Abstract
Background:
Intraplaque hemorrhage (IPH) is associated with plaque progression and ischemic events and plaque lipid content (% lipid core) predicts the residual atherosclerotic cardiovascular disease (ASCVD) risk. This study examined the impact of IPH on lipid content change in the setting of intensive lipid-lowering therapy.
Methods:
214 AIM-HIGH participants with clinically established ASCVD and low high-density lipoprotein cholesterol (HDL-C) received carotid MRI at baseline and 2 years to assess changes in carotid morphology and composition. Patients were randomized to extended-release niacin (ERN) or placebo, and all received simvastatin with optional ezetimibe as necessary to lower low-density lipoprotein cholesterol (LDL-C) to 40–80 mg/dL. Changes in lipid content and carotid morphology were tested using the Wilcoxon signed-rank test. Differences between subjects with and without IPH and between subjects assigned ERN or placebo were tested using the Wilcoxon rank-sum test. Linear regression was used to test the association of IPH and lipid content changes after adjusting for clinical risk factors.
Results:
Among 156 patients (61±9 years; 81% male) with complete MRI, prior statin use: <1 year, 26%; 1 to 5 years, 37%; >5 years, 37%. Triglycerides and ApoB decreased significantly, while HDL-C and ApoA1 increased significantly over time. Plaque lipid content was significantly reduced (−0.5±2.4 %/year, p=0.017) without a significant difference between the two treatment groups. However, the lipid content increased in plaques with IPH but regressed in plaques without IPH (1.2±2.5 %/year vs. −1.0±2.2, p=0.006). Additionally, IPH was associated with a decrease in lumen area (−0.4±0.9 mm2/year vs. 0.3±1.4, p=0.033). IPH remained significantly associated with increase in lipid content in multivariable analysis (54.4%, 95% CI: 26.8, 88.0, p<0.001).
Conclusions:
Carotid plaques under continued intensive lipid-lowering therapy moved towards stabilization. However, plaques with IPH showed greater increases in lipid content and greater decreases in lumen area than plaques without IPH.
Keywords: atherosclerotic cardiovascular disease, carotid artery, magnetic resonance imaging, plaque lipid content, intraplaque hemorrhage
Clinical summary
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death in the US. Despite guidelines promoting aggressive anti-atherosclerotic therapies, there is near 5%/year residual ASCVD risk in patients who achieve profound LDL-C lowering (median 30 mg/dL) with combined statin and PCSK9 inhibitor therapy. Intraplaque hemorrhage (IPH) is a common feature of advanced atherosclerotic lesions and a critical element leading to accelerated plaque progression, plaque instability and ischemic vascular events in humans. The AIM-HIGH MRI sub-study showed that continued intensive lipid-lowering therapy over 2 years was associated with a significant reduction in plaque lipid content, no significant change in lumen area, and an increase in wall area. IPH was independently and significantly associated with increased plaque lipid content in the setting of continued intensive lipid-lowering therapy. These data have provided new evidence that IPH is an important factor contributing to residual cardiovascular risk under intensive lipid-lowering therapy. Further investigations are needed to uncover mechanisms in IPH pathogenesis and to discover potential therapeutic targets with a goal of reducing residual ASCVD risk.
Introduction
Low-density lipoprotein (LDL) cholesterol lowering therapy, particularly with statins, is a cornerstone in the prevention of cardiovascular events including myocardial infarction and ischemic stroke. Nonetheless, there remains a near 5% per year residual risk in patients with atherosclerotic cardiovascular disease (ASCVD) under intensive LDL-lowering therapy. In the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) study (1), 16% of patients with LDL-C at 62 mg/dL experienced at least 1 major adverse cardiovascular event during a 3-years follow-up. The event rate was 33% over 7 years in subjects treated with simvastatin plus ezetimibe to lower LDL-C to 53 mg/dL in IMPROVE-IT (2). More recently, treatment with a combination of statin and PCSK9 inhibitor to LDL-C lowering to 30 mg/dL in FOURIER showed an event rate of 13% over 2.2 years (3).
It is well established that rupture or erosion of atherosclerotic plaque with intraluminal thrombosis or thromboembolization is the primary cause of ASCVD events. Histopathological studies identified morphological features that predispose plaques to rupture, including increased plaque volume, large lipid-rich necrotic core, thin or ruptured cap, intraplaque hemorrhage (IPH), and increased inflammatory infiltration (4). These high-risk plaque features can be detected and/or quantified in vivo using high-resolution carotid magnetic resonance imaging (MRI) (5–8). Among these plaque risk features, IPH is a common feature of advanced atherosclerotic lesions and a critical element leading to accelerated plaque progression (9–11), plaque instability (12–14) and ischemic vascular events (15–19). However, the impact of IPH on plaque lipid content under lipid-lowering therapy remains unclear. In this study, we investigate the role of IPH as a risk feature leading to increased plaque lipid content despite intensive lipid-lowering therapy using the MRI sub-study of the AIM-HIGH study.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding Xue-Qiao Zhao at xueqiao@uw.edu.
Study Population
The AIM-HIGH Carotid MRI Sub-study (NCT01178320; https://clinicaltrials.gov) was a multicenter investigation on carotid plaque progression embedded within AIM-HIGH, leveraging the existing infrastructure, resources, and standardized interventions of the parent study (1). The AIM-HIGH trial randomized 3,414 patients (1:1 ratio) to extended-release niacin (ERN, 1,500 or 2,000 mg per day) or its active placebo. Eligible patients were ≥45 years old with stable ASCVD and atherogenic dyslipidemia, who tolerated ≥1500 mg ERN per day during the run-in phase. ASCVD needed to be clinically established, including documented coronary artery, cerebrovascular, or peripheral artery disease. Atherogenic dyslipidemia was defined as low HDL cholesterol (≤40 mg/dl for men and ≤50 mg/dl for women) and high triglycerides (150 to 400 mg/dl). LDL cholesterol needed to be ≤180 mg/dl for patients not taking statins with commensurate adjustments in statin users. In addition to the randomized intervention, all subjects in AIM-HIGH received background lipid-lowering therapy with simvastatin and optional use of ezetimibe to meet the on-trial LDL cholesterol target of 40 to 80 mg/dl (1.0 to 2.1 mmol/L). At the time of the MRI sub-study which was initiated approximately 2 years behind the main trial, 447 AIM-HIGH subjects at 21 clinical sites with access to carotid MRI capability were available. Subjects with any contraindication for MRI or gadolinium contrast (e.g. metal implants, claustrophobia, estimated glomerular infiltration rate <60 ml/min/1.73 m2) were excluded. A total of 232 eligible subjects consented and received carotid MRI at 10 imaging centers, of which 214 had acceptable image quality for analysis of plaque burden and tissue composition (19). Institutional review board approval was obtained at all participating sites and subjects gave informed consent.
