Abstract
Since 2010, five new antineoplastic therapies have been FDA approved for the treatment of metastatic prostate cancer. With additional treatment options, questions arose about the optimal sequence of these agents. Until recently, chemotherapy has been deferred until later in the disease course in favor of next-generation androgen deprivation therapy. Prior to the development of abiraterone acetate and enzalutamide, clinical trials were opened investigating the combination of chemotherapy with androgen deprivation therapy in patients with metastatic hormone-sensitive disease. With the development of new oral therapies used to treat castration-resistant disease, these trials were largely forgotten or felt to be obsolete. Recently, two trials have been reported showing an overall survival benefit of the early use of chemotherapy in patients with hormone-naive prostate cancer, changing the treatment paradigm for metastatic disease. Here we review the history of chemotherapy in treating prostate cancer and the emerging evidence favoring its use as first-line therapy against metastatic hormone-sensitive disease.
Keywords: Docetaxel, Hormone sensitive, Androgen deprivation therapy
Introduction
Prostate cancer is the most common non-skin cancer in men with over 180,000 new cases expected to be diagnosed in 2016.1 Local therapies for early-stage disease are effective leading to favorable clinical outcomes. When metastatic disease develops, patients are initially treated with androgen deprivation therapy (ADT). Although response rates to ADT are near 80%, inevitably, the cancer learns to grow in a low testosterone environment, leading to a clinical state of castration resistance. Docetaxel chemotherapy was among the first treatments to be Food and Drug Administration (FDA) approved for metastatic castration-resistant prostate cancer (mCRPC) based on a survival benefit. When docetaxel became the standard-of-care (SOC) treatment for mCRPC in 2004, several trials were subsequently launched to assess the effect of docetaxel in metastatic hormone-naive disease, but it would be a decade before the data matured. During the years when the trials were ongoing, preclinical research focused on mCRPC and reaffirmed that androgen receptor (AR) signaling continued to drive tumor growth upon developing castration resistance. The next generation of ADT (i.e. abiraterone acetate, enzalutamide) was then developed and shown to prolong survival in mCRPC patients. AR-targeted therapies gained favor over chemotherapy based on perceived safety profile and novelty, a trend many clinicians felt would continue. Docetaxel, and subsequently cabazitaxel, were then deferred until late-stage disease and trials involving chemotherapy in hormone-sensitive disease were all but forgotten.
Recently, these chemotherapy trials in patients with metastatic hormone-naive prostate cancer have been published. The long-awaited evidence favored the use of chemotherapy with ADT, which has changed the treatment paradigm for metastatic prostate cancer. Here we review the history of chemotherapy in prostate cancer and discuss the evidence for its use as frontline therapy in combination with ADT for metastatic disease.
History of chemotherapy in patients with mCRPC
After the pioneering work of Charles Huggins showed an inhibitory effect of testosterone suppression on prostate cancer growth, the history of treatment for metastatic prostate cancer began with surgical castration (i.e. orchiectomy).2 In the 1960s, drug therapy evolved after the Veterans Administration Cooperative Urologic Research Group (VACURG) demonstrated that oral estrogen, in the form of diethylstilbestrol, was as effective as orchiectomy in treating prostate cancer.3 Although orchiectomy remained the gold standard of treatment through the 1980s, the VACURG findings fostered the notion of medical castration as an alternative to orchiectomy. Flutamide, an oral, non-steroidal antiandrogen was then developed, which could inhibit AR signaling through receptor blockade. During the 1970s, flutamide showed clinical efficacy against prostate cancer.4 Subsequently, the structure of luteinizing hormone-releasing hormone (LHRH) was identified. In 1980, intranasal buserelin was administered to the first patient with prostate cancer, which resulted in a significant decrease in serum testosterone.5 However, a transient rise in serum androgens was noted which caused concern for possible disease flare. The combination of an antiandrogen (flutamide) with an LHRH agonist (LHRH ethylamide [HOE-766]) prevented this flare phenomenon, resulting in a decrease in prostatic acid phosphatase levels and symptomatic improvement in 9 of 10 patients.6 In the decades that followed, several generations of antiandrogens (nilutamide, bicalutamide) and LHRH agonists (leuprolide, goserelin, triptorelin) have been used to treat prostate cancer. Androgen suppression via LHRH agonists has since remained the backbone of therapy for metastatic hormone-sensitive disease. Once progression occurs on AR targeted therapies, treatment options prior to 2004 were limited. This led several investigators to test different therapeutic approaches, including chemotherapy, in patients with mCRPC.
Clinical trials involving the use of cytotoxic chemotherapy in advanced prostate cancer began in the 1950s with alkylating agents. The response rates to chemotherapy were typically low and results were difficult to interpret due to the small number of patients and lack of meaningful endpoints.7 This prompted many thought leaders in the field to suggest that prostate cancer was a chemotherapy-resistant disease.7–9 Due to the perceived lack of survival benefit, chemotherapy trials were then designed to assess palliation endpoints. In 1996, mitoxantrone in combination with prednisone was the first FDA-approved chemotherapy for mCRPC based on symptomatic improvement.10 Mitoxantrone is a topoisomerase II inhibitor given intravenously (12 mg/m2) on a 3-week schedule. Side effects range from mild (nausea, alopecia) to more severe (dose-dependent cardiomyopathy). Prostate-specific antigen (PSA) responses were low and no survival benefit was shown.10–12 Although mitoxantrone achieved palliation benefits, there was no chemotherapeutic agent that improved overall survival in prostate cancer until 2004.
