Abstract
Culture supernatants prepared from reactogenic strains of Vibrio cholerae cause a decrease in the transcellular epithelial resistance of T84 intestinal cells. This decrease correlates with the presence of hemagglutinin/protease but not with the presence of other potential accessory toxins or proteases. These data suggest a possible role for hemagglutinin/protease in reactogenicity, although other factors may also contribute.
The potentially life-threatening disease cholera is caused by toxigenic strains of the gram-negative organism Vibrio cholerae. The hallmark symptom of cholera, profuse, watery diarrhea, is caused primarily by cholera toxin (CT). The genes encoding CT, ctxAB, are carried on a transducing phage, CTXΦ, that integrates into the V. cholerae genome (29). The “core” element of the phage genome carries four genes in addition to ctxAB: cep, orfU, ace, and zot (29). Both zot and orfU are known to be essential for phage morphogenesis (29). In addition, the recombinant products of the zot and ace genes have also been associated with changes in intestinal tissue conductance, suggesting that these genes encode accessory toxins of V. cholerae (6, 27, 28).
The development of a safe and effective vaccine to protect against cholera is a multifaceted problem. An ideal cholera vaccine must confer long-lasting protective immunity, be inexpensive, and be easy to use. A vaccine strategy employing live attenuated V. cholerae strains should meet each of these requirements (15, 17). However, production of a safe, attenuated strain has been problematic. Shortly after the discovery of the genes zot and ace, one would have predicted that a core deletion, resulting in mutants unable to produce CT, zonula occludens toxin (Zot), and accessory cholera enterotoxin (Ace), would produce ideal vaccine candidate strains. However, even with the core element deleted, strains CVD110, CVD111, and CVD112 were still “reactogenic,” causing residual side effects in volunteer recipients, including mild diarrhea, nausea, vomiting, abdominal cramps, and fever (21, 23). Similarly, vaccine strains with deletions of the entire integrated CTXΦ element (attRS deletions) are also mildly reactogenic (5, 24). These data indicate that these strains encode additional reactogenic factors encoded at loci other that the integrated CTXΦ prophage. Curiously, the reactogenic effect of these undefined factors is absent from vaccine candidate strains that have additional defects in motility (5, 11, 24).
This paper describes experiments using transcellular epithelial resistance (TER) across polarized T84 epithelial cells to monitor potential reactogenic factors in various vaccine strains. We show that an activity associated with the zot gene is not detected in this system. However, we find that a decrease in TER correlates with the presence of the genes for production of hemagglutinin/protease (HA/protease), indicating that HA/protease may be a significant contributor to the reactogenicity of some V. cholerae vaccine strains.
Addition of supernatant fluids to polarized T84 intestinal cell monolayers.
To investigate the utility of in vitro polarized intestinal monolayers for the study of accessory cholera toxins, we examined the effect of adding supernatant fluids of several different vaccine strains to the apical surface of polarized T84 intestinal epithelial cells. To prepare supernatant fluids, V. cholerae strains were grown overnight from single colonies or from frozen stocks with constant aeration at 30°C in the appropriate antibiotic, except where noted otherwise. Cells from 5-ml overnight cultures were pelleted by centrifugation. Supernatant fluids were removed and dialyzed overnight against phosphate-buffered saline (PBS) pH 7.4, using Spectrapor-2 dialysis tubing (molecular weight cutoff, 12,000 to 14,000) with a final dilution of at least 1:105. After dialysis, the supernatant fluids were either used on the same day or frozen at −80°C until used.
T84 cells obtained from the American Type Culture Collection were cultured as previously described in 0.33-cm2 Transwell inserts (Costar Laboratories, Cambridge, Mass.) coated with a dilute collagen solution (13). TERs attained stable levels (> 1,000 ohms · cm2) after 7 days.
