Abstract
Simple Summary
Maternal behavior involves active and passive responses associated with the willingness to nurse and protect the young. In some species, its expression is very selective toward individuals that are recognized as their own and may be long-lasting, whereas in other species the expression is not as selective or may be short-lasting. Brain processes of acceptance, social recognition, inhibition of rejection/fear, and increase in care motivation mediate its expression. The neurocircuitry of maternal behavior is activated upon exposure to the right natural stimuli, such as those that occur during pregnancy, parturition, and lactation. However, even virgin females and males can respond with maternal behaviors if they develop sensitization to the offspring via cohabitation or cross-sensitization via mating. Herein, we discuss behavioral expression in different species, the natural triggering stimuli, and the putative neurocircuitries of acceptance, social recognition, motivation, and rejection during maternal behavior.
Abstract
Among the different species of mammals, the expression of maternal behavior varies considerably, although the end points of nurturance and protection are the same. Females may display passive or active responses of acceptance, recognition, rejection/fear, or motivation to care for the offspring. Each type of response may indicate different levels of neural activation. Different natural stimuli can trigger the expression of maternal and paternal behavior in both pregnant or virgin females and males, such as hormone priming during pregnancy, vagino-cervical stimulation during parturition, mating, exposure to pups, previous experience, or environmental enrichment. Herein, we discuss how the olfactory pathways and the interconnections of the medial preoptic area (mPOA) with structures such as nucleus accumbens, ventral tegmental area, amygdala, and bed nucleus of stria terminalis mediate maternal behavior. We also discuss how the triggering stimuli activate oxytocin, vasopressin, dopamine, galanin, and opioids in neurocircuitries that mediate acceptance, recognition, maternal motivation, and rejection/fear.
Keywords: preoptic area, parturition, amygdala, oxytocin, dopamine, recognition, brain, motivation, bond
1. Introduction
Survival of the mammal offspring depends on the correct expression of maternal behaviors, particularly during the early postnatal period. Newborns must be a powerful source of incentive sensory stimulation to the dam, and in return, they must be capable of responding either actively or passively to such stimuli, expressing acceptance and motivation to invest energy and time and willingness to risk their physical safety. Hence, the capacity to express maternal behavior depends on the sensitivity to respond to the right stimuli under certain physiological, ontogenic, or cognitive conditions. Accordingly, to understand the neurobiology of this behavior, we must consider neural systems involved in acceptance, social recognition, motivation, and fear/rejection. This review aims to provide information on the neurobiology of such processes in nonhuman mammals. We begin by describing the objective measures of active and passive responses in different species. Then, we discuss the natural stimuli that facilitate the expression of maternal behavior and the putative neurocircuitries. Accordingly, scientific articles related to maternal behavior were analyzed. The electronic databases PubMed, GoogleScholar, and SciELO were searched using the following keywords in English: maternal behavior; animals; preoptic area; parturition; amygdala; oxytocin; dopamine; recognition; brain; motivation; bond. The exploration included studies on laboratory and domestic animals.
2. Active and Passive Maternal Behaviors
Maternal behavior involves the facilitation of acceptance, recognition, and motivation, along with the inhibition of rejection and fear toward offspring. Acceptance is inferred from behaviors that allow proximity to any newborn, whereas recognition involves selective acceptance of specific individuals. Thus, females may passively accept unfamiliar newborns (i.e., allowing nursing to any young) and recognize/accept only familiar ones. Similarly, rejection may involve active responses (i.e., aggression/infanticide) to discourage contact, whereas fear may be expressed via passive avoidance (i.e., not approaching them). Furthermore, care motivation involves active behaviors that indicate willingness to nurse and protect the young. So, although the expression of maternal behaviors varies considerably among species, the endpoints served are the same. For instance, some precocial species, such as sheep and horses, express very selective maternal behavior toward offspring they accept/recognize as theirs during the very first hours postpartum [1,2]. That kind of maternal selectivity requires strict mechanisms of acceptance/social recognition that occur via imprinting (i.e., associative learning) during a brief period following parturition. Disturbance of recognition between the dam and her offspring during the early imprinting period may result in rejection (perhaps fear), despite all the hormonal input or whelping experience. By contrast, altricial species with massive reproductive strategies, like rats, canids, or pigs, are considered less strict because they may accept alien offspring during extended periods [3,4,5]. In addition, some ungulates [6] and nonhuman primates [7,8] may display extensive maternal repertoires daily for weeks or months, whereas others, such as lagomorphs, will display only a few minutes of nursing once a day [9].
Some behaviors that start before parturition might be considered indirect maternal behaviors. For example, nest-building (e.g., digging, shredding paper, straw carrying, and hair pulling) and isolation from the pack or herd [10,11,12,13]. Other early behaviors, such as those observed in pregnant dogs, including restlessness, reduced appetite, lack of attention, drowsiness, aggression, anxiety, fickleness, capriciousness, irritation, and increase in attention request, may only reflect an imminent parturition [14,15]. Indeed, nest-building and isolation are associated with searching for and selecting the appropriate birthplace [16,17]. It is possible that nest-building is more likely observed in altricial species, whereas isolation from others may occur in precocial ones.
Direct maternal behaviors are observed after parturition, during the first contact with the newborns. Dogs will actively bite and tear the fetal membranes and cut the umbilical cord, which functions to prevent asphyxiation of the pups [18]. The dam actively licks the head and the mouth of the newborn to stimulate respiration and orient the pups toward the mammary gland [13,15]. They also lick the anogenital area to facilitate urination and excretion during the first 2 postnatal weeks [19]. Rats also express anogenital licking, especially toward males during the first 10 postnatal days [20]. Preference to lick males is evoked by attractive odors from preputial compounds, such as dodecyl propionate [21], which depend on the levels of systemic steroids. If female pups are treated with androgens (i.e., testosterone and dihydrotestosterone) on the day of birth, they receive an equivalent amount of active anogenital licking as males [22]. Enhanced anogenital stimulation appears to have positive long-term effects on male reproductive behavior [23]. Dams display other active responses as well, such as retrieving the pups, oral consumption of the placenta, and defense from predators and conspecifics, and passive behaviors such as huddling and crouching to regulate body temperature or allowing nursing. In the beginning, passive behaviors may depend on acceptance, whereas active behaviors may depend on enhanced motivation to care. Other species, such as cows, also express intense active licking for the first hour and will be very protective if someone approaches. Passive acceptance may occur within the first 30–120 min postpartum when the calf stands and searches for the udder [16] (Table 1).
Table 1.
Examples of active and passive maternal behaviors that are associated with processes of acceptance, recognition, motivation, and rejection/fear.
| Responses | Acceptance | Recognition | Motivation | Rejection/Fear |
|---|---|---|---|---|
| Passive | Allow proximity | Selective proximity | Staying in nest | Avoidance |
| Nursing | Selective nursing | Crouching over | ||
| Active | Directed calls | Licking | Aggression | |
| Directed attention | Retrieving | Infanticide | ||
| Placenta consumption | ||||
| Defense | ||||
| Nest-building |
The lack of maternal behaviors represents a serious problem that jeopardizes not only the survival of the offspring but also a very important mechanism of early socialization, cognitive development, and epigenetic changes associated with resilience to stress [24,25]. Good maternal behavior is associated with the so-called stress hyporesponsive period [24,26], which refers to a delay of the timing of glucocorticoid elevation in infants, associated with reduced stress response in adulthood [26]. Inappropriate maternal behaviors may occur in 50% of primiparous dogs, especially following cesarean section [27] or as a result of early separation during the postpartum period [28].
