Abstract
Background
Adequate bowel preparation before colonoscopy is crucial. Unfortunately, 25% of colonoscopies have inadequate bowel cleansing. From a patient perspective, bowel preparation is the main obstacle to colonoscopy. Several low-volume bowel preparations have been formulated to provide more tolerable purgative solutions without loss of efficacy.
Objectives
Investigate efficacy, safety, and tolerability of Sodium Picosulphate plus Magnesium Citrate (SPMC) vs. Polyethylene Glycol plus Ascorbic Acid (PEG-ASC) solutions in patients undergoing diagnostic colonoscopy.
Materials and methods
In this phase 4, randomized, multicenter, two-arm trial, adult outpatients received either SPMC or PEG-ASC for bowel preparation before colonoscopy. The primary aims were quality of bowel cleansing (primary endpoint scored according to Boston Bowel Preparation Scale) and patient acceptance (measured with six visual analogue scales). The study was open for treatment assignment and blinded for primary endpoint assessment. This was done independently with videotaped colonoscopies reviewed by two endoscopists unaware of study arms. A sample size of 525 patients was calculated to recognize a difference of 10% in the proportion of successes between the arms with a two-sided alpha error of 0.05 and 90% statistical power.
Results
Overall 550 subjects (279 assigned to PEG-ASC and 271 assigned to SPMC) represented the analysis population. There was no statistically significant difference in success rate according to BBPS: 94.4% with PEG-ASC and 95.7% with SPMC (P = 0.49). Acceptance and willing to repeat colonoscopy were significantly better for SPMC with all the scales. Compliance was less than full in 6.6 and 9.9% of cases with PEG-ASC and SPMC, respectively (P = 0.17). Nausea and meteorism were significantly more bothersome with PEG-ASC than SPMC. There were no serious adverse events in either group.
Conclusion
SPMC and PEG-ASC are not different in terms of efficacy, but SPMC is better tolerated than PEG-ASC. SPMC could be an alternative to low-volume PEG based purgative solutions for bowel preparation.
Clinical trial registration
[ClinicalTrials.gov], Identifier [NCT01649674 and EudraCT 2011–000587–10].
Keywords: bowel preparation, sodium picosulphate plus magnesium citrate, polyethylene glycol plus ascorbic acid, patients compliance, colonoscopy, randomized controlled trial
Introduction
The goal of colonoscopy should be safe, accurate, and complete examination of the entire colon. Therefore, colon cleansing before colonoscopy is crucial. Unfortunately, up to 25% of all colonoscopies have an inadequate bowel cleansing, which is due to different factors ranging from patient-related variables (compliance with preparation and/or medical conditions) to scheduling of colonoscopy (waiting times, timing of the exam, etc.) (1–3).
The ideal preparation for colonoscopy should clean the colon, with no alteration of colonic mucosa and without causing patient discomfort or shifts in fluids, as well as electrolytes (1–4). High quality bowel cleansing is obtained using high-volume (4 L) solutions of Polyethylene Glycol with electrolytes (PEG-ELS) (1, 2). However, 15% of patients do not complete the bowel preparation with this solution because of its large volume (4). Low-volume (2 L) PEG-ELS solutions were formulated to provide a more tolerable bowel preparation with similar efficacy (5–7). Nevertheless, still 5–10% patients do not assume the entire purgative solution (5–8). Sodium Mono Phosphate (NaP), a very low-volume (500 ml), osmotically active agent, was used to improve patient compliance for bowel preparation, but its use is not recommended because of risk for serious adverse events (8, 9). An alternative to NaP is Sodium Picosulfate and Magnesium Citrate (SPMC), another very low-volume (300 ml) bowel preparation (10). Previous studies comparing SPMC with low-volume PEG-ELS solutions demonstrated the non-inferiority of SPMC in bowel cleansing and suggested that SPMC is more tolerable than PEG-based solutions (11–20), even if one study showed that patient overall tolerance was higher for PEG-ASC than SPMC (12). However, the majority of these studies had a single-center design and evaluated a relatively small number of patients (10–16), with the exception of the two SEE CLEAR studies (19, 20).
This paper reports the results of a large, phase 4, randomized, multicenter study that investigated efficacy, safety, and tolerability of SPMC vs. PEG-ELS plus ascorbic acid (PEG-ASC) solutions in patients undergoing colonoscopy.
Materials and methods
This non-profit multicenter, phase 4, randomized, two-arm trial compared SPMC versus PEG-ASC, in patients undergoing colonoscopy. The primary aim was to compare the quality of bowel preparation. The co-primary aim was to evaluate patient acceptance of the purgative solutions. The secondary aim was to compare patient compliance for bowel preparation. The study protocol was approved by the Ethics Committee of the coordinating center (March 29, 2011), and by the Ethics Committees of the other participating centers. The study was conducted in accordance with the Principles of the Declaration of Helsinki, Good Clinical Practice, and applicable regulatory requirements. All participants provided written informed consent at the time of the enrollment and randomization, which were coincident with the booking of colonoscopy. The trial was registered at ClinicalTrials.gov (NCT01649674) and EudraCT (2011—000587—10).
The study was open for the treatment assignment (both the endoscopist and the patient were aware of the type of preparation), but it was blind for the independent assessment of the primary end-point (reviewers were unaware of the bowel preparation assumed by patients). Patients were registered and randomized via a web platform1 at the Clinical Trial Unit of the coordinating center which collected all the data. Randomization was performed by using a minimization procedure that included center, indication for colonoscopy (screening vs. previous clinical or radiological suspicion of cancer vs. other), previous colonoscopy (no vs. yes), and modality of assumption (standard vs. split), as stratification variables. Sample size was calculated with the aim of recognizing a difference of 10% (considered as a clinically relevant minimum value) in the proportion of successes (adequate bowel preparation) between the two purgative solutions. Considering a success rate in the less effective arm equal to 80%, and a two-sided alpha error of 0.05, the study was going to guarantee 90% power in highlighting the expected difference of 10%, with the enrollment of 525 patients. With this sample size, and the same alpha error, the study has more than 99% power to recognize a difference of 1 point on the patients’ acceptance scale.