As previously published (19), compared to the rest of AIM-HIGH cohort, the 214 subjects in the MRI sub-study had the following statistically significant differences: they were younger (mean: 61 vs. 64 years, p<0.001), more likely to be non-white (12% vs. 7%, p=0.02), more likely to have hypertension (83% vs. 71%, p<0.001), less likely to be treated with statin for one year or longer (72% vs. 82%, p=0.001), and less likely to have diabetes (25% vs. 35%, p=0.004). They also had smaller BMI (mean: 30 vs. 31 kg/m2, p=0.009) and lower triglycerides (median: 158 vs. 165 mg/dl, p=0.03).
Carotid MRI
MRI scans were performed at baseline and 2 years using the same 3T scanner (GE or Philips) and commercially available phased-array carotid coil (GE: 6-channel, Neocoil LLC, Pewaukee, Wisconsin; Philips: 8-channel, Shanghai Chenguang Medical Technologies, Shanghai, China) (20). A standardized multicontrast protocol was used, which included time-of-flight (TOF), T1-/T2-/intermediate-weighted (T1w/T2w/PDw) turbo spin echo, and magnetization prepared rapid acquisition gradient echo (MP-RAGE). T1-weighted (T1w) turbo spin echo was repeated about 5 minutes after administrating gadolinium contrast (Magnevist, Bayer Healthcare) to acquire contrast-enhanced images. All acquisitions were in the axial plane with imaging slab centered at the bifurcation level of the index carotid artery, which was selected at baseline as the one with greater wall thickness. All images had a spatial resolution of 0.625×0.625×2 mm3. Detailed parameters and scan-rescan reproducibility of this protocol have been reported previously (21). Total scan time was approximately 45 minutes.
Image analysis
Image analysis was performed by blinded readers in a core lab using a custom-designed image analysis software package (CASCADE, University of Washington, Seattle, Washington) with the following workflow: 1) matching: different image series representing different contrast weightings were aligned using the carotid flow divider between the proximal internal and external carotid arteries as a fiducial landmark; 2) arterial wall boundary detection: lumen and outer wall boundaries were delineated in the T1w images with reference to other contrast weightings; 3) image registration: lumen and outer wall contours in the T1w images were copied to other image series, which were used to precisely register different contrast weightings; 4) tissue component classification: plaque components were detected based on histology-validated criteria (7, 22). Briefly, calcification was hypointense on all contrast weightings; lipid core defined as non-calcified areas that had no or little enhancement on contrast-enhanced T1w; IPH was recorded, within a lipid core, as signal hyperintensities on MP-RAGE.
Morphological measurements were automatically calculated by CASCADE for each imaging slice, including maximum wall thickness, mean wall thickness, outer wall area, lumen area, wall area (outer wall area – lumen area), outer wall area, calcification area, and lipid core area. Measurements on slices were aggregated to obtain mean lumen area, mean wall area, mean outer wall area, lipid core volume ( mm3, i.e. cross-sectional slice thickness), percent (%) lipid core volume (), calcification volume ( mm3), and percent (%) calcification volume (). Plaque progression was determined as annualized changes in imaging measurements between baseline and follow-up scans.
Statistical analysis
Categorical variables were summarized as count (percentage) and continuous variables were summarized as mean ± standard deviation or median (inter-quartile range). The median (inter-quartile) range was used to summarize continuous variable with substantial right-skewness, based on graphical assessment using histograms. Changes in lipid levels were expressed as differences (follow up - baseline) and changes in plaque morphology and composition were expressed as annualized differences. Changes in measurements between baseline and follow up were assessed using the Wilcoxon signed-rank test. Changes were assessed in the cohort as a whole as well as within subgroups defined by IPH presence/absence and by the AIM-HIGH treatment assignment (statin alone vs. statins plus ERN). Groups of subjects were compared using Fisher’s exact test or the Wilcoxon rank-sum test, as appropriate, including between subjects with and without IPH and between subjects assigned to statin alone and statin plus ERN. Univariable associations of individual baseline clinical variables, IPH status, and other imaging variables with annualized changes in lipid core and % lipid core were explored using linear regression without p-value adjustments for multiple comparisons. These models were adjusted for baseline lipid core or % lipid core and treatment assignment. Multivariable models with IPH status and other imaging and clinical factors were also examined. Covariates with p <0.05 in the univariable analysis were included in the multivariable models. Highly right-skewed variables were log-transformed prior to inclusion in the models. All statistical calculations were conducted with the statistical computing language R (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as p<0.05 (two-sided).
Results
Baseline characteristics
Of 214 subjects enrolled, 164 (77%) completed the 2-year follow-up scan. Reasons for subject drop-out were withdrawal of consent (n=21), early termination of the parent study (n=28), and other reasons (n=1). Another 8 subjects were excluded due to poor image quality. A total of 156 (73%) subjects were included in the analysis.
As previously described (20), the sub-study participants were younger (mean age: 61±9 vs. 64±9 years), had lower BMI (30±4 vs. 31±5 kg/m2) and a higher percentage of non-Caucasians (13% vs. 8%) than other AIM-HIGH participants, while the prevalence of clinically established coronary artery disease was comparable (96% vs. 92%). Most subjects had prior statin use for over a year (<1 year, 26%; 1 to 5 years, 37%; >5 years, 37%). There were no significant differences in clinical characteristics between those who completed the study and those who dropped out prematurely (data not shown).
