Abstract
Herein, we report practical Cu(BF4)2/activated carbon-catalyzed amination of various anilines, isoquinolinone, and naphthyridinone with aryl boronic acids. The ultrasonic and rotary evaporation treatment of the mixture of aq. Cu(BF4)2 and activated carbon in methanol afforded a novel Cu(II)-catalyst, which is air-stable and can be effectively applied in the Chan–Lam coupling reaction. The products of N-arylation were isolated in good to excellent yields at low catalytic loading. And Cu(BF4)2/AC also showed good reusability.
Introduction
Substituted isoquinolinones and naphthyridinones form an important class of compounds in the pharmaceutical industry.1,2 Particularly, substituted isoquinolin-1(2H)-one, which constitutes the core scaffold of marketed or reported small molecular inhibitors, such as Duvelisib, compound 4, and Eganelisib, made this heterocycle a popular pharmacophore for synthetic chemists (Scheme 1, compounds 1, 3, and 4).3−5 Naphthyridinone also acted as a small molecular inhibitor against some medicinal targets (compounds 2, 5, 6).6−8 The Ullmann coupling reaction or multistep cyclization are commonly used to construct N-phenyl-isoquinolinone segments.9,10 However, these suffer from several limitations, such as expensive copper salts, air-sensitive ligands, and harsh reaction conditions, thus hampering the application of conventional synthetic methods.11
Scheme 1. Chemical Structures of Reported Isoquinolinone or Naphthyridinone-Based Inhibitors.

Although the Buchwald–Hartwig coupling is another well-known C–N bond formation method and is dramatically used in synthetic chemistry,12,13 some drawbacks still restrict its wide application.14 Since its first discovery in 1998, the Chan–Lam amination methodology has emerged as one of the three most prominent cross-coupling reactions for the formation of the C–N bond. This route uses the aryl organoboron derivatives to react with nucleophiles under facile reaction conditions (open flask or O2 balloon, room temperature, weak base).15,16 Compared to the routinely exploited Ullmann and Buchwald–Hartwig coupling, the Chan–Lam amination was a more practical method to construct the C–N bond.
Aryl boronic acids served as an acceptor and were widely applied in Chan–Lam coupling, which has been extensively studied in mechanistic description:17 (1) A weak base or an essential additive always played an important role in the process of deprotonation in the reaction. (2) Different copper salts work differently in the presence of various additives. (3) Small amounts of H2O in the solvent might facilitate the coupling reaction. Many modifications have been reported to improve the reaction efficiency using different copper salts, such as Cu(OAc)2, CuOAc, CuCl, Cu(OTf)2, and [Cu(OH)·TMEDA]2Cl2.18−21 However, apart from its application in oxidative C–C coupling with pyrene derivatives, Cu(BF4)2·nH2O-mediated C–N formation has rarely been explored (Scheme 2a).22 Its high hygroscopicity maybe restrict the applications.23
Scheme 2. Cu(BF4)2·nH2O Catalyzed Oxidative C–C Coupling Reaction and Copper-Catalyzed C–N Bond Formation.

Several heterogeneous copper catalysts have been prepared to expand the scope of substrates and improve the efficiency of the Chan–Lam reaction (Scheme 2b). For example, Kantam reported copper fluorapatite (CuFAP) facile-catalyzed N-arylation of imidazole and anilines at room temperature.24 Beletskaya designed copper nanoparticles supported on zeolite to catalyze the formation of C–C, C–S, and C–N bonds.25 Thereafter, various heterogeneous catalyst systems were disclosed, such as CuSO4/Fe3O4-EDTA,26 Cu(OAc)2/graphene oxide,27 Cu(OAc)2/MCIP-1,28 and Cu (II)/chitosan,29 etc. But these reported methods still suffered from some drawbacks, such as large catalyst usage, expensive ligands or base, substrate specificity, complex post-processing, and side products.30 Therefore, the development of an efficient, economical, and environmental-friendly catalyst system for the Chan–Lam coupling reaction has drawn promising interest.
Activated carbon-supported transition metal catalysts, such as Pd/AC, Pt/AC, and Co/AC, have been widely used in numerous organic transformations.31−33 However, Cu(II)/AC catalysts have not been well utilized in the industry. Herein, an effective, easy-prepared, and economical activated carbon-supported Cu(BF4)2 catalyst was developed for efficient amination of anilines, isoquinolin-1(2H)-one, and 1,6-naphthyridin-5(6H)-one with aryl boronic acids (Scheme 2c).
Experimental Section
Materials and Methods
Unless otherwise noted, all starting materials, reagents, and solvents were commercially available and used without further purification. The intermediates and end-products were purified by flash column chromatography with silica gel 60 (200–300 mesh). Chemical reactions were monitored by thin-layer chromatography (TLC) or liquid chromatography (LC)/mass spectrometry (MS). LC/MS was performed on an Agilent HPLC1260-MS6120 system (column: Agilent-SB-C18, 2.5 mm × 30 mm, 3.5 μm). 1H NMR and 13C NMR spectra were obtained using CDCl3 or DMSO-d6 as a solvent on a Bruker Avance III 400 or 600 M frequency spectrometer. The coupling constant J is given in hertz. NMR data are reported as follows: chemical shift (TMS as an internal standard), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br. = broad, m = multiplet, dt = double triplet), coupling constants, and integration. Specific surface areas were calculated using Brunauer–Emmett–Teller (BET) theory. Scanning electron microscopy (SEM) was performed on a field emission Hitachi SU8220 electron microscope. X-ray diffraction (XRD) patterns were collected using a Bruker D8 Advance X-ray powder diffractometer (Cu Kα1) over a 2θ range of 5–60 with Bragg–Brentano geometry. For X-ray photoelectron spectroscopy (XPS), the sample was pressed onto indium foil and monitored on a Thermo Fisher Scientific K-α X-ray photoelectron spectrometer.
