Abstract
Aims
Treatment response to vericiguat, based on baseline N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) subgroups specified in the protocol, was evaluated in the heart failure (HF) VICTORIA trial population by post hoc analysis of combined lower three quartiles [Q1–Q3] vs. the upper quartile [Q4].
Methods and results
VICTORIA participants with available baseline NT‐proBNP levels (n = 4805; 95.1% of total) were included. Compared with patients in Q1–Q3 (NT‐proBNP: Q1, ≤1556 pg/mL; Q2, >1556–2816 pg/mL; and Q3, >2816–5314 pg/mL), patients in Q4 (NT‐proBNP: >5314 pg/mL) were older (69.2 ± 12.0 vs. 66.6 ± 12.1 years), had lower mean ejection fraction (27.2 ± 8.3% vs. 29.5 ± 8.2%; P < 0.0001), and were more likely to be in New York Heart Association (NYHA) Class III (51.8 vs. 35.6%) or IV (2.4 vs. 1.0%). Compared with Q1–Q3, patients in Q4 had higher mean Meta‐Analysis Global Group in Chronic Heart Failure risk score (27.3 ± 6.6 vs. 23.5 ± 6.4; P < 0.0001), had lower mean estimated glomerular filtration rate (eGFR; 51.5 ± 25.5 vs. 65.0 ± 26.8 mL/min/1.73 m2; P < 0.0001) and haemoglobin (12.8 ± 2.0 vs. 13.6 ± 1.9 g/dL; P < 0.0001), and more had atrial fibrillation (48.7% vs. 43.1%; P = 0.0007) and were randomized while hospitalized for HF (14.8 vs. 9.9%; P < 0.0001). Target dose was achieved in 72.3 and 63.7% of patients in Q1–Q3 and Q4, respectively (P < 0.0001). Primary outcome (composite of time to cardiovascular death or first HF hospitalization) rates were 24.5 and 31.7 per 100 patient‐years for vericiguat and placebo in Q1–Q3 [hazard ratio (HR) 0.78; 95% confidence interval (CI) 0.69–0.88, P < 0.001] and 73.6 and 63.6 in Q4 (HR 1.15; 95% CI 0.99–1.34, P = 0.070).
Serious adverse events were more frequent in NT‐proBNP Q4 (total population) compared with Q1–Q3 (38.3 vs. 32.3%; P = 0.0001), driven mainly by the placebo group. Adverse events leading to death were more frequent in Q4 than Q1–Q3 (5.8 vs. 2.4%; P < 0.0001).
Conclusions
Plasma NT‐proBNP may help identify patients with worsening HF with reduced ejection fraction, in whom the beneficial effects of vericiguat may be highest. Patients with highest NT‐proBNP values are probably too far advanced, suffering more co‐morbidities, or still clinically unstable after decompensation to derive benefit from vericiguat.
Keywords: Heart failure, Heart failure with reduced ejection fraction, NT‐proBNP
Introduction
Increased N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) values are associated with worse outcomes in patients with heart failure (HF). 1 Primary analysis of the VICTORIA trial confirmed that, in patients with HF, vericiguat reduced the risk of the primary composite endpoint of cardiovascular (CV) death or time to first HF hospitalization, 2 with generally consistent results across subgroups of baseline characteristics, including patients in New York Heart Association (NYHA) Class I or II, Class III or IV, and in patients receiving sacubitril/valsartan.
The results of the pre‐specified primary analysis showed potential heterogeneity of treatment effect by NT‐proBNP at randomization; patients who received vericiguat in each of the lower three baseline NT‐proBNP quartiles appeared to experience a numerically different treatment effect to that seen in patients in the highest baseline NT‐proBNP quartile. 2 In a post hoc model to evaluate the relationship of increasing NT‐proBNP values vs. hazard ratio (HR) for the primary composite, the treatment effect of vericiguat compared with placebo on the primary composite endpoint was greatest in patients with NT‐proBNP levels <8000 pg/mL at randomization, which was further amplified if these levels were <4000 pg/mL. 3 The US Food and Drug Administration‐approved label indicates that, among patients in the highest baseline NT‐proBNP quartile (>5314 pg/mL), the estimated HRs for both CV death and first HF hospitalization were unfavourable, in contrast to the estimated HRs for patients in the three quartiles with lower NT‐proBNP levels (≤5314 pg/mL). 4 To better understand the discrepancies in outcomes and safety in response to vericiguat, a post hoc analysis of outcomes, safety, and clinical profiles of patients in the combined lower baseline quartiles (Q1–Q3) and the highest NT‐proBNP quartile (Q4) was conducted.
Methods
Study patients
The design, baseline characteristics, and results of the VICTORIA trial have been previously reported. 2 , 5 In brief, 5050 patients with symptomatic worsening chronic HF, left ventricular ejection fraction (LVEF) <45%, and elevated natriuretic peptide levels, who were receiving guideline‐directed medical therapy, were randomized, within 6 months after a HF decompensation, in a 1:1 ratio to receive vericiguat or placebo. Eligibility criteria mandated brain natriuretic peptide (BNP) levels of ≥300 pg/mL or NT‐proBNP levels of ≥1000 pg/mL for patients in sinus rhythm, and, for patients with atrial fibrillation, higher levels of BNP (≥500 pg/mL) or NT‐proBNP (≥1600 pg/mL) were required. The study protocol encouraged investigators to use guideline‐based HF therapies, including sacubitril/valsartan.
Natriuretic peptide sampling and assessment
This analysis was restricted to the subset of patients who had evaluable levels of NT‐proBNP, as measured by a central laboratory using a Roche Elecsys assay system (Roche Diagnostics, Mannheim, Germany) during screening, 30 days prior to randomization. Baseline NT‐proBNP levels used in this analysis were those measured at randomization. The sensitivity of the assay was 10–175 000 pg/mL (175 000 pg/mL is not a truly measured NT‐proBNP value in any of the patients, but was extrapolated for patients above the upper limit of detection).
Clinical outcomes
The primary outcome of the VICTORIA trial was the composite endpoint of time to CV death or first HF hospitalization. In addition, the individual components of the composite outcome (CV death and HF hospitalization), as well as total HF hospitalizations (first and recurrent), CV hospitalization, and all‐cause death, were analysed. Time to pre‐specified index events (qualifying worsening HF event) was also monitored.
Safety assessments
Adverse events (AEs) and laboratory changes were reported by NT‐proBNP subgroup, overall and by region (America, Asia, and Europe). The following events were considered events of clinical interest: symptomatic hypotension, syncope, and hepatic events.
Statistical analysis
Patients were divided into two groups, with NT‐proBNP Quartiles 1–3 combined into one group and Quartile 4 into a separate group. The NT‐proBNP quartiles were pre‐specified subgroups; however, the analysis of Q1–Q3 and Q4 was post hoc. Baseline characteristics were summarized as percentages, means and standard deviation (SD), or medians. Comparisons of baseline characteristics, AEs, and laboratory changes between NT‐proBNP subgroups, and between treatment and NT‐proBNP subgroups, were conducted based on F‐test for continuous variables and chi‐squared test for discrete variables. In addition, we evaluated the relationship between time to index event and randomization.
Kaplan–Meier (KM) estimates for time‐to‐event data for the primary outcome and its components were reported. HRs with 95% confidence intervals (CIs) were calculated and added to a forest plot. Treatment group and the stratification factors (defined by region and race) used for randomization were included in the model as fixed effects: Eastern Europe plus Israel and South Africa, Western Europe, North America (Black), North America (non‐Black), Latin and South America, and Asia Pacific (including Australia). Efficacy and safety of vericiguat, according to NT‐proBNP quartile group (Q1–Q3 or Q4), were examined. Analyses were conducted with SAS version 9.4.