Serum lipids and apolipoprotein levels
Over the study period, decreases were seen in LDL cholesterol (from 76±27 mg/dl to 72±23 mg/dl, p=0.082) and triglycerides [from 162 (127–206) mg/dl to 144 (112–200) mg/dl, p=0.011], while HDL cholesterol increased significantly from 35±6 mg/dl to 40±9 mg/dl (p<0.001) (Table 1). There were corresponding changes in ApoB (p<0.001) and ApoA-I (p<0.001), leading to a significantly decrease in ApoB:ApoA-I ratio (p<0.001). In addition, Lp(a) decreased moderately from 32 (14–149) nmol/L to 28 (10–109) nmol/L (p<0.001). Similar to the main study (1), these changes in serum lipids and apolipoproteins were observed in both the ERN and placebo group (data not shown), although the changes were greater in the statin plus ERN group.
Table 1.
Changes in serum lipids and apolipoproteins.
| Variable | Baseline | Follow-up | P-value* |
|---|---|---|---|
|
| |||
| Total cholesterol†, mg/dl | 145 ± 31 | 144 ± 28 | 0.73 |
| LDL cholesterol†, mg/dl | 76 ± 27 | 72 ± 23 | 0.082 |
| Triglycerides†, mg/dl | 162 (127 – 206) | 144 (112 – 200) | 0.011 |
| HDL cholesterol†, mg/dl | 35 ± 6 | 40 ± 9 | <0.001 |
| ApoB‡, mg/dl | 86 ± 23 | 77 ± 19 | <0.001 |
| ApoA-I‡, mg/dl | 122 ± 17 | 131 ± 20 | <0.001 |
| ApoB:ApoA-I ratio‡ | 0.72 ± 0.20 | 0.60 ± 0.17 | <0.001 |
| Lp(a)‡§, nmol/L | 32 (14 – 149) | 28 (10 – 109) | <0.001 |
LDL: Low-density lipoprotein, HDL; high-density lipoprotein. Values are mean ± standard deviation or median (inter-quartile range) unless otherwise specified;
Wilcoxon signed-rank test comparing baseline and follow-up measurements.
One subject missing the follow-up value was excluded;
Three subjects missing baseline or follow-up values were excluded;
Follow-up measurements were performed at 1 year.
Treatment effects on plaque burden and lipid content
Both treatment groups had a significant progression in plaque burden, which manifested as an increase in wall area without a decrease in lumen area (Table 2). However, % lipid core for both treatment groups was significantly reduced (−0.5±2.4 %/y, p=0.017; representative example shown in Figure 1). This % lipid core reduction was not significantly different between the statin alone and the statin plus ERN groups, p=0.44 for comparison between the 2 groups (Table 2).
Table 2.
Changes in plaque burden and lipid content over 2 years by treatment groups.
| All subjects (N=156) | Statin alone (n=89) | Statin + ERN (n=67) | P-value for between-group differences† | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Variable | Baseline | Follow-up | Baseline | Follow-up | Baseline | Follow-up | |
|
| |||||||
| Mean lumen area, mm2 | 43 ± 15 | 44 ± 16 | 43 ± 16 | 43 ± 16 | 43 ± 15 | 44 ± 15 | 0.85 |
| Mean wall area, mm2 | 30 ± 8 | 31 ± 8* | 30 ± 8 | 31 ± 8* | 30 ± 8 | 31 ± 8* | 0.68 |
| Mean outer wall area, mm2 | 73 ± 20 | 74 ± 20* | 73 ± 21 | 74 ± 21* | 73 ± 19 | 75 ± 19* | 0.51 |
| Lipid core volume, mm3 | 31 (17 – 71) | 27 (14 – 60) | 29 (16 – 53) | 26 (15 – 50) | 32 (18 – 98) | 32 (15 – 98) | 0.50 |
| Lipid core, % | 7 (4 – 12) | 6 (3 – 11)* | 6 (4 – 11) | 5 (3 – 9)* | 9 (5 – 16) | 7 (4 – 19) | 0.44 |
ERN: extended-release niacin. Values are mean ± standard deviation or median (inter-quartile range) unless otherwise specified;
: p<0.05 by Wilcoxon signed-rank test comparing baseline and follow-up measurements;
Wilcoxon rank-sum test comparing annualized changes from baseline to follow-up between treatment groups.
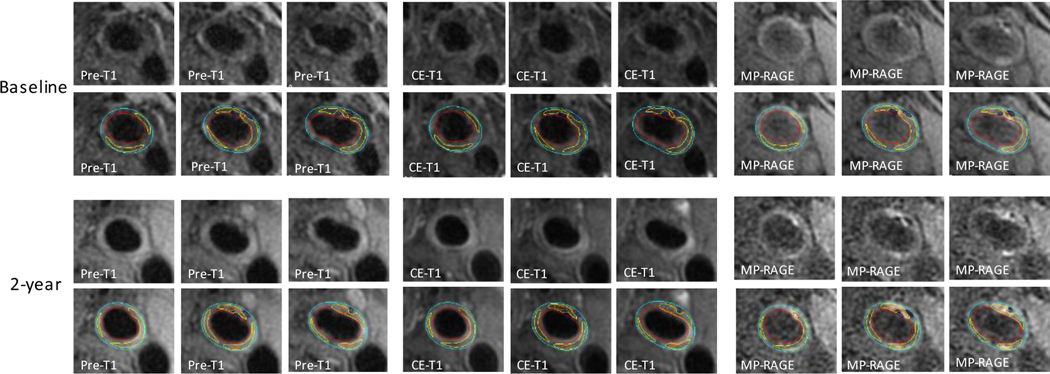
Figure 1: Plaque lipid depletion under intensive lipid-lowering therapy.
Upper (baseline) and lower (2-year follow-up) rows of panels show consecutive cross-sectional images of a mixed carotid plaque. Contours have been added in the lower row of each time point (red: lumen boundary; azure: outer wall boundary). Hypointense areas indicate calcifications (navy blue contours). Non-/less-enhanced areas as compared to adjacent fibrous tissue indicate lipid cores (yellow contours). Hyperintense area on CE-T1 indicate loose matrix (purple contour). Changes in MR signals between baseline and 2-year follow-up scans suggest a reduction in lipid content with an increase in non-lipid content. Pre-T1 = T1-weighted; CE-T1 = contrast-enhanced T1-weighted.
