Abstract
Objective
Patients hospitalized with COVID-19 and hyperglycemia require frequent glucose monitoring, usually performed with glucometers. Continuous glucose monitors (CGMs) are common in the outpatient setting but not yet approved for hospital use. We evaluated CGM accuracy, safety for insulin dosing, and CGM clinical reliability in 20 adult patients hospitalized with COVID-19 and hyperglycemia.
Methods
Study patients were fitted with a remotely monitored CGM. CGM values were evaluated against glucometer readings. The CGM sensor calibration was performed if necessary. CGM values were used to dose insulin, without glucometer confirmation.
Results
CGM accuracy against glucometer, expressed as mean absolute relative difference (MARD), was calculated using 812 paired glucometer-CGM values. The aggregate MARD was 10.4%. For time in range and grades 1 and 2 hyperglycemia, MARD was 11.4%, 9.4%, and 9.1%, respectively, with a small variation between medical floors and intensive care units. There was no MARD correlation with mean arterial blood pressure levels, oxygen saturation, daily hemoglobin levels, and glomerular filtration rates. CGM clinical reliability was high, with 99.7% of the CGM values falling within the “safe” zones of Clarke error grid. After CGM placement, the frequency of glucometer measurements decreased from 5 to 3 and then 2 per day, reducing nurse presence in patient rooms and limiting viral exposure.
Conclusion
With twice daily, on-demand calibration, the inpatient CGM use was safe for insulin dosing, decreasing the frequency of glucometer fingersticks. For glucose levels >70 mg/dL, CGMs showed adequate accuracy, without interference from vital and laboratory values.
Key words: CGM accuracy, continuous glucose monitor, COVID-19 pneumonia, inpatient
Abbreviations: ARD, absolute relative difference; CEG, Clarke error grid; CGM, continuous glucose monitor; CT, computed tomography; DM, diabetes mellitus; FDA, U.S. Food and Drug Administration; ICU, intensive care unit; IV, intravenous; MARD, mean absolute relative difference; POC, point of care; PPE, personal protected equipment; SQ, subcutaneous; TIR, time in range
Highlights
-
•
Continuous glucose monitors (CGMs) were accurate for glucose monitoring in hospitalized patients with COVID-19 pneumonia
-
•
With twice daily on-demand calibration, CGMs proved safe for insulin dosing
-
•
CGM mean absolute relative difference did not correlate with blood pressure, Spo 2, daily hemoglobin, and daily glomerular filtration rate
-
•
Patients and hospital nursing staff were satisfied with the CGM use
Clinical Relevance
This study emphasizes the safety and accuracy of continuous glucose monitors (CGMs) in the inpatient setting. For glucose levels >70 mg/dl, CGMs had adequate accuracy, and no accuracy variation with daily vital signs and laboratory values. With on-demand calibration, CGMs can be used for insulin dosing on medical floors, thus decreasing the frequency of glucometer fingersticks and improving patients’ satisfaction.
Introduction
Hyperglycemia, caused by both stress and diabetes mellitus (DM), can be present in 32.2% of patients in intensive care unit (ICU) and 32.0% of non-ICU patients.1 The inpatient standard of care for glucose monitoring consists of capillary glucose measurement using point-of-care (POC) glucometers. Continuous glucose monitors (CGM) were first approved by the U.S. Food and Drug Administration (FDA) in 1999 to monitor glucose and to dose insulin in the outpatient setting. For hospitalized patients, the use of this technology is not yet approved.
Patients with DM are at increased risk of severe COVID-19 and hospitalization.2 COVID-19 treatment involves the use of high doses of corticosteroids with subsequent hyperglycemia and the need for higher doses of insulin to control the glucose. This dosing requires more frequent glucose monitoring with POC glucometer checks, which exposes nurses to patients with viral infection. Acknowledging these special circumstances, the FDA temporarily permitted hospitals to use CGMs “to allow staff to remotely monitor glucose in patients to reduce patient interaction, limiting viral exposure by hospital staff and conserving personal protected equipment (PPE).”3
The COVID-19 pandemic, together with this FDA exception, and the support from manufacturers have accelerated the inpatient use of CGMs under research protocols. Studies detailing CGM accuracy in the ICU and non-ICU hospital setting have been published.4, 5, 6, 7, 8, 9
We report the findings of our pilot study evaluating the use of CGMs for hospitalized patients with COVID-19. The study goal was to evaluate CGMs accuracy compared with that of POC glucometers and to determine whether CGM readings could be safely used to replace some of POC glucometer measurements for insulin dosing. We aimed to reduce medical staff exposure to patients with COVID-19. We evaluated the effect of vital signs and biochemical parameters on CGM accuracy and recorded patient satisfaction with the CGM use.
Methods
This prospective open-label, single-arm, single-center pilot study was approved by Mayo Clinic institutional review board (ClinicalTrials.gov: NCT04756141).