Results
Patient characteristics
Of the 5050 randomized patients in the VICTORIA trial, 4805 (95.1%) patients had evaluable NT‐proBNP levels at randomization and were included in this analysis, 3604 had baseline NT‐proBNP levels ≤75th percentile (Q1, ≤1556 pg/mL; Q2, >1556–2816 pg/mL; and Q3, >2816–5314 pg/mL), and 1201 had baseline NT‐proBNP levels >75th percentile (Q4, >5314 pg/mL). Table 1 shows the patient demographics and clinical baseline characteristics by quartile group. At randomization, patients in Q4, as compared with patients in Q1–Q3, tended to be older (69.2 vs. 66.6 years) and have a lower body mass index (BMI; 26.3 vs. 28.2 kg/m2). Mean baseline ejection fraction was lower in patients in Q4 than those in Q1–3 (27.2 vs. 29.5%; P < 0.0001), and more patients in Q4 had ejection fraction ≤40% compared with Q1–Q3 (94.4 vs. 92.0%; P = 0.0062). Mean heart rate was higher at baseline in patients in Q4 compared with those in Q1–Q3 (74.9 vs. 72.5 bpm; P < 0.0001). Patients in Q4, compared with patients in Q1–Q3, were also more likely to have a lower mean estimated glomerular filtration rate (eGFR; 51.5 vs. 65.0 mL/min/1.73 m2; P < 0.0001) and an eGFR of ≤30 mL/min/1.73m2 (20.1 vs. 6.6%). Haemoglobin (mean 12.8 vs. 13.6 g/dL; P < 0.0001) and baseline haematocrit (40.1 vs. 42.3%; P < 0.0001) were lower in patients in Q4 than those in Q1–Q3. In comparison with those in Q1–Q3, patients in Q4 were more likely to be classified as NYHA Class III (51.8 vs. 35.6%) or Class IV (2.4 vs. 1.0%), respectively, and have a higher Meta‐Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score (27.3 vs. 23.5; P < 0.0001). Compared with patients in Q1–Q3, patients in Q4 were more likely to have atrial fibrillation (48.7 vs. 43.1%; P = 0.0007). Overall, median NT‐proBNP was 2694, 2892, and 2881 pg/mL for America, Asian, and Europe regions, respectively. In each region, median NT‐proBNP was similar between patients randomized to vericiguat and placebo (Table S1 ).
Table 1.
Patient characteristics according to baseline NT‐proBNP levels in Quartiles 1–3 (Q1–Q3) and Quartile 4 (Q4)
| Patients with baseline NT‐proBNP in Q1–Q3 | Patients with baseline NT‐proBNP in Q4 | |||||
|---|---|---|---|---|---|---|
| Characteristics | Vericiguat n = 1798 | Placebo n = 1806 | Total n = 3604 | Vericiguat n = 616 | Placebo n = 585 | Total n = 1201 |
| Age (years), mean (SD) | 66.7 (12.2) | 66.6 (12.1) | 66.6 (12.1) | 69.4 (12.0) | 69.0 (11.9) | 69.2 (12.0) |
| Male, n (%) | 1380 (76.8) | 1380 (76.4) | 2760 (76.6) | 458 (74.4) | 428 (73.2) | 886 (73.8) |
| BMI (kg/m2), mean (SD) | 28.2 (6.0) | 28.3 (6.2) | 28.2 (6.1) | 26.2 (5.0) | 26.3 (5.5) | 26.3 (5.3) |
| Race, n (%) | ||||||
| White | 1319 (63.3) | 1135 (62.8) | 2274 (63.1) | 390 (63.3) | 368 (62.9) | 758 (63.1) |
| Black | 83 (4.6) | 89 (4.9) | 172 (4.8) | 31 (5.0) | 29 (5.0) | 60 (5.0) |
| Asian | 433 (24.1) | 418 (23.1) | 851 (23.6) | 132 (21.4) | 135 (23.1) | 267 (22.2) |
| Other | 143 (8.0) | 164 (9.1) | 307 (8.5) | 63 (10.2) | 53 (9.1) | 116 (9.7) |
| Geographic region, n (%) | ||||||
| Eastern Europe | 588 (32.7) | 604 (33.4) | 1192 (33.1) | 217 (35.2) | 193 (33.0) | 410 (34.1) |
| Western Europe | 296 (16.5) | 285 (15.8) | 581 (16.1) | 116 (18.8) | 111 (19.0) | 227 (18.9) |
| Asia Pacific | 447 (24.9) | 437 (24.2) | 884 (24.5) | 135 (21.9) | 142 (24.3) | 277 (23.1) |
| Latin America | 252 (14.0) | 273 (15.1) | 525 (14.6) | 101 (16.4) | 81 (13.8) | 182 (15.2) |
| North America | 215 (12.0) | 207 (11.5) | 422 (11.7) | 47 (7.6) | 58 (9.9) | 105 (8.7) |
| Randomized while hospitalized, n (%) | 189 (10.5) | 166 (9.2) | 355 (9.9) | 91 (14.8) | 87 (14.9) | 178 (14.8) |
| Index event, n (%) | ||||||
| HF hospitalization within 3 months | 1148 (63.8) | 1183 (65.5) | 2331 (64.7) | 450 (73.1) | 434 (74.2) | 884 (73.6) |
| HF hospitalization within 3–6 months | 332 (18.5) | 305 (16.9) | 637 (17.7) | 99 (16.1) | 85 (14.5) | 184 (15.3) |
| IV diuretic for HF (without hospitalization) within 3 months | 318 (17.7) | 318 (17.6) | 636 (17.6) | 67 (10.9) | 66 (11.3) | 133 (11.1) |
| Medical history | ||||||
| Ejection fraction, n | 1794 | 1803 | 3,597 | 612 | 585 | 1197 |
| Mean (SD) % | 29.7 (8.2) | 29.4 (8.3) | 29.5 (8.2) | 27.2 (8.3) | 27.2 (8.4) | 27.2 (8.3) |
| Ejection fraction ≤40%, n (%) | 1654 (92.2) | 1656 (91.8) | 3310 (92.0) | 582 (95.1) | 548 (93.7) | 1130 (94.4) |
| NYHA class at baseline, n (%) | ||||||
| Class I | 0 (0.0) | 1 (0.1) | 1 (0.0) | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| Class II | 1139 (63.3) | 1146 (63.5) | 2285 (63.4) | 275 (44.6) | 274 (46.8) | 549 (45.7) |