Plaque changes in the presence/absence of IPH
Table 3 summarizes changes in plaque morphological and compositional measurements in subjects with (n=18) and without IPH (n=138). The subjects with IPH included 15 with IPH present at baseline and 3 that developed IPH by the follow-up scan. All 15 subjects with IPH present at baseline still had IPH present on their follow-up scans. Compared to subjects with lipid core but without IPH, IPH was associated with an increase in plaque lipid content (change in lipid core volume: −3.8±11.7 mm3/y vs. 7.8±31.7 mm3/y, p=0.022; change in % lipid core: −1.0±2.2 %/y vs. 1.2±2.5 %/y, p=0.006) (Figure 2). Furthermore, plaques with IPH had greater decreases in lumen area (mean: −0.4±0.9 vs. 0.3±1.4 mm2/y, p=0.033) than plaques without IPH. The greater lumen restriction in plaques with IPH occurred without notable outer wall expansion (mean change: 0.1±1.7 mm2/y, p=0.70), consistent with a relatively constrictive remodeling pattern, while the plaques without IPH had significant outer wall area expansion (mean: 0.7±1.9 mm2/y, p<0.001), consistent with outward remodeling (Table 3). Both plaques with and without IPH tended to have increases in absolute calcification volume (mean change: 1.1±3.6 mm3/y, p=0.095 and 1.6±3.2 mm3/y, p=0.003, respectively), but, not in % calcification (Table 3).
Table 3.
Plaque morphology and composition in subjects with and without IPH.
| Without IPH (N=138) | With IPH (N=18) | P-value* | |
|---|---|---|---|
|
| |||
| Mean lumen area | |||
| Baseline, mm2 | 44 ± 16 | 38 ± 11 | 0.18 |
| Follow-up, mm2 | 44 ± 16 | 38 ± 11 | 0.098 |
| Annualized change, mm2/y | 0.3 ± 1.4 | −0.4 ± 0.9 | 0.033 |
| P-value† | 0.073 | 0.11 | |
| Mean wall area | |||
| Baseline, mm2 | 29 ± 6 | 40 ± 11 | <0.001 |
| Follow-up, mm2 | 30 ± 7 | 41 ± 11 | <0.001 |
| Annualized change, mm2/y | 0.4 ± 1.1 | 0.5 ± 1.6 | 0.57 |
| P-value† | <0.001 | 0.11 | |
| Mean outer wall area | |||
| Baseline, mm2 | 73 ± 20 | 78 ± 17 | 0.075 |
| Follow-up, mm2 | 74 ± 21 | 78 ± 17 | 0.11 |
| Annualized change, mm2/y | 0.7 ± 1.9 | 0.1 ± 1.7 | 0.25 |
| P-value† | <0.001 | 0.70 | |
| Lipid core volume ‡ | |||
| Baseline, mm3 | 26 (15 – 41) | 167 (71 – 333) | <0.001 |
| Follow-up, mm3 | 22 (13 – 32) | 195 (108 – 315) | <0.001 |
| Annualized change, mm3/y | −3.8 ± 11.7 | 7.8 ± 31.7 | 0.022 |
| P-value† | 0.002 | 0.14 | |
| % lipid core ‡ | |||
| Baseline, % | 6 (3 – 9) | 24 (10 – 32) | <0.001 |
| Follow-up, % | 4 (3 – 7) | 24 (19 – 31) | <0.001 |
| Annualized change, %/y | −1.0 ± 2.2 | 1.2 ± 2.5 | 0.006 |
| P-value† | <0.001 | 0.12 | |
| Calcification volume § | |||
| Baseline, mm3 | 14 (5 – 31) | 9 (5 – 24) | 0.67 |
| Follow-up, mm3 | 15 (6 – 30) | 14 (2 – 27) | 0.57 |
| Annualized change, mm3/y | 1.1 ± 3.7 | 1.6 ± 3.2 | 0.86 |
| P-value† | 0.003 | 0.095 | |
| % calcification § | |||
| Baseline, % | 4 (1 – 7) | 1 (1 – 3) | 0.053 |
| Follow-up, % | 4 (2 – 6) | 1 (1 – 3) | 0.021 |
| Annualized change, %/y | 0.0 ± 0.8 | 0.1 ± 0.5 | 0.59 |
| P-value† | 0.12 | 0.80 | |
IPH: Intraplaque hemorrhage.
P-values are for the comparison of the subjects with and without IPH using the Wilcoxon rank-sum test;
P-values are for the comparison of baseline and follow-up measurements using the Wilcoxon signed-rank test;
Based on cases with lipid core at either baseline or follow-up (n=80);
Based on cases with calcification at either baseline or follow-up (n=81).
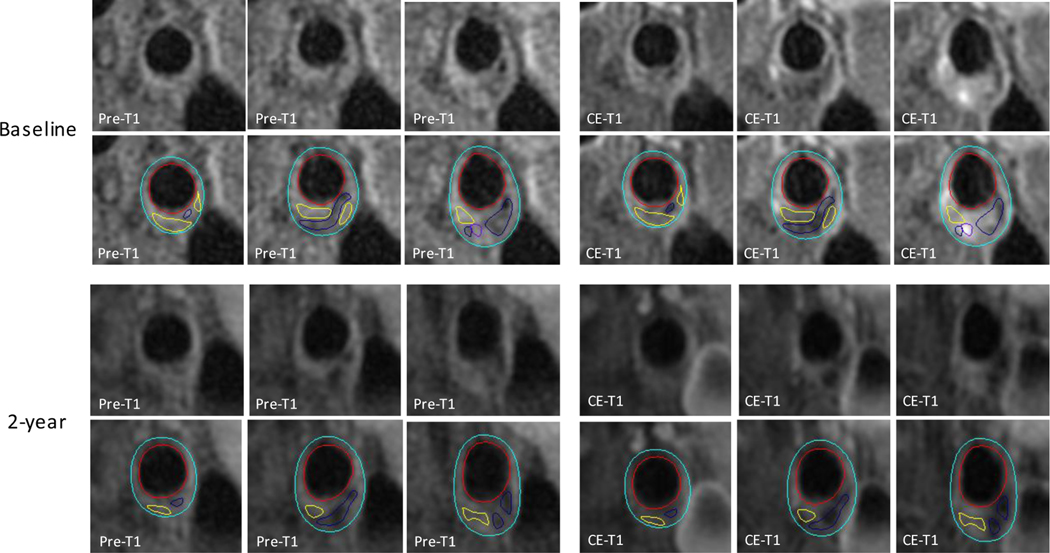
Figure 2: Intraplaque hemorrhage and increase in plaque lipid content despite intensive lipid-lowering therapy.