For electrophysiology studies, confluent monolayers in Transwell inserts were transferred to Hanks balanced salt solution. Aliquots (100 μl) of dialyzed supernatant fluids were placed directly onto the apical side of confluent T84 cell monolayers. Resistance and short-circuit current were measured at various time intervals using a dual-voltage clamp device and 25-μA current pulses as previously described (13).
Supernatant preparations from V. cholerae strains cause a decrease in TER across a T84 monolayer.
Zot has been associated with intestinal secretion following the opening of tight junctions (6–8). In the T84 model system, changes in the integrity of tight junctions can be monitored by measuring changes in TER across the polarized monolayer. To test if a change in resistance dependent upon the zot gene could be detected in T84 monolayers, V. cholerae E1 Tor strain Bah-2 was used as the control strain. This strain is the recA+ parent of reactogenic vaccine strain Bah-3, which carries the ΔattRS deletion eliminating the entire integrated prophage, including genes for CT, Zot, and Ace (24). This large deletion also inactivates the gene for the recently described toxin called RtxA (14). Into this strain, pCTXΦ-Km (29), the replicative form of CTXΦ marked with kanamycin resistance and with ctxAB deleted, was introduced as a source of zot. As a control, pMW101, a derivative of pCTXΦ-Km with an interruption at the MluI site within the zot gene, was also introduced (29).
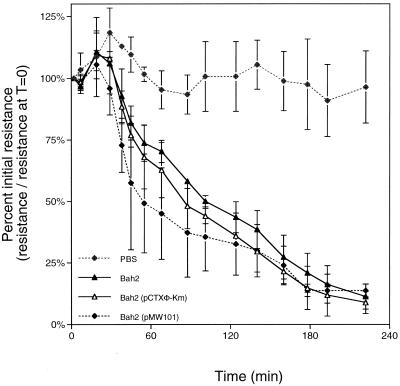
Supernatant fluids prepared from these Bah-2-derived strains were added to the apical surface of the T84 monolayers, and TER across the intact monolayers was measured. Surprisingly, a significant change in TER across the monolayer was measured for all of the strains tested, including control strain Bah-2 (Fig. 1). For all three Bah-2 strains, the TER dropped at least 80% over 3 h after addition of the culture supernatant fluids compared to initial resistance values (Fig. 1). Cells inoculated with PBS showed little change in TER (Fig. 1).
FIG. 1.
Culture supernatants from V. cholerae Bah-2 and derivatives cause a decrease in TER. Each point represents the average and standard deviation of duplicate samples.
In addition, a similar trend was observed with polarized Caco-2 cells, indicating a general, rather than a cell line-specific, effect (data not shown). These data suggest that Bah-2, which does not encode Zot, Ace, CT, or RtxA, contains an accessory factor that affects the integrity of both T84 and Caco-2 monolayers.
Decrease in TER is observed in other V. cholerae vaccine strains.
To assess whether other vaccine strains may export this accessory factor, supernatant fluids from other strains varying in reactogenicity were tested. The strains included O395-N1, a classical strain carrying a deletion of ctxAB which has previously been reported to be defective in Zot production (1, 16); Peru-3, a derivative of the El Tor strain C6709 with the ΔattRS deletion (24); and Bengal-3, a derivative of O139 strain MO10 also with the ΔattRS deletion (24). As noted above, these ΔattRS strains no longer possess genetic sequences corresponding to zot, ace, and ctxAB and also contain a partial deletion of rtxA. When tested in volunteers, these strains exhibited mild-to-moderate reactogenicity, suggesting that they may export factors other than CT, Zot, Ace, or RtxA that elicit diarrhea or other adverse symptoms (24). Peru-15 and Bengal-15 are spontaneous nonmotile derivatives of Peru-3 and Bengal-3, respectively, that are not reactogenic in human volunteers (5, 11).
