Abstract
Study objective
The coronavirus disease 2019 (COVID-19) outbreak has caused a severe burden on medical professionals, as the rapid disposition of patients is important. Therefore, we aimed to develop a new clinical assessment tool based on the shock index (SI) and age-shock index (ASI). We proposed the hypoxia-age-shock index (HASI) and determined the usability of triage for COVID-19 infected patients in the first scene.
Methods
The predictive power for three indexes on mortality, intensive care unit (ICU) admission, and endotracheal intubation rate was evaluated using the receiver operating curve (ROC). We used DeLong's method for comparing the ROCs.
Results
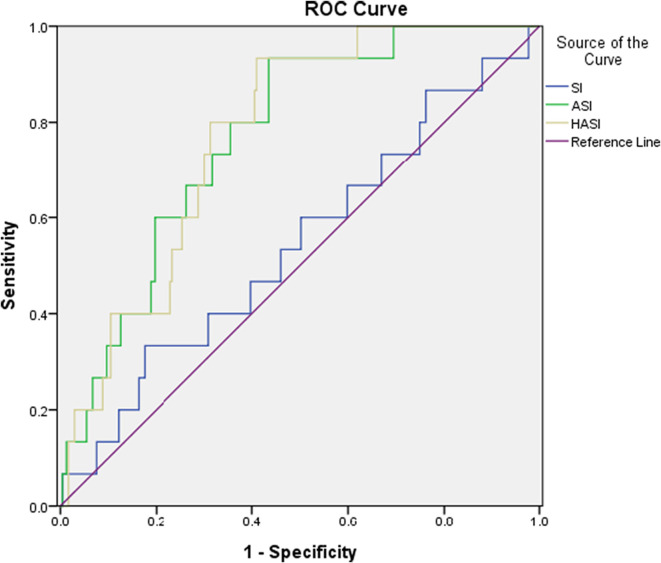
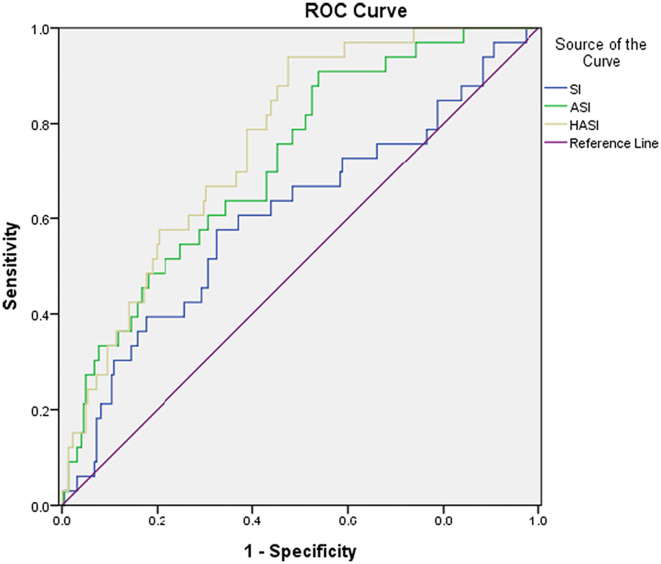
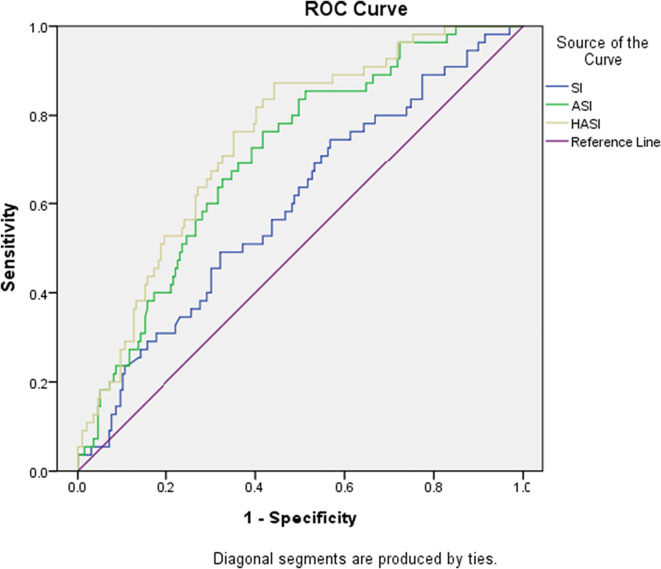
The area under the curve (AUC) for ROC on mortality for SI, ASI, and HASI were 0.546, 0.771, and 0.773, respectively. The AUC on ICU admission mortality for SI, ASI, and HASI were 0.581, 0.700, and 0.743, respectively. The AUC for intubation for SI, ASI, and HASI were 0.592, 0.708, and 0.757, respectively. The AUC differences between HASI and SI showed statistically significant (P = 0.001) results on mortality, ICU admission, and intubation. Additionally, statistically significant results were found for the AUC difference between the HASI and ASI on ICU admission and intubation (P = 0.001 and P = 0.004, respectively).