3. Natural Stimuli That Facilitate the Expression of Maternal Behavior
3.1. Hormones
Gregarious species have a natural predisposition to care for the young. However, the capacity to express appropriate levels of maternal behavior develops gradually with hormonal changes that occur throughout pregnancy. Then, drastic changes during parturition are needed to trigger the expression of behavior. In rats, for example, concentrations of progesterone (P4) gradually start to increase from the very first day of pregnancy, reaching a peak at day 15, and are followed by a drastic reduction during the last 3 days before delivery. By contrast, the levels of estradiol (E2) and prolactin (PRL) stay relatively low at the beginning but increase dramatically during those last 3 days. The reduction of P4 and increase in hormones such as E2, PRL, oxytocin (OT), and corticosteroids, are the main hormonal drastic changes during parturition [29,30,31] and therefore are associated with sensitization of maternal behavior. For example, in the rabbit doe, digging is stimulated by changes in E2 and P4, while straw carrying and hair pulling are under the control of PRL. In the rat, the reduction in P4 and the increase in E2 and PRL levels facilitate active licking, retrieving, and gathering of pups. Pharmacological blockade of estrogen in the medial preoptic area (mPOA) and small interfering RNA silencing of estrogen receptors (ERα) disrupts maternal behavior in mice [32,33], whereas specific activation of ERα-positive mPOA neurons enhances pup retrieval [34,35]. Likewise, the blockade of PRL receptors within the mPOA in mice abolishes pup retrieval [36].
3.2. Vagino-Cervical Stimulation
In the ewe, licking, low-pitched bleats, and nursing are also evoked by changes in P4/E2 ratio and by the release of OT triggered by vagino-cervical (VCS) and nipple stimulation [37]. VCS caused by the passing of the young through the pelvic canal must be considered a powerful triggering stimulus to evoke maternal behavior after hormonal sensitization during pregnancy. For instance, artificial VCS (pressure on and stretching of the neck of the cervix provided by hand) in ewes can facilitate maternal acceptance toward an alien lamb up to 27.5 h postpartum [38,39]. This also occurs in other species, such as rats, in which normal expression of maternal behavior depends on the interaction between hormonal priming in the mPOA and VCS evoked by parturition. One study showed that, 24 h before parturition, only a few pregnant females exposed to pups (from a different female) expressed active pup retrieval, but 12 h before parturition, up to 80% of them retrieved pups. In addition, that study explored the maternal behavior of pregnant females implanted bilaterally in the mPOA with the antiestrogen 4-hydroxytamoxifen (OH-TAM). Accordingly, 12 h before parturition, none of the OH-TAM females expressed retrieving behavior, and in the absence of parturitional experience (delivery by cesarean section), maternal behavior was almost absent upon exposure to their own pups. By contrast, those OH-TAM females that were allowed to undergo normal parturition (with natural VCS) expressed normal retrieving behavior upon exposure to their pups [40]. Hence, hormones and VCS play a synergistic role in evoking the whole repertoire of maternal behaviors. Indeed, pseudopregnant female rats and mice that go through all the hormonal changes without parturition express only a few indirect maternal behaviors such as nest-building [41,42]. Similarly, pseudopregnant dogs (i.e., pseudocyesis) can also express some maternal behaviors (e.g., nesting, defense) toward pup-looking puppets [43]. In one case report, a sudden decrease in systemic P4 following ovariohysterectomy during the luteal phase of diestrus was reported as the triggering stimulus for maternal behavior, evoking a parturition-like drastic reduction of P4. Similarly, sudden maternal behavior has also been observed in pregnant rats following hysterectomy [44]. Males artificially exposed to E2 and P4 also expressed paternal behavior [45]. When those males received lesions in the mPOA, their behavior was significantly reduced, indicating the mPOA mediates parental behavior in both males and females.
Hormones and physical stimuli (VCS, nipple stimulation) that occur during parturition and lactation are the best natural stimuli that induce maternal behavior. Upon stimulation, magnocellular neurons in the supraoptic (SON) and paraventricular nuclei (PVN) fire high-frequency bursts of action potentials. Each burst generates a large pulse of OT release into the bloodstream to evoke contractions of the uterus and milk ejection [46]. Likewise, parvocellular neurons release OT toward the central nervous system. As our study shows, OT modulates acceptance, social recognition, learning, memory, emotions, reward, eating, drinking, sleep, wakefulness, nociception, analgesia, and sexual and maternal behaviors [47].
3.3. Exposure to Pups
Interestingly, exposure to pups can also result in sensitization of maternal behavior in male and nonpregnant female rats. One week of daily exposure to pups induces both active (e.g., nest-building, retrieving, licking) and passive behaviors (e.g., nursing posture) [48,49,50]. This indicates that gradual exposure sensitizes parental behavior without the need for any hormonal priming. This type of sensitization also occurs when juvenile rats are exposed to infant rats [51], and watching a conspecific perform maternal behavior (i.e., retrieval) activates OT neurons in the observer [52]. Accordingly, the putative neurocircuitry that mediates maternal behavior might be gradually activated and sensitized by daily exposure to pups, but hormones, parturition, and lactation function as triggering stimuli that accelerate its activation.
3.4. Mating
Similarly, copulation can also sensitize the neurocircuitry of parental behavior. In male rats [53] and mice [54], copulation blocks infanticide behavior (an expression of rejection) and facilitates active retrieving (an expression of care motivation) in future encounters with pups [55]. More than 90% of male mice will normally commit infanticide if exposed to pups between 1–4 days after mating with any female, indicating that during those immediate days males reject the pups. However, between 80–90% of those males will behave parentally and will not kill the pups if they are exposed to them 12–50 days after copulating to ejaculation. The actual mechanisms for sex-induced parental behavior in males are unknown but appear to require changes in the mPOA. This area is sensitive to mounts, intromissions, and ejaculations [56,57], such that consecutive copulatory series increase the number of firing neurons in the mPOA [58] and lesions impair consummatory sexual behavior [59]. Those changes may modify plasticity within the mPOA to facilitate parental behavior.
3.5. Maternal Experience
Former maternal experience also improves the expression of maternal responses. For example, multiparous dogs express more time of body contact with pups and constant maternal care during the 21-day postpartum period, whereas primiparous females show a gradual increase in licking, nursing, and contact with the puppies from day 1 to 21 [60]. Multiparous cows also express more maternal defense than primiparous [61] and isolate less from the herd [62], probably related to less intense fear, considering that multiparous female rats are less anxious in open field tests, compared to primiparous females [63]. In sheep, former maternal experience is associated with increased suckling, following, grooming, and low-pitched bleating and decreased aggressive behavior [64].
3.6. Environmental Enrichment
Environmental enrichment (EE) also improves maternal behavior. In one study with rats, EE condition consisted of housing seven females per cage; the EE cage (120 × 100 × 70 cm) was designed with four floors with lid ramps and contained plastic balls, tubes, and bedding material. The interactive objects and location of food were rearranged every three days to increase novelty and complexity, which resulted in a highly stimulating sensory and social environment with other females. Following parturition, EE females expressed less anxiety and displayed more licking, grooming, and crouching over pups during the first postpartum week as compared to females living in standard cages [65]. During that period, EE mothers also showed more aggressiveness to an intruder female. Associated with offspring-directed behaviors, EE females expressed more neural activity in the mPOA, PVN, and medial amygdala (MeA) but less activity in the basolateral amygdala (BLA) than standard-housed females [66]. As we discuss in the following sections, those brain areas are associated with maternal motivation and rejection, respectively. Taken together, the data indicate that the capacity to express maternal behavior develops as a consequence of hormonal priming but is triggered by stimuli such as parturition and lactation. In addition, cohabitation, copulation, former experience, and environmental enrichment facilitate its expression (Table 2).