Population
Between March 3, 2021, and July 31, 2021, we enrolled 20 patients admitted to Mayo Clinic in Florida Hospital with COVID-19 diagnosis and hyperglycemia requiring insulin therapy. We excluded patients with diabetic ketoacidosis, hyperosmolar hyperglycemic states, patients initially admitted to the ICU, pregnant or breastfeeding patients, and patients with end stage renal disease requiring dialysis. If enrolled patients were subsequently transferred to the ICU, the CGM was kept on, but the CGM values were not used for insulin dosing. After patients were transferred out of the ICU, the CGM values were used again to dose insulin. Data from all patients at all levels of care (medical floor, progressive medical floor, and ICU) were included.
Protocol for Glucose Monitoring and Insulin Therapy
The diabetes educator, endocrinology nurse practitioners, and provider champions were involved with the study protocol design, staff education, sensor insertion, and equipment setup (Supplementary Material).
Enrolled patients were fitted with a Dexcom G6 CGM sensor/transmitter. A smartphone connected to the CGM transmitted glucose readings to the nursing station where it was displayed on an iPad by Dexcom Follow application.
Protocols for subcutaneous (SQ) or intravenous (IV) insulin therapy were designed. Owing to challenges with implementation, the IV protocol was used only once during the study. The protocols were divided into 2 phases. During the initial “adjustment phase,” CGM readings were compared against glucometer values for the first 4 POC readings if patients were receiving SQ insulin or for the first 12 hourly POC readings if patients were receiving IV insulin. If the CGM values were found to be within ±15% of POC glucometer values, the CGM was deemed acceptable for monitoring, and the “utilization phase” was initiated: for patients receiving SQ insulin, the POC check frequency was decreased to twice per day (before breakfast and at bedtime), and CGM values were used to dose insulin before lunch and dinner. For patients receiving an IV insulin infusion, every other glucometer reading was substituted by the timed CGM value. CGM values were used for glucose monitoring between POC checks. A CGM value out of range would trigger a POC check. The insulin drip rate changes were only based on POC glucometer values.
During both adjustment and utilization phases, the CGMs were calibrated if their values fell outside a predetermined ±15% range of the POC value. Calibration was mandatory on the medical floors, but it was not required in the ICU because, in the ICU, CGM values were not used for insulin dosing.
The CGM system (sensor and transmitter) did not have to be removed for computed tomography (CT) or x-ray unless it would interfere with the scanned field, but it had to be removed and replaced for any magnetic resonance imaging.
Outcomes
The primary outcomes were CGM accuracy and clinical reliability when compared with POC glucometer readings. Accuracy was assessed with a mean absolute relative difference (MARD). We reviewed MARD for POC glucose readings below the range (<70 mg/dL), within the range (70-180 mg/dL) and above the range (>180 mg/dL). The Clarke error grid (CEG) was used to assess clinical reliability.
Using CGMs values to dose insulin instead of bedside glucometer checks, our secondary aim was to decrease nurses’ exposure to COVID-19. Another secondary outcome studied was MARD variation with mean arterial blood pressure, Spo 2, daily hemoglobin, and daily glomerular filtration rate (GFR) levels. Finally, patient satisfaction with CGM system use was evaluated with a postinterventional survey.
Materials
The CGM G6 system, the transmitting smartphones, and the monitoring software (G6 application and Follow application) were provided at no cost by Dexcom. The 2 iPads (Apple) and the funding for statistical analysis were provided by the Department of Medicine. POC glucometers used were StatStrip (Nova Biomedical).
Statistical Analysis
Data were analyzed using SAS version 9.4M7 (SAS Institute) and R version 4.0.3 (R Foundation for Statistical Computing). Plots of the CEG analysis were created using the ega R package (version 2.0.0).10 Absolute relative difference (ARD) was calculated as the absolute difference between POC glucose measurement and the nearest CGM glucose measurement (within 5 minutes of POC), divided by the POC glucose value and expressed as a percentage. Average MARD for each patient and aggregate MARD were summarized with mean and SD values. Patient-averaged median ARD and aggregated median ARD were listed with IQR. The sample median (minimum, 25th percentile; and maximum, 75th percentile) was used to summarize POC frequency on days 1, 2, and 3 after CGM initiation and the percentage of time the CGM was within, below, and above the range.
Results
Twenty patients hospitalized with COVID-19 pneumonia and hyperglycemia requiring insulin therapy were included in the study. Their demographic characteristics are summarized in Table 1 .
Table 1.