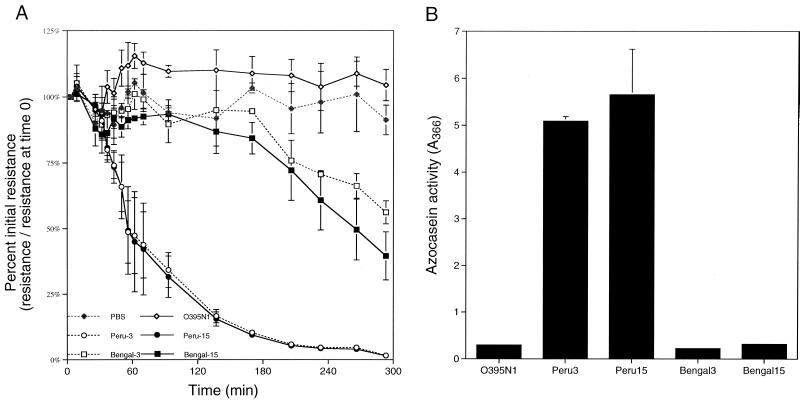
We found that supernatant fluids prepared from these strains caused a range of responses when added to T84 monolayers (Fig. 2). A significant decrease in TER was elicited by supernatant fluids prepared from El Tor strain Peru-3 and its nonmotile counterpart Peru-15 (Fig. 2A), suggesting that these strains produce the same accessory factor as the Bah-2 strain. By contrast, supernatant fluids prepared from O139 vaccine strains Bengal-3 and Bengal-15 elicited only small decreases in TER (Fig. 2C) while supernatant fluids from O395-N1 did not elicit a response different from that of a PBS control (Fig. 2A). These data show that the accessory factor that disrupts the TER of T84 monolayers is present in both El Tor and O139 strains but is more highly expressed in El Tor strains such as Peru-3 and Bah-2.
FIG. 2.
Decrease in the TER of various vaccine strains correlates with protease activity. Both the ability to elicit a decrease in TER (A) and total protease activity (B) were measured in culture supernatants prepared from the strains indicated. The values reported are averages and standard deviations of two to five independent monolayers (A) or supernatant preparations (B).
Decrease in TER is not due to production of Zot.
Since O395-N1 does not produce the accessory factor Zot, we repeated our previous experiment for detection of Zot in T84 monolayers using strain O395-N1 as the genetic background. Addition of supernatant fluids prepared from O395-N1 carrying pCTXΦ-Km as a source of zot did not elicit a decrease in resistance. Even 3 h after addition of the supernatant fluid to the monolayer, the T84 monolayer maintained 100% of its initial resistance while supernatant fluids from O395-N1 alone showed a slight drop that was also observed in the PBS control. Thus, a decrease in the resistance of T84 monolayers does not correlate with acquisition of the zot gene (data not shown).
Interestingly, supernatant fluids from strain Peru-15 carrying pCTXΦ-Km also did not elicit a drop in TER. Three hours after addition of supernatant fluids from strain Peru-15 carrying pCTXΦ-Km, the T84 monolayers remained stable (data not shown). This result is surprising, since supernatant fluids from strain Peru-15 without the phage elicited a drop to 35% of the initial resistance, consistent with prior experiments (Fig. 2A). Given that zot is an essential CTXΦ replication gene and strain Peru-15 carrying pCTXΦ-Km produces 105 to 106 phage particles per ml (W. Lin and J. J. Mekalanos, unpublished results), we assume that zot expression by this strain is at least as high as, if not higher than, that of the other strains in which zot is produced from the integrated prophage. These data suggest that, in this particular strain, active expression of zot does not cause a decrease in TER. Further, expression of phage genes or CTXΦ assembly itself inhibits the production of the alternate factor responsible for the loss of TER of T84 cells. However, it is important to note that this may be a strain-specific inhibition since a similar inhibition was not observed in the Bah-2 strain (Fig. 1).
In all, these data show that Zot itself does not cause a decrease in TER in the T84 monolayer system. These data may further suggest that phage genes or phage production may inhibit production or secretion of an alternate factor that does affect the stability of the T84 monolayer.
