Abstract
During the coronavirus disease 2019 (COVID‐19) pandemic, it has become difficult to provide centre‐based cardiac rehabilitation for heart failure patients. Digital therapeutics is a novel concept proposed in recent years that refers to the use of evidence‐based therapeutic interventions driven by high‐quality software programs to treat, manage, or prevent a medical condition. However, little is known about the use of this technology in heart failure patients. This study aims to explore the safety and efficacy of digital therapeutics‐based cardiac rehabilitation in heart failure patients and to provide new insights into a new cardiac rehabilitation model during the COVID‐19 era. To identify technologies related to digital therapeutics, such as the use of medical applications, wearable devices, and the Internet, all relevant studies published on PubMed, EMBASE, Cochrane database, and China National Knowledge Internet were searched from the time the database was established until October 2021. The PEDro was used to assess the quality of included studies. We ultimately identified five studies, which included 1119 patients. The mean age was 66.37, the mean BMI was 25.9, and the NYHA classification ranged from I to III (I = 232, II = 157, III = 209). The mean 6‐min walk distance was 397.7 m. The PEDro scores included in the study ranged from 4 to 8, with a mean of 5.8. Exercise training was performed in four studies, and psychological interventions were conducted in three studies. No death or serious adverse events were observed. Adherence was reported in three studies, and all exceeded 85%. The results of most studies showed that digital therapeutics‐based cardiac rehabilitation significantly increases exercise capacity and quality of life in heart failure patients. Overall, although this study suggests that digital therapeutics‐based cardiac rehabilitation may be a viable intervention for heart failure patients during the COVID‐19 era, the efficacy of this new model in routine clinical practice needs to be further validated in a large clinical trial.
Keywords: Heart failure, Digital therapeutics, Cardiac rehabilitation, COVID‐19
Introduction
Heart failure (HF) is the terminal stage of various cardiovascular diseases. Despite advanced interventions such as pharmacological treatment, cardiac synchronous treatment, and heart transplantation, HF patients still have a poor quality of life (QoL) and low survival rates. 1 The overall survival rates were 81.3, 51.5, and 29.5% at 1, 5, and 10 years, respectively. 2 Previous research studies showed that the readmission rate of HF patients within 1 year was 30.9, 28.4, and 24.3% in HF with reduced ejection fraction, HF mid‐range ejection fraction, and HF with preserved ejection fraction, respectively, which was not only a sign of poor prognosis but also brings a heavy financial burden to the family and society. 3 How to enhance the comprehensive management of HF patients has become an urgent issue.
Cardiac rehabilitation (CR) is an effective treatment that promotes exercise capacity, reduces cardiovascular risk, and improves health‐related QoL. 4 The core components of guideline‐directed therapy in CR included baseline assessment, nutritional counselling, risk factor modification psychosocial interventions, physical activity guidance, and exercise training. 5 In recent years, there have been systematic reviews and meta‐analyses demonstrating the benefits of CR for HF patients, and this evidence has been incorporated into the latest clinical guidelines. 6 , 7 , 8 The recently published ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease provided the optimal exercise training prescription for patients with chronic HF, including aerobic exercise and resistance exercise. 9 Current evidence suggests that participation in CR can reduce mortality and morbidity in HF patients by approximately 25%, yet unfortunately CR utilization is low worldwide. 10 Challenges associated with CR compliance include chronic fatigue and shortness of breath; ageing; gender, especially females; more co‐morbidities; cognitive impairment; depression; economic conditions and regional traffic restrictions; limited health insurance; and many other factors. 11
Traditionally, the model of CR started as acute inpatient rehabilitation and shifted from hospital‐based to community‐based and home‐based CR. However, the COVID‐19 pandemic has become normal and put the viability of centre‐based CR in serious jeopardy. The model of CR has gradually transitioned from centre‐based CR to home‐based CR, as for the hospital‐to‐home model (H‐to‐H model). Multiple studies have demonstrated that home‐based and centre‐based models of CR are equally effective in improving clinical symptoms, health‐related QoL, reducing mortality, and readmission rates in patients with cardiovascular disease. 12 , 13 Home‐based CR has the advantage of being free from traffic and weather limitations, but its safety, compliance, and whether patients are following the exercise prescription are difficult to guarantee. 13
Digital therapeutics (DTx) is a novel intervention proposed in recent years that refers to the use of evidence‐based therapeutic interventions driven by high‐quality software programs to treat, manage, or prevent a medical condition. 14 A more valuable feature that differentiates DTx from traditional medicine or therapy is the use of artificial intelligence and machine learning systems to monitor and predict information such as individual patient symptom data through digital biomarkers in a clinical feedback loop to provide a precision medicine approach to healthcare. 15 Artificial intelligence platforms can use a large number of individual variables to learn and predict effective interventions to deliver customized, more targeted treatment plans. 16 It enables healthcare to be more personalized and actively adapted to patients' individual clinical needs, goals, and lifestyles.
A systematic review of digital health interventions in cardiovascular disease found that digital technology offers the potential to address the challenges associated with traditional, facility‐based CR. 5 However, to our knowledge, there is no systematic review that explicitly addresses the application of DTx‐based CR in HF patients. The purpose of this study was to explore the safety and efficacy of DTx‐based CR in HF patients and to provide a new CR model during the COVID‐19 era.
