Abstract
In view of the composition characteristics of lithium, calcium, and bromine rich in Nanyishan oilfield water of the Qaidam Basin, Qinghai Province, the phase equilibrium relationships of quaternary system LiBr–NaBr–KBr–H2O and its ternary subsystems LiBr–NaBr–H2O and LiBr–KBr–H2O at 348.15 K were studied by the isothermal solution equilibrium method, and the equilibrium solid-phase crystallization regions and composition of each invariant point in each system were determined. The results show that there is no complex salt or solid solution in ternary systems LiBr–NaBr–H2O and LiBr–KBr–H2O at 348.15 K, and the phase diagram contains only one invariant point, two isothermal univariate curves, and two equilibrium solid-phase crystallization regions. The quaternary system LiBr–NaBr–KBr–H2O also has no formation of complex salt or solid solution, and the phase diagram contains only one invariant point, three isothermal univariate curves, and three equilibrium solid-phase crystallization regions. Meanwhile, the phase equilibrium relationships and change laws of each component of the above-mentioned systems at different temperatures were compared and discussed.
1. Introduction
As an important liquid mineral resource enrichment area in China, Qinghai is not only rich in Salt Lake oil, natural gas, nonferrous metals, and other resources but also rich in underground brine resources. In particular, the Nanyishan area of western Qinghai Province is not only rich in oilfield water resources but also widely distributed, which is considered an important reserve resource for potassium extraction in the future.1,2 According to the existing geological survey data, Nanyishan oilfield water belongs to the brine system of the calcium chloride type. In addition to the conventional sodium chloride and calcium chloride resources, it also contains abnormally rich key strategic resources such as potassium, boron, bromine, iodine, lithium, rubidium, and cesium. If it is comprehensively developed and utilized, it can not only provide the urgently needed resource guarantee for national economic development but also produce obvious economic and social benefits.3
As we all know, different brine resources can be divided into different brine composition systems due to their different effective mineral compositions, such as the classical seawater system (Na–K–Mg–Ca–Cl–SO4–H2O), carbonate-type brine system (Na–K–Mg–Cl–SO4–CO3–H2O), nitrate-type brine system (Na–K–Mg–Cl–SO4–NO3–H2O),4 and so forth. The different brine composition systems often correspond to different salt mineral compositions, and it is often of great significance for guiding the rational and effective development of brine resources. According to the ion species contained in Nanyishan oilfield water, the brine composition can be simply summarized as the complex system of Li–Na–K–Mg–Ca–Sr–Cl–Br–B2O3–H2O. A series of in-depth studies on this complex system can not only reveal the migration differentiation convergence mineralization mechanism of this brine resource but also provide important theoretical guidance for the comprehensive development and utilization of this brine resource.5,6
Referring to the current situation of comprehensive development and utilization of brine resources at home and abroad, it is often necessary to rely on specific brine compositions, guided by phase equilibrium research, and achieve comprehensive development and utilization of brine resources through a series of phase separation technologies such as evaporation, crystallization, salting out, heating, cooling, and dissolution.7 After the separation and enrichment of Nanyishan oilfield water in the early stage, with the continuous precipitation and separation of major components, lithium, potassium, bromine, and other elements with small contents in brine will be further concentrated and enriched. It can be regarded as an important and valuable old brine resource for the preparation of lithium, potassium, and bromine salts. According to its brine composition, it can be simply summarized as the complex system of Li–Na–K–Ca–Cl–Br–H2O. It is worth noting that the research on multitemperature phase equilibria and phase diagrams of this complex brine system is seldom carried out. Therefore, it is necessary to systematically carry out the research on multitemperature phase equilibria and phase diagrams of this complex brine system from a simple system to a multicomponent system.
At present, some research results have been achieved in the research on the Nanyishan oilfield water system. In the early stage, our research group conducted normal-temperature evaporation experiments according to the composition characteristics of Nanyishan oilfield water and summarized the crystallization behavior of various salts under normal-temperature conditions. In view of the composition characteristics of lithium, potassium, and strontium rich in Nanyishan oilfield water, Sun et al. carried out a study on the stable-phase equilibria of quinary system LiCl–NaCl–KCl–SrCl2–H2O and related subsystems at a temperature of 298.15 K.8−11 Deng et al. carried out studies on metastable and stable-phase equilibria of a series of subsystems of multicomponent system Li–Na–K–Ca–Sr–Cl–B4O7–H2O; meanwhile, the theoretical simulations of the relevant subsystem were carried out by using the Pitzer electrolyte solution theoretical model.12−18 To carry out in-depth research on relevant systems, on the basis of full reference to previous studies and in view of the composition characteristics of lithium, potassium, and bromine rich in Nanyishan oilfield water of Qinghai Province, our research group continued to carry out experimental research on stable-phase equilibria of quaternary system Li–Na–K–Br–H2O, Li–Na–Mg–Br–H2O, Li–K–Mg–Br–H2O, Li–Na–Br–SO4–H2O, Li–K–Br–SO4–H2O, and quinary system Li–Na–K–Br–SO4–H2O at 298.15 K.19−22
In summary, most of the current studies on Nanyishan oilfield water are focused on chloride systems, but the studies on bromine-containing systems, especially lithium bromide-containing systems, are relatively less. However, most of the existing studies focus on normal-temperature conditions and rarely involve high temperature, which obviously has certain deficiencies in building a comprehensive thermodynamic database of lithium bromide-containing systems from low temperature to high temperature. Therefore, on the basis of continuing the existing work of our research group and in view of the characteristics of lithium, potassium, and bromine rich in Nanyishan oilfield water, this paper will continue to study the solid–liquid stable-phase equilibrium and phase diagram of quaternary system LiBr–NaBr–KBr–H2O and its related ternary subsystem at 348.15 K and determine the equilibrium liquid-phase composition of each system and draw the corresponding phase diagram. The research results of this paper will be conducive to the establishment of a complex brine system of Nanyishan oilfield water and provide important basic theoretical data for the comprehensive development and utilization of Nanyishan oilfield water. At the same time, it will have important practical significance for guiding the comprehensive development and utilization of this brine resource.
