Abstract
Segmented filamentous bacteria (SFB) are autochthonous bacteria colonizing the ileum of many young animals by attaching to intestinal epithelial cells. These nonpathogenic bacteria strongly stimulate the mucosal immune system and induce intestinal epithelial cells to express major histocompatibility complex class II molecules. We tried to discover whether SFB are phagocytized and intracellularly processed by the host cells, which is indicative of antigen processing. The middle part of the ileum was extracted from 10- and 20-day-old broiler chicks (Gallus gallus domesticus). Samples were processed and examined by scanning and transmission electron microscopy (SEM and TEM, respectively). In SEM, no, few, medium, and dense SFB colonization levels were classified. In TEM of cells from animals with medium or dense SFB colonization levels, we could observe extracellular particles ranging from those only indenting the cell membrane to particles found in the cytoplasmatic area beyond the terminal web. These particles had a structural similarity with SFB that were floating freely in the intestinal lumen. Furthermore, we observed unlacing of the membrane and septum surrounding the extracellular particles and their incorporation into host cytoplasmatic components, which strongly suggests that these particles are phagocytized and intracellularly processed SFB. This conclusion is supported by TEM analysis of samples with no or few SFB, in which we failed to find these characteristic morphologies. The phagocytosis process described here could be an important trigger for the stimulating effect of SFB on the mucosal immune system.
Segmented filamentous bacteria (SFB) are known to be nonpathogenic, gram-positive, anaerobic, spore-forming bacteria that inhabit the intestinal tract. SFB are characterized by their long filamentous shape with defined septa between each segment and their attachment to epithelial cells. Although SFB have been reported in many animal species, an official taxonomic name has not been established as yet due to the lack of an in vitro culturing method. Using 16S ribosomal DNA analysis, the phylogenetic positions of SFB in mice, rats, and chickens have been reported as being closely related to each other and distantly related to members of the genus Clostridium (19). However, SFB of chickens are different from those of mice, because SFB of mice are longer, wider, and have slightly different morphological characteristics than those of chickens (1). Furthermore, SFB in chickens, rats, and mice are reported to be host specific (1, 24).
SFB adhere to intestinal epithelial cells with holdfasts, and filaments are usually found only at the ileal villus tip of young animals (4, 6, 10, 12, 16, 17). Recently, SFB have been reported to have a potential antagonistic effect against gastrointestinal infections (8). Observations by transmission electron microscopy (TEM) have revealed that the host cells to which SFB are anchored do not show any cytopathologic changes, and no inflammatory reactions have been observed in the lamina propria (6, 27). SFB are therefore not pathogenic. Nevertheless, SFB are the most potent indigenous bacteria to stimulate the intestinal immune system (2, 11, 23, 25, 26), intestinal motility (18), and the proliferation of intestinal epithelial cells (25) of germ-free mice to a physiologically normal state. The mechanism of this stimulation is unknown. SFB are able to colonize Peyer's patches although attachment to M cells is rare (9). In mice, intestinal epithelial cells start to express major histocompatibility complex class II molecules after SFB colonization (25), which suggests antigen uptake and processing by these cells. Although SFB are host specific (1, 24), there are at present no indications that host responses to SFB are different in mice or chickens. Attachment of SFB was recognized by a distorted cell membrane and a thickened and more electron-dense underlying area of the host cell (4, 7, 12, 16, 27), which has later been identified as an accumulation of actin filaments at the attachment site (9). These morphological changes indicate a definite host reaction and imply a cell-metabolic response.
The processes of adherence and the subsequent forming of new filaments are not understood. Previously, we observed that the bacterial membrane at its apex seems to undergo lysis, and this suggested a possibility that host cells may take up a part of SFB and digest it (27). Intracellular processing of phagocytized SFB might explain why these bacteria are such potent activators of the mucosal immune system. The data presented here strongly suggest that SFB are phagocytized and intracellularly processed by chicken epithelial cells.
Chicks and experimental design. One hundred ten newly hatched male broiler (Marshall Chunky) chicks (Gallus gallus domesticus) were obtained from a commercial hatchery and maintained in a battery-type brooder in an environmentally controlled room on a 14-h photoperiod (6:00 a.m. to 8:00 p.m.). Birds were given ad libitum access to water and a standard starter G-mash diet for broiler chicks (crude protein, 23.5%; metabolizable energy, 3,050 kcal/kg). Seventeen and 12 chicks were selected at random at 10 and 20 days of age, respectively. At the end of each experiment, birds were sacrificed by decapitation under light anesthesia with ether. All experiments were performed according to the humane care guidelines provided by the Kagawa Medical School.