Methods
The systematic review was undertaken by following the guideline of recording results followed by the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement (Table S1 ). 17 The protocol was registered in the PROSPERO system review registry (CRD42022315078).
Search strategy
All relevant articles published on PubMed, EMBASE, Cochrane database, and China National Knowledge Internet were searched from the time the database was established until October 2021. Search strategies were developed based on PICOs model, which consisted of participants (e.g. HF) and interventions such as DTx, mobility, technology, and CR ( Tables S2 and S3 ). A manual search was also conducted to include any relevant studies that may not have been identified in the original search.
Selection criteria
At the preliminary stage, the authors screened all titles and abstracts based on predetermined inclusion. The inclusion criteria included (i) adult participants with HF; (ii) any of the following components, such as baseline patient assessments, nutritional counselling, risk factor modification, psychosocial interventions, cognitive behavioural therapy, physical activity guidance, or exercise training; (iii) original research study using high‐quality or specialized software programs to treat, manage, or prevent a medical condition; (iv) reported results for feasibility, usability, or clinical outcomes; and (v) English or Chinese language. The exclusion criteria included (i) meeting abstracts, letters, reviews, protocols, and case reports; (ii) text message intervention alone or telephone intervention studies; and (iii) qualitative studies or studies without clinical endpoints. Concerning the conflicting assessments, a third senior author was consulted to resolve the dispute, and a majority decision was taken.
Data extraction
Data relating to key characteristics of this systematic review including information about the objectives, participants, intervention features, outcomes assessed, comparisons performed, and main conclusions were extracted using an electronic form that was developed for this systematic review. Two authors independently extracted data according to the designed table. The following data were collected: (i) demographic and clinical information of the study population such as gender; (ii) detailed intervention parameters including devices and CR components; and (iii) the details of outcomes. Differences are resolved in small groups.
Quality assessment
Two authors assessed the quality of each study for methodological rigour and risk of bias using the Physiotherapy Evidence Database (PEDro). 18 PEDro is an 11‐item scale used to assess the internal validity and bias control of trials. PEDro scores of 0–3 are considered poor, 4–5 fair, 6–8 good, and 9–10 excellent. Any disagreement between two reviewers on quality scores was decided by an independent third reviewer.
Statistical analysis
Continuous data were presented with mean and standard deviation (SD). Data integration was conducted following the standardized procedures. Means at baseline were calculated by combining means of the intervention and control groups, weighted by the number of participants in each study group. SDs at baseline were estimated by combining SDs of the intervention and control groups with the following formula: SD = √[(N1 − 1) × SD12 + (N2 − 1) × SD22 + N1 × N2/N1 + N2 × (M12 + M22 − 2 × M1 × M2)] and weighted by the number of participants in each study group. 19
Results
Study selection
Figure 1 summarized the process of identifying eligible studies. A total of 2739 relevant studies were identified in the initial database search. After excluding the duplicate, 2509 articles remained, and 2461 studies were excluded after screening the title and abstract screening. Forty‐eight studies were reviewed in full text, 10 studies were excluded because they were protocols, 12 studies were excluded because they were reviews or meta‐analyses, one study was excluded because of a letter, 17 studies were excluded because they were irrelevant, and three studies were excluded because they were from the same laboratory. Finally, five studies were included for systematic review. 20 , 21 , 22 , 23 , 24
Figure 1.
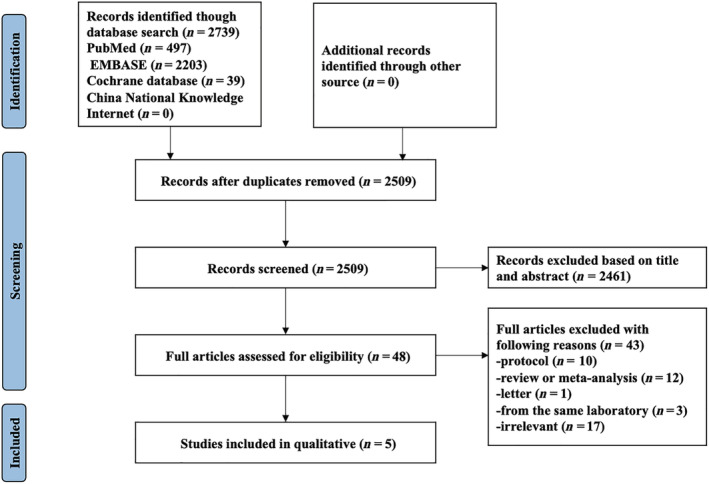
Flow chart of selecting studies for inclusion in this systematic review.