Conclusion
HASI can provide a better prediction compared to ASI on ICU admission and endotracheal intubation. HASI was more sensitive in mortality, ICU admission, and intubation prediction than the ASI.
Keywords: Critical care medicine, Respiratory distress, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19), a viral illness resulting from severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections, caused >6 million deaths worldwide [1]. Since the outbreak of COVID-19 pandemic, the health-care system has been stressed to overcome the relentless wave of patients. The burden of dealing with this illness usually falls on the emergency department. Therefore, a rapid deposition strategy is urgently required to resolve massive numbers of incoming patients efficiently.
The shock index (SI) and age shock index (ASI) have been proven valid for mortality prediction in patients with septic shock [2]. SI was initially proposed in 1967 by Allgöwer et al. [3] and was proven useful in predicting traumatic hypovolemic shock and septic shock patients [4]. While the discrimination made by SI for probability of the intensive care unit (ICU) admission for COVID-19 patients remains controversial [5], there is evidence demonstrating the utility of in-hospital mortality of the COVID-19 infected population. There is no doubt that there are correlations between vital signs during the presentation and the prognosis of COVID-19 patients. Previous literature also confirmed the validity of shock index on predicting the mortality of COVID infected patients [6,7].
A cardinal manifestation of COVID-19 is respiratory distress, which often presents as hypoxemia. However, this key element was not included in the SI. Medical professionals also found hypoxia, a critical prognostic factor, could be insidious until decompensation occurred [8,9]. The importance of oxygen saturation cannot be overestimated. However, this variable was not included in the classical shock index. Based on these premises, we aimed to develop a clinical tool for assessing the risk of poor outcome with the severity of hypoxia and SI.
For a more rapid deposition and efficient management of patients, we aimed to develop a convenient and reliable score to evaluate incoming patients. Therefore, we constructed a reliable measure based on age, respiratory distress, and shock index while identifying an appropriate cut-off point for judgment of future severity.
2. Methods
2.1. Study design and setting
This is a retrospective cohort study performed at Far Eastern Memorial Hospital in New Taipei City, Taiwan, from May 1, 2021 to July 31, 2021. Our study was approved by the Institutional Review Board of Far Eastern Memorial Hospital (case number: 110233-E).
2.2. Patient selection
We included patients admitted to our hospital with a confirmed diagnosis via SARS-CoV-2 RT-PCR via oropharyngeal or nasopharyngeal swabs with age over 18 years old. Additionally, we double confirmed the diagnosis of COVID-19 with the ICD-10 record by another personnel. Whereas exclusion criteria were as follows: previous refusal to participate in scientific research, inter-facility transfer of patients initially admitted to another hospital, patients with multiple infectious diseases (COVID-19 combined with other infectious diseases), patients with COVID-19 and other medical emergencies (surgical emergencies or pathologies with a high risk of fatal outcome), and patients discharged from or who died at the emergency department. Informed consent was not necessary because our study was observational and retrospective.