Patient Characteristics
| Attribute | n | Median (range) or n (%) |
|---|---|---|
| Baseline characteristics | ||
| Female sex | 20 | 8 (40) |
| Age at admission, y | 20 | 59 (29-85) |
| Race | 20 | |
| White | 17 (85) | |
| Black or African American | 2 (10) | |
| Other | 1 (5) | |
| Hispanic or Latino ethnicity | 20 | 4 (20) |
| Body mass index, kg/m2 | 20 | 31.1 (21.6-52.4) |
| Type of hyperglycemia | 20 | |
| Type 2 DM—oral/SQ medication, no insulin | 7 (35) | |
| Type 2 DM—MDI insulin or insulin pump | 6 (30) | |
| Before admission medication-induced hyperglycemia | 1 (5) | |
| COVID-19 therapy–related hyperglycemia only | 4 (20) | |
| New DM type 2 diagnosis during current hospitalization | 2 (10) | |
| Last HbA1C (%; mmol/mol) | 16 | 7.0 (4.9-11.9); 53 (30-107) |
| Post-CGM placement information | ||
| Insulin administered while CGM was active | 20 | |
| SQ insulin only | 15 (75) | |
| Both IV and SQ insulin | 5 (25) | |
| No. of times SQ insulin was dosed based on CGM values | 20 | 8 (1-39) |
| No. of times CGM value was not documented in EPIC while SQ insulin was dosed based on CGM value | 20 | 1 (0-6) |
| Medication(s) received for treatment of COVID-19 | 20 | |
| Remdesivir | 19 (95) | |
| Dexamethasone | 19 (95) | |
| Convalescent plasma | 11 (55) | |
| Tocilizumab | 8 (40) | |
| Research trial—camostat vs placebo | 1 (5) | |
| No. of CGM calibrations on medical floors and ICU | 20 | 2.5 (0-25) |
| No. of CGM calibrations on medical floors | 20 | 2.5 (0-25) |
| No. of CGM calibrations in ICU | 6 | 1 (0-2) |
Abbreviations: CGM = continuous glucose monitor; DM = diabetes mellitus; MDI = multiple daily injections; ICU = intensive care unit; IV = intravenous; SQ = subcutaneous.
The median age was 59 years (29-85 years), 85% of participants were White, and 60% were men. Most of them (n = 13, 65%) reported a previous diagnosis of type 2 DM, 2 patients (10%) were diagnosed newly with type 2 DM on admission, and 5 patients (25%) presented with corticosteroid-induced hyperglycemia. Six patients (30%) previously used SQ insulin at home, and no patient used a CGM before admission.
The CGM was used for a median of 173 hours (84-386 hours) per patient, with 130 hours on the medical floor and 106 hours in the ICU (Table 2 ). Most of the patients (n = 14, 70%) used only 1 sensor. The median duration of hospitalization was 11 days (4-61 days). Two patients did not complete the study (inaccurate readings or left the study against medical advice). Five patients (25%) received an IV insulin infusion for 89 hours while the CGM was active. Six patients (30%) were transferred to the ICU while having the CGM in place. Four episodes of hypoglycemia were prevented, and there was no report of sensor insertion site associated side effects.
Table 2.
Total Hours of CGM Follow-Up
| Patient Location | No. of Patients | Median (IQR) | Range |
|---|---|---|---|
| Medical + ICU | 20 | 173 (84-386) | 43-812 |
| Medical only | 20 | 130 (84-336) | 43-812 |
| ICU only | 6 | 106 (63-142) | 36-213 |
Abbreviations: CGM = continuous glucose monitor; ICU = intensive care unit.
Although unintended as part of our study, the Dexcom G6 sensor proved to be durable. Four patients (20%) underwent a CT scan of the abdomen while the CGM was on. There was no significant sensor/transmitter interference with the acquired images, and there was no sensor/transmitter malfunction after CT scan. Nine patients (45%) underwent other CT scans (chest, head, and cervical spine) with an unshielded sensor, and no CGM malfunction was noted. No patients were ordered magnetic resonance imaging that would have required CGM removal and replacement.
Primary Outcomes
For all 20 patients, 812 pairs of POC-CGM values were retrieved; 596 pairs were recorded on medical/progressive care unit floors. The rest of 216 pairs were recorded in the ICU. The patient-averaged and the aggregate MARD, together with the patient-averaged and the aggregate median ARD, are summarized in Table 3 . The aggregate MARD was 10.4% ± 9.4% based on all paired CGM-POC glucose readings, and the average MARD per patient was 10.1% ± 2.3%. The aggregate median ARD was 8.1% (IQR: 3.4%-14.7%) for all POC-CGM pairs. We noted a slight decrease of MARD from 10.2% on day 1 to 9.8% on day 2 and 9.1% on day 3 (Table 4 ).
Table 3.
