 27.70 | 21.10 | 0.422 |
| Severe, % | 23.70 | 36.40 | 24.50 | 9.50 | 0.000 |
| PH severity (n = 288, 96%) | |||||
| No, % | 72.60 | 58.20 | 71.30 | 88.50 | 0.000 |
| Mild, % | 13.90 | 19.40 | 13.80 | 8.30 | 0.084 |
| Moderate, % | 11.50 | 18.40 | 12.80 | 3.10 | 0.003 |
| Severe, % | 2.10 | 4.10 | 2.10 | 0.00 | 0.138 |
ACEI, ACE inhibitor; ARB, angiotensin II receptor blocker; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, brain natriuretic peptide; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic pressure at presentation; HDL‐C, blood high density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C reactive protein; LBBB, left bundle branch block; LDL‐C, blood low density lipoprotein cholesterol; MR, mitral regurgitation; PH, pulmonary hypertension; RBBB, right bundle branch block; SBP, systolic pressure at presentation; TC, total cholesterol; TG, triglyceride.
Bold items indicate P‐value < 0.05.
Table 2.
CMR characteristics stratified by tertiles of LA‐εs
| Characteristics | Total | LA εs < 8.0% | LA εs8% to 15.9% | LA εs > 15.9% | P‐value |
|---|---|---|---|---|---|
| CMR‐LV functional parameters | |||||
| CMR‐LVEDV, median (IQR), mL | 241.1 (178.21, 321.14) | 242.50 (185.56, 323.29) | 229.65 (171.21, 297.02) | 282.08 (173.62, 348.66) | 0.484 |
| CMR‐LVSV, median (IQR), mL | 46.85 (36.11, 62.34) | 44.65 (34.00, 58.98) | 50.79 (36.5, 65.17) | 49.45 (42.01, 66.06) | 0.052 |
| CMR‐LVESV, median (IQR), mL | 190.24 (136.57, 268.25) | 192.37 (143.36, 273.19) | 184.48 (120.16, 246.20) | 224.09 (140.24, 284.75) | 0.293 |
| CMR‐LVEF, median (IQR), % | 19.88 (14.75, 27.40) | 18.77 (13.55, 24.91) | 22.54 (15.71, 30.70) | 22.09 (16.53, 29.28) | 0.009 |
| CMR‐LVMASS, median (IQR), g | 131.58 (107.03, 165.75) | 136.44 (110.00, 168.89) | 129.69 (105.29, 166.47) | 118.70 (106.86, 153.15) | 0.327 |
| CMR‐LVPGLS, median (IQR), % | −5.40 (−7.32, −3.60) | −4.70 (−6.48, −3.20) | −6.30 (−8.77, −4.37) | −6.80 (−8.20, −6.22) | 0.000 |
| CMR‐LVPGCS, median (IQR), % | −6.56 (−8.99, −4.6) | −6.00 (−8.32, −4.21) | −7.83 (−10.42, −5.10) | −6.70 (−8.25, −4.75) | 0.001 |
| CMR‐LA functional parameters | |||||
| CMR‐LAVmax, median (IQR), mL | 84.67 (60.48, 111.76) | 95.39 (64.96, 119.34) | 73.36 (56.15, 95.93) | 78.60 (57.76, 106.81) | 0.000 |
| CMR‐LAVmin, median (IQR), mL | 51.00 (31.40, 79.01) | 61.43 (35.80, 87.88) | 40.62 (27.21, 62.8) | 32.88 (26.00, 63.75) | 0.000 |
| CMR‐LAEF, median (IQR), % | 37.72 (24.99, 50.31) | 30.74 (22.46, 46.11) | 45.62 (36.21, 53.79) | 48.51 (34.93, 57.12) | 0.000 |
| CMR‐LA‐εs, median (IQR), % | 7.10 (4.16, 10.19) | 4.95 (2.73, 6.64) | 10.55 (9.00, 12.50) | 22.40 (17.63, 24.50) | 0.000 |
| CMR‐LA‐εe, median (IQR), % | 3.00 (1.35, 4.59) | 1.83 (0.86, 3.03) | 4.85 (3.65, 6.25) | 8.05 (4.50, 12.10) | 0.000 |
| CMR‐LA‐εa, median (IQR), % | 3.65 (2.21, 5.78) | 2.60 (1.40, 3.55) | 5.90 (4.90, 6.90) | 12.90 (9.65, 17.58) | 0.000 |
| CMR‐LA‐SRs, median (IQR), s | 4.00 (2.05, 5.74) | 2.65 (1.55, 4.14) | 6.35 (4.95, 7.50) | 9.65 (2.08, 13.75) | 0.000 |
| CMR‐LA‐SRe, median (IQR), s | −0.68 (−0.95, −0.4) | −0.60 (−0.90, −0.35) | −0.75 (−1.00, −0.50) | −0.95 (−1.10, −0.63) | 0.004 |
| CMR‐LA‐SRa, median (IQR), s | −0.80 (−1.40, −0.45) | −0.68 (−1.15, −0.35) | −1.05 (−1.60, −0.65) | −1.55 (−2.03, −0.90) | 0.000 |
| CMR‐RA functional parameters | |||||
| CMR‐RAVmax, median (IQR), mL | 54.07 (40.04, 78.89) | 57.83 (41.40, 86.10) | 49.92 (37.92, 71.73) | 51.35 (44.39, 73.68) | 0.038 |
| CMR‐RAVmin, median (IQR), mL | 29.05 (17.42, 44.14) | 32.42 (22.10, 50.00) | 23.00 (15.25, 36.52) | 18.50 (15.40, 38.89) | 0.000 |