2.3. Data extraction
We described the demographic data obtained from the emergency department triage and the laboratory data of the patients. The vital sign used in our analysis was obtained at the outdoor triage station of the emergency department. The SI presented the ratio of heart rate to systolic blood pressure at emergency department triage. The ASI is the product of the age in years and SI. In addition, we combined the variables of age, SI, and oxygen saturation into the hypoxia-age-shock index (HASI) using the following formula:
Laboratory data were collected including leukocyte count, hemoglobin level, platelet count, alanine transaminase (ALT), creatinine, C-reactive protein (CRP), D-dimer, sodium, potassium, and ferritin levels.
2.4. Outcome measured
The outcome of interest in the included patients was the power of the prediction of SI, ASI, and HASI on ICU admission, endotracheal intubation, and in-hospital mortality. ICU admission was defined as either immediate ICU admission from the emergency department or in-hospital transferal from the quarantine ward to ICU due to deterioration.
2.5. Statistical analysis
Descriptive statistics were used to describe the data from the included studies. Individuals were classified into the ICU admission and non-ICU admission groups. To measure intergroup differences, an independent t-test was used for continuous variables. The Chi-square test was used for the analysis of categorical variables. Whereas Fisher's exact test was applied for categorical variables with fewer than five objects in individual disease entities.
Receiver operating curves (ROC) for SI, ASI, and HASI were sketched to assess the power of prediction for the rates of ICU admission, intubation, and in-hospital mortality. To compare the respective powers of prediction, the area under the curve (AUC) was compared for each ROC curve of SI, ASI, and HASI using the DeLong's method. Additionally, the best cut-off point for each index was determined by the Youden method [10], which provides the optimal standard of index for categorizing the status of the outcome [10]. Statistical significance was set at p-value <0.05. Statistical analyses were performed using MedCalc statistical software (MedCalc Software Ltd., 2022) and IBM SPSS statistical software (version 24.0; SPSS Inc., Chicago, 136 IL).
3. Results
The demographic data of the 262 patients (18–90; mean age 56.19) included in our analysis is shown in Table 1 . The study included 145 male patients and 117 female patients. The distribution of major medical history was not significantly different between the ICU and non-ICU admission groups. Among all patients, 56 were admitted to the ICU. Thirty-three out of 34 intubated patients were transferred to the ICU, while one patient died before transfer. Additionally, 15 patients died in the study population.
Table 1.
Demographic data of included population.
| Total | ICU admission | Non-ICU admission | P value | ||
|---|---|---|---|---|---|
| Total patients | 262 (100%) | 56 | 206 | ||
| Age | 57 ± 17.97 | 64.02 ± 10.84 | 54.10 ± 19.09 | <0.001** | |
| Sex | Male | 145 | 36 | 109 | 0.130 |
| Female | 117 | 20 | 97 | ||
| Body weight | 65.91 ± 16.93 | 69.70 ± 15.22 | 64.86 ± 17.31 | 0.058 | |
| Past medical history | |||||
| Nil | 85 | 16 | 69 | 0.485 | |
| Hypertension | 112 | 26 | 86 | 0.531 | |
| Cardiac diseases | 27 | 9 | 18 | 0.109 | |
| Diabetes mellitus | 59 | 17 | 42 | 0.113 | |
| Cerebrovascular accident/other neurological disorder | 9 | 1 | 8 | 0.689 | |
| Impairment of renal function | 7 | 3 | 4 | 0.170 | |
| Respiratory system/Airway disorder | 6 | 1 | 5 | 1.000 | |
| Liver diseases | 10 | 3 | 7 | 0.450 | |
| Malignancy | 12 | 3 | 9 | 0.723 | |
| Others | 32 | 6 | 26 | 0.699 | |
| Index | |||||
| Shock index | 0.80 ± 0.22 | 0.855 ± 0.252 | 0.784 ± 0.208 | 0.033* | |
| Age-shock index | 44.63 ± 17.44 | 53.948 ± 17.057 | 42.024 ± 16.684 | <0.001** | |
| Hypoxia-age-shock index | 0.48 ± 0.22 | 0.638 ± 0.257 | 0.446 ± 0.193 | <0.001** | |
| Outcome | |||||
| Intubation | 35 | 34 | 1 | <0.001** | |
| mortality | 15 | 11 | 4 | <0.001** | |
*P value < 0.05, **P value < 0.01.