Patients, aged 18 years or older, were eligible if they were scheduled for a colonoscopy due to screening, diagnosis and follow-up after polypectomy. Subjects who had previously undergone bowel resection or had severe inflammatory bowel disease, renal failure, heart failure (NYHA classes III and IV), hepatic insufficiency (class B or C, according to the Child-Pugh classification), severe dehydration, as well as, pregnant women, individuals assuming lithium, and subjects with other known contraindications, either to bowel preparation (i.e., hypermagnesemia, rhabdomyolysis) or to execution of colonoscopy, were excluded.
The purgative solutions used for bowel preparation were respectively PEG-ASC and SPMC. Both bowel preparations were used in clinical practice at the time of the study. PEG-ASC (Moviprep, Norgine, Harefield, UK) is composed of PEG-3350, sodium sulfate, sodium chloride and ascorbic acid (2, 21). Ascorbic acid cannot be absorbed and functions as osmotic laxative, reducing the volume of the purgative solution to 2 L (4, 22). However, an additional volume of 1 L of clear fluid is required to reduce the risk of dehydration (4). SPMC (Citrafleet, Casen Recordati S. L., Zaragoza, Spain) is composed of sodium picosulfate and magnesium citrate which act as stimulant laxative, and osmotic laxative, respectively (2, 23). Despite the very low-volume (300 ml) of purgative solution, there is a need of an additional 3 L of clear liquids to avoid dehydration (4). Bowel preparation in both arms was done according to two different assumption modalities: standard (purgative solution was assumed entirely the evening before colonoscopy) or split (purgative solution was divided between the evening before and the morning of the colonoscopy). The modality of bowel preparation (standard or split) was declared before randomization and was considered among the stratification variables in the minimization procedure. Bowel preparation in the PEG-ASC - standard assumption arm consisted of 1 L of purgative solution followed by 0.5 L of clear liquids assumed twice, over a period of 90 min, respectively from 5:00 to 6:30 pm and from 8:00 to 9:30 pm, on the day before colonoscopy. The colonoscopy had to be performed the next morning, before 2:00 pm. Bowel preparation in the PEG-ASC–split assumption group consisted of 1 L of purgative solution followed by 0.5 L of clear liquids assumed twice, over a period of 90 min, respectively from 8:00 to 9:30 pm on the evening before colonoscopy, and from 7:00 to 8:30 am on the day of colonoscopy. Colonoscopy had to be performed in the afternoon. Bowel preparation in the SPMC arm - standard assumption, consisted of 150 ml of purgative solution followed by 1.5 L of clear liquids over a 90 min period, assumed twice, respectively at 2:00 pm and at 8:00 pm, on the day before colonoscopy. Colonoscopy had to be performed the next morning, before 2:00 pm. Bowel preparation in the SPMC–split assumption arm, consisted of 150 ml of purgative solution followed by 1.5 L of clear liquids over 90 min, assumed twice, respectively at 8:00 pm on the night before colonoscopy, and at 7:00 am on the day of colonoscopy. Colonoscopy had to be performed in the afternoon. In both arms, patients were asked to (i) follow a low-fiber diet and assume at least 2 L of water for 4 days before colonoscopy; (ii) assume a liquid diet on the day before colonoscopy and (iii) fast on the day of colonoscopy. All these instructions and the steps of bowel preparation were reviewed by the research nurse with each single subject at the time of enrollment.
The primary end-point of the study was the quality of bowel preparation. This was assessed according to the “Boston Bowel Preparation Scale” (BBPS), whose rating scale is between 0 and 3. The assessment was carried out prior to water flushing for the following colonic segments: right colon (caecum and ascending colon); transverse colon (including hepatic flexure and splenic flexure); left colon (descending and sigmoid colon, rectum). Consequently, the total score (0 to 3 for each segment) ranged from 0 to 9. Success was a total score between 6 and 9, with at least a score of 2 for each colonic segment (24, 25).
Colonoscopy was performed according to standard of practice of each participating center. All the exams were videotaped in their entirety with HD recorders and the CDs obtained were collected by the data manager of the Clinical Trial Unit for central blinded review. Five endoscopists (VDA, MDB, EDG, PM, and GBR) acted as blinded reviewers for the assessment of bowel preparation of the recorded colonoscopies in their totality. Each CD was reviewed and the quality of the bowel preparation was scored independently by two of them, who were selected by the trial coordinator at the Clinical Trial Unit. The chosen reviewers were unaware respectively of the bowel preparation assumed by the patient and the results of each other evaluation. Success was defined as a BBPS score ≥ 6, with at least a score of two for each colonic segment. In case of contrasting evaluation, a third reviewer was involved. In any case, a reviewer could not review an exam performed by her/himself. The outcome of each colonoscopy was described according to completeness of exploration (complete vs. incomplete) and duration time; causes of incomplete exploration were described.
Evaluation of patient acceptability of each bowel preparation was a co-primary end-point. The patients were provided a diary that was completed and returned to the research nurse before colonoscopy. The diary included visual analogic scales (VAS) from 0 to 10 (lower score representing a better outcome) and regarded the impact of purgative solutions on the following items: food intake (no impact up to inability to eat), taste (no impact up to very bad taste), simplicity of assumption (very simple up to very difficult), personal activity (no effect up to inability to perform personal activities), work activity (no effect up to inability to perform work activities), general perception (excellent up to very bad). Patients were also required to record whether all the purgative solution was assumed within the required time period and whether the correct amount of clear liquids was consumed. Finally, patients were asked if they were willing to repeat the same bowel preparation (no/yes). Compliance was calculated from patient diaries that inquired about the adherence to the prescribed diet, timing of assumptions, assumed volumes of both purgative solutions and clear liquids. Compliance was defined as full (all prescriptions respected), or less-than-full (one or more prescriptions not respected).
Statistical analysis was based on a modified intention-to-treat strategy (mITT), excluding patients who did not actually start bowel preparation. Success rate in the two arms was compared using the chi-square test. In the primary analysis, cases in which colonoscopy was interrupted for reasons other than bowel cleansing were considered failures. A sensitivity analysis was planned excluding those cases in which colonoscopy was interrupted before the evaluation of all three colonic segments for reasons independent of bowel cleansing (i.e., stricture of the colon). Success rate in the two arms was described for the subgroups defined according the meaningful clinical characteristics (gender, age, bowel segment, administration modality, and patients experience with a previous colonoscopy). No cutoff score for acceptability was defined in the protocol, outcomes of the scales in the two arms were compared as central measures by the Wilcoxon rank sum test. General perception was considered as a global outcome measure and the individual scores of the different dimensions were considered as secondary outcome measures. Compliance rate in the two arms was compared using the chi-square test.