| CMR‐RAEF, mean (SD), % | 46.79 ± 15.01 | 42.98 ± 14.84 | 52.26 ± 13.23 | 54.99 ± 14.23 | 0.000 |
| CMR‐RA‐εs, median (IQR), % | 15.25 (9.00, 21.23) | 11.00 (6.25, 14.45) | 22.50 (19.60, 26.60) | 39.00 (34.80, 46.70) | 0.000 |
| CMR‐RA‐εe, median (IQR), % | 6.90 (4.00, 4.90) | 4.90 (2.53, 6.80) | 11.70 (8.80, 13.50) | 20.40 (14.55, 28.65) | 0.000 |
| CMR‐RA‐εa, median (IQR), % | 7.55 (3.80, 11.075) | 4.90 (2.70, 7.80) | 11.90 (9.40, 14.50) | 18.80 (8.85, 26.95) | 0.000 |
| CMR‐RA‐SRs, median (IQR), s | 1.00 (0.70, 1.50) | 0.80 (0.60, 1.10) | 1.40 (1.10, 1.80) | 2.10 (1.55, 3.90) | 0.000 |
| CMR‐RA‐SRe, median (IQR), s | −0.80 (−1.10, −0.50) | −0.60 (−0.80, −0.40) | −1.10 (−1.40, −0.80) | −1.90 (−3.30, −1.30) | 0.000 |
| CMR‐RA‐SRa, median (IQR), s | −1.10 (−1.50, −0.63) | −0.80 (−1.20, −0.50) | −1.40 (−1.80, −1.00) | −2.10 (−2.70, −1.15) | 0.000 |
| CMR‐LGE | |||||
| LGE present, % | 67.70 | 63.30 | 75.30 | 64.70 | 0.012 |
| LGE extent, median (IQR), % | 6.85 (0.00, 22.49) | 4.60 (0, 22.58) | 10.00 (2.10, 23.50) | 4.80 (0.00, 19.83) | 0.296 |
| ICM, % | 43.70 | 37.20 | 55.30 | 41.20 | 0.097 |
CMR, cardiac magnetic resonance; ICM, ischaemic cardiomyopathy; LAVmin, minimum left atrial volume; LAEF, left atrial ejection fraction; LGE, late gadolinium enhancementLVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; LVSV, left ventricular stroke volume; LVEF, left ventricular ejection fraction; LVMASS, left ventricular mass; LVpGLS, left ventricular peak global longitudinal strain; LVpGCS, left ventricular peak global circumferential strain; LAVmax, maximum left atrial volume; RAEF, right atrial ejection fraction; RAVmax, maximum right atrial volume; RAVmin, minimum right atrial volume; SRe, peak early negative strain rate; SRa, peak late negative strain rate; SRs, peak positive strain rate; εs, reservoir strain; εe, conduit strain; εa, booster strain.
Bold items indicate P‐value < 0.05.
Inter‐observer and intra‐observer variability
The reproducibility of atrial strain and SR parameters was good (ICC = 0.83–0.98, P < 0.05) (Supporting Information, Table S2 ). And the reliability of the strain indexes of LA was higher than the strain and SR of the RA.
Primary outcomes
A total of 94 patients experienced MACEs, including 42 with ICM and 52 with NIDCM. The median follow‐up time was 3.84 (IQR: 2.4–5.4) years.
Univariate and multivariate analysis and incremental prognostic value
Supporting Information, Table S3 summarizes the results of the univariate analysis for all patients. AUC was highest for LA‐εs (0.765), followed by LA‐εe (0.753) and LA‐SRs (0.751) (Figure 2 ). The results of correlation analysis are shown in Supporting Information, Table S4 . We included the covariates in Table 3 in the Cox regression model based on their clinical value, previous references, and correlation between parameters. After adjustment for clinical and radiographic risk factors, LA‐εs remained a significant independent predictor of endpoint events (HR = 0.87/%; P < 0.001). Additionally, for each 1% decrease in LA‐εs, the risk of MACEs increased by 13% (Table 3 ). Meanwhile, LGE extent (HR = 1.04/%; P < 0.001) and LA ejection fraction (LAEF) (HR = 1.03/%; P < 0.001) is significantly associated with MACEs. The addition of LA‐εs to the model with clinical and radiographic predictors resulted in a significant increase in C statistic from 0.776 to 0.801 (P < 0.0001) and a combined differential improvement of 0.261 (95% CI: 0.0722–0.1301) compared with the original model with an NRI of 0.075 (95% CI: 0.0262–0.1301).