A summary of laboratory findings is presented in Table 2 . For all included laboratory data, leukocyte count, platelet count, D-dimer, ALT, ferritin, and CRP levels were significantly different between the two groups. Furthermore, the difference in CRP levels was the most profound (P < 0.001).
Table 2.
Laboratory findings between groups
| Total patients | ICU admission | Non-ICU admission | P value | |
|---|---|---|---|---|
| Leukocyte count (10^3/μL) | 7.04 ± 2.93 | 7.89 ± 3.91 | 6.80 ± 2.55 | 0.049* |
| Hb level (g/dL) | 13.56 ± 1.82 | 13.55 ± 1.77 | 13.56 ± 1.85 | 0.948 |
| Platelet count (10^3/μL) | 209.12 ± 97.83 | 181.55 ± 67.34 | 216.80 ± 103.60 | 0.017* |
| D-dimer (μg/mL FEU) | 873.90 ± 1040.01 | 1300.97 ± 1363.41 | 765.51 ± 513.51 | 0.011* |
| ALT (U/L) | 31.79 ± 39.71 | 54.02 ± 70.27 | 25.70 ± 21.55 | 0.005** |
| Creatinine (mg/dL) | 1.30 ± 4.45 | 1.24 ± 1.26 | 1.32 ± 4.99 | 0.897 |
| Ferritin (ng/mL) | 1112.28 ± 2173.83 | 2046.63 ± 3691.98 | 837.75 ± 1353.72 | 0.018* |
| CRP (mg/dL) | 5.61 ± 7.40 | 9.91 ± 7.18 | 4.38 ± 7.01 | <0.001*** |
| Sodium (mmol/L) | 133.97 ± 9.49 | 133.12 ± 4.57 | 134.21 ± 10.48 | 0.446 |
| Potassium (mmol/L) | 3.74 ± 0.59 | 3.86 ± 0.68 | 3.70 ± 0.56 | 0.072 |
*P value < 0.05, **P value <0.01, ***P value <0.001.
We also compared the power of prediction between SI, ASI, and HASI for predicting the outcomes of COVID-19 patients, which are demonstrated in Table 3, Table 4 and Fig. 1, Fig. 2, Fig. 3 . ASI and HASI both showed better results than SI, with an AUC > 0.7 in the prediction of intubation rate, ICU admission, and mortality rate. Additionally, from the perspective of intubation and ICU admission, HASI had better performance on detection (ICU admission: AUC of HASI = 0.743 vs. AUC of ASI = 0.700, p < 0.05; intubation: AUC of HASI = 0.757 vs. AUC of ASI = 0.708, p < 0.05).
Table 3.
Summary of ROCs for SI, ASI, and HASI for prediction for rate of ICU admission, intubation, and mortality.
| Cutoff point | Sensitivity | Specificity | PPV | NPV | AUC (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| ICU admission | |||||||
| SI | 0.72 | 72.73% | 43.00% | 25.98% | 85.15% | 0.581 (0.518–0.643) | 0.031* |
| ASI | 42.46 | 74.55% | 58.50% | 33.07% | 89.31% | 0.700 (0.639–0.756) | <0.001*** |
| HASI | 0.43 | 87.50% | 55.78% | 35.24% | 94.19% | 0.743 (0.685–0.796) | <0.001*** |
| Intubation | |||||||
| SI | 0.85 | 55.88% | 67.57% | 20.88% | 90.91% | 0.592 (0.529–0.653) | 0.047* |
| ASI | 39.55 | 88.24% | 46.40% | 20.13% | 96.26% | 0.708 (0.648–0.763) | <0.001*** |
| HASI | 0.43 | 94.29% | 54.29% | 24.01% | 98.41% | 0.757 (0.699–0.808) | <0.001*** |
| Mortality | |||||||
| SI | 0.98 | 33.33% | 82.57% | 10.64% | 95.21% | 0.546 (0.482–0.608) | 0.568 |
| ASI | 44.35 | 93.33% | 56.85% | 11.87 | 99.27 | 0.771 (0.725–0.821) | <0.001*** |
| HASI | 0.48 | 93.33% | 58.92% | 12.39% | 99.30% | 0.773 (0.716–0.823) | <0.001 |
*P value < 0.05, **P value < 0.01, ***P value < 0.001.