Table 2.
Natural stimuli that trigger maternal behavior. Estrogen (E2), progesterone (P4), prolactin (PRL), oxytocin (OT), vaginocervical stimulation (VCS). ⇑ = increase, ⇓ = decrease.
| Stimuli | Effect | Behavioral Response | Representative References |
|---|---|---|---|
| Hormones E2 P4 PRL OT |
⇑ ⇑ ⇑ ⇑ |
Pup retrieval Retrieving Nest-building Nursing |
[34,35] [45] [36] [47] |
| Parturition VCS |
⇑ |
Nursing Retrieving |
[38,39] [40] |
| Mating | ⇓ | Infanticide | [54,55] |
| Exposure to pups | ⇑ | Nest-building, retrieving, licking, crouching posture |
[48,49,50] |
| Experience Multiparous |
⇑ ⇑ ⇑ ⇑ ⇓ |
Licking, nursing, contact Defense Suckling Following, grooming Rejection |
[60] [61] [64] |
| Environmental enrichment | ⇓ ⇑ |
Anxiety Licking, grooming, crouching posture, defense |
[65] [66] |
4. Neural Pathways Underlying Maternal Behavior
4.1. Areas Involved in Acceptance and Social Recognition
Maternal behavior requires at least three neural processes to mediate: (1) increase in acceptance/social recognition, (2) decrease in rejection/fear, and (3) increase in care motivation [67]. Acceptance/recognition occurs when a sensitized brain is exposed to the right stimulus. For example, during the postpartum period, bitches will accept any pup scented with amniotic fluid. Washing the pups immediately after birth results in a lack of acceptance. However, if pups are bathed in amniotic fluid, the process of acceptance is restored, even hours later [28]. Similarly, washing a newborn lamb impairs acceptance/recognition behaviors in sheep (low bleats, acceptance at the udder, nursing, and licking time) and increases rejection/fear behaviors (high-pitched bleats, rejection at the udder, and aggressive behavior). Preventing contact with the mother during the first 4 h postpartum worsens rejection. Interestingly, washing an alien lamb also improves its acceptance [68]. Indeed, both acceptance and rejection behaviors depend on the olfactory system because anosmic ewes (treated with intranasal zinc sulfate) fail to express any acceptance or rejection behavior [69]. However, if tested many hours after birth, anosmic sheep will be capable of accepting/recognizing their lamb through visual or auditory cues that have been learned during the first postpartum hours [70].
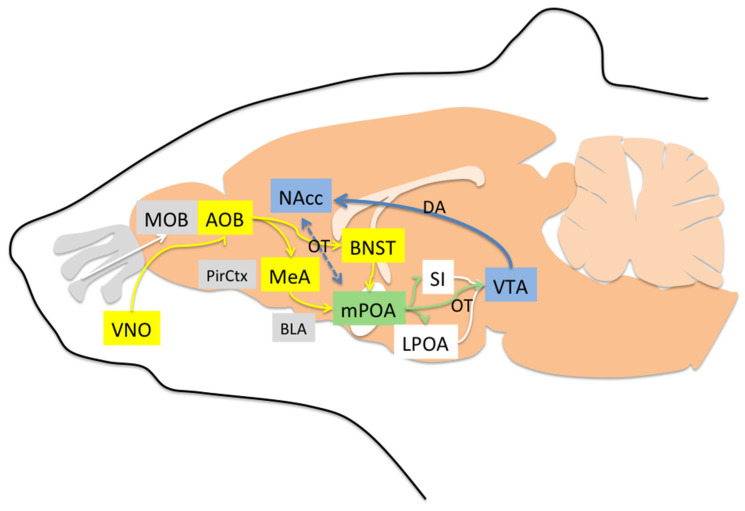
Olfactory social recognition is perhaps the most relevant sensory system in many mammals and depends on both main and accessory olfactory pathways. Nonvolatile odor molecules are detected by the accessory pathway via the vomeronasal organ (VNO), located in the soft tissue of the nasal septum. The VNO projects to the accessory olfactory bulb (AOB), which innervates both the bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA). BNST and MeA project to the mPOA, which in turn projects to the lateral preoptic area (LPOA) and substantia innominata (SI). LPOA and SI also send efferents to the ventral tegmental area (VTA), which sends dopaminergic projections to the nucleus accumbens (NAcc) [71,72]. With regard to volatile odor molecules (those that have a low boiling point and evaporate easily at room temperature), they are detected in the roof of the nasal cavity by the main olfactory epithelium (MOE), where olfactory sensory neurons are located. These neurons project to the main olfactory bulb (MOB), and MOB has efferents to the piriform cortex (PirCtx), and from there to the NAcc and mPOA [71]. Thus, both volatile odors and nonvolatile odors not only access the social recognition system but also the mesolimbic pathway of motivation. Within these pathways, social recognition depends on the nonapeptides arginine vasopressin (AVP) and oxytocin (OT). Mice with depletion of AVP V1a receptor (V1aRKO) exhibit profound social recognition impairment. They can remember the odors of food but fail to remember the odors of individuals they just spent time with [73,74]. Likewise, OT knockout mice (OTKO) express impaired discrimination to odors of conspecifics [75]. Following a social encounter, OTKO mice express less activity in MeA, BNST, and mPOA, but an infusion of OT in the MeA is sufficient to restore social recognition [74,76,77]. Interestingly, during the postpartum period, OT receptor (OTR) binding increases not only in the MeA but also in the BNST, mPOA [78], and dopaminergic regions of the brain stem [74,79,80,81,82,83]. The increase in OTR is mainly the result of exposure to E2 [84]. Thus, OT and OTR start to play a gradual role in the acceptance and olfactory social recognition via MeA starting two days before parturition when E2 increases. Experiments in sheep have shown that infusion of local anesthesia in the MeA or cortical amygdala (CoA) impairs recognition of the lamb during the early postpartum period, but this was not found with infusions in the BLA region [85]. As we describe later, BLA mediates rejection/fear responses, whereas MeA and CoA mediate olfactory acceptance/recognition, mainly influenced by OT, AVP, and dopamine (DA) (Figure 1).
Figure 1.
Sagittal drawing of brain olfactory pathways involved in acceptance, offspring social recognition, care motivation, and inhibition of rejection. Nonvolatile odor molecules are processed by the accessory pathway (yellow) via the vomeronasal organ (VNO), accessory olfactory bulb (AOB), bed nucleus of the stria terminalis (BNST), medial amygdala (MeA), and medial preoptic area (mPOA, green). The mPOA projects to the lateral preoptic area (LPOA), substantia innominata (SI), and ventral tegmental area (VTA, blue), which sends dopaminergic projections to the nucleus accumbens (NAcc, blue). Volatile odor molecules are processed by the main (gray) olfactory epithelium (MOE), main olfactory bulb (MOB), and piriform cortex (PirCtx), and from there to the NAcc and mPOA (which interact via oxytocin “OT” and dopamine “DA”). The basolateral (BLA) amygdala and prefrontal cortex (not shown) participate in rejection and fear.