Study characteristic
The characteristics of the study were summarized in Table 1 . Of the 1119 patients, 948 (84.7%) were males. The mean age was 66.37, the mean BMI was 25.9, and the NYHA classification ranged from I to III (I = 232, II = 157, III = 209). Only three included studies reported left ventricular ejection fraction and 6‐min walk distance (6MWD), with a mean left ventricular ejection fraction of 35.5% and a mean 6MWD of 397.7 m before intervention. 20 , 21 , 24 The components of CR in included studies are baseline assessment, 20 , 21 , 22 , 23 , 24 exercise training, 20 , 21 , 23 , 24 education, 20 , 21 and psychological management. 20 , 21 , 23 Three studies addressed other CR core components of nutrition counselling, 20 physical activity guidance, 23 and risk factor modification. 22
Table 1.
The characteristics of the included studies
| Author/year | Country | Study type | Age | Gender (Male/female) | No. of patients | BMI | NYHA (I/II/III/IV) | LVEF | 6MWD | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Clays et al. 2021 | Belgium | RCT | 63.1 ± 10.5 | 43/13 |
IG: 34 CG: 22 |
29.0 ± 5.3 | 0/46/7/0 | 32.2 ± 6.3 | 396 ± 124.1 | |
| Piotrowicz et al. 2020 | Poland | RCT | 62.4 ± 10.5 | 751/97 |
UC: 425 HCTR: 425 |
28.9 ± 4.9 | 104/577/169/0 | 30.5 ± 7.0 | 414.0 ± 101.7 | |
| Guo et al. 2019 | China | Single arm | 69.35 ± 11.15 | 34/32 | HCF: 66 | 22.17 ± 13.73 | 11/32/23/0 | / | / |
|
| Frederix et al. 2015 | Belgium | RCT | 61 ± 8 | 114/25 |
IG: 69 CG: 70 |
28 ± 5 | 115/16/8/0 | / | / | |
| Kikuchi et al. 2021 | Japan | Single arm | 76 ± 7 | 6/4 | IG: 10 | 21.3 ± 3.3 | 2/6/2/0 | 44 ± 20 | 383 ± 94 |
6MWD, 6‐min walk distance; CG, control group; CPET, cardiopulmonary exercise testing; HCF, hospital–community–family; HCTR, hybrid comprehensive telerehabilitation; HRQL, health‐related quality of life; IG, intervention group; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; RCT, randomized control trial; SF‐36, 36‐Item Short Form Survey; UC, usual care; VT, ventilation threshold.
P < 0.05.
Intervention details
Intervention details were listed in Table 2 . Clays et al. used the ‘HeartMan’ personal health system to remotely monitor and intervene in HF patients. 20 The hardware included a wristband sensor, an upper arm blood pressure monitor, and a pill box organizer. The main software was the HeartMan application, which was designed to remind the patient to take measurements with the above hardware provided, and the results of the measurements were fed back to the physician. The interventions were mainly carried out through HeartMan application and include physical health interventions and psychological support. In the research initiated by Piotrowicz et al., a special remote device for tele‐electrocardiogram (ECG)‐monitored and supervised exercise training device was used to monitor exercise training. 21 The hardware component consisted of an electrocardiographic monitoring device (EHO mini device) and a manometer. The system also supported the patient's remote event dynamic ECG function. In the study by Guo et al., the hardware component consisted of an electrocardiogram, peripheral capillary oxygen saturation and blood pressure, long‐term wearable ECG monitors, and mobile ECG monitors. 22 The software included a remote monitoring service platform for collecting and integrating patient data and a personal health tracking mobile application. The primary function of the platform was to store patient history information and monitor data collected by the device. The main function of the software was to send health information, monitor clinical signs, and provide direct supervision and guidance to patients. In Frederix et al., the researcher used a semiautomatic tele‐coaching system to monitor physical activity in HF patients to provide feedback to patients (once a week) via email and text messaging services to encourage patients to gradually reach pre‐determined exercise training goals. 23 The primary device was an accelerometer and associated password‐protected web service. An integrated telerehabilitation platform including an ergometer and a wireless ECG monitoring device and an android compatible tablet was applied in the study by Kikuchi et al. 24 The platform was primarily designed to provide supervision and feedback during exercise training.
Table 2.
The details of delivery digital device
| Author/year | Study design | Delivery digital device | ||||
|---|---|---|---|---|---|---|
| Intervention | Control | Duration | Web | Wearable | Special‐purpose software | |
| Clays et al. 2021 | Mobile personal health system | Usual care | 3–6 months | Y | Y | Y |
| Piotrowicz et al. 2020 | Hybrid comprehensive telerehabilitation (HCTR) | Usual care | 9 weeks | Y | Y | Y |
| Guo et al. 2019 | Hospital–community–family (HCF)‐based telehealth programme | NA | 4 months | Y | Y | Y |
| Frederix et al. 2015 | Internet‐based telerehabilitation programme | Conventional cardiac rehabilitation | 24 weeks | N | Y | Y |
| Kikuchi et al. 2021 | Integrated telerehabilitation platform | NA | 12 weeks | Y | Y | Y |
N, no; NA, not applicable; Y, yes.
Study quality
The PEDro scores included in the study ranged from 4 to 8, with a mean of 5.8 (Table 3 ). The overall quality was fair. The weakest component was the blinding of therapists and participants (criterion 5 and criterion 7). Allocation concealment (criterion 3) was often unreported or poorly described. The lowest scoring component of the analysis was the overall quality of the included studies, with the blinding being the weakest component.