Decrease in TER correlates with protease activity in V. cholerae supernatant fluids.
Since genes from the core of CTXΦ do not appear to be associated with decreases in TER in the T84 monolayer system, we sought to identify the accessory factor that is responsible by using a genetic approach. V. cholerae is known to export a number of proteases into the culture supernatant, so we considered that protease activity might be affecting the T84 monolayers. Thus, supernatant fluids prepared from the O395-N1, Peru, and Bengal vaccine strains were tested for protease activity using an azocasein digestion assay.
This was a modified azocasein hydrolysis assay (26). Briefly, 50 μl of appropriately diluted supernatant fluid was added to 800 μl of azocasein at 2 mg/ml in 0.1 M Tris-HCl (pH 8.0). After 1 h of incubation at 37°C, the reaction was stopped by adding 160 μl of 50% trichloroacetic acid to each tube. Samples were immediately centrifuged at 16,000 × g for 15 min. The absorbance of the solution was read at 366 nm on a Spectronic Genesys 5 spectrophotometer.
While supernatant fluids from strains O395-N1, Bengal-3, and Bengal-15 have little or no appreciable protease activity, both strains Peru-3 and Peru-15 exhibit significant protease activity (Fig. 2B). For all of the vaccine strains tested, there is a strong correlation between protease activity and the strains that showed a decrease in TER (Fig. 2A and B).
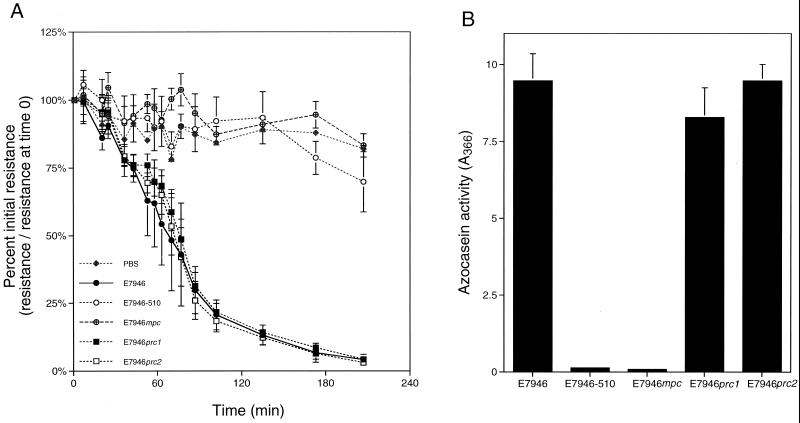
We extended this line of inquiry into other, nonvaccine V. cholerae strains. Strains E7946-510 and E7946mpc are derivatives of El Tor strain E7946 that carry mutations resulting in the loss of expression of 11 electrophoretically different proteases by zymograph analysis (3). Parent strain E7946 showed high protease activity, while both of the multiprotease mutants exhibited little or no protease activity in the azocasein hydrolysis assay (Fig. 3B). Correspondingly, supernatant fluids prepared from the parent E7946 elicited a decrease in TER while supernatant fluids from E7946-510 and E7946mpc had little or no effect on TER (Fig. 3A). These trends were also shown when supernatant fluids were added to Caco-2 cells (data not shown). Thus, for these E7946 derivatives and the vaccine strains, the ability to disrupt the TER of T84 and Caco-2 monolayers correlates with protease activity.
FIG. 3.
Decrease in TER of El Tor strain E7946 correlates with total protease activity but not leucine aminopeptidase activity. Both the ability to elicit a decrease in TER (A) and total protease activity (B) were measured in culture supernatants prepared from the strains indicated. The values reported are averages and standard deviations of four or five independent monolayers (A) or supernatant preparations (B). The PBS control values are for a single sample.
Decrease in TER is not due to aminopeptidase.