Results
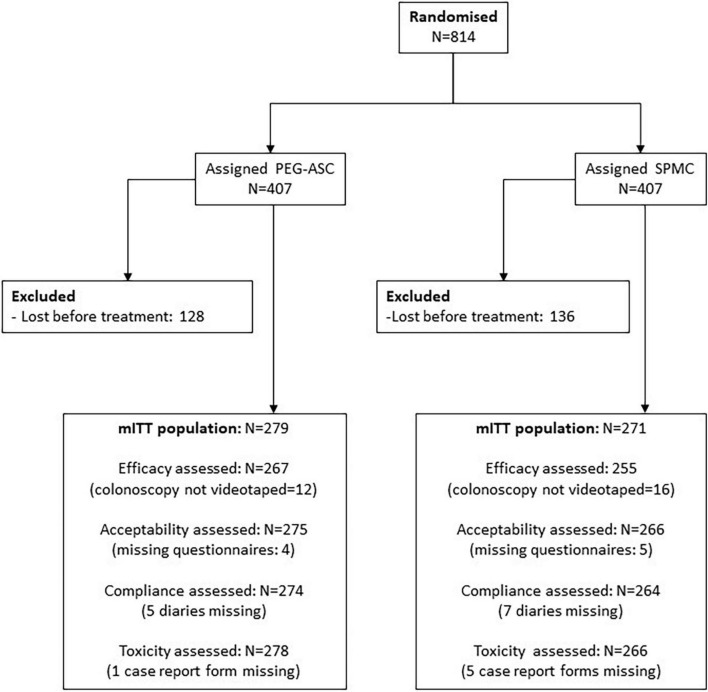
From November 7th, 2011, to February 16th, 2015, 814 subjects were enrolled in seven Italian centers and assigned to PEG-ASC (n = 407) or SPMC (n = 407): they represented the ITT population. Overall, 264 subjects were excluded because they were lost before starting bowel preparation and the remaining 550 cases represented the modified ITT (mITT) population (Figure 1). Details of both ITT and mITT are summarized in Table 1. ITT and mITT populations were similar in terms of baseline characteristics (Table 1). In the mITT population, the median age was 54 (range 18—94); 297/550 (54%) individuals were males; 83/550 (15%) subjects had already undergone colonoscopy. The most frequent indication for colonoscopy was diagnosis (351/550, 64%), followed by screening (134/550, 24%) and follow-up after polypectomy (62/550, 12%). Two-thirds of the bowel preparations were performed according to standard modality in both study arms, while one third of the purgative solutions was administered with the split modality approach.
FIGURE 1.
Patients flow.
TABLE 1.
Baseline data of intention-to-treat (ITT) and modified intention-to-treat (mITT) populations by assigned arm.
| Intention-to-treat |
Modified intention-to-treat |
|||
| PEG-ASC | SPMC | PEG-ASC | SPMC | |
| (N = 407) | (N = 407) | (N = 279) | (N = 271) | |
| n (%) | n (%) | n (%) | n (%) | |
| Gender, n (%) | ||||
| Male | 216 (53.1%) | 208 (51.1%) | 148 (53.0%) | 149 (55.0%) |
| Female | 191 (46.9%) | 199 (48.9%) | 131 (47.0%) | 122 (45.0%) |
| Age, years | ||||
| Median | 54 | 55 | 54 | 54 |
| Range | 18–86 | 18–94 | 18–86 | 18–94 |
| IQR | 45–66 | 44–66 | 44–66 | 44–64 |
| Previous colonoscopy, n (%) | 51 (12.5%) | 60 (14.7%) | 41 (14.7%) | 42 (15.5%) |
| Aim of the colonscopy, n (%) | ||||
| Diagnosis | 260 (63.9%) | 259 (63.6%) | 176 (63.1%) | 175 (64.6%) |
| Screening | 100 (24.6%) | 102 (25.1%) | 72 (25.8%) | 62 (22.9%) |
| Follow-up after colonoscopy | 47 (11.5%) | 46 (11.3%) | 31 (11.1%) | 34 (12.5%) |
| Scheme of administration, n (%) | ||||
| Standard | 252 (61.9%) | 260 (63.9%) | 181 (64.9%) | 178 (65.7%) |
| Split | 155 (38.1%) | 147 (36.1%) | 98 (35.1%) | 93 (34.3%) |
In the mITT population, 522 cases were assessible for efficacy evaluation because colonoscopy was not videotaped in 28 cases. There was no statistically significant difference in the rate of bowel cleansing according to BBPS between the two arms: in 252/267 (94.4%) subjects of PEG-ASC arm and 244/255 individuals (95.7%) of SPMC group overall colon cleansing was successful with a BBPS > 6, and a minimum score of 2 in each bowel segment (P = 0.49). Table 2 shows that the success rate of both bowel preparations was not influenced by gender, age, bowel segment, administration modality, and patients experience with a previous colonoscopy.
TABLE 2.
Success of bowel preparation.
| PEG-ASC | SPMC | P | |||
| Overall success | 252/267 | 94.4% | 244/255 | 95.7% | 0.49 |
| By gender | |||||
| Males | 134/141 | 95.0% | 135/140 | 96.4% | |
| Females | 118/126 | 93.7% | 109/115 | 94.7% | |
| By age | |||||
| = 54 | 123/130 | 94.6% | 118/123 | 95.9% | |
| > 54 | 129/137 | 94.2% | 126/132 | 95.5% | |
| By previous colonoscopy | |||||
| No previous colonoscopy | 217/229 | 94.8% | 204/214 | 95.3% | |
| Already done previous colonoscopy | 35/38 | 92.1% | 40/41 | 97.6% | |
| By administration scheme | |||||
| Standard scheme | 160/172 | 93.0% | 157/165 | 95.2% | |
| Split scheme | 92/95 | 96.8% | 87/90 | 96.7% | |
| By bowel side | |||||
| Right bowel | 252/267 | 94.4% | 244/255 | 95.7% | |
| Transverse bowel | 256/267 | 95.9% | 249/255 | 97.6% | |
| Left bowel | 266/267 | 99.6% | 255/255 | 100% | |
Success was defined as a Boston Bowel Prep Score > 6, with a minimum score of 2 in each of the 3 bowel segments.