Preparation of the Cu(BF4)2/AC Heterogeneous Catalyst
Commercially available 45 wt % aq. Cu(BF4)2 solution (1.0 g) was added to a mixed solution of methanol (50 mL) and dichloromethane (100 mL). Subsequently, active carbon (5.0 g) was added to the mixture. The ultrasonic (5 min) and rotary evaporation treatment of the mixture solution gave a black powder Cu(BF4)2/AC (5.8 g, 7.7 mass %).
Typical Procedure for Preparing 3a-m by N-Arylation of Aniline with Substituted Phenylboronic Acid
In a typical experimental procedure, di-tert-butyl peroxide (2.0 mmol) was dropwise added to a mixture of aniline (1.0 mmol), Cu(BF4)2/AC (10 mol %), 3 Å. MS (1.2 g), and substituted phenylboronic acid (2.5 mmol) in methanol (2 mL) at room temperature, and the mixture was stirred for 12 h. After TLC showed the disappearance of the starting material disappeared, the reaction mixture was filtered and the filtrate was concentrated in vacuo to give a yellow oil. The oil was purified by column chromatography on silica gel (eluent:ethyl acetate/petroleum ether = 0–1/1) to afford the N-phenylaniline derivatives 3a-m.
Typical Procedure for Preparing 3n-s, 3g-h, and 3k-l by N-Arylation of Substituted Aniline with Phenylboronic Acid
In a typical experimental procedure, di-tert-butyl peroxide (2.0 mmol) was dropwise added to a mixture of substituted aniline (1.0 mmol), Cu(BF4)2/AC (10 mol %), 3 Å. MS (1.2 g), and phenylboronic acid (2.5 mmol) in methanol (2 mL) at room temperature, and the mixture was stirred for 12 h. After TLC showed the disappearance of the starting material, the reaction mixture was filtered and the filtrate was concentrated in vacuo to give a yellow oil. The oil was purified by column chromatography on silica gel (eluent:ethyl acetate/petroleum ether = 0–1/1) to afford the N-phenylaniline derivatives 3n-s, 3g-h, and 3k-l.
Typical Procedure for Preparing 6a-x by N-Arylation of Isoquinolin-1(2H)-one and 1,6-Naphthyridin-5(6H)-one with Various Substituted Phenylboronic Acids
In a typical experimental procedure, Cu(BF4)2/AC (10 mol %) was added to a mixture of isoquinolinone or naphthyridinone (1.0 mmol), TMEDA (3.0 mmol), and aryl boronic acid (2.5 mmol) in a mixed solvent (10.0 mL, THF/MeOH = 4.0/6.0 mL) at room temperature and the mixture was stirred until no starting material was detected by TLC. The reaction mixture was filtered, and the filtrate was concentrated in vacuo to give a yellow oil. The oil was purified by column chromatography on silica gel with an eluent (ethyl acetate /petroleum ether = 0–3/1) to afford the desired product 6a-x.
Results and Discussion
To gain an optimized protocol for the synthesis of N-phenylaniline, we initiated our studies on phenylamine (1a) and phenylboronic acid (2a) in the presence of different doses of aq. Cu(BF4)2 (45 wt % in water). As shown in Table 1, when 3% of the copper salt was applied, only 41% product was performed (entry 1). A moderately increased yield was observed after increased usage of the copper salt (entry 2). Lastly, a stoichiometric amount of catalyst (entry 3) led to the formation of 3a in an 87% yield. Subsequently, we carefully optimized the solvent and base in the presence of 10% copper salt. Relatively, MeOH, EtOAc, and 1,4-dioxane were preferable (entries 4–8). In contrast, TMEDA was a better organic base choice (entry 9 vs 12; Table S1, entry 3). Compared to TMEDA or K2CO3, the strong basicity of DBU or KOH did not indicate the benefits (entries 10 and 11). All of these indicated that TMEDA and K2CO3 both appeared to be the optimized bases for efficient reactivity. And these data also demonstrated that aq. Cu(BF4)2 could be used as a moderately effective catalyst for Chan–Lam coupling.