Table 3.
PEDro risk of bias assessment
| Author/year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clays et al. 2021 | ✓ | ✓ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | 6 |
| Piotrowicz et al. 2020 | ✓ | ✓ | ✕ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✓ | 8 |
| Guo et al. 2019 | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | 4 |
| Frederix et al. 2015 | ✓ | ✓ | ✕ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | 6 |
| Kikuchi et al. 2021 | ✓ | ✕ | ✕ | ✓ | ✕ | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | 5 |
Key outcomes
Safety
The TELEREH‐HF trial showed no deaths or other serious adverse events during telemonitored exercise training sessions, with only two cases of syncope and two episodes of complex ventricular tachycardia. 21 Kikuchi et al. found that no serious cardiovascular events were reported during the home‐based rehabilitation, including exacerbation of HF leading to unscheduled hospitalization, and no patient used a provided emergency alarm button throughout the study. 24
Feasibility
Two studies have reported the feasibility. Guo et al. demonstrated that participants were generally satisfied with the ease and usefulness of the interventions. 22 Another study reported that 97% (67/69) of patients reported the motion sensor was easy to use. 23
Adherence
The multicentre clinical trial demonstrated high adherence during the 9‐week training period, in which 350 patients were adherent (88.4%), 39 were partially adherent (9.8%), and only seven patients were non‐adherent (1.8%). As for the UC group, 51 patients (12.0%) participated in CR programmes. 21 During follow‐up, 32 participants (3.8%) lost follow‐up, but these participants were examined at the last contact. 21 Adherence in this study was defined as the use of the app or the access to a web platform more than once a week and the visit to specialist clinics as scheduled during the study period. 22 This study investigating the feasibility more than 60% (40/66). Most patients adhere to remote monitoring at home, 94.7% adhere to remote monitoring, and only 5.3% adhere to partial remote monitoring. 25
Satisfaction
One study reported that patients were either very satisfied (30/69) or satisfied (51%) in the study. Eighty‐nine percent of patients (61/69) would like to use the system upon completion of their studies. 23 Kikuchi et al. stated that all patients enrolled in intervention group were satisfied with the availability and usability of the system. 24
Effectiveness
Major cardiovascular events
Only one of the included studies reported the differences in all‐cause mortality, CV mortality, CV hospitalizations, HF hospitalizations, or composite endpoints between intervention and control group. 21 But in this study, there were no differences observed. This study also showed no increase in survival or days to discharge in the intervention group. In contrast, the results of another randomized controlled trial showed a significant increase in LVEF and a significant decrease in the predicted 1‐year risk of death score in the intervention group. 20
Function capacity
Four studies reported the effects of DTx on exercise capacity. In research by Piotrowicz et al., the intervention improved peak oxygen consumption (VO2 peak) [0.95 (95% CI, 0.65–1.26) mL/kg/min vs. 0.00 (95% CI, −0.31–0.30) mL/kg/min; P < 0.001] and 6MWD range of 30.0 m (95% CI, 24.7–35.3) vs. 20.7 m (95% CI, 15.4–26.0) (P = 0.01) compared with the usual group. 21 Frederix et al. showed an increase in the average VO2 peak from baseline to 24 weeks in the intervention group (P < 0.01) and no significant change in the average VO2 peak in the control group (P = 0.09). And between‐group analysis of aerobic capacity was significant after 24 weeks (P < 0.001) in favour of the intervention group. Similar changes were observed over time in ventilation thresholds (Watt), oxygen uptake efficiency slope (mL/min/ [log mL/min]), and Watt (predicted percentage). 23 Kikuchi et al. reported a significant improvement in 6MWD from 383 ± 94 to 432 ± 83 m (P = 0.003). 24 Piotrowicz et al. reported the change in 6MWD was in intervention group 30.0 (95% CI, 24.7–35.3) m vs. 20.7 (95% CI, 15.4–26.0) m in control group (P = 0.01). 21 However, one study showed 6MWT motor ability with no observed intervention effect. 20 As for self‐reported physical activity, the International Physical Activity Questionnaire showed a significant improvement in the DTx group (P = 0.01) compared with conventional CR alone (P = 0.01). 23
Quality of life
Piotrowicz et al. found that the intervention can significantly improve QoL measured by Survey Short Form‐36 questionnaire score at 9 weeks (P = 0.008). 21 However, the HeartMan trial showed that patients with health‐related QoL as measured by the Minnesota Heart Failure Patient Questionnaire for 21 diseases were not significant compared with the standard care group. 20 Another study, which also assessed health‐related QoL using offline cardiac quality questionnaires from 14 projects, found that 24‐week intervention significantly improved health‐related QoL (P = 0.01) compared with conventional CR alone. 23
Lifestyle and health behaviours
One randomized study reported positive effects of DTx programme on patient self‐management, including healthy eating (P = 0.046), eating more fruits and vegetables (P = 0.02), weight monitoring (P = 0.002), blood pressure controlling (P = 0.001), correct timing (P = 0.049), and daily dosages (P = 0.006) of medicine adherence. 22 Only one study reported cardiovascular risk factors, and there was no significant difference in body weight (P = 0.69), BMI (P = 0.63), and diastolic blood pressure (P = 0.48) or systolic blood pressure (P = 0.26) between telerehabilitation programmes combined with conventional CR group and conventional CR alone. During the study period, fasting glucose levels, HbA1c, and LDL cholesterol did not change in the intervention group (P = 0.67, P = 0.18, and P = 0.20, respectively) or in the control group (P = 0.25, P = 0.51, and P = 0.31, respectively). 23
Discussion
The global healthcare landscape changed during the pandemic of COVID‐19. One of the main changes was the discontinuation of some routine clinical services at many institutions, which made the viability of centre‐based CR extremely challenging. This makes the already low CR participation worse. Therefore, there is an urgent need to find a CR model that is both safe and effective. DTx is a novel intervention proposed in recent years. It has been used in hypertensive and diabetic patients, and its safety and efficacy have been initially demonstrated. 26 , 27 In recent years, some investigators have applied this model to HF patients for improving their comprehensive management patterns. 21 , 25 To the best of our knowledge, this is the first systematic review evaluating the safety and efficacy of DTx‐based CR in HF patients, and its results suggest that DTx‐based CR is a promising therapeutic approach in the COVID‐19 era. The study also highlights that DTx‐based CR is a novel, individualized intervention with a favourable safety profile in HF patients, significantly improving participation and adherence, as well as exercise capacity and QoL.
