Abstract
In the postantibiotic era, prostatic abscesses (PAs) are rare, affecting primarily immunocompromised men and/or caused by atypical drug-resistant pathogens, raising both diagnostic and management challenges. PA caused by methicillin-resistant Staphylococcus aureus (MRSA) is an uncommon condition and also a primary source of bacteremia. Nevertheless, the continued pattern of increase in reported cases, due especially to community-associated strains, is a growing concern regarding the significant morbidity and mortality. Besides proper antibiotics, drainage of a PA may be required, which is usually transrectal or transurethral. Herein, we describe the case of MRSA PA extending into the penis with concomitant MRSA bacteremia of unknown origin, whereupon diabetes mellitus was newly diagnosed in a previously healthy man residing in a community setting, and managed successfully by a transperineal drainage with good outcome. This case also highlights that individuals diagnosed with such rare deep-seated MRSA infections should be assessed for undiagnosed comorbidities. To the best of our knowledge, this is the first reported case of percutaneous drainage of a PA by using a double-lumen catheter.
Keywords: Prostatic abscess, Methicillin-resistant Staphylococcus aureus, Drainage, Diabetes mellitus
Case history
A 51-year-old man, with unknown comorbidities, presented with mild lower urinary tract symptoms (LUTS), pelvic pain and fever (38.4 centigrade) of 3 weeks’ duration. His physical examination revealed a painful swelling of the penis, with an enlarged, tender and fluctuant prostate gland on digital rectal exam. The urine dipstick test was positive for leucocyte esterase with glycosuria, but negative results for nitrites and ketones.
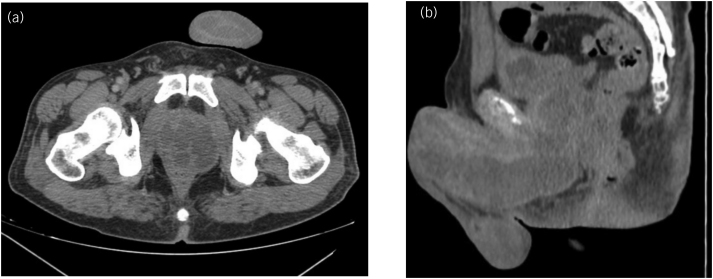