Table 1. Screening Reaction Conditionsa.
| entry | X | atm. | base | solvent | yieldd |
|---|---|---|---|---|---|
| 1 | 3 | air | K2CO3 | 1,4-dioxane | 41% |
| 2 | 10 | air | K2CO3 | 1,4-dioxane | 62% |
| 3 | 100 | air | K2CO3 | 1,4-dioxane | 87% |
| 4 | 10 | air | K2CO3 | tert-butanol | 4% |
| 5 | 10 | air | K2CO3 | MeOH | 59% |
| 6 | 10 | air | K2CO3 | THF | 51% |
| 7 | 10 | air | K2CO3 | EtOAc | 65% |
| 8 | 10 | air | K2CO3 | DCM | 14% |
| 9 | 10 | air | TMEDA | 1,4-dioxane | 67% |
| 10 | 10 | air | DBU | 1,4-dioxane | 19% |
| 11 | 10 | air | KOH | 1,4-dioxane | 14% |
| 12 | 10 | air | DIPEA | 1,4-dioxane | 12% |
| 13 | 10 | N2 | K2CO3 | 1,4-dioxane | NR |
| 14 | 10b | air | K2CO3 | 1,4-dioxane | 82% |
| 15 | 10c | air | K2CO3 | 1,4-dioxane | 80% |
Unless otherwise noted, all of the reactions were run with 1a (2.1 mmol), 2a (2.5 equiv), and base (2.0 equiv) under air in 10.0 mL of solvent. Equivalents (equiv) refer to the mole ratio to aniline.
Content value of Cu(BF4)2/AC was 7.7 mass %.
Content value of Cu(BF4)2/AC was 15.4 mass %.
Isolated yields.
During the reaction proceeding, the addition of phenylboronic acid in a ratio of 1:1.5 to 1:3 (1a: 2a) did not afford improvement in the yield (Table S1, entries 1–4). The reason for this phenomenon is the formation of several byproducts, such as azobenzene, phenol, and diphenyl ether, which could inversely be heightened by H2O in the catalyst or atmosphere (Scheme 3). On this basis, to eliminate the influence of H2O, a heterogeneous catalyst system containing activated carbon and Cu(BF4)2 was subsequently prepared. And with a lower catalyst content, a slightly higher yield of 82% was obtained (entries 14 and 15) with no open flask and no diphenylamine (entry 13).
Scheme 3. Control Experiments.

In expanding the substrate range, employing methyl, ethyl, or bromo atom as the ortho-substituent in phenylboronic acid, its reaction resulted in low yields (Table S2, 3b, 3c, 3i, 38, 27, 28%). The phenylation of 2-fluoroaniline showed a trace product. And the formation of 1-phenyl-3,4-dihydroquinolin-2(1H)-one was found to be in a moderate yield (Table 4, 6w, 34%). In addition, using TMEDA as a base, N-phenylation of 1,6-naphthyridin-5(6H)-one showed good reactivity with an excellent yield of 99% (Table S3, 6m). The changes in the yield of benzamide derivatives may be due to the nucleophilicity of abundant NH-amides. Therefore, we knew that the Cu(BF4)2/AC catalytic system could not be further applied to the N-arylation of o-substituent anilines unless a big change was made.
Table 4. Cu(BF4)2/AC-Catalyzed the Coupling of 1,6-Naphthyridin-5(6H)-one or Isoquinolin-1(2H)-one with Substituted Phenylboronic Acidsa,b.
All reactions were performed with 5 (2.5 equiv to 1.0 mmol of 4), 10 mol % Cu(BF4)2/AC, TMEDA (3.0 equiv), THF (4.0 mL), and MeOH (6.0 mL).
Only amide compound. All yields were isolated.
Normally, oxygen (O2) is considered an economic and environmentally friendly oxidant for Chan–Lam coupling. As shown in Table 2, the O2 balloon did slightly increase the yield to 41% (entries 1 and 2). Using di-tert-butyl peroxide (DTBP), a significant increase in yield was observed even in the absence of a base (entry 3). Compared with 1,4-dioxane, the coupling reaction was carried out with higher efficiency in methanol in the presence of molecular sieves (entries 4 and 5). This might be due to the higher solubility of ortho-tolylboronic acid in methanol.
Table 2. Conditions Screening of the Cu(BF4)2/AC-Catalyzed Oxidative Coupling Reaction of Phenylamine with ortho-Tolylboronic Acida.
| entry | oxidantb | additivec | solventd | yield (%) |
|---|---|---|---|---|
| 1 | air | K2CO3 | 1,4-dioxane | 38 |
| 2 | O2 | K2CO3 | 1,4-dioxane | 41 |
| 3 | DTBP | no base | 1,4-dioxane | 57 |
| 4 | DTBP | M.s. 3 Å | 1,4-dioxane | 58 |
| 5 | DTBP | M.s. 3 Å | methanol | 93 |
Unless otherwise noted, all of the reactions were run with 1a (1.0 mmol), 2b (2.5 mmol).
Reaction performed using DTBP (2.0 mmol), O2 balloon, air = open flask.
K2CO3 (2.0 mmol).
1,4-Dioxane (10.0 mL), methanol (2.0 mL).
With the optimized conditions and oxidant in hand, we examined the substrate scope of the Cu(BF4)2/AC-catalyzed Chan–Lam coupling reaction between phenylamines with phenylboronic acids under open-flask conditions. These results are summarized in Table 3. All products were obtained as yellow or colorless oil and characterized by 1H NMR, 13C NMR, and mass spectra.