Safety is a prerequisite for all treatments. With the pandemic of COVID‐19 and efforts to prevent the spread of infection by maintaining social distance, the safety and security of the shift from centre‐based to home‐based and remote treatment have been a contentious issue. High‐quality, specialized software combined with smart monitoring devices can re‐establish real‐time contact between patients and medical staff, which can further ensure the safety of home rehabilitation. To date, published studies have not reported the occurrence of significant adverse events or complications during participation in DTx‐based CR, such as death or readmission. 21 , 25 , 28 Only the sub‐analysis of the TELEREH‐HF trial have reported mild skin reactions in some patients to the electrode pads of monitoring devices. 25 So how does DTx ensure the safety of participants during CR? Guo et al. used an innovative digital device to monitor patients' vital signs such as blood pressure, heart rate, and activity levels in real time. The system can also optimize home management strategies for HF patients based on the daily symptom logs uploaded by patients and medication adherence. 22 When basic physiological parameters are outside the acceptable range, patients will receive a video call or text message through the app to ensure patient safety during participation. 22 The feasibility of DTx is the basis for clinical dissemination, and our review also found that patients did not reject the use of smartphone apps and monitoring devices. A study of an intervention using a remote monitoring service platform, a mobile app, and a smart health tracking device found that 97% patients thought the app were easy to use. 20 In this review, we also found that many patients were satisfied with the DTx‐based CR programme. This suggests that DTx‐based CR is safe and feasible in HF patients and have a high level of satisfaction.
Poor compliance is a major obstacle in the treatment of most chronic diseases. Improving the patient's compliance in CR is also a more pressing issue nowadays. Home‐based CR programmes are unable to determine whether patients have completed their tasks as prescribed; nevertheless, this will not be present in DTx. Nakayama et al. found that tele‐CR during the pandemic of COVID‐19 was a good alternative to outpatient CR and significantly increased patient participation. 29 In this study, adherence refers to the patient achieving the set training session according to the exercise prescription. The included study also found that 94.7 and 88.4% adherent to DTx programme. 21 , 22 A possible explanation for this finding is that the user‐friendliness of digital devices and home‐targeted interventions may play an important role in promoting patient adherence. However, group‐based exercise training in the community or medical centre has unique advantages because it can help patients get good encouragement and psychological support from each other. 30 In the included studies, only one‐to‐one or multiple patients were guided by the same team and did not provide a channel for patients to communicate with each other.
Current evidence suggests that CR may reduce long‐term clinical events in cardiovascular disease. Results from landmark clinical studies (HF‐ACTION) showed that CR can reduce the combined endpoint of all‐cause mortality and hospitalization risk in HF patients by about 11% and reduce the combined endpoint of cardiovascular‐related death and HF‐related hospitalization risk by about 15%. 31 Only one of the included studies reported the impact of DTx‐based CR on long‐term clinical events in HF patients; the results showed that the intervention group did not increase the percentage of HF survival and hospital discharge days, nor did it reduce long‐term follow‐up mortality or hospitalization. 21 Possible explanations are that the two studies differed in both trial protocol and demographic characteristics and 12.0% of patients in the usual care group were reported to have participated in the rehabilitation program in the TELEREH‐HF study, which may have diluted the effect of DTx and thus prevented a long‐term effect. Three of the included studies reported the impact of DTx‐based CR on the efficacy of exercise training in HF patients. VO2 peak is an authoritative indicator of exercise capacity in HF patients. 32 Both Piotrowicz et al. and Frederix et al. found that VO2 peak was significantly improved compared with the control group (P < 0.05). 21 , 23 However, similar results were not observed in the study conducted by Kikuchi et al. 24 The TELEREH‐HF study showed a mean improvement in 6MWD of 30 m in the intervention group. 21 The study by Kikuchi et al. also showed an average improvement of 49 m in 6MWD after the intervention. 24 The improvement in 6MWD in both studies exceeded the minimum clinical difference value of 30 m. 33 From the limited evidence available, these results showed that DTx‐based CR appears to improve exercise training outcomes that are superior to those observed in the HF‐ACTION study. 31 The goals of a comprehensive intervention for HF patients also include improvement in QoL, which reflects the subjective perception of the intervention on the patient's symptoms and treatment, relies on patient‐reported outcomes, and is a patient‐centred reflection. Patient‐reported outcomes such as self‐reported health‐related QoL and health status have shown a pathophysiological basis and are predictors of clinical events in HF patients. 25 The research by Piotrowicz et al. found a significant improvement in QoL in the intervention group compared with the control group (P < 0.05).