Colonoscopy was reported less than complete in 40 cases, respectively 21/267 (8.0%) and 19/255 (7.7%) in the two arms (P = 0.86). These results were independent of the assumption modality of both bowel preparations. Among subjects assuming purgative solutions with standard modality, there was a success rate of 93.0% with PEG-ASC and 95.2% with SPMC, respectively; among subjects receiving the split dose, PEG-ASC was effective in 96.8% of cases and SPMC in 96.7% of patients, respectively. In the two arms, the duration of colonoscopy was comparable, with a median time of 21 min (interquartile range [IQR] 16—30) for procedure. Similarly, the withdrawal time of the scope during colonoscopy was equivalent with a median duration of 10 min in both study groups (IQR 8—15).
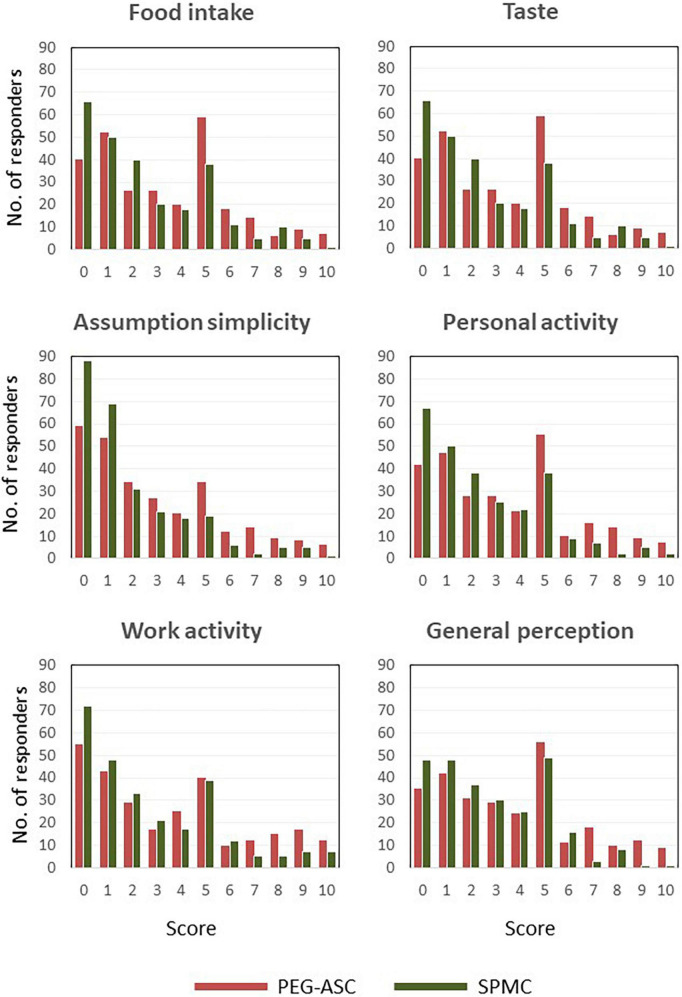
The impact of bowel preparation on patient acceptance was significantly better with SPMC in all the explored scales. Perception of preparation had a mean score of 3.7 (SD ± 0.2) vs. 2.8 (SD ± 0.1) (P < 0.0001), respectively for PEG-ASC and SPMC. Details of mean (SD) scores for patient-reported effect on food intake, taste, ease of administration, working activities are summarized in Table 3. Graphical representations of score distributions are reported in Figure 2. The rate of patients willing to repeat the bowel preparation was significantly lower with PEG-ASC (81.3%), in comparison to SPMC (92.3%, P < 0.001).
TABLE 3.
Impact of bowel preparation on patients’ acceptance (mean–SD).
| PEG - ASC N = 275 | SPMC N = 266 | P | |||
| Impact on food intake, mean (SD) | 3.5 | (0.2) | 2.6 | (0.1) | < 0.0001 |
| Impact on taste, mean (SD) | 3.6 | (0.2) | 2.3 | (0.1) | < 0.0001 |
| Easiness, mean (SD) | 3.0 | (0.2) | 1.9 | (0.1) | < 0.0001 |
| Impact on daily activities, mean (SD) | 3.5 | (0.2) | 2.5 | (0.1) | < 0.0001 |
| Impact on working activities, mean (SD) | 3.6 | (0.2) | 2.7 | (0.2) | < 0.0001 |
| Perception of preparation, mean (SD) | 3.7 | (0.2) | 2.8 | (0.1) | < 0.0001 |
FIGURE 2.
Score distribution.
Compliance with assumption of purgative solutions and the subsequent amount of clear liquids was less than complete, respectively, in 18/274 (6.6%) subjects assuming PEG-ASC and in 26/264 (9.9%) individuals using SPMC bowel preparation (P = 0.17).
Side effects of bowel preparation by study arm are summarized in Table 4. Nausea and meteorism were significantly more bothersome with PEG-ASC than SPMC, while vomiting, abdominal cramps and anal irritation were comparable in both arms. Headache, chills, insomnia and asthenia were similar with both purgative solutions. Side effects were not influenced by the modality of assumption (i.e., standard vs. split) of both purgative solutions. There were no serious adverse events leading to discontinuation of bowel preparation in either group.
TABLE 4.