In dogs, salivary OT (sOT) increases gradually during the postpartum period [86]. High levels of sOT are negatively correlated with the frequency of sniffing behavior toward the pups, perhaps because high sOT facilitates olfactory recognition, and recognized pups require less sniffing. Given that sniffing and time spent out of the whelping box have been positively correlated, low sOT is also a predictor of time away from the pups. Therefore, treatment with OT should improve maternal recognition and performance. In fact, rats that undergo cesarean section lack a naturally powerful stimulus to release OT, which results in reduced maternal behavior. However, intranasal OT in those rats restores pup retrieval and anogenital licking [87]. Similarly, intranasal OT facilitates paternal motivation, as observed in male marmosets that express shorter latency to respond with an approach to infant stimuli [88]. Dogs treated with intranasal OT turn more social toward humans [89] and more playful with other dogs [90]. In female rats, OT improves depression-like behaviors [91] and induces conditioned place preference (CPP) in the presence of a conspecific (social-CPP). Social-CPP reflects emotional memories of a place where cohabitation occurred, and it has been reported in rats that receive MDMA “Ecstasy” but not in those that receive AVP. Accordingly, both OT and AVP regulate olfactory recognition, but only OT augments the rewarding effects of social interaction [92].
In dogs, polymorphisms of the OTR gene have been associated with variations in the maternal behavior and with higher levels of sOT [93]. However, to date, no one has shown the potential good effects of intranasal OT in dogs with poor maternal behavior. Certainly, systemic injections of OT at birth are commonly indicated to facilitate uterine contractions, but only 1% of such systemic OT crosses the blood–brain barrier (BBB) [94]. Nevertheless, it has been demonstrated that fetal voles from mothers injected with OT at birth expressed increased methylation of OTR in the brain. In adulthood, OT-exposed voles are more gregarious, with increased alloparental caregiving toward pups and increased close social contact with other adults [95]. Thus, OT treatment at birth (even if injected) may facilitate both immediate and future social encounters.
4.2. Areas Mainly Involved in Increasing Motivation
The NAcc and mPOA are the main areas involved in maternal motivation [96]. In rats, broad lesions in the mPOA disrupted pup retrieval and reduced Fos-immunoreactivity in the NAcc evoked by exposure to pups [97]. However, specific lesions in the ventromedial region of the preoptic area (vmPOA) disrupt nest-building in mice but do not affect pup retrieval, whereas specific lesions in the central part of the mPOA (cmPOA) disrupt all maternal behaviors [98]. Lesions in the NAcc also decrease pup retrieval and bar pressing to gain access to pups [99], whereas lesions in the VTA (where NAcc DA afferents originate) disrupt the frequency of approaches and interaction with pups [100,101].
Between mPOA and NAcc, there is a synergistic role OT and DA. One study showed that female rats that display high levels of active maternal behavior (i.e., licking/grooming) express more OT-positive neurons in the mPOA and PVN, which in turn, project to the VTA. Thus, OT in the VTA facilitates DA release into the NAcc, which also enhances motivation [102]. The mPOA itself is a large and complex region formed by many subnuclei, such as the medial preoptic nucleus (MPN), median preoptic nucleus (MnPO), posterodorsal preoptic nucleus (PD), and ventrolateral preoptic nucleus (VLPO) in mice. Within all these nuclei, there is a vast diversity of neuron populations based on gene expression for transporters or neurotransmitters that are not found in the lateral preoptic area (LPOA). For instance, at least fifteen different markers of cell populations have been reported in the mice mPOA, including glutamic acid decarboxylase (GAD67), galanin, calbindin, a novel marker of sexually dimorphic nuclei (Moxd1), cocaine- and amphetamine-regulated transcript (CART), vesicular glutamate transporter (VGLUT2), vesicular GABA transporter (Vgat), proenkephalin (Penk), brain-derived neurotrophic factor (BDNF), leptin, cholecystokinin (CCK), neurotensin, neuropeptide tachykinin 2 (Tac2), prodynorphin (Pdyn), thyrotropin-releasing hormone (TRH), and oxytocin (reviewed in [103]). Many of these markers are known for controlling mechanisms of body temperature, wake–sleep cycle, or sexual behavior and appear not to have any direct role in maternal behavior. However, galaninergic neurons within the lateral part of the mouse mPOA (lmPOA) and MPN project toward MeA, VTA, PVN, and periaqueductal gray (PAG) and play a major role in motor coordination, motivation, and social recognition during maternal behavior by integrating inputs from many areas in the brain [104]. More than 70% of galanin-positive neurons in the mPOA also express alpha estrogen receptors (ERα) and androgen receptors [105] and therefore may become more active during the final phase of pregnancy. Optogenetic activation of mPOA galanin-positive neurons projecting to the PAG enhances pup grooming, whereas inhibition of those neurons impairs grooming. Likewise, activation of galanin neurons that project to VTA promotes interaction with pups but not pup retrieval, and their inhibition suppresses interaction [104]. The mPOA also receives input from hypocretin-1-containing neurons (HCRT-1, also known as orexin A) from the postero-lateral hypothalamus. Activation of HCRT-1 metabotropic receptors within the mPOA promotes active maternal behaviors such as licking and retrieving but also passive responses such as nursing [106]. The mPOA also receives inhibitory inputs via agouti-related neuropeptide (AGRP) neurons from the arcuate nucleus, which respond in conditions of caloric needs and normally produce hunger. Activation of AGRP inhibitory neurons or its projections to mPOA results in less maternal nest-building, without affecting pup retrieval, partly recapitulating suppression of maternal behaviors during food restriction [107]. Thus, retrieving pups and nest-building are associated with different neuronal activity patterns in the mPOA, such that neurons that are activated during pup retrieval tend to be inhibited during nest-building [34]. Some AGRP neurons that project directly to mPOA express Vgat. Optogenetic stimulation of Vgat neurons in the mPOA elicits both pup retrieval and nest-building, whereas inhibition of Vgat neurons results in decreased nest-building. Thus, GABA activation in mPOA can inhibit inhibitory AGRP neurons and facilitate all maternal behaviors. However, if stimulation is exclusively directed toward the subpopulation of Vgat neurons that express ERα, then only pup-retrieval is elicited, and not nest-building [107] (Table 3). In addition, ERα-positive neurons in the mouse mPOA project inhibitory efferents to nondopaminergic neurons in the VTA that promote maternal pup retrieval through disinhibition of dopaminergic neurons [34]. Accordingly, galanin-positive, ERα-positive, and Vgat-positive neurons may be involved in maternal motivation (pup retrieval) via mPOA and VTA [103] (Figure 2).
Like the mPOA, the NAcc also facilitates motivation toward recognized/accepted individuals. The NAcc is also important for the consolidation of social bonds. For example, neurons of the NAcc in monogamous voles express more OT receptors than in the polygamous voles [108]. Infusion of an OT antagonist into the NAcc of monogamous voles disrupts pair bonds facilitated by DA agonists, whereas OT antagonists disrupt bonds facilitated by DA agonists [109]. Pair bonds that develop after sex are disrupted by infusions of DA antagonists (i.e., haloperidol) in the NAcc, and by contrast, low doses of DA agonists (i.e., apomorphine) facilitate their development even in absence of sex [110,111]. Thus, having a working DA system is necessary, but not sufficient (OT is needed) to induce selective social bonds. During exposure to pups, maternal rats release more DA in the NAcc [112], as well as during licking and grooming of the pups [113]. Likewise, infusions of general DA antagonists such as flupenthixol into the NAcc of lactating rats impair maternal behavior (retrieval and licking) [114]. Specific injections of a D1-type antagonist (SCH23390) in the NAcc impairs pup retrieval, whereas a D2-type antagonist (eticlopride) does not impair such behavior [115]. Thus, maternal behavior is mainly facilitated by D1-type receptors and OT.