Table 4.
Comparison between ROC of SI, ASI, and HASI on prediction of ICU admission, intubation, and mortality.
| AUC difference | Standard error | 95% confidence interval | P value | |
|---|---|---|---|---|
| ICU admission | ||||
| SI-ASI | 0.119 | 0.037 | 0.047–0.190 | 0.001** |
| SI-HASI | 0.162 | 0.039 | 0.086–0.238 | <0.001*** |
| ASI-HASI | 0.044 | 0.012 | 0.019–0.068 | <0.001*** |
| Intubation | ||||
| SI-ASI | 0.116 | 0.039 | 0.040–0.192 | 0.003** |
| SI-HASI | 0.165 | 0.0432 | 0.080–0.249 | <0.001*** |
| ASI-HASI | 0.049 | 0.017 | 0.015–0.082 | 0.004** |
| Mortality | ||||
| SI-ASI | 0.226 | 0.056 | 0.116–0.336 | <0.001*** |
| SI-HASI | 0.227 | 0.060 | 0.109–0.345 | <0.001*** |
| ASI-HASI | 0.001 | 0.014 | −0.026-0.029 | 0.921 |
*P value < 0.05, **P value < 0.01, ***P value < 0.00.
Fig. 1.
The ROCs of SI, ASI, and HASI for mortality.
Fig. 2.
The ROCs of SI, ASI, and HASI for intubation.
Fig. 3.
The ROCs of SI, ASI, and HASI for ICU admission.
3.1. Limitations
However, our study has limitations that are worth mentioning. First, the retrospective and single-center nature of our study cause inevitable selection bias. In addition, the vital signs for calculating the shock index series may fluctuate dramatically upon arrival at the emergency department. Several factors, including medication history, environmental stimulation, and even waiting time, may influence the measurement and result in uncertainty.
4. Discussion
Shock indices have been widely used to evaluate acutely ill patients. Considering hypoxia, it was assumed that the new modified HASI could provide a better prediction of grave outcomes. Indeed, our study showed that HASI could offer a better prediction of the rate of intubation and ICU admission than ASI.
Previous studies have shown a correlation between low oxygen saturation levels and decreased survival rates [11]. Other risk factors that may be associated with mortality include, but are not limited to old age [12], chronic illness, obesity, or abnormality in laboratory results [7]. Baccolini et al. [13] have found that COVID infected patients may be more vulnerable to health-care-associated infections, contributing to the higher in-hospital mortality rate. Image findings had also been considered as in-hospital mortality indicators [14].
The rates of intubation and ICU admission were less profound for HASI than for death in our study. Nonetheless, we believe that the necessity for invasive mechanical ventilation and intensive care will cause difficulties for clinicians in patient disposition and resource allocation. Using the cutoff point of the HASI for vital signs acquired at the emergency department triage, we can categorize the patient as requiring intensive monitoring and aggressive intervention procedures as early as possible.
Numerous tools have been used to assess clinical outcomes. Chen et al. [15] used the CANPT score to predict severe disease with the presence of comorbidities, body temperature, and other laboratory findings. Ji et al. [16] applied the CALL score for disease progression using albumin, creatinine, and the neutrophil-to-lymphocyte ratio. In one Taiwanese study, Chen et al. [17] compared the other scoring system for predicting patient outcomes with the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) score, Coronavirus Clinical Characterisation Consortium Mortality (4C Mortality) score, SpO2, Obesity, Age, Respiratory rate, Stroke history (SOARS) score, and Veterans Health Administration COVID-19 (VACO) index. The 4C Mortality score has an AUC of 0.8 for predicting mortality rate in patients >60 years old. In contrast, our study proposed a more convenient and non-inferior HASI, for which medical history and laboratory data are unnecessary. It is especially useful in an overloaded emergency department during a pandemic. Moreover, our index also provides comprehensive predictive power for mortality, intubation, and ICU admission, which can assist clinical decisions in crowded emergency room scenarios.