| Class III | 641 (35.7) | 642 (35.5) | 1283 (35.6) | 325 (52.8) | 297 (50.8) | 622 (51.8) |
| Class IV | 18 (1.0) | 17 (0.9) | 35 (1.0) | 16 (2.6) | 13 (2.2) | 29 (2.4) |
| Blood pressure, n | 1798 | 1803 | 3601 | 616 | 584 | 1200 |
| Systolic blood pressure (mmHg), mean (SD) | 121.0 (15.45) | 121.4 (15.6) | 121.2 (15.5) | 121.5 (16.4) | 121.5 (16.5) | 121.5 (16.4) |
| Diastolic blood pressure (mmHg), mean (SD) | 72.5 (11.0) | 73.0 (10.5) | 72.8 (10.8) | 72.4 (11.4) | 73.3 (12.3) | 72.9 (11.8) |
| Heart rate, n | 1798 | 1805 | 3603 | 616 | 585 | 1201 |
| Mean (SD) bpm | 72.4 (12.8) | 72.7 (12.6) | 72.5 (12.7) | 74.6 (13.3) | 75.3 (14.2) | 74.9 (13.8) |
| Atrial fibrillation, n (%) | 744 (41.4) | 809 (44.8) | 1553 (43.1) | 295 (47.9) | 290 (49.6) | 585 (48.7) |
| Diabetes mellitus, n (%) | 864 (48.1) | 799 (44.2) | 1663 (46.1) | 312 (50.6) | 279 (47.7) | 591 (49.2) |
| COPD, n (%) | 289 (16.1) | 318 (17.6) | 607 (16.8) | 119 (19.3) | 91 (15.6) | 210 (17.5) |
| CAD, n (%) | 1079 (60.0) | 1009 (55.9) | 2088 (57.9) | 365 (59.3) | 351 (60.0) | 716 (59.6) |
| History of smoking, n (%) | 1063 (59.1) | 1082 (59.9) | 2145 (59.5) | 346 (12.0) | 326 (55.7) | 672 (56.0) |
| Time from diagnosis of any HF to randomization (years), mean (SD) | 5.2 (5.7) | 5.3 (5.6) | 5.2 (5.6) | 5.1 (5.8) | 5.2 (6.1) | 5.1 (5.9) |
| Guideline‐directed medical therapy, n (%) | ||||||
| ACE‐I or ARB | 1346 (74.9) | 1372 (76.0) | 2718 (75.4) | 426 (69.2) | 396 (67.7) | 822 (68.4) |
| Sacubitril/valsartan | 263 (14.6) | 262 (14.5) | 525 (14.6) | 74 (12.0) | 85 (14.5) | 159 (13.2) |
| Beta‐blocker | 1682 (93.5) | 1687 (93.4) | 3369 (93.5) | 566 (91.9) | 536 (91.6) | 1102 (91.8) |
| MRA | 1283 (71.4) | 1334 (73.9) | 2617 (72.6) | 398 (64.6) | 374 (63.9) | 772 (64.3) |
| Triple therapy | 1104 (61.4) | 1161 (64.3) | 2265 (62.8) | 316 (51.3) | 293 (50.1) | 609 (50.7) |
| ICD | 495 (27.5) | 505 (28.0) | 1000 (27.7) | 166 (26.9) | 158 (27.0) | 324 (27.0) |
| Biventricular pacing | 257 (14.3) | 251 (13.9) | 508 (14.1) | 94 (15.3) | 92 (15.7) | 186 (15.5) |
| Laboratory results | ||||||
| Haemoglobin, n | 1732 | 1742 | 3474 | 601 | 564 | 1165 |
| Mean (SD) g/dL | 13.6 (1.9) | 13.6 (1.9) | 13.6 (1.9) | 12.8 (1.9) | 12.8 (2.0) | 12.8 (2.0) |
| Haematocrit, n | 1732 | 1742 | 3474 | 601 | 564 | 1165 |
| Mean (SD) % | 42.2 (5.84) | 42.4 (5.82) | 42.2 (5.83) | 40.1 (6.17) | 40.1 (6.12) | 40.1 (6.14) |
| eGFR, mL/min/1.73 m2, n (%) | ||||||
| ≤30 | 122 (6.8) | 115 (6.4) | 237 (6.6) | 124 (20.1) | 117 (20.0) | 241 (20.1) |
| >30 to ≤60 | 724 (40.3) | 715 (39.6) | 1439 (39.9) | 293 (47.6) | 295 (50.4) | 588 (49.0) |
| >60 | 932 (51.8) | 965 (53.4) | 1897 (52.6) | 197 (32.0) | 170 (29.1) | 367 (30.6) |
| Mean (SD) mL/min/1.73 m2 | 64.7 (26.6) | 65.3 (27.1) | 65.0 (26.8) | 51.9 (26.0) | 51.1 (25.0) | 51.5 (25.5) |
| MAGGIC risk score, n | 1770 | 1780 | 3550 | 610 | 574 | 1184 |
| Mean (SD) | 23.50 (6.4) | 23.40 (6.4) | 23.45 (6.4) | 27.40 (6.7) | 27.17 (6.6) | 27.29 (6.6) |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter defibrillator; IQR, interquartile range; IV, intravenous; MAGGIC, Meta‐Analysis Global Group in Chronic Heart Failure; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Patients in Q4, compared with those in Q1–Q3, were more likely to have experienced HF hospitalization within 3 months prior to randomization (73.6 vs. 64.7%; P < 0.0001) and be randomized while hospitalized (14.8 vs. 9.9%; P < 0.0001), respectively. Patients randomized while hospitalized had higher median baseline NT‐proBNP values relative to outpatients (3742 and 2679 pg/mL for vericiguat, respectively, and 3483 and 2785 pg/mL for placebo, respectively).
Overall, median time from index event of HF hospitalization to randomization was similar in both quartile groups [median (range): 34.5 (2–621) days and 32.0 (2–253) days for Q1–Q3 and Q4, respectively]. Similarly, median time from index event of intravenous diuretics treatment to randomization was 25.0 (range, 2–180) days and 28.0 (range, 2–89) days for Q1–Q3 and Q4, respectively.
Primary outcomes by NT‐proBNP baseline quartile group
KM curves of the primary outcome (CV death or first hospitalization for HF) and its components by baseline NT‐proBNP group are presented in Figure 1 . The primary outcome occurred in 506 (28.1%) patients receiving vericiguat and in 619 (34.3%) patients receiving placebo in the Q1–Q3 group (HR 0.78; 95% CI 0.69–0.88, P < 0.001) (Figure 1 A ). The primary outcome occurred in 355 patients (57.6%) receiving vericiguat and in 302 patients (51.6%) receiving placebo (HR 1.15; 95% CI 0.99–1.34, P = 0.070) in Q4 (Figure 1 B ).
Figure 1.