Figure 2.
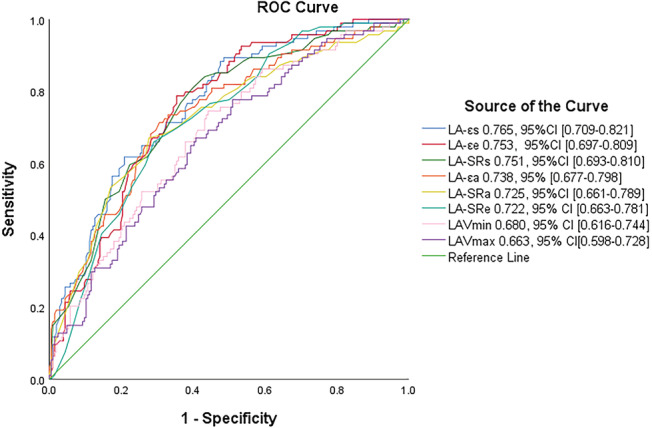
ROC curve. The ROC curve for the overall performance of left atrial indexes for MACEs.
Table 3.
Multivariate Cox proportional hazards model analysis for predicting overall survival in patients with HFrEF but without AF
| HR | 95% CI | P‐value | |
|---|---|---|---|
| Age, years | 1.02 | (1.00–1.04) | 0.041 |
| Male, % | 1.07 | (0.57–2.03) | 0.828 |
| BMI, kg/m2 | 0.99 | (0.93–1.06) | 0.861 |
| SBP, mmHg | 0.99 | (0.97–1.01) | 0.172 |
| QRS duration, ms | 1.01 | (1.00–1.02) | 0.050 |
| Diabetes mellitus, % | 0.99 | (0.60–1.65) | 0.971 |
| Medication: ACEI/ARB | 0.96 | (0.50–1.82) | 0.889 |
| BNP, pg/mL | 1.00 | (1.00–1.00) | 0.039 |
| ALT, U/L | 1.00 | (1.00–1.00) | 0.084 |
| Creatinine, μmol/L | 1.00 | (0.99–1.01) | 0.682 |
| Cl−, mmol/L | 1.00 | (1.00–1.01) | 0.177 |
| Red blood cell, g/L | 0.67 | (0.49–0.92) | 0.012 |
| CMR‐LVEDV, mL | 1.00 | (1.00–1.00) | 0.521 |
| CMR‐LVPGLS, % | 0.92 | (0.83–1.03) | 0.149 |
| CMR‐LVPGCS, % | 0.98 | (0.89–1.09) | 0.718 |
| CMR‐LAVmax, mL | 1.01 | (1.00–1.01) | 0.086 |
| CMR‐LAEF, % | 1.03 | (1.01–1.06) | 0.007 |
| CMR‐LA‐εs, % | 0.87 | (0.82–0.92) | 0.000 |
| CMR‐RAVmax, mL | 1.01 | (1.00–1.01) | 0.083 |
| LGE extent, % | 1.04 | (1.02–1.05) | 0.000 |
ACEI, ACE inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement LVEDV, left ventricular end‐diastolic volume; LVpGLS, left ventricular peak global longitudinal strain; LVpGCS, left ventricular peak global circumferential strain; LAVmax, maximum left atrial volume; LAEF, left atrial ejection fraction; RAVmax, maximum right atrial volume; SBP, systolic pressure at presentation; εs, reservoir strain.
Bold items indicate P‐value < 0.05.
Outcomes stratified by left atrial‐εs and late gadolinium enhancement
LA‐εs was divided into three groups according to the tertile method (<8, 8–15.9, and >15.9). Kaplan–Meier survival analysis (Figure 3A ) showed a significant difference between patients stratified by LA‐εs values; as the LA‐εs tertile worsened, the risk of MACE increased significantly (log‐rank P < 0.0001). According to Kaplan–Meier analysis, the presence of LGE was significantly associated with an increased risk of MACEs (log‐rank P = 0.0001) (Figure 4A ). Kaplan–Meier analysis stratified by the highest and lowest LA‐εs tertile for those with LGE and without LGE showed that patients in the worst LA‐εs tertile faced a significantly increased risk of MACEs irrespective of LGE (log‐rank P < 0.0001) (Figure 4B ).
Figure 3.
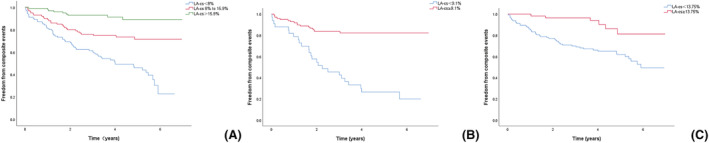
Kaplan–Meier survival curves. (A) Stratified by tertiles of LA‐εs in all patients. (B) Stratified by dichotomous of LA‐εs in ICM. (C) Stratified by dichotomous of LA‐εs in NIDCM.