Absolute Relative Difference in CGM Glucose Levels Compared With That in POC Glucose Levels
| Patient Location | Patient-averaged ARD |
Aggregated ARD |
||||
|---|---|---|---|---|---|---|
| N | Mean ARD ± SD, % | Median ARD (IQR), % | No. pairs | Mean ARD ± SD, % | Median ARD (IQR), % | |
| All CGM-POC pairs | ||||||
| Medical + ICU | 20 | 10.1 ± 2.3 | 9.8 (8.3-11.4) | 812 | 10.4 ± 9.4 | 8.1 (3.4-14.7) |
| Medical | 20 | 10.0 ± 2.3 | 9.5 (8.3-10.8) | 596 | 10.2 ± 9.0 | 8.5 (3.7-14.3) |
| ICU | 6 | 12.5 ± 5.4 | 10.1 (8.6-15.5) | 216 | 10.9 ± 10.5 | 7.2 (2.9-16.8) |
| POC <70 mg/dL | ||||||
| Medical + ICU | 4 | 22.1 ± 10.0 | 20.8 (13.7-29.1) | 6 | 25.8 ± 15.9 | 22.0 (15.1-27.0) |
| Medical | 2 | 16.4 ± 3.7 | 16.4 (15.1-17.7) | 2 | 16.4 ± 3.7 | 16.4 (15.1-17.7) |
| ICU | 3 | 27.2 ± 13.6 | 27.7 (20.6-34.1) | 4 | 30.6 ± 18.1 | 26.4 (22.1-34.8) |
| POC 70-180 mg/dL | ||||||
| Medical + ICU | 19 | 10.9 ± 5.0 | 11.0 (8.1-14.1) | 380 | 11.4 ± 11.1 | 8.5 (3.4-15.7) |
| Medical | 19 | 10.7 ± 5.3 | 9.3 (7.9-13.9) | 245 | 11.6 ± 11.2 | 9.6 (4.1-15.3) |
| ICU | 6 | 13.3 ± 6.9 | 12.3 (9.2-16.8) | 135 | 11.2 ± 11.1 | 7.5 (2.5-17.6) |
| POC 181-250 mg/dL | ||||||
| Medical + ICU | 20 | 9.1 ± 1.8 | 9.3 (8.1-10.6) | 241 | 9.4 ± 7.4 | 8.3 (3.1-13.6) |
| Medical | 20 | 9.1 ± 2.1 | 9.4 (7.4-10.8) | 193 | 9.2 ± 7.1 | 8.6 (3.4-13.1) |
| ICU | 5 | 9.7 ± 5.9 | 8.7 (6.9-9.9) | 48 | 10.0 ± 8.8 | 7.0 (2.5-16.6) |
| POC >250 mg/dL | ||||||
| Medical + ICU | 19 | 8.9 ± 4.0 | 8.3 (7.1-10.2) | 185 | 9.1 ± 6.6 | 7.5 (3.7-14.0) |
| Medical | 19 | 9.1 ± 4.1 | 8.3 (7.3-10.2) | 156 | 9.2 ± 6.6 | 7.8 (3.5-14.3) |
| ICU | 5 | 5.9 ± 4.4 | 5.0 (3.7-9.1) | 29 | 8.4 ± 6.4 | 6.7 (3.8-12.2) |
Abbreviations: ARD = absolute relative difference; CGM = continuous glucose monitor; ICU = intensive care unit; POC = point of care.
Table 4.
Aggregated Mean ARD in First 72 Hours
| Time after CGM placement, h | No. of CGM-POC pairs | Aggregated Mean ARD ± SD, % |
|---|---|---|
| 0-24 | 116 | 10.2 ± 8.7 |
| 24-48 | 68 | 9.8 ± 10.2 |
| 48-72 | 50 | 9.1 ± 6.8 |
Abbreviations: ARD = absolute relative difference; CGM = continuous glucose monitor; POC = point of care.
The median number of CGM calibrations on the medical floors was 2.5 (2-25) per patient. CGMs were rarely calibrated in the ICU because this was not required by the protocol because the CGM values were used only to monitor and not to dose insulin.
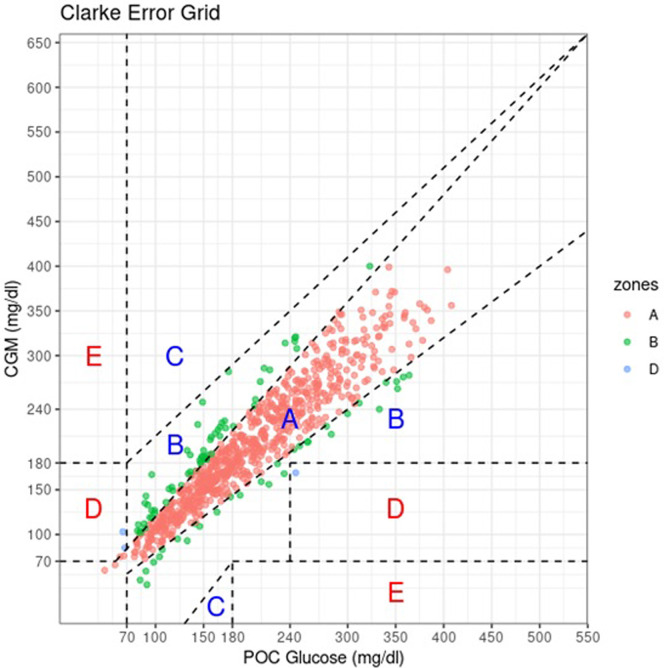
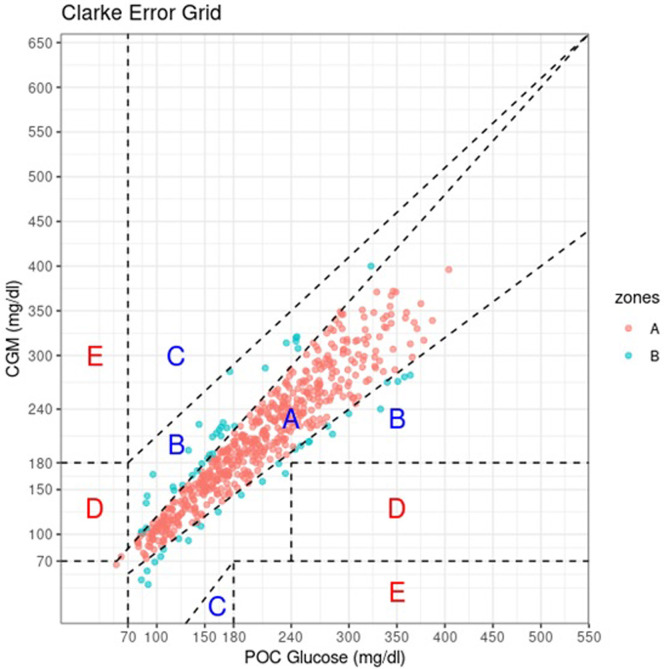
CEG plotting defines 5 clinical safety zones. Zone A plotted CGM values within 20% of POC values, zone B has CGM values >20% POC that would not lead to inappropriate treatment. Therapy based on CGM values from Zone C would lead to overcorrecting for an acceptable blood glucose levels. Values in zone D showed failure of the CGM to detect hypoglycemia or hyperglycemia that would need treatment. Zone E values would confound hypoglycemia for hyperglycemia and vice versa. Of all 812 CGM-POC value pairs, 715 pairs (88.1%) were in zone A, 94 (11.6%) in zone B, 3 (0.4%) in zone D, and none in zone E. For the medical floors, all values (100%) fell either in zone A or zone B (Fig 1, Fig. 2, Fig. 3, Fig. 4 ).