4.3. Areas Mainly Involved in Reducing Rejection/Fear
In addition to being motivated, individuals must reduce their rejection and fear of pups during the first postpartum encounters. This represents a dichotomy in excitatory/inhibitory neurocircuitries and appears to depend on the positive interaction of mPOA/BNST and a negative mechanism mediated by the anterior hypothalamic nucleus (AHN)/ventromedial nucleus (VMN)/PAG, amygdala, and some cortical areas [116]. Thus, activation of mPOA appears to facilitate maternal behavior upon its release from the inhibitory effects of the amygdala [117]. For instance, lesions of the vomeronasal inputs (amygdala) to the mPOA facilitate maternal behavior even in nulliparous female rats [118,119,120], mainly due to BLA projections that mediate fear responses [121,122]. BLA increases its expression of OTR during the postpartum period [108], and it can be inhibited by endogenous opioids as well [123]. Therefore, the elevation of OT and endogenous opioids during parturition (i.e., during VCS) may help reduce fear/rejection toward pups [124]. Opioids also regulate the release of both OT [125] and DA into the prefrontal cortex (PFC) [126]. PFC plays a key role in attention, memory, and negative emotions [127]. When activity in PFC is reduced, sheep express less aggressive behavior toward alien lambs without affecting the social recognition of their own lambs [128]. PFC also expresses more ERα and OTR in lactating rats, which appear to be associated with decreased anxiety [129].
The central region of mPOA and MPN also mediate the inhibition of some rejection responses. These regions express galanin, and direct optogenetic stimulation of galanin-positive neurons in male mice suppresses pup-directed aggression [130]. Within the vmPOA, some neurons mediate sickness symptoms [131]. However, it is unknown whether those neurons may induce rejection or fear toward pups.
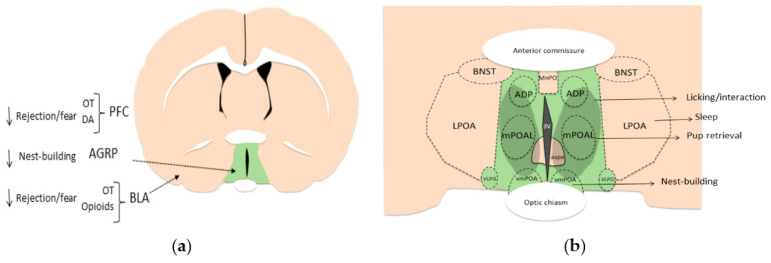
Figure 2.
(a) Coronal drawing of a rat brain (modified from [132]). The green region represents the medial preoptic area (mPOA) as the main generator of maternal motivation via oxytocin (OT) and dopamine (DA) activity. Agouti-related peptide (AGRP) facilitates rejection in mPOA, but OT, DA, and opioids reduce rejection via other regions such as prefrontal cortex (PFC, not shown) and basolateral amygdala (BLA). (b) Amplification of the mPOA and subregions in the rat brain. Darker green spot represents putative galanin/ER+ neurons that mediate the expression of licking and pup retrieval in the lateral (mPOAL) and dorsal (ADP) parts of mPOA, where OT neurons are also found (modified from [103]). Nest-building depends on the activity of the ventral portion of the mPOA (vmPOA). Also shown are the bed nucleus of stria terminalis (BNST), median preoptic nucleus (MnPO), ventrolateral nucleus (vlPO), anteroventral periventricular nucleus (avpv), and lateral preoptic area (LPOA) that mediates sleep. Different mPOA subregions mediate diverse maternal behaviors interacting with mesolimbic regions.
Table 3.
Brain areas and neurochemicals associated with maternal behaviors. Medial amygdala (MeA), bed nucleus of stria terminalis (BNST), cortical amygdala (CoA), medial preoptic area (mPOA), ventral tegmental area (VTA), basolateral amygdala (BLA), nucleus accumbens (NAc), prefrontal cortex (PFC), oxytocin (OT), estrogen (E2), prolactin (PRL), dopamine (DA), vesicular GABA transporter (Vgat), hypocretin-1 neurons (HCRT-1), agouti related protein (AGRP).
| Brain Area | Neurochemical | Effect | Representative References |
|---|---|---|---|
| MeA BNST CoA |
OT | Acceptance, social recognition | [74,79,83] |
| mPOA | E2 PRL OT DA Galanin Vgat HCRT-1 AGRP |
Pup retrieval Pup retrieval, nest-building Acceptance, licking/grooming Motivation, licking, grooming Motor coordination, motivation, recognition, grooming, inhibition of aggression Nest-building Licking/retrieval Inhibition of nest-building |
[32,33,40] [36] [102,103] [103,104,130] [130] [106] [107] |
| VTA | OT | Approach, interaction | [74,79,86] |
| BLA | OT opioids |
Inhibit rejection/fear | [85,108,121,124] |
| NAc | DA OT |
Motivation, approach, interaction, pup retrieval | [99,100,101,112] |
| PFC | OT DA |
Inhibit rejection/fear | [125,126] |
5. Conclusions
The capacity to express maternal behavior develops gradually as a consequence of hormonal priming during pregnancy. Around parturition time, sudden changes in hormones and physical stimuli trigger the expression of acceptance, recognition, and motivation at a time that reduces rejection and fear. The neurocircuitries of maternal behavior can be also sensitized by cohabitation, sex, and experience. Olfactory recognition depends mainly on the activity of OT in the MeA. Motivation is mainly mediated by OT and DA in NAcc and subregions of the mPOA, in which ventral parts mediate nest-building, and central and dorsal parts mediate licking retrieval and interaction with pups. Finally, BLA, PFC, and other hypothalamic regions (i.e., arcuate nucleus) mediate rejection and fear responses. These areas mediate discrimination of stimuli that might have an incentive value per se (i.e., amniotic fluid) or stimuli related to past experiences (i.e., memories of sexual reward, maternal reward, cohabitation, etc.).