2. Experimental Section
The isothermal dissolution equilibrium method was used to determine the solubilities in this paper. The chemicals used in this work are given in Table 1. The resistivity of ultrapure water was greater than 17 MΩ·cm.
Table 1. CAS Registry Numbers, Sources, and Purity of Chemicals.
| chemical name | sources | CAS number | mass fractiona |
|---|---|---|---|
| LiBr | Shanghai Aladdin Reagent Company | 7550-35-8 | ≥0.995 |
| KBr | Shanghai Aladdin Reagent Company | 7758-02-3 | ≥0.995 |
| NaBr | Shanghai Aladdin Reagent Company | 7647-15-6 | ≥0.995 |
The data are provided by the suppliers.
According to the composition of invariant points of subsystems, another new salt was added in a gradient manner. Each sample was placed in a rigid plastic bottle and kept at 348.15 ± 0.02 K using a thermostatic water bath oscillator. During the whole experiment, the samples were kept in a state of continuous oscillation. At the same time, the upper liquid phases were sampled and analyzed many times, and the results of sampling and analysis with more than three consecutive intervals remain unchanged as a sign of reaching equilibrium. According to the analysis results of the liquid phase during the experiment, the time to reach equilibrium is approximately 20 days. The solid and liquid phases are not separated until equilibrium is reached. After equilibrium, the liquid phase and solid phase were taken for analysis and identification, respectively.
The composition of the equilibrium liquid phase is determined by the chemical analysis method commonly used in brine analysis; that is, the standard sample is used for inspection first, and at least two parallel samples and blank samples are analyzed at the same time to ensure the accuracy of experimental data. The composition of the solid phase is determined by the wet residue method of Schreinemakers.23 Of these, Li+ was determined by inductively coupled plasma-optical emission spectrometry (ICP-OES), K+ was determined by the sodium tetraphenylborate method, Br– was measured by the mercury method, and Na+ was obtained by the ion balance difference subtraction method. The relative standard uncertainties of ion analysis are ur[w(Li+)] = 0.005, ur[w(Na+)] = 0.005, ur[w(K+)] = 0.005, and ur[w(Br–)] = 0.003. Meanwhile, to further determine the compositions of the equilibrium solid phase, X-ray diffraction was used for identification. The main instrument and equipment used in this experiment are shown in Table 2.
Table 2. Main Instrument and Equipment Used in the Experiment.
| name | manufacturer | model | accuracy |
|---|---|---|---|
| thermostatic water bath oscillator | Julabo Labortechnik GmbH | SW23 | 0.02 K |
| immersion magnetic stirrer | Julabo Labortechnik GmbH | CR40215 | |
| ultrapure water machine | Sichuan Youpu Ultrapure Technology Co., LTD. | UPL-H/U | |
| electronic analytical balance | Sartorius Scientific Instruments (Beijing) Co., LTD. | BSA124S | 0.0001 g |
| X-ray diffractometer | PANalytical B.V. | X-Pert PRO | |
| ICP-OES spectrometer | PerkinElmer Co., Ltd | PE Avio 200 |
3. Results and Discussion
3.1. Ternary System LiBr–NaBr–H2O
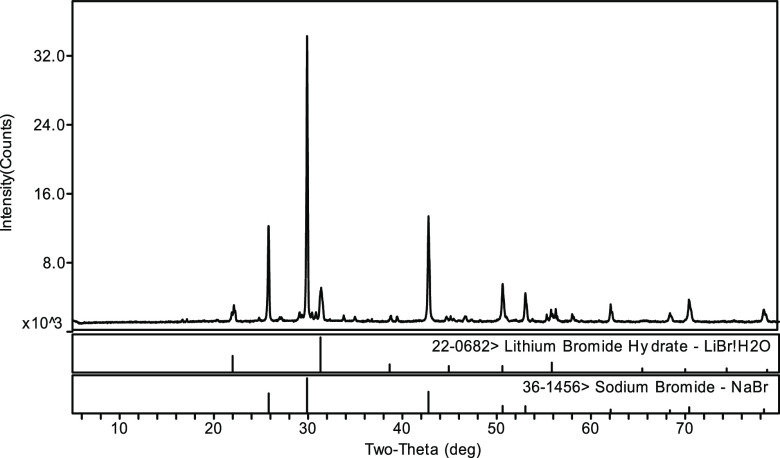
The experimental results of stable-phase equilibrium of ternary system LiBr–NaBr–H2O at 348.15 K are listed in Table 3. The data cover the solubility data of LiBr and NaBr, compositions of the wet residue, and composition of the equilibrium solid phase at each experimental point. According to the data in Table 3, the equilibrium phase diagram of this ternary system at 348.15 K was drawn, as shown in Figure 1. To further identify the compositions of the equilibrium solid phase at the invariant point (E1) of this ternary system, the X-ray powder diffraction method was used for identification, and the results are shown in Figure 2.
Table 3. Experimental Results of Phase Equilibria in Ternary System LiBr–NaBr–H2O at 348.15 K and 0.077 MPaa.
| composition
of liquid phase 100·w(B) |
composition of wet residue 100·w(B) |
||||
|---|---|---|---|---|---|
| no. | LiBr | NaBr | LiBr | NaBr | equilibrium solids |
| 1, A1 | 67.45 | 0.00 | LiBr·H2O | ||
| 2 | 67.25 | 0.17 | LiBr·H2O | ||
| 3 | 66.57 | 0.49 | LiBr·H2O | ||
| 4, E1 | 66.04 | 1.00 | 92.17 | 1.98 | LiBr·H2O + NaBr |
| 5 | 62.47 | 1.23 | 13.26 | 78.41 | NaBr |
| 6 | 56.65 | 2.01 | 8.66 | 85.67 | NaBr |
| 7 | 45.42 | 6.96 | 7.90 | 84.51 | NaBr |
| 8 | 40.87 | 11.57 | 9.79 | 79.01 | NaBr |
| 9 | 18.62 | 33.79 | NaBr | ||
| 10 | 9.33 | 45.21 | 2.55 | 86.43 | NaBr |
| 11 | 4.66 | 49.80 | 1.14 | 89.07 | NaBr |
| 12 | 1.40 | 53.00 | NaBr | ||
| 13, B1 | 0.00 | 54.34 | NaBr | ||
Standard uncertainties u are u(T) = 0.02 K and u(P) = 0.002 MPa. Relative standard uncertainty of the measurement ur[w(LiBr)] = 0.005 and ur[w(NaBr)] = 0.005.