In conclusion, we developed a new clinical assessment tool, the HASI, and found a superior prediction of intubation and ICU admission rates. Abnormalities in biochemical and hematological surveys are related to the rate of ICU admission. Further studies are required to obtain robust results for HASI, so that it can be applied in future clinical scenarios.
Funding
This study was supported by a grant from Far Eastern Memorial Hospital (FEMH-2022-C-035).
Declaration of Competing Interest
No conflict of interest.
Acknowledgement
We pay tribute to all dedicated frontline health care professionals during the COVID-19 pandemic.
Special thanks go to Dr. Man-Ju Ting, Dr Jen-Tang Sun, Dr. Chien-Min Fan and Professor Chung-Ta Chang for useful comments and for the study on age shock index in COVID-19.
This article was subsidized for English editing by National Taiwan University under the Excellence Improvement Program for Doctoral Students (grant number 108-2926-I-002-002-MY4), sponsored by National Science and Technology Council, Taiwan.
References
- 1.Cascella M., Rajnik M., Aleem A., et al. StatPearls Publishing; Treasure Island (FL): June 30, 2022. Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls. [PubMed] [Google Scholar]
- 2.Berger T., Green J., Horeczko T., et al. Shock index and early recognition of sepsis in the emergency department: pilot study. West J Emerg Med. 2013;14:168–174. doi: 10.5811/westjem.2012.8.11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allgöwer M., Burri C. “Schockindex” [“Shock index”] Dtsch Med Wochenschr. 1967;92:1947–1950. doi: 10.1055/s-0028-1106070. [DOI] [PubMed] [Google Scholar]
- 4.Al Jalbout N., Balhara K.S., Hamade B., et al. Shock index as a predictor of hospital admission and inpatient mortality in a US national database of emergency departments. Emerg Med J. 2019;36:293–297. doi: 10.1136/emermed-2018-208002. [DOI] [PubMed] [Google Scholar]
- 5.van Rensen I.H.T., Hensgens K.R.C., Lekx A.W., et al. Early detection of hospitalized patients with COVID-19 at high risk of clinical deterioration: utility of emergency department shock index. Am J Emerg Med. 2021;49:76–79. doi: 10.1016/j.ajem.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doğanay F., Elkonca F., Seyhan A.U., Yılmaz E., Batırel A., Ak R. Shock index as a predictor of mortality among the Covid-19 patients. Am J Emerg Med. 2021;40:106–109. doi: 10.1016/j.ajem.2020.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jouffroy R., Brami E., Scannavino M., et al. Association between prehospital shock index and mortality among patients with COVID-19 disease. Am J Emerg Med. 2022;56:133–136. doi: 10.1016/j.ajem.2022.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman A., Tabassum T., Araf Y., et al. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep. 2021;48:3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo Marin B., Aghagoli G., Lavine K., et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31:1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruopp M.D., Perkins N.J., Whitcomb B.W., et al. Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430. doi: 10.1002/bimj.200710415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie J., Covassin N., Fan Z., et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Belda A.B., Fernández-Garcés M., Mateo-Sanchis E., et al. COVID-19 in older adults: what are the differences with younger patients? Geriatr Gerontol Int. 2021;21:60–65. doi: 10.1111/ggi.14102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baccolini V., Migliara G., Isonne C., et al. The impact of the COVID-19 pandemic on healthcare-associated infections in intensive care unit patients: a retrospective cohort study. Antimicrob Resist Infect Control. 2021;10:87. doi: 10.1186/s13756-021-00959-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y., Xiao A., Yu X., et al. Development and validation of a prognostic nomogram based on clinical and CT features for adverse outcome prediction in patients with COVID-19. Korean J Radiol. 2020;21:1007–1017. doi: 10.3348/kjr.2020.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Zhou X., Yan H., et al. CANPT score: a tool to predict severe COVID-19 on admission. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.608107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji D., Zhang D., Xu J., et al. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71:1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T.-H., Chen C., Jo S.-Y., et al. Discriminant ability of in-hospital mortality based on prognostic scores of elderly patients with COVID-19: a Taiwan medical center study. Int J Gerontol. 2022;16:202–206. doi: 10.6890/IJGE.202207_16(3).0007. [DOI] [Google Scholar]