Figure 4.
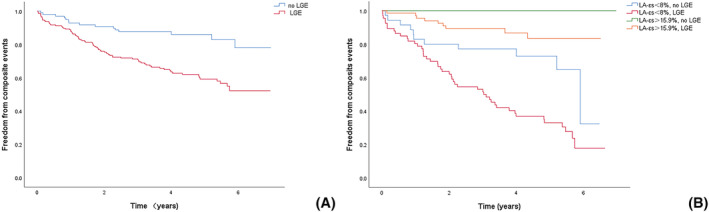
Kaplan–Meier survival curves. (A) Stratified by LGE present in all patients. (B) Stratified by tertiles of LA‐εs and LGE present in all patients.
Prognostic value of left atrial‐εs in ischaemic and non‐ischaemic dilated cardiomyopathy
In both ICM and NIDCM, the dichotomization of LA‐εs values was assessed by Kaplan–Meier survival analysis (Figure 3B, C ), which showed a significant difference between the two groups of patients. In both ICM and NIDCM patients, LA‐εs was significantly associated with the risk of MACEs after adjusting for clinical and radiographic risk factors (ICM: HR = 0.94/%; P = 0.002; NIDCM: HR = 0.88/%; P = 0.001) (Table 4 ). Subsequently, LA‐εs remained a critical, independent predictor of the occurrence of MACEs when LGE presence was used instead of extent (Table 4 ).
Table 4.
Multivariate Cox proportional hazards model analysis for predicting overall survival in patients with ICM or NIDCM
| Multivariable model using LGE extent | Multivariable model using LGE present | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICM | NIDCM | ICM | NIDCM | |||||||||
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age, years | 1.03 | (0.96–1.03) | 0.808 | 1.04 | (1.01–1.07) | 0.016 | 1.00 | (0.97–1.04) | 0.953 | 1.04 | (1.01–1.07) | 0.013 |
| Male, % | 2.91 | (0.18–2.91) | 0.648 | 1.44 | (0.60–3.47) | 0.416 | 0.55 | (0.16–1.96) | 0.358 | 1.64 | (0.65–4.13) | 0.298 |
| BMI, kg/m2 | 1.04 | (0.81–1.04) | 0.160 | 1.02 | (0.95–1.11) | 0.575 | 0.91 | (0.82–1.02) | 0.122 | 1.04 | (0.96–1.12) | 0.343 |
| SBP, mmHg | 1.06 | (0.98–1.06) | 0.364 | 0.98 | (0.96–1.01) | 0.154 | 1.00 | (0.97–1.04) | 0.985 | 0.99 | (0.96–1.01) | 0.161 |
| QRS duration, ms | 1.03 | (1.00–1.03) | 0.036 | 1.00 | (0.99–1.02) | 0.716 | 1.02 | (1.01–1.03) | 0.005 | 1.00 | (0.99–1.02) | 0.778 |
| Diabetes mellitus, % | 2.38 | (0.43–2.38) | 0.976 | 0.98 | (0.40–2.40) | 0.960 | 0.87 | (0.38–1.96) | 0.733 | 0.67 | (0.25–1.79) | 0.422 |
| Medication: ACEI/ARB | 2.64 | (0.29–2.64) | 0.809 | 0.78 | (0.33–1.84) | 0.563 | 0.75 | (0.25–2.29) | 0.612 | 0.67 | (0.29–1.54) | 0.343 |
| BNP, pg/mL | 1.00 | (1.00–1.00) | 0.075 | 1.00 | (1.00–1.00) | 0.330 | 1.00 | (1.00–1.00) | 0.049 | 1.00 | (1.00–1.00) | 0.321 |
| ALT, U/L | 1.02 | (1.00–1.02) | 0.140 | 1.00 | (1.00–1.00) | 0.162 | 1.01 | (1.00–1.02) | 0.065 | 1.00 | (1.00–1.00) | 0.043 |
| Creatinine, μmol/L | 1.02 | (0.99–1.02) | 0.972 | 1.00 | (0.99–1.01) | 0.965 | 1.00 | (0.99–1.02) | 0.963 | 1.00 | (0.99–1.01) | 0.988 |
| Cl−, mmol/L | 1.16 | (0.91–1.16) | 0.713 | 1.00 | (1.00–1.01) | 0.307 | 1.02 | (0.91–1.15) | 0.708 | 1.00 | (1.00–1.01) | 0.051 |
| Red blood cell, g/L | 1.19 | (0.18–1.19) | 0.109 | 0.68 | (0.46–0.99) | 0.043 | 0.42 | (0.17–1.01) | 0.052 | 0.79 | (0.55–1.13) | 0.201 |
| CMR‐LVEDV, mL | 1.01 | (0.99–1.01) | 0.826 | 1.00 | (1.00–1.01) | 0.405 | 1.00 | (0.99–1.00) | 0.556 | 1.00 | (1.00–1.01) | 0.571 |
| CMR‐LVPGLS, % | 1.34 | (0.87–1.34) | 0.502 | 0.88 | (0.77–1.01) | 0.077 | 1.06 | (0.86–1.31) | 0.596 | 0.91 | (0.79–1.05) | 0.204 |
| CMR‐LVPGCS, % | 1.13 | (0.78–1.13) | 0.473 | 0.99 | (0.87–1.12) | 0.836 | 0.98 | (0.81–1.18) | 0.801 | 0.96 | (0.85–1.08) | 0.460 |
| CMR‐LAVmax, mL | 1.02 | (0.99–1.02) | 0.693 | 1.01 | (1.00–1.02) | 0.229 | 1.00 | (0.99–1.02) | 0.547 | 1.01 | (1.00–1.01) | 0.296 |
| CMR‐LAEF, % | 1.09 | (1.00–1.09) | 0.036 | 1.02 | (0.99–1.06) | 0.177 | 1.04 | (1.00–1.08) | 0.073 | 1.01 | (0.98–1.05) | 0.482 |
| CMR‐LA‐εs, % | 0.94 | (0.78–0.94) | 0.002 | 0.88 | (0.81–0.95) | 0.001 | 0.84 | (0.76–0.92) | 0.000 | 0.89 | (0.83–0.96) | 0.002 |
| CMR‐RAVmax, mL | 1.02 | (0.99–1.02) | 0.398 | 1.01 | (1.00–1.02) | 0.242 | 1.01 | (0.99–1.02) | 0.515 | 1.00 | (0.99–1.01) | 0.400 |
| LGE extent, % | 1.07 | (1.01–1.07) | 0.005 | 1.04 | (1.01–1.06) | 0.001 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| LGE present, % | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | 3.24 | (1.51–6.95) | 0.003 |
ACEI, ACE inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; CMR, cardiac magnetic resonance; LAEF, left atrial ejection fraction; LGE, late gadolinium enhancement; LAVmax, maximum left atrial volume; LVEDV, left ventricular end‐diastolic volume; LVpGCS, left ventricular peak global circumferential strain; LVpGLS, left ventricular peak global longitudinal strain; RAVmax, maximum right atrial volume; SBP, systolic pressure at presentation; εs, reservoir strain.