Fig 1.
Clarke error grid plot of all CGM-POC paired glucose measures. Of the 812 CGM-POC pairs, 715 (88.1%) were in zone A, 94 (11.6%) in zone B, and 3 (0.4%) in zone D. CGM = continuous glucose monitor; POC = point of care.
Fig. 2.
Clarke error grid plot of in-hospital CGM-POC paired glucose measures. Of the 596 in-hospital CGM-POC pairs, 536 (89.9%) were in zone A and 60 (10.1%) in zone B. CGM = continuous glucose monitor; POC = point of care.
Fig. 3.
Clarke error grid plot of in-ICU CGM-POC paired glucose measures. Of the 216 CGM-POC pairs in the ICU, 179 (82.9%) were in zone A, 34 (15.7%) in zone B, and 3 (1.4%) in zone D. CGM = continuous glucose monitor; ICU = intensive care unit; POC = point of care.
Fig. 4.
Clarke error grid plot of CGM-POC paired glucose measures while receiving IV Insulin. Of the 151 CGM-POC pairs measured while receiving IV insulin, 134 (88.7%) were in zone A, 16 (10.6%) were in zone B, and 1 (0.7%) was in zone D. CGM = continuous glucose monitor; IV = intravenous; POC = point of care.
For POC glucose <70 mg/dL, the aggregate MARD was 25.8% (30.6% for ICU patients and 16.4% for patients on the medical floor). MARD for hypoglycemia was calculated on a minimal sample (6 POC-CGM pairs). When POC readings were within the range (71-180 mg/dL) and for grade 1 (181-250 mg/dL) and grade 2 (>250 mg/dL) hyperglycemia, MARD was 11.4%, 9.4%, and 9.1% respectively, with no large value variation between the medical floors and ICU.
Secondary Outcomes
Patients wore the CGM between 43 and 812 hours (median: 173 hours; IQR: 84-386 hours) and they spent approximately 39% of the time with CGM values within the range (70-180 mg/dL). In the ICU, 50.6% of the recorded time had values within the range. For the rest of the time, CGM was mostly above the range (Table 5 ).
Table 5.
Frequency of POC Glucose Measurements Days 1-3 After Initial CGM Value
| Day Number | No. of Patients | Median (IQR) | Range |
|---|---|---|---|
| Day 1 | 20 | 5 (4-5) | 3-19 |
| Day 2 | 20 | 3 (2-4) | 1-12 |
| Day 3 | 19 | 2 (2-3) | 1-6 |
Abbreviations: CGM = continuous glucose monitor; POC = point of care.
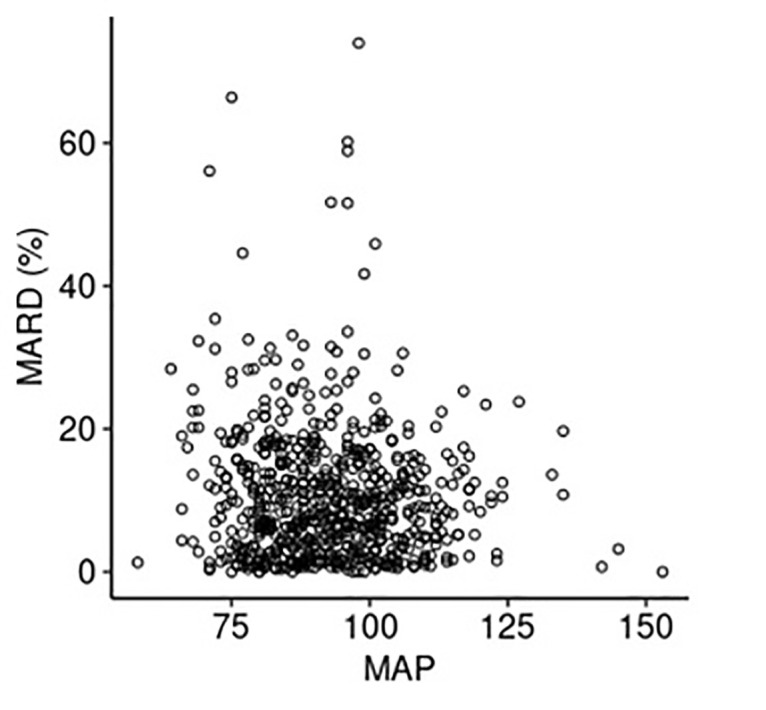
MARD variation with mean arterial blood pressure, oxygen saturation, daily hemoglobin and daily GFR levels was plotted. There was no evidence of MARD correlation with any of the 4 vitals or laboratory values that were assessed (all |Spearman correlation coefficients| ≤ 0.07) (Supplementary Figure).