Upper (baseline) and lower (2-year follow-up) rows of panels show consecutive cross-sectional images of a carotid plaque with intraplaque hemorrhage. Contours have been added in the lower row of each time point (red: lumen boundary; azure: outer wall boundary). Non-/less-enhanced areas on CE-T1 as compared to adjacent fibrous tissue indicate lipid cores (yellow contours). Hyperintense areas on MP-RAGE indicate intraplaque hemorrhage (orange contours). Hypointense areas across the three contrast-weightings indicate calcification (navy blue contours). Changes in MR signals between baseline and 2-year follow-up scans suggest an increase in lipid content with an expansion in intraplaque hemorrhage. Pre-T1 = T1-weighted; CE-T1 = contrast-enhanced T1-weighted; MP-RAGE = magnetization-prepared rapid acquisition gradient echo.
Influence of IPH and clinical characteristics on change in plaque lipid content
Age (p=0.037), race (p=0.023), and total cholesterol (p=0.035) were significantly associated with relative change in % lipid core in univariable analyses, adjusted for baseline % lipid core and randomized treatment assignment (Table 4). Specifically, younger subjects, non-Caucasians, and those with lower cholesterol levels were more likely to show plaque lipid depletion. ApoB levels were also positively associated with change in % lipid core in univariable analyses (p=0.018). IPH was significantly associated with an increase in % lipid core (percent difference in relative % lipid core change: 65.5% / year, 95% CI: 38.5, 97.8, p<0.001) in the same analysis. In the multivariable analysis, IPH was independently associated with a relative increase in % lipid core (percent difference in relative % lipid core change: 54.4% / year, 95% CI: 26.8, 88.0, p<0.001) after further adjusting for age (p = 0.47 in the same multivariable model), race (p=0.33), and total cholesterol (p=0.31), whereas none of the clinical factors remained significantly associated with increase in % lipid core. Results were similar when relative change in lipid core volume was analyzed instead of relative change in % lipid core (Table 4). IPH was independently associated with a relative increase in lipid core volume (percent difference in relative lipid core volume change: 50.3% / year, 95% CI: 19.4, 89.2, p<0.001) after multivariable adjustments.
Table 4.
Associations of each clinical, laboratory and plaque characteristics with relative change in lipid core volume and % lipid core.
| Relative change in lipid core volume | Relative change in % lipid core | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Variable* | SD | %Δ† | 95% CI | P-Value | %Δ† | 95% CI | P-Value |
|
| |||||||
| Lipid core‡§ | § | −3.1 | (−10.4, 4.8) | 0.42 | −7.9 | (−14.4, −0.8) | 0.030 |
| On-study ERN use | − | −2.3 | (−16.4, 14.2) | 0.77 | 0.6 | (−13.2, 16.5) | 0.94 |
| Age, years | 9 | 9.2 | (1.1, 18.0) | 0.025 | 7.9 | (0.5, 15.8) | 0.037 |
| Male sex | − | −9.5 | (−32.2, 20.7) | 0.49 | −8.4 | (−29.4, 18.9) | 0.51 |
| Caucasian | − | 49.3 | (8.9, 104.5) | 0.013 | 41.1 | (4.9, 89.6) | 0.023 |
| Total cholesterol, mg/dl | 30 | 10.4 | (2.3, 19.2) | 0.012 | 8.2 | (0.6, 16.4) | 0.035 |
| LDL cholesterol, mg/dl | 26 | 7.8 | (−0.1, 16.5) | 0.054 | 6.6 | (−0.9, 14.6) | 0.084 |
| Triglycerides‡, log(mg/dl) | 0.5 | 6.5 | (−1.8, 15.4) | 0.13 | 4.2 | (−3.3, 12.4) | 0.27 |
| HDL cholesterol, mg/dl | 5 | 5.4 | (−2.6, 13.9) | 0.19 | 3.2 | (−4.2, 11.2) | 0.40 |
| ApoB, mg/dl | 24 | 11.0 | (2.7, 19.9) | 0.009 | 9.4 | (1.6, 17.7) | 0.018 |
| ApoA-1, mg/dl | 16 | 1.5 | (−6.4, 10.0) | 0.72 | −1.5 | (−8.8, 6.4) | 0.70 |
| Lipoprotein(a)‡, log(nmol/L) | 2.2 | 5.7 | (−2.3, 14.5) | 0.17 | 5.1 | (−2.3, 13.2) | 0.18 |
| Calcification | − | 15.7 | (−1.8, 36.3) | 0.080 | 8.5 | (−7.5, 27.4) | 0.31 |
| Intraplaque hemorrhage | − | 67.4 | (33.9, 109.2) | <0.001 | 65.5 | (38.5, 97.8) | <0.001 |
ERN: extended-release niacin, LDL: low-density lipoprotein, HDL: high-density lipoprotein.
Linear regression model with the baseline lipid core variable (log-transformed) and on-study ERN use;
Percent difference in annualized relative change of lipid core volume or % lipid core per 1-SD increase (continuous baseline variables) or between groups (categorical baseline variables);
Log-transformed prior to inclusion in the model;
Baseline lipid core volume for change in lipid core volume (SD: 2 log(mm3)) and baseline % lipid core for change in % lipid core (SD: 1.4 log(%)).
The univariable and multivariable analyses were also repeated using absolute change in lipid core volume and % lipid volume as the outcome variable instead of relative change (Table S1). In these multivariable analyses, IPH was independently associated with an absolute increase in lipid core volume (difference in absolute lipid core change: 26.4 mm3 / year, 95% CI: 14.5, 38.3, p < 0.001) and absolute increase in % lipid core (difference in absolute % lipid core change: 4.0% / year, 95% CI: 2.7, 5.2, p < 0.001) after adjusting for baseline lipid core volume or % lipid volume, randomized treatment assignment, and other clinical covariates significantly associated with lipid changes in the univariable analysis (race and lp(a)).