Bold items indicate P‐value < 0.05.
Discussion
The primary findings of our study were as follows: (i) patients with HFrEF and without AF had reduced reservoir, conduit, and booster deformation indexes (strain and SR) in LA and RA. LA‐εs was closely related to LA‐εe, LA‐εa, LA‐SRs, and LA‐SRe (r > 0.5). (ii) LA‐εs, LAEF, and LGE extent were independent predictors of MACEs, and LA‐εs increased the incremental predictive value of common clinical and CMR imaging risk factors for outcomes in patients with HFrEF. (iii) LA‐εs was an independent predictor of outcome in ICM and NIDCM, respectively, even after adjusting for clinical, laboratory, and CMR variables.
The atrium is a container for storing blood and has been under intensive focus for its role in cardiac function. During the cardiac cycle, it has three main functions: (i) the atrial storage function of pulmonary venous blood flow during ventricular systole; (ii) the conduit function of delivering blood flow into the ventricle during early diastole; (iii) the systolic function of pumping the remaining blood into the ventricle by end‐diastole auto‐systole. 22 Impaired atrial function indicates an elevated ventricular filling pressures, and the diastolic function of the ventricles determines the degree of relative atrial contribution to ventricular filling. 23 Previous studies suggested that abnormal LA function in the early stages of HF is an early indicator of LV diastolic dysfunction. 24 , 25 Depletion of compensatory mechanisms translates into overt HF or deteriorated clinical condition. 26 In the advanced stage of HF, LA is enlarged and remodelled to varying degrees with impaired function, which is usually associated with chronic and severe symptoms. 27 , 28 With the progression of HF, atrial dysfunction may be a late consequence of systemic and pulmonary circulatory changes caused by HF. Melenovsky et al. 29 demonstrated that HFrEF is characterized by significant eccentric LA remodelling. The degree of LA deformation is of great value for the prognostic evaluation of patients with HFrEF. The measurement of LA strain by speckle tracking is challenging due to the low signal‐to‐noise ratio and a thin atrial wall. CMR has a high spatial resolution and enables rapid and reliable quantification of LA functional parameters with short measurement times and high reproducibility. Leng et al.'s prospective study showed that LA strain measured using CMR can be used as a predictor of long‐term outcome in ST‐elevation myocardial infarction (STEMI), thus providing additional prognostic information for the established predictors of STEMI. 24 Cojan‐Minzat et al. reported that LA total strain had independent predictive value for incremental outcome in NIDCM. 11 Dick et al. assessed the diagnostic value of LA and RA strain parameters for acute myocarditis and demonstrated that LA SRe is the best predictor of condition. 20 To the best of our knowledge, this is the first study to analyse the long‐term prognostic value of atrial indexes obtained by CMR in patients with ICM and NIDCM of HFrEF without AF, with a median follow‐up of up to 3.84 years. Herein, we showed that LA strain provides additional prognostic information beyond clinical and CMR parameter models in both categories of patients. LAEF was significantly associated with increased filling pressure of LV. 30 As reported by Pellicori et al., in patients with HF, LAEF predicts the adverse outcomes independently of other measures of cardiac dysfunction. 31 The aforementioned conclusions were verified in our study too. When the culprit vessel of ICM causes the three‐vessel coronary artery disease and the symptoms of HF are distinct, the cavity will enlarge and the overall movement of the wall will be weakened, which can be easily misdiagnosed as DCM; thus, the distribution of LGE is the key differential point between the two phenomena. Previous studies have shown that the presence and extent of LGE can predict the prognosis of patients with HF caused by ICM and DCM and is an independent predictor of death 32 , 33 ; these findings were confirmed in the current study. In patients with and without LGE, the grouping of LA‐εs is valuable for the evaluation of prognostic risk stratification. Romano et al. 32 demonstrated that LV longitudinal strain is an independent predictor of death in patients with ICM or DCM. In this study, the