Tissue sampling. Immediately after decapitation, the ileal middle part between Meckel's diverticulum and the ileo-cecal-colonic junction was taken and flushed with phosphate-buffered saline. A 2-cm length of the ileum was fixed in a mixture of 3% glutaraldehyde and 4% paraformaldehyde fixative solution in 0.1 M cacodylate buffer (pH 7.4). Tissue specimens were postfixed with 1% osmium tetroxide in the same ice-cold buffer for 2 h. Then, the specimens for observation by scanning electron microscopy (SEM) were subjected to critical-point drying (Hitachi HCP-1) using liquid carbon dioxide as the medium. The dried specimens were coated with platinum (RMC-Eiko RE vacuum coater) and examined with a Hitachi S-800 scanning electron microscope at 8 kV. The specimens for observation by TEM were stained enbloc with 0.5% uranyl acetate overnight and embedded in Spurr's plastic mixture. Silver to gray ultrathin sections were cut, stained with lead nitrate, and examined under a Hitachi H-7100 transmission electron microscope (Hitachi, Ibaragi) at 75 kV.
SFB colonization evaluated by SEM and TEM. At first, all ileal samples were checked for their SFB colonization level using SEM. Figure 1 shows examples of no (10 birds) (A), few (8 birds) (B), medium (7 birds) (C), and dense (4 birds) (D) SFB colonization levels among 29 birds. Next, to evaluate the correlation between SFB colonization in SEM and the presence of extracellular particles in TEM, we randomly selected samples of 5, 2, 2, and 4 birds with no, few, medium, and dense SFB colonization levels, respectively.
FIG. 1.
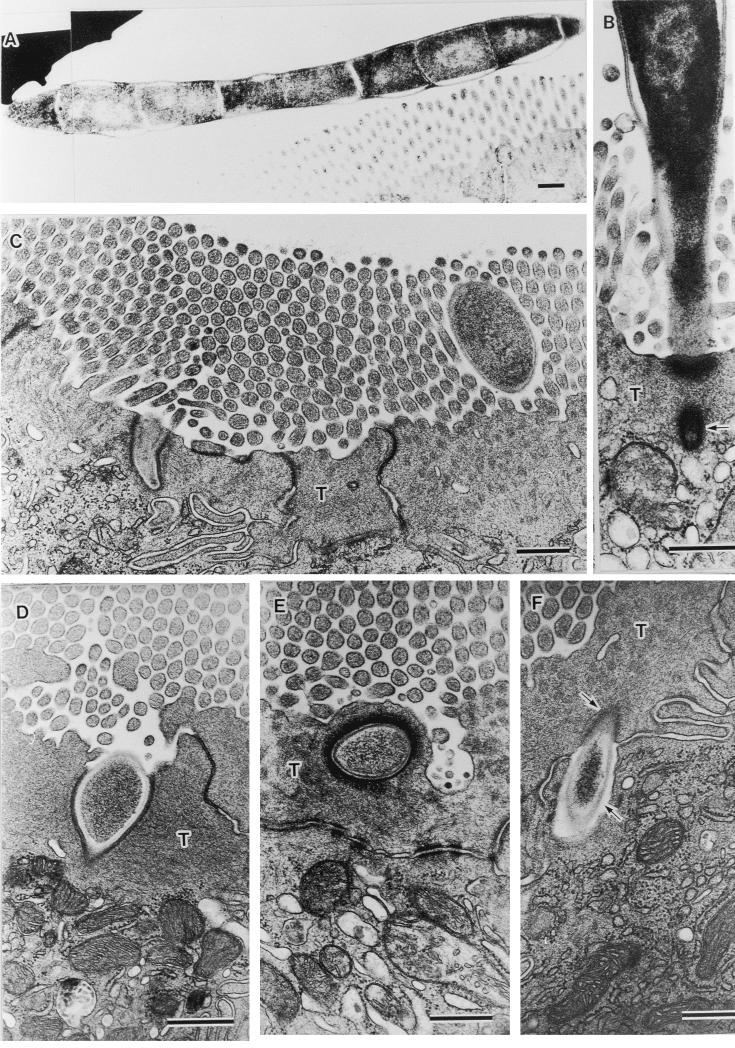
Examples of no (A), few (B), medium (C), and dense (D) SFB colonization on the ileal villi. Bar, 50 μm; magnification, ×210.