Docetaxel and paclitaxel were developed in the early 1990s.13 In prostate cancer, preclinical data suggested a potential synergistic effect of estramustine and docetaxel.14 Several early-phase trials found the combination to be safe and clinically active against castration-resistant prostate cancer.15–17 SWOG9916 was a randomized Phase III trial in mCRPC patients, which compared docetaxel + estramustine to mitoxantrone with both arms containing a steroid backbone. The 21-day cycle of docetaxel (60 mg/m2 given on Day 2) with estramustine (280 mg three times daily on Days 1–5) significantly increased the median overall survival by 1.9 months and included improved PSA and objective responses.18 Due to concerns about increased thromboembolic events caused by estramustine, docetaxel was studied as a single agent and shown to have activity in advanced prostate cancer.19,20
Tannock et al., in the pivotal Phase III TAX327 trial involving mCRPC patients, showed a 2.4-month increase in overall survival in the docetaxel (given every 3 weeks) + prednisone arm compared with mitoxantrone + prednisone.21 Docetaxel (75 mg/m2) given every 3 weeks also had a significant survival advantage over weekly docetaxel (30 mg/m2). Use of docetaxel either weekly or every 3 weeks had increased adverse events (peripheral edema, neuropathy, gastrointestinal toxicities) compared with mitoxantrone, but significant reductions in pain and improved quality of life measures were noted. Although use of single-agent docetaxel was FDA approved in 2004 and most clinicians stopped using estramustine, it was not until 2008 that docetaxel was compared head-to-head with docetaxel + estramustine. One hundred and fifty men with mCRPC were randomly assigned to docetaxel (35 mg/m2 on Days 2 and 9 every 21 days) and prednisone (10 mg daily) with or without estramustine (280 mg on Days 1–5 and 8–12 every 21 days), which resulted in no difference in PSA response or overall survival.22 The combination also resulted in increased toxicities confirming the clinical practice to use docetaxel as a single agent. Both SWOG9916 and TAX327 are credited with establishing docetaxel given on a 3-week schedule as first-line chemotherapy in metastatic prostate cancer after developing disease progression on conventional hormonal therapy.
The exact mechanism of action of docetaxel in prostate cancer is unclear. Docetaxel is generally known to stabilize microtubules preventing cellular division and resulting in cell-cycle arrest. However, more recent evidence suggests that docetaxel can inhibit the nuclear translocation of AR in prostate cancer.23 Resistance to docetaxel may develop through several mechanisms including drug efflux, alterations in microtubule structure, activation of survival pathways, and changes in the tumor vasculature.24–27 In an effort to overcome resistance, investigators looked at combining targeted therapies with docetaxel. Multi-tyrosine kinase inhibitors and VEGF-targeted therapies did not show significant efficacy in several trials against prostate cancer.28–32 Although pilot studies suggested thalidomide may have added benefit when given with docetaxel, a randomized Phase III trial with lenalidomide did not increase survival.33–35 Natural products (i.e. calcitriol, vitamin D analogues), small molecule inhibitors, and vaccination have not improved upon the survival advantage of docetaxel alone.36–43 With no clear combination strategy evident, attempts were made to identify a second-line chemotherapy for castration-resistant prostate cancer. Several Phase II studies explored combinations of platinum agents with and without antimetabolites, but without a larger Phase III study, these treatments have not been clinically utilized.44–46 It would not be until 2010 that a chemotherapy would become FDA approved for mCRPC following progression on docetaxel.
Cabazitaxel, a semi-synthetic member of the taxane family, was developed as a derivative of docetaxel. Two potential advantages of cabazitaxel over docetaxel are: (1) the diminished affinity for P-glycoprotein, an important drug efflux pump and (2) the ability to cross the blood–brain-barrier.47 In the Phase III trial, TROPIC, men with mCRPC and prior exposure to a docetaxel-containing regimen were randomized to receive cabazitaxel (25 mg/m2 every 3 weeks) or mitoxantrone (12 mg/m2 every 3 weeks), with both groups receiving prednisone. Cabazitaxel significantly improved overall survival by 2.4 months, which was the primary endpoint of the study.48 With additional follow-up, 2-year survival was also improved in the cabazitaxel group with similar palliation benefits observed when compared with mitoxantrone.49 Based on the results from TROPIC, cabazitaxel became the FDA approved, second-line chemotherapy after progression on docetaxel. Mitoxantrone was relegated to third-line chemotherapy.