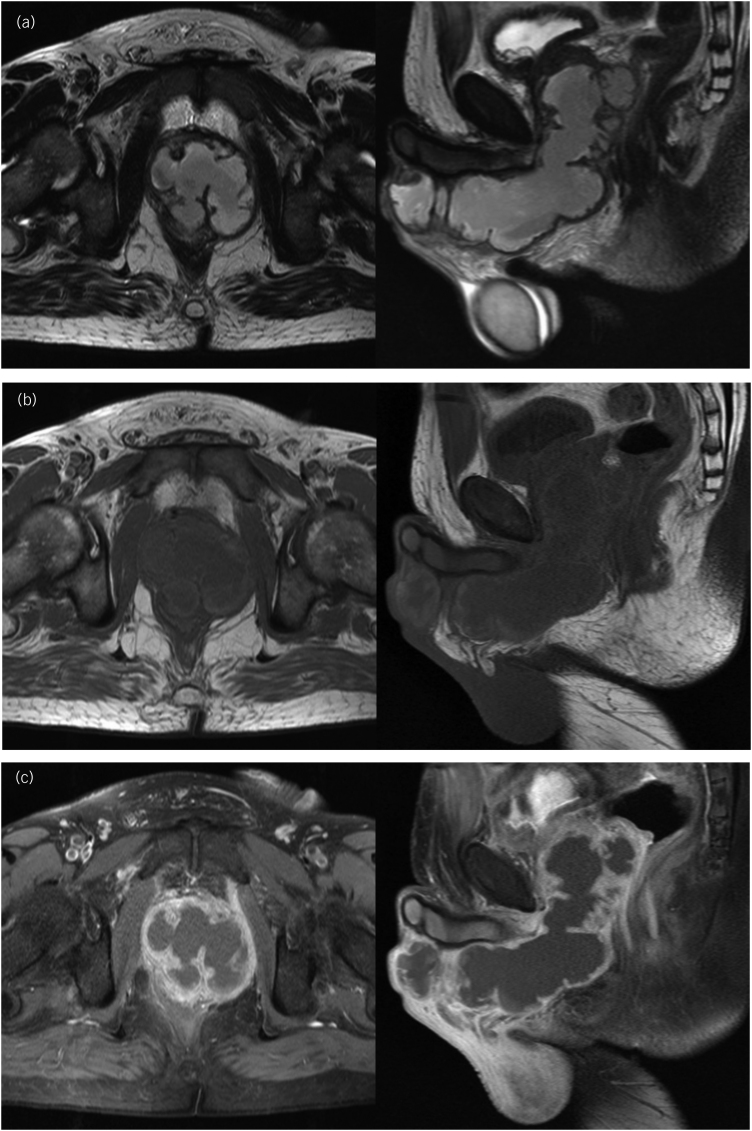
Transrectal ultrasonography (TRUS) showed a heterogeneous hypoechoic collection area with a thick fluid in the prostate suggesting abscess. Computed tomography (CT) scan of the pelvis demonstrated a defined prostatic fluid collection of 6.1 × 6.0 × 7.6cm3 expanding into the penis (Figure 1). In order to investigate further, magnetic resonance imaging (MRI) of the pelvis showed a singular nonseptate prostatic fluid collection that spread to the base of the penis to form a collection of 13.0×4.5×4.6cm3 along its ventral aspect (Figure 2). Blood tests showed leukocytosis with marked neutrophilia (93.6%), and elevated C-reactive protein. A diagnosis of prostatic abscess (PA) was considered. Urine and blood specimens were obtained for culture, and human immunodeficiency virus (HIV) was negative. His laboratory tests were otherwise normal but revealed elevated blood glucose level (17.8mmol/l) and glycated haemoglobin HbA1c (110.9mmol/mol), consistent with diabetes mellitus (DM), albeit without ketoacidosis.
Figure 1 .
Axial (a) and sagittal (b) pelvic CT showing a defined prostatic fluid collection expanding into the anterior part of the perineum and along the penis. CT = Computed tomography.
Figure 2 .
Axial (left) and sagittal (right) pelvic MRI images showing a nonseptate PA that spread into the base of the penis to form a collection of along the ventral aspect of the penis, with neither urethral stricture nor fistulae. The abscess is seen as a heterogeneous hyperintense signal on T2-weighted images (a), and as a hypointense signal on T1-weighted images (b) with peripheral contrast enhancement (c). The seminal vesicles were involved but the anterior rectal wall was intact. MRI = Magnetic resonance imaging; PA = prostatic abscess.