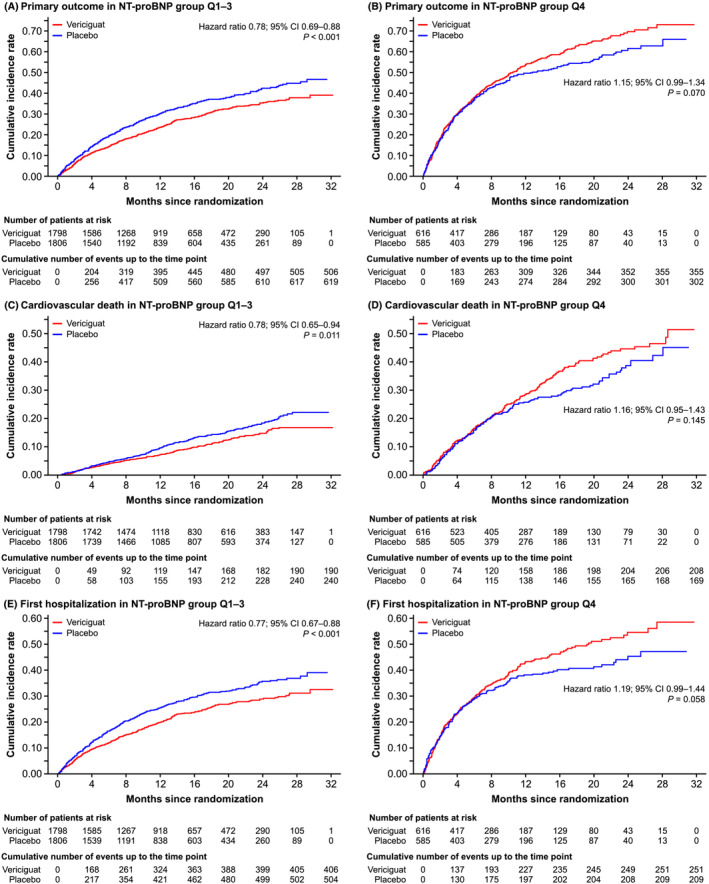
Kaplan–Meier curves of clinical outcomes for the primary composite endpoint and its components in patients with baseline NT‐proBNP levels in Quartiles 1–3 (Q1–Q3) and Quartile 4 (Q4). Primary outcome was composite of death from cardiovascular causes or first hospitalization for HF. Cumulative incidences of composite endpoint by NT‐proBNP group (Q1–Q3 and Q4) are shown in Panels A and B, respectively. Cumulative incidences of cardiovascular death by NT‐proBNP group (Q1–Q3 and Q4) are shown in Panels C and D, respectively. Cumulative incidences of first hospitalization by NT‐proBNP group (Q1–Q3 and Q4) are shown in Panels E and F, respectively. CI, confidence interval; CV, cardiovascular; HF, heart failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Death from CV causes occurred in 190 (10.6%) patients receiving vericiguat and in 240 (13.3%) patients receiving placebo (HR 0.78; 95% CI 0.65–0.94, P = 0.011) within the Q1–Q3 group (Figure 1 C ). In the Q4 group, death from CV causes occurred in 208 (33.8%) and 169 (28.9%) patients receiving vericiguat and placebo, respectively (HR 1.16; 95% CI 0.95–1.43, P = 0.145) (Figure 1 D ). In the Q1–Q3 group, first hospitalization occurred in 406 (22.6%) patients receiving vericiguat and in 504 (27.9%) patients receiving placebo (HR 0.77; 95% CI 0.67–0.88, P < 0.001) (Figure 1 E ). In the Q4 group, first hospitalization occurred in 251 (40.7%) patients receiving vericiguat and in 209 (35.7%) patients receiving placebo (HR 1.19; 95% CI 0.99–1.44, P = 0.058) (Figure 1 D ). For total HF hospitalization events, the annualized rate in the Q1–Q3 group was 30.2% in patients receiving vericiguat and 37.7% in patients receiving placebo (HR 0.80; 95% CI 0.72–0.89, P < 0.001). In the Q4 group, the annualized rate was 66.8% in patients receiving vericiguat and 63.1% in patients receiving placebo (HR 1.08; 95% CI 0.93–1.25, P = 0.329) (Table 2 ).
Table 2.
Event rates per 100 patient‐years, annualized absolute risk reduction, and elevation for 1 year of treatment with vericiguat are shown for the primary composite endpoint, total HF hospitalizations (first and recurrent), CV hospitalization, and all‐cause death
| N (%) | Per 100 patient‐years | N (%) | Per 100 patient‐years | Per 100 patient‐years | ||
|---|---|---|---|---|---|---|
| Vericiguat (n = 1798) | Placebo (n = 1806) | Annualized ARR (%) | ||||
| Patients with baseline NT‐proBNP in Q1–Q3 | Primary composite | 506 (28.1) | 24.5 | 619 (34.3) | 31.7 | 7.2 |
| CV death | 190 (10.6) | 7.9 | 240 (13.3) | 10.1 | 2.2 | |
| HF hospitalization (first) | 406 (22.6) | 19.6 | 504 (27.9) | 25.8 | 6.2 | |
| Total HF hospitalizations (first and recurrent) | 725 (40.3) | 30.2 | 891 (49.3) | 37.7 | 7.5 | |
| CV hospitalization | 594 (33.0) | 31.4 | 698 (38.6) | 39.7 | 8.3 | |
| All‐cause death | 237 (13.2) | 9.8 | 286 (15.8) | 12.1 | 2.3 | |
| Vericiguat (n = 616) | Placebo (n = 585) | Annualized ARR (%) | ||||
| Patients with baseline NT‐proBNP in Q4 | Primary composite | 355 (57.6) | 73.6 | 302 (51.6) | 63.6 | −10.0 |
| CV death | 208 (33.8) | 32.0 | 169 (28.9) | 27.1 | −4.9 | |
| HF hospitalization (first) | 251 (40.7) | 52.0 | 209 (35.7) | 44.0 | −8.0 | |
| Total HF hospitalization (first and recurrent) | 432 (70.1) | 66.8 | 392 (67.0) | 63.1 | −3.7 | |
| CV hospitalization | 304 (49.4) | 68.0 | 271 (46.3) | 63.0 | −5.0 | |
| All‐cause death | 256 (41.6) | 39.4 | 211 (36.1) | 33.8 | −5.6 | |
ARR, annualized absolute risk reduction; CV, cardiovascular; HF, heart failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
In Table 2 , event rates per 100 patient‐years and annualized absolute risk differences for 1 year of treatment with vericiguat are shown for the primary composite endpoint, total HF hospitalizations (first and recurrent), CV hospitalization, and all‐cause death. Event rates for the primary composite endpoint were 24.5 and 31.7 per 100 patient‐years for vericiguat and placebo, respectively, in Q1–Q3 and 73.6 and 63.6 for vericiguat and placebo, respectively, in Q4.
CV death and time to first HF hospitalization, as well as total HF hospitalization, CV hospitalization, and all‐cause mortality, in patients with baseline NT‐proBNP levels in Q1–Q3 were significantly lower in the vericiguat group than with placebo. A trend (not statistically significant) for increased incidence of the primary outcome was noted in the Q4 population (Figure 2 ).
Figure 2.
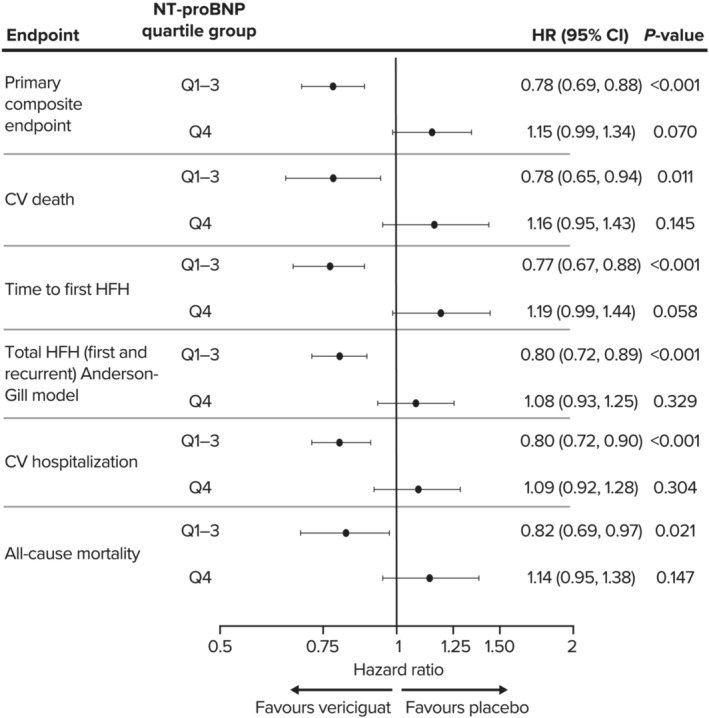
Forest plot of hazard ratios with 95% confidence intervals are shown for the primary composite endpoint and its components, CV death and time to first HF hospitalization, as well as total HF hospitalization, CV hospitalization, and all‐cause mortality, in patients with baseline NT‐proBNP levels in Quartiles 1–3 (Q1–Q3) and Quartile 4 (Q4). CI, confidence interval; CV, cardiovascular; HF, heart failure; HFH, heart failure hospitalization; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide. *Calculated using the Anderson–Gill model.