Figure 1.
(a) Equilibrium phase diagram of ternary system LiBr–NaBr–H2O at 348.15 K and (b) partially enlarged diagram of (a).
Figure 2.
X-ray diffraction pattern of invariant point E1 (LiBr·H2O, NaBr) in ternary system LiBr–NaBr–H2O at 348.15 K.
It can be seen from Figure 1 and Table 3 that there is no solid solution or complex salt formation in ternary system LiBr–NaBr–H2O at 348.15 K; only single salt NaBr and hydrated salt LiBr·H2O form, which belongs to a simple system. The liquid-phase compositions of ternary invariant point E1 are w(LiBr) = 66.04%, w(NaBr) = 1.00%, and w(H2O) = 32.96%, and the corresponding equilibrium solid phases are identified as LiBr·H2O and NaBr, respectively. The two isothermal univariate curves are A1E1 and B1E1, and the corresponding equilibrium solid phases are LiBr·H2O and NaBr, respectively. Among them, the solid-phase crystallization region of NaBr is much larger than that of LiBr·H2O, indicating that the solubility of NaBr in a saturated solution of this ternary system is much smaller than that of LiBr·H2O, and it is easier to crystallize and precipitate from the saturated solution.
3.2. Ternary System LiBr–KBr–H2O
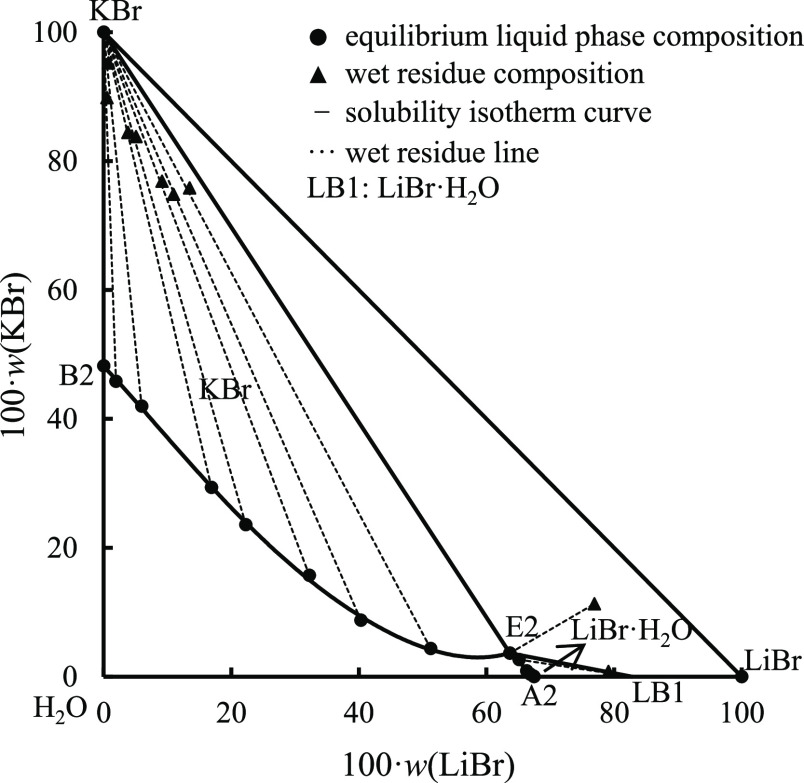
The experimental results of stable-phase equilibrium of ternary system LiBr–KBr–H2O at 348.15 K are listed in Table 4. The data in table are given in the form of the mass percentage. Based on the data in Table 4, the equilibrium phase diagram of the ternary system at 348.15 K was drawn, as shown in Figure 3. Figure 4 is an X-ray powder diffraction pattern of the equilibrium solid phase of this ternary system at the invariant point (E2).
Table 4. Experimental Results of Phase Equilibria in Ternary System LiBr–KBr–H2O at 348.15 K and 0.077 MPaa.
| composition
of liquid phase 100·w(B) |
composition of wet residue 100·w(B) |
||||
|---|---|---|---|---|---|
| no. | LiBr | KBr | LiBr | KBr | equilibrium solids |
| 1, A2 | 67.45 | 0.00 | LiBr·H2O | ||
| 2 | 67.39 | 0.08 | LiBr·H2O | ||
| 3 | 66.84 | 0.41 | LiBr·H2O | ||
| 4 | 66.29 | 0.90 | LiBr·H2O | ||
| 5 | 65.10 | 2.65 | 79.10 | 0.78 | LiBr·H2O |
| 6, E2 | 63.68 | 3.66 | 76.89 | 11.28 | LiBr·H2O + KBr |
| 7 | 51.25 | 4.36 | 13.46 | 75.82 | KBr |
| 8 | 40.31 | 8.77 | 10.93 | 74.81 | KBr |
| 9 | 32.23 | 15.73 | 9.14 | 76.87 | KBr |
| 10 | 22.28 | 23.58 | 5.07 | 83.77 | KBr |
| 11 | 16.89 | 29.37 | 3.80 | 84.42 | KBr |
| 12 | 5.95 | 41.96 | 0.73 | 95.22 | KBr |
| 13 | 1.93 | 45.84 | 0.53 | 89.82 | KBr |
| 14, B2 | 0.00 | 48.21 | KBr | ||
Standard uncertainties u are u(T) = 0.02 K and u(P) = 0.002 MPa. Relative standard uncertainty of the measurement ur[w(LiBr)] = 0.005 and ur[w(KBr)] = 0.005.
Figure 3.