Side effects of bowel preparation.
| PEG-ASC (N = 278) |
SPMC (N = 266) |
P
|
|||||||||||||||||||
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||||||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | ||
| Nausea | 242 | (87.1%) | 18 | (6.5%) | 13 | (4.7%) | 3 | (1.1%) | 2 | (0.7%) | 230 | (86.5%) | 30 | (11.3%) | 5 | (1.9%) | 1 | (0.4%) | 0 | (0.0%) | 0.048 |
| Vomiting | 265 | (95.3%) | 8 | (2.9%) | 2 | (0.7%) | 2 | (0.7%) | 1 | (0.4%) | 261 | (98.1%) | 3 | (1.1%) | 2 | (0.8%) | 0 | (0.0%) | 0 | (0.0%) | 0.28 |
| Meteorism | 237 | (85.3%) | 21 | (7.6%) | 12 | (4.3%) | 7 | (2.5%) | 1 | (0.4%) | 233 | (87.6%) | 26 | (9.8%) | 7 | (2.6%) | 0 | (0.0%) | 0 | (0.0%) | 0.047 |
| Abdominal cramps | 246 | (88.5%) | 22 | (7.9%) | 6 | (2.2%) | 3 | (1.1%) | 1 | (0.4%) | 242 | (91.0%) | 18 | (6.8%) | 6 | (2.3%) | 0 | (0.0%) | 0 | (0.0%) | 0.38 |
| Anal discomfort | 248 | (89.2%) | 16 | (5.8%) | 9 | (3.2%) | 4 | (1.4%) | 1 | (0.4%) | 242 | (91.0%) | 13 | (4.9%) | 7 | (2.6%) | 4 | (1.5%) | 0 | (0.0%) | 0.85 |
| Headache | 255 | (91.7%) | 17 | (6.1%) | 2 | (0.7%) | 3 | (1.1%) | 1 | (0.4%) | 243 | (91.4%) | 15 | (5.6%) | 7 | (2.6%) | 0 | (0.0%) | 1 | (0.4%) | 0.20 |
| Asthenia | 244 | (87.8%) | 30 | (10.8%) | 3 | (1.1%) | 0 | (0.0%) | 1 | (0.4%) | 233 | (87.6%) | 23 | (8.6%) | 8 | (3.0%) | 2 | (0.8%) | 0 | (0.0%) | 0.18 |
| Chills | 255 | (91.7%) | 14 | (5.0%) | 6 | (2.2%) | 2 | (0.7%) | 1 | (0.4%) | 251 | (94.4%) | 6 | (2.3%) | 7 | (2.6%) | 2 | (0.8%) | 0 | (0.0%) | 0.40 |
| Insomnia | 267 | (96.0%) | 8 | (2.9%) | 2 | (0.7%) | 0 | (0.0%) | 1 | (0.4%) | 256 | (96.2%) | 2 | (0.8%) | 7 | (2.6%) | 1 | (0.4%) | 0 | (0.0%) | 0.08 |
Discussion
Accurate bowel cleansing and patient acceptability of bowel preparation are the two pillars of quality for colonoscopy (4, 7, 26–28). We chose these two parameters as co-primary endpoints of this trial that compares two low-volume purgative solutions, routinely used in clinical practice. The results of our study confirm that both SPMC and PEG-ASC are highly efficacious in terms of bowel cleansing, independently of the administration modality. Our findings are consistent with those of eight smaller randomized clinical trials which compared SPMC with PEG-ASC (Table 5; 11–18). However, these studies had a single-center design and evaluated a relatively small number of patients. The current study is a large prospective, randomized trial which was needed to add further and conclusive evidence that SPMC is as effective as PEG-ASC and shows better tolerability. Similarly, three recent meta-analyses showed that PEG-based purgative solutions and SPMC have comparable efficacy in bowel cleansing, either when assumed with standard modality or with split regimen (28–30). Moreover, a phase 3 clinical trial comparing a novel very low (1 L) PEG-based bowel preparation with SPMC showed that there is no difference in bowel cleansing efficacy between the two purgative solutions, when both are assumed with standard modality (31).
TABLE 5.
Randomized trials comparing sodium picosulphate plus magnesium citrate (SPMC) with polyethylene glycol plus ascorbic acid (PEG-ASC).
| Authors | Ref. | Country | Design | #pts (ITT/PP) | SPMC (ITT/PP) | PEG-ASC (ITT/PP) | Bowel cleansing | Patients compliance | Patients acceptance |
| Manes et al. (11) | 11 | Italy | Multicenter | 293/285 | 145/140 | 148/145 | 75.7% SPMC Vs. 76.5% PEG-ASC | 83.6% SPMC Vs. 77.9% PEG-ASC | *97.8% SPMC Vs. 83.4% PEG-ASC |
| Choi et al. (12) | 12 | South Korea | Single-center | 220/200 | 110/102 | 110/98 | 88.2% SPMC Vs. 85.7% PEG-ASC | 98% SPMC Vs. 99% PEG-ASC | 83.3% SPMC Vs. 85.7% PEG-ASC |
| Jeon et al. (13) | 13 | South Korea | Single-center | 388/356 | 193/165 | 195/191 | 90.3% SPMC Vs. 89.5% PEG-ASC | *87% SPMC Vs. 99% PEG-ASC | 92.1% SPMC Vs. 90.6% PEG-ASC |
| Sahebally et al. (14) | 14 | Ireland | Single-center | 130/130 | 64/64 | 66/66 | 75% SPMC Vs. 1.8% PEG-ASC | 93.8% SPMC Vs. 92.4% PEG-ASC | *95.3% SPMC Vs. 84.9% PEG-ASC |
| Worthington et al. (15) | 15 | UK | Single-center | 70/65 | 33/33 | 32/30 | 72.7% SPMC Vs. 84.4% PEG-ASC | 100% SPMC Vs. 96.9% PEG-ASC | NR% SPMC Vs. NR% PEG-ASC |
| Yoo et al. (16) | 16 | South Korea | Single-center | 200/200 | 100/100 | 100/100 | 80% SPMC Vs. 82% PEG-ASC | 94% SPMC Vs. 88% PEG-ASC | NR% SPMC Vs. NR% PEG-ASC |
| Seo et al. (17) | 17 | South Korea | Single-center | 223/223 | 114/114 | 109/109 | 93.8% SPMC Vs. 93.5% PEG-ASC | *84.2% SPMC Vs. 55.9% PEG-ASC | *92.1% SPMC Vs. 83.4% PEG-ASC |
| Mathus-Vliegen et al. (18) | 18 | Netherlands | Single-center | 354/337 | 177/171 | 177/166 | 75.8% SPMC Vs. 81.4% PEG-ASC | NR% SPMC Vs. NR% PEG-ASC | 94% SPMC Vs. 60% PEG-ASC |
| D’Angelo et al. | Italy | Multicenter | 550/522 | 271/254 | 279/268 | 95.7% SPMC Vs. 94.4% PEG-ASC | 90.1% SPMC Vs. 93.4% PEG-ASC | *92.8% SPMC Vs. 81.3% PEG-ASC |
ITT = number of randomized patients (intention to treat); PP = number of treated patients (per protocol).