The progression of HF is usually associated not only with a decline in exercise capacity but also with a decline in mental health, with depression and anxiety becoming more prevalent. Both physical and psychological deterioration are common and adversely affect the patient's daily life, QoL, and ultimately hospitalization and mortality. 34 , 35 HF patients are more reluctant to exercise than to comply with other recommendations (e.g. diet and medications) and are only likely to adhere to a home‐based exercise programme with supervision. DTx‐based CR can be used as a way of continuous monitoring and management allowing face‐to‐face interventions with patients and their families in a familiar setting and as part of daily life. Reducing patient anxiety and stress can enhance exercise compliance and ultimately improve health‐related QoL. 35 , 36 Therefore, an increasing number of studies are now focusing on the mental health of HF patients. 34 Our review found that DTx‐based CR was more effective than conventional CR or usual care in improving mental health. 21 , 24 Patients using DTx‐based CR benefit from exercise training in addition to psychological remote support and remote assistance from a remote monitoring team (including nurses, physicians, and physical therapists). A recent systematic review by Scherrenberg et al. suggests that even patients with COVID‐19 should be allowed early CR, and if face‐to‐face contact is not an option, telerehabilitation should be considered. 37 Moreover, the pandemic situation itself is generating more patients eligible for exercise programmes and that the DTx‐based CR improves the availability of gradual and carefully monitored exercise programmes for patients after COVID‐19 disease.
Limitations
There were several limitations in this systematic review. Firstly, although the included studies met the definition of DTx, the hardware and software utilized varied dramatically, and the intervention methods and intervention goals of these studies were different. Some studies focused on exercise training, whereas others focused on cognitive behavioural therapy and comprehensive management. This also made the data from the included studies inconsistent and unable quantitative analysis. Secondly, the included studies with limited levels of evidence, two of which were non‐randomized controlled studies. More well‐designed multicentre randomized controlled studies are needed. Thirdly, although we used the definition based on the DTx alliance as the inclusion criteria, it is still difficult to avoid distinguishing DTx from general health applications. The main development direction of DTx is currently the use of artificial intelligence and deep learning techniques, but unfortunately, these techniques are not maturely used in CR, expecting future studies to focus on this area. Finally, the mean age of the patients included in this study was 66 years, and the proportion of female patients was only 15%. Therefore, the reported adherence and patient acceptance of DTx in this study do not reflect the overall picture, and the question of whether DTx is accepted by older and female patients was not adequately demonstrated in this study.
Conclusions
In conclusion, although this study suggests that DTx‐based CR may be an attractive approach for patients with HF, especially in the light of the normalization of the COVID‐19 pandemic, further studies are needed to confirm the efficacy of a novel model of rehabilitation in routine clinical practice.
Conflict of interest
There were no financial or competing conflicts of interest in relation to this work.
Funding
This study was supported by the Major Project of the Science and Technology Department in Sichuan province China (grant number 2022YFS0112).
Supporting information
Table S1. The PRISMA statement of this systematic review.
Table S2. Search strategy for PubMed.
Table S3. The PICOs principles of the systematic review.
Acknowledgement
The author would like to gratefully acknowledge the mentorship of Yuqiang Wang.
Zhang, X. , Luo, Z. , Yang, M. , Huang, W. , and Yu, P. (2022) Efficacy and safety of digital therapeutics‐based cardiac rehabilitation in heart failure patients: a systematic review. ESC Heart Failure, 9: 3751–3760. 10.1002/ehf2.14145.