Equilibrium phase diagram of ternary system LiBr–KBr–H2O at 348.15 K.
Figure 4.
X-ray diffraction pattern of invariant point E2 (KBr, LiBr·H2O) in ternary system LiBr–KBr–H2O at 348.15 K.
Compared with ternary systems LiBr–NaBr–H2O and NaBr–KBr–H2O, the ternary system LiBr–KBr–H2O also has no solid solution and complex salt formation at 348.15 K, belonging to a simple system. It can be seen from Figures 3 and 4 that the equilibrium phase diagram of the ternary system contains a ternary invariant point, two isothermal univariate curves (A2E2, B2E2), and two equilibrium solid-phase crystallization regions (LiBr·H2O, KBr). The percentages of LiBr, KBr, and H2O at the invariant point E2 were 63.68, 3.66, and 32.66%, respectively. The equilibrium solid phases were identified as LiBr·H2O and KBr.
Similar to ternary system LiBr–NaBr–H2O, affected by the salting out of LiBr, the solid-phase crystallization region of LiBr·H2O of ternary system LiBr–KBr–H2O is much smaller than that of KBr, indicating that the solubility of LiBr·H2O in a saturated solution is much larger than that of KBr, and it is difficult to crystallize from the saturated solution of the ternary system.
3.3. Quaternary System LiBr–NaBr–KBr–H2O
The experimental results of stable-phase equilibrium of quaternary system LiBr–NaBr–KBr–H2O at 348.15 K are listed in Table 5, of which the solubility data are expressed in the form of the mass fraction w(B) and the Jänecke index J(B). J(B) is calculated according to J(LiBr) + J(NaBr) + J(KBr) = 100 g, and the specific calculation formulas are as follows
| 1 |
| 2 |
| 3 |
| 4 |
Table 5. Experimental Results of Phase Equilibria in Quaternary System LiBr–NaBr–KBr–H2O at 348.15 K and 0.077 MPaa.
| Jänecke index of dry salt/(g/100 g) |
||||||||
|---|---|---|---|---|---|---|---|---|
| composition of solution 100·w(B) |
J(LiBr) + J(NaBr) + J(KBr) = 100 |
|||||||
| no. | LiBr | NaBr | KBr | LiBr | NaBr | KBr | H2O | equilibrium solids |
| 1, E1 | 66.04 | 1.00 | 0.00 | 98.51 | 1.49 | 0.00 | 49.15 | LB1 + NB |
| 2 | 64.40 | 0.97 | 1.99 | 95.61 | 1.44 | 2.95 | 48.46 | LB1 + NB |
| 3, E2 | 63.68 | 0.00 | 3.66 | 94.56 | 0.00 | 5.44 | 48.49 | LB1 + KB |
| 4, F1 | 63.28 | 0.90 | 3.61 | 93.35 | 1.33 | 5.33 | 47.51 | LB1 + NB + KB |
| 5, E3 | 0.00 | 46.58 | 12.26 | 0.00 | 79.16 | 20.84 | 69.95 | NB + KB |
| 6 | 1.84 | 44.51 | 11.71 | 3.17 | 76.66 | 20.17 | 72.24 | NB + KB |
| 7 | 5.05 | 41.33 | 10.96 | 8.81 | 72.08 | 19.11 | 74.40 | NB + KB |
| 8 | 9.73 | 36.84 | 9.84 | 17.25 | 65.31 | 17.44 | 77.28 | NB + KB |
| 9 | 15.53 | 31.15 | 9.12 | 27.83 | 55.82 | 16.34 | 79.19 | NB + KB |
| 10 | 28.47 | 19.86 | 7.47 | 51.02 | 35.59 | 13.39 | 79.22 | NB + KB |
| 11 | 36.95 | 13.51 | 6.10 | 65.33 | 23.89 | 10.79 | 76.80 | NB + KB |
| 12 | 38.38 | 12.71 | 5.77 | 67.50 | 22.35 | 10.15 | 75.84 | NB + KB |
| 13 | 40.89 | 11.09 | 5.48 | 71.16 | 19.30 | 9.54 | 74.02 | NB + KB |
| 14 | 53.43 | 3.89 | 4.02 | 87.10 | 6.34 | 6.55 | 63.02 | NB + KB |
| 15 | 56.53 | 2.73 | 3.74 | 89.73 | 4.33 | 5.94 | 58.74 | NB + KB |
Abbreviations: LB1 = LiBr·H2O, KB = KBr, and NB = NaBr. Standard uncertainties u are u(T) = 0.02 K and u(P) = 0.002 MPa. Relative standard uncertainty of the measurement ur[w(LiBr)] = 0.005, ur[w(NaBr)] = 0.005, and ur[w(KBr)] = 0.005.