Interaction analysis of outcomes by quartile category (Q1, Q2, Q3, and Q4) and treatment indicated a statistically significant interaction for the primary composite endpoint (P = 0.0010), HF hospitalization (P = 0.0021), and all‐cause death or HF hospitalization (P = 0.0016). Interactions approached statistical significance for CV death (P = 0.0503) and all‐cause death (P = 0.0544). When this analysis was restricted to Q1, Q2, and Q3 only, no interactions were observed for any outcome.
Study medication dose following titration is shown in Table S2 for both subgroups. In Q1–Q3, 2335/3604 (64.8%) patients were titrated to the target dose of 10 mg and remained at this dose for 80% of the treatment period (63.5% of the vericiguat arm and 66.2% of the placebo arm). In Q4, 680/1201 (56.7%) patients (56.8% of the vericiguat arm and 56.5% in the placebo arm) were titrated to vericiguat 10 mg. Overall, a larger proportion of patients in Q1–Q3 than Q4 reached the target dose of 10 mg (vericiguat and placebo; 72.4 vs. 63.8%, P < 0.0001) and spent a greater number of days at the target dose [median (range): 289 (0–960) days vs. 179 (0–916) days; P < 0.0001].
Safety by baseline NT‐proBNP quartile group
Table 3 presents safety events (AEs including symptomatic hypotension, syncope, and hepatic events and changes in laboratory parameters), by baseline NT‐proBNP subgroup.
Table 3.
Summary of AEs and laboratory changes from baseline in patients with baseline NT‐proBNP levels in Quartiles 1–3 (Q1–Q3) and Quartile 4 (Q4)—all safety patients
| Patients with baseline NT‐proBNP in Q1–A3 | Patients with baseline NT‐proBNP in Q4 | P value a | P value b | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Vericiguat n = 1798 | Placebo n = 1803 | Total n = 3601 | Vericiguat n = 616 | Placebo n = 584 | Total n = 1200 | ||
| AEs, n (%) | n = 1798 | n = 1803 | n = 3601 | n = 616 | n = 584 | n = 1200 | ||
| AEs | 1449 (80.6) | 1459 (80.9) | 2908 (80.8) | 491 (79.7) | 474 (81.2) | 965 (80.4) | NS | NS |
| Death c | 41 (2.3) | 44 (2.4) | 85 (2.4) | 38 (6.2) | 32 (5.5) | 70 (5.8) | <0.0001 | NS |
| SAEs | 582 (32.4) | 580 (32.2) | 1162 (32.3) | 211 (34.3) | 248 (42.5) | 459 (38.3) | 0.0001 | 0.0101 |
| Drug‐related AEs | 271 (15.1) | 205 (11.4) | 476 (13.2) | 74 (12.0) | 69 (11.8) | 143 (11.9) | NS | NS |
| Drug‐related SAEs | 20 (1.1) | 14 (0.8) | 34 (0.9) | 8 (1.3) | 4 (0.7) | 12 (1.0) | NS | NS |
| AEs that led to treatment discontinuation | 111 (6.2) | 97 (5.4) | 208 (5.8) | 49 (8.0) | 51 (8.7) | 100 (8.3) | 0.0017 | NS |
| SAEs that led to treatment discontinuation | 52 (2.9) | 48 (2.7) | 100 (2.8) | 18 (2.9) | 34 (5.8) | 52 (4.3) | 0.0217 | 0.0254 |
| Events of clinical interest, n (%) | n = 1798 | n = 1803 | n = 3601 | n = 616 | n = 584 | n = 1201 | ||
| Symptomatic hypotension | 174 (9.7) | 131 (7.3) | 305 (8.5) | 44 (7.1) | 53 (9.1) | 97 (8.1) | NS | |
| Syncope | 77 (4.3) | 62 (3.4) | 139 (3.9) | 21 (3.4) | 21 (3.6) | 42 (3.5) | NS | |
| Hepatic events | 14 (0.8) | 11 (0.6) | 25 (0.7) | 9 (1.5) | 2 (0.3) | 11 (0.9) | NS | |
| Changes in laboratory values, n/N (%) | ||||||||
| eGFR decrease ≥25% | 407/1583 (25.7) | 395/1621 (24.4) | 802/3204 (25.0) | 147/460 (32.0) | 121/448 (27.0) | 268/908 (29.5) | 0.008 | NS |
| eGFR decrease ≥50% | 42/1583 (2.7) | 43/1621 (2.7) | 85/3204 (2.7) | 28/460 (6.1) | 16/448 (3.6) | 44/908 (4.8) | 0.0025 | NS |
| Haematocrit decrease by 10 percentage points and value <LLN | 54/1538 (3.5) | 32/1566 (2.0) | 86/3104 (2.8) | 25/447 (5.6) | 10/431 (2.3) | 35/878 (4.0) | NS | NS |
| Haemoglobin decrease ≥3.0 g/dL and value <LLN | 70/1538 (4.6) | 48/1566 (3.1) | 118/3104 (3.8) | 30/447 (6.7) | 11/431 (2.6) | 41/878 (4.7) | NS | NS |
AE, adverse event; eGFR, estimated glomerular filtration rate; ITT, intent‐to‐treat; LLN, lower limit of normal; NS, not statistically significant; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SAE, serious adverse event.
Includes events/measurements from the day of first dose of study drug to 14 days after the last dose of study drug.
Based on chi‐square test for comparing the total between Q1–Q3 and Q4.
Based on the Chi‐square test for comparing among vericiguat/placebo and NT‐proBNP subgroups.
Adverse event associated with a fatal outcome.
Overall, the proportion of patients with an AE was similar between treatments and NT‐proBNP subgroups. The proportion of AEs leading to death was higher in patients in Q4 compared with Q1–Q3 (5.8% vs. 2.4%; P < 0.0001), and it was similar between patients treated with vericiguat and placebo. Compared with Q1–Q3, larger proportions of patients in Q4 had a serious AE (SAE; 38.3 vs 32.3%; P = 0.0001), mainly driven by a larger proportion of SAEs in the Q4 placebo group (42.5 vs. 32.2, 32.4, and 34.3% for Q1–Q3 placebo, Q1–Q3 vericiguat, and Q4 vericiguat, respectively; P = 0.0101). There were more treatment discontinuations due to an AE in Q4 than in Q1–Q3 (8.3 vs 5.8%) and more treatment discontinuations due to a SAE in the placebo Q4 subgroup compared with other subgroups (5.8 vs. 2.9, 2.7, and 2.9% for Q1–Q3 vericiguat, Q1–Q3 placebo, and Q4 vericiguat, respectively; P = 0.0254). These differences were not related to AEs of clinical interest (Table 3 ), and the proportions of drug‐related AEs and SAEs were similar between Q1–Q3 and Q4 and between vericiguat and placebo. For events of clinical interest, the proportion of patients with symptomatic hypotension and syncope did not differ significantly between Q1–Q3 and Q4 overall. The proportion of patients experiencing syncope or symptomatic hypotension was increased with vericiguat relative to placebo (P = 0.002) in Q1–Q3, as expected. 2 , 6 However, no differences in symptomatic hypotension were observed in Q4 (P = 0.210).