References
- 1. Fanaroff AC, DeVore AD, Mentz RJ, Daneshmand MA, Patel CB. Patient selection for advanced heart failure therapy referral. Crit Pathw Cardiol. 2014; 13: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taylor CJ, Ryan R, Nichols L, Gale N, Hobbs FR, Marshall T. Survival following a diagnosis of heart failure in primary care. Fam Pract. 2017; 34: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd‐Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011; 123: 933–944. [DOI] [PubMed] [Google Scholar]
- 4. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016; 67: 1–12. [DOI] [PubMed] [Google Scholar]
- 5. Wongvibulsin S, Habeos EE, Huynh PP, Xun H, Shan R, Porosnicu Rodriguez KA, Wang J, Gandapur YK, Osuji N, Shah LM, Spaulding EM, Hung G, Knowles K, Yang WE, Marvel FA, Levin E, Maron DJ, Gordon NF, Martin SS. Digital health interventions for cardiac rehabilitation: Systematic literature review. J Med Internet Res. 2021; 23: e18773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, Dalal H, Rees K, Singh SJ, Taylor RS. Exercise‐based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. 2019; 1: Cd003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor RS, Walker S, Ciani O, Warren F, Smart NA, Piepoli M, Davos CH. Exercise‐based cardiac rehabilitation for chronic heart failure: The EXTRAMATCH II individual participant data meta‐analysis. Health Technol Assess. 2019; 23: 1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoemaker MJ, Dias KJ, Lefebvre KM, Heick JD, Collins SM. Physical therapist clinical practice guideline for the management of Individuals with heart failure. Phys Ther. 2020; 100: 14–43. [DOI] [PubMed] [Google Scholar]
- 9. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos‐Hesselink JW, Graham Stuart A, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M, ESC Scientific Document Group , Guazzi M, la Gerche A, Aboyans V, Adami PE, Backs J, Baggish A, Basso C, Biffi A, Bucciarelli‐Ducci C, Camm AJ, Claessen G, Delgado V, Elliott PM, Galderisi M, Gale CP, Gray B, Haugaa KH, Iung B, Katus HA, Keren A, Leclercq C, Lewis BS, Mont L, Mueller C, Petersen SE, Petronio AS, Roffi M, Savonen K, Serratosa L, Shlyakhto E, Simpson IA, Sitges M, Solberg EE, Sousa‐Uva M, van Craenenbroeck E, van de Heyning C, Wijns W, Gati S, Bäck M, Börjesson M, Caselli S, Collet JP, Corrado D, Drezner JA, Halle M, Hansen D, Heidbuchel H, Myers J, Niebauer J, Papadakis M, Piepoli MF, Prescott E, Roos‐Hesselink JW, Stuart AG, Taylor RS, Thompson PD, Tiberi M, Vanhees L, Wilhelm M, Tahmi M, Zelveian PH, Berger T, Gabulova R, Sudzhaeva S, Lancellotti P, Sokolović Š, Gruev I, Velagic V, Nicolaides E, Tuka V, Rasmusen H, Khamis H, Viigimaa M, Laukkanen JA, Bosser G, Hambrecht R, Kasiakogias A, Merkely B, Gunnarsson GT, McAdam B, Keren A, Perrone‐Filardi P, Bajraktari G, Mirrakhimov E, Rozenštoka S, Marinskis G, Banu C, Abela M, Vataman E, Belada N, Belghiti H, Jorstad HT, Srbinovska‐Kostovska E, Haugaa K, Główczyńska R, Dores H, Mitu F, Smolensky A, Foscoli M, Nedeljkovic I, Farsky S, Fras Z, Boraita A, Sörenssen P, Schmied C, Bsata W, Zakhama L, Uzun M, Nesukay E, Rakhit D. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021; 42: 17–96. [DOI] [PubMed] [Google Scholar]
- 10. Grace SL, Kotseva K, Whooley MA. Cardiac rehabilitation: Under‐utilized globally. Curr Cardiol Rep. 2021; 23: 118. [DOI] [PubMed] [Google Scholar]
- 11. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW, American Heart Association Science Advisory and Coordinating Committee . Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: A presidential advisory from the American Heart Association. Circulation. 2011; 124: 2951–2960. [DOI] [PubMed] [Google Scholar]
- 12. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS, Cochrane Heart Group . Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017; 6: Cd007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zwisler AD, Norton RJ, Dean SG, Dalal H, Tang LH, Wingham J, Taylor RS. Home‐based cardiac rehabilitation for people with heart failure: A systematic review and meta‐analysis. Int J Cardiol. 2016; 221: 963–969. [DOI] [PubMed] [Google Scholar]
- 14. Hong JS, Wasden C, Han DH. Introduction of digital therapeutics. Comput Methods Programs Biomed. 2021; 209: 106319. [DOI] [PubMed] [Google Scholar]
- 15. Palanica A, Docktor MJ, Lieberman M, Fossat Y. The need for artificial intelligence in digital therapeutics. Digit Biomark. 2020; 4: 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dang A, Arora D, Rane P. Role of digital therapeutics and the changing future of healthcare. J Family Med Prim Care. 2020; 9: 2207–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust J Physiother. 2009; 55: 129–133. [DOI] [PubMed] [Google Scholar]
- 19. Pattyn N, Beulque R, Cornelissen V. Aerobic interval vs. continuous training in patients with coronary artery disease or heart failure: An updated systematic review and meta‐analysis with a focus on secondary outcomes. Sports Med. 2018; 48: 1189–1205. [DOI] [PubMed] [Google Scholar]