Figure 2 shows various stages of the infection process of SFB. Figure 2A is an example of a free form of SFB as found in the intestinal lumen. It has an electron-dense homogeneous cytoplasm with an electron-pale nuclear area that corresponds with the initial developmental stage (10). In the terminal web of epithelial cells at the villus tip, a tip of the first segment of SFB was observed tearing from the SFB body (arrow in Fig. 2B). In the epithelial cells located on the lower villi, we observed extracellular particles penetrating the terminal web (Fig. 2C and D), embedded into the terminal web (Fig. 2E), and completely engulfed by host cytoplasm beyond the terminal web (Fig. 2F). As a characteristic feature, the engulfed particle was surrounded by an electron-dense layer in the terminal web (upper arrow in Fig. 2F) but surrounded by an electron-pale layer beyond the terminal web (lower arrow in Fig. 2F). Structures of these extracellular particles as revealed by TEM resembled those of floating particles of SFB in the intestinal lumen (Fig. 2A through C).
FIG. 2.
Fine structural demonstration of phagocytosis pathway of SFB into ileal epithelial cells. (A) SFB sectioned through its floating part in the intestinal lumen tangentially (magnification, ×11,520). (B) SFB attached to host cell distributing on villus tip, a tip of which is tearing from the SFB body (arrow; ×32,400). C through F, extracellular particles torn on the terminal web (T) (magnifications: C, 23,040; D, 38,250) embedded into T (E: magnification, ×27,000), and engulfed into the cytoplasm beyond T (F: magnification, ×28,800) of host cells distributing on the lower villi. In T, an engulfed particle is surrounded by electron-dense host cytoplasm (upper arrow in F), but in the cytoplasmic area beyond T, it is surrounded by an electron-pale layer (lower arrow in F). Note a similarity in the images of extracellular particles with that of SFB and successive pictures, from the tearing of SFB to its engulfment into host cytoplasm. All bars, 0.5 μm.
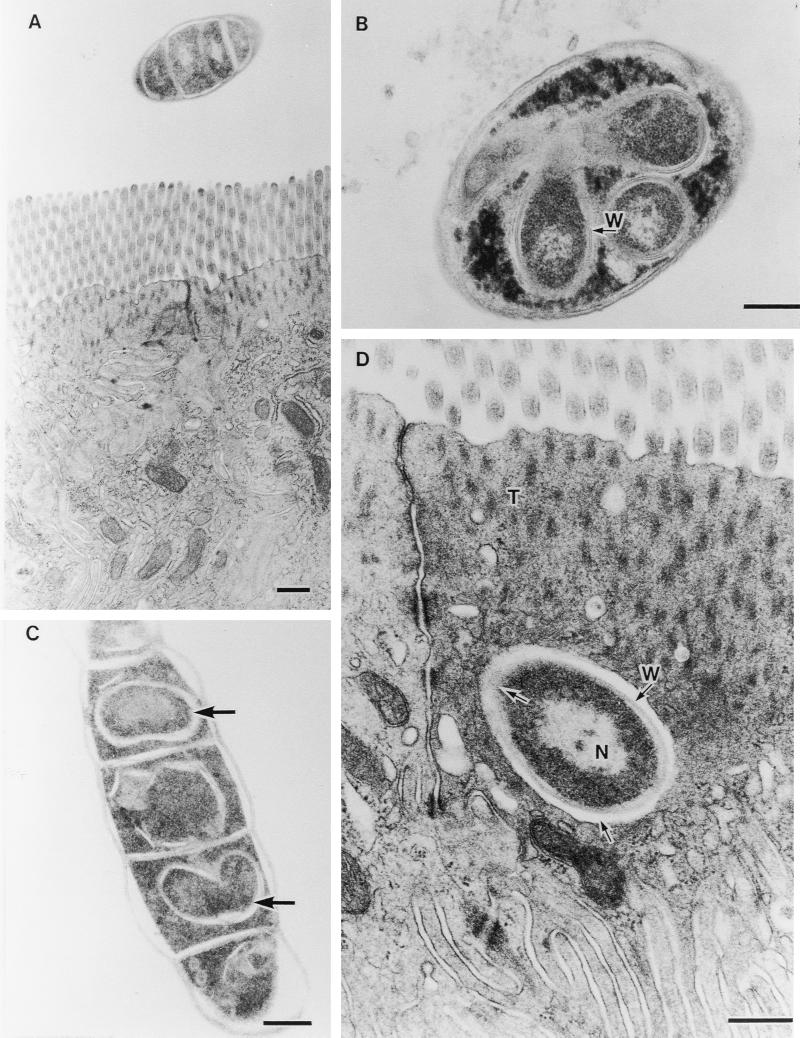
Other segments of SFB contained intrasegmental bodies. It is thought that these bodies are new holdfast particles that can infect epithelial cells or alternatively form spores (10). Figure 3 shows examples of this type of SFB sectioned obliquely (Fig. 3A), transversely (Fig. 3B), and tangentially (Fig. 3C). Intrasegmental bodies in Fig. 3B had a nuclear area and a cell wall (arrow with W). An electron-dense cytoplasmic area around these bodies seems to correspond with the electron-dense homogeneous cytoplasm of SFB in Fig. 2B. In Fig. 3C, cell mitosis of these bodies was observed (lower arrow). Like in Fig. 2F, this engulfed extracellular particle was found in the area beyond the terminal web (Fig. 3D). It was also surrounded by an electron-pale layer (lower arrow) except for the upper left part that was merging into the host cell (upper arrow). The structure of the present extracellular particle as observed by TEM resembled the intrasegmental bodies in Fig. 3A through C and those of floating SFB reported elsewhere (4, 7, 8, 16). We failed to find these extracellular particles in seven chicks colonized with no and few SFB, whereas in six birds with high levels of SFB, extracellular particles were frequently found.
FIG. 3.
Fine structural similarity of images of extracellular particles in the ileal epithelial cell to that of intracellular bodies in SFB. (A) Obliquely cut SFB (magnification, ×14,400). (B) Transversely cut SFB including 3 clear intracellular bodies with nucleus and cell wall (arrow with W) (magnification, ×30,000). (C) Tangentially cut SEB including intracellular bodies (arrows) (magnification, ×23,500). (D) Extracellular particle with nucleus (N) and cell wall (arrow with W) engulfed into the host cytoplasm immediately beneath the terminal web (T). Extracellular particles are surrounded by an electron-pale layer (lower arrow) except for the upper left part, which is shown merging into the host cell (upper arrow) (magnification, ×38,400). All bars, 0.5 μm.
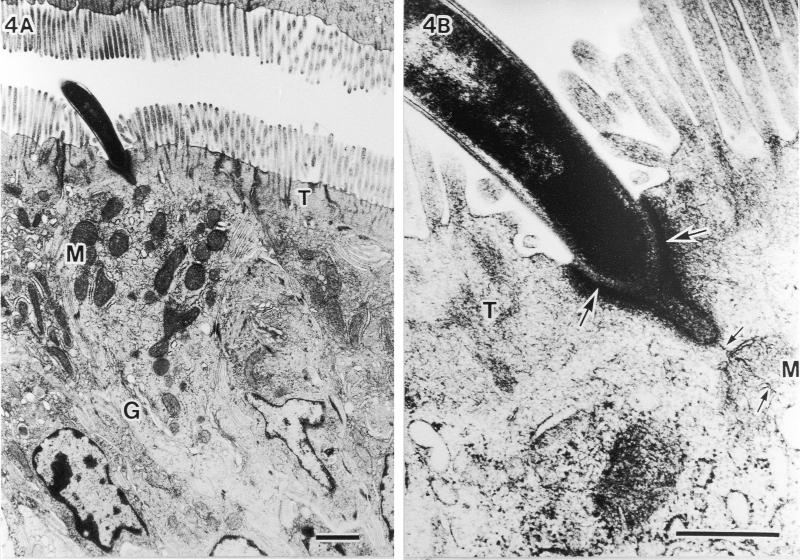
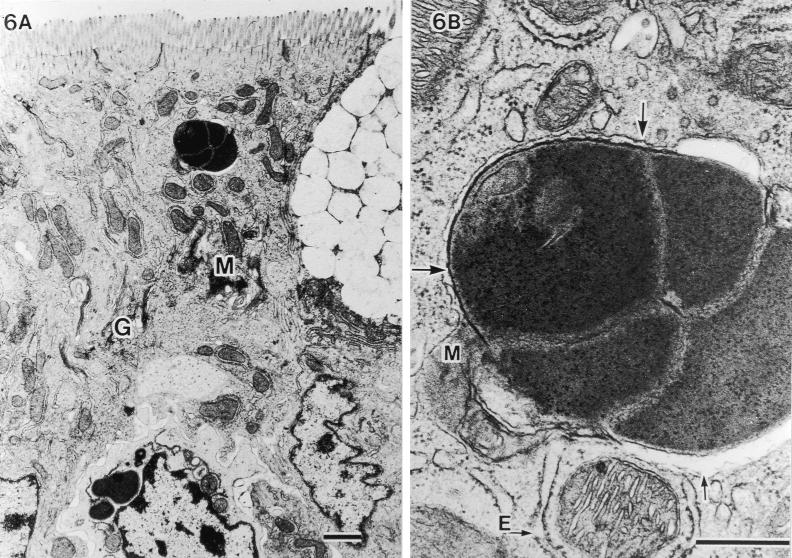
TEM analysis of the host cell response. No specific cytopathologic changes, acute inflammatory reactions, or morphological alterations of organella were observed in epithelial cells penetrated by SFB. Nevertheless, the microvilli were found to be pushed aside (Fig. 4A), and the underlying host cytoplasm was apparently thickened and more electron dense (large arrows in Fig. 4B). These TEM structural changes have also been reported by others (4, 7, 10, 16). In addition, we observed a strange fine structure at the attachment area of SFB; a plasma membranelike structure of SFB that was unlacing from the holdfast segment and was connected with the host mitochondria (small arrows in Fig. 4B). In the host-cytoplasmic area attached by SFB, many aggregated mitochondria and well-developed Golgi bodies were observed, suggesting that the host cell is activated due to attachment of SFB.
FIG. 4.
Fine structural alterations of ileal epithelial cells attached by SFB. (A) Aggregated mitochondria (M) and well-developed Golgi bodies (G) (bar, 1 μm; magnification, ×9,000). (B) Higher magnification of thickened and more electron-dense host-cytoplasmic area around the holdfast segment of bacteria (large arrows) and SFB membranes (small arrows) extending more deeply to contact mitochondria (M) (bar, 0.5 μm; magnification, ×45,000). T, terminal web.
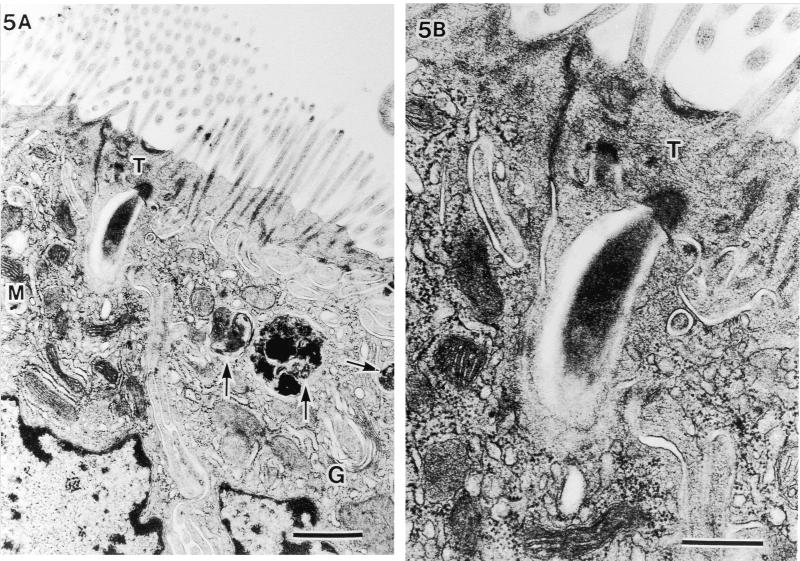
In the epithelial cells located on the lower parts of the villi, extracellular particles were observed in the deeper cytoplasmic area beyond the terminal web (Fig. 5 through 7). Figure 5A is an example of an extracellular particle with an SFB-like shape and of phagosomes thought to be a final stage of the intracellular process of SFB (arrows). An electron-pale layer with no cytoplasm was observed alongside SFB (Fig. 5B). Figures 6 and 7 are examples of electron-dense extracellular particles. This seems to correspond with the electron-dense cytoplasm after exposure from SFB in murine as was described by Davis and Savage (4). In Fig. 6, an unlacing of the outer-limit membrane (large arrows), an unlaced septum, an electron-pale outer layer of SFB (small arrows), and the mitochondrial membrane coalescing directly into SFB (M) were observed. Furthermore, we observed that a double-helix-like septum of SFB was unlacing and merging into another electron-dense particle at the upper side surface of SFB (Fig. 7A and arrows in Fig. 7B). Also in this epithelial cell, an electron-pale layer without cytoplasm and phagosomes could be seen. In these cells in Fig. 5A, 6A, and 7A, many aggregated mitochondria and well-developed Golgi bodies were observed (M and G, respectively). These characteristic TEM structures could not be observed in chicks without SFB by using SEM.
FIG. 5.
Intracellular digestion of SFB phagocytized into the host cell area beyond the terminal web (T). (A) Phagosomes thought to be a final stage of intracellular digestion of SFB (arrows) (bar, 1 μm; magnification, ×14,400). G, Golgi body; M, mitochondria. (B) Higher magnification of electron-dense area in T and electron-pale layer in host cytoplasm immediately beneath T (bar, 0.5 μm; magnification, ×36,000).
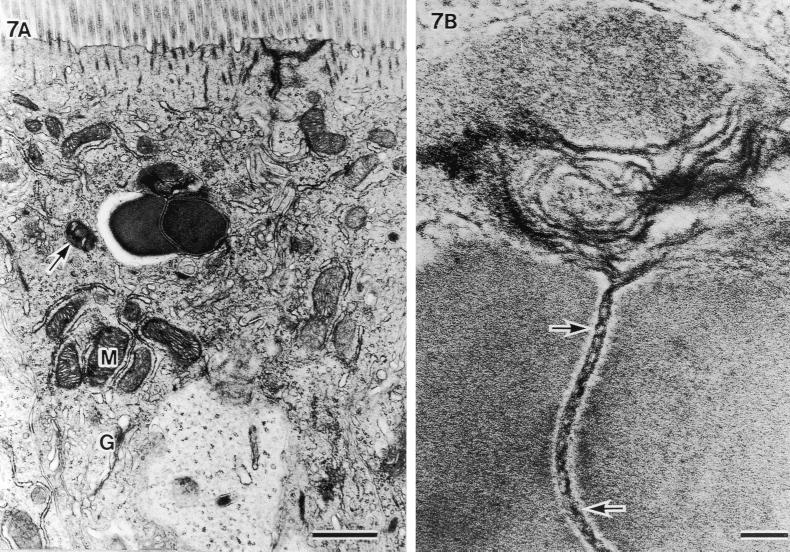
FIG. 7.
Another example of an SFB particle phagocytized into the host cell area beneath the terminal web and cut at the septum. (A) Aggregated mitochondria under the bacteria (M) and well-developed Golgi bodies (G) (scale bar, 1 μm; ×14,400). (B) Higher magnification of an unlacing of septum showing a double-helix-like structure (arrows) and melting at the upper side (scale bar, 0.1 μm; ×108,000).
FIG. 6.
An example of an SFB particle phagocytized into the host cell area beneath the terminal web and cut at the septum level. (A) Aggregated mitochondria around the bacteria (M) and well-developed Golgi bodies (G) (bar, 1 μm; magnification, ×7,200). (B) Higher magnification of an unlacing of limited outer membrane (large arrows), an unlaced septum, and a digested electron-pale layer (small arrows). One mitochondrion (M) is fusing with an SFB membrane and septum, and the lowest mitochondrion is wrapped by isolated envelope with a double membrane (arrow with E) (bar, 0.5 μm; magnification, ×45,000).
In general, phagocytized extracellular particles are intracellularly processed by autophagolysosomes (3). The first indication of the autophagolysosomes is the presence of the isolated envelope with a double membrane. This structure becomes surrounded by a Golgi body to form a nascent autophagic vacuole, which is subsequently fused with a preexisting lysosome to create the autophagolysosomes. Some mitochondria are coalesced directly into the autophagolysosomes. During this period, the Golgi bodies evidently produce lysosomal enzymes, and autophagosomes show an activated acid phosphatase reaction. In our study, we also observed such morphological features. These narrow electron-pale areas surrounding the SFB have also been reported in other infections (21, 22). Histochemically, such a narrow space between the double membrane of an autophagous vacuole is shown to be filled with reaction products of acid phosphatase (15). In the case of the yeast Saccharomyces-cerevisiae, the vacuole was shown to contain hydrolases that digested the target materials by fusing their membranes with outer membrane materials (14). The present electron-pale layer alongside the SFB might therefore be caused by hydrolysis of the phagocytized SFB due to acid phosphatase during lysosomal intracellular digestion. Electron-dense bodies including various levels of densities (phagosome) (arrows in Fig. 5A and 7B) might be at the final stage of the intracellular digestion of extracellular particles. The fact that such an activated cell function likely needs much energy from mitochondria correlates well with the present results that many aggregated mitochondria and well-developed Golgi bodies were observed in the host cytoplasm. Since all of these characteristic TEM structures were found only in chicks confirmed to be colonized with SFB and were absent in SFB-free chicks, we suggest that they show the intracellular processing of phagocytized SFB.