NR = % not reported in full text; *p < 0.05.
In both study arms, the quality of bowel preparation was not influenced by gender, age, bowel segment, administration modality, and previous colonoscopy (Table 2). However, an excellent bowel preparation (BBPS ≥ 8) was accomplished in only 13% of individuals, independently of the bowel preparation used. These results suggest that bowel cleansing is not influenced by the above-mentioned parameters, which are usually considered crucial for the effectiveness of bowel preparation in clinical trials. The majority of colonoscopies (82.5%) had satisfactory bowel cleansing (6 ≤ BBPS ≤ 7), which is consistent with the average evaluation of bowel cleansing usually documented in the majority of colonoscopies routinely performed in clinical practice.
The use of patient-reported questionnaires allowed us to assess patient perception of the impact of the two bowel preparations. Indeed, SPMC was significantly better than PEG-ASC in all the domains explored: food intake, simplicity of assumption, personal activity, work activity, and general perception. In addition, the number of patients willing to repeat bowel preparation was significantly higher with SPMC than with PEG-ASC, even if patient completion rate was similar for both purgative solutions. Although the real volume of solution ingested was not recorded in the patient diaries, it was requested to report complete vs. incomplete assumption of purgative solutions. This information correlated with the success of bowel preparation, which was obtained in 95% of subjects assuming the entirety of either one or the other purgative solution, independently of assumption modality (i.e., standard vs. split). We acknowledge that there are data showing that compliance may not be linked with the volume of purgative solutions but the routine use of low volume purgative solutions for bowel preparation in clinical practice supports our results (32). Volume reduction and improved palatability of purgative solutions increase patient compliance for bowel preparation before colonoscopy, despite the fact that they still have to assume an additional volume (2–3 L) of clear liquids, as recommended to the patients enrolled in the current study (1, 2). This was evident for both PEG-ASC and SPMC, with the latter being more acceptable and preferred by the patients recruited in the trial. Similar results were reported by the majority of the studies which compared SPMC to PEG-ASC: SPMC was usually rated a favorable purgative solution, with good taste and easability for consumption (11, 13–20). Only one study reported that patient overall tolerance was higher for PEG-ASC than SPMC (12). These differences might be due to several reasons, including patients age, race or prior use of specific bowel cleansing solutions.
In the current study, the type and the incidence of adverse events were similar to those reported in other studies and they were independent of the modality of assumption. The most frequent adverse events were gastrointestinal symptoms, with nausea and meteorism being significantly bothersome in patients assuming PEG-ASC. No patients reported adverse events resulting from dehydration, which can result in electrolyte imbalance and hypotension in elderly and/or frail subjects assuming SPMC (13, 16, 17, 19, 20). According to a recent meta-analysis, SPMC is not recommended in patients with renal insufficiency, end-stage liver disease, heart failure and electrolyte abnormalities (30). These relative contraindications could limit the use of SPMC for bowel preparation in an open access system, even if a recent study reported no SPMC related adverse events in a physically disabled population (18).
Our study has some strengths that are worth emphasizing. First, this is one of the largest trial comparing PEG-ASC with SPMC, and its results are comparable in terms of efficacy and safety with those of other smaller randomized trials conducted in both Eastern and Western countries (11–18). Second, the evaluation of the primary endpoint was done by two independent blind reviewers who reviewed each single recorded colonoscopy in its entirety. Therefore, the bowel preparation was scored without the possible bias of the endoscopist who performed the colonoscopy, ensuring the quality of the results and the reliability of our findings. To our knowledge, a similarly robust study design was planned only in another randomized trial (22). Third, PEG-ASC is the most used low-volume PEG purgative solution and it has clearly shown to have similar efficacy and better tolerability than high-volume PEG-ELS solution (5, 6, 26). Therefore, the comparison of SPMC with PEG-ASC is a warranty of non-inferiority for SPMC, which is equally effective as bowel preparation. Forth, the evaluation of patient’s acceptance of bowel preparation has been thoroughly investigated in the current study, allowing to conclude that SPMC is better tollerated and accepted than low–volume PEG solutions with few exceptions (12). Volume reduction and palatability of SPMC increases patient compliance for this bowel preparation, despite the fact that they still have to assume an additional volume (2–3 L) of clear liquids (1, 2). Finally, the study was conducted in a manner that reflects current clinical practice, since colonoscopies were performed according to the standard practice of each participating center which used different modalities of execution of the exam (e.g., different regimen of sedation, single or double operator, different brands of endoscopes).
On the other hand, our study has several limitations that merit discussion. First, this study was affected by a larger (30%) than expected dropout of enrolled subjects due to the fact that at the time of the study patients were allowed to book the colonoscopy simultaneously at different institutions and subsequently decide where to undergo the procedure, on the basis of the fastest waiting list. This caused a longer than planned duration of enrollment and forced us to apply a modified-intention-to-treat strategy for the analysis. Second, we are publishing our data quite late after the end of the enrollment: this was substantially due to the time necessary for collecting the blinded revisions required by the study design, and the subsequent delay in the evaluation of the data. Third, we did not randomize the modality of assumption of bowel preparation; the majority of our patients received the bowel preparation in standard modality and this is not consistent with the routine use of split regimen. The latter has been shown to be superior, regardless of type of bowel preparation and dose (33). Our choice aimed to simplify patient management at participating centers, since there is no hypothesis of interaction between the modality of assumption and the type of bowel preparation (28–30). We recognize that our findings of comparable results in terms of bowel cleanness with standard and split preparations are limited because of lack of randomization and tend to be inconsistent with the literature data that favors split modality (2). However, we believe that the reliability of our findings are warranted by the blinded revision on which our results are based. Fourth, at the time of the execution of the study, it was standard of practice to recommend a low-fiber diet for 4 days before colonoscopy and the assumption of a liquid diet on the day before colonoscopy. Nowadays, patients are instructed to follow a regular diet until lunch on the day before colonoscopy (2). However, the results of our study were not affected by the quality of the diet assumed by the patients in the days preceding the colonscopy. Fifth, the effects of the two regimens on intravascular volume and electrolyte balance were not addressed in the current study, but previous studies have demonstrated that SPMC is safe if high risk patients are excluded (16, 19, 20). Finally, adenoma detection rate was not recorded. However, the definition of success according to BBPS is the most important premise for optimal colonoscopy and we decided to use only BBPS for the comparison of the two bowel preparations (24, 25).