The incidence of haemoglobin decrease ≥3.0 g/dL and value <lower limit of normal (LLN) were similar in Q4 and Q1–Q3; however, the proportion of patients with the AE anaemia was lower in Q4 than Q1–Q3 (P = 0.0224). eGFR deceases of ≥50% were more frequent in patients in Q4 than in Q1–Q3 (4.8 vs. 2.7%; P = 0.0025). There was a trend for more frequent eGFR deceases of ≥50% in patients treated with vericiguat compared with placebo in Q4 (6.1 vs. 3.6%) that was not apparent in Q1–Q3 (2.7 vs. 2.7%); however, this difference between treatments and subgroups was not statistically significant.
Analyses of safety parameters for each region separately were generally consistent with the findings overall (Table S3 ).
Discussion
This post hoc analysis, using a combination of the NT‐proBNP quartile subgroups that were pre‐specified in the protocol, adds to our understanding of the results of the VICTORIA trial in patients with worsening HF. It is complementary to findings in a post hoc analysis of this landmark study published previously by Ezekowitz et al., 3 in which NT‐proBNP was analysed as a continuous variable. In their study, Ezekowitz et al. reported an association between treatment effects in patients with NT‐proBNP levels <4,000 pg/mL, which remained evident up to 8000 pg/mL, with both cut‐offs being based on visual inspection of the continuous relationship between outcomes and NT‐proBNP, and where the curve or the upper bound of the CI hits HR = 1.
Our analysis provides relevant novel insights into the data from VICTORIA in two respects. First, the NT‐proBNP cut‐offs in the analysis by Ezekowitz et al. were derived from post‐hoc analysis of the data. By contrast, the cut‐off in this analysis (5314 pg/mL) results from the prospectively pre‐specified NT‐proBNP quartiles in the study protocol, of which Q1, Q2, and Q3 were very similar in terms of outcomes but differed from Q4. Second, we explore the patient characteristics of Q1–Q3 and Q4 and the safety outcomes according to NT‐proBNP subgroup in greater detail, thereby extending findings published in Armstrong et al. 2 Understanding the clinical profile of patients who comprise NT‐proBNP Q1–Q3 and Q4 provides important insights to help us recognize under which clinical circumstances patients with HFrEF and a recent decompensation will benefit from vericiguat.
Outcomes
In line with previous analyses of the VICTORIA trial, 2 , 3 this post hoc evaluation demonstrated a reduction in the risk of CV death and HF hospitalization under vericiguat treatment in patients with baseline NT‐proBNP ≤5314 pg/mL (the Q1–Q3 group); and no significant difference in outcomes in patients with baseline NT‐proBNP >5314 pg/mL (the Q4 group). No interaction between treatment and NT‐proBNP quartile was observed across Quartiles 1–3, confirming that the effect of treatment differed in Q4 only.
The benefit of vericiguat on HF hospitalization and CV death in the Q1–Q3 group was similar in magnitude to the benefit in patients below the inflection point reported by Ezekowitz et al. (<4000 pg/mL). At the same time, Ezekowitz et al. demonstrated that clinical benefit remains evident and statistically significant up to a cut‐off of 8000 pg/mL, indicating that many patients in our protocol pre‐specified Q4 group still derived benefit from treatment with vericiguat. Although these results further corroborate the notion that plasma NT‐proBNP levels may help to identify patients with worsening HF with reduced ejection fraction (HFrEF) in whom the beneficial effects of vericiguat may be highest, they also add to the understanding that there is no single cut‐off value that can reliably identify patients who do not respond to treatment. This is consistent with the knowledge that NT‐proBNP levels are subject to dynamic intra‐individual changes but are also sensitive to factors unrelated to HF status, such as the deterioration of renal function. 7 , 8
Safety
A possible trend for increased incidence of the primary outcome was observed in the vericiguat group relative to placebo. Although the potential for risk of adverse outcomes with vericiguat in patients with the highest baseline levels of NT‐proBNP cannot be excluded based on these data, the HR for Q4 did not reach statistical significance, despite analyses not being corrected for multiplicity.
The rates of SAEs and discontinuations due to a SAE were higher in Q4 than in Q1–Q3, and this was not related to AEs of clinical interest associated with the mode of action of vericiguat. The higher rate of SAEs in Q4 overall was driven mainly by the placebo group; however, this finding may have been confounded by differences in the mortality rate between the treatment groups. The notably higher occurrence of SAEs in the placebo group in Q4 when compared with the vericiguat group warrants investigation in future analyses evaluating specific events by organ systems.
A non‐statistically significant trend for more frequent decreases in eGFR ≥50% in patients treated with vericiguat compared with those receiving placebo was observed in Q4. In a separate analysis of the VICTORIA trial, which addressed whether the beneficial effects of vericiguat were maintained across the full spectrum of eGFR, no significant interaction between baseline eGFR and the overall reduction of the primary endpoint by vericiguat was observed. 9 This analysis also demonstrated that the beneficial effects of vericiguat on the primary endpoint were similar, both in those patients who developed worsening renal function and those who did not. The higher rate of eGFR decreases of ≥50% with vericiguat in our analysis is consistent with a small initial eGFR decrease observed with other drugs, including sodium–glucose co‐transporter 2 (SGLT2) inhibitors, renin–angiotensin system blockers, and mineralocorticoid receptor blockers. 10 , 11 , 12 , 13 This decrease is believed to reflect a haemodynamic effect, occurs more often in patients with advanced kidney disease, 10 and does not negatively affect the benefit of treatment on long‐term clinical outcomes. 10 , 11 , 12 , 13
Of note, the titration regimen in the VICTORIA trial was blood pressure and symptom guided, and no differences in blood pressure changes or hypotension were seen in our analysis. In Q4, the lack of a treatment effect on symptomatic hypotension, an AE of clinical interest related to the mechanism of action of vericiguat, supports the conclusion that vericiguat is unlikely to pose a haemodynamic safety risk. This is consistent with the findings of Lam et al., who found vericiguat to be safe and haemodynamically tolerated, even in high‐risk subgroups of the VICTORIA trial population. 6
Factors relating to outcomes and safety
Differences in baseline characteristics between Q1–Q3 and Q4 indicated that patients in Q4 comprised a higher risk subgroup, which makes them more difficult to treat. This is evidenced by the fact that they were older, with lower eGFR, lower haemoglobin levels, higher heart rate, and higher incidence of atrial fibrillation, comprised a slightly larger proportion of patients with NYHA Class III/IV, and had a higher MAGGIC risk score. They were also more likely to be randomized while still hospitalized for HF. None of these differences was large enough individually to plausibly explain the observed large difference in treatment effect. Of note, a prognostic model developed using the VICTORIA dataset that included these factors as covariates did not identify any evidence of treatment modification stronger than that observed with baseline NT‐proBNP. 2 , 3 The higher rates of the primary outcome and its components, as well as all‐cause deaths in Q4 compared with Q1–Q3, which were apparent in both the placebo and vericiguat groups, also indicate a greater severity of disease in patients in Q4. The data presented in this paper demonstrate that, while the entire population in the VICTORIA trial was a high‐risk population (having had a recent HF decompensation), the subgroup of patients in Q4 were at particularly high risk.