Discussion
Recent advances in cardiovascular imaging have allowed for in vivo quantification of plaque lipid core in coronary and carotid arteries. Plaque lipid content measured in a focal plaque using different imaging methods has been consistently shown to predict systemic cardiovascular outcomes (23–25). This study examined serial changes in carotid plaque lipid core by MRI in AIM-HIGH participants to understand what factors influenced plaque lipid content change under intensive lipid-lowering therapy. The main findings are: 1) in patients with clinically established ASCVD, continued intensive lipid-lowering therapy over 2 years was associated with a significant reduction in plaque lipid content, no significant change in lumen area, and an increase in wall area; 2) despite the favorable effects on the lipid profile, the addition of ERN to statin therapy had no significant effect on plaque lipid content; 3) IPH was independently and significantly associated with increased plaque lipid content in the setting of continued intensive lipid-lowering therapy.
Atherosclerosis regression under lipid-lowering therapy
In addition to the evidence that plaque slow progression and regression induced by lipid-lowering therapies in previous studies using intravascular ultrasound (26–28), CT angiography (29,30), or black-blood MRI (31, 32), a number of studies also have shown that plaque lipid content can be depleted during intensive lipid-lowering (33–36). However, the majority of these studies were conducted in patients without extended lipid-lowering treatment history. The current study showed a reduction of plaque lipid content in ASCVD patients who have been treated with statin therapy prior to AIM-HIGH and received continued, intensified lipid-lowering during AIM-HIGH. We also provided new insights into the relationship between changes in plaque morphology and lipid content under lipid-lowering therapy. Despite a reduction in plaque lipid content, there was a small yet statistically significant increase in mean wall area but no worsening in lumen restriction, indicating positive vascular remodeling. Whether the plaque stabilization with reduced lipid content without a further reduction in plaque burden can translate to decreased cardiovascular event risk remains to be determined, although prospective studies have indicated that plaque lipid content may be more closely associated with both local and systemic cardiovascular outcomes than plaque burden measurements (23–25).
Incremental benefit of niacin
Total and LDL cholesterol levels remained relatively unchanged and comparable between the two groups, but patients in the ERN group had larger increases in HDL cholesterol and ApoA-1 and larger decreases in ApoB and lipoprotein(a). However, these ERN-associated lipid changes did not translate into favorable modification of carotid plaque morphology and lipid content. Previously, Lee et al (37) studied the effect of niacin on carotid wall area in patients with low HDL cholesterol (<40 mg/dl) under statin therapy and found the changes in carotid wall area were −1.1±2.6 mm2 in the niacin group versus 1.2±3.0 mm2 in the placebo group (p=0.03 for difference) over 12 months. In addition to HDL cholesterol, LDL cholesterol was also significantly changed in the niacin group (from 85±23 mg/dl to 69±21 mg/dl) but not in the placebo group (from 84±32 mg/dl to 80±28 mg/dl). In an elderly population, Sibley et al (38) found wall volume reduction in the internal carotid artery as LDL cholesterol was reduced, which was seen in both statin alone and statin plus niacin groups (p=0.49 for difference in wall volume reduction). These previous studies suggested that plaque burden reduction in the niacin studies could be attributed to additional LDL lowering effect. In the present study, LDL cholesterol remained at a relatively constant level in the low 70’s in both treatment groups. Without the interfering effect from further lowering LDL cholesterol, our data have provided new evidence from plaque imaging that raising HDL cholesterol with niacin is unlikely to be beneficial in patients who receive intensive LDL-lowering therapy.
IPH induced plaque progression despite intensive lipid-lowering therapy
While modest plaque lipid depletion was observed at the group level, serial changes in plaque lipid content varied dramatically at the individual level. The determinants of progression or regression in plaque burden and lipid content under intensive lipid-lowering therapy are largely unknown. Despite a relatively narrow range of LDL cholesterol levels in this study as a result of the AIM-HIGH on-trial target of 40–80 mg/dl, higher levels of total cholesterol, LDL cholesterol, and ApoB were associated with lesser reduction in % lipid core. This relationship between LDL cholesterol and % lipid core was reinforced by a recent study demonstrating that further LDL cholesterol reduction with a PCSK9 inhibitor, alirocumab, resulted in significant % lipid core reduction (36). Nonetheless, the present study demonstrates that IPH has the strongest, independent effect on increasing in plaque lipid core and decreasing lumen area. Previous natural history studies (9, 39) also identified that IPH is associated with lipid core enlargement and plaque progression that are most likely driven by the deleterious effects of IPH (40). In addition, our serial data demonstrate that development of IPH is associated with accelerated plaque progression (10). The adverse association of IPH with plaque lipid content is consistent with clinical observations indicating a 4- to 10-fold increased risk for cerebrovascular events in patients with carotid plaques with IPH as compared to those without (15–17, 41). Our data have provided new evidence that IPH is an important factor contributing to residual cardiovascular risk under intensive lipid-lowering therapy. Histopathological studies suggested neoangiogenesis with compromised structural integrity (fragile and leaky) is a major source for IPH (42,43). A more recent study showed that CD163+ macrophages, induced by IPH, further promote vascular permeability, which may mediate a positive feedback loop that connects increased vascular permeability and IPH and reveals a non-lipid-driven pathway of plaque progression (44). Further investigation of this non-lipid-driven pathway is needed, for example, to assess whether local anti-inflammatory mechanisms are present within the plaque that might suppress IPH-mediated plaque progression, and to examine whether such mechanisms may be leveraged into novel therapeutic approaches.
Study limitations
The sample size of this study was relatively small compared to traditional clinical trials using clinical endpoints, with only 18 plaques containing IPH. Nonetheless, it is still one of the largest serial MRI studies to-date demonstrating differential changes in plaque lipid content in plaques with and without IPH. Further, this differential progression in the setting of intensive lipid-lowering therapy suggests an under-recognized mechanism for residual cardiovascular risk that may have implications for clinical management and new drug development. Another limitation is related to the multi-contrast MRI technique. Although in vivo quantification of plaque lipid content by MRI has been histologically validated, segmentation of multiple-contrast-weighted images only allows the detection of relatively large, confluent lipid areas. The recent development of quantitative vessel wall T1 and T2 mapping techniques may provide more sensitive and reproducible measurements than T1- and T2-weighted MRI (45).