corresponding parameters of the LV did not show independent prognostic value in multivariate Cox analysis. This could be because for patients with advanced HF, the degree of impaired LV systolic function loses its ability for survival prediction. HF can lead to secondary MR and PH. 34 MR and PH have previously been reported in the literature to be associated with the prognosis in patients with HF. 35 However, our results showed that the degrees of MR, PH, and LA‐εs were weakly correlated. This correlation could be attributed to the relationship between the severity of HF in our included patients, and the presence of some MR and PH in the three groups of patients categorized by LA‐εs. In the subsequent research, we should collect HF with preserved ejection and mildly reduced ejection to verify this aspect. Nonetheless, some patients with advanced HF can benefit from advanced treatments, such as heart transplantation and LV assist device implantation. 36 The present study has potential clinical implications in the use of LA strains to reclassify such patient populations that would help in making precise HF management decisions in the future.
Limitations
Nevertheless, the present study has some limitations. First, the proportion of men was relatively high. We grouped the baseline characteristics (clinical and CMR characteristics, including LA εs) by sex and showed statistically significant differences for the six parameters of left bundle branch block, intraventricular block, smoking, drinking, CMR‐RA‐εe, and CMR‐RA‐SRa (Supporting Information, Table S5 ). Inclusion of the aforementioned variables in the univariate analysis showed that they were not significantly associated with the outcome. Sex was not statistically significant in univariate analysis. Furthermore, we included the sex indicator in the multivariate Cox model, and after multivariate adjustment, the results showed that there was no significant association between sex and the outcome. We tried to reduce the impact of selection bias via the aforementioned work. Second, this was a single‐centre study, and the number of patients was limited, which might affect the accuracy of the results. Third, collecting clinical data was not comprehensive. For example, 10% of patients had missing data for BNP. We input the missing data using statistical methods for multiple interpolation and confirmed our hypothesis of the missing data pattern by demonstrating that BNP was an independent predictor of MACEs before and after data input (P < 0.05). In addition, similar to ultrasound tracking techniques, 37 algorithmic differences were detected between different CMR FT software. Further investigation of the applicability of other FT vendors is essential. Finally, our data were all derived from 3 T magnetic resonance, and in subsequent studies, we intend to use 1.5 T magnetic resonance to verify our results, thereby enhancing the generalizability of the conclusions.
Conclusion
LA strain is a robust predictor of MACEs in patients with ischaemic and non‐ischaemic dilated cardiomyopathy, independent of common clinical and CMR imaging markers. In HFrEF, LA‐εs adds incremental prognostic information on outcomes from common clinical and CMR risk factors (including LGE). The current findings may exert a significant impact on management decisions based on risk stratification in these individuals and contribute to the dynamic assessment of prognosis in patients with HFrEF.
Funding
This study was supported by grants from the National Natural Science Foundation of China (U1908211) and the Capital's Funds for Health Improvement and Research Foundation of China (2020‐1‐1052).
Conflict of interest
Kairui Bo, Yifeng Gao, Xuelian Gao, Zhen Zhou, Tong Liu, Hongkai Zhang, Qing Li, Hui Wang, and Lei Xu declare that they have no conflict of interest.
Supporting information
Table S1. Baseline characteristics stratified by ICM.
Table S2. Reproducibility of atrial strain parameter measurements.
Table S3. Univariate analysis of major adverse events in 5‐year follow‐up of patients with ICM or NIDCM.
Table S4. The correlations between covariates.
Table S5. Baseline characteristics stratified by sex.
Bo, K. , Gao, Y. , Zhou, Z. , Gao, X. , Liu, T. , Zhang, H. , Li, Q. , Wang, H. , and Xu, L. (2022) Incremental prognostic value of left atrial strain in patients with heart failure. ESC Heart Failure, 9: 3942–3953. 10.1002/ehf2.14106.