In conclusion, the most significant finding of this study is the validation of previous results by means of a large, prospective, randomized, multicenter trial which confirms that SPMC is equally effective and shows increased tolerability in comparison with PEG-ASC. Therefore, SPMC could be used as an alternative to standard low-volume PEG based purgative solutions for bowel preparation in adult, healthy outpatients who do not tolerate either high or low-volume purgative solutions.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: 10.5281/zenodo.6957443.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato Etico dell ‘IRCCS Istituto Nazionale per lo Studio e la Cura dei Tumori Fondazione Giovanni Pascale di Napoli. The patients/participants provided their written informed consent to participate in this study.
Author contributions
VDA, MDM, MCP, and MDB managed the overall project. FP and CG analyzed all the data. MDB and FP prepared the first draft and finalized the article on the basis of comments from the other authors. CG, MDM, MCP, and VDA reviewed the first draft. All other authors provided data, reviewed results and contributed to the final version of the article.
Acknowledgments
The authors acknowledge the contributions of the following collaborators that aided their efforts: Manuela Florio, Laura Arenare, Simona Bevilacqua, Giuliana Canzanella, Antonia Del Giudice, Giovanni De Matteis, Marilena Martino, Maria Teresa Ribecco, Fiorella Romano, Alfonso Savio, Lucia Sparavigna, Veronica Tudisco, Serena Bellofatto, Rosaria Aprea, Margherita De Rosa, Francesco De Falco, Pasquale Del Prete, Marco Esposito, Giuseppe Amiranda, Antonio Del Core, Francesca Mauro, and Antonella Serrao. The authors are also grateful to Alessandra Trocino, Librarian at Istituto Nazionale Tumori–IRCCS, Fondazione G. Pascale, Napoli, Italy, for bibliographic assistance.
Footnotes
Funding
This work was supported by the Italian Ministry of Health, Ricerca Corrente Funds, Linea 4/10.
Conflict of interest
VDA, CG, EDG, PM, GBR, CC, AT, CB, MCT, MR, AM, DT, GS, PE, RS, RP, FRDP, EDV, and MDB have nothing to disclose regarding financial and personal relationships with other people or organizations, even if it does not directly relate to the submitted work. MDM reports personal fees from Merck Sharp, Dohme, Bristol Myers Squibb, Eisai, Janssen, Astellas, AstraZeneca, and Pfizer; grants from Tesaro, outside the submitted work. FP reports personal fees from non-financial support from Bayer; personal fees from Sandoz, Celgene, Pierre Fabre, and Janssen Cilag; grants and personal fees from Incyte and Astra Zeneca, grants from Roche and Pfizer, outside the submitted work. MCP reports personal fees from Daiichi Sankyo, Glaxo, Astra Zeneca, and MSD; non-financial support from Bayer; grants from Astra Zeneca, outside the submitted work.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1013804/full#supplementary-material
References
- 1.ASGE Standards Of Practice Committee. Bowel preparation before colonoscopy. Gastrointest Endosc. (2015) 81:781–94. [DOI] [PubMed] [Google Scholar]
- 2.Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, et al. Bowel preparation for colonoscopy: ESGE Guideline – Update 2019. Endoscopy. (2019) 51:775–94. 10.1055/a-0959-0505 [DOI] [PubMed] [Google Scholar]
- 3.Spada C, Cannizzaro C, Bianco MA, Conigliaro R, Di Giulio E, Hassan C, et al. Preparation for colonoscopy: recommendations by an expert panel in Italy. Digestive Liver Dis. (2018) 50:1124–32. 10.1016/j.dld.2018.07.036 [DOI] [PubMed] [Google Scholar]
- 4.Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the U.S. Multi-society task force on colorectal cancer. Gastrointest Endosc. (2014) 80:543–62. 10.1016/j.gie.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 5.Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. (2010) 45:1380–6. 10.3109/00365521003734158 [DOI] [PubMed] [Google Scholar]
- 6.Zorzi M, Valiante F, Bastianello G, Baldassarre G, Coria B, Rinaldi M, et al. Comparison between different colon cleansing products for screening colonoscopy. A noninferiority trial in population-based screening programs in Italy. Endoscopy. (2016) 48:223–31. 10.1055/s-0035-1569574 [DOI] [PubMed] [Google Scholar]
- 7.Barkun AN, Martel M, Epstein IL, Hallé P, Hilsden RJ, James PD, et al. The Bowel CLEANsing national initiative: a low-volume same-day polyethylene glycol (PEG) preparation vs low-volume split-dose PEG with bisacodyl or high-volume split-dose PEG preparations—a randomized controlled trial. Am J Gastroenterol. (2020) 115:2068–76. 10.14309/ajg.0000000000000760 [DOI] [PubMed] [Google Scholar]
- 8.Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. (2006) 25:373–84. 10.1111/j.1365-2036.2006.03212.x [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Vanner SJ. Colon cleansing before colonoscopy: does oral sodium phosphate solution still make sense? Can J Gastroenterol. (2009) 23:210–4. 10.1155/2009/417296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dakkak M, Aziz K, Bennett JR. Short report: comparison of two orally administered bowel preparations for colonoscopy-polyethylene glycol and sodium picosulphate. Aliment Pharmacol Ther. (1992) 6:513–9. 10.1111/j.1365-2036.1992.tb00566.x [DOI] [PubMed] [Google Scholar]
- 11.Manes G, Amato A, Arena M, Pallotta S, Radaelli F, Masci E. Efficacy and acceptability of sodium picosulphate/magnesium citrate vs low-volume polyethylene glycol plus ascorbic acid for colon cleansing: a randomized controlled trial. Colorectal Dis. (2013) 15:1145–53. 10.1111/codi.12246 [DOI] [PubMed] [Google Scholar]