Conclusions
In a serial MRI study embedded in the AIM-HIGH trial, carotid plaque lipid depletion was observed with continued lipid-lowering therapy. Although there was a concurrent increase in carotid wall area, there was not a worsening in luminal area. Plaque lipid depletion did not appear to be influenced by niacin use. Plaques with IPH showed greater increases in lipid core and greater decreases in lumen area than plaques without IPH, suggesting a more constrictive remodeling pattern in the plaques with IPH. Thus, IPH, with its deleterious effects on plaque progression, may be an under-recognized mechanism for the residual cardiovascular risk under intensive lipid-lowering therapy.
Supplementary Material
Acknowledgements:
We appreciate support from the AIM-HIGH Study Principal Investigators: B. Gregory Brown, MD, PhD (passed away), Jeffrey L. Probstfield, MD, William E. Boden, MD. We would like to thank the investigators who were instrumental in recruiting subjects and obtaining the MR images.
Financial support:
This study was supported in part by the National Institutes of Health (R01 HL088214, R01 HL089504, R01 HL103609, R01 NS083503, R01 NS092207, UL1 TR002319) and the American Heart Association (17MCPRP33671077). Carotid coils were provided by GE Healthcare and Philips Healthcare. The content is solely the responsibilities of the authors and do not necessarily represent the official views of the National Institutes of Health or the US Government.
Disclosures:
Mr. Hippe has received research grants from GE Healthcare, Philips Healthcare, and Toshiba America Medical Systems. Dr. Balu has received research grants from Philips Healthcare. Dr. Hatsukami has received research grants from Philips Healthcare. Dr. Yuan has received research grants from Philips Healthcare. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Abbreviation list
- AIM-HIGH
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes
- ASCVD
atherosclerotic cardiovascular disease
- ERN
extended-release niacin
- HDL
high-density lipoprotein
- IPH
intraplaque hemorrhage
- LDL
low-density lipoprotein
- MRI
magnetic resonance imaging
Footnotes
Competency in Medical Knowledge
Intensive lipid lowering therapy was associated with a reduction in carotid plaque lipid content despite an increase in carotid wall area. However, plaques with IPH showed greater increases in lipid core and greater decreases in lumen area than plaques without IPH.
Translational outlook
Identifying modifiable risk factors for incident IPH and developing therapies targeting the deleterious effects of IPH on plaque progression may help to reduce residual cardiovascular risk.
Clinical Trial Registration Information: https://clinicaltrials.gov/ct2/show/NCT00880178
References
- 1.Investigators AIM-HIGH, Boden WE Probstfield JL, Anderson T Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. Erratum in: N Engl J Med. 2012;367:189. [DOI] [PubMed] [Google Scholar]
- 2.Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. , Investigators I-I. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. , Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 4.Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, Ohayon J, Pettigrew R, Sabatine MS, Tearney GJ, et al. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–73. [DOI] [PubMed] [Google Scholar]
- 6.Takaya N, Cai J, Ferguson MS, Yarnykh VL, Chu B, Saam T, Polissar NL, Sherwood J, Cury RC, Anders RJ, et al. Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging. 2006;24:203–10. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–44. [DOI] [PubMed] [Google Scholar]
- 8.Qi H, Sun J, Qiao H, Chen S, Zhou Z, Pan X, Wang Y, Zhao X, Li R, Yuan C, et al.Carotid Intraplaque Hemorrhage Imaging with Quantitative Vessel Wall T1 Mapping: Technical Development and Initial Experience. Radiology. 2018;287:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111(21):2768–75. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Underhill HR, Hippe DS, Xue Y, Yuan C, Hatsukami TS. Sustained acceleration in carotid atherosclerotic plaque progression with intraplaque hemorrhage: a long-term time course study. JACC Cardiovasc Imaging. 2012;5:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson RJ, Akwei S, Hosseini AA, MacSweeney ST, Auer DP, Altaf N. MR imaging-detected carotid plaque hemorrhage is stable for 2 years and a marker for stenosis progression. AJNR Am J Neuroradiol. 2015;36:1171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Underhill HR, Yuan C, Yarnykh VL, Chu B, Oikawa M, Dong L, Polissar NL, Garden GA, Cramer SC, Hatsukami TS. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol. 2010;31:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altaf N, Goode SD, Beech A, Gladman JR, Morgan PS, MacSweeney ST, Auer DP. Plaque hemorrhage is a marker of thromboembolic activity in patients with symptomatic carotid disease. Radiology. 2011;258:538–45. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk AC, Truijman MT, Hussain B, Zadi T, Saiedie G, de Rotte AA, Liem MI, van der Steen AF, Daemen MJ, Koudstaal PJ, et al. Intraplaque Hemorrhage and the Plaque Surface in Carotid Atherosclerosis: The Plaque At RISK Study (PARISK). AJNR Am J Neuroradiol. 2015;36:2127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, Tran N, Polissar NL, Isaac C, Ferguson MS, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006;37:818–23. [DOI] [PubMed] [Google Scholar]
- 16.Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62:1081–1091. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44:3071–7. [DOI] [PubMed] [Google Scholar]
- 18.Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, de Vries JP, Seldenrijk KA, De Bruin PC, Vink A, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. 2010;121:1941–50. [DOI] [PubMed] [Google Scholar]
- 19.Bos D, Arshi B, van den Bouwhuijsen QJ, Ikram MK, Selwaness M, Vernooij MW, Kavousi M, van der Lugt A. Atherosclerotic carotid plaque composition and incident stroke and coronary events. Journal of the American College of Cardiology. 2021;77:1426–35. [DOI] [PubMed] [Google Scholar]