The number of times the insulin was dosed solely on the CGM value ranged from 1 to 39 times with a median of 8 times per patient. The median frequency of POC measurements after CGM start decreased from 5/d on day 1 to 3/d on day 2 and 2/d on day 3 (Table 6 ). Because performing a glucose check using glucometer requires use of PPE for direct patient contact, the decrease in the frequency of glucometer checks resulted in decreased staff exposure to SARS-CoV-2 and, potentially, less PPE use.
Table 6.
Glucose Control Based on CGM Readings
| Patient Location | No. of Patients | Median (IQR) | Range |
|---|---|---|---|
| Percentage of time with CGM within the range (70-180 mg/dL) | |||
| Medical + ICU | 20 | 39.0 (22.0-50.6) | 0.0-72.2 |
| Medical only | 20 | 37.5 (22.0-50.4) | 0.0-72.2 |
| ICU only | 6 | 50.6 (40.7-60.0) | 19.8-95.4 |
| Percentage of time with CGM below the range (<70 mg/dL) | |||
| Medical + ICU | 20 | 0.0 (0.0-0.1) | 0.0-0.5 |
| Medical only | 20 | 0.0 (0.0-0.0) | 0.0-0.5 |
| ICU only | 6 | 0.0 (0.0-0.4) | 0.0-4.6 |
| Percentage of time with CGM above the range (>180 mg/dL) | |||
| Medical + ICU | 20 | 60.4 (48.8-78.0) | 27.8-100.0 |
| Medical only | 20 | 62.0 (49.6-78.0) | 27.8-100.0 |
| ICU only | 6 | 44.0 (34.5-58.6) | 0.0-80.2 |
Abbreviations: CGM = continuous glucose monitor; ICU = intensive care unit.
Sixteen patients (80%) completed the postinterventional survey, 2 declined it, and 2 died. All 16 patients reported minimal or no pain with the CGM use. Twelve patients (75.1%) reported being satisfied or very satisfied with the CGM use. Eleven patients (68.8%) reported confidence with staff monitoring their glucose levels. Eight patients (56.3%) perceived fewer fingersticks, 10 (62.5%) felt there were fewer care interruptions, 8 (64.3%) believed they experienced better DM control, and 6 (54.6%) perceived early hypoglycemic detection. The results are summarized in Supplementary Table.
Discussion
The main goal of this study was to assess the accuracy of CGMs used to monitor blood glucose for patients admitted with COVID-19 pneumonia and to determine whether CGM readings could be safely used to replace some POC glucometer measurements for insulin dosing. Accuracy was assessed with MARD values. To our knowledge, this study is the first to present a protocol with on-demand CGM calibration for lack of correlation between CGM and POC values. The MARD correlation with vitals and daily laboratory values and the report on patient satisfaction with CGM use were rarely found in the literature describing CGM use in inpatient setting.
We observed an averaged MARD of 10.1% per patient and an aggregate MARD of 10.4% for all CGM-POC pairs. Our reported MARDs (10.4% combined, 10.2% for medical floors, and 10.9% for patients in ICU) were higher than the outpatient reported MARD of 9%11 but in line with previous reported MARD for hospital use. Most inpatient studies reported a MARD higher than the outpatient MARD, and this tendency was maintained for COVID-19 and non–COVID-19 patients either in the ICU or on medical floors. Reutrakul et al9 found a MARD of 9.77% in 9 non–ICU COVID-19 patients, whereas Agarwal et al12 studied COVID-19 patients in the ICU found a MARD of 12.8%. Moreover, the study by Faulds et al5 reported a MARD of 13.9% for patients in ICU with COVID-19. Longo et al13 reviewed 28 patients with COVID-19, both in the ICU and on medical floors. The reported MARD was 13.9% overall, with 14% MARD for floor patients and 12.1% for patients in ICU. Davis et al14 cumulated data from 3 studies and reported MARD for 218 non-ICU, non–COVID-19 patients to be 12.8%.
The CGM system in our study showed a higher MARD for the first day when compared with days 2 and 3 of wear. This was similar to that noted in previous outpatient studies (MARD 10.7% initially and 9% total)15 and other inpatient studies (initial MARD 14.4% and 12.8% total).14
This study reported a lower MARD for both non-ICU and patients in ICU when compared with a study that enrolled a larger number of patients.14 The study by Longo et al,13 with a similar protocol to this study, reported a higher overall MARD, with a lower MARD for patients in ICU. Longo et al13 and Davis et al14 did not report device calibration in the hospital setting. Our study protocol required twice daily POC to CGM value comparison and on-demand CGM calibration if the CGM values would fall outside ±15% of the reported POC readings. This calibration was required by the protocol on the medical floors, but it was optional in the ICU, possibly leading to our higher MARD observed in the ICU than on the medical floors. It is possible that this mandatory monitoring and device calibration is responsible for our lower MARD values. The G6 system is factory calibrated to account for sensor drift, and it does not routinely require calibration. Dexcom recommends calibration for some sensors on the first 24 hours or as needed if CGM values are higher or <20% POC values.16 A future prospective study protocol for CGM accuracy should include daily CGM to POC value comparison and on-demand calibration. Such calibration would better align CGM and POC values, potentially mitigating the sensor drift and variation in CGM sensitivity with vitals and laboratory parameters.