Author Contributions
Conceptualization, G.A.C.-A.; investigation, G.A.C.-A., D.H.-C., L.I.G., R.T., M.E.H., P.P.-R., A.A.C.-M. and J.M.; writing—original draft preparation, G.A.C.-A.; writing—review and editing, G.A.C.-A., D.H.-C., L.I.G., R.T., M.E.H., P.P.-R., A.A.C.-M. and J.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kendrick K.M., Hinton M.R., Atkins K., Haupt M.A., Skinner J.D. Mothers determine sexual preferences. Nature. 1998;395:229–230. doi: 10.1038/26129. [DOI] [PubMed] [Google Scholar]
- 2.Crowell-Davis S.L., Houpt K.A. Maternal Behavior. Veter. Clin. N. Am. Equine Pract. 1986;2:557–571. doi: 10.1016/S0749-0739(17)30706-X. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X., Wang M., He T., Long S., Guo Y., Chen Z. Effect of Different Cross-Fostering Strategies on Growth Performance, Stress Status and Immunoglobulin of Piglets. Animals. 2021;11:499. doi: 10.3390/ani11020499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grota L.J. Effects of litter size, age of young, and parity on foster mother behaviour in Rattus norvegicus. Anim. Behav. 1973;21:78–82. doi: 10.1016/S0003-3472(73)80042-9. [DOI] [PubMed] [Google Scholar]
- 5.Scharis I., Amundin M. Cross-fostering in gray wolves (Canis lupus lupus) Zoo Biol. 2015;34:217–222. doi: 10.1002/zoo.21208. [DOI] [PubMed] [Google Scholar]
- 6.von Keyserlingk M.A., Weary D.M. Maternal behavior in cattle. Horm. Behav. 2007;52:106–113. doi: 10.1016/j.yhbeh.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Dienske H., Van Vreeswijk W. Regulation of nursing in chimpanzees. Dev. Psychobiol. 1987;20:71–83. doi: 10.1002/dev.420200110. [DOI] [PubMed] [Google Scholar]
- 8.Davenport R.K., Jr., Menzel E.W., Jr., Rogers C.M. Maternal care during infancy: Its effect on weight gain and mortality in the chimpanzee. Am. J. Orthopsychiatry. 1961;31:803–809. doi: 10.1111/j.1939-0025.1961.tb02178.x. [DOI] [PubMed] [Google Scholar]
- 9.Schulte I., Hoy S. Nursing and suckling behavior and mother-child contacts in domestic rabbits. Berl. Munch. Tierarztl. Wochenschr. 1997;110:134–138. [PubMed] [Google Scholar]
- 10.Kleiman D. Reproduction in the Canidae. Int. Zoo Yearb. 1968;8:3–8. doi: 10.1111/j.1748-1090.1968.tb00419.x. [DOI] [Google Scholar]
- 11.Denenberg V.H., Zarrow M., Taylor R.E. Maternal Behavior in the Rat: An Investigation and Quantification of Nest Building. Behaviour. 1969;34:1–16. doi: 10.1163/156853969X00369. [DOI] [PubMed] [Google Scholar]
- 12.Benedek I., Altbäcker V., Zsolnai A., Molnár T. Exploring the Genetic Background of the Differences in Nest-Building Behavior in European Rabbit. Animals. 2020;10:1579. doi: 10.3390/ani10091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleicher N. Behavior of the bitch during parturition. J. Am. Veter. Med. Assoc. 1962;140:1076–1082. [PubMed] [Google Scholar]
- 14.Ferrari J., Monteiro-Filho E.L.A. Canid Familiaris-Comparative Analysis of pre and Postpartum Behavioral Patterns. Universidade Federal do Paraná; Curitiba, Brazil: 2004. [Google Scholar]
- 15.Santos N.R., Beck A., Fontbonne A. A review of maternal behaviour in dogs and potential areas for further research. J. Small Anim. Pract. 2019;61:85–92. doi: 10.1111/jsap.13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lidfors L. Parental Behavior in Bovines. Adv. Neurobiol. 2022;27:177–212. doi: 10.1007/978-3-030-97762-7_6. [DOI] [PubMed] [Google Scholar]
- 17.Rørvang M.V., Nielsen B.L., Herskin M.S., Jensen M.B. Prepartum Maternal Behavior of Domesticated Cattle: A Comparison with Managed, Feral, and Wild Ungulates. Front. Veter. Sci. 2018;5:45. doi: 10.3389/fvets.2018.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweizer C.M., Meyers-Wallen V.M. Medical managment of dystocia and indications of cesarean section in the bitch. In: Bonagura W.B., editor. Current Veterinary Therapy XIII. Saunders Co; Philadelphia, PA, USA: 2000. pp. 933–939. [Google Scholar]
- 19.Rheingold H.L. Maternal behavior in the dog. In: Mammals I., Reingold H.L., editors. Maternal Behaviors. John Wiley and Sons; New York, NY, USA: 1963. pp. 169–202. [Google Scholar]
- 20.Moore C.L., Morelli G.A. Mother rats interact differently with male and female offspring. J. Comp. Physiol. Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 21.Lévy F., Keller M., Poindron P. Olfactory regulation of maternal behavior in mammals. Horm. Behav. 2004;46:284–302. doi: 10.1016/j.yhbeh.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Moore C. Maternal behavior of rats is affected by hormonal condition of pups. J. Comp. Physiol. Psychol. 1982;96:123–129. doi: 10.1037/h0077866. [DOI] [PubMed] [Google Scholar]
- 23.Moore C.L. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev. Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa M., Shibata Y., Yonezawa A., Takahashi T., Kanai M., Ohtsuka H., Suenaga Y., Yabana Y., Mogi K., Kikusui T. Basal cortisol concentrations related to maternal behavior during puppy development predict post-growth resilience in dogs. Horm. Behav. 2021;136:105055. doi: 10.1016/j.yhbeh.2021.105055. [DOI] [PubMed] [Google Scholar]
- 25.Weaver I.C., Cervoni N., Champagne F.A., D’Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epi-genetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 26.Nagasawa M., Shibata Y., Yonezawa A., Morita T., Kanai M., Mogi K., Kikusui T. The behavioral and endocrinological development of stress response in dogs. Dev. Psychobiol. 2013;56:726–733. doi: 10.1002/dev.21141. [DOI] [PubMed] [Google Scholar]
- 27.Santos N.R., Beck A., Maenhoudt C., Billy C., Fontbonne A. Profile of Dogs’ Breeders and Their Considerations on Female Reproduction, Maternal Care and the Peripartum Stress—An International Survey. Animals. 2021;11:2372. doi: 10.3390/ani11082372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abitbol M.L., Inglis S.R. Role of amniotic fluid in newborn acceptance and bonding in canines. J. Matern.-Fetal Med. 1997;6:49–52. doi: 10.1002/(SICI)1520-6661(199701/02)6:13.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblatt J.S., Mayer A.D., Giordano A.L. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 30.Nelson R.J. An Introduction to Behavioral Endocrinology. 2nd ed. Sinauer Associates; Sunderland, MA, USA: 2000. [Google Scholar]
- 31.Bridges R.S. The role of lactogenic hormones in maternal behavior in female rats. Acta Paediatr. Suppl. 1994;83:33–39. doi: 10.1111/j.1651-2227.1994.tb13263.x. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro A.C., Musatov S., Shteyler A., Simanduyev S., Arrieta-Cruz I., Ogawa S., Pfaff D.W. siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc. Natl. Acad. Sci. USA. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catanese M., Vandenberg L.N. Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology. 2016;158:516–530. doi: 10.1210/en.2016-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fang Y.-Y., Yamaguchi T., Song S.C., Tritsch N.X., Lin D. A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron. 2018;98:192–207.e10. doi: 10.1016/j.neuron.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei Y.-C., Wang S.-R., Jiao Z.-L., Zhang W., Lin J.-K., Li X.-Y., Li S.-S., Zhang X., Xu X.-H. Medial preoptic area in mice is capable of mediating sexually dimorphic behaviors regardless of gender. Nat. Commun. 2018;9:279. doi: 10.1038/s41467-017-02648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown R.S.E., Aoki M., Ladyman S.R., Phillipps H.R., Wyatt A., Boehm U., Grattan D.R. Prolactin action in the medial preoptic area is necessary for postpartum maternal nursing behavior. Proc. Natl. Acad. Sci. USA. 2017;114:10779–10784. doi: 10.1073/pnas.1708025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lévy F. Neuroendocrine control of maternal behavior in non-human and human mammals. Ann. d’Endocrinol. 2016;77:114–125. doi: 10.1016/j.ando.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Kendrick K., Lévy F., Keverne E. Importance of vaginocervical stimulation for the formation of maternal bonding in primiparous and multiparous parturient ewes. Physiol. Behav. 1991;50:595–600. doi: 10.1016/0031-9384(91)90551-X. [DOI] [PubMed] [Google Scholar]