It is possible that this effect is due to the V. cholerae leucine aminopeptidase, an exported protease of V. cholerae (3, 25). Strains E7946prc1 and E7946prc2 have insertional disruptions in the gene for the leucine aminopeptidase causing the loss of a single 54-kDa band on zymograph analysis (3). However, these mutants still show high levels of protease activity in the azocasein hydrolysis assay despite the lapA mutations (Fig. 3B). In addition, a corresponding rapid decrease in TER on T84 monolayers shows that the lapA gene product does not specifically contribute to the disruption of T84 cells (Fig. 3A).
Decrease in TER is due to HA/protease.
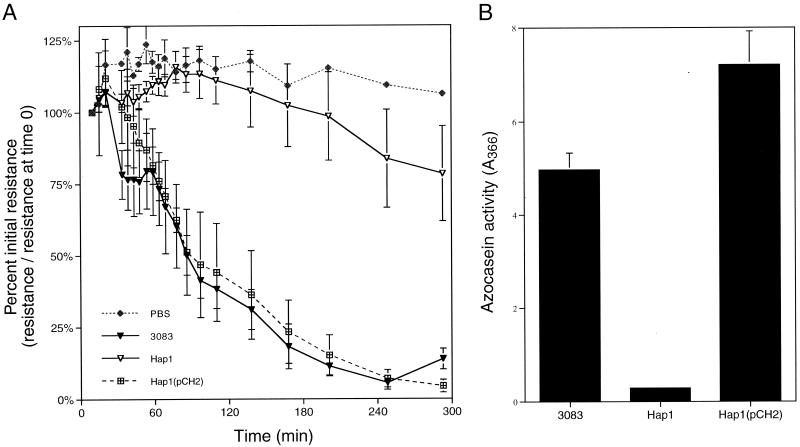
Another major protease of V. cholerae that may cause this decrease in TER is the HA/protease, originally characterized by Häse and Finkelstein (10). To test if HA/protease is the protease that causes a decrease in TER, supernatant fluids from V. cholerae mutants bearing a deletion within hapA, the gene encoding HA/protease, were tested for protease activity and a decrease in TER. Supernatant fluids from wild-type strain 3083 (9) both showed high protease activity and elicited a rapid and dramatic decrease in the TER of T84 monolayers (Fig. 4). By contrast, the deletion mutant Hap1 (9) had a significant decrease in extracellular protease activity compared to the parent 3083 (Fig. 4B) and supernatant fluids from this strain produced only a slight decrease in TER (Fig. 4A). However, when the plasmid pCH2 harboring hapA was provided in trans (9), protease activity and the ability to elicit a decrease in TER were restored (Fig. 4). In these experiments, there is a strong correlation between the presence of HA/protease and the subsequent decrease in TER.
FIG. 4.
Loss of TER is due to HA/protease. Both the ability to elicit a decrease in TER (A) and total protease activity (B) were measured in culture supernatants prepared from the indicated strains grown at 37°C. The resistance values are averages and standard deviations from one (PBS), two [Hap1(pCH2)], or six (3083 and Hap1) independent monolayers. The protease values shown are averages and standard deviations of four independent cultures.
Other proteases do not affect the TER of T84 monolayers.
Our data are consistent with a report of Wu et al. (30), that HA/protease purified from V. cholerae supernatant fluids can cause a loss of TER in MDCK cells. Also in concordance with their results, our observed decrease in TER across T84 monolayers is temperature sensitive and can be inhibited with Zincov, a zinc-metalloprotease inhibitor (data not shown). However, our genetic data allow us to further conclude that HA/protease may have a specific effect on cells that differs from those of other proteases of V. cholerae. Zymogram analysis of E7946 has shown that as many as 11 proteases are exported by V. cholerae (3). It seems plausible that any or all of these proteases could contribute to disruption of T84 monolayers. In fact, HA/protease seems to have the predominating effect. Hap1, bearing a mutation of only hapA in a wild-type background, shows only a slight decrease in TER compared to mock-infected controls (Fig. 4A). In a corollary experiment, sterile supernatant fluids prepared from a hapA deletion in the Bah-2 genetic background shows no detectable change in TER even if the supernatants are concentrated 50-fold by precipitation in 60% ammonium sulfate (K.J.F., unpublished results). Thus, the decrease in TER of T84 monolayers is likely due specifically to the activity of HA/protease and not to random proteolytic activity.