The International Organization for Standardization (ISO) has listed criteria for blood glucose monitoring systems for self-testing in managing DM (ISO 15197:2013). For hospital use, these accuracy criteria requires that >99% of measured glucose values to be in zones A and B17 of CEG. Clinical reliability showed that most CGM readings (99.7%) in this study were in the “safe zones” A and B, with only 3 (0.3%) values that fell within zone D. All these 3 values were measured in the ICU, one during IV insulin infusion, and all during rapid glycemic fluctuation with CGM being affected by lag time. As Agarwal et al12 noted and Perez-Guzman et al18 recommended, CGMs may not be ready to be used as a sole measure for glucose monitoring in the ICU; a hybrid model would be safer. For glucose levels <85 mg/dL, these experts recommend doing POC testing instead of using the CGM value.18
The lack of data regarding CGM accuracy with vital signs and physiologic parameters is a theoretical barrier to CGM implementation in the hospital setting.19 Davis et al14 noted a higher MARD in patients with an admission hemoglobin <7 g/dL. Conversely, our study looked at MARD correlation with daily hemoglobin levels, as opposed to admission hemoglobin level and disproved any negative correlation. The lack of correlation between MARD and blood pressure, oxygenation, hemoglobin level, and GFR found in this study is encouraging and sets the base for a future prospective research study.
Our recorded CGM time in range (TIR) on the medical floors was low at 37.5% (IQR: 22.0%-50.4%). In the ICU, where IV insulin was used more frequently to control hyperglycemia, the TIR was better at 50.6% (IQR: 40.7%-60%). Of the 59,741 CGM glucose values, the mean glucose was 199 mg/dL (SD = 65 mg/dL), and the median glucose was 194 mg/dL (IQR: 150-244 mg/dL). Published reports indicate that TIR for patients with COVID-19 is lower than that in non–COVID-19 patients.20 Despite CGM daily glycemic profile being used for insulin dosing adjustment, our TIR was significantly lower than other published results. Gómez et al7 reported TIR of 72% for non-ICU and 73% for patients in ICU with COVID-19. It is possible that our lower TIR and higher time above the range (60.4%) were related to the use of high and very high dose (20 mg dexamethasone daily) of corticosteroids in almost all (95%) enrolled patients.
Patients did not perceive any harm from the use of CGMs, and most were satisfied with CGM use despite only about half of them perceiving fewer fingersticks. An unanticipated outcome was nurse satisfaction and acceptance of the CGM monitoring system. They welcomed and requested expansion of the “blood glucose telemetry” that allowed them to remotely monitor patient glucose levels without direct viral exposure. Faulds et al6 noted similar nursing staff reaction to CGM implementation in ICU setting.
Limitations
The main limitations to this study were the observational nature and its small cohort size. Implementation of this novel monitoring protocol was challenging initially, with notable differences among nursing staff. While patients were under ICU care, CMGs were only used for monitoring, and calibration was performed sparingly. This lack of timely calibration may have affected CGM accuracy in the ICU.
Our patients experienced a very low prevalence of hypoglycemia. This may have been related not only to a small cohort size but also to our difficulty with glycemic control (TIR was only 39%, and almost 0% time below the range). With such a small sample, we were unable to assess CGM accuracy for blood glucose values <70 mg/dL.
Having to manually document CGM readings in the electronic medical records led to some CGM values not being recorded (Table 1). An automatic synchronization of CGM data to the electronic medical records would reduce errors in further studies.
Similar nursing staff challenges with CGM use, protocol implementation, and CGM values documentation were described by Faulds et al.6
Conclusion
Despite all challenges with implementation, execution, and data recording, we consider that our pilot study reached its goals. We demonstrated that CGM accuracy in the inpatient setting is not that different from outpatient CGM accuracy. CGMs were accurate, even in our special population—sick patients with moderate-to-severe COVID-19 pneumonia, treated with high dose of glucocorticoids. We were able to show that CGMs can be used safely to measure and dose insulin on the medical floors. No direct patient harm was noted. Although the Dexcom G6 system is factory calibrated, POC and CGM values comparison and on-demand device calibration, twice per day on day 1 and then once daily, may be needed to ensure a better CGM accuracy in the hospital. Dexcom G6 transmitter functionality was not affected by direct exposure to CT scans or x-rays on our short-term follow-up. We managed to decrease POC check frequency after the CGM start, with a resultant decrease in staff exposure to COVID-19 and possibly decreasing the use of PPE. Our hospitalized patients and the nursing staff were satisfied with the use of these devices. Staff training and familiarity with CGMs is imperative for any future deployment of these devices in the hospital. It is imperative to deploy large randomized prospective studies to validate the CGMs accuracy and their safety for use in the inpatient setting.