Table 3. Investigation of the Scope of Substituted Phenylboronic Acids and Aniline Derivativesa.
| entry | Ar1 | Ar2 | Pd. | yield (%)b |
|---|---|---|---|---|
| 1 | phenyl (1a) | phenyl (2a) | 3a | 94 |
| 2 | phenyl (1a) | 2-Me-C6H4 (2b) | 3b | 93 |
| 3 | phenyl (1a) | 2-Et-C6H4 (2c) | 3c | 79 |
| 4 | phenyl (1a) | 2,6-di-Me-C6H4 (2d) | 3d | 52 |
| 5 | phenyl (1a) | 3,5-di-Me-C6H4 (2e) | 3e | 72 |
| 6 | phenyl (1a) | 3-Me-C6H4 (2f) | 3f | 82 |
| 7 | phenyl (1a) | 4-Me-C6H4 (2g) | 3g | 96 |
| 8 | phenyl (1a) | 2-F-C6H4 (2h) | 3h | 24 |
| 9 | phenyl (1a) | 2-Br-C6H4 (2i) | 3i | 43 |
| 10 | phenyl (1a) | 4-F-C6H4 (2j) | 3j | 85 |
| 11 | phenyl (1a) | 4-Cl-C6H4 (2k) | 3k | 76 |
| 12 | phenyl (1a) | 3-NO2-C6H4 (2l) | 3l | 80 |
| 13 | phenyl (1a) | 3-MF-C6H4 (2m) | 3m | 94 |
| 14 | 4-NO2-C6H4 (1n) | phenyl (2a) | 3n | 74 |
| 15 | 4-Br-C6H4 (1o) | phenyl (2a) | 3o | 85 |
| 16 | 4-tert-butyl-C6H4 (1p) | phenyl (2a) | 3p | 86 |
| 17 | 4-CF3-C6H4 (1q) | phenyl (2a) | 3q | 59 |
| 18 | 3-Br-4-Cl-C6H4 (1r) | phenyl (2a) | 3r | 68 |
| 19 | 4-Me-C6H4 (1g) | phenyl (2a) | 3g | 86 |
| 20 | 4-Cl-C6H4 (1k) | phenyl (2a) | 3k | 80 |
| 21 | 2-F-C6H4 (1h) | phenyl (2a) | 3h | 62 |
| 22 | 3-NO2-C6H4 (1l) | phenyl (2a) | 3l | 83 |
| 23 | 4-piperidin-C6H4(1s) | phenyl (2a) | 3s | 75 |
Unless otherwise noted, all of the reactions were run with 1 (1.0 mmol), 2 (2.5 mmol), 10 mol % Cu(BF4)2/AC in 2.0 mL of MeOH. The reaction was performed using DTBP (2.0 mmol), open flask.
Isolated yields.
All ortho-alkyl on the aryl boronic acid provided the corresponding diphenylamine derivatives in excellent yields. The amination of phenylamine (1a) with ortho/para-tolyl phenylboronic acid (2b, 2g) gave yields similar to phenylboronic acid (entries 1, 2, and 7). The reaction of 2-ethylphenylboronic acid (2c) with aniline gave 3c in a lower yield (79%) than 3b (entry 3). However, when methyl was located in the meta-position, the yield of 3f dropped slightly to 82% (entry 6). With more steric hindrance existing, the yields significantly decreased (3d, 3e vs 3b). Di-methyl at the m-position performed better than at the o-position (3e vs 3d). Some sorts of m-substituted aromatic boronic acids undergo reaction smoothly, such as methyl, nitro, and methyl formate (MF) groups (3f, 3l, 3m). Entirely different yields from the same substituted group but in a different position indicated that the electron-withdrawing effect by o-F or Br significantly affected the efficiency of C–N bond formation (3h vs 3j, 3i vs 3k).
Next, we turned our attention to exploring the scope of various substituted anilines (entries 14–21). For para-substituted anilines, both electron-donating (tert-butyl, methyl) and electron-withdrawing groups (NO2, Br, and Cl) can be equally applied with acceptable yields (3n, 3o, 3p, 3g, 3k). And the yield of 3q was decreased to 59% in the presence of a strong electron-withdrawing group, trifluoromethyl. In contrast, two halogen atom-substituted product 3r was obtained in a relatively lower yield (68%, 3r vs 3o and 3k). And N-arylation of 4-(piperidin-1-yl)aniline proceeded well (3s, entry 23). Interestingly, for the synthesis of 2-F-N-phenylaniline, two different yields in two ways indicated that the effect of ortho-F might be more powerful in transmetallation than that of the reductive elimination process (3h, entry 8 vs 21).
The scope of Cu(BF4)2/AC-catalyzed C–N bond formation was further extended to the coupling reaction of substituted aryl boronic acids with isoquinolin-1(2H)-one and 1,6-naphthyridin-5(6H)-one (Table 4). TMEDA was a good organic base choice for Chan–Lam amination (3a, Table 1). And we were gratified to find that using TMEDA and a mixed solvent of THF and methanol for both electron-withdrawing and electron-donating substituents on aryl boronic acids were well tolerated to give corresponding products with good to excellent yields.