Discussion.
Production of a safe vaccine against V. cholerae has been problematic due to residual reactogenicity. CVD110 is a derivative of E7946 with deletions in the CTXΦ core region, as well as a disruption in the hlyA gene (23), yet this strain is still highly reactogenic in volunteers (23). More surprisingly, these volunteers show a diarrheal disease more inflammatory than normal cholera, suggesting that the absence of CT enhances the activity of undefined factors that elicit local inflammation (20).
HA/protease is an attractive candidate for the reactogenicity factor. In this study, we have show that strains with the hapA protease gene deleted, even in a wild-type background, show little effect on the TER of T84 monolayers. It has also been shown that addition of purified HA/protease to MDCK cells causes a decrease in TER and a concurrent release of nitric oxide, an elicitor of localized immune responses (30, 31). Further, HA/protease could have a greater impact in the absence of the cholera toxin genes. HA/protease is exported by the same secretory apparatus as CT; thus, elimination of CT may indirectly cause an increase in HA/protease export (19). In concordance with this idea, strains with deletions in the core region of the phage, including CT, produce more HA/protease than do the wild-type parent strains (12). Thus, a core deletion, as found in CVD110, could export more HA/protease than the parent, eliciting a more inflammatory form of diarrhea, as was observed.
However, a recent volunteer study suggests that the overall pathogenesis of V. cholerae is even more complex. Benítez et al. (2) constructed El Tor vaccine strain 638, which has both a core deletion of CTXΦ and an insertional interruption in hapA. Although volunteers showed fewer symptoms and a decrease in diarrhea compared to those in previous studies utilizing core deletion strains, 9.5% of the volunteers still had diarrhea and as many as 25% had other reactogenic symptoms, including abdominal cramps and vomiting (2). Thus, strain 638 in not asymptomatic, indicating that even more reactogenic factors have yet to be described.
It is notable that the method used to construct the core deletion in strain 638 would not have eliminated the neighboring toxin gene, rtxA, as was done for attRS deletion strains such as Bah-3, Peru-3, and Bengal-3 (14). Thus, strain 638 likely still expresses this newly discovered toxic factor. Further, this strain did not have a deletion of the hemolysin gene hlyA, as did CVD110. It has recently been shown that V. cholerae hemolysin has a cytotoxic cell vacuolating activity, reviving interest in its role as a pathogenic factor (4, 18).
In all, a strain with all of the possible reactogenic factors deleted has never been tested in human volunteers. CVD110 bears the core deletion and an interruption in hlyA, but rtxA and hapA remain intact. Bah-3, Peru-3, and Bengal-3 bear a large deletion eliminating the core region and rtxA but produce both hemolytic and HA/protease activities (Fig. 2 and data not shown). Finally, 638 has the core region and hapA deleted but rtxA and hlyA are presumably intact. Interestingly, of the El Tor or O139 vaccine candidates, only the nonmotile derivatives of Peru-3 and Bengal-3 showed no reactogenicity (5, 11), even though these strains produce HA/protease in vitro (Fig. 2B). If HA/protease does cause reactogenicity in volunteers, then this observation suggests that delivery of this toxin is impeded by the motility defect or that expression of HA/protease in vivo is blocked in the motility mutants.
We propose that reactogenicity is a complex issue and that no single reactogenicity factor will be described. Only a full understanding of the contribution of the entire battery of potential toxigenic factors, motility, and adherence to disease will lead to definition of a genetic “blueprint” for construction of safe live attenuated vaccines against cholera in any parental V. cholerae background.