Lei Xu and Hui Wang have contributed equally to this work and share corresponding authorship.
Contributor Information
Hui Wang, Email: hugeren@126.com.
Lei Xu, Email: leixu2001@hotmail.com.
References
- 1. Bauersachs J, de Boer RA, Lindenfeld J, Bozkurt B. The year in cardiovascular medicine 2021: Heart failure and cardiomyopathies. Eur Heart J. 2022; 43: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the heart failure association (HFA) of the ESC. Rev Esp Cardiol (Engl Ed). 2022; 75: 523. [DOI] [PubMed] [Google Scholar]
- 3. Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta‐analysis global Group in Chronic (MAGGIC) heart failure risk score: Validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018; 7: e009594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson JL, Horne BD, Pennell DJ. Atrial dimensions in health and left ventricular disease using cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2005; 7: 671–675. [DOI] [PubMed] [Google Scholar]
- 5. Liu T, Ma X, Liu W, Ling S, Zhao L, Xu L, Song D, Liu J, Sun Z, Fan Z, Luo T, Kang J, Liu X, Dong J. Late gadolinium enhancement amount as an independent risk factor for the incidence of adverse cardiovascular events in patients with stage C or D heart failure. Front Physiol. 2016; 7: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu T, Gao Y, Wang H, Zhou Z, Wang R, Chang SS, Liu Y, Sun Y, Rui H, Yang G, Firmin D, Dong J, Xu L. Association between right ventricular strain and outcomes in patients with dilated cardiomyopathy. Heart. 2020; 107: 1233–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cameli M, Lisi M, Mondillo S, Padeletti M, Ballo P, Tsioulpas C, Bernazzali S, Maccherini M. Left atrial longitudinal strain by speckle tracking echocardiography correlates well with left ventricular filling pressures in patients with heart failure. Cardiovasc Ultrasound. 2010; 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jain S, Kuriakose D, Edelstein I, Ansari B, Oldland G, Gaddam S, Javaid K, Manaktala P, Lee J, Miller R, Akers SR, Chirinos JA. Right atrial phasic function in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging. 2019; 12: 1460–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gulati A, Ismail TF, Jabbour A, Ismail NA, Morarji K, Ali A, Raza S, Khwaja J, Brown TD, Liodakis E, Baksi AJ, Shakur R, Guha K, Roughton M, Wage R, Cook SA, Alpendurada F, Assomull RG, Mohiaddin RH, Cowie MR, Pennell DJ, Prasad SK. Clinical utility and prognostic value of left atrial volume assessment by cardiovascular magnetic resonance in non‐ischaemic dilated cardiomyopathy. Eur J Heart Fail. 2013; 15: 660–670. [DOI] [PubMed] [Google Scholar]
- 10. Modin D, Sengeløv M, Jørgensen PG, Olsen FJ, Bruun NE, Fritz‐Hansen T, Andersen DM, Jensen JS, Biering‐Sørensen T. Prognostic value of left atrial functional measures in heart failure with reduced ejection fraction. J Card Fail. 2019; 25: 87–96. [DOI] [PubMed] [Google Scholar]
- 11. Cojan‐Minzat BO, Zlibut A, Muresan ID, Orzan RI, Cionca C, Horvat D, David L, Visan AC, Florea M, Agoston‐Coldea L. Left atrial geometry and phasic function determined by cardiac magnetic resonance are independent predictors for outcome in non‐Ischaemic dilated cardiomyopathy. Biomedicine. 2021; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002; 39: 210–218. [DOI] [PubMed] [Google Scholar]
- 13. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non‐dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2016; 37: 1850–1858. [DOI] [PubMed] [Google Scholar]
- 14. Martinez YV, Benett I, Lewington AJP, Wierzbicki AS. Chronic kidney disease: Summary of updated NICE guidance. BMJ. 2021; 374: n1992. [DOI] [PubMed] [Google Scholar]
- 15. Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013; 14: 611–644. [DOI] [PubMed] [Google Scholar]
- 16. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Rev Esp Cardiol (Engl Ed). 2015; 69: 177. [DOI] [PubMed] [Google Scholar]
- 17. Fisher MR, Forfia PR, Chamera E, Housten‐Harris T, Champion HC, Girgis RE, Corretti MC, Hassoun PM. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009; 179: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013; 15: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kowallick JT, Kutty S, Edelmann F, Chiribiri A, Villa A, Steinmetz M, Sohns JM, Staab W, Bettencourt N, Unterberg‐Buchwald C, Hasenfuß G, Lotz J, Schuster A. Quantification of left atrial strain and strain rate using cardiovascular magnetic resonance myocardial feature tracking: A feasibility study. J Cardiovasc Magn Reson. 2014; 16: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dick A, Schmidt B, Michels G, Bunck AC, Maintz D, Baessler B. Left and right atrial feature tracking in acute myocarditis: A feasibility study. Eur J Radiol. 2017; 89: 72–80. [DOI] [PubMed] [Google Scholar]