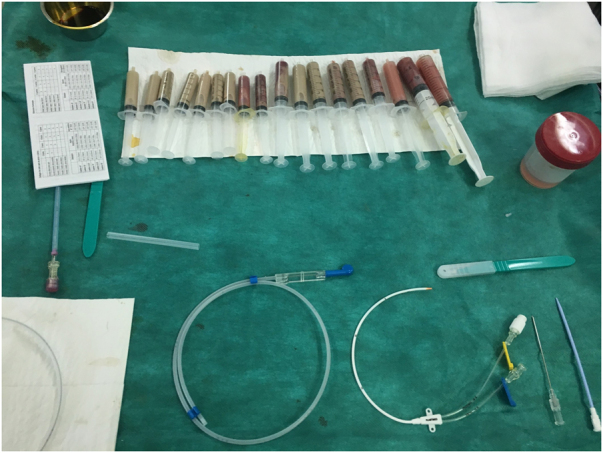
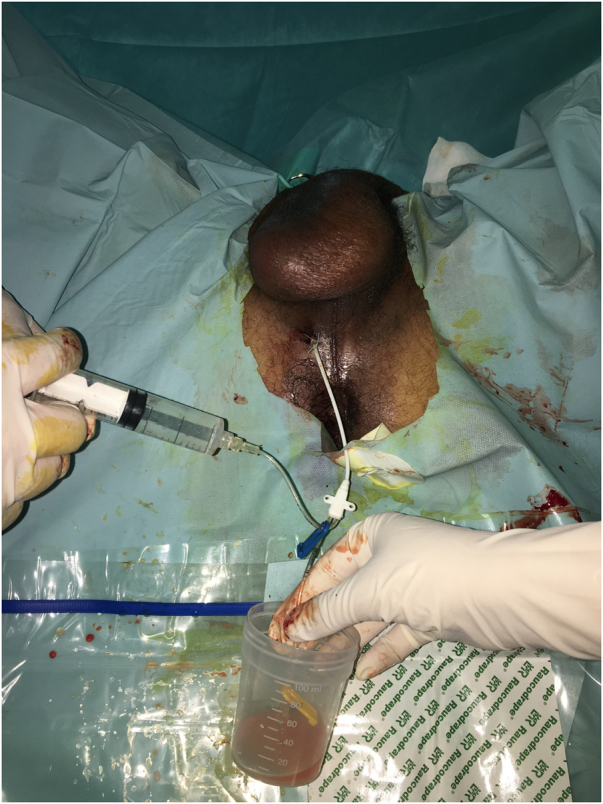
The patient was started on intravenous (IV) empirical antibiotics (ceftriaxone and gentamicin) and insulin. Thereafter, he underwent TRUS-guided transperineal catheter drainage of the abscess. Puncture of the abscess showed frank pus and specimens were taken for cultures (Figure 3). As the first aspirate was too thick, use of a double-lumen catheter (DLC) was decided in order to perform regular washing of the abscess cavity. A 7 French (2.4mm)×20cm double-lumen central venous catheter (CVC) (16/16 Gauge) was inserted using the Seldinger technique (Figure 4), and approximately 190ml of pus was aspirated manually (Figure 5). Saline with gentamicin injections were performed through its two lumens and continued twice a day (Figure 6). The immediate postoperative course was uneventful; his symptoms improved dramatically and glycaemia was corrected. Urine culture was negative, but pus and blood cultures grew a methicillin-resistant Staphylococcus aureus (MRSA). The patient was then switched to IV vancomycin based on sensitivity.
Figure 3 .
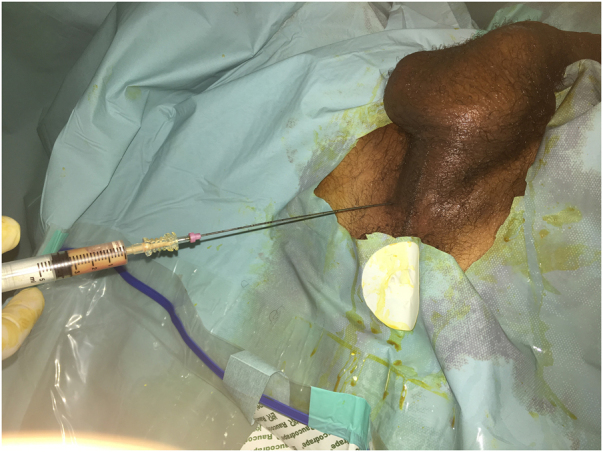
Puncture of the abscess performed via the perineum with aspiration of samples of thick frank pus using 18 gauge Chiba-type needle in lithotomy position under spinal anesthesia
Figure 4 .