Acknowledgments
We thank Claudia Häse for providing strains and Margaret Ferguson-Maltzman for technical assistance.
This work was supported by NIH grants AI-18045 to J.J.M. and DK-48106 to W.I.L. S.F.M. was funded by a fellowship from the Cancer Research Institute. K.J.F. was supported by NRSA postdoctoral fellowship AI-10385.
REFERENCES
- 1.Baudry B, Fasano A, Ketley J, Kaper J B. Cloning of a gene (zot) encoding a new toxin produced by Vibrio cholerae. Infect Immun. 1992;60:428–434. doi: 10.1128/iai.60.2.428-434.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benítez J A, García L, Silva A, García H, Fando R, Cedré B, Pérez A, Campos J, Rodríguez B L, Pérez J L, Valmaseda T, Pérez O, Pérez A, Ramírez M, Ledón T, Jidy M D, Lastre M, Bravo L, Sierra G. Preliminary assessment of the safety and immunogenicity of a new CTXΦ-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect Immun. 1999;67:539–545. doi: 10.1128/iai.67.2.539-545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bortner S R. Ph.D. dissertation. Boston, Mass: Harvard University; 1988. [Google Scholar]
- 4.Coelho A, Andrade J R C, Vicente A C P, DiRita V J. Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin. Infect Immun. 2000;68:1700–1705. doi: 10.1128/iai.68.3.1700-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coster T S, Killeen K P, Waldor M K, Beattie D T, Spriggs D R, Kenner J R, Trofa A, Sadoff J C, Mekalanos J J, Taylor D N. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet. 1995;345:949–952. doi: 10.1016/s0140-6736(95)90698-3. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper J B, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum S E. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Investig. 1995;96:710–720. doi: 10.1172/JCI118114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology. 1997;112:839–846. doi: 10.1053/gast.1997.v112.pm9041245. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein R A, Boesman-Finkelstein M, Chang Y, Häse C C. Vibrio cholerae hemagglutinin/protease, colonial variation, virulence, and detachment. Infect Immun. 1992;60:472–478. doi: 10.1128/iai.60.2.472-478.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Häse C C, Finkelstein R A. Bacterial extracellular zinc-containing metalloproteases. Microbiol Rev. 1993;57:823–837. doi: 10.1128/mr.57.4.823-837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kenner J R, Coster T S, Taylor D N, Trofa A F, Barrera-Oro M, Hyman T, Adams J M, Beattie D T, Killeen K P, Spriggs D R, Mekalanos J J, Sadoff J C. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J Infect Dis. 1995;172:1126–1129. doi: 10.1093/infdis/172.4.1126. [DOI] [PubMed] [Google Scholar]
- 12.Kimsey H H, Waldor M K. Vibrio cholerae hemagglutinin/protease inactivates CTXΦ. Infect Immun. 1998;66:4025–4029. doi: 10.1128/iai.66.9.4025-4029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lencer W I, Delp C, Neutra M R, Madara J L. Mechanism of cholera toxin action on a polarized human intestinal epithelial line: role of vesicular traffic. J Cell Biol. 1992;117:1197–1209. doi: 10.1083/jcb.117.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mekalanos J J, Sadoff J C. Cholera vaccines: fighting an ancient scourge. Science. 1994;265:1387–1389. doi: 10.1126/science.8073279. [DOI] [PubMed] [Google Scholar]
- 16.Mekalanos J J, Swartz D J, Perason G D N, Harford N, Groyne F, de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983;306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 17.Mekalanos J J, Waldor M K, Gardel C L, Coster T S, Kenner J, Killeen K P, Beattie D T, Trofa A, Taylor D N, Sadoff J C. Live cholera vaccines: perspectives on their construction and safety. Bull Inst Pasteur. 1995;93:255–262. [Google Scholar]