- 20.Zhao XQ, Hatsukami TS, Hippe DS, Sun J, Balu N, Isquith DA, Crouse JR 3rd, Anderson T, Huston J 3rd, Polissar N,et al. AIM-HIGH Carotid MRI Sub-study Investigators. Clinical factors associated with high-risk carotid plaque features as assessed by magnetic resonance imaging in patients with established vascular disease (from the AIM-HIGH Study). Am J Cardiol. 2014;114:1412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Zhao XQ, Balu N, Hippe DS, Hatsukami TS, Isquith DA, Yamada K, Neradilek MB, Cantón G, Xue Y, et al. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: a multicenter reproducibility study. Int J Cardiovasc Imaging. 2015;31:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. [DOI] [PubMed] [Google Scholar]
- 23.Schuurman AS, Vroegindewey M, Kardys I, Oemrawsingh RM, Cheng JM, de Boer S, Garcia-Garcia HM, van Geuns RJ, Regar ES, Daemen J, et al. Near-infrared spectroscopy-derived lipid core burden index predicts adverse cardiovascular outcome in patients with coronary artery disease during long-term follow-up. Eur Heart J. 2018;39:295–302. [DOI] [PubMed] [Google Scholar]
- 24.Xing L, Higuma T, Wang Z, Aguirre AD, Mizuno K, Takano M, Dauerman HL, Park SJ, Jang Y, Kim CJ, et al. Clinical Significance of Lipid-Rich Plaque Detected by Optical Coherence Tomography: A 4-Year Follow-Up Study. J Am Coll Cardiol. 2017;69:2502–2513. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Zhao XQ, Balu N, Neradilek MB, Isquith DA, Yamada K, Cantón G, Crouse JR 3rd, Anderson TJ, Huston J 3rd, et al. Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging. 2017;10:241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, et al. REVERSAL Investigators. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–80. [DOI] [PubMed] [Google Scholar]
- 27.Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, Raichlen JS, Uno K, Borgman M, Wolski K, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–87. [DOI] [PubMed] [Google Scholar]
- 28.Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, et al. Effect of Evolocumab on Progression of Coronary Disease in Statin-Treated Patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 29.Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, Sanda Y, Anno H, Kondo T, Wong ND. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3:691–8. [DOI] [PubMed] [Google Scholar]
- 30.Shin S, Park HB, Chang HJ, Arsanjani R, Min JK, Kim YJ, Lee BK, Choi JH, Hong GR, Chung N. Impact of Intensive LDL Cholesterol Lowering on Coronary Artery Atherosclerosis Progression: A Serial CT Angiography Study. JACC Cardiovasc Imaging. 2017;10:437–446. [DOI] [PubMed] [Google Scholar]
- 31.Corti R, Fuster V, Fayad ZA, Worthley SG, Helft G, Chaplin WF, Muntwyler J, Viles-Gonzalez JF, Weinberger J, Smith DA, et al. Effects of aggressive versus conventional lipid-lowering therapy by simvastatin on human atherosclerotic lesions: a prospective, randomized, double-blind trial with high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2005;46:106–12 [DOI] [PubMed] [Google Scholar]
- 32.Yonemura A, Momiyama Y, Fayad ZA, Ayaori M, Ohmori R, Higashi K, Kihara T, Sawada S, Iwamoto N, Ogura M, et al. Effect of lipid-lowering therapy with atorvastatin on atherosclerotic aortic plaques detected by noninvasive magnetic resonance imaging. J Am Coll Cardiol. 2005;45:733–42. [DOI] [PubMed] [Google Scholar]
- 33.Underhill HR, Yuan C, Zhao XQ, Kraiss LW, Parker DL, Saam T, Chu B, Takaya N, Liu F, Polissar NL, et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J. 2008;155:584.e1–8. doi: Erratum in: Am Heart J. 2008;155:1127. [DOI] [PubMed] [Google Scholar]
- 34.Zhao XQ, Dong L, Hatsukami T, Phan BA, Chu B, Moore A, Lane T, Neradilek MB, Polissar N, Monick D, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging. 2011;4:977–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kini AS, Baber U, Kovacic JC, Limaye A, Ali ZA, Sweeny J, Maehara A, Mehran R, Dangas G, Mintz GS, et al. Changes in plaque lipid content after short-term intensive versus standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol. 2013;62:21–9. [DOI] [PubMed] [Google Scholar]
- 36.Lepor NE, Sun J, Canton G, Contreras L, Hippe DS, Isquith DA, Balu N, Kedan I, Simonini AA, Yuan C, et al. Regression in carotid plaque lipid content and neovasculature with PCSK9 inhibition: A time course study. Atherosclerosis. 2021;327:31–38. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, Digby JE, Bannister T, Handa A, Wiesmann F, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–94. [DOI] [PubMed] [Google Scholar]
- 38.Sibley CT, Vavere AL, Gottlieb I, Cox C, Matheson M, Spooner A, Godoy G, Fernandes V, Wasserman BA, Bluemke DA, et al. MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin in a randomised controlled trial: the NIA plaque study. Heart. 2013;99:1675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J, Balu N, Hippe DS, Xue Y, Dong L, Zhao X, Li F, Xu D, Hatsukami TS, Yuan C. Subclinical carotid atherosclerosis: short-term natural history of lipid-rich necrotic core--a multicenter study with MR imaging. Radiology. 2013;268:61–8. [DOI] [PubMed] [Google Scholar]
- 40.Michel JB, Virmani R, Arbustini E, Pasterkamp G. Intraplaque haemorrhages as the trigger of plaque vulnerability. Eur Heart J. 2011;32:1977–85, 1985a, 1985b, 1985c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, Singh N, Kwee RM, Kurosaki Y, Yamagata S, et al. Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging. 2020;13:395–406. [DOI] [PubMed] [Google Scholar]
- 42.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. [DOI] [PubMed] [Google Scholar]
- 43.Sluimer JC, Kolodgie FD, Bijnens AP, Maxfield K, Pacheco E, Kutys B, Duimel H, Frederik PM, van Hinsbergh VW, Virmani R, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo L, Akahori H, Harari E, Smith SL, Polavarapu R, Karmali V, Otsuka F, Gannon RL, Braumann RE, Dickinson MH, et al. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J Clin Invest. 2018;128:1106–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi H, Sun J, Qiao H, Zhao X, Guo R, Balu N, Yuan C, Chen H. Simultaneous T1 and T2 mapping of the carotid plaque (SIMPLE) with T2 and inversion recovery prepared 3D radial imaging. Magn Reson Med. 2018;80:2598–2608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.