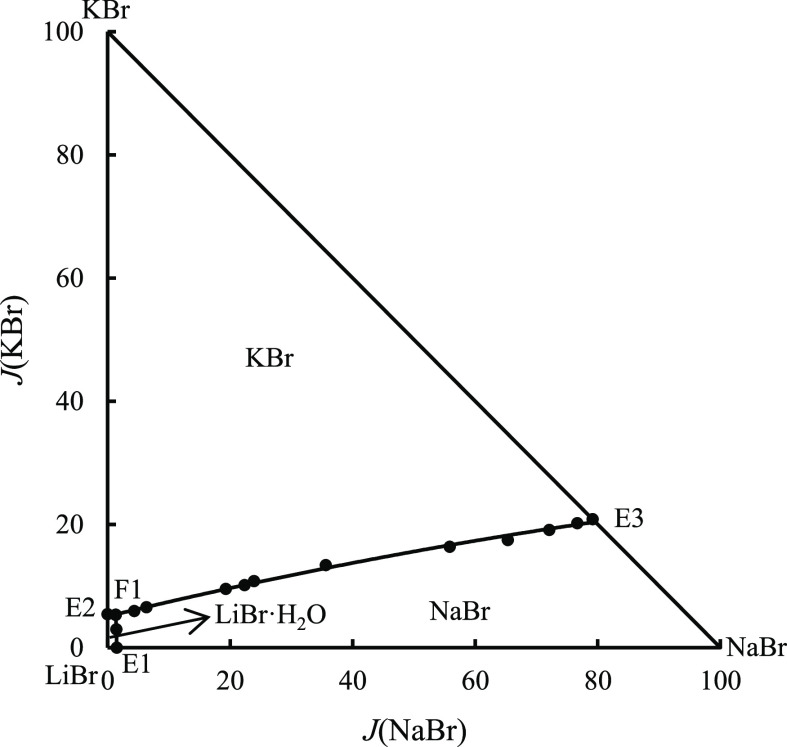
According to the data listed in Table 5, the equilibrium phase diagram and water content diagram of the quaternary system were drawn, as shown in Figures 5 and 6. The equilibrium solid-phase compositions at invariant point F1 were identified by X-ray powder diffraction, as shown in Figure 7. It can be seen from Figures 5 and 7 and Table 5 that the quaternary system LiBr–NaBr–KBr–H2O has no solid solution or complex salt formation at 348.15 K, and its equilibrium phase diagram belongs to a simple phase diagram. The equilibrium phase diagram of this quaternary system at 348.15 K includes a quaternary invariant point (F1), three isothermal univariate curves (corresponding to E1f1, E2F1, and E3F1), and three equilibrium solid-phase crystallization regions. The compositions of the equilibrium solid phase and liquid phase at quaternary invariant point F1 are as follows: (1) The corresponding equilibrium solid phases at invariant point F1 are LiBr·H2O, NaBr, and KBr. (2) The corresponding equilibrium liquid-phase compositions are w(LiBr) = 63.28%, w(NaBr) = 0.90%, w(KBr) = 3.61, and w(H2O) = 32.21%. The equilibrium solid phases corresponding to three isothermal univariate curves are (1) E1F1/LiBr·H2O, NaBr; (2) E2F1/LiBr·H2O, KBr; and (3) E3F1/NaBr, KBr.
Figure 5.
Equilibrium phase diagram of quaternary system LiBr–NaBr–KBr–H2O at 348.15 K.
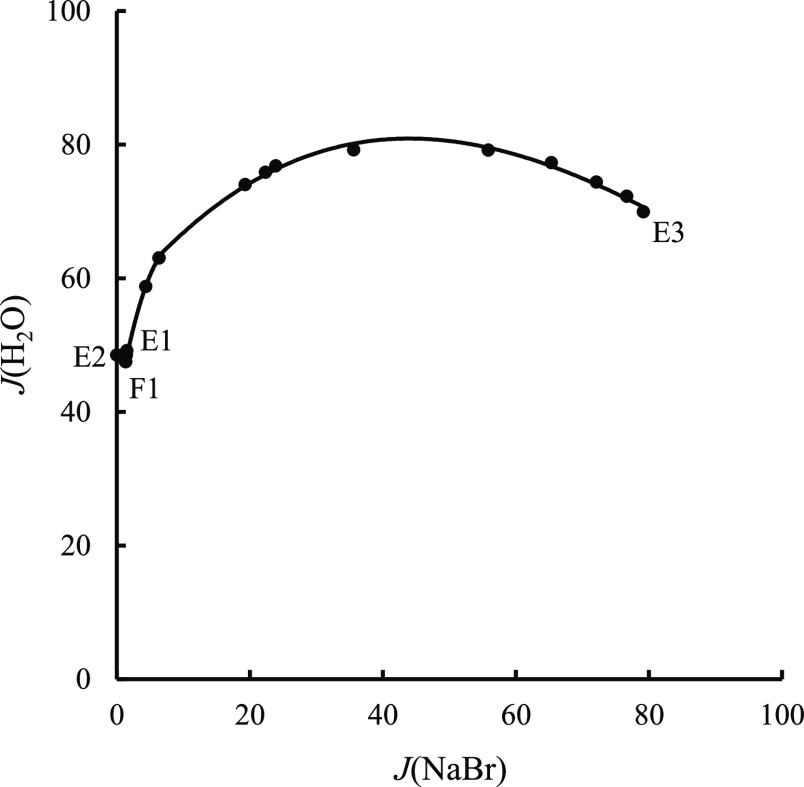
Figure 6.
Water contents of saturated solutions in quaternary system LiBr–NaBr–KBr–H2O at 348.15 K.
Figure 7.
X-ray diffraction pattern of invariant point F1 (KBr, LiBr·H2O, NaBr) in quaternary system LiBr–NaBr–KBr–H2O at 348.15 K.
The size of the three equilibrium solid-phase crystallization regions increases in the order of LiBr·H2O, NaBr, and KBr. Among them, KBr has the largest equilibrium solid-phase crystallization region, accounting for more than 50%, indicating that KBr has the smallest solubility in a saturated solution, which easily crystallizes and precipitates from the saturated solution of the above-mentioned quaternary system. However, LiBr·H2O has the smallest solid-phase crystallization region and largest solubility in the abovementioned saturated solution. Compared with the other two equilibrium solid phases, it is more difficult to crystallize and precipitate from the saturated solution. Compared with ternary subsystems, there is no new solid phase in the equilibrium solid phase of this quaternary system. Figure 6 is the diagram of water content versus composition of quaternary system LiBr–NaBr–KBr–H2O. As seen from Figure 6, the water content reaches the minimum at point F1 and then increases with an increase in the J(NaBr) value.