For 1,6-naphthyridin-5(6H)-one, not many differences in 6a-6d and 6m indicated a little impact of steric hindrance exerted by an electron-rich ortho-alkyl substituent. Similarly, coupling of isoquinolin-1(2H)-one with ortho-substituted aryl boronic acids also afforded the products in about 99% yields (6n-6p). Both electron-donating and electron-withdrawing groups at the meta-position, including double methyl-substituted (6e, 6t), methyl (6f), Br (6g, 6v), NO2 (6h, 6r), and methoxycarbonyl (6i, 6s), worked well across the two different substrates. The strong electron-withdrawing effect of ortho-Br reduced the efficiency (6j, 6u). In addition, ortho-hydroxy (6k, 6q) substituted aryl boronic acids were also well reactive. Interestingly, hydrolysis completely proceeded to the amide group in situ when using ortho-CN-phenylboronic acid (6l). This result indicated that our heterogeneous catalyst can be used for cyanide hydrolysis.
N-Arylation of naphthyridinone proceeded with a better yield than that of isoquinolinone with the same substituents. The proposed reason might be that the elimination of nitrogen atoms from naphthyridinone reduces the nucleophilicity of amide-NH. Otherwise, the phenylation of pyridazine-3(2H)-one and quinolin-2(1H)-one was significantly reduced, which could be due to the π-deficient effect (6w, 6x).
Next, control experiments were carried out to understand the plausible reaction mechanism (Scheme 3). Only azobenzene formation was observed within the aq. Cu(BF4)2-catalyzed reaction (Scheme 3i and Table S1, entry 5). After using the molecule sieves, biphenyl became the main byproduct, and the ratio to azobenzene was close to 4:1 (Scheme 3ii and Table S4). A small number of byproducts containing phenol, anisole, and diphenyl ether also existed (Table S4) in the Cu(BF4)2/AC-catalyzed reaction (Scheme 3iii). The results showed biphenyl, phenol, and diphenyl ether perhaps derived from phenylboronic acid. We extended the rotary evaporation time in the preparation of Cu(BF4)2/AC to eliminate the effect of H2O. The formation of biphenyl was still observed under open-flask conditions (TLC detection, Table 1, entry 14, and Scheme 3iv). Further performing the reaction with DTBP, the homocoupling of phenylboronic acid did not occur (not detected in TLC, Scheme 3v). The reaction could also be carried out under a N2 atmosphere. However, there were more byproducts in a frightful mess (Scheme 3vi, TLC detection). Finally, in the absence of air and DTBP, the formation of diphenylamine and biphenyl was not observed (Scheme 3vii), proving that an oxidant is essential for the Chan–Lam coupling and homocoupling of phenylboronic acid.
The currently accepted catalytic cycle for Chan–Lam coupling is the one proposed by Stahl, in which the arylated Cu(II) complex is oxidized to arylated Cu(III) by another Cu(II) prior to reductive elimination.17,34−36 And based on the experimental results and previous literature about the possible mechanism for phenylboronic acid homocoupling,37 a tentative mechanism is shown in Scheme 4. The first step is the reaction of Cu(BF4)2/AC and aryl boronic acids, resulting in the formation of complex A. Next, oxidation of A to the Cu(III) complex B occurs by the disproportionation reaction, which liberates a Cu(I) intermediate. Reductive elimination of arylamines affords N-arylated products and gives the Cu(I) intermediate. The oxidative dimerization mechanism of aryl boronic acids involved the Cu(I) species. And compared to O2, DTBP might facilitate the reoxidation of the Cu(I) intermediate to the Cu(II) catalyst. It is not clear but possibly be the reason for less biphenyl formation.
Scheme 4. Proposed Mechanism.
The recyclability of the prepared catalyst was tested using aniline and phenylboronic acid as substrates (Table 3, entry 1). The catalyst was easily separated from the reaction mixture by filtration. The recovered catalyst was washed with ethyl acetate and dichloromethane, then dried and reused for the same reaction. No significant decrease in catalyst activity was observed for up to 5 runs (Figure 1). Furthermore, when the conversion was 60% (4 h), we separated Cu(BF4)2/AC from the reaction mixture and washed it with methanol. The filtrate was stirred for an additional 6 h. No further reactivity was observed in the reaction solution. These results indicated the heterogeneous characteristics of Cu(BF4)2/AC.
Figure 1.

Recyclability of the catalyst.