Most previous publications have commented on attachment as the final stage of the interaction between SFB and epithelial cells. Attachment of SFB filaments is recognized by morphological changes in the epithelial cell such as the displacement of microvilli and actin accumulation around the attachment site (4, 7, 9, 12, 16, 27). However, no information on the adhesion process itself, including the possibility of phagocytosis, has been reported. On the contrary, some authors have reported that the absence of bacteria inside the host cells implies that adhesion of bacteria to the host cell is a phenomenon unrelated to the translocation of bacteria to cells (13). Others have claimed that there is no evidence that SFB penetrate beyond the host cell membrane (5, 6, 17) or that they are phagocytized by either epithelial cells or macrophages (4). Only one report has claimed that parts of indigenous microbes (possibly SFB) were observed immediately beneath the terminal web, but none of these bacteria penetrated the cell deeper than 2 μm (16). The reason that no information on the phagocytosis of SFB has been reported is related to the fact that studies on the interaction between SFB and epithelial cells are limited to morphological observations, which is due to lack of an in vitro culturing method. Confirmation that extracellular particles in the host cell are indeed SFB, for example by an immunocytochemical procedure using gold-labeled antibodies, is currently impossible due to the lack of specific antibodies against SFB. The identity of the particles can be established only by comparison of morphological characteristics. Our successive range of images of SFB from their indenting the host cytoplasm to being engulfed by the cell, together with their structural similarity with SFB found in the intestinal lumen, strongly suggests the possibility of phagocytosis of SFB into host cells. This is supported by the absence of such particles in the cytoplasm of epithelial cells in chicks without SFB.
It is known that SFB are present in high numbers only shortly after weaning in mice (4, 10, 20) and for about 10 days after hatching in chicks (27). During this period, the mucosal immune system is strongly stimulated by SFB. Klaasen et al. (11) were the first to report an increase in immunoglobulin A (IgA)-secreting cells in the gut lamina propria after colonization of SFB. Umesaki et al. (25) reported an increase in α-β T-cell receptors bearing intraepithelial lymphocytes. By comparing immunodeficient athymic mice with normal mice, it has been suggested that the immune activation results in a reduction of SFB colonization levels (20). The reason that SFB may induce this powerful stimulation of the mucosal immune system has not been determined but may be related to their interaction with intestinal epithelial cells. Umesaki et al. (25) described the induction of major histocompatibility complex class II molecules on the surface of intestinal epithelial cells after contamination of germ-free mice with SFB. Expression of these molecules is usually a characteristic of antigen-presenting cells and requires uptake and processing of SFB antigen after which immune activation can take place. We speculate that the uptake and intracellular processing of SFB rather than adhesion is a stimulating trigger for this. The finding that, besides immune activation, proliferation of epithelial cells is also stimulated (25) might indicate another host mechanism for clearance of adhering bacteria, including SFB and pathogens.
In conclusion, we were the first to observe that the SFB were phagocytized into the ileal epithelial cells and intracellularly processed by lysosomal heterophagy. Phagocytosis could be the first triggering step for the immunological response to SFB, resulting in increasing numbers of IgA-producing cells and α-β intraepithelial lymphocytes in the intestinal mucosa.
REFERENCES
- 1.Allen P C. Comparative study of long, segmented, filamentous organisms in chickens and mice. Lab Anim Sci. 1992;42:542–547. [PubMed] [Google Scholar]
- 2.Cebra J J, Periwal S B, Lee G, Lee F, Shroff K E. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol. 1998;6:13–18. doi: 10.1155/1998/68382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng H-W, Chiang A-S. Autophagy and acid phosphatase activity in the corpora allata of adult mated females of Diploptera punctata. Cell Tissue Res. 1995;281:109–117. [Google Scholar]
- 4.Davis C P, Savage D C. Habitat, succession, attachment, and morphology of segmented, filamentous microbes indigenous to the murine gastrointestinal tract. Infect Immun. 1974;10:948–956. doi: 10.1128/iai.10.4.948-956.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller R, Turvey A. Bacteria associated with the intestinal wall of the fowl (Gallus domesticus) J Appl Bacteriol. 1971;34:617–622. doi: 10.1111/j.1365-2672.1971.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 6.Glick B, Holbrook K A, Olah I, Perkins W D, Stinson R. A scanning electron microscope study of the caecal tonsil: the identification of a bacterial attachment to the villi of the caecal tonsil and the possible presence of lymphatics in the caecal tonsil. Poultry Sci. 1978;57:1408–1416. doi: 10.3382/ps.0571408. [DOI] [PubMed] [Google Scholar]