3.4. Comparison and Discussion under Different Temperature Conditions
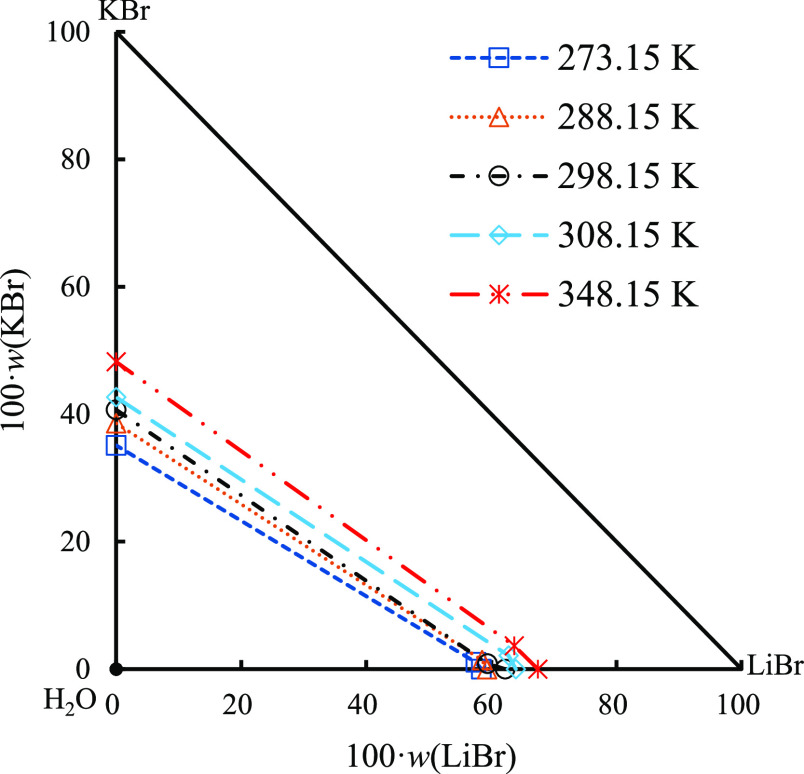
At present, the studies on stable-phase equilibria of the quaternary system (LiBr–NaBr–KBr–H2O) and its ternary subsystems (LiBr–NaBr–H2O, LiBr–KBr–H2O) under different temperature conditions have been reported in the literature, and the research temperatures are mainly focused on the range of 273.15–323.15 K.19,24−26 Although the reported data are limited, the change characteristics of the equilibrium phase diagram and the change rule of the equilibrium solid phase of this quaternary system and its ternary subsystems can also be seen through the comparison under different temperature conditions. For this reason, this paper lists the liquid-phase compositions and corresponding equilibrium solid phases of the above-mentioned three systems at invariant points, as shown in Table 6. Figures 8–10 show the comparison of equilibrium phase diagrams of the above-mentioned three systems at different temperatures.
Table 6. Solubility Data of Invariant Points of Quaternary System LiBr–NaBr–KBr–H2O and Its Ternary Subsystems LiBr–NaBr–H2O and LiBr–KBr–H2O at Different Temperaturesa.
| liquid-phase
composition of invariant points 100·w(B) |
||||||
|---|---|---|---|---|---|---|
| systems | T/K | LiBr | NaBr | KBr | equilibrium solids | refs |
| LiBr–NaBr–H2O | 288.15 | 58.81 | 0.56 | 0.00 | LB2 + NB | (24) |
| 40.72 | 8.69 | 0.00 | NB2 + NB | |||
| 298.15 | 60.60 | 0.90 | 0.00 | LB2 + NB | (19) | |
| 33.60 | 16.40 | 0.00 | NB2 + NB | |||
| 323.15 | 63.65 | 0.75 | 0.00 | LB1 + NB | (26) | |
| 19.27 | 32.39 | 0.00 | NB2 + NB | |||
| 348.15 | 66.04 | 1.00 | 0.00 | LB1 + NB | this work | |
| LiBr–KBr–H2O | 273.15 | 57.54 | 0.00 | 1.07 | LB2 + KB | (25) |
| 288.15 | 58.50 | 0.00 | 1.41 | LB2 + KB | (24) | |
| 298.15 | 59.40 | 0.00 | 0.83 | LB2 + KB | (19) | |
| 308.15 | 62.74 | 0.00 | 2.13 | LB2 + KB | (25) | |
| 348.15 | 63.68 | 0.00 | 3.66 | LB1 + KB | this work | |
| LiBr–NaBr–KBr–H2O | 288.15 | 58.40 | 0.56 | 1.42 | LB2 + NB + KB | (24) |
| 39.78 | 8.52 | 2.50 | NB2 + NB + KB | |||
| 298.15 | 59.10 | 0.91 | 0.88 | LB2 + NB2 + KB | (19) | |
| 29.60 | 16.50 | 4.25 | NB2 + NB + KB | |||
| 348.15 | 63.28 | 0.90 | 3.61 | LB1 + NB + KB | this work | |
Abbreviations: LB1 = LiBr·H2O, LB2 = LiBr·2H2O, NB = NaBr, NB2 = NaBr·2H2O, and KB = KBr.
Figure 8.
Comparison of equilibrium phase diagrams of ternary system LiBr–NaBr–H2O at different temperatures (288.15 K,24 298.15 K,19 323.15 K,26 and 348.15 K).
Figure 10.
(a) Comparison of equilibrium phase diagrams of quaternary system LiBr–NaBr–KBr–H2O at different temperatures (288.15 K,24 298.15 K,19 and 348.15 K) and (b) partially enlarged diagram of (a).
It can be seen from Table 6 and Figure 8 that the phase diagrams of ternary system LiBr–NaBr–H2O at 288.15, 298.15, and 323.15 K are similar to each other, and no solid solution or complex salt is formed. The phase diagrams contain three isothermal univariate curves, three equilibrium solid-phase crystallization regions, and two invariant points. Different from the above-mentioned three temperatures, the equilibrium phase diagram of this ternary system changes at 348.15 K. With the increase in temperature, the solid-phase crystallization region of LiBr·2H2O and NaBr·2H2O disappears gradually. When the temperature increases to 323.15 K, LiBr·2H2O loses water and changes into LiBr·H2O, while NaBr·2H2O remains unchanged, but when the temperature increases to 348.15 K, NaBr·2H2O completely loses water and becomes NaBr.
As seen from Figure 9, different from those of ternary system LiBr–NaBr–H2O, the phase diagrams of ternary system LiBr–KBr–H2O at 273.15, 288.15, 298.15, 323.15, and 348.15 K are similar, and there is no solid solution or complex salt formation. The phase diagram consists of two isothermal univariate curves, two equilibrium solid-phase crystallization regions, and one invariant point. Among them, the isothermal univariate curves show basically the same trend, and the solubility of LiBr and KBr shows a certain increasing trend with the continuous increase in temperature. It is worth noting that with the increase in temperature, the phenomenon of LiBr·2H2O losing water to LiBr·H2O also exists in this ternary system.