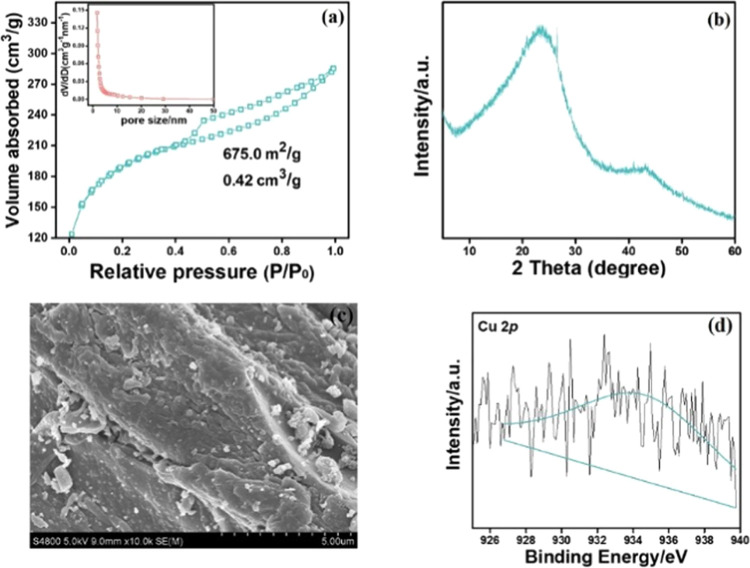
Finally, the catalyst was further profiled by the Brunauer–Emmett–Teller (BET) method, X-ray diffractometer (XRD), scanning electron microscope (SEM), and X-ray photoelectron spectroscopy (XPS).38,39 Our catalyst has a Brunauer–Emmett–Teller (BET) specific surface area of 675.0 m2 g–1. And the pore volume was 0.42 m3 g–1 (Figure 2a). Due to the low content of copper salt, the graph showed the amorphous nature of the material as a major portion of the material constitutes activated carbon (Figure 2b). However, the scanning electron microscopy (SEM) images and the wide-scan XPS spectrum of Cu(BF4)2/AC shown in Figure 2c,d revealed that copper salt was coated on activated carbon successfully. And the chemical state of copper mainly existed as Cu2+ valence and was assigned to Cu 2p3/2 at a binding energy of 932.1 eV.
Figure 2.
Characterization of the Cu(BF4)2/AC (a) specific surface area and pore size determined by the BET method. (b) XRD of Cu(BF4)2/AC. (c) SEM image. (d) XPS of Cu(BF4)2/AC.
Conclusions
In summary, an efficient activated carbon-supported Cu(BF4)2-catalyzed Chan–Lam coupling method has been developed. During the synthesis of substituted diphenylamine, the amination method with phenylboronic acids involving base-free and DTBP as the oxidant showed broad substrate scope and produced the corresponding coupling products in moderate to excellent yields with low catalytic loadings at room temperature. This methodology could also be successfully applied for the synthesis of novel 2-phenylisoquinolin-1(2H)-one and 6-phenyl-1,6-naphthyridin-5(6H)-one derivatives. Further detailed study of the mechanism of this reaction is ongoing, and results will be reported in due course.
Acknowledgments
The authors acknowledge the financial support from the National Nature Sciences Foundation of China (General Program: 21877036), the Science and Technology Innovation Program of Hunan Province (2020RC2051), the Hunan Natural Science Foundation (2021JJ40335), and the Science and Technology Planning Project of Hunan Province (2018TP1017). This work was also funded by the Natural Science Foundation of Guangdong Province (2022A1515011737) and the GDAS′ Project of Science and Technology Development (2021GDASYL-20210102010). We also thank all supporting functions, including the compound management team, Minxiong Li, and Fuxian Wang.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04299.
Experimental procedures, NMR spectra data, and MS of all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cappelli A.; Mohr G. P.; Giuliani G.; Galeazzi S.; Anzini M.; Mennuni L.; Ferrari F.; Makovec F.; Langer T.; Vomero S.; et al. Further Studies on Imidazo[4,5-b]pyridine AT1 Angiotensin II Receptor Antagonists. Effects of the Transformation of the 4-Phenylquinoline Backbone into 4-Phenylisoquinolinone or 1-Phenylindene Scaffolds. J. Med. Chem. 2006, 49, 6451–6464. 10.1021/jm0603163. [DOI] [PubMed] [Google Scholar]
- Sun H.; Zhou L.; Dong H.; Huang W.; She N. Discovery of 8-Amino-Substituted 2-Phenyl-2,7-Naphthyridinone Derivatives as New c-Kit/VEGFR-2 Kinase Inhibitors. Molecules 2019, 24, 4461–4474. 10.3390/molecules24244461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair H. A. Duvelisib: First Global Approval. Drugs 2018, 78, 1847–1853. 10.1007/s40265-018-1013-4. [DOI] [PubMed] [Google Scholar]
- Tong L.; Wang P.; Li X.; Dong X.; Hu X.; Wang C.; liu Tao.; Li J.; Zhou Y. Identification of 2-Aminopyrimidine Derivatives as FLT3 Kinase Inhibitors with High Selectivity over c-KIT. J. Med. Chem. 2022, 65, 3229–3248. 10.1021/acs.jmedchem.1c01792. [DOI] [PubMed] [Google Scholar]
- Evans C. A.; Liu T.; Nair S. J.; Grenier L.; Pradeilles J. A.; Glenadel Q.; Tibbitts T.; Rowley A. M.; Tremblay M. R.; Castro A. C.; et al. Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate. ACS Med. Chem. Lett. 2016, 7, 862–867. 10.1021/acsmedchemlett.6b00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C.; Rao Z.; Pan Q.; An W. Z. updated patent review of small-molecule c-Met kinase inhibitors (2018-present). Expert Opin. Ther. Pat. 2022, 32, 279–298. 10.1080/13543776.2022.2008356. [DOI] [PubMed] [Google Scholar]