- 21. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008; 27: 157–172 discussion 207‐12. [DOI] [PubMed] [Google Scholar]
- 22. Kebed KY, Addetia K, Lang RM. Importance of the left atrium: More than a bystander? Heart Fail Clin. 2019; 15: 191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. Am J Cardiol. 1998; 82: 756–761. [DOI] [PubMed] [Google Scholar]
- 24. Leng S, Ge H, He J, Kong L, Yang Y, Yan F, Xiu J, Shan P, Zhao S, Tan RS, Zhao X, Koh AS, Allen JC, Hausenloy DJ, Mintz GS, Zhong L, Pu J. Long‐term prognostic value of cardiac MRI left atrial strain in ST‐segment elevation myocardial infarction. Radiology. 2020; 296: 299–309. [DOI] [PubMed] [Google Scholar]
- 25. Kowallick JT, Lotz J, Hasenfuß G, Schuster A. Left atrial physiology and pathophysiology: Role of deformation imaging. World J Cardiol. 2015; 7: 299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bandera F, Mollo A, Frigelli M, Guglielmi G, Ventrella N, Pastore MC, Cameli M, Guazzi M. Cardiac imaging for the assessment of left atrial mechanics across heart failure stages. Front Cardiovasc Med. 2021; 8: 750139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leite L, Mendes SL, Baptista R, Teixeira R, Oliveira‐Santos M, Ribeiro N, Coutinho R, Monteiro V, Martins R, Castro G, Ferreira MJ, Pego M. Left atrial mechanics strongly predict functional capacity assessed by cardiopulmonary exercise testing in subjects without structural heart disease. Int J Cardiovasc Imaging. 2017; 33: 635–642. [DOI] [PubMed] [Google Scholar]
- 28. Rosca M, Lancellotti P, Popescu BA, Piérard LA. Left atrial function: Pathophysiology, echocardiographic assessment, and clinical applications. Heart. 2011; 97: 1982–1989. [DOI] [PubMed] [Google Scholar]
- 29. Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circ Heart Fail. 2015; 8: 295–303. [DOI] [PubMed] [Google Scholar]
- 30. Posina K, McLaughlin J, Rhee P, Li L, Cheng J, Schapiro W, Gulotta RJ, Berke AD, Petrossian GA, Reichek N, Cao JJ. Relationship of phasic left atrial volume and emptying function to left ventricular filling pressure: A cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2013; 15: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pellicori P, Zhang J, Lukaschuk E, Joseph AC, Bourantas CV, Loh H, Bragadeesh T, Clark AL, Cleland JG. Left atrial function measured by cardiac magnetic resonance imaging in patients with heart failure: Clinical associations and prognostic value. Eur Heart J. 2015; 36: 733–742. [DOI] [PubMed] [Google Scholar]
- 32. Romano S, Judd RM, Kim RJ, Kim HW, Klem I, Heitner JF, Shah DJ, Jue J, White BE, Indorkar R, Shenoy C, Farzaneh‐Far A. Feature‐tracking global longitudinal strain predicts death in a multicenter population of patients with ischemic and nonischemic dilated cardiomyopathy incremental to ejection fraction and late gadolinium enhancement. JACC Cardiovasc Imaging. 2018; 11: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El Aidi H, Adams A, Moons KG, Den Ruijter HM, Mali WP, Doevendans PA, Nagel E, Schalla S, Bots ML, Leiner T. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: A systematic review of prognostic studies. J Am Coll Cardiol. 2014; 63: 1031–1045. [DOI] [PubMed] [Google Scholar]
- 34. Malagoli A, Rossi L, Zanni A, Sticozzi C, Piepoli MF, Benfari G. Quantified mitral regurgitation and left atrial function in heart failure with reduced ejection fraction: Interplay and outcome implications. Eur J Heart Fail. 2022; 24: 694–702. [DOI] [PubMed] [Google Scholar]
- 35. Damy T, Goode KM, Kallvikbacka‐Bennett A, Lewinter C, Hobkirk J, Nikitin NP, Dubois‐Randé JL, Hittinger L, Clark AL, Cleland JG. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J. 2010; 31: 2280–2290. [DOI] [PubMed] [Google Scholar]
- 36. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, Barrabés JA, Boriani G, Braunschweig F, Brignole M, Burri H, Coats AJS, Deharo JC, Delgado V, Diller GP, Israel CW, Keren A, Knops RE, Kotecha D, Leclercq C, Merkely B, Starck C, Thylén I, Tolosana JM. ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J. 2021; 42: 3427–3520.34455430 [Google Scholar]
- 37. Pedrizzetti G, Claus P, Kilner PJ, Nagel E. Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson. 2016; 18: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics stratified by ICM.
Table S2. Reproducibility of atrial strain parameter measurements.
Table S3. Univariate analysis of major adverse events in 5‐year follow‐up of patients with ICM or NIDCM.
Table S4. The correlations between covariates.
Table S5. Baseline characteristics stratified by sex.