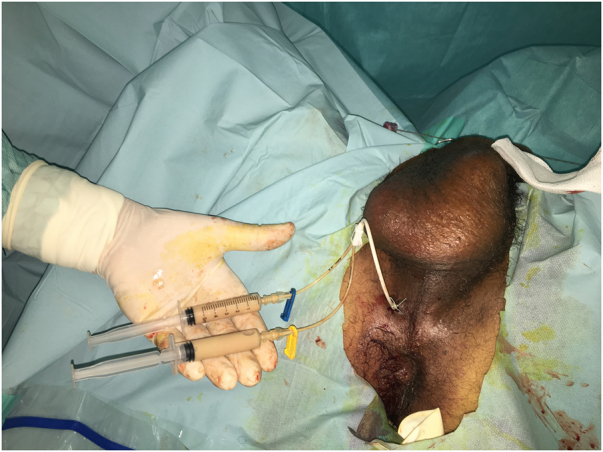
Percutaneous drainage of the OA with a 7 French central venous DLC that was placed transperinally using the Seldinger technique. DLC = Double-lumen catheter; PA = prostatic abscess.
Figure 5 .
Approximately 190ml of pus was aspirated via the two lumens of the central venous DLC. DLC = Double-lumen catheter.
Figure 6 .
Bidirectional washing of the abscess, which is flushed regularly with saline and antibiotics
Follow-up pelvic CT performed 4 days later demonstrated complete resolution of the abscess. The catheter was then removed and the patient discharged on the 11th day with trimethoprim/sulfamethoxazole, insulin and multivitamins. At 6 months, our insulin-treated diabetic patient denied any voiding or sexual difficulties, achieved good glycemic control (HbA1c of 43.2mmol/mol) and was followed up regularly with TRUS, which showed collapsed cavity.
Discussion
PA is an uncommon condition, reported in 0.5 to 2.5% of all prostatitis, often caused by Escherichia coli and other Gram-negative bacteria.1,2 However, PA is an unusual complication of Staphylococcus aureus infections; only 40 cases have been documented in the literature through January 2017.3 Since then, the continued pattern of increase in Staphylococcal PA cases is a growing concern; MRSA has been involved more frequently.3,4
MRSA is a Gram-positive bacteria that has developed resistance to virtually most β-lactam antibiotics. It originally emerged as a healthcare-associated infection that usually involved skin, soft tissue, bones and heart. Recently, MRSA has become increasingly prevalent in community settings as a significant cause of bacteremia with concurrent occult deep-seated infections in previously healthy individuals.3,4 In this case, a community-associated strain of MRSA (CA-MRSA) was thought to be implicated, which is also the culprit in most published reports of staphylococcal PA.3–5
In the postantibiotic era, men with PA are typically debilitated or immunologically compromised, with >50% of all patients having DM.1,2 Although our patient was considered to be previously healthy, he was diagnosed with DM. Actually, newly diagnosed diabetics represent 17–25% of PA cases.1 Others reported risk factors for MRSA PA are associated skin and soft tissue infections, immunodeficiency state, intravenous drugs use, hepatitis C infection and history of genitourinary disease or instrumentation.3–5
PA commonly presents with a wide range of nonspecific complaints, including LUTS, pain, urinary retention, hematuria, chills, purulent urethral discharge, tenesmus and fever. Thus, it is often confused with prostatitis or chronic pelvic pain syndrome.1,2 In this case, the insidious onset was thought to be the result of an atypical micro-organism that affected a debilitated patient. This subclinical presentation could fail to improve or worsen with empirical antibiotics that usually aim to cover Gram-negative bacteria to treat an acute prostatitis. Consequently, MRSA PA should be suspected in case of such a pattern and outcome, especially in at-risk subjects. Imaging with cultures are then required to avoid diagnostic errors or delayed treatment.
A PA can usually be diagnosed by TRUS; yet, transabdominal or transperineal ultrasound can be attempted in thin patients. CT and MRI are useful when TRUS is contraindicated or inconclusive, and when emphysematous PA or extraprostatic spread are suspected.1,2 As urine studies are often inaccurate,1 culture of the aspirate was helpful in this case. Furthermore, rapid identification of MRSA bloodstream infection was also crucial for improving our patient's outcome.