It is possible to hypothesize that some patients in Q4 may have been too far advanced in their HF pathophysiology to derive significant benefit from pharmacotherapy. The outcomes in the highest quartile are comparable to the INTERMACS study, Profiles 5–6. 14 By contrast, time‐to‐event rates and absolute risk reduction (ARR) for CV death in Q1–Q3 are similar to those reported in lower risk trials. 15 , 16 , 17 The improvement in clinical outcomes in Q1–Q3 only, and lack of clinical response in Q4, underlines our limited understanding of the trajectory of patients in the range of baseline NT‐proBNP in Q4, as they are not frequently seen in clinical practice and are under‐represented in other recent HF trials (PARADIGM‐HF, DAPA‐HF, and EMPEROR‐REDUCED), 15 , 16 , 17 , 18 or proving refractory to treatment, as in the advanced HF trial LIFE (although the patients in LIFE still had far lower NT‐proBNP than the patients in Q4 of the current analysis). 19 Consistent with the hypothesis that patients with very advanced disease are unable to benefit from pharmacotherapy, clinical trials with other HF therapies have shown that patients in NYHA Class IV are less likely to benefit from treatment. 16 , 20 The VICTOR study (NCT05093933) will further examine the efficacy and safety of vericiguat in patients with HFrEF without a recent decompensation event, who may be considered at lower risk than the VICTORIA population.
The magnitude of benefit relative to proximity to the index event and whether the patient was clinically stable following decompensation are further interesting questions. A separate analysis of VICTORIA trial data that examined outcomes by pre‐specified index event subgroups (<3 months after HF hospitalization, 3–6 months after HF hospitalization, and those requiring outpatient intravenous diuretic therapy only for worsening HF), as well as more finely divided subgroups, indicated that the risk of the primary outcome was greater in patients closest to their index hospitalization. 21 Vericiguat reduced the risk of the primary outcome in all pre‐specified subgroups, without evidence of treatment heterogeneity; however, analysis of the continuous association between time since index event and treatment effect showed a trend towards increased benefit of vericiguat with longer time since index event. In the VICTORIA trial, patients after a HF decompensation event were required to be stabilized on adequate background HF therapy according to local standard of care, prior to initiation of vericiguat therapy. Rapid changes in haemodynamics and fluid status in this period may have influenced the treatment outcomes. As patients in Q4 were more likely to have experienced HF hospitalization within 3 months and be randomized while hospitalized, a larger proportion of patients in this quartile may have been insufficiently clinically stabilized to derive a benefit from treatment. Trial data are not available to fully elucidate changes in therapy in this narrow time window. Future analyses should elucidate whether more thorough optimization of background HF therapy in the Q1–Q3 group was associated with the more favourable treatment benefit with vericiguat.
A further potential reason for lower drug efficacy in the Q4 group could be suboptimal dosing. The proportion of patients able to reach the maximum dose of 10 mg of study medication was lower in Q4 than in the Q1–Q3 group. As the rates of target‐dose achievement were similar for vericiguat and placebo in both Q1–Q3 and Q4, the lower rate in Q4 was likely a result of greater severity of HF compared with Q1–Q3. Minimal differences in the pharmacokinetics of vericiguat were reported in Q4 relative to other NT‐proBNP quartiles, 4 with individual predicted areas under the concentration–time curve being comparable across the four quartiles (results pending publication). These findings indicate that the heterogeneity of treatment effect did not result from a difference in exposure to vericiguat in Q4.
Limitations
The cut‐off used to compare patients below vs. those above the 75th percentile of baseline NT‐proBNP was among pre‐specified quartiles in the original study protocol and analysis plan. Nevertheless, it is a post hoc analysis and results presented must be regarded as exploratory and hypothesis‐generating. We presented analyses adjusted for the stratification factors, as in the original analysis plan, and did not conduct multivariable analyses. However, it is unlikely that such analysis would have changed the conclusions, given that Ezekowitz et al. 3 confirmed NT‐proBNP as the strongest independent predictor of treatment effect in the VICTORIA trial, after multiple adjustments (including the MAGGIC score and the VICTORIA risk model that that adjusted for a comprehensive list of baseline parameters).
We did not evaluate pharmacokinetics to relate drug exposure to treatment effect and the influence of up‐titration and down‐titration of study drug after the initial period. Analysis of change in NT‐proBNP is limited by intercurrent events in this high‐mortality population. There is also a lack of data on congestion and oedema, which would be clinically relevant in optimizing patient volume status after a worsening chronic HF event. Differences between the Q1–Q3 and Q4 groups in the number of dose adjustments of other HF‐related medications may have affected the findings. Comprehensive analysis of these dose adjustments is beyond the scope of this article; however, it will be addressed in a future dedicated manuscript. There may be additional confounders of the relationship between vericiguat treatment effect and NT‐proBNP and other biomarkers. All these factors should be subject to future analyses.
Conclusion
Evidence of the positive clinical benefit on reducing HF hospitalization and CV mortality observed in the overall high‐risk HFrEF study population appears strongest in patients with baseline plasma NT‐proBNP below the 75th percentile in the VICTORIA trial. These results further corroborate the notion that plasma NT‐proBNP levels may help to identify patients with worsening HFrEF in whom the beneficial effects of vericiguat may be highest. There is no single cut‐off value that can reliably identify patients who do not respond to treatment. We hypothesize that patients with the highest NT‐proBNP values are probably too far advanced, suffering more co‐morbidities, or may not have been sufficiently clinically stabilized to derive benefit from vericiguat. Therefore, very high NT‐proBNP may indicate the requirement to first optimize the volume status before initiation of vericiguat will be beneficial. Moreover, the patient clinical journey with potential changes in NT‐proBNP plasma levels over time should be taken into consideration.
Conflict of interest
RN, CF, VMV, and LR are employees of Bayer AG and may own stock in the company. ACS has received honoraria from Bayer AG and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), during the conduct of the study; and personal fees from Novartis, Abbott, Sanofi, Vifor, Astra Zeneca, Servier, Leo Pharma, and Boehringer Ingelheim, outside the submitted work. JLS has received research grants from MSD, Angem, Pfizer, and Bayer AG; honoraria from Menarini; support for attending meetings from Pfizer; and support for participation on advisory boards from Bayer AG. MS has received consulting fees from Bayer AG and MSD. PP has received consulting fees from Boehringer Ingelheim, AstraZeneca, Vifor Pharma, Amgen, Servier, Novartis, Bayer, MSD, Pfizer, Cibiem, Impulse Dynamics, Renal Guard Solutions, and BMS. PP has also received honoraria from Boehringer Ingelheim, AstraZeneca, Vifor Pharma, Amgen, Servier, Novartis, Berlin Chemie, Bayer, Pfizer, Impulse Dynamics, Renal Guard Solutions, BMS, and Abbott Vascular for lectures, presentations, speakers' bureaus, manuscript writing, or educational events. BP has received research funds from Bayer Healthcare, Servier, and AstraZeneca, as well as speakers honoraria/committee membership fees from Novartis, Bayer Healthcare, Daiichi‐Sankyo, MSD, Stealth Peptides, AstraZeneca, Sanofi, Vifor, and Servier.
Funding
This research was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, in collaboration with Bayer AG, Wuppertal, Germany.
Supporting information
Table S1. Baseline NT‐proBNP (pg/mL) by region.
Table S2. Study medication dose at end of initial titration period.
Table S3. Summary of AEs and laboratory changes from baseline in patients with baseline NT‐proBNP levels in quartiles 1–3 (Q1–3) and quartile 4 (Q4) in America, Asia, and Europe – all patients as treated.