Figure 9.
Comparison of equilibrium phase diagrams of ternary system LiBr–KBr–H2O at different temperatures (273.15 K,25 288.15 K,24 298.15 K,19 308.15 K,25 and 348.15 K).
As shown in Figure 10, the equilibrium phase diagrams of quaternary system LiBr–NaBr–KBr–H2O at 288.15 and 298.15 K have some similarities; that is, the phase diagrams contain five isothermal univariate curves, two invariant points, and four equilibrium solid-phase crystallization regions, and neither of them has solid solution or double salt formation. Different from the above-mentioned two temperatures, the equilibrium phase diagram of this quaternary system at 348.15 K only contains three isothermal univariate curves, one invariant point, and three equilibrium solid-phase crystallization regions. Moreover, with the increase in temperature, the solid-phase crystallization regions of LiBr·2H2O and NaBr·2H2O disappear which is consistent with the change rule of its ternary subsystems. In addition, compared with 288.15 and 298.15 K, the solid-phase crystallization region of LiBr·2H2O in this quaternary system completely disappears at 348.15 K and is replaced by the solid-phase crystallization region of LiBr·H2O which is consistent with the temperature of dehydration transition of the LiBr hydrate.27 It can be seen from the comparison of the above-mentioned three systems at different temperatures that the solubility of each salt shows a certain increasing trend with the increase in temperature in which the solubility of LiBr is significantly higher than that of NaBr and KBr, indicating that LiBr is more difficult to crystallize and precipitate from the saturated solution than NaBr and KBr under both high- and low-temperature conditions.
4. Conclusions
As one of the important liquid mineral resources in China, the Nanyishan oilfield water resources which are rich in lithium, potassium, and bromine in the western Qaidam Basin of Qinghai Province are not only rich in reserves but also excellent in quality, with broad prospects for comprehensive development and utilization. According to the composition characteristics of this brine, the stable-phase equilibria of quaternary LiBr–NaBr–KBr–H2O and its ternary subsystem LiBr–NaBr–H2O and LiBr–KBr–H2O containing lithium, potassium, and bromine were carried out at 348.15 K, and the solubility data at the corresponding temperature were obtained. The ternary systems LiBr–NaBr–H2O and LiBr–KBr–H2O have no complex salt or solid solution formation. The phase diagram consists of one invariant point, two isothermal univariate curves, and two equilibrium solid-phase crystallization regions. The quaternary system LiBr–NaBr–KBr–H2O also has no double salt or solid solution formation, and the phase diagram contains only one invariant point, three isothermal univariate curves, and three equilibrium solid-phase crystallization regions. The comparison of the above-mentioned three systems at different temperatures shows that compared with NaBr and KBr, LiBr is more difficult to crystallize and precipitate from the saturated solution under either low- or high-temperature conditions. The research results of this paper not only supplement the basic thermodynamic data of the complex multitemperature system of Nanyishan oilfield water but also provide important theoretical guidance for the comprehensive development and utilization of this brine resource and the same type of brine resource.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (no. 41903051), the Natural Science Foundation of Qinghai Province (no. 2022-ZJ-965Q), “Thousand Talents Program for High-end Innovative Talents” of Qinghai Province and CAS “Light of West China” Program, and Thousand Talents Plan in Qinghai.
The authors declare no competing financial interest.
References
- Li W.; Dong Y. P.; Song P. S.. The Development and Utilization of Salt Lake Brine Resources; Chemical Industry Press: Beijing, 2012. [Google Scholar]
- Song P. S.; Yao Y.; Sun B.; Li W. Pitzer Model of Thermodynamics for the Li+, Na+, K+, Mg2+/Cl–, SO42–-H2O System. Sci. Sin.: Chim. 2010, 40, 1286–1296. [Google Scholar]
- Zheng X. Y.; Zhang M. G.; Xu C.; Li B. X.. Salt Lake Annals of China; Science Press: Beijing, 2002. [Google Scholar]
- Usdowski E.; Dietzel M.. Atlas and Data of Solid-Solution Equilibria of Marine Evaporites; Springer Verlag: Berlin, 1998. [Google Scholar]
- Yao Y.; Song P. S.; Li J. Thermodynamics and Phase Equilibria of the Salt Lake Brine System at 25 °C. Prog. Chem. 2000, 12, 255–267. [Google Scholar]
- Song P. S. The Phase Diagram of Salt-Water Systems and Utilization of Salt Lake Resources. J. Salt Lake Res. 2016, 24, 35–49. [Google Scholar]
- Peng-Sheng S.; Wu L.; Bai S.; Zhen N.; Ling-Zhong B.; Yun-Sheng W. Recent Development on Comprehensive Utilization of Salt Lake Resources. Chin. J. Inorg. Chem. 2011, 27, 801–815. [Google Scholar]
- Sun B.; Song P. S.; Li W.; Guo L. J. Thermodynamics and Phase Equilibria of the Oil Field Brine with Sr System at 25 °C I. Sr, Na, K, Li//Cl-H2O system. J. Salt Lake Res. 2015, 23, 50–58. [Google Scholar]
- Bi Y. J.; Sun B.; Zhao J.; Song P. S.; Li W. Phase Equilibrium in Ternary System SrCl2-CaCl2-H2O at 25 °C. Chin. J. Inorg. Chem. 2011, 27, 1765–1771. [Google Scholar]
- Ding X. P.; Sun B.; Shi L. J.; Yang H. T.; Song P. S. Study on Phase Equilibria in NaCl-SrCl2-H2O Ternary System at 25 °C. Inorg. Chem. Ind. 2010, 42, 9–10. [Google Scholar]
- Shi L. J.; Sun B.; Ding X. P.; Song P. S. Phase Equilibria in Ternary System KCl-SrCl2-H2O at 25 °C. Chin. J. Inorg. Chem. 2010, 26, 333–338. [Google Scholar]
- Yu X. P.; Wang Q.; Guo Y. F.; Deng T. L. Metastable Phase Equilibrium in the Reciprocal Quaternary System LiCl+MgCl2+Li2SO4+MgSO4+H2O at 348.15 K and 0.1 MPa. Chem. Res. Chin. Univ. 2018, 34, 798–802. 10.1007/s40242-018-7407-8. [DOI] [Google Scholar]
- Wang S. Q.; Guo Y. F.; Liu D. F.; Deng T. L. Phase equilibria in system LiCl-NaCl-H2O at 308 and 348 K. Russ. J. Phys. Chem. A 2016, 90, 2532–2537. 10.1134/s0036024416130161. [DOI] [Google Scholar]
- Wang S. Q.; Han X. N.; Jing Y.; Guo Y. F.; Zhao M. L.; Deng T. L. Phase Equilibria in the Ternary System (LiCl + Li2SO4 + H2O) at T = (288.15 and 308.15) K: Experimental Determination and Model Simulation. J. Chem. Eng. Data 2016, 61, 1155–1161. 10.1021/acs.jced.5b00805. [DOI] [Google Scholar]
- Wang S.; Guo Y.; Li D.; Zhao F.; Qiao W.; Deng T. Solid-Liquid Phase Equilibria in the Ternary Systems (LiCl + MgCl2 + H2O) and (Li2SO4 + MgSO4 + H2O) at 288.15 K. J. Chem. Eng. Data 2015, 60, 821–827. 10.1021/je500946w. [DOI] [Google Scholar]
- Meng L. Z.; Gruszkiewicz M.; Deng T. L.; Guo Y. F.; Li D. Isothermal Evaporation Process Simulation Using the Pitzer Model for the Quinary System LiCl-NaCl-KCl-SrCl2-H2O at 298.15 K. Ind. Eng. Chem. Res. 2015, 54, 8311–8318. 10.1021/acs.iecr.5b01897. [DOI] [Google Scholar]
- Meng L.; Wang X.; Li D.; Deng T. L.; Guo Y. F.; Yang L. Experimental Determination and Thermodynamic Modeling of Solid-Liquid Equilibria in the Quaternary System NaCl-KCl-SrCl2-H2O at 288.15 K. J. Chem. Eng. Data 2018, 63, 4410–4417. 10.1021/acs.jced.8b00510. [DOI] [Google Scholar]
- Deng T. L.; Li D. Solid–Liquid Metastable Equilibria in the Quaternary System (NaCl + LiCl + CaCl2 + H2O) at 288.15 K. J. Chem. Eng. Data 2008, 53, 2488–2492. 10.1021/je8000798. [DOI] [Google Scholar]
- Cui R. Z.; Li W.; Dong Y. P.; Li J. Phase Equilibrium and Phase Diagram for the Quaternary System LiBr-NaBr-KBr-H2O at 298.15 K. J. Chem. Eng. Data 2020, 65, 3021–3028. 10.1021/acs.jced.0c00048. [DOI] [Google Scholar]
- Cui R. Z.; Li W.; Dong Y. P.; Li J. Measured and Predicted Solubility Phase Diagrams of Quaternary Systems LiBr-NaBr-MgBr2-H2O and LiBr-KBr-MgBr2-H2O at 298.15 K. Chem. Res. Chin. Univ. 2020, 36, 1234–1240. 10.1007/s40242-020-0154-7. [DOI] [Google Scholar]
- Cui R. Z.; Zhang Y. M.; Dong Y. P.; Li W. Solid – Liquid equilibria of two quaternary systems LiBr-NaBr-Li2SO4-Na2SO4-H2O and LiBr-KBr-Li2SO4-K2SO4-H2O at 298.15 K. J. Chem. Thermodyn. 2022, 165, 106665. 10.1016/j.jct.2021.106665. [DOI] [Google Scholar]
- Guo L. R.; Nie G. L.; Cui R. Z.; Li W.; Zhang Y.-M. Solid-Liquid Equilibria in Reciprocal Quinary System Li+, Na+, K+/Br-, and SO42--H2O at 298.15 K. J. Chem. Eng. Data 2022, 67, 1500–1512. 10.1021/acs.jced.2c00030. [DOI] [Google Scholar]
- Fosbøl P. L.; Thomsen K.; Stenby E. H. Reverse Schreinemakers method for experimental analysis of mixed-solvent electrolyte systems. J. Solution Chem. 2009, 38, 1–14. 10.1007/s10953-008-9353-4. [DOI] [Google Scholar]
- Qi X.-Y.; He C. X.; Sang S. H.; Liu J.; Gao Y.-Y. Solid-liquid equilibria in the quaternary system LiBr-NaBr-KBr-H2O and its two ternary subsystems at 288.15 K. Asia-Pac. J. Chem. Eng. 2021, 16, e2595 10.1002/apj.2595. [DOI] [Google Scholar]
- Ye C.; Wu Z. Z.; Sang S. H.; Qi X. Y.; Liu X. Solid-Liquid Phase Equilibria of Ternary System KBr-LiBr-H2O at 273 and 308 K. J. Chem. Eng. Data 2019, 64, 5288–5294. 10.1021/acs.jced.9b00517. [DOI] [Google Scholar]
- Sang S. H.; Zhang H. Z.; Jiang P. H.; Zhao L. R.; Cui R. Z. Studies on Phase Equilibria of the Quaternary System LiBr-KBr-SrBr2-H2O and Ternary System LiBr-NaBr-H2O at 323 K. J. Chem. Eng. Data 2022, 67, 500–509. 10.1021/acs.jced.1c00646. [DOI] [Google Scholar]
- Duvall K. N.; Dirksen J. A.; Ring T. A. Ostwald-meyers metastable region in LiBr crystallization-comparison of measurements with predictions. J. Colloid Interface Sci. 2001, 239, 391–398. 10.1006/jcis.2001.7619. [DOI] [PubMed] [Google Scholar]