Herein, a MRSA PA resulted in bacteremia and extension into a neighbouring organ; the penis. Untreated, it can also lead to infertility, rupture, fistula, ascending urinary tract infections, distant visceral infections, overwhelming sepsis and even death.1–5
There are currently no formal guidelines on the management of PA. Besides proper antibiotics, drainage may be required and changes according to abscess’s characteristics (size and shape), clinical status and associated conditions. It can include TRUS-guided aspiration, transurethral resection or deroofing (TUD) of the abscess using wire loop, Collin’s knife or Holmium laser, and open or TRUS-guided percutaneous perineal drainage.1,2 In the current case, TUD combined with open perineal incision could have been feasible and effective, but potential risk factors include ejaculatory dysfunction, impotence, incontinence and fistulae. Consequently, transperineal percutaneous drainage is a good treatment option for this condition, especially in sexually active men wishing to preserve continence and fertility.
Despite recent published data encouraging its use,4 the transperineal route is not commonly practiced,2 and usually performed with single-lumen catheters (nephrostomy, suprapubic or simple J catheters). Nevertheless, its growing utilisation in prostatic biopsies may certainly lead to an increasingly routine use for PA drainage in the near future.2
In this case, a DLC was inserted transperineally. Unlike single-lumen catheters, its adequate position in the abscess was confirmed by aspirating pus from the proximal lumen without removing the safety guide wire from the distal one. Moreover, it allowed bidirectional washings of the abscess cavity, which might be helpful during the waiting period of antibacterial sensitivity test, especially when a drug resistant pathogen is involved or suspected. By mobilising thick residual pus, such a modality could hasten recovery, and may prevent some recurrences reported in the literature.1,2 Furthermore, washings are performed without abscess overdistension, which made the repeated procedure much more bearable and largely painless in our patient, and would also minimise risks of bacteremia or spread into surrounding tissues.
Conclusion
MRSA is an uncommon pathogen that causes PA; yet, the prostate gland should be considered as a site of primary infection in cases of MRSA bacteremia of unknown source, especially in at-risk groups. As reports of CA-MRSA PA increase, and given the risks of delayed treatment, individuals presenting with such condition should be assessed for underlying comorbidities and treated with empirical antibiotic regimens covering also Gram-positive organisms. Adequate treatment may also require drainage, which can be transrectal or transurethral. Percutaneous drainage is underutilised, yet is usually performed with single-lumens catheters. This case is interesting and unique; use of a DLC has never been described, but seems to be easy, safe and effective.
Acknowledgements
We gratefully thank Driss Touiti for his expert advice, knowledge and exacting attention to detail throughout this research, as well as Kamal Moufid for his contribution during paper preparation.
References
- 1.Ackerman AL, Parameshwar PS, Anger JT. Diagnosis and treatment of patients with prostatic abscess in the post-antibiotic era. Int J Urol 2018; 25: 103–110. 10.1111/iju.13451 [DOI] [PubMed] [Google Scholar]
- 2.Khudhur H, Brunckhorst O, Muir Get al. Prostatic abscess: a systematic review of current diagnostic methods, treatment modalities and outcomes. Turk J Urol 2020; 46: 262–273. 10.5152/tud.2020.19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carroll DE, Marr I, Huang GKLet al. Staphylococcus aureus prostatic abscess: a clinical case report and a review of the literature. BMC Infect Dis 2017; 17: 509. 10.1186/s12879-017-2605-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker B, Heidel E, Shorman M. Clinical characteristics and outcome of Staphylococcus aureus prostate abscess, Ten-year experience at a tertiary care center. Open Forum Infect Dis 2019; 6: ofz372. 10.1093/ofid/ofz372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vyas V, Endy T. A rare case of prostatic and bilateral renal abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus infection. Cureus 2020; 12: e7046. 10.7759/cureus.7046 [DOI] [PMC free article] [PubMed] [Google Scholar]