Disclosure
A.D. holds shares in Dexcom, Sensionics, Tandem, and Insulet. M.P. holds shares in Dexcom and Insulet. A.M. holds shares in Medtronics. The other authors have no multiplicity of interest to disclose.
Acknowledgment
This work was supported by Department of Medicine, Mayo Clinic in Florida via STARDOM Award. ClinicalTrials.gov NCT04756141.
Supplementary Material
Suplemental Table Patient Satisfaction.
Supplemental Figure Panel A.

Supplemental Figure Panel B.

Supplemental Figure Panel C.

References
- 1.Swanson C.M., Potter D.J., Kongable G.L., Cook C.B. Update on inpatient glycemic control in hospitals in the United States. Endocr Pract. 2011;17(6):853–861. doi: 10.4158/EP11042.OR. [DOI] [PubMed] [Google Scholar]
- 2.Bode B., Garrett V., Messler J., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [Erratum: J Diabetes Sci Technol. 2020:1932296820932678] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DEXCOM. Information for healthcare providers: Dexcom G6 CGM system in the hospital. https://www.dexcom.com/hospitalcovid-19 Accessed June 28, 2022.
- 4.Chow K.W., Kelly D.J., Rieff M.C., et al. Outcomes and healthcare provider perceptions of real-time continuous glucose monitoring (rtCGM) in patients with diabetes and COVID-19 admitted to the ICU. J Diabetes Sci Technol. 2021;15(3):607–614. doi: 10.1177/1932296820985263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faulds E.R., Boutsicaris A., Sumner L., et al. Use of continuous glucose monitor in critically ill COVID-19 patients requiring insulin infusion: an observational study. J Clin Endocrinol Metab. 2021;106(10):e4007–e4016. doi: 10.1210/clinem/dgab409. [DOI] [PubMed] [Google Scholar]
- 6.Faulds E.R., Jones L., McNett M., et al. Facilitators and barriers to nursing implementation of continuous glucose monitoring (CGM) in critically ill patients with COVID-19. Endocr Pract. 2021;27(4):354–361. doi: 10.1016/j.eprac.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gómez A.M., Henao D.C., Muñoz O.M., et al. Glycemic control metrics using flash glucose monitoring and hospital complications in patients with COVID-19. Diabetes Metab Syndr. 2021;15(2):499–503. doi: 10.1016/j.dsx.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadhu A.R., Serrano I.A., Xu J., et al. Continuous glucose monitoring in critically ill patients with COVID-19: results of an emergent pilot study. J Diabetes Sci Technol. 2020;14(6):1065–1073. doi: 10.1177/1932296820964264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reutrakul S., Genco M., Salinas H., et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care. 2020;43(10):e137–e138. doi: 10.2337/dc20-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke W.L., Cox D., Gonder-Frederick L.A., Carter W., Pohl S.L. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care. 1987;10(5):622–628. doi: 10.2337/diacare.10.5.622. [DOI] [PubMed] [Google Scholar]
- 11.Shah V.N., Laffel L.M., Wadwa R.P., Garg S.K. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–433. doi: 10.1089/dia.2018.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal S., Mathew J., Davis G.M., et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care. 2021;44(3):847–849. doi: 10.2337/dc20-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Longo R.R., Elias H., Khan M., Seley J.J. Use and accuracy of inpatient CGM during the COVID-19 pandemic: an observational study of general medicine and ICU patients. J Diabetes Sci Technol. 2022;16(5):1136–1143. doi: 10.1177/19322968211008446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis G.M., Spanakis E.K., Migdal A.L., et al. Accuracy of dexcom G6 continuous glucose monitoring in non-critically ill hospitalized patients with diabetes. Diabetes Care. 2021;44(7):1641–1646. doi: 10.2337/dc20-2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DEXCOM Evaluation of automatic class III designation for Dexcom G6 Continuous Glucose Monitoring System. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN170088.pdf Accessed June 28, 2022.
- 16.DEXOM G6 Continuous glucose monitoring system. User guide. https://s3-us-west-2.amazonaws.com/dexcompdf/G6-CGM-Users-Guide.pdf Accessed June 28, 2022.
- 17.Online Browsing Platform. In vitro diagnostic test systems — Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. Accessed June 28, 2022. https://www.iso.org/obp/ui/#iso:std:iso:15197:ed-2:v1:en
- 18.Perez-Guzman M.C., Shang T., Zhang J.Y., Zhang J.Y., Jornsay D., Klonoff D.C. Continuous glucose monitoring in the hospital. Endocrinol Metab (Seoul) 2021;36(2):240–255. doi: 10.3803/EnM.2021.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galindo R.J., Aleppo G., Klonoff D.C., et al. Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol. 2020;14(4):822–832. doi: 10.1177/1932296820932903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapoor R., Timsina L.R., Gupta N., et al. Maintaining blood glucose levels in range (70-150 mg/dL) is difficult in COVID-19 compared to non-COVID-19 ICU patients—a retrospective analysis. J Clin Med. 2020;9(11):3635. doi: 10.3390/jcm9113635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.