- Quambusch L.; Depta L.; Landel I.; Lubeck M.; Kirschner T.; Nabert J.; Uhlenbrock N.; Levy L. M.; Glanemann F.; Rauh D.; et al. Cellular model system to dissect the isoform-selectivity of Akt inhibitors. Nat. Commun. 2021, 12, 5297 10.1038/s41467-021-25512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T.; Xue Y.; Lu J. Chuanfei Jin. Synthesis and biological evaluation of 1-(4-(piperazin-1-yl)phenyl)pyridin-2(1H)-one derivatives as potential SSRIs. Eur. J. Med. Chem. 2021, 223, 113644 10.1016/j.ejmech.2021.113644. [DOI] [PubMed] [Google Scholar]
- Filipski K. J.; Kohrt J. T.; Casimiro-Garcia A.; Van Huis C. A.; Dudley D. A.; Cody W. L.; Bigge C. F.; Desiraju S.; Sun Shaoyi.; Edmunds J. J.; et al. A versatile copper-catalyzed coupling reaction of pyridin-2(1H)-ones with aryl halides. Tetrahedron Lett. 2006, 47, 7677–7680. 10.1016/j.tetlet.2006.08.112. [DOI] [Google Scholar]
- Grøn N. N.; Karsten J. Conjugate Addition-SNAr Domino Reaction for the Synthesis of Benzo- or Pyridyl-Fused Lactams and Sultams. Synthesis 2010, 24, 4273–4281. 10.1055/s-0030-1258302. [DOI] [Google Scholar]
- Zhixiang C.; Dawei M. Cu/N,N′-Dibenzyloxalamide-Catalyzed N-Arylation of Heteroanilines. Org. Lett. 2019, 21, 6874–6878. 10.1021/acs.orglett.9b02509. [DOI] [PubMed] [Google Scholar]
- Fors B. P.; Buchwald S. L. A Multiligand Based Pd Catalyst for C–N Cross-Coupling Reactions. J. Am. Chem. Soc. 2010, 132, 15914–15917. 10.1021/ja108074t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q.; Hartwig J. F. [(CyPF-tBu)PdCl2]: An Air-Stable, One-Component, Highly Efficient Catalyst for Amination of Heteroaryl and Aryl Halides. Org. Lett. 2008, 10, 4109–4112. 10.1021/ol801615u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Castillo P.; Buchwald S. L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. 10.1021/acs.chemrev.6b00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D. M. T.; Monaco K. L.; Wang R.-P.; Winters M. P. New N- and O-arylations with phenylboronic acids and cupric acetate. Tetrahedron Lett. 1998, 39, 2933–2936. 10.1016/S0040-4039(98)00503-6. [DOI] [Google Scholar]
- Lam P. Y. S.; Clark C. G.; Saubern S.; Adams J.; Winters M. P.; Chan D. M. T.; Combs A. New aryl/heteroaryl C–N bond cross-coupling reactions via arylboronic acid/cupric acetate arylation. Tetrahedron Lett. 1998, 39, 2941–2944. 10.1016/S0040-4039(98)00504-8. [DOI] [Google Scholar]
- Duparc V. H.; Bano G. L.; Schaper F. Chan–Evans–Lam Couplings with Copper Iminoarylsulfonate Complexes: Scope and Mechanism. ACS Catal. 2018, 8, 7308–7325. 10.1021/acscatal.8b01881. [DOI] [Google Scholar]
- Antilla J. C.; Buchwald S. L. Copper-Catalyzed Coupling of Arylboronic Acids and Amines. Org. Lett. 2001, 3, 2077–2079. 10.1021/ol0160396. [DOI] [PubMed] [Google Scholar]
- Lan J.-B.; Chen Li.; Yu X.-Q.; You J.-S.; Xie R. A simple copper salt catalysed the coupling of imidazole with arylboronic acids in protic solvent. Chem. Commun. 2004, 188–189. 10.1039/b307734a. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Zhang G.; Zhang M.; Cheng J. Cu(OTf)2-Mediated Chan–Lam Reaction of Carboxylic Acids to Access Phenolic Esters. J. Org. Chem. 2010, 75, 7472–7474. 10.1021/jo101558s. [DOI] [PubMed] [Google Scholar]
- Collman J. P.; Zhong M. An Efficient Diamine·Copper Complex-Catalyzed Coupling of Arylboronic Acids with Imidazoles. Org. Lett. 2000, 2, 1233–1236. 10.1021/ol000033j. [DOI] [PubMed] [Google Scholar]
- Yamashita K.-i.; Nakamura A.; Sugiura K.-i. Regioselective Oxidative Oligomerization Reaction of 2-tert-Alkylpyrene and Isolation of Structurally Well-defined 1,3-Pyrenylenes. Chem. Lett. 2015, 44, 303–305. 10.1246/cl.141029. [DOI] [Google Scholar]
- Rajbangshi S.; Sugiura K.-i. An Alternative Synthesis of Bipyrenol: A High-Yield Oxidative Coupling Reaction of a Pyrene Derivative with Cu(BF4)2·nH2O. Synthesis 2017, 49, 3145–3148. 10.1055/s-0036-1588819. [DOI] [Google Scholar]