- 12.Choi HS, Chung JW, Lee JW, Lim MY, Park DK, Kim YJ, et al. Polyethylene glycol plus ascorbic acid is as effective as sodium picosulfate with magnesium citrate for bowel preparation: a randomized trial. J Dig Dis. (2016) 17:268–73. 10.1111/1751-2980.12337 [DOI] [PubMed] [Google Scholar]
- 13.Jeon SR, Kim HG, Lee JS, Jin-Oh Kim J-O, Lee TH, Cho J-H, et al. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Color Dis. (2015) 30:251–8. 10.1007/s00384-014-2066-9 [DOI] [PubMed] [Google Scholar]
- 14.Sahebally SM, Burke JP, Chu S, Mabadeje O, Geoghegan J. A randomized controlled trial comparing polyethylene glycol + ascorbic acid with sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Ir J Med Sci. (2015) 184:819–23. 10.1007/s11845-014-1182-4 [DOI] [PubMed] [Google Scholar]
- 15.Worthington J, Thyssen M, Chapman G, Chapman R, Geraint M. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin. (2008) 24:481–8. 10.1185/030079908x260844 [DOI] [PubMed] [Google Scholar]
- 16.Yoo IK, Lee JS, Chun HJ, Jeen YT, Keum B, Kim ES, et al. A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis. (2015) 47:131–7. 10.1016/j.dld.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 17.Seo SI, Kang JG, Kim HS, Jang MK, Kim HY, Shin WG. Efficacy and tolerability of 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate: a randomized controlled trial. Int J Colorectal Dis. (2018) 33:541–8. 10.1007/s00384-018-2989-7 [DOI] [PubMed] [Google Scholar]
- 18.Mathus-Vliegen EMH, van der Vliet K, Wignand-van der Storm IJ, Stadwijk JS. Efficacy and safety of sodium picosulfate/magnesium citrate for bowel preparation in a physically disabled outpatient population: a randomized, endoscopist-blinded comparison with ascorbic acid-enriched polyethylene glycol solution plus bisacodyl (The PICO-MOVI Study). Dis Colon Rectum. (2018) 61:239–49. 10.1097/DCR.0000000000000956 [DOI] [PubMed] [Google Scholar]
- 19.Rex DK, Katz PO, Bertiger G, Vanner S, Hookey LC, Alderfer V, et al. Split-dose administration of a dual-action, low volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest Endosc. (2013) 78:132–41. 10.1016/j.gie.2013.02.024 [DOI] [PubMed] [Google Scholar]
- 20.Katz PO, Rex DK, Epstein M, Grandhi NK, Vanner S, Hookey LC, et al. A dual-action, low-volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II Study. Am J Gastroenterol. (2013) 108:401–9. 10.1038/ajg.2012.441 [DOI] [PubMed] [Google Scholar]
- 21.Frommer D. Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum. (1997) 40:100–4. 10.1007/BF02055690 [DOI] [PubMed] [Google Scholar]
- 22.Ell C, Fischbach W, Bronisch H-J, Dertinger S, Layer P, Rünzi M, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. (2008) 103:883–93. 10.1111/j.1572-0241.2007.01708.x [DOI] [PubMed] [Google Scholar]
- 23.Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate. Drugs (2009) 69:123–36. 10.2165/00003495-200969010-00009 [DOI] [PubMed] [Google Scholar]
- 24.Lai EJ, Calderwood AH, Doros G. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. (2009) 69:620–5. 10.1016/j.gie.2008.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc (2010) 72:686–92. 10.1016/j.gie.2010.06.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLachlan SA, Clements A, Austoker J. Patients’ experiences and reported barriers to colonoscopy in the screening context – a systematic review of the literature. Patient Educ Couns. (2012) 86:137–46. 10.1016/j.pec.2011.04.010 [DOI] [PubMed] [Google Scholar]
- 27.Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, et al. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS One. (2014) 9:e99092. 10.1371/journal.pone.0099092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and metaanalysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur J Clin Pharmacol. (2016) 72:523–32. 10.1007/s00228-016-2013-5 [DOI] [PubMed] [Google Scholar]
- 29.van Lieshout I, Munsterman ID, Eskes AM, Maaskant JM, van der Hulst R. Systematic review and meta-analysis: sodium picosulphate with magnesium citrate as bowel preparation for colonoscopy. U Eur Gastroenterol J. (2017) 5:917–43. 10.1177/2050640616684696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Paula Rocha RS, Ribeiro IB, Hourneaux de Moura DT, Marques Bernardo W, Kazuyoshi Minata M, Ananias Morita FH, et al. Sodium picosulphate or polyethylene glycol before elective colonoscopy in outpatients? A systematic review and meta-analysis. World J Gastrointest Endosc. (2018) 10:422–41. 10.4253/wjge.v10.i12.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber S, Baumgart DC, Drenth JPH, Filip RS, Clayton LB, Hylands K, et al. on behalf of the DAYB Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus sodium picosulfate with magnesium citrate: a randomized phase 3 trial. Endoscopy. (2019) 51:73–84. 10.1055/a-0639-5070 [DOI] [PubMed] [Google Scholar]
- 32.Kojecky V, Matous J, Keil R, Dastych M, Kroupa R, Zadorova Z, et al. A head-to-head comparison of 4-L polyethylene glycol and low-volume solutions before colonoscopy: which is the best? A multicentre, randomized trial. Int J Colorectal Dis. (2017) 32:1763–6. 10.1007/s00384-017-2901-x [DOI] [PubMed] [Google Scholar]
- 33.Bucci C, Rotondano G, Hassan C, Rea M, Bianco MA, Cipolletta L, et al. Optimal bowel cleansing for colonoscopy: split the dose! A series of meta-analyses of controlled studies. Gastrointest Endosc. (2014) 80:566–76. 10.1016/j.gie.2014.05.320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: 10.5281/zenodo.6957443.