Despite advances in chemotherapy for castration-resistant disease, researchers continued to focus their efforts on targeting the AR pathway based on the observation that prostate cancer, following progression on conventional ADT, still continued to have an intact AR signaling axis.50 Subsequently, therapeutic agents that attenuated AR signaling via alternative mechanisms were explored. Inhibitors of extratesticular androgen synthesis, including ketoconazole and abiraterone acetate, suppressed androgen production from the adrenal glands. Ketoconazole can inhibit adrenal androgen synthesis via its effect on multiple cytochrome p450 enzymes and was shown to decrease PSA levels in patients with prostate cancer.51 However, the ability of ketoconazole to inhibit cytochrome P450 (CYP) – 3A may result in significant drug interactions, particularly with psychotropic medications.52 Abiraterone acetate is an irreversible inhibitor of the CYP-17, a key enzyme in androgen biosynthesis. After initially being FDA approved for use in mCRPC patients following chemotherapy, abiraterone acetate (1000 mg by mouth daily with prednisone 5 mg twice daily) demonstrated a survival benefit versus placebo (prednisone alone) in patients without prior docetaxel exposure.53 Side effects of abiraterone acetate include mineralocorticoid excess (i.e. fluid retention, hypokalemia, hypertension), liver function test elevation, and cardiac abnormalities such as tachycardia and atrial fibrillation. Enzalutamide (160 mg by mouth daily), a second-generation antiandrogen, similarly received FDA approval for the treatment of mCRPC in both the pre- and post-chemotherapy setting after demonstrating an increase in overall survival.54,55 The most common adverse event reported was fatigue in half of the patients. The enthusiasm for an orally administered drug such as abiraterone acetate and enzalutamide is offset by the cost, which is estimated to be approximately $20,000 USD per 90-day supply for each. However, the clinical development and implementation of abiraterone acetate and enzalutamide supported the notion that next-generation targeting of AR signaling would further defer the use of chemotherapy for mCRPC, particularly in an older population of prostate cancer patients.
Radiopharmaceutical treatment and immunotherapy have also been shown to prolong survival in select populations of mCRPC patients. A calcium mimic, Radium-223, is an alpha-emitting radioisotope which is readily taken up at sites of osseous metastases. In patients with bone-only disease, previously treated with docetaxel or chemotherapy ineligible, Radium-223 led to an increase in overall survival versus placebo.56 Radium-223 is not indicated for patients with lymph node and/or visceral metastases. Sipuleucel-T is an autologous dendritic cell vaccine, which is administered following dendritic cell priming with a GM-CSF-prostatic acid phosphatase fusion protein.57 In mCRPC patients with asymptomatic or minimally symptomatic disease, sipuleucel-T administration led to a 4.1-month increase in median survival versus placebo.58 Whereas chemotherapy and AR-directed therapy are more broadly used in the treatment of mCRPC, both Radium-223 (bone-only) and sipuleucel-T (asymptomatic/minimally symptomatic) are only FDA approved for select mCRPC patients who meet the eligibility criteria.
Evidence for early use of chemotherapy
With the recent development of new drugs for mCRPC, a focus of clinical trials and retrospective analyses has been on the most effective sequence of therapies. To date, several retrospective studies have provided little guidance on the proper timing of chemotherapy versus next-generation ADT in patients with mCRPC.59–63 The sequence of abiraterone acetate, enzalutamide and chemotherapy largely depends on patient characteristics, side-effect profile, as well as patient and provider preference (Fig. 1).
Fig. 1.
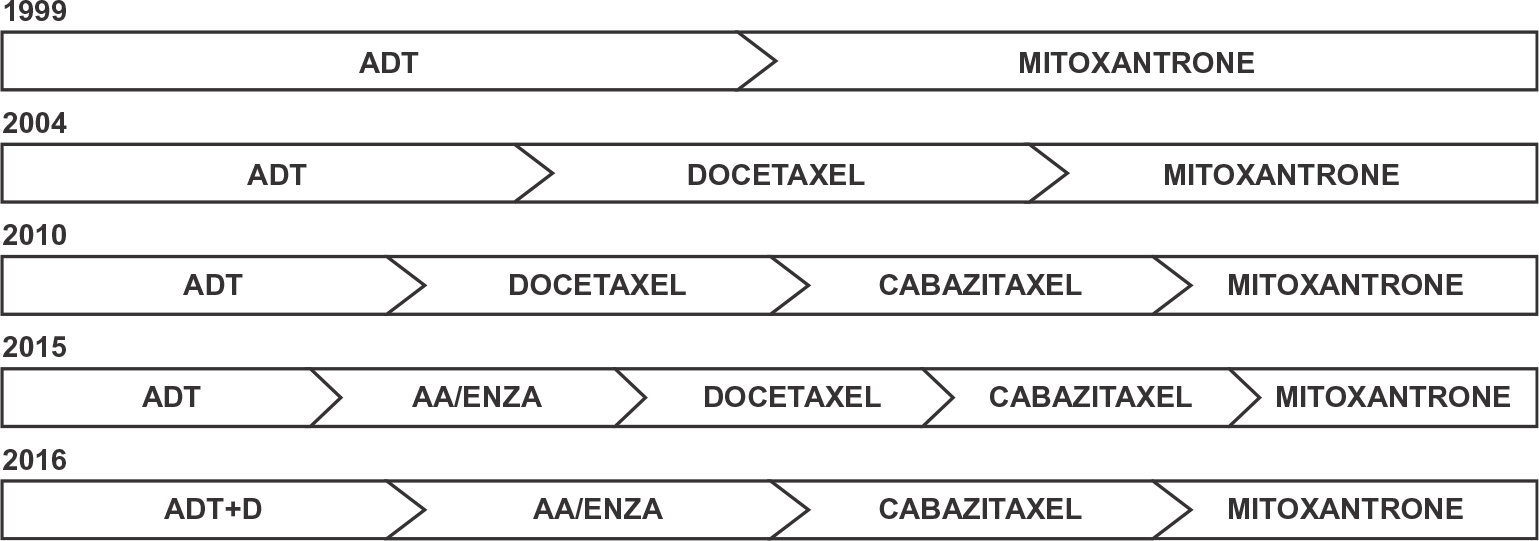
History of treatment paradigms for metastatic prostate cancer. The treatment of metastatic prostate cancer has evolved since 1999 with the development of new therapeutic agents. Recent evidence now favors the use of chemotherapy early in the course of treatment for metastatic hormone-sensitive prostate cancer. D = Docetaxel, AA = Abiraterone Acetate, Enza = Enzalutamide.