- 18.Mitra R, Figueroa P, Mukhopadhyay A K, Shimada T, Takeda Y, Berg D E, Nair G B. Cell vacuolation, a manifestation of the El Tor hemolysin of Vibrio cholerae. Infect Immun. 2000;68:1928–1933. doi: 10.1128/iai.68.4.1928-1933.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandkvist M, Michel L O, Hough L P, Morales V M, Bagdasarian M, Koomey M, DiRita V J, Bagdasarian M. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J Bacteriol. 1997;179:6994–7003. doi: 10.1128/jb.179.22.6994-7003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva T M J, Schuleupner M A, Tacket C O, Steiner T S, Kaper J B, Edelman R, Guerrant R L. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholerae vaccines and by El Tor and O139 Vibrio cholerae. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tacket C O, Losonsky G, Nataro J P, Comstock L, Michalski J, Edelman R, Kaper J B, Levine M M. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J Infect Dis. 1995;172:883–886. doi: 10.1093/infdis/172.3.883. [DOI] [PubMed] [Google Scholar]
- 22.Tacket C O, Kotloff K L, Losonsky G, Nataro J P, Michalski J, Kaper J B, Edelman R, Levine M M. Volunteer studies investigating the safety and efficacy of live oral El Tor Vibrio cholerae O1 vaccine strain CVD111. Am J Trop Med Hyg. 1997;56:533–537. doi: 10.4269/ajtmh.1997.56.533. [DOI] [PubMed] [Google Scholar]
- 23.Tacket C O, Losonsky G, Nataro J P, Cryz S J, Edelman R, Fasano A, Michalski J, Kaper J B, Levine M M. Safety and immunogenicity of live oral cholera vaccine candidate CVD110, a ΔctxA Δzot, Δace derivative of El Tor Ogawa Vibrio cholerae. J Infect Dis. 1993;168:1536–1540. doi: 10.1093/infdis/168.6.1536. [DOI] [PubMed] [Google Scholar]
- 24.Taylor D N, Killeen K P, Hack D C, Kenner J R, Coster T S, Beattie D T, Ezzell J, Hyman T, Trofa A, Sjogren M H, Friedlander A, Mekalanos J J, Sadoff J C. Development of a live, oral, attenuated vaccine against El Tor cholera. J Infect Dis. 1994;170:1518–1523. doi: 10.1093/infdis/170.6.1518. [DOI] [PubMed] [Google Scholar]
- 25.Toma C, Honma Y. Cloning and genetic analysis of the Vibrio cholerae aminopeptidase gene. Infect Immun. 1996;64:4495–4500. doi: 10.1128/iai.64.11.4495-4500.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomarelli R M, Charney J, Harding M L. The use of azoalbumin as a substrate in the colorimetric determination of peptic and tryptic activity. J Lab Clin Med. 1949;34:428–433. [PubMed] [Google Scholar]
- 27.Trucksis M, Conn T L, Fasano A, Kaper J B. Production of Vibrio cholerae accessory cholera enterotoxin (Ace) in the yeast I. Infect Immun. 1997;65:4984–4988. doi: 10.1128/iai.65.12.4984-4988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trucksis M, Galen J E, Michalski J, Fasano A, Kaper J B. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc Natl Acad Sci USA. 1993;90:5267–5271. doi: 10.1073/pnas.90.11.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Milton D, Nybom P, Sjö A, Magnusson K-E. Vibrio cholerae hemagglutinin/protease (HA/protease) causes morphological changes in cultured epithelial cells and perturbs their paracellular barrier function. Microb Pathog. 1996;21:111–123. doi: 10.1006/mpat.1996.0047. [DOI] [PubMed] [Google Scholar]
- 31.Wu Z, Nybom P, Sudqvist T, Magnusson K-E. Endogenous nitric oxide in MDCK-I cells modulates the Vibrio cholerae haemagglutinin/protease (HA/P)-mediated cytoxicity. Microb Pathog. 1998;24:321–326. doi: 10.1006/mpat.1998.0201. [DOI] [PubMed] [Google Scholar]