- 39.Keverne E.B., Levy F., Poindron P., Lindsay D.R. Vaginal Stimulation: An Important Determinant of Maternal Bonding in Sheep. Science. 1983;219:81–83. doi: 10.1126/science.6849123. [DOI] [PubMed] [Google Scholar]
- 40.Ahdieh H.B., Mayer A.D., Rosenblatt J.S. Effects of Brain Antiestrogen Implants on Maternal Behavior and on Postpartum Estrus in Pregnant Rats. Neuroendocrinology. 1987;46:522–531. doi: 10.1159/000124875. [DOI] [PubMed] [Google Scholar]
- 41.Gandelman R., McDermott N.J., Kleinman M., DeJianne D. Maternal nest building by pseudopregnant mice. Reproduction. 1979;56:697–699. doi: 10.1530/jrf.0.0560697. [DOI] [PubMed] [Google Scholar]
- 42.Steuer M.A., Thompson A.C., Doerr J.C., Youakim M., Kristal M.B. Induction of maternal behavior in rats: Effects of pseudopregnancy termination and placenta-smeared pups. Behav. Neurosci. 1987;101:219–227. doi: 10.1037/0735-7044.101.2.219. [DOI] [PubMed] [Google Scholar]
- 43.Misner T.L., A Houpt K. Animal behavior case of the month. Aggression that began 4 days after ovariohysterectomy. J. Am. Veter. Med. Assoc. 1998;213:1260–1262. [PubMed] [Google Scholar]
- 44.Rosenblatt J.S., Siegel H.I. Hysterectomy-induced maternal behavior during pregnancy in the rat. J. Comp. Physiol. Psychol. 1975;89:685–700. doi: 10.1037/h0077052. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblatt J.S., Hazelwood S., Poole J. Maternal Behavior in Male Rats: Effects of Medial Preoptic Area Lesions and Presence of Maternal Aggression. Horm. Behav. 1996;30:201–215. doi: 10.1006/hbeh.1996.0025. [DOI] [PubMed] [Google Scholar]
- 46.Perkinson M.R., Kim J.S., Iremonger K.J., Brown C.H. Visualising oxytocin neurone activity in vivo: The key to unlocking central regulation of parturition and lactation. J. Neuroendocr. 2021;33:e13012. doi: 10.1111/jne.13012. [DOI] [PubMed] [Google Scholar]
- 47.Wang P., Wang S.C., Liu X., Jia S., Wang X., Li T., Yu J., Parpura V., Wang Y.-F. Neural Functions of Hypothalamic Oxytocin and its Regulation. ASN Neuro. 2022;14:17590914221100706. doi: 10.1177/17590914221100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terkel J., Rosenblatt J.S. Humoral factors underlying maternal behavior at parturition: Corss transfusion between freely moving rats. J. Comp. Physiol. Psychol. 1972;80:365–371. doi: 10.1037/h0032965. [DOI] [PubMed] [Google Scholar]
- 49.Rosenblatt J.S. Nonhormonal Basis of Maternal Behavior in the Rat. Science. 1967;156:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 50.Jakubowski M., Terkel J. Transition from pup killing to parental behavior in male and virgin female albino rats. Physiol. Behav. 1985;34:683–686. doi: 10.1016/0031-9384(85)90364-6. [DOI] [PubMed] [Google Scholar]
- 51.Harding K.M., Lonstein J.S. Extensive juvenile “babysitting” facilitates later adult maternal responsiveness, decreases anxiety, and increases dorsal raphe tryptophan hydroxylase-2 expression in female laboratory rats. Dev. Psychobiol. 2016;58:492–508. doi: 10.1002/dev.21392. [DOI] [PubMed] [Google Scholar]
- 52.Carcea I., Caraballo N.L., Marlin B.J., Ooyama R., Riceberg J.S., Navarro J.M.M., Opendak M., Diaz V.E., Schuster L., Torres M.I.A., et al. Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596:553–557. doi: 10.1038/s41586-021-03814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mennella J.A., Moltz H. Infanticide in rats: Male strategy and female counter-strategy. Physiol. Behav. 1988;42:19–28. doi: 10.1016/0031-9384(88)90254-5. [DOI] [PubMed] [Google Scholar]
- 54.vom Saal F.S. Time-contingent change in infanticide and parental behavior induced by ejaculation in male mice. Physiol. Behav. 1985;34:7–15. doi: 10.1016/0031-9384(85)90069-1. [DOI] [PubMed] [Google Scholar]
- 55.Dulac C., O’Connell L.A., Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coolen L.M., Peters H.J., Veening J.G. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: A sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- 57.Malsbury C.W. Facilitation of male rat copulatory behavior by electrical stimulation of the medial preoptic area. Physiol. Behav. 1971;7:797–805. doi: 10.1016/0031-9384(71)90042-4. [DOI] [PubMed] [Google Scholar]
- 58.Shimura T., Yamamoto T., Shimokochi M. The medial preoptic area is involved in both sexual arousal and performance in male rats: Re-evaluation of neuron activity in freely moving animals. Brain Res. 1994;640:215–222. doi: 10.1016/0006-8993(94)91875-9. [DOI] [PubMed] [Google Scholar]
- 59.Arendash G.W., Gorski R.A. Effects of discrete lesions of the sexually dimorphic nucleus of the preoptic area or other medial preoptic regions on the sexual behavior of male rats. Brain Res. Bull. 1983;10:147–154. doi: 10.1016/0361-9230(83)90086-2. [DOI] [PubMed] [Google Scholar]
- 60.Guardini G., Bowen J., Raviglione S., Farina R., Gazzano A. Maternal behaviour in domestic dogs: A comparison between primiparous and multiparous dogs. Dog Behav. 2015;1:23–33. doi: 10.4454/DB.V1I1.4. [DOI] [Google Scholar]
- 61.Vicentini R.R., El Faro L., Ujita A., Lima M.L.P., Oliveira A.P., Sant’Anna A.C. Is maternal defensiveness of Gyr cows (Bos taurus indicus) related to parity and cows’ behaviors during the peripartum period? PLoS ONE. 2022;17:e0274392. doi: 10.1371/journal.pone.0274392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jensen M.B., Webb L.E., Vaarst M., Bokkers E. The effect of hides and parity on behavior of periparturient dairy cows at pasture. J. Dairy Sci. 2022;105:6196–6206. doi: 10.3168/jds.2021-21614. [DOI] [PubMed] [Google Scholar]
- 63.Aguggia J.P., Suárez M.M., Rivarola M.A. Multiparity Dampened the Neurobehavioral Consequences of Mother–Pup Separation Stress in Dams. Neuroscience. 2019;416:207–220. doi: 10.1016/j.neuroscience.2019.07.042. [DOI] [PubMed] [Google Scholar]
- 64.Lv S.-J., Yang Y., Li F.-K. Parity and litter size effects on maternal behavior of Small Tail Han sheep in China. Anim. Sci. J. 2015;87:361–369. doi: 10.1111/asj.12441. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Y.-M., Cheng Y.-Z., Wang Y.-T., Wei R.-M., Ge Y.-J., Kong X.-Y., Li X.-Y. Environmental Enrichment Reverses Maternal Sleep Deprivation-Induced Anxiety-Like Behavior and Cognitive Impairment in CD-1 Mice. Front. Behav. Neurosci. 2022;16:943900. doi: 10.3389/fnbeh.2022.943900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Núñez-Murrieta M.A., Noguez P., Coria-Avila G.A., García-García F., Santiago-García J., Bolado-García V.E., Corona-Morales A.A. Maternal behavior, novelty confrontation, and subcortical c-Fos expression during lactation period are shaped by gestational environment. Behav. Brain Res. 2021;412:113432. doi: 10.1016/j.bbr.2021.113432. [DOI] [PubMed] [Google Scholar]