- 7.Hampton J C, Rosario B. The attachment of microorganisms to epithelial cells in the distal ileum of the mouse. Lab Investig. 1965;14:1464–1481. [PubMed] [Google Scholar]
- 8.Heczko U, Abe A, Finlay B B. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J Infect Dis. 2000;181:1027–1033. doi: 10.1086/315348. [DOI] [PubMed] [Google Scholar]
- 9.Jepson M A, Clark M A, Simmons N L, Hirst B H. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect Immun. 1993;61:4001–4004. doi: 10.1128/iai.61.9.4001-4004.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klaasen H L B M, Koopman J P, Poelma F G J, Beynen A C. Intestinal, segmented, filamentous bacteria. FEMS Microbiol Rev. 1992;88:165–179. doi: 10.1111/j.1574-6968.1992.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 11.Klaasen H L B M, Van der Heijden P J, Stok W, Poelma F J G, Koopman J P, Van der Brink M E, Bakker M H, Eling W M C, Beynen A C. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect Immun. 1993;61:303–306. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowden S, Heath T. Segmented filamentous bacteria associated with lymphoid tissues in the ileum of horses. Res Vet Sci. 1995;59:272–274. doi: 10.1016/0034-5288(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 13.McNab J M. The avian caeca: a review. World Poultry Sci J. 1973;29:251–263. [Google Scholar]
- 14.Noda T, Matsuura A, Wada Y, Ohsumi Y. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem Bioph Res Commun. 1995;210:126–132. doi: 10.1006/bbrc.1995.1636. [DOI] [PubMed] [Google Scholar]
- 15.Paavola L G. The corpus luteum of the guinea pig. III. Cytochemical studies on the Golgi complex and GERL during normal postpartum regression of luteal cells, emphasizing the origin of lysosomes and autophagic vacuoles. J Cell Biol. 1978;79:59–73. doi: 10.1083/jcb.79.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reimann H A. Microbic phagocytosis by enteric epithelial cells. J Am Med. 1965;192:100–103. doi: 10.1001/jama.1965.03080260018005. [DOI] [PubMed] [Google Scholar]
- 17.Sanford S E. Light and electron microscopic observations of a segmented filamentous bacterium attached to the mucosa of the terminal ileum of pigs. J Vet Diagn Investig. 1991;3:328–333. doi: 10.1177/104063879100300410. [DOI] [PubMed] [Google Scholar]
- 18.Snel J, Van den Brink M E, Bakker M H, Poelma F G J, Heidt P J. The influence of indigenous segmented filamentous bacteria on small intestinal transit in mice. Microb Ecol Health Dis. 1996;9:207–214. [Google Scholar]
- 19.Snel J, Heinen P P, Blok H J, Carman R J, Duncan A J, Allen P C, Collins M D. Comparison of 16S rRNA sequences of segmented filamentous bacteria isolated from mice, rats, and chickens and proposal of “Candidatus arthromitus.”. Int J Syst Bacteriol. 1995;45:780–782. doi: 10.1099/00207713-45-4-780. [DOI] [PubMed] [Google Scholar]
- 20.Snel J, Hermsen C C, Smits H J, Bos N A, Eling W M C, Cebra J J, Heidt P J. Interactions between gut-associated lymphoid tissue and colonization levels of indigenous, segmented, filamentous bacteria in the small intestine of mice. Can J Microbiol. 1998;44:1177–1182. doi: 10.1139/cjm-44-12-1177. [DOI] [PubMed] [Google Scholar]
- 21.Speek C A, Dubey J P. Ultrastructure of early stages of infections in mice fed Toxoplasma gondii oocysts. Parasitology. 1998;116:35–42. doi: 10.1017/s0031182097001959. [DOI] [PubMed] [Google Scholar]
- 22.Steinhagen D. Ultrastructural observations on merogonic and gamogonic stages of Goussia carpelli (Apicomplexa, Coccidia) in experimentally infected common carp Cyprinus carpio. Eur J Protistol. 1991;27:71–78. doi: 10.1016/S0932-4739(11)80429-3. [DOI] [PubMed] [Google Scholar]
- 23.Talham G L, Jiang H-Q, Bos N A, Cebra J J. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tannock G W, Miller J R, Savage D C. Host specificity of filamentous, segmented microorganisms adherent to the small bowel epithelium in mice and rats. Appl Environ Microbiol. 1984;47:441–442. doi: 10.1128/aem.47.2.441-442.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umesaki Y, Okada Y, Matsumoto S, Imaoka A, Setoyama H. Segmented filamentous bacteria are indigenous intestinal bacteria that activate intraepithelial lymphocytes and induce MHC class II molecules and fucosyl asialo GM1 glycolipids on the small intestinal epithelial cells in the ex-germ-free mouse. Microbiol Immunol. 1995;39:555–562. doi: 10.1111/j.1348-0421.1995.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 26.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–3511. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamauchi K, Isshiki Y, Zhou Z-X, Nakahiro Y. Scanning and transmission electron microscopic observations of bacteria adhering to the ileal epithelial cells in growing broiler and White Leghorn chickens. Br Poultry Sci. 1990;31:129–137. doi: 10.1080/00071669008417238. [DOI] [PubMed] [Google Scholar]