- Kantam M. L.; Venkanna G. T.; Sridhar C.; Sreedhar B.; Choudary B. M. An Efficient Base-Free N-Arylation of Imidazoles and Amines with Arylboronic Acids Using Copper-Exchanged Fluorapatite. J. Org. Chem. 2006, 71, 9522–9524. 10.1021/jo0614036. [DOI] [PubMed] [Google Scholar]
- Mitrofanov A. Y.; Murashkina A. V.; Martín-García I.; Alonso F.; Beletskaya I. P. Formation of CC, C–S and C–N bonds catalysed by supported copper nanoparticles. Catal. Sci. Technol. 2017, 7, 4401–4412. 10.1039/C7CY01343D. [DOI] [Google Scholar]
- Mostafalu R.; Kaboudin B.; Kazemi F.; Yokomatsu T. N-arylation of amines: C–N coupling of amines with arylboronic acids using Fe3O4 magnetic nanoparticles-supported EDTA-Cu(II) complex in water. RSC Adv. 2014, 4, 49273–49279. 10.1039/C4RA08137D. [DOI] [Google Scholar]
- Kumar A.; Layek S.; Agrahari B.; Kujur S.; Pathak D. D. Graphene Oxide Immobilized Copper(II) Schiff Base Complex [GO@AF-SB-Cu]: A Versatile Catalyst for Chan-Lam Coupling Reaction. ChemistrySelect 2019, 4, 1337–1345. 10.1002/slct.201803113. [DOI] [Google Scholar]
- Biffis A.; Filippi F.; Palma G.; Lora S.; Maccà C.; Corain B. Metallation of functional resins with copper acetate: control of metal speciation and catalytic activity in C–N coupling reactions. J. Mol. Catal. A: Chem. 2003, 203, 213–220. 10.1016/S1381-1169(03)00260-7. [DOI] [Google Scholar]
- Anuradha; Kumari S.; Pathak D. D. Synthesis and development of Chitosan anchored Copper(II) Schiff base complexes as heterogeneous catalysts for N-arylation of amines. Tetrahedron Lett. 2015, 56, 4135–4142. 10.1016/j.tetlet.2015.05.049. [DOI] [Google Scholar]
- Miceli M.; Frontera P.; Macario A.; Malara A. Recovery/Reuse of Heterogeneous Supported Spent Catalysts. Catalysts 2021, 11, 591–608. 10.3390/catal11050591. [DOI] [Google Scholar]
- Mao S.; Shi X.; Soulé J.-F.; Doucet H. Pd/C as Heterogeneous Catalyst for the Direct Arylation of (Poly)fluorobenzenes. Chem. - Eur. J. 2019, 25, 9504–9513. 10.1002/chem.201900921. [DOI] [PubMed] [Google Scholar]
- Xu S.; Kim Y.; Park J.; Higgins D.; Shen S.-J.; Schindler P.; Thian D.; Provine J.; Schladt T. D.; Park J.; et al. Extending the limits of Pt/C catalysts with passivation-gas-incorporated atomic layer deposition. Nat. Catal. 2018, 1, 624–630. 10.1038/s41929-018-0118-1. [DOI] [Google Scholar]
- Wei J.; Chen Y.; Ma Y.; Shi X.; Zhang X.; Shi C.; Hu M.; Liu J. Precisely Engineering Architectures of Co/C Sub-Microreactors for Selective Syngas Conversion. Small 2021, 17, 2100082 10.1002/smll.202100082. [DOI] [PubMed] [Google Scholar]
- Vantourout J. C.; Miras H. N.; Isidro-Llobet A.; Sproules S.; Watson A. J. B. Spectroscopic Studies of the Chan-Lam Amination: A MechanismInspired Solution to Boronic Ester Reactivity. J. Am. Chem. Soc. 2017, 139, 4769–4779. 10.1021/jacs.6b12800. [DOI] [PubMed] [Google Scholar]
- Huffman L. M.; Huffman; Stahl S. S. Carbon-Nitrogen Bond Formation Involving Well-Defined Aryl-Copper(III) Complexes. J. Am. Chem. Soc. 2008, 130, 9196–9197. 10.1021/ja802123p. [DOI] [PubMed] [Google Scholar]
- King A. E.; Ryland B. L.; Brunold T. C.; Shannon S. S. Kinetic and Spectroscopic Studies of Aerobic Copper(II)-Catalyzed Methoxylation of Arylboronic Esters and Insights into Aryl Transmetalation to Copper(II). Organometallics 2012, 31, 7948–7957. 10.1021/om300586p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir A. S.; Reis Ö.; Emrullahoglu M. Role of Copper Species in the Oxidative Dimerization of Arylboronic Acids: Synthesis of Symmetrical Biaryls. J. Org. Chem. 2003, 68, 10130–10134. 10.1021/jo034680a. [DOI] [PubMed] [Google Scholar]
- Liu Q.; cao H.; Xu W.; Li J.; Zhou Q.; Tao W.; Zhu H.; Cao X.; Zhong L.; Wu J.; et al. Vacancy engineered polymeric carbon nitride nanosheets for enhanced photoredox catalytic efficiency. Cell Rep. Phys. Sci. 2021, 2, 100491 10.1016/j.xcrp.2021.100491. [DOI] [Google Scholar]
- Liu Q.; Cheng H.; Chen T.; Lo T. W. B.; Xiang Z.; Wang F. Regulating the* OCCHO intermediate pathway towards highly selective photocatalytic CO2 reduction to CH3CHO over locally crystallized carbon nitride. Energy Environ. Sci. 2022, 15, 225–233. 10.1039/D1EE02073K. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.