- 20. Clays E, Puddu PE, Luštrek M, Pioggia G, Derboven J, Vrana M, de Sutter J, le Donne R, Baert A, Bohanec M, Ciancarelli MC, Dawodu AA, de Pauw M, de Smedt D, Marino F, Pardaens S, Schiariti MS, Valič J, Vanderheyden M, Vodopija A, Tartarisco G. Proof‐of‐concept trial results of the HeartMan mobile personal health system for self‐management in congestive heart failure. Sci Rep. 2021; 11: 5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, Orzechowski P, Szalewska D, Pluta S, Glówczynska R, Irzmanski R, Oreziak A, Kalarus Z, Lewicka E, Cacko A, Mierzynska A, Piotrowicz R. Effects of a 9‐week hybrid comprehensive telerehabilitation program on long‐term outcomes in patients with heart failure: The telerehabilitation in heart failure patients (TELEREH‐HF) randomized clinical trial. JAMA Cardiol. 2020; 5: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo X, Gu X, Jiang J, Li H, Duan R, Zhang Y, Sun L, Bao Z, Shen J, Chen F. A hospital‐community‐family‐based telehealth program for patients with chronic heart failure: Single‐arm, prospective feasibility study. JMIR Mhealth Uhealth. 2019; 7: e13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, van Craenenbroeck E, van Driessche N, Dendale P. Medium‐term effectiveness of a comprehensive internet‐based and patient‐specific telerehabilitation program with text messaging support for cardiac patients: Randomized controlled trial. J Med Internet Res. 2015; 17: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kikuchi A, Taniguchi T, Nakamoto K, Sera F, Ohtani T, Yamada T, Sakata Y. Feasibility of home‐based cardiac rehabilitation using an integrated telerehabilitation platform in elderly patients with heart failure: A pilot study. J Cardiol. 2021; 78: 66–71. [DOI] [PubMed] [Google Scholar]
- 25. Piotrowicz E, Zieliński T, Bodalski R, Rywik T, Dobraszkiewicz‐Wasilewska B, Sobieszczańska‐Małek M, Stepnowska M, Przybylski A, Browarek A, Szumowski Ł, Piotrowski W, Piotrowicz R. Home‐based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. Eur J Prev Cardiol. 2015; 22: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 26. Kario K, Nomura A, Kato A, Harada N, Tanigawa T, So R, Suzuki S, Hida E, Satake K. Digital therapeutics for essential hypertension using a smartphone application: A randomized, open‐label, multicenter pilot study. J Clin Hypertens (Greenwich). 2021; 23: 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nordyke RJ, Appelbaum K, Berman MA. Estimating the impact of novel digital therapeutics in type 2 diabetes and hypertension: Health economic analysis. J Med Internet Res. 2019; 21: e15814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Piotrowicz E, Baranowski R, Bilinska M, Stepnowska M, Piotrowska M, Wójcik A, Korewicki J, Chojnowska L, Malek LA, Klopotowski M, Piotrowski W, Piotrowicz R. A new model of home‐based telemonitored cardiac rehabilitation in patients with heart failure: Effectiveness, quality of life, and adherence. Eur J Heart Fail. 2010; 12: 164–171. [DOI] [PubMed] [Google Scholar]
- 29. Nakayama A, Takayama N, Kobayashi M, Hyodo K, Maeshima N, Takayuki F, Morita H, Komuro I. Remote cardiac rehabilitation is a good alternative of outpatient cardiac rehabilitation in the COVID‐19 era. Environ Health Prev Med. 2020; 25: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desveaux L, Harrison S, Lee A, Mathur S, Goldstein R, Brooks D. “we are all there for the same purpose”: Support for an integrated community exercise program for older adults with HF and COPD. Heart Lung. 2017; 46: 308–312. [DOI] [PubMed] [Google Scholar]
- 31. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goulart CDL, Dos Santos PB, Caruso FR, Arêas GPT, Marinho RS, Camargo PF, Alexandre TD, Oliveira CR, da Silva AL, Mendes RG, Roscani MG. The value of cardiopulmonary exercise testing in determining severity in patients with both systolic heart failure and COPD. Sci Rep. 2020; 10: 4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Täger T, Hanholz W, Cebola R, Fröhlich H, Franke J, Doesch A, Katus HA, Wians FH Jr, Frankenstein L. Minimal important difference for 6‐minute walk test distances among patients with chronic heart failure. Int J Cardiol. 2014; 176: 94–98. [DOI] [PubMed] [Google Scholar]
- 34. Celano CM, Villegas AC, Albanese AM, Gaggin HK, Huffman JC. Depression and anxiety in heart failure: A review. Harv Rev Psychiatry. 2018; 26: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sokoreli I, de Vries JJG, Pauws SC, Steyerberg EW. Depression and anxiety as predictors of mortality among heart failure patients: Systematic review and meta‐analysis. Heart Fail Rev. 2016; 21: 49–63. [DOI] [PubMed] [Google Scholar]
- 36. Kulcu DG, Kurtais Y, Tur BS, Gülec S, Seckin B. The effect of cardiac rehabilitation on quality of life, anxiety and depression in patients with congestive heart failure. A randomized controlled trial, short‐term results. Eura Medicophys. 2007; 43: 489–497. [PubMed] [Google Scholar]
- 37. Scherrenberg M, Wilhelm M, Hansen D, Völler H, Cornelissen V, Frederix I, Kemps H, Dendale P. The future is now: A call for action for cardiac telerehabilitation in the COVID‐19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2020; 28: 524–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The PRISMA statement of this systematic review.
Table S2. Search strategy for PubMed.
Table S3. The PICOs principles of the systematic review.