Acknowledgements
The authors wish to thank the investigators, staff, and participants in the VICTORIA trial. Medical writing support was provided by Laila Guzadhur, PhD, and editorial support was provided by Ian Norton, PhD, both of Scion, London, UK, supported by Bayer AG, Wuppertal, Germany, and Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, according to Good Publication Practice guidelines (http://annals.org/aim/article/2424869/good‐publication‐practice‐communicating‐company‐sponsored‐medical‐research‐gpp3). The Sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Open Access funding enabled and organized by Projekt DEAL. [Correction added on 29 July 2022, after first online publication: Projekt DEAL funding statement has been added.]
Senni, M. , Lopez‐Sendon, J. , Cohen‐Solal, A. , Ponikowski, P. , Nkulikiyinka, R. , Freitas, C. , Vlajnic, V. M. , Roessig, L. , and Pieske, B. (2022) Vericiguat and NT‐proBNP in patients with heart failure with reduced ejection fraction: analyses from the VICTORIA trial. ESC Heart Failure, 9: 3791–3803. 10.1002/ehf2.14050.
References
- 1. Gaggin HK, Truong QA, Rehman SU, Mohammed AA, Bhardwaj A, Parks KA, Sullivan DA, Chen‐Tournoux A, Moore SA, Richards AM, Troughton RW, Lainchbury JG, Weiner RB, Baggish AL, Semigran MJ, Januzzi JL Jr. Characterization and prediction of natriuretic peptide “nonresponse” during heart failure management: Results from the ProBNP outpatient tailored chronic heart failure (PROTECT) and the NT‐proBNP‐assisted treatment to lessen serial cardiac readmissions and death (BATTLESCARRED) study. Congest Heart Fail. 2013; 19: 135–142. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O'Connor CM. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020; 382: 1883–1893. [DOI] [PubMed] [Google Scholar]
- 3. Ezekowitz JA, O'Connor CM, Troughton RW, Alemayehu WG, Westerhout CM, Voors AA, Butler J, Lam CSP, Ponikowski P, Emdin M, Patel MJ, Pieske B, Roessig L, Hernandez AF, Armstrong PW. N‐terminal pro‐B‐type natriuretic peptide and clinical outcomes: Vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020; 8: 931–939. [DOI] [PubMed] [Google Scholar]
- 4. Food and Drug Administration . Verquvo™ (vericiguat) tablets. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf. Accessed Feb 08, 2021.
- 5. Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: The VICTORIA trial. JACC Heart Fail. 2018; 6: 96–104. [DOI] [PubMed] [Google Scholar]
- 6. Lam C, Mulder H, Lopatin Y, Vazquez‐Tanus JB, Siu D, Ezekowitz J, Pieske B, O'Connor CM, Roessig L, Patel MJ, Anstrom KJ, Hernandez AF, Armstrong PW, VICTORIA Study Group . Blood pressure and safety events with Vericiguat in the VICTORIA trial. J Am Heart Assoc. 2021; 10: e021094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berge K, Lyngbakken MN, Myhre PL, Brynildsen J, Røysland R, Strand H, Christensen G, Høiseth AD, Omland T, Røsjø H. High‐sensitivity cardiac troponin T and N‐terminal pro‐B‐type natriuretic peptide in acute heart failure: Data from the ACE 2 study. Clin Biochem. 2021; 88: 30–36. [DOI] [PubMed] [Google Scholar]
- 8. Anwaruddin S, Lloyd‐Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL Jr. Renal function, congestive heart failure, and amino‐terminal pro‐brain natriuretic peptide measurement. J Am Coll Cardiol. 2006; 47: 91–97. [DOI] [PubMed] [Google Scholar]
- 9. Voors AA, Mulder H, Reyes E, Cowie MR, Lassus J, Hernandez AF, Ezekowitz JA, Butler J, O'Connor CM, Koglin J, Lam CSP, Pieske B, Roessig L, Ponikowski P, Anstrom KJ, Armstrong PW, for the VICTORIA Study Group . Renal function and the effects of vericiguat in patients with worsening heart failure with reduced ejection fraction: Insights from the VICTORIA (Vericiguat global study in subjects with HFrEF) trial. Eur J Heart Fail. 2021; 23: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kraus BJ, Weir MR, Bakris GL, Mattheus M, Cherney DZI, Sattar N, Heerspink HJL, Ritter I, von Eynatten M, Zinman B, Inzucchi SE, Wanner C, Koitka‐Weber A. Characterization and implications of the initial estimated glomerular filtration rate ‘dip’ upon sodium‐glucose cotransporter‐2 inhibition with empagliflozin in the EMPA‐REG OUTCOME trial. Kidney Int. 2021; 99: 750–762. [DOI] [PubMed] [Google Scholar]
- 11. Oshima M, Jardine MJ, Agarwal R, Bakris G, Cannon CP, Charytan DM, de Zeeuw D, Edwards R, Greene T, Levin A, Lim SK, Mahaffey KW, Neal B, Pollock C, Rosenthal N, Wheeler DC, Zhang H, Zinman B, Perkovic V, Heerspink HJL. Insights from CREDENCE trial indicate an acute drop in estimated glomerular filtration rate during treatment with canagliflozin with implications for clinical practice. Kidney Int. 2021; 99: 999–1009. [DOI] [PubMed] [Google Scholar]
- 12. Holtkamp FA, de Zeeuw D, Thomas MC, Cooper ME, de Graeff PA, Hillege HJL, Parving HH, Brenner BM, Shahinfar S, Lambers Heerspink HJ. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long‐term renal function. Kidney Int. 2011; 80: 282–287. [DOI] [PubMed] [Google Scholar]
- 13. Morales E, Millet VG, Rojas‐Rivera J, Huerta A, Gutierrez E, Gutierrez‐Solis E, Egido J, Praga M. Renoprotective effects of mineralocorticoid receptor blockers in patients with proteinuric kidney diseases. Nephrol Dial Transplant. 2013; 28: 405–412. [DOI] [PubMed] [Google Scholar]
- 14. Samman‐Tahhan A, Hedley JS, McCue AA, Bjork JB, Georgiopoulou VV, Morris AA, Butler J, Kalogeropoulos AP. INTERMACS profiles and outcomes among non‐inotrope‐dependent outpatients with heart failure and reduced ejection fraction. JACC Heart Fail. 2018; 6: 743–753. [DOI] [PubMed] [Google Scholar]
- 15. Butler J, Anstrom K, Armstrong P. Comparing the benefit of novel therapies across clinical trials. Circulation. 2020; 142: 717–719. [DOI] [PubMed] [Google Scholar]
- 16. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 17. Anker SD, Butler J, Filippatos G, Khan MS, Marx N, Lam CSP, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, Bocchi EA, Ponikowski P, Perrone SV, Januzzi JL, Verma S, Böhm M, Ferreira JP, Pocock SJ, Zannad F, Packer M, On behalf of the EMPEROR‐Reduced Trial Committees and Investigators . Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: Results from the EMPEROR‐Reduced trial. Circulation. 2021; 143: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 19. Mann DL. LCZ696 in advanced heart failure ‐ LIFE. American College of Cardiology Virtual Annual Scientific Session 2021.
- 20. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐la Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 21. Lam CSP, Giczewska A, Sliwa K, Edelmann F, Refsgaard J, Bocchi E, Ezekowitz JA, Hernandez AF, O'Connor CM, Roessig L, Patel MJ. Clinical outcomes and response to vericiguat according to index heart failure event: Insights from the VICTORIA trial. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline NT‐proBNP (pg/mL) by region.
Table S2. Study medication dose at end of initial titration period.
Table S3. Summary of AEs and laboratory changes from baseline in patients with baseline NT‐proBNP levels in quartiles 1–3 (Q1–3) and quartile 4 (Q4) in America, Asia, and Europe – all patients as treated.