- 67.Coria-Avila G.A., Manzo J., Garcia L.I., Carrillo P., Miquel M., Pfaus J.G. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014;43:173–182. doi: 10.1016/j.neubiorev.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Poindron P., Otal J., Ferreira G., Keller M., Guesdon V., Nowak R., Lévy F. Amniotic fluid is important for the maintenance of maternal responsiveness and the establishment of maternal selectivity in sheep. Animal. 2010;4:2057–2064. doi: 10.1017/S1751731110001126. [DOI] [PubMed] [Google Scholar]
- 69.Levy F., Poindron P., Le Neindre P. Attraction and repulsion by amniotic fluids and their olfactory control in the ewe around parturition. Physiol. Behav. 1983;31:687–692. doi: 10.1016/S0031-9384(83)80004-3. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira G., Terrazas A., Poindron P., Nowak R., Orgeur P., Lévy F. Learning of olfactory cues is not necessary for early lamb recognition by the mother. Physiol. Behav. 2000;69:405–412. doi: 10.1016/S0031-9384(00)00211-0. [DOI] [PubMed] [Google Scholar]
- 71.Rymer T.L. The Role of Olfactory Genes in the Expression of Rodent Paternal Care Behavior. Genes. 2020;11:292. doi: 10.3390/genes11030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fallon J.H., Moore R.Y. Catecholamine innervation of the basal forebrain IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J. Comp. Neurol. 1978;180:545–579. doi: 10.1002/cne.901800310. [DOI] [PubMed] [Google Scholar]
- 73.Bielsky I.F., Hu S.-B., Szegda K.L., Westphal H., Young L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-Like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology. 2003;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 74.Bielsky I.F., Young L.J. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Kavaliers M., Colwell D., Choleris E., Ågmo A., Muglia L.J., Ogawa S., Pfaff D.W. Impaired discrimination of and aversion to parasitized male odors by female oxytocin knockout mice. Genes Brain Behav. 2003;2:220–230. doi: 10.1034/j.1601-183X.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 76.Ferguson J.N., Aldag J.M., Insel T.R., Young L.J. Oxytocin in the Medial Amygdala is Essential for Social Recognition in the Mouse. J. Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dumais K.M., Bredewold R., Mayer T.E., Veenema A.H. Sex differences in oxytocin receptor binding in forebrain regions: Correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 78.Caughey S.D., Klampfl S.M., Bishop V.R., Pfoertsch J., Neumann I.D., Bosch O.J., Meddle S.L. Changes in the Intensity of Maternal Aggression and Central Oxytocin and Vasopressin V1a Receptors Across the Peripartum Period in the Rat. J. Neuroendocr. 2011;23:1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- 79.Young L.J., Lim M.M., Gingrich B., Insel T.R. Cellular Mechanisms of Social Attachment. Horm. Behav. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 80.Fuchs A.-R., Fuchs F., Husslein P., Soloff M.S., Fernström M.J. Oxytocin Receptors and Human Parturition: A Dual Role for Oxytocin in the Initiation of Labor. Science. 1982;215:1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- 81.Gimpl G., Fahrenholz F. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 82.Insel T.R., Gingrich B.S., Young L.J. Oxytocin: Who needs it? Prog. Brain Res. 2001;133:59–66. doi: 10.1016/s0079-6123(01)33005-4. [DOI] [PubMed] [Google Scholar]
- 83.Grieb Z.A., Lonstein J.S. Oxytocin receptor expression in the midbrain dorsal raphe is dynamic across female reproduction in rats. J. Neuroendocr. 2021;33:e12926. doi: 10.1111/jne.12926. [DOI] [PubMed] [Google Scholar]
- 84.Quiñones-Jenab V., Jenab S., Ogawa S., Adan R.A., Burbach P.H., Pfaff D.W. Effects of Estrogen on Oxytocin Receptor Messenger Ribonucleic Acid Expression in the Uterus, Pituitary, and Forebrain of the Female Rat. Neuroendocrinology. 1997;65:9–17. doi: 10.1159/000127160. [DOI] [PubMed] [Google Scholar]
- 85.Keller M., Perrin G., Meurisse M., Ferreira G., Lévy F. Cortical and medial amygdala are both involved in the formation of olfactory offspring memory in sheep. Eur. J. Neurosci. 2004;20:3433–3441. doi: 10.1111/j.1460-9568.2004.03812.x. [DOI] [PubMed] [Google Scholar]
- 86.Ogi A., Mariti C., Pirrone F., Baragli P., Gazzano A. The Influence of Oxytocin on Maternal Care in Lactating Dogs. Animals. 2021;11:1130. doi: 10.3390/ani11041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li T., Jia S.-W., Hou D., Liu X., Li D., Liu Y., Cui D., Wang X., Hou C., Brown C.H., et al. Intranasal Oxytocin Restores Maternal Behavior and Oxytocin Neuronal Activity in the Supraoptic Nucleus in Rat Dams with Cesarean Delivery. Neuroscience. 2021;468:235–246. doi: 10.1016/j.neuroscience.2021.06.020. [DOI] [PubMed] [Google Scholar]
- 88.Taylor J.H., French J.A. Oxytocin and vasopressin enhance responsiveness to infant stimuli in adult marmosets. Horm. Behav. 2015;75:154–159. doi: 10.1016/j.yhbeh.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Turcsán B., Román V., Lévay G., Lendvai B., Kedves R., Petró E., Topál J. Intranasal Oxytocin Improves Social Behavior in Laboratory Beagle Dogs (Canis familiaris) Using a Custom-Made Social Test Battery. Front. Veter. Sci. 2022;9:785805. doi: 10.3389/fvets.2022.785805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romero T., Nagasawa M., Mogi K., Hasegawa T., Kikusui T. Intranasal administration of oxytocin promotes social play in domestic dogs. Commun. Integr. Biol. 2015;8:e1017157. doi: 10.1080/19420889.2015.1017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ji H., Su W., Zhou R., Feng J., Lin Y., Zhang Y., Wang X., Chen X., Li J. Intranasal oxytocin administration improves depression-like behaviors in adult rats that experienced neonatal maternal deprivation. Behav. Pharmacol. 2016;27:689–696. doi: 10.1097/FBP.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 92.Ramos L., Hicks C., Caminer A., Goodwin J., McGregor I.S. Oxytocin and MDMA (‘Ecstasy’) enhance social reward in rats. Psychopharmacology. 2015;232:2631–2641. doi: 10.1007/s00213-015-3899-9. [DOI] [PubMed] [Google Scholar]
- 93.Ogi A., Naef V., Santorelli F.M., Mariti C., Gazzano A. Oxytocin Receptor Gene Polymorphism in Lactating Dogs. Animals. 2021;11:3099. doi: 10.3390/ani11113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ermisch A., Rühle H.-J., Landgraf R., Hess J. Blood—Brain Barrier and Peptides. J. Cereb. Blood Flow Metab. 1985;5:350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 95.Kenkel W.M., Perkeybile A.-M., Yee J.R., Pournajafi-Nazarloo H., Lillard T.S., Ferguson E.F., Wroblewski K.L., Ferris C.F., Carter C.S., Connelly J.J. Behavioral and epigenetic consequences of oxytocin treatment at birth. Sci. Adv. 2019;5:eaav2244. doi: 10.1126/sciadv.aav2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh C.J., Fleming A.S., Lee A., Magnusson J.E. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav. Neurosci. 1996;110:134–153. doi: 10.1037/0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- 97.Stack E.C., Balakrishnan R., Numan M.J., Numan M. A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav. Brain Res. 2002;131:17–36. doi: 10.1016/S0166-4328(01)00370-9. [DOI] [PubMed] [Google Scholar]
- 98.Tsuneoka Y., Maