Abstract
Klebsiella pneumoniae is a multidrug-resistant opportunistic human pathogen related to various infections. As such, synthetic peptides have emerged as potential alternative molecules. Mo-CBP3-PepI has presented great activity against K. pneumoniae by presenting an MIC50 at a very low concentration (31.25 µg mL−1). Here, fluorescence microscopy and proteomic analysis revealed the alteration in cell membrane permeability, ROS overproduction, and protein profile of K. pneumoniae cells treated with Mo-CBP3-PepI. Mo-CBP3-PepI led to ROS overaccumulation and membrane pore formation in K. pneumoniae cells. Furthermore, the proteomic analysis highlighted changes in essential metabolic pathways. For example, after treatment of K. pneumoniae cells with Mo-CBP3-PepI, a reduction in the abundance of protein related to DNA and protein metabolism, cytoskeleton and cell wall organization, redox metabolism, regulation factors, ribosomal proteins, and resistance to antibiotics was seen. The reduction in proteins involved in vital processes for cell life, such as DNA repair, cell wall turnover, and protein turnover, results in the accumulation of ROS, driving the cell to death. Our findings indicated that Mo-CBP3-PepI might have mechanisms of action against K. pneumoniae cells, mitigating the development of resistance and thus being a potent molecule to be employed in producing new drugs against K. pneumoniae infections.
Keywords: multidrug-resistant bacteria, proteomic analysis, synthetic peptides, antibacterial peptides
1. Introduction
The emergence of multidrug-resistant bacteria (MDRB) is a challenge to public health worldwide, leading to lengthy hospital stays, high healthcare costs around USD 1 million, and a death rate of 46.31% [1,2,3]. K. pnuemoniae is a Gram-negative bacterium that colonizes the human gastrointestinal system and often is found in feces [4]. Additionally, K. pneumoniae is a bacterium belonging to the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) group of MDRB that are pathogenic to humans [5,6].
K. pneumoniae is an opportunistic MDRB only able to infect people with a compromised immune system [4]. K. pneumoniae presents resistance against polymyxins, carbapenems, fluoroquinolones, aminoglycosides, tetracyclines, third-generation cephalosporins, and is pan drug-resistant [7,8,9]. The clinical manifestations of K. pneumoniae present as an acute bacterial skin infection, bacteremia, pneumonia, and osteoarticular infection [10,11,12].
The multidrug resistance acquired by K. pneumonia made this bacterium an important pathogen threatening human health. Based on that, it is emergent and imperative to seek new molecules to fight back in two ways: (1) to develop a new drug to produce a new treatment; or (2) to produce a new molecule that could act synergistically with commercial drugs making them effective again [1]. In this scenario, antimicrobial peptides represent a notorious group of molecules that could help scientists quickly find a way to cope with antimicrobial resistance [13]. However, natural antimicrobial peptides have presented problems in clinical trials, such as toxicity to host cells, low resistance to proteolysis, and sometimes high cost of obtention [13,14].
Synthetic antimicrobial peptides emerged as a solution to solve the problems presented by natural antimicrobial peptides. Synthetic antimicrobial peptides are designed using a natural model sequence to have higher antimicrobial activity, greater resistance to proteolysis, and no toxicity to host cells. Recently, our research group has designed synthetic peptides called Mo-CBP3-PepI using the sequence of a chitin-binding protein from Moringa oleifera [15]. Mo-CBP3-PepI is a non-hemolytic small cationic peptide with a net charge +1, a molecular mass of 893.10 Da, and a hydrophobic ratio of 62%. It has a secondary structure as α-helix confirmed by circular dichroism assays [15].
Regarding the antimicrobial potential, Mo-CBP3-PepI presents great anticandidal activity against Candida albicans, C. parapsilosis, C. krusei, and C. tropicalis. The mechanisms of action of Mo-CBP3-PepI against the Candida genus were revealed using C. albicans as a model [15,16,17]. Regarding the antibacterial activity, Mo-CBP3-PepI, the only relevant activity, was evaluated against K. pneumoniae. Therefore, this study employed fluorescence and scanning electron microscopes and proteomic analysis to provide new insights into the mechanism of antibacterial action of Mo-CBP3-PepI against K. pneumoniae. Additionally, toxicity tests were performed against human cells to produce new data on the safety of Mo-CBP3-PepI.
2. Material and Methods
2.1. Biological Material
The human-pathogenic Gram-negative bacteria K. pneumoniae (ATCC 10031) strain was obtained from the Laboratory of Plant Toxins (LABTOX) of the Federal University of Ceará (UFC).
2.2. Peptide Synthesis
The synthetic peptide Mo-CBP3-PepI (CPAIQRCC) [15] was chemically synthesized by the company ChemPeptide (Shanghai, China), where its purity and quality of it were tested by mass spectrometry and reverse-phase high-performance liquid chromatography (Figure S1 (Supplementary Materials)).
2.3. Cell Viability by MTT Assay
The cell viability assay was performed as described by Lima et al. [17], with some adjustments. After the antibacterial assay was completed as described by Oliveira et al. [15], the wells containing the treated cells with Mo-CBP3-PepI at 1 mg mL−1 and control cells (50 µL) were incubated for 3 h in the dark at 37 °C with 50 µL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (2 mg mL−1, MTT). After incubation, 100 µL of 100% DMSO was added to the wells, and the plate was slowly shaken to dissolve the formazan crystals. The absorbance was measured using a microplate reader (Epoch, BioTek) with a wavelength of 495 nm. The controls used for this assay were 5% of dimethyl sulfoxide (DMSO) prepared in 0.15 M NaCl (saline) solution and ciprofloxacin (1000 µg mL−1) prepared in 5% ethanol in sterile saline solution.
2.4. Antibiofilm Assay
The antibiofilm assay was conducted in flat-bottom 96-well polystyrene microplates as described by Neto et al. [18]. A single colony of K. pneumoniae was collected in stock Petri plates containing inoculated Mueller–Hinton agar. The colony was used as inoculum for 5 mL of Mueller–Hinton broth, and the media was incubated for 24 h in the dark at 37 °C. In sequence, the O.D. of the cell suspension was measured using a microplate reader (Epoch, BioTek) and adjusted to 0.1 at 630 nm with Mueller–Hinton broth. For the inhibition of biofilm formation, 50 µL of the cell (2.5 × 10−5 CFU mL−1) suspension was incubated with 50 µL of the peptide (31.25 μg mL−1) solution and 50 µL of the respective controls described above. The incubation lasted for 48 h at 37 °C. For the biofilm degradation assay, 50 µL of the cell suspension was incubated for 24 h at 37 °C. After this, 50 µL of the peptide solution and the respective controls were added to the wells containing the preformed biofilm and incubated for 24 h at 37 °C.
After incubation, both wells of biofilm formation inhibition and biofilm degradation were washed once with a sterile saline solution. Then, the wells were fixated with 100 µL methanol 99% for 15 min. After the methanol was removed and the plates were dried at 37 °C, then, the wells were stained with 200 µL of 0.1% violet crystal solution for 20 min. In sequence, the wells were washed three times with distilled water. Then, the crystals stained on the biofilm were diluted with 200 µL of 33% acetic acid, and the O.D was measured using a wavelength of 600 nm. The assay was executed in triplicate, with three independent biological experiments.
2.5. Mechanism of Action Evaluation by Fluorescence Microscopy
2.5.1. Cell Membrane Integrity by Propidium Iodide (PI) and FITC-Dextran Uptake
The assay was conducted as described by Oliveira et al. [15], with some modifications. Preparation of cell suspension was completed as mentioned above. After, 50 µL of the diluted bacteria solution was incubated in the dark for 24 h, at 37 °C, with 50 µL of the peptide solution (31.25 µg mL−1) previously prepared with 5% of dimethyl sulfoxide (DMSO) diluted in 0.15 M NaCl solution. The assay was conducted in 1.5 mL microtubes. The diluted bacteria solution was incubated only with the DMSO-NaCl solution for control. After incubation, the microtubes were centrifuged (5000× g, 5 min, 4 °C) and washed three times. Next, the washed cells were incubated with 1 µM propidium iodide for 30 min, in the dark, at 37 °C. After this, the cells were washed two times with saline solution to remove the excess fluorophore and observed with a fluorescence microscope (Olympus System BX 60; excitation wavelength, 488 nm; emission wavelength, 525 nm).
Additionally, to know the size of the pore formed, in a new experiment completed precisely as above, cells were incubated with FITC-Dextran (fluorescein isothiocyanate (FITC)-Dextran) with a size of 10-kDa. After incubation for 30 min, cells were washed with 0.15 M of NaCl and visualized in light and fluorescence microscope Olympus System BX 60 with an excitation wavelength of 490 nm and emission wavelength of 520 nm.
2.5.2. Detection of Peptide-Induced Overproduction of Reactive Oxygen Species (ROS)
This assay was conducted in the same way as above but with a few differences. The preparation of cell suspension and the antimicrobial assay was performed in the same way described above. After the three rounds of centrifugation and washes, the cells were incubated with 1 mM 2,7-dichlorofluorescein diacetate (DCFH-DA) [19] for 30 min, in the dark, at 37 °C. After the washes, the cells were also observed under a fluorescence microscope (Olympus System BX60; excitation wavelength, 488 nm; emission wavelength, 525 nm).
2.5.3. Scanning Electronic Microscopy (SEM) Analysis
SEM analysis was conducted as described by Staniszewska et al. [20]. After the antibacterial assay, cells were centrifuged (5000× g, 5 min, 4 °C), the supernatant was removed, cells resuspended, and fixated for 5 h in a fixation solution (2.5% glutaraldehyde (v/v) in 0.15 M Na-phosphate buffer, pH: 7.2). After centrifugations as described above, the cells were washed three times with 0.15 M Na-phosphate buffer, pH: 7.2. For dehydration, the samples were incubated and dried with ethanol (30%, 50%, 70%, 100%, 100% (v/v)) for 10 min each, and centrifugation as described above after each incubation time. Lastly, the samples were incubated with 50/50 ethanol/hexamethyldisilane (HMDS) for 10 min and centrifuged. Then, the pellet was washed with 100% HMDS and transferred to a coverslip to dry out. After complete drying, the coverslips were assembled on stubs and coated with a 20 nm gold layer using a PET coating machine (EMITECH—Q150TES; Quorum Technologies, Lewes, England). The SEM analyses were completed with an Inspect™ 50 FEI Scanning Electron Microscope, equipped with a low energy detector (Everhart Thornley detector), using an acceleration beam voltage of 20,000 kV and 20,000× detector magnification.
2.6. Protein Extraction and Gel-Free Proteomic Analysis
Initially, an antibacterial assay was performed within 24 h of incubation, using the best inhibitory concentration of Mo-CBP3-PepI (31.25 µg mL−1) [15]. After this, samples were washed twice with 50 mM sodium acetate pH 5.2, with centrifugations at 12,000× g for 15 min at 4 °C. At the end of the washes, the samples were resuspended in 300 µL in the same buffer and frozen for 24 h. Then, the frozen samples were sonicated for 30 min to break the cell wall and membrane, the samples were centrifuged again, and the supernatant was collected.
After that, the Bradford assay was performed to determine the protein concentration in the samples. This step was followed by adding a 10 mM DTT solution under incubation for 1 h at 37 °C to reduce the proteins. Then iodoacetamide was added to a final concentration of 15 mM and incubated for 30 min in a dark room to alkylate the reduced proteins. The proteins reduced and alkylated were digested using trypsin gold (Promega, Madison, WI, USA) to a final concentration of 1:20 (w/w) as described by manufacturers. The trypsin digestion was performed for 16 h at 37 °C. Finally, the samples were dried in a speed vacuum (Eppendorf, Hamburg, Germany) for 3 h and analyzed by ESI-QUAD-TOF mass spectrometer.
2.7. Protein Identification
Tandem mass spectra were extracted into PKL files for both samples, and the proteins were searched using MASCOT MS/MS ions search from MATRIX SCIENCE (https://www.matrixscience.com/cgi/search_form.pl?FORMVER=2&SEARCH=MIS (accessed on 15 September 2022)) against UP625_E_coli_K12 (AA), UP808_K_pneumoniae and SwissProt databases (the taxonomy was set in bacteria). The terms for the search were: fixed modifications to Carbamidomethyl (C); variable modifications to Oxidation (O); the Peptide tolerance was set to 1.2 DA (with 1% FDR); the MS/MS tolerance was set to 0.6 DA; the peptide charge was set to 2+, 3+, and 4+; and finally, the instrument was set to ESI-QUAD-TOF. The proteins identified in both samples were searched for in UNIPROT and separated into 3 sets (unique from control, unique from the cells treated with Mo-CBP3-PepI, and Mo-CBP3-PepI x control shared proteins).
The proteins with a fold-change value ≥ 1.5 (p < 0.05, Tukey’s test) that were up-accumulated (increased the abundance), and proteins with a fold-change value ≤ 0.5 (p < 0.05, Tukey’s test) that were down-accumulated (decreased the abundance) were taken into consideration for comparisons. For each protein, its corresponding FASTA file was downloaded. Then, the blast2go program (https://www.blast2go.com/ (accessed on 30 September 2022)) was used to categorize the proteins detected by Gene Ontology (GO) annotation according to Molecular function, Biological Activity, and subcellular location.
2.8. Cytotoxicity Assay
The cytotoxicity assay was assessed by measuring the ability of live cells to convert a yellow dye, 3-(4,5-dimethyl-2-thiozolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), to formazan. The cell lines used were L929 (murine fibroblasts, ATCC number CCL-1), MRC-5 (human lung fibroblasts, ATCC number CCL-171), and HaCaT (human keratinocytes) following the methodology described by Souza et al. [14]. Cells were treated with Mo-CBP3-PepI at 1 mg mL−1. The alkylating agent methyl methanesulfonate (MMS) at 4 × 10−5 M was used as the positive control. The MTT formazan product was dissolved in 150 µL of DMSO, and the absorbance was measured using a multiplate reader (Spectra Count, Packard, Mississauga, ON, Canada). The drug effect was quantified as the percentage of control absorbance of the reduced dye at 595 nm.
2.9. Comet Assay
For this assay, MMS at 4 × 10−5 M was used as the positive control for DNA damage, and peptides were assayed at 1 mg mL−1. The standard alkaline comet assay (single-cell gel electrophoresis) after treatment (24 h). Cells were washed with ice-cold PBS, trypsinized, and resuspended in a complete medium. Thus, 20 µL of cell suspension (0.7 × 105 cells/mL) was dissolved in 0.75% low-melting-point agarose. Slides were incubated in ice-cold lysis solution (2.5 M NaCl, 0.01 M Tris, 0.1 M EDTA, 1% Triton X-100, and 10% DMSO, pH 10.0) at 4 °C for at least 1 h to remove cell membranes, leaving DNA as “nucleoids”. Afterward, the slides were placed in a horizontal electrophoresis unit and incubated with fresh buffer solution (0.3 M NaOH, 0.001 M EDTA, pH 13.0) at 4 °C for 20 min to allow DNA to unwind and the expression of alkali-labile sites. Electrophoresis was conducted for 20 min at 25 V (94 V/cm). All the above steps were performed in the dark to prevent additional DNA damage. Slides were neutralized (0.4 M Tris, pH 7.5) and stained using 20 µg mL of ethidium bromide. Three hundred cells (100 cells from each of the three replicate slides for each treatment) were selected, coded, and blindly analyzed for DNA migration. These cells were visually scored according to tail length into five classes:
Class 0: undamaged, without a tail;
Class 1: with a tail shorter than the diameter of the head nucleus;
Class 2: with tail length 1–2× the diameter of the head;
Class 3: with a tail longer than 2× the diameter of the head;
Class 4: comets with no heads.
The index of damage (ID) value was calculated for each sample. The ID was defined as an arbitrary score based on the number of cells in the different damage classes, which are visually scored by measuring DNA migration length and the amount of DNA in the tail. DI ranges from 0 (no tail: 100 cells × 0) to 400 (with maximum migration: 100 cells × 4).
2.10. Morphological Analysis of Apoptotic Cells
For this assay, the concentration of peptides was 1 mg mL−1. DMSO-NaCl was the negative control for damage, and MMS at 4 × 10−5 M was used as the positive control for DNA damage. Peptides and control solutions were incubated as described above. Cells with the morphological characteristics of apoptosis, such as apoptotic bodies, peripheral condensation of chromatin, fragmented nucleus, and small cell volume, were evaluated after 24 h of treatment, either with controls or peptides. The evaluation was executed using acridine orange (AO)/ethidium bromide (EB) staining assay. A total of 50 µL of the cell suspension was mixed with 1 µL of the staining solution (100 µg/mL AO + 100 µg/mL EB in PBS) and spread on a slide, where 300 cells were counted per data point.
3. Results and Discussion
3.1. Cell Viability and Antibiofilm Activity of Mo-CBP3-PepI against K. pneumoniae
As reported in a previous work by Oliveira et al. [15], Mo-CBP3-PepI presented an MIC50 against K. pneumoniae at 31.25 µg mL−1. The experiments we repeated here led to the same results, corroborating the data presented by Oliveira et al. [15]. The concentration of Mo-CBP3-PepI to reach an MIC50 against K. pneumoniae is very low compared to other synthetic peptides [21,22]. For example, Fleeman et al. [21] showed that the synthetic peptide called PepC, which presented an MIC50 at a concentration of 350 µg mL−1 11 times higher than the concentration presented by Mo-CBP3-PepI. Additionally, Tincho et al. [22] tested three synthetic peptides, and all presented an MIC50 against K. pneumoniae at a concentration of 500 µg mL−1, 16.07 times higher than Mo-CBP3-PepI. These results revealed that Mo-CBP3-PepI is much more effective against K. pneumoniae than other synthetic peptides.
We further evaluated the cell viability of K. pneumoniae cells and biofilm formation after treatment with Mo-CBP3-PepI. The first was to evaluate the number of viable cells after treatment with Mo-CBP3-PepI (Table 1). The MTT assay revealed that only 47.54% ± 0.008 of K. pneumoniae cells were viable after incubation with Mo-CBP3-PepI, which agrees with the data of MIC50. These results prove that Mo-CBP3-PepI kills half of the cells at the tested concentration. Otherwise, 100% of K. pneumoniae cells were viable in control treated with 5% DMSO in 0.15 M NaCl (Table 1).
Table 1.
Cell viability and antibiofilm potential of Mo-CBP3-PepI against K. pneumoniae.
| a MIC50 of Mo-CBP3-PepI toward K. pneumoniae | ||||
|---|---|---|---|---|
| Treatments | Antibiofilm Potential | |||
| Cell Viability (%) | Inhibition of Biofilm Formation (%) | Degradation of Preformed Biofilm (%) | ||
| DMSO | 100 ± 0.005 | 0 | 0 | |
| Mo-CBP3-PepI | 47.54 ± 0.008 | 11.87 ± 0.001 | 0 | |
a The MIC50 concentration was 31.25 μg mL−1, as defined by Oliveira et al. [1]. Cell viability assay was executed with planktonic cells.
Moving forward, the antibiofilm potential of Mo-CBP3-PepI toward K. pneumoniae was evaluated. Mo-CBP3-PepI barely inhibited the biofilm formation of K. pneumoniae, only 11.87% ± 0.001, and presented no activity against the preformed biofilm (Table 1). As expected, the control solution was ineffective in inhibiting the formation or degrading the preformed biofilms of K. pneumoniae (Table 1).
3.2. Toxicity of Mo-CBP3-PepI to Human Cells
Before we move forward with the study to understand the mechanisms of action of Mo-CBP3-PepI against K. pneumoniae, toxicity tests against human cells were needed to provide information that might indicate the application of Mo-CBP3-PepI. For example, Oliveira et al. [15] presented a safe Mo-CBP3-PepI based on the absence of hemolytic activity against human red blood cells even at concentrations (120 µg mL−1) four times higher than MIC50 concentration [15].
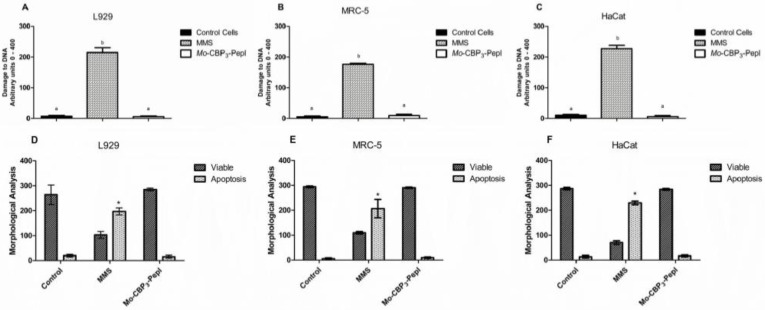
In this study, we went further in the analysis of toxicity. Mo-CBP3-PepI was assayed against other human cells (Figure 1). MTT assay and morphological analysis of human fetal lung fibroblast (MRC-5 line), human keratinocytes (HaCaT line), and L929 fibroblast cells from mice revealed that Mo-CBP3-PepI did not affect either cell viability and morphology of those cells even at a concentration of 1 mg mL−1, which is 32 times higher than MIC50 concentration. In contrast, the positive control for damage methyl methanesulfonate (MMS) (4 × 10–5 M) led to the death of all cells. It caused severe damage to DNA and nuclei structure indicating the establishment of cell death (Figure 1).
Figure 1.
Assessment of toxicity of Mo-CBP3-PepI to human cell lines. (A) L929, (B) HaCaT, and (C) MRC-5 lines were incubated with synthetic peptide at a concentration of 1 mg mL−1 to evaluate the damage to DNA by comet assay. (D–F) Cell lines were incubated with peptides as described and evaluated for viable cells and cells in apoptosis. MMS (4 × 10−5 M) was employed as a positive control for cell toxicity, and healthy cells as a negative control for toxicity. Data are shown as mean ± standard deviation of three independent experiments. * p < 0.05. Small letters indicate statistical significance.
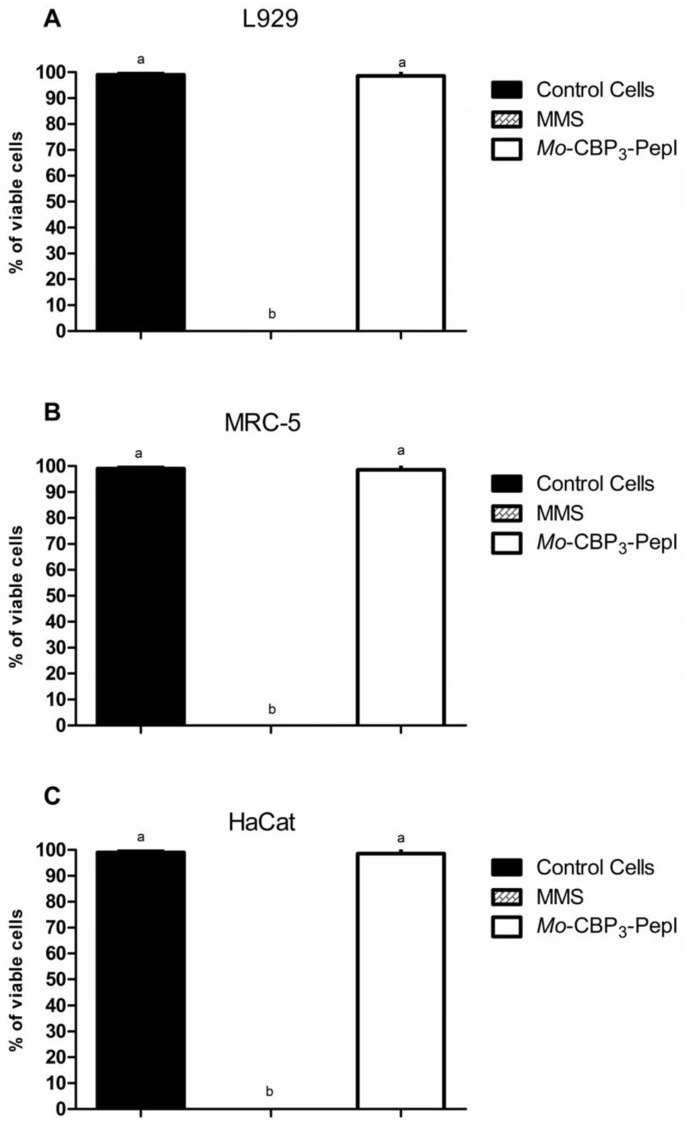
Another experiment to evaluate damage and fragmentation of DNA caused by Mo-CBP3-PepI was evaluated by Comet assay (Figure 2) against the same cell lines and at the same concentration. The assay revealed that the DNA of cells treated with peptides presented no damage. In contrast, cells treated with MMS presented with completely damaged DNA (Figure 2). These results assure that the peptide is either safe or presents a low risk for human cells. Other peptides have presented high toxicity to human red blood cells and other human-type cells, such as WRL-68 (liver cells) and NL-20 (lung cells) [23].
Figure 2.
Evaluation of toxicity of Mo-CBP3-PepI to human cell lines by MTT viability assay. In (A) L929, (B) HaCaT, and (C) MRC-5 lines were incubated with synthetic peptides at a concentration of 1 mg mL−1. Methyl methanesulfonate (MMS; 4 × 10−5 M) was employed as a positive control for cell toxicity, and healthy cells as a negative control for toxicity. Data are shown as mean ± standard deviation of three independent experiments with significance of p < 0.05. Small letters indicate statistical significance.
The synthetic peptides RN7-IN10, RN7-IN9, RN7-IN8, RN7-IN7, and RN7-IN6, derived from indolicidin and ranalexin, presented 50% of hemolytic activity, respectively, at 62.5, 62.5, 125, and 125 µg mL−1 [23]. Additionally, the authors revealed that the synthetic peptides RN7-IN10, RN7-IN9, RN7-IN8, RN7-IN7, and RN7-IN6 were toxic to human cell lines such as WRL-68 (liver cells) and NL-20 (lung cells) at a concentration of 125 µg mL−1 [23]. These concentrations show that those peptides are more toxic than Mo-CBP3-PepI. Therefore, based on the results of the toxicity of Mo-CBP3-PepI, we decided to move forward in the experiment of the mechanism of action.
3.3. Mechanism of Action of Mo-CBP3-PepI against K. pneumoniae
3.3.1. Membrane Pore Formation and ROS Overproduction
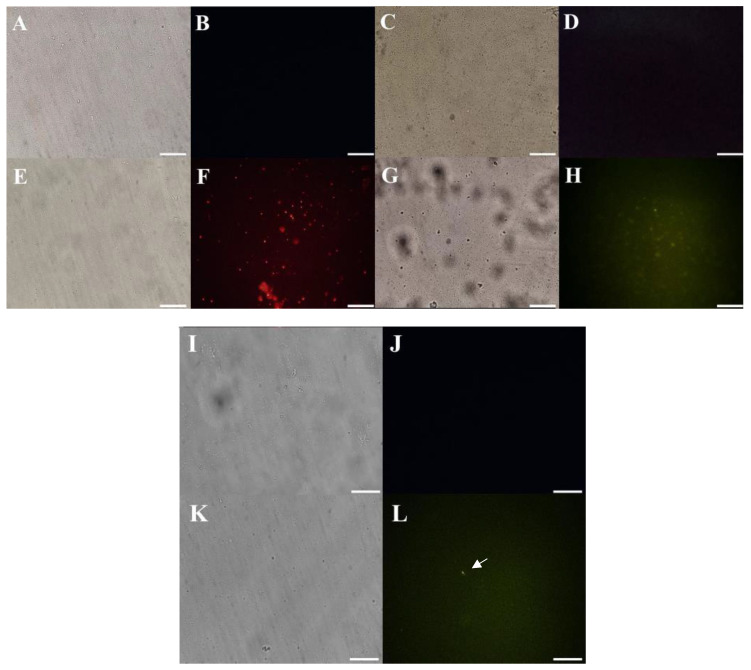
The mechanisms employed by Mo-CBP3-PepI against K. pneumoniae were evaluated against planktonic lifestyle, given that the biofilm activity was not satisfactory (Table 1). The assay to evaluate the pore in the membrane by PI uptake revealed that the treatment with Mo-CBP3-PepI induced the pore formation in the membrane of K. pneumoniae cells, as revealed by the detected red fluorescence (Figure 3A,B,E,F). The green fluorescence of Dextran-FITC (Figure 3C,D,G,H) indicated that the pore formed by Mo-CBP3-PepI in the membrane of K. pneumoniae cells is at least 10 kDa because the size fluorophore used is 10 kDa.
Figure 3.
Mechanism of action of Mo-CBP3-PepI assessed by fluorescence microscopy. In all experiments, Mo-CBP3-PepI was used at a concentration of 31.26 μg mL−1. (A,B) control K. pneumoniae cells for PI uptake assay. (E,F) Mo-CBP3-PepI-treated K. pneumoniae cells for PI uptake assay. Red fluorescence (F) indicates pore formation induced by Mo-CBP3-PepI. (C,D) control K. pneumoniae cells for FITC-Dextran uptake assay. (G,H) Mo-CBP3-PepI-treated K. pneumoniae cells for FITC-Dextran uptake assay. Green fluorescence (H) indicates 10-kDa -sized pore formation induced by Mo-CBP3-PepI. (I,J) control K. pneumoniae cells for ROS overproduction assay. (K,L) Mo-CBP3-PepI-treated K. pneumoniae cells for ROS overproduction assay. Green fluorescence (L) indicates pore formation induced by Mo-CBP3-PepI. Bars indicate 100 μm.
Membranes are the main target of synthetic or natural peptides [16,24]. This is different from antibiotics, which generally affect a protein leading to the rapid development of resistance. By targeting the membrane of K. pneumoniae, Mo-CBP3-PepI imposes a challenging problem for cells to overcome. Membranes are a complex structure, highly conserved during cell evolution. So, remodeling plasma membrane upon external stress is metabolically expensive and dangerous to cells [14].
Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII are synthetic peptides derived from a chitin-binding protein purified from Moringa oleifera [15]. In the same way as Mo-CBP3-PepII and Mo-CBP3-PepIII, Mo-CBP3-PepI can interact with chitin and induce membrane pores in important human pathogenic fungi such as Candida spp., Cryptococcus neoformans, and Trichophyton mentagrophytes [16,17,25]. Here, the mechanisms of action behind the antibacterial activity of Mo-CBP3-PepI against K. pneumoniae cells were evaluated. The movement of PI (Figure 3A,B,E,F) through the membrane of K. pneumoniae revealed by red fluorescence indicates the permeabilization by establishing a pore size of 692.50 Da [26,27]. The size is estimated to be 10 kDa (Figure 3C,D,G,H), given the green fluorescence released by Dextran-FITC.
The questions are, how does Mo-CBP3-PepI induce pore formation in the membrane of K. pneumoniae cells, and why is the pore so big? To answer the first question, it is necessary to revert to the design process of Mo-CBP3-PepI. During the design process, the Mo-CBP3-PepI sequence was achieved for reaching three essential features for antimicrobial activity, positive net charge (+1), hydrophobic potential (62%), and probability of 100% to produce a secondary structure in α-helix [13,15,28]. First, the positive net charge of Mo-CBP3-PepI given by the presence of an arginine residue is essential for the ionic attraction of Mo-CBP3-PepI to negatively charged peptides in the outlier of K. pneumoniae membrane and initial insertion on it [29]. Second, the hydrophobic potential critical for interaction with the hydrophobic core of the membrane’s lipid bilayer is conferred by the presence of apolar amino-acid residues [30]. Third, a secondary structure in α-helix is essential to the attraction and insertion of Mo-CBP3-PepI into the K. pneumoniae [28]. Bioinformatics analysis revealed that Mo-CBP3-PepI is a cell-penetrating peptide [15]. It is important to notice that K. pneumoniae is a Gram-negative bacterium with an outer membrane completely exposed to the Mo-CBP3-PepI attack by the above mechanism.
To answer the second question, it is necessary to understand an essential characteristic of antimicrobial peptides, self-association [31]. Self-association is the ability of antimicrobial peptides to interact during the insertion into the membrane, allowing the formation of a huge pore [31]. Based on this result, it is hypothesized the establishment of a barrel-stave model induces pore formation. In this model, a peptide interacts with lipids in the membrane, as described above, to perform the insertion into the membrane. Then, the peptide molecules interact between them to form a huge pore on the membrane [31,32,33]. Lima et al. [16] showed that Mo-CBP3-PepI induces the formation of a pore of 10 kDa in C. albicans cells.
In the same way, the evaluation of ROS overproduction in K. pneumoniae cells was evaluated. The treatment of K. pneumoniae cells with Mo-CBP3-PepI led to a slight accumulation of ROS within cells. In contrast, control cells treated with DMSO did not present any ROS production, which was an expected result (Figure 3I,J). ROS are essential to cell development. However, the levels of ROS in the cell have to be regulated because higher accumulation than necessary could be lethal for the cell. The induction of overproduction of ROS could be lethal to cells (Figure 3K,L). The undesired and uncontrolled ROS production and accumulation are associated with damage in important cell life molecules such as proteins, lipids, and DNA, driving cells to death [26]. It is known that Mo-CBP3-PepI can induce ROS overproduction in C. albicans cells [16]. However, the ability to induce ROS in bacterial cells is revealed for the first time. Rowe-Magnus et al. [34] showed that synthetic Cathelicidin-derived peptides induced ROS overproduction in the Gram-negative Vibrio cholera. The authors discuss that induction of ROS accumulation was mediated by damage caused in the membrane. Here, we showed that Mo-CBP3-PepI could also induce pore formation in the membrane of K. pneumoniae and ROS overproduction. So, it is possible to correlate the ROS overproduction induced by Mo-CBP3-PepI in K. pneumoniae cells with the pore that was formed.
3.3.2. Scanning Electron Microscopy (SEM)
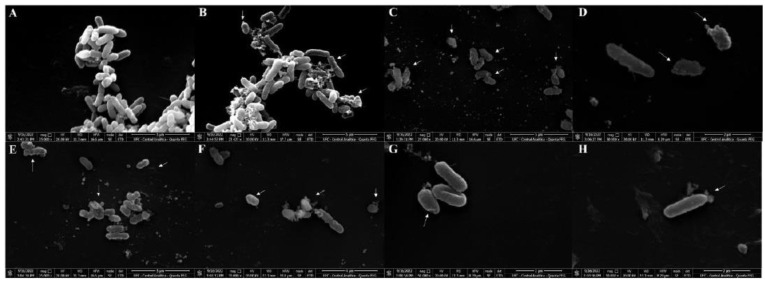
SEM analysis was employed to provide more insights into the effect of Mo-CBP3-PepI on K. pneumoniae morphology (Figure 4). As expected, control K. pneumoniae cells treated with DMSO presented a healthy morphology with no damage on the surface (Figure 4A). However, Mo-CBP3-PepI-treated K. pneumoniae cells presented several lethal damages on the surface (Figure 4B–H). After treatment with Mo-CBP3-PepI, K. pneumoniae cells presented cells completely broken with damage to the cell wall, such as depressions, abnormal morphology, roughness, and an irregular cell surface (Figure 4B,C—white arrows), and in many cases, it is possible to see that the extravasation of cytoplasmic content may occur due to the pores formed in the membrane (Figure 4D–F,H—white arrows). Interestingly, Mo-CBP3-PepI-treated K. pneumoniae cells presented structure-like depressions, broken cell walls, and contorted cells (Figure 4G).
Figure 4.
Scanning electron microscope of K. pneumoniae cells treated with Mo-CBP3-PepI. (A) control K. pneumonia cells treated with DMSO-NaCl solution. (B–H) K. pneumoniae cells treated with Mo-CBP3-PepI 31.26 μg mL−1.
SEM analysis corroborates the damage suggested by fluorescence microscopy. As revealed by fluorescence microscopy, K. pneumoniae cells present pores in the membrane after treatment with Mo-CBP3-PepI. SEM analysis revealed several areas of damage to the structure of K. pneumoniae, including the loss of internal content mediated by pores. Mo-CBP3-PepI induced the same damage in Candida cells [16]. For example, Mo-CBP3-PepIII, a closely related peptide of Mo-CBP3-PepI, also damaged the morphology of Staphylococcus aureus [15].
3.4. Proteomic Profile of K. pneumoniae Cells Treated with Mo-CBP3-PepI
3.4.1. Overview
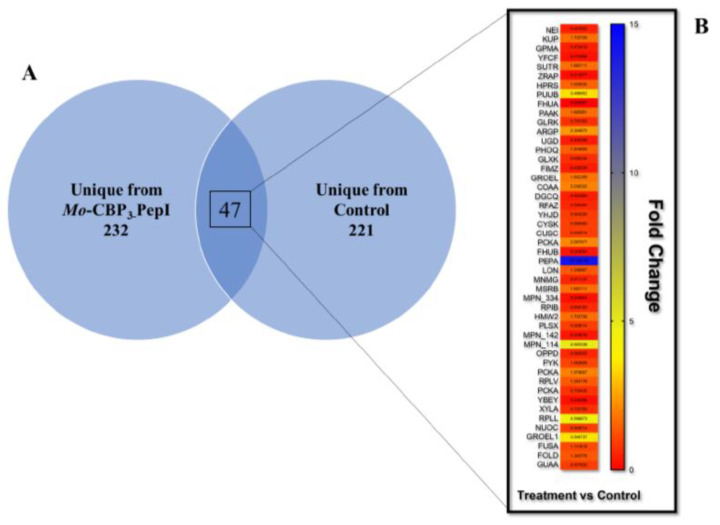
Proteomic analysis is a powerful technique employed to provide an overview of what happens in cells after treatment with peptides [35,36,37,38,39]. The proteomic response to peptides has been analyzed in Escherichia coli K12 [40], Bacillus subtilis [35], and Clostridioides difficile [38]. In many cases, proteomic analysis has been performed to understand the behavior of the multidrug-resistant bacteria’s response to antibiotics [36]. Here, proteomic analysis was employed to overview protein changes in K. pneumoniae after treatment with Mo-CBP3-PepI. In total, 547 were successfully identified (Figure 5). In total, 279 proteins were identified in Mo-CBP3-PepI-treated K. pneumoniae cells and 268 in control K. pneumoniae cells (Figure 3A; Tables S1 and S2). Among exclusive proteins, 232 unique proteins were from Mo-CBP3-PepI-treated K. pneumoniae cells and 221 were unique from control K. pneumoniae cells (Figure 5A; Tables S1 and S2).
Figure 5.
Distribution of K. pneumoniae proteins obtained after treatment with Mo-CBP3-PepI. (A) Venn diagram shows the dispersion of total proteins of control and Mo-CBP3-PepI-treated cells. (B) Represents the fold-change value of overlapping proteins, which were found in both groups. The vertical bar indicates the color scale according to each protein’s fold-change value.
Besides the unique proteins, which are those exclusively found in one group, there were proteins detected in both groups, and they were shared proteins. To understand the patterns of differential accumulation of these proteins, a fold-change rule was applied, taking into account the intensity of protein Mo-CBP3-PepI/Control K pneumoniae cells.
Among the overlapping proteins, which were found in both groups, proteins with a fold-change value ≥ 1.5 (p < 0.05, Tukey’s test) [41] were considered up-accumulated (increased the abundance), and proteins with a fold-change value ≤ 0.5 (p < 0.05, Tukey’s test) were considered down-accumulated (decreased the abundance). For example, endonuclease 8 has a fold-change value of 0.287 and considered with a reduced abundance in cells treated with Mo-CBP3-PepI compared to control cells.
Forty-seven proteins common to both groups, 19 up-accumulated, 19 down-accumulated, and 9 did not change when comparing Mo-CBP3-PepI-treated with control cells (Figure 5B).
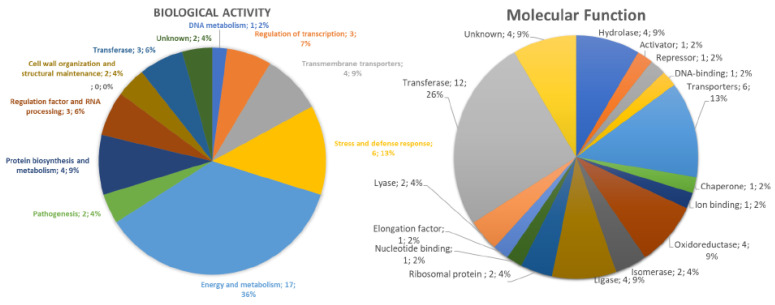
The Gene ontology classification of proteins shared by Mo-CBP3-PepI- and control- K. pneumoniae cells revealed 11 and 16 groups of proteins, respectively, for biological activity and molecular function (Figure 6). Regarding the biological activity, the group that held the highest number of identified proteins was Energy and Metabolism with 36% of total proteins and the DNA metabolism group held the lowest number with 2% of the total identified proteins (Figure 6). In the case of molecular function, the transferase group possessed the highest amount, 26%, of identified proteins. Many groups, such as chaperone and ion binding, have a small number of proteins, 2% of the total identified included (Figure 6).
Figure 6.
Classification proteins from K. pneumoniae cells identified by LC-ESI-MS/MS analysis. The overlapping proteins (found in both control and treated) were classified based on biological process and molecular function.
The protein groups involved in regulating transcription, transmembrane transporters, stress, and Defense Response, Energy and Metabolism, Pathogenesis, Protein Biosynthesis, Metabolism, Cell wall organization, and structural maintenance and transferase were composed of proteins that are both up- and down-accumulated (Table 2). In contrast, the groups Regulation Factor and RNA Processing and DNA metabolism are composed of proteins that decrease the abundance in K. pneumoniae cells after treatment with Mo-CBP3-PepI (Table 2 and Figure 5B Fold-Change). On the other hand, the downregulated proteins were related to regulation factors and transferase (Figure 5B—Fold-Change).
Table 2.
Differentially accumulated proteins identified by ESI-LC-MS/MS.
| Protein Name | ID (Uniprot) | Organism Reference | Cellular Compartment | Fold Change Mo-CBP3-PepI vs. DMSO |
|---|---|---|---|---|
| DNA metabolism | ||||
| Endonuclease 8 | P50465 | Escherichia coli (strain K12) | Cytoplasm | 0.287 |
| Regulation of transcription | ||||
| HTH-type transcriptional regulator SutR | P77626 | Escherichia coli (strain K12) | Cytoplasm | 2.566 |
| HTH-type transcriptional regulator ArgP | P0A8S1 | Escherichia coli (strain K12) | Cytoplasm | 2.364 |
| Fimbriae Z protein | P0AEL8 | Escherichia coli (strain K12) | Cytoplasm | 0.690 |
| Transmembrane transporters | ||||
| Low affinity potassium transport system protein kup | P63183 | Escherichia coli (strain K12) | Plasma membrane | 1.704 |
| Ferrichrome outer membrane transporter/phage receptor | P06971 | Escherichia coli (strain K12) | Plasma membrane | 1.063 |
| Cation efflux system protein CusC | P77211 | Escherichia coli (strain K12) | cell outer membrane | 0.920 |
| Iron (3+)-hydroxamate import system permease protein FhuB | P06972 | Escherichia coli (strain K12) | Plasma membrane | 0.948 |
| Stress and Defense Response | ||||
| Zinc resistance-associated protein | P0AAA9 | Escherichia coli (strain K12) | Periplasm space | 1.682 |
| Chaperonin GroEL | P0A6F5 | Escherichia coli (strain K12) | Cytoplasm | 1.444 |
| Undecaprenyl-diphosphatase | Q2KX31 | Bordetella avium (strain 197N) | Plasma membrane | 1.340 |
| Peptide methionine sulfoxide reductase MsrB | P75129 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 14.104 |
| Periplasmic trehalase | Q4UZ12 | Xanthomonas campestris pv. campestris (strain 8004) | Periplasmatic space | 0.937 |
| Lon protease | P78025 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 0.342 |
| Energy and Metabolism | ||||
| 2.3-bisphosphoglycerate-dependent phosphoglycerate mutase | P62707 | Escherichia coli (strain K12) | Cytoplasm | 0.519 |
| Gamma-glutamylputrescine oxidoreductase | P37906 | Escherichia coli (strain K12) | Cytoplasm | 0.985 |
| 2-succinyl-5-enolpyruvyl-6-hydroxy-3-cyclohexene-1-carboxylate synthase | P17109 | Escherichia coli (strain K12) | Plasma membrane | 3.486 |
| UDP-glucose 6-dehydrogenase | P76373 | Escherichia coli (strain K12) | Cytoplasm | 0.902 |
| Glycerate 3-kinase | P77364 | Escherichia coli (strain K12) | Cytoplasm | 0.745 |
| Phenylacetate-coenzyme A ligase | P76085 | Escherichia coli (strain K12) | Cytoplasm | 1.666 |
| Pantothenate kinase | P0A6I3 | Escherichia coli (strain K12) | Cytoplasm | 0.548 |
| Pyruvate kinase | P78031 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Membrane | 3.139 |
| Phosphoenolpyruvate carboxykinase (ATP) | A8AQV7 | Escherichia coli (strain K12) | Cytoplasm | 0.990 |
| Xylose isomerase | B5ZQV6 | Rhizobium leguminosarum bv. trifolii (strain WSM2304) | Cytoplasm | 1.062 |
| Bifunctional protein FolD | Q88WM8 | Lactiplantibacillus plantarum (strain ATCC BAA-793/NCIMB 8826/WCFS1) | Periplasmic space | 0.726 |
| GMP synthase (glutamine-hydrolyzing) | Q6APU2 | Desulfotalea psychrophila (strain LSv54/DSM 12343) | Cytoplasm | 4.596 |
| Sensor histidine kinase GlrK | P52101 | Escherichia coli (strain K12) | Cell inner membrane | 0.752 |
| Sensor protein PhoQ | P23837 | Escherichia coli (strain K12) | Plasma membrane | 0.823 |
| Putative ABC transporter ATP-binding protein MPN_334 | P75444 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Plasma membrane | 1.336 |
| Oligopeptide transport ATP-binding protein OppD | P75552 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Plasma membrane | 0.948 |
| Pantothenate synthetase | B8I2Z3 |
Ruminiclostridium cellulolyticum
(strain ATCC 35319/DSM 5812/JCM 6584/H10) |
Cytoplasm | 1.142 |
| Pathogenesis | ||||
| Lipopolysaccharide core biosynthesis protein RfaZ | P27241 | Escherichia coli (strain K12) | Cytoplasm | 2.038 |
| Inner membrane protein YhjD | P37642 | Escherichia coli (strain K12) | Plasma membrane | 0.362 |
| Protein Biosynthesis and Metabolism | ||||
| 50S ribosomal protein L22 | A5IYY1 | Mycoplasmopsis agalactiae | Large ribosomal subunit | 0.187 |
| 50S ribosomal protein L7/L12 | P0A466 | Aquifex aeolicus (strain VF5) | Large ribosomal subunit | 1.979 |
| Diaminopimelate decarboxylase | Q8K9C4 |
Buchnera aphidicola subsp. Schizaphis graminum (strain Sg) |
Cytoplasm | 3.546 |
| Cysteine synthase A | P0ABK5 | Escherichia coli (strain K12) | Cytoplasm | 0.596 |
| Regulation Factor and RNA Processing | ||||
| tRNA uridine 5-carboxymethylaminomethyl modification enzyme MnmG | P75221 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 0.487 |
| Elongation factor G | Q7NAV3 | Mycoplasma gallisepticum (strain R(low/passage 15/clone 2)) | Cytoplasm | 0.234 |
| Methylenetetrahydrofolate--tRNA-(uracil-5-)-methyltransferase TrmFO | A4WRQ2 | Cereibacter sphaeroides (strain ATCC 17025/ATH 2.4.3) | Cytoplasm | 0.948 |
| Cell wall organization and structural maintenance | ||||
| Cytadherence high molecular weight protein 2 | P75471 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 1.682 |
| Mgp-operon protein 3 | Q50341 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Plasma membrane | 0.654 |
| Transferase | ||||
| Glutathione S-transferase YfcF | P77544 | Escherichia coli (strain K12) | Cytoplasm | 0.273 |
| Phosphate acyltransferase | P75232 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 3.096 |
| Sensor histidine kinase HprS | P76339 | Escherichia coli (strain K12) | Plasma membrane | 0.213 |
| Unknown | ||||
| Probable cytosol aminopeptidase | P75206 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Cytoplasm | 2.097 |
| Putative acetyltransferase MPN_114 | P75448 | Mycoplasma pneumoniae (strain ATCC 29342/M129) | Unknown | 1.703 |
3.4.2. DNA Metabolism-Related Proteins
In this group, one protein was found in both Mo-CBP3-PepI and DMSO groups, Endonuclease 8 (Table 2), which was down-accumulated in K. pneumoniae cells treated with Mo-CBP3-PepI compared to DMSO cells (control). Endonuclease 8 is an enzyme involved in the DNA repair process after damage by oxidation by ROS [42,43,44]. The reduction in the abundance of Endonuclease 8 in cells treated with Mo-CBP3-PepI is attractive because it noticed a higher accumulation of ROS (Figure 3) in those cells. So, the high accumulation of ROS and reduction in accumulation of the Endonuclease 8 suggest that DNA from K. pneumoniae cells is being damaged by ROS induced by Mo-CBP3-PepI [26].
The proteins UvrA, UvrB, and UvrC, were exclusive from K. pneumoniae cells treated with Mo-CBP3-PepI (Table S1). Based on that, it is feasible to suggest that Mo-CBP3-PepI induced damage in the DNA of K. pneumoniae because these enzymes repair both strands of DNA [45]. Interestingly, only in the control cells (Table S2), but not in treated cells, the protein DNA repair protein RecN was detected as the cell’s first line to protect the DNA from damage [46]. Somehow, Mo-CBP3-PepI induces a down-accumulation in K. pneumoniae cells that, combined with ROS accumulation, leads to DNA damage and cell death.
3.4.3. Stress and Defense Response Related Proteins
In this group, one protein deserved attention, Peptide methionine sulfoxide reductase MsrB was highly accumulated in K. pneumoniae cells treated with Mo-CBP3-PepI compared to control cells with a fold-change value of 14.104 (Table 2). The MsrB protein is a highly conserved protein essential in the cell defense mechanism against high ROS levels, and it works in the repair of inactivated protein by ROS [47]. The increase in MsrB in cells treated with Mo-CBP3-PepI compared to control agrees with the high accumulation of ROS in cells (Figure 3). Proteins and other vital molecules in the cell are attacked and inactivated by ROS [26]. The increased abundance of MsrB protein indicates severe damage in proteins of K. pneumoniae cells after treatment with Mo-CBP3-PepI and that cells are trying to recover from this stress. The MsrB proteins are involved in recovering proteins oxidized by ROS species [26].
Proteomic analysis of cells revealed a reduction in the accumulation of an important protein, Lon protease, in cells treated with Mo-CBP3-PepI compared to control cells (Table 2). Lon protease is a multifunctional, highly conserved ATP-dependent serine protease involved in protein turnover in bacterial cells [48,49]. Lon protease degrades either natural or ROS-induced misfolded proteins leading to free amino acids to produce new functional proteins [48,50]. The reduction in the abundance of Lon protease in Mo-CBP3-PepI-treated cells may suggest an accumulation of misfolded proteins that mitigate the chance of responding to the stress imposed by Mo-CBP3-PepI. Additionally, the Lon protease is essential to encapsulation, motility, heat-shock response, persister formation and drug resistance, and virulence factor production [51,52,53,54,55,56]. By inducing the reduction in accumulation of Lon protease in K. pneumoniae cells, Mo-CBP3-PepI dramatically reduced the chances of the cell to respond to stress and inhibit several essential processes to cell normal function leading to death.
The proteins unique from control cells were identified, such as multidrug resistance protein MdtN, UPF0194 membrane protein YbhG, and multidrug resistance protein EmrK (Table S2). MdtN is a protein involved in the resistance against puromycin and acriflavine [55], EmrK is a part of the efflux pump involved in multidrug resistance [57], and YbhG is involved in resistance to chloramphenicol [58]. This is an exciting result because the absence of these in the Mo-CBP3-PepI-treated cells indicates that they became susceptible to these antibiotics again, which is an important outcome.
3.4.4. Protein Biosynthesis and Metabolism Related Proteins
The analysis of proteins related to protein biosynthesis and metabolism revealed a quite complex scenario in K. pneumoniae cells after treatment with Mo-CBP3-PepI (Table 2; Tables S1 and S2). For example, among the overlapping proteins, the 50S ribosomal protein L22 and 50S ribosomal protein L7/L12, respectively, showed a reduction and increased abundance in K. pneumoniae cells treated with Mo-CBP3-PepI compared to control cells (Table 2). The 50S ribosomal protein L22 is a vital core protein of bacterial ribosomes involved in the aggregation and stabilization of ribosomal proteins to form the ribosome in bacteria [59,60]. The L22 subunit is so important to bacterial ribosomes that it is the target of antibiotics such as macrolides [59,60]. The reduction in abundance in this protein induced by Mo-CBP3-PepI indicates a destabilization of bacterial ribosomes leading to the inhibition of protein synthesis in bacteria.
An increase in the abundance of 50S ribosomal protein L7/L12 in cells treated with Mo-CBP3-PepI (Table 2) was seen, compared to the control. The increase in this protein is perhaps a mechanism of the cell to supply the deficiency of the ribosomal protein L22. However, the L7/L12 is a GTPase protein involved in the process such as translation initiation, elongation, and termination by the mature 70S ribosome [61]. However, increasing this protein will not help cells perform protein synthesis without the L22 subunit.
An interesting result came out by evaluating the unique proteins, Mo-CBP3-PepI and control cells (Table 1 and Table 2). Among the proteins exclusively detected in K. pneumoniae cells treated with Mo-CBP3-PepI are Cysteine-tRNA ligase, Leucine-tRNA ligase, Serine-tRNA ligase, Valine-tRNA ligase, Glutamine-tRNA, Phenylalanine-tRNA ligase alpha subunit, Valine-tRNA ligase, Proline-tRNA ligase, Alanine-tRNA ligase, and Threonine-tRNA ligase (Table S1). All these proteins are involved in amino acid delivery to ribosomes during protein synthesis. The increase in these proteins’ abundance indicates a cell attempt to either increase or maintain the protein synthesis at normal levels to allow cells to fight back against insults imposed by Mo-CBP3-PepI.
Proteins are important to all living organisms, and bacteria must understand what is happening in their environment to respond accordingly. Proteins make all this happen. To respond to stress agents, such as Mo-CBP3-PepI, K. pneumoniae must reprogram all its protein profiles to produce defense proteins [62]. For example, K. pneumoniae cells should produce scavenger proteins to defend themselves from ROS overproduction, but that is impossible. That happens because Mo-CBP3-PepI reduced the abundance of an important protein for ribosomal activity. As a consequence, K. pneumoniae cells could not produce scavenger proteins, thus leading to ROS accumulation and damage to DNA (as reported above) and damage to other proteins leading the cell to death as revealed by the damage present in fluorescence and scanning electron microscopy.
3.4.5. Regulation Factor and RNA Processing Related Proteins
In this group of proteins, the protein elongation factor (Q7NAV3) G that decreases in abundance in K. pneumoniae treated with Mo-CBP3-PepI deserves attention (Table 2). By looking into unique proteins from control cells (Table S2), one isoform of elongation factor G (Q492B1) and other factors, such as Elongation factor Tu 1 and Elongation factor 4, are present but disappear completely in treated versus control cells. Elongation factor G is important for the translocation process during prokaryotic protein synthesis, and it uses the energy held in GTP to interact with tRNA and mRNA [63]. The decrease in the abundance of Elongation factor G led to the shutdown of protein synthesis in K. pneumoniae cells. As discussed above in the protein metabolism section, the protein synthesis in K. pneumoniae cells is dramatically affected by Mo-CBP3-PepI, giving them no chance to respond to the stress imposed by the peptide.
3.4.6. Cell Wall Organization and Structure Maintenance Related Proteins
In this group of proteins, important proteins were exclusively found in K. pneumoniae cells treated with Mo-CBP3-PepI (Table S1), which are soluble lytic murein transglycosylase, D-alanyl-D-alanine carboxypeptidase DacB, Cell shape-determining protein MreB, Probable L, D-transpeptidase ErfK/SrfK, Cell shape-determining protein MreC, Murein tetrapeptide carboxypeptidase, Murein DD-endopeptidase MepH, Sensor protein LytS, D-alanine–D-alanine ligase, and Inner membrane protein YdcZ. All are involved in cell wall turnover, cell structure maintenance, and shape stabilization [64,65,66]. The increased abundance of these proteins indicates that K. pneumoniae cells are suffering from stress in the cell wall imposed by Mo-CBP3-PepI and are trying to overcome the stress. However, as revealed by SEM analysis (Figure 4), K. pneumoniae cells treated with Mo-CBP3-PepI presented damage to the cell wall and cell morphology. It has been related that Mo-CBP3-PepI can bind to the cell wall of the yeast C. albicans [16].
The bacterial cell wall is a crucial component of the cell involved in mechanical defense against several environmental stresses [67]. During stress, the cell must recover the cell wall every time that damage occurs. The exclusive detection of this protein in cells treated with Mo-CBP3-PepI indicates that it is imposing stress on the cell wall and is trying to recover, but as revealed by microscopic analysis (Figure 3 and Figure 4).
3.4.7. Transferase-Related Proteins
In this group, one protein is relevant, the sensor histidine kinase HprS, which presented a reduced abundance in cells treated with Mo-CBP3-PepI compared to the control cells (Table 2). Histidine kinase sensors are environmental elements employed by bacteria to sense the environment and respond accordingly [68,69]. These sensors are mainly responsible for perceiving and responding to oxidative stress [70]. Here, the reduction in the abundance of HprS agreed with the high accumulation of ROS (Figure 3) in K. pneumoniae cells treated with Mo-CBP3-PepI. This result shows that Mo-CBP3-PepI imposes two stresses on K. pneumoniae cells. First is the induction of accumulation of ROS at higher levels; second is the reduction in the protein accumulation involved in the perception and response of stress caused by ROS. In this case, Mo-CBP3-PepI simultaneously induces stress and inhibits the cell’s ability to develop a response to it.
3.4.8. Cell Redox Homeostasis-Related Proteins
This group is particularly important given the scenario of high levels of ROS accumulation in K. pneumoniae cells induced by Mo-CBP3-PepI. In this group, where no overlapping proteins found, only exclusive proteins either from Mo-CBP3-PepI-treated or control K. pneumoniae cells (Tables S1 and S2). For example, Alkyl hydroperoxide reductase C and Alkyl hydroperoxide reductase subunit F were only detected in control K. pneumoniae (Table S2). Thiol-peroxidases are responsible for the scavenging of H2O2, protecting the bacterial cell from toxic levels of endogenously H2O2 [71,72].
Interestingly, these enzymes were not detected in cells treated with Mo-CBP3-PepI, indicating a complete depletion of these enzymes. The absence of these enzymes in treated cells might lead to the accumulation of H2O2 (Figure 3), as revealed by fluorescence microscopy. To cope with the high levels of ROS induced by peptides, K. pneumoniae cells increase the abundance of a catalase-peroxidase enzyme, only detected in treated cells, which is involved in the defense mechanism against high levels of ROS [71]. Our data revealed that even though K. pneumoniae cells treated with Mo-CBP3-PepI increased the abundance of a catalase enzyme to remove the excess ROS, those scavenger enzymes are not enough to prevent the damage produced by high levels of H2O2 once microscopic analysis shows damage to the cell structure and many enzymes suggest damage in DNA and proteins.
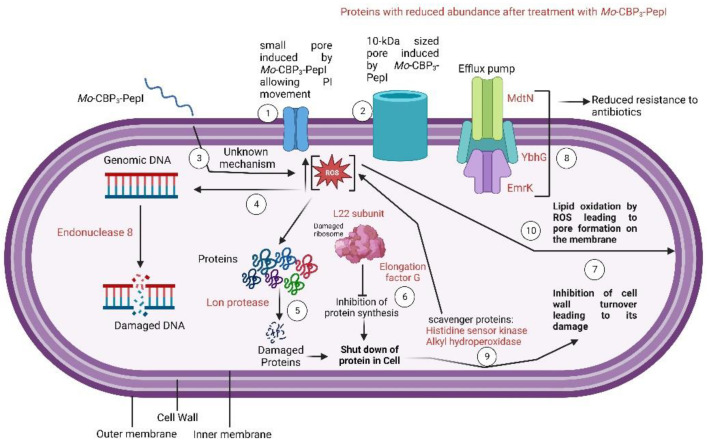
The mechanisms by which Mo-CBP3-PepI induced damage and death in K. pneumoniae cells are many and quite complex. Based on that, a scheme (Figure 7) was produced to provide an overview of all processes induced by Mo-CBP3-PepI that lead K. pneumoniae to death. (1) As revealed by PI uptake, Mo-CBP3-PepI can induce the formation of tiny pores on the membrane of K. pneumoniae. Additionally, FITC-Dextran revealed the presence of a 10-kDa sized pore (2); (3) the interaction of Mo-CBP3-PepI led to the accumulation of high levels of ROS inside the cell; (4) ROS accumulation led to damage to DNA; (5) The high levels of ROS led to damage to proteins leading to misfolding and degradation; (6) As revealed by proteomic analysis, Mo-CBP3-PepI induced a reduction in the abundance of proteins related to proteins and, together with the events in (5), led to a shutdown in protein in K. pneumoniae cells; (7) The shutdown in protein inhibits the cell wall turnover leading to damage, as revealed by SEM analysis (Figure 4); (8) the shutdown in protein levels is also responsible for reducing proteins related to antibiotic resistance; (9) There is also a reduction in a group of proteins involved in early response to ROS accumulation leading to the accumulation of ROS at higher levels; (10) The higher levels of ROS led to damage to DNA (3), proteins (5), and lipids on the membrane (10).
Figure 7.
Scheme showing the mechanisms of antibacterial action of Mo-CBP3-PepI against K. pneumoniae. This scheme was produced only with proteins that decreased in abundance. The numbers explain important process. All them are explained in the text.
4. Conclusions
Altogether the data presented here indicate an intricated and coordinated sequence of events induced by Mo-CBP3-PepI that could drive K. pneumoniae cells to death. All these complex mechanisms of action are also difficult for K. pneumoniae to develop resistance because they present multiple targets simultaneously. Based on that, it is feasible to suggest that our peptide is a potential candidate for developing new strategies to cope with K. pneumoniae resistance.
Acknowledgments
We are also grateful to the staff of the central analytical facilities of UFC, Brazil.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11121753/s1, Figure S1: Purity and quality of synthesis process of Mo-CBP3-PepI. (A) reverse-phase high performance liquid chromatography to the purity of Mo-CBP3-PepI. (B) MS/MS data analysis corroborating the result of HPLC analysis; Table S1: Mo-CBP3-PepI group unique proteins identified by ESI-LC-MS/MS.
Author Contributions
All authors made substantial contributions. The conception and design of the study and acquisition of data, analysis, and interpretation were performed by L.A.C.B., P.F.N.S., N.A.S.N., T.K.B.A., A.F.B.S., R.F.C., C.S.N., F.P.M., L.B.L. and C.D.T.F.; Microscopic analyses were carried out by L.A.C.B., P.F.N.S., N.A.S.N. and T.K.B.A.; Writing or revising the article was completed by L.A.C.B. and P.F.N.S.; P.F.N.S. completed the final approval and submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this study’s findings are available on request from the corresponding author.
Conflicts of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and the writing of the paper.
Funding Statement
Special thanks to CAPES for providing the postdoctoral grant to Pedro F. N. Souza (grant number 88887.318820/2019-00).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Government of the United Kingdom; London, UK: 2016. [Google Scholar]
- 2.About Antibiotic Resistance|CDC. [(accessed on 11 November 2022)]; Available online: https://www.cdc.gov/drugresistance/about.html.
- 3.Murray C.J., Ikuta K.S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebsiella Pneumoniae in Healthcare Settings|HAI|CDC. [(accessed on 11 November 2022)]; Available online: https://www.cdc.gov/hai/organisms/klebsiella/klebsiella.html.
- 5.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Oliveira D.M.P., Forde B.M., Kidd T.J., Harris P.N.A., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020;33:e00181-19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Livermore D.M., Macgowan A.P., Wale M.C.J. Surveillance of Antimicrobial Resistance. Volume 317. World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
- 8.Tzouvelekis L.S., Markogiannakis A., Piperaki E., Souli M., Daikos G.L. Treating Infections Caused by Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Infect. 2014;20:862–872. doi: 10.1111/1469-0691.12697. [DOI] [PubMed] [Google Scholar]
- 9.Zowawi H.M., Forde B.M., Alfaresi M., Alzarouni A., Farahat Y., Chong T.M., Yin W.F., Chan K.G., Li J., Schembri M.A., et al. Stepwise Evolution of Pandrug-Resistance in Klebsiella Pneumoniae. Sci. Rep. 2015;5:15082. doi: 10.1038/srep15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeil J.C., Vallejo J.G., Hultén K.G., Kaplan S.L. Osteoarticular Infections Following Open or Penetrating Trauma in Children in the Post-Community-Acquired Methicillin-resistant Staphylococcus aureus Era: The Impact of Enterobacter cloacae. Pediatr. Infect. Dis. J. 2018;37:1204–1210. doi: 10.1097/INF.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey G.R., Arhin F.F., Wikler M.A., Sahm D.F., Kreiswirth B.N., Mediavilla J.R., Good S., Fiset C., Jiang H., Moeck G., et al. Pooled Analysis of Single-Dose Oritavancin in the Treatment of Acute Bacterial Skin and Skin-Structure Infections Caused by Gram-Positive Pathogens, Including a Large Patient Subset with Methicillin-Resistant Staphylococcus Aureus. Int. J. Antimicrob. Agents. 2016;48:528–534. doi: 10.1016/j.ijantimicag.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein E., Kollef M.H., Nathwani D. Pneumonia Caused by Methicillin-Resistant Staphylococcus Aureus. Clin. Infect. Dis. 2008;46:S378–S385. doi: 10.1086/533594. [DOI] [PubMed] [Google Scholar]
- 13.Lima A.M., Azevedo M.I.G., Sousa L.M., Oliveira N.S., Andrade C.R., Freitas C.D.T., Souza P.F.N. Plant Antimicrobial Peptides: An Overview about Classification, Toxicity and Clinical Applications. Int. J. Biol. Macromol. 2022;214:10–21. doi: 10.1016/j.ijbiomac.2022.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Souza P.F.N., Marques L.S.M., Oliveira J.T.A., Lima P.G., Dias L.P., Neto N.A.S., Lopes F.E.S., Sousa J.S., Silva A.F.B., Caneiro R.F., et al. Synthetic Antimicrobial Peptides: From Choice of the Best Sequences to Action Mechanisms. Biochimie. 2020;175:132–145. doi: 10.1016/j.biochi.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira J.T.A., Souza P.F.N., Vasconcelos I.M., Dias L.P., Martins T.F., Van Tilburg M.F., Guedes M.I.F., Sousa D.O.B. Mo-CBP3-PepI, Mo-CBP3-PepII, and Mo-CBP3-PepIII Are Synthetic Antimicrobial Peptides Active against Human Pathogens by Stimulating ROS Generation and Increasing Plasma Membrane Permeability. Biochimie. 2019;157:10–21. doi: 10.1016/j.biochi.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Lima P.G., Souza P.F.N., Freitas C.D.T., Oliveira J.T.A., Dias L.P., Neto J.X.S., Vasconcelos I.M., Lopes J.L.S., Sousa D.O.B. Anticandidal Activity of Synthetic Peptides: Mechanism of Action Revealed by Scanning Electron and Fluorescence Microscopies and Synergism Effect with Nystatin. J. Pept. Sci. 2020;26:e3249. doi: 10.1002/psc.3249. [DOI] [PubMed] [Google Scholar]
- 17.Souza P.F.N., Lima P.G., Freitas C.D.T., Sousa D.O.B., Neto N.A.S., Dias L.P., Vasconcelos I.M., Freitas L.B.N., Silva R.G.G., Sousa J.S., et al. Antidermatophytic Activity of Synthetic Peptides: Action Mechanisms and Clinical Application as Adjuvants to Enhance the Activity and Decrease the Toxicity of Griseofulvin. Mycoses. 2020;63:979–992. doi: 10.1111/myc.13138. [DOI] [PubMed] [Google Scholar]
- 18.Neto N.A.S., Oliveira J.T.A., Aguiar T.K.B., Bezerra L.P., Branco L.A.C., Mesquita F.P., Freitas C.D.T., Souza P.F.N. Synergistic Antibiofilm Activity between Synthetic Peptides and Ciprofloxacin against Staphylococcus Aureus. Pathogens. 2022;11:995. doi: 10.3390/pathogens11090995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima P.G., Freitas C.D.T., Oliveira J.T.A., Neto N.A.S., Amaral J.L., Silva A.F.B., Sousa J.S., Franco O.L., Souza P.F.N. Synthetic Antimicrobial Peptides Control Penicillium Digitatum Infection in Orange Fruits. Food Res. Int. 2021;147:110582. doi: 10.1016/j.foodres.2021.110582. [DOI] [PubMed] [Google Scholar]
- 20.Staniszewska M., Bondaryk M., Swoboda-Kopec E., Siennicka K., Sygitowicz G., Kurzatkowski W. Candida Albicans Morphologies Revealed by Scanning Electron Microscopy Analysis. Braz. J. Microbiol. 2013;44:813–821. doi: 10.1590/S1517-83822013005000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleeman R.M., Macias L.A., Brodbelt J.S., Davies B.W. Defining Principles That Influence Antimicrobial Peptide Activity against Capsulated Klebsiella Pneumoniae. Proc. Natl. Acad. Sci. USA. 2020;117:27620–27626. doi: 10.1073/pnas.2007036117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tincho M.B., Morris T., Meyer M., Pretorius A. Antibacterial Activity of Rationally Designed Antimicrobial Peptides. Int. J. Microbiol. 2020;2020:2131535. doi: 10.1155/2020/2131535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jindal H.M., Le C.F., Yusof M.Y.M., Velayuthan R.D., Lee V.S., Zain S.M., Isa D.M., Sekaran S.D. Antimicrobial Activity of Novel Synthetic Peptides Derived from Indolicidin and Ranalexin against Streptococcus Pneumoniae. PLoS ONE. 2015;10:e0128532. doi: 10.1371/journal.pone.0128532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parra A.L.C., Freitas C.D.T., Souza P.F.N., von Aderkas P., Borchers C.H., Beattie G.A., Silva F.D.A., Thornburg R.W. Ornamental Tobacco Floral Nectar Is a Rich Source of Antimicrobial Peptides. Plant Sci. 2022;324:111427. doi: 10.1016/j.plantsci.2022.111427. [DOI] [PubMed] [Google Scholar]
- 25.Aguiar T.K.B., Neto N.A.S., Freitas C.D.T., Silva A.F.B., Bezerra L.P., Malveira E.A., Branco L.A.C., Mesquita F.P., Goldman G.H., Alencar L.M.R., et al. Antifungal Potential of Synthetic Peptides against Cryptococcus Neoformans: Mechanism of Action Studies Reveal Synthetic Peptides Induce Membrane–Pore Formation, DNA Degradation, and Apoptosis. Pharmaceutics. 2022;14:1678. doi: 10.3390/pharmaceutics14081678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang K., Dang W., Xie J., Zhu R., Sun M., Jia F., Zhao Y., An X., Qiu S., Li X., et al. Antimicrobial Peptide Protonectin Disturbs the Membrane Integrity and Induces ROS Production in Yeast Cells. Biochim. Biophys. Acta-Biomembr. 2015;1848:2365–2373. doi: 10.1016/j.bbamem.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee M.T., Hung W.C., Chen F.Y., Huang H.W. Mechanism and Kinetics of Pore Formation in Membranes by Water-Soluble Amphipathic Peptides. Proc. Natl. Acad. Sci. USA. 2008;105:5087–5092. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lima P.G., Oliveira J.T.A., Amaral J.L., Freitas C.D.T., Souza P.F.N. Synthetic Antimicrobial Peptides: Characteristics, Design, and Potential as Alternative Molecules to Overcome Microbial Resistance. Life Sci. 2021;278:119647. doi: 10.1016/j.lfs.2021.119647. [DOI] [PubMed] [Google Scholar]
- 29.Hristova K., Wimley W.C. A Look at Arginine in Membranes. J. Membr. Biol. 2011;239:49. doi: 10.1007/s00232-010-9323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campelo F., McMahon H.T., Kozlov M.M. The Hydrophobic Insertion Mechanism of Membrane Curvature Generation by Proteins. Biophys. J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y., Huang J., Chen Y. Alpha-Helical Cationic Antimicrobial Peptides: Relationships of Structure and Function. Protein Cell. 2010;1:143–152. doi: 10.1007/s13238-010-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shai Y. Mechanism of the Binding, Insertion and Destabilization of Phospholipid Bilayer Membranes by Alpha-Helical Antimicrobial and Cell Non-Selective Membrane-Lytic Peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/S0005-2736(99)00200-X. [DOI] [PubMed] [Google Scholar]
- 33.Jean-François F., Elezgaray J., Berson P., Vacher P., Dufourc E.J. Pore Formation Induced by an Antimicrobial Peptide: Electrostatic Effects. Biophys. J. 2008;95:5748–5756. doi: 10.1529/biophysj.108.136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rowe-Magnus D.A., Kao A.Y., Prieto A.C., Pu M., Kao C. Cathelicidin Peptides Restrict Bacterial Growth via Membrane Perturbation and Induction of Reactive Oxygen Species. mBio. 2019;10:e02021-19. doi: 10.1128/mBio.02021-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wenzel M., Chiriac A.I., Otto A., Zweytick D., May C., Schumacher C., Gust R., Albada H.B., Penkova M., Krämer U., et al. Small Cationic Antimicrobial Peptides Delocalize Peripheral Membrane Proteins. Proc. Natl. Acad. Sci. USA. 2014;111:1409–1418. doi: 10.1073/pnas.1319900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsakou F., Jersie-Christensen R., Jenssen H., Mojsoska B. The Role of Proteomics in Bacterial Response to Antibiotics. Pharmaceuticals. 2020;13:214. doi: 10.3390/ph13090214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moyer T.B., Purvis A.L., Wommack A.J., Hicks L.M. Proteomic Response of Escherichia Coli to a Membrane Lytic and Iron Chelating Truncated Amaranthus Tricolor Defensin. BMC Microbiol. 2021;21:110. doi: 10.1186/s12866-021-02176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maaß S., Bartel J., Mücke P.A., Schlüter R., Sura T., Zaschke-Kriesche J., Smits S.H.J., Becher D. Proteomic Adaptation of Clostridioides Difficile to Treatment with the Antimicrobial Peptide Nisin. Cells. 2021;10:372. doi: 10.3390/cells10020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senges C.H.R., Stepanek J.J., Wenzel M., Raatschen N., Ay Ü., Märtens Y., Prochnow P., Hernández M.V., Yayci A., Schubert B., et al. Comparison of Proteomic Responses as Global Approach to Antibiotic Mechanism of Action Elucidation. Antimicrob. Agents Chemother. 2021;65:e01373-20. doi: 10.1128/AAC.01373-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H., Xie Y., Zhang H., Jin J., Zhang H. Quantitative Proteomic Analysis Reveals the Influence of Plantaricin BM-1 on Metabolic Pathways and Peptidoglycan Synthesis in Escherichia Coli K12. PLoS ONE. 2020;15:e0231975. doi: 10.1371/journal.pone.0231975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noronha Souza P.F., Abreu Oliveira J.T., Vasconcelos I.M., Magalhães V.G., Albuquerque Silva F.D., Guedes Silva R.G., Oliveira K.S., Franco O.L., Gomes Silveira J.A., Leite Carvalho F.E. H2O2Accumulation, Host Cell Death and Differential Levels of Proteins Related to Photosynthesis, Redox Homeostasis, and Required for Viral Replication Explain the Resistance of EMS-Mutagenized Cowpea to Cowpea Severe Mosaic Virus. J. Plant Physiol. 2020;245:153110. doi: 10.1016/j.jplph.2019.153110. [DOI] [PubMed] [Google Scholar]
- 42.Burgess S., Jaruga P., Dodson M.L., Dizdaroglu M., Stephen Lloyd R. Determination of Active Site Residues in Escherichia Coli Endonuclease VIII. J. Biol. Chem. 2002;277:2938–2944. doi: 10.1074/jbc.M110499200. [DOI] [PubMed] [Google Scholar]
- 43.Jiang D., Hatahet Z., Blaisdell J.O., Melamede R.J., Wallace S.S. Escherichia Coli Endonuclease VIII: Cloning, Sequencing, and Overexpression of the Nei Structural Gene and Characterization of Nei and Nei Nth Mutants. J. Bacteriol. 1997;179:3773–3782. doi: 10.1128/jb.179.11.3773-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oshima T., Aiba H., Baba T., Fujita K., Hayashi K., Honjo A., Ikemoto K., Inada T., Itoh T., Kajihara M., et al. A 718-Kb DNA Sequence of the Escherichia Coli K-12 Genome Corresponding to the 12.7–28.0 Min Region on the Linkage Map. DNA Res. 1996;3:137–155. doi: 10.1093/dnares/3.3.137. [DOI] [PubMed] [Google Scholar]
- 45.Verhoeven E.E.A., Wyman C., Moolenaar G.F., Goosen N. The Presence of Two UvrB Subunits in the UvrAB Complex Ensures Damage Detection in Both DNA Strands. EMBO J. 2002;21:4196–4205. doi: 10.1093/emboj/cdf396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kidane D., Sanchez H., Alonso J.C., Graumann P.L. Visualization of DNA Double-Strand Break Repair in Live Bacteria Reveals Dynamic Recruitment of Bacillus Subtilis RecF, RecO and RecN Proteins to Distinct Sites on the Nucleoids. Mol. Microbiol. 2004;52:1627–1639. doi: 10.1111/j.1365-2958.2004.04102.x. [DOI] [PubMed] [Google Scholar]
- 47.Delaye L., Becerra A., Orgel L., Lazcano A. Molecular Evolution of Peptide Methionine Sulfoxide Reductases (MsrA and MsrB): On the Early Development of a Mechanism That Protects against Oxidative Damage. J. Mol. Evol. 2007;64:15–32. doi: 10.1007/s00239-005-0281-2. [DOI] [PubMed] [Google Scholar]
- 48.Luo S., McNeill M., Myers T.G., Hohman R.J., Levine R.L. Lon Protease Promotes Survival of Escherichia Coli During Anaerobic Glucose Starvation. Arch. Microbiol. 2008;189:181. doi: 10.1007/s00203-007-0304-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He L., Luo D., Yang F., Li C., Zhang X., Deng H., Zhang J.R. Multiple Domains of Bacterial and Human Lon Proteases Define Substrate Selectivity. Emerg. Microbes Infect. 2018;7:1–18. doi: 10.1038/s41426-018-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stadtman E.R., Levine R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 51.Torres-Cabassa A., Gottesman S., Frederick R.D., Dolph P.J., Coplin D.L. Control of Extracellular Polysaccharide Synthesis in Erwinia Stewartii and Escherichia Coli K-12: A Common Regulatory Function. J. Bacteriol. 1987;169:4525. doi: 10.1128/jb.169.10.4525-4531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee S., Bree A.C., Liu J., Patrick J.E., Chien P., Kearns D.B. Adaptor-Mediated Lon Proteolysis Restricts Bacillus Subtilis Hyperflagellation. Proc. Natl. Acad. Sci. USA. 2015;112:250–255. doi: 10.1073/pnas.1417419112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bissonnette S.A., Rivera-Rivera I., Sauer R.T., Baker T.A. The IbpA and IbpB Small Heat-Shock Proteins Are Substrates of the AAA+ Lon Protease. Mol. Microbiol. 2010;75:1539. doi: 10.1111/j.1365-2958.2010.07070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shan Y., Gandt A.B., Rowe S.E., Deisinger J.P., Conlon B.P., Lewis K. ATP-Dependent Persister Formation in Escherichia Coli. mBio. 2017;8:e02267-16. doi: 10.1128/mBio.02267-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricci V., Blair J.M.A., Piddock L.J.V. RamA, Which Controls Expression of the MDR Efflux Pump AcrAB-TolC, Is Regulated by the Lon Protease. J. Antimicrob. Chemother. 2014;69:643. doi: 10.1093/jac/dkt432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbst K., Bujara M., Heroven A.K., Opitz W., Weichert M., Zimmermann A., Dersch P. Intrinsic Thermal Sensing Controls Proteolysis of Yersinia Virulence Regulator RovA. PLoS Pathog. 2009;5:1000435. doi: 10.1371/journal.ppat.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.EmrK—Probable Multidrug Resistance Protein EmrK—Escherichia Coli (Strain K12)|UniProtKB|UniProt. [(accessed on 7 November 2022)]. Available online: https://www.uniprot.org/uniprotkb/P52599/entry.
- 58.YbhG—UPF0194 Membrane Protein YbhG—Escherichia Coli (Strain K12)|UniProtKB|UniProt. [(accessed on 7 November 2022)]. Available online: https://www.uniprot.org/uniprotkb/P75777/entry.
- 59.Moore S.D., Sauer R.T. Revisiting the Mechanism of Macrolide-Antibiotic Resistance Mediated by Ribosomal Protein L22. Proc. Natl. Acad. Sci. USA. 2008;105:18261–18266. doi: 10.1073/pnas.0810357105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davydova N., Streltsov V., Wilce M., Liljas A., Garber M. L22 Ribosomal Protein and Effect of Its Mutation on Ribosome Resistance to Erythromycin. J. Mol. Biol. 2002;322:635–644. doi: 10.1016/S0022-2836(02)00772-6. [DOI] [PubMed] [Google Scholar]
- 61.Carlson M.A., Haddad B.G., Weis A.J., Blackwood C.S., Shelton C.D., Wuerth M.E., Walter J.D., Spiegel P.C. Ribosomal Protein L7/L12 Is Required for GTPase Translation Factors EF-G, RF3 and IF2 to Bind in Their GTP State to 70S Ribosomes. FEBS J. 2017;284:1631. doi: 10.1111/febs.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Starosta A.L., Lassak J., Jung K., Wilson D.N. The Bacterial Translation Stress Response. FEMS Microbiol. Rev. 2014;38:1172. doi: 10.1111/1574-6976.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C., Cui X., Beausang J.F., Zhang H., Farrell I., Cooperman B.S., Goldman Y.E. Elongation Factor G Initiates Translocation through a Power Stroke. Proc. Natl. Acad. Sci. USA. 2016;113:7515–7520. doi: 10.1073/pnas.1602668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Divakaruni A.V., Baida C., White C.L., Gober J.W. The Cell Shape Proteins MreB and MreC Control Cell Morphogenesis by Positioning Cell Wall Synthetic Complexes. Mol. Microbiol. 2007;66:174–188. doi: 10.1111/j.1365-2958.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- 65.Dik D.A., Marous D.R., Fisher J.F., Mobashery S. Lytic Transglycosylases: Concinnity in Concision of the Bacterial Cell Wall. Crit. Rev. Biochem. Mol. Biol. 2017;52:503–542. doi: 10.1080/10409238.2017.1337705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hung W.C., Jane W.N., Wong H.C. Association of a D-Alanyl-D-Alanine Carboxypeptidase Gene with the Formation of Aberrantly Shaped Cells during the Induction of Viable but Nonculturable Vibrio Parahaemolyticus. Appl. Environ. Microbiol. 2013;79:7305–7312. doi: 10.1128/AEM.01723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mueller E.A., Levin P.A. Bacterial Cell Wall Quality Control during Environmental Stress. mBio. 2020;11:e02456-20. doi: 10.1128/mBio.02456-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szurmant H., Bu L., Brooks C.L., Hoch J.A. An Essential Sensor Histidine Kinase Controlled by Transmembrane Helix Interactions with Its Auxiliary Proteins. Proc. Natl. Acad. Sci. USA. 2008;105:5891–5896. doi: 10.1073/pnas.0800247105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheung J., Le-Khac M., Hendrickson W.A. Crystal structure of a histidine kinase sensor domain with similarity to periplasmic binding proteins. Proteins. 2009;77:235–241. doi: 10.1002/prot.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang H.W., Chung C.H., Ma T.Y., Wong H.C. Roles of Alkyl Hydroperoxide Reductase Subunit C (AhpC) in Viable but Nonculturable Vibrio Parahaemolyticus. Appl. Environ. Microbiol. 2013;79:3734–3743. doi: 10.1128/AEM.00560-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen L., Xie Q.W., Nathan C. Alkyl Hydroperoxide Reductase Subunit C (AhpC) Protects Bacterial and Human Cells against Reactive Nitrogen Intermediates. Mol. Cell. 1998;1:795–805. doi: 10.1016/S1097-2765(00)80079-9. [DOI] [PubMed] [Google Scholar]
- 72.Yuan F., Yin S., Xu Y., Xiang L., Wang H., Li Z., Fan K., Pan G. The Richness and Diversity of Catalases in Bacteria. Front. Microbiol. 2021;12:485. doi: 10.3389/fmicb.2021.645477. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available on request from the corresponding author.