Until recently, ADT alone had been the gold standard of treatment for metastatic, hormone-naive prostate cancer. Given FDA approval of docetaxel in 2004 and no other known life-prolonging therapies at the time, a number of trials were launched investigating the combination of ADT with docetaxel in patients with newly metastatic disease. Following the waves of new drugs for mCRPC, these trials were easy to forget and almost rendered obsolete. The conventional wisdom was to incorporate these new agents at the time of castration resistance pushing chemotherapy further down in the lines of treatment. Chemotherapy would then be considered following the use of abiraterone acetate and/or enzalutamide. Three clinical trials (GETUG-AFU15, CHAARTED, and STAMPEDE) have challenged this paradigm looking at the effect of ADT with concurrent chemotherapy for metastatic hormone-naive disease. Investigators hypothesized that chemotherapy may be better tolerated earlier in the disease course rather than in the latter stages of disease when the performance status tends to be worse. Moreover, the cytotoxic effect of chemotherapy may enhance ADT-mediated apoptosis resulting in synergistic cell kill. As these trials matured, the findings identified a clinical benefit of using chemotherapy in hormone-sensitive disease and forced providers to reexamine how metastatic prostate cancer is treated. We seek to describe the results of each trial and how the field of chemo-hormonal therapy has since evolved (Table 1).
Table 1.
Overall survival of patients with metastatic prostate cancer treated with chemohormonal therapy versus androgen deprivation therapy alone in three clinical trials.
| Clinical trial | ADT + chemo (months) | ADT (months) | HR (95% Cl) | P-value | Date published |
|---|---|---|---|---|---|
|
| |||||
| GETUG-AFU 15 | 58.9 | 54.2 | 1.01 (0.75–1.36) | 0.955 | 2013 |
| CHAARTED | 57.6 | 44 | 0.61 (0.47–0.80) | <0.001 | 2015 |
| STAMPEDE | 81 | 71 | 0.78 (0.66–0.93) | 0.006 | 2015 |
In 2004, the first Phase III trial, GETAUG-AFU15, began accruing patients in an attempt to answer the question of whether chemotherapy when combined with ADT could affect survival in patients with newly metastatic prostate cancer. The trial randomized 385 men with metastatic prostate cancer to receive ADT alone or ADT + docetaxel (75 mg/m2 every 3 weeks, up to 9 cycles) with a primary endpoint of median overall survival.64 In 2013, the trial results showed a non-significant increase in median overall survival of 4.7 months with the addition of chemotherapy in patients with metastatic hormone-naive disease. Although progression-free survival was significantly increased with a trend towards improved overall survival in the chemotherapy group, the trial was viewed as a negative study. At the time, the lack of perceived benefit from early chemotherapy fit with conventional wisdom.
In 2014, the initial results from the randomized, Phase III CHAARTED trial were reported at the annual American Society of Clinical Oncology (ASCO) meeting, which contradicted the GETUG-AFU15 findings. Opened in 2006, CHAARTED (E3805) accrued a total of 790 men with metastatic hormone-naive disease who were randomized to ADT or ADT + docetaxel (75 mg/m2 every 3 weeks for 6 cycles).65 The trial was originally designed to look at men with high-volume disease only, defined as visceral metastases and/or four or more bone metastases. During accrual, an amendment was approved which allowed men with low volume disease to enroll. Median overall survival was significantly increased by 13.6 months in the group receiving docetaxel together with ADT. This survival increase was largely driven by the effect seen in the high-volume disease cohort, which represented approximately 2/3 of all patients participating in the trial. The high-volume group had a 17-month increase in overall survival with the addition of chemotherapy to conventional ADT. The median overall survival in the low-volume disease cohort was not reached in either arm of the study (Table 2). Since the high-volume cohort was accrued earlier and had poorer outcomes, the effect on survival was readily apparent at the time of analysis. Given that the survival advantage of ADT + docetaxel remained significant in all patients on study, low-volume patients are still likely to benefit from the combination. However, the magnitude of benefit is potentially less and may become significant with further follow-up. Although the findings of the CHAARTED trial were impressive, the negative results in the GETUG-AFU15 trial led investigators to critically analyze both trials in order to get clarification of the role of early chemotherapy.
Table 2.
Overall survival of patients with low- and high-volume metastatic prostate cancer treated with chemohormonal therapy versus androgen deprivation therapy alone.
| Clinical trial | Disease volume | ADT + chemo (months) | ADT (months) | HR (95% CI) | P-value | Follow-up (months) |
|---|---|---|---|---|---|---|
|
| ||||||
| GETUG-AFU 15 | High | 39.8 | 35.1 | 0.78 (0.56–1.09) | 0.14 | 83.9 |
| Low | NR | 83.4 | 1.02 (0.67–1.55) | 0.9 | ||
| CHAARTED | High | 49.2 | 32.2 | 0.60 (0.45–0.81) | <0.001 | 28.9 |
| Low | NR | NR | 0.60 (0.32–1.13) | 0.11 | ||
The first key issue with GETUG-AFU15 is the lack of statistical power to detect a survival difference. Seven hundred and ninety patients were randomized in CHAARTED compared with only 385 in GETUG-AFU15. Had GETUG-AFU15 accrued more patients, the overall survival difference with chemo-hormonal therapy may have been significant. Irrespective of the statistical concerns, the GETUG-AFU15 trial findings hinted at a benefit of adding docetaxel to ADT, which is consistent with the CHAARTED trial. A second issue with these trials is the difference in the number of high-volume disease patients. In GETUG-AFU15, less than half of patients randomized were considered to have high-volume disease versus nearly two thirds in CHAARTED. Since high-volume patients had the most benefit from chemo-hormonal therapy, having a larger proportion of these patients in CHAARTED increased the likelihood of achieving a survival difference. Gravis et al., did a post hoc analysis of the GETUG-AFU15 trial, subdividing patients into low- and high-volume disease to better mirror the population in the CHAARTED study (Table 2).66 In retrospect, a non-significant improvement in overall survival was noted in the high-volume group of patients receiving chemotherapy with ADT. However, the lack of statistical power and retrospective analysis make further interpretation difficult. One minor difference between trials is that fewer patients discontinued docetaxel due to toxicity in the CHAARTED trial, which may have led to more favorable outcomes. After all issues were considered, many practitioners favored the results of the CHAARTED study over GETUG-AFU15. However, some still sought clarification before giving chemotherapy to patients with hormone-naive prostate cancer.
A third trial, STAMPEDE, was key to settling the debate about the role of early chemotherapy in metastatic hormone-naive prostate cancer. In STAMPEDE, nearly 3000 men with high-risk locally-advanced or metastatic prostate cancer were randomized to SOC (i.e. ADT), SOC + zolendronic acid, SOC + docetaxel, or SOC + docetaxel + zolendronic acid.67 The findings were reported at the 2015 ASCO meeting and showed a 10-month increase in overall survival with the addition of chemotherapy to SOC (HR for death: 0. 78, P = 0.006) compared with SOC alone. In STAMPEDE, volume of disease was not pre-specified, supporting the CHAARTED conclusion that docetaxel will benefit patients with both high- and low-volume disease. The results of STAMPEDE favor the early use of chemotherapy in combination with ADT in metastatic prostate cancer.
Future directions
Although the use of early docetaxel in the treatment of prostate cancer is becoming more commonplace, clinical questions still remain. For instance, in the above three trials, patients were generally younger (median age < 65 years old) with an excellent performance status. In real-world practice, many patients with newly metastatic disease are older and less physically fit. These trials do not answer the question of how to treat elderly patients in this context. Older patients or those with a poorer functional status may still benefit from chemotherapy with ADT, but concerns about safety are significant and must be considered. In such patients, volume of disease may be considered since those with high-volume disease may benefit the most from chemotherapy.
With respect to volume of disease, many providers remain apprehensive about treating low-volume disease patients with docetaxel. In CHAARTED, the addition of chemotherapy to ADT prolonged survival in patients with metastatic prostate cancer, with a trend towards improved overall survival in a subgroup analysis of those patients with low-volume disease. Should this trend in improved survival remain not statistically significant with additional follow-up, we must defer to the findings of STAMPEDE which did not pre-specify volume status as an inclusion criteria and prospectively supports the use of chemohormonal therapy in all M1 patients. However, we may need to reconsider the current definition of low- and high-volume disease to better capture those low-volume patients more likely to benefit from the addition of chemotherapy. For instance, certain oligometastatic prostate cancer may respond more favorably to local therapies such as stereotactic body radiation therapy with curative intent.68 The remaining patients with low-volume, metastatic disease may be more effectively treated with a palliative, systemic approach (i.e. ADT + docetaxel).
The role of cabazitaxel in metastatic hormone-naive disease is yet to be determined. FIRSTANA was a Phase III trial comparing cabazitaxel to docetaxel in patients with chemotherapy-naive mCRPC. The results of FIRSTANA were recently reported at the 2016 ASCO meeting, which showed that cabazitaxel was not superior to docetaxel for overall survival.69 However, cabazitaxel given at 25 mg/m2 yielded a significant improvement in tumor response compared to docetaxel. Consideration may be given to testing cabazitaxel + ADT in patients with metastatic hormone-sensitive disease. Currently, cabazitaxel should not be used in this context.
Following chemohormonal therapy in hormone sensitive metastatic disease, it remains to be determined whether retreatment with docetaxel will yield clinical benefit upon developing castration resistance. A practical approach for these patients with mCRPC is to consider docetaxel retreatment following progression on second-generation ADT. However, patients should be carefully followed after each cycle and treatment could be changed to cabazitaxel at first signs of progression. Prospective data will be needed to determine whether docetaxel retreatment or use of cabazitaxel following disease progression on second generation ADT is a more favorable therapeutic strategy.
The optimal sequence of therapies for metastatic prostate cancer remains unknown. The recent trials involving docetaxel + ADT in metastatic hormone-naive prostate cancer supports its use in properly selected patients and also shows that the SOC treatment is constantly being challenged and subject to change. Several clinical trials are ongoing looking at various combinations of chemohormonal and androgen deprivation therapies (Table 3). As the data from these trials are published and the findings of CHAARTED and STAMPEDE mature, we should expect further changes in how we treat metastatic, hormone sensitive prostate cancer. Both researchers and clinicians must keep an open mind not only to the type of therapy available, but also where it may be best utilized within the treatment paradigm.
Table 3.
Ongoing clinical trials enrolling patients with metastatic, hormone sensitive prostate cancer.
| Clinical Trials for Metastatic Hormone Sensitive Prostate Cancer |
|---|
|
|
| Chemotherapy |
| ODM-201 in Addition to Standard ADT and Docetaxel in Metastatic Castration Sensitive Prostate Cancer – NCT02799602 |
| Docetaxel and PROSTVAC for Metastatic Castration-Sensitive Prostate Cancer – NCT02649855 |
| A Phase III of ADT ± Docetaxel ± Local RT ± Abiraterone Acetate in Metastatic Hormone-naïve Prostate Cancer (PEACE1) – NCT01957436 |
| AR Targeted Therapies |
| A Study of Enzalutamide Plus Androgen Deprivation Therapy (ADT) Versus Placebo Plus ADT in Patients With Metastatic Hormone Sensitive Prostate Cancer (mHSPC) – NCT02677896 |
| Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer (ENZAMET) – NCT02446405 |
| Dose Finding Study of BKM 120 in Combination With LH-RH Agonists and Bicalutamide in Men With Non Castrate Metastatic Prostate Cancer (NCMPC-BKM) – NCT02487823 |
| LHRH Analogue Therapy With Enzalutamide or Bicalutamide in Treating Patients With Metastatic Hormone Sensitive Prostate Cancer – NCT02058706 |
| Enzalutamide Versus Standard Androgen Deprivation Therapy for the Treatment Hormone Sensitive Prostate Cancer – NCT02278185 |
| A Study of Apalutamide (JNJ-56021927, ARN-509) Plus Androgen Deprivation Therapy (ADT) Versus ADT in Participants With mHSPC (TITAN) – NCT02489318 |
| A Phase II Study of Androgen Deprivation Therapy With or Without Palbociclib in RB-Positive Metastatic Prostate Cancer – NCT02059213 |
| Bicalutamide and Goserelin or Leuprolide Acetate With or Without Cixutumumab in Treating Patients With Newly Diagnosed Metastatic Prostate Cancer – NCT01120236 |
| S1216, Phase III ADT + TAK-700 vs. ADT + Bicalutamide for Metastatic Prostate Cancer (S1216) – NCT01809691 |
| Ipilimumab + Androgen Deprivation Therapy in Prostate Cancer – NCT01377389 |
Acknowledgments
The project described was supported by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins NIH Grants P30 CA006973. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflict of interests statement
The authors were solely responsible for the development of content for this manuscript. Formatting and proofing services were provided by MediTech Media, funded by Sanofi Genzyme. The authors have no conflicts of interest to report.
Disclosures
The authors were solely responsible for the development of content for this manuscript. Formatting and proofing services were provided by MediTech Media, funded by Sanofi Genzyme.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol 2002;168:9–12. [DOI] [PubMed] [Google Scholar]
- [3].Treatment and survival of patients with cancer of the prostate. The Veterans Administration Co-operative Urological Research Group. Surgery, Gynecology & Obstetrics 1967; 124: 1011–17. [PubMed] [Google Scholar]
- [4].Liao S, Howell DK, Chang TM. Action of a nonsteroidal antiandrogen, flutamide, on the receptor binding and nuclear retention of 5 alpha-dihydrotestosterone in rat ventral prostate. Endocrinology 1974;94:1205–9. [DOI] [PubMed] [Google Scholar]
- [5].Labrie F, Cusan L, Seguin C, et al. Antifertility effects of LHRH agonists in the male rat and inhibition of testicular steroidogenesis in man. Int J Fertility 1980;25:157–70. [PubMed] [Google Scholar]
- [6].Labrie F, Dupont A, Belanger A, et al. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med 1982;5:267–75. [PubMed] [Google Scholar]
- [7].Eisenberger MA, Simon R, O’Dwyer PJ, Wittes RE, Friedman MA. A reevaluation of nonhormonal cytotoxic chemotherapy in the treatment of prostatic carcinoma. J Clin Oncol: Official J Am Soc Clin Oncol 1985;3:827–41. [DOI] [PubMed] [Google Scholar]
- [8].Tannock IF. Is there evidence that chemotherapy is of benefit to patients with carcinoma of the prostate? J Clin Oncol: Official J Am Soc Clin Oncol 1985;3:1013–21. [DOI] [PubMed] [Google Scholar]
- [9].Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer 1993;71:1098–109. [DOI] [PubMed] [Google Scholar]
- [10].Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol: Official J Am Soc Clin Oncol 1996;14:1756–64. [DOI] [PubMed] [Google Scholar]
- [11].Kantoff PW, Halabi S, Conaway M, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol: Official J Am Soc Clin Oncol 1999;17:2506–13. [DOI] [PubMed] [Google Scholar]
- [12].Berry W, Dakhil S, Modiano M, Gregurich M, Asmar L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J Urol 2002;168:2439–43. [DOI] [PubMed] [Google Scholar]
- [13].Pazdur R, Kudelka AP, Kavanagh JJ, Cohen PR, Raber MN. The taxoids: paclitaxel (Taxol) and docetaxel (Taxotere). Cancer Treat Rev 1993;19:351–86. [DOI] [PubMed] [Google Scholar]
- [14].Kreis W, Budman DR, Calabro A. Unique synergism or antagonism of combinations of chemotherapeutic and hormonal agents in human prostate cancer cell lines. Br J Urol 1997;79:196–202. [DOI] [PubMed] [Google Scholar]
- [15].Petrylak DP, Macarthur RB, O’Connor J, et al. Phase I trial of docetaxel with estramustine in androgen-independent prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 1999;17:958–67. [DOI] [PubMed] [Google Scholar]
- [16].Kreis W, Budman DR, Fetten J, Gonzales AL, Barile B, Vinciguerra V. Phase I trial of the combination of daily estramustine phosphate and intermittent docetaxel in patients with metastatic hormone refractory prostate carcinoma. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 1999;10:33–8. [DOI] [PubMed] [Google Scholar]
- [17].Petrylak DP, Macarthur R, O’Connor J, et al. Phase I/II studies of docetaxel (Taxotere) combined with estramustine in men with hormone-refractory prostate cancer. Semin Oncol 1999;26:28–33. [PubMed] [Google Scholar]
- [18].Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. New Engl J Med 2004;351:1513–20. [DOI] [PubMed] [Google Scholar]
- [19].Picus J, Schultz M. Docetaxel (Taxotere) as monotherapy in the treatment of hormone-refractory prostate cancer: preliminary results. Semin Oncol 1999;26:14–8. [PubMed] [Google Scholar]
- [20].Friedland D, Cohen J, Miller R Jr, et al. A phase II trial of docetaxel (Taxotere) in hormone-refractory prostate cancer: correlation of antitumor effect to phosphorylation of Bcl-2. Semin Oncol 1999;26:19–23. [PubMed] [Google Scholar]
- [21].Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. New Engl J Med 2004;351:1502–12. [DOI] [PubMed] [Google Scholar]
- [22].Machiels JP, Mazzeo F, Clausse M, et al. Prospective randomized study comparing docetaxel, estramustine, and prednisone with docetaxel and prednisone in metastatic hormone-refractory prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 2008;26:5261–8. [DOI] [PubMed] [Google Scholar]
- [23].Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulintargeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res 2010;70:7992–8002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kyle AH, Huxham LA, Yeoman DM, Minchinton AI. Limited tissue penetration of taxanes: a mechanism for resistance in solid tumors. Clin Cancer Res: Official J Am Assoc Cancer Res 2007;13:2804–10. [DOI] [PubMed] [Google Scholar]
- [25].Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000;92:1295–302. [DOI] [PubMed] [Google Scholar]
- [26].Berrieman HK, Lind MJ, Cawkwell L. Do beta-tubulin mutations have a role in resistance to chemotherapy? Lancet Oncol 2004;5:158–64. [DOI] [PubMed] [Google Scholar]
- [27].Yamanaka K, Rocchi P, Miyake H, et al. Induction of apoptosis and enhancement of chemosensitivity in human prostate cancer LNCaP cells using bispecific antisense oligonucleotide targeting Bcl-2 and Bcl-xL genes. BJU Int 2006;97:1300–8. [DOI] [PubMed] [Google Scholar]
- [28].Tannock IF, Fizazi K, Ivanov S, et al. Aflibercept versus placebo in combination with docetaxel and prednisone for treatment of men with metastatic castration-resistant prostate cancer (VENICE): a phase 3, double-blind randomised trial. Lancet Oncol 2013;14:760–8. [DOI] [PubMed] [Google Scholar]
- [29].Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol: Official J Am Soc Clin Oncol 2012;30:1534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): a randomised, double-blind phase 3 trial. Lancet Oncol 2013;14:1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zurita AJ, George DJ, Shore ND, et al. Sunitinib in combination with docetaxel and prednisone in chemotherapy-naive patients with metastatic, castration-resistant prostate cancer: a phase 1/2 clinical trial. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 2012;23:688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Horti J, Widmark A, Stenzl A, et al. A randomized, double-blind, placebo-controlled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biotherapy Radiopharm 2009;24:175–80. [DOI] [PubMed] [Google Scholar]
- [33].Figg WD, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res: Official J Am Assoc Cancer Res 2001;7:1888–93. [PubMed] [Google Scholar]
- [34].Dahut WL, Gulley JL, Arlen PM, et al. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 2004;22:2532–9. [DOI] [PubMed] [Google Scholar]
- [35].Petrylak DP, Vogelzang NJ, Budnik N, et al. Docetaxel and prednisone with or without lenalidomide in chemotherapy-naive patients with metastatic castration-resistant prostate cancer (MAINSAIL): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol 2015;16:417–25. [DOI] [PubMed] [Google Scholar]
- [36].Hainsworth JD, Meluch AA, Spigel DR, et al. Weekly docetaxel and bortezomib as first-line treatment for patients with hormone-refractory prostate cancer: a Minnie Pearl Cancer Research Network phase II trial. Clin Genitourinary Cancer 2007;5:278–83. [DOI] [PubMed] [Google Scholar]
- [37].Sinibaldi VJ, Elza-Brown K, Schmidt J, et al. Phase II evaluation of docetaxel plus exisulind in patients with androgen independent prostate carcinoma. Am J Clin Oncol 2006;29:395–8. [DOI] [PubMed] [Google Scholar]
- [38].Sternberg CN, Dumez H, Van Poppel H, et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 2009;20:1264–9. [DOI] [PubMed] [Google Scholar]
- [39].Scher HI, Jia X, Chi K, et al. Randomized, open-label phase III trial of docetaxel plus high-dose calcitriol versus docetaxel plus prednisone for patients with castration-resistant prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 2011;29:2191–8. [DOI] [PubMed] [Google Scholar]
- [40].Attia S, Eickhoff J, Wilding G, et al. Randomized, double-blinded phase II evaluation of docetaxel with or without doxercalciferol in patients with metastatic, androgen-independent prostate cancer. Clin Cancer Res: Official J Am Assoc Cancer Res 2008;14:2437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Quinn DI, Tangen CM, Hussain M, et al. Docetaxel and atrasentan versus docetaxel and placebo for men with advanced castration-resistant prostate cancer (SWOG S0421): a randomised phase 3 trial. Lancet Oncol 2013;14:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Fizazi K, Higano CS, Nelson JB, et al. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 2013;31:1740–7. [DOI] [PubMed] [Google Scholar]
- [43].Harrop R, Chu F, Gabrail N, Srinivas S, Blount D, Ferrari A. Vaccination of castration-resistant prostate cancer patients with TroVax (MVA-5T4) in combination with docetaxel: a randomized phase II trial. Cancer Immunol, Immunother: CII 2013;62:1511–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buonerba C, Federico P, D’Aniello C, et al. Phase II trial of cisplatin plus prednisone in docetaxel-refractory castration-resistant prostate cancer patients. Cancer Chemother Pharmacol 2011;67:1455–61. [DOI] [PubMed] [Google Scholar]
- [45].Gasent Blesa JM, Giner Marco V, Giner-Bosch V, Cerezuela Fuentes P, Alberola Candel V. Phase II trial of oxaliplatin and capecitabine after progression to first-line chemotherapy in androgen-independent prostate cancer patients. Am J Clin Oncol 2011;34:155–9. [DOI] [PubMed] [Google Scholar]
- [46].Dorff TB, Tsao-Wei DD, Groshen S, et al. Efficacy of oxaliplatin plus pemetrexed in chemotherapy pretreated metastatic castration-resistant prostate cancer. Clin Genitourinary Cancer 2013;11:416–22. [DOI] [PubMed] [Google Scholar]
- [47].Paller CJ, Antonarakis ES. Cabazitaxel: a novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des, Dev Therapy 2011;5:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–54. [DOI] [PubMed] [Google Scholar]
- [49].Bahl A, Oudard S, Tombal B, et al. Impact of cabazitaxel on 2-year survival and palliation of tumour-related pain in men with metastatic castration-resistant prostate cancer treated in the TROPIC trial. Ann Oncol: Official J Eur Soc Med Oncol/ESMO 2013;24:2402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Small EJ, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol: Official J Am Soc Clin Oncol 2004;22:1025–33. [DOI] [PubMed] [Google Scholar]
- [52].Greenblatt DJ, Zhao Y, Venkatakrishnan K, et al. Mechanism of cytochrome P450–3A inhibition by ketoconazole. J Pharm Pharmacol 2011;63:214–21. [DOI] [PubMed] [Google Scholar]
- [53].Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. New Engl J Med 2013;368:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- [55].Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. New Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. New Engl J Med 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- [57].Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol 2010;10:580–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl J Med 2010;363:411–22. [DOI] [PubMed] [Google Scholar]
- [59].Maughan BL, Xhou XC, Suzman DL, et al. Optimal sequencing of docetaxel and abiraterone in men with metastatic castration-resistant prostate cancer. Prostate 2015;75:1814–20. [DOI] [PubMed] [Google Scholar]
- [60].Suzman DL, Luber B, Schweizer MT, Nadal R, Antonarakis ES. Clinical activity of enzalutamide versus docetaxel in men with castration-resistant prostate cancer progressing after abiraterone. Prostate 2014;74:1278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nadal R, Zhang Z, Rahman H, et al. Clinical activity of enzalutamide in Docetaxel-naive and Docetaxel-pretreated patients with metastatic castration-resistant prostate cancer. Prostate 2014;74:1560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Azad AA, Eigl BJ, Murray RN, Kollmannsberger C, Chi KN. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur Urol 2015;67:23–9. [DOI] [PubMed] [Google Scholar]
- [63].Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol 2014;65:30–6. [DOI] [PubMed] [Google Scholar]
- [64].Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol 2013;14:149–58. [DOI] [PubMed] [Google Scholar]
- [65].Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New Engl J Med 2015;373:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gravis G, Boher JM, Joly F, et al. Androgen Deprivation Therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol 2015. [DOI] [PubMed] [Google Scholar]
- [67].James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Singh D, Yi WS, Brasacchio RA, et al. Is there a favorable subset of patients with prostate cancer who develop oligometastases? Int J Radiat Oncol Biol Phys 2004;58:3–10. [DOI] [PubMed] [Google Scholar]
- [69].Sartor O, Oudard S, Sengelov L, Daugaard G, Saad F. Cabazitaxel vs docetaxel in chemotherapy-naive (CN) patients with metastatic castration-resistant prostate cancer (mCRPC): a three-arm phase III study (FIRSTANA). J Clin Oncol: Official J Am Soc Clin Oncol 2016;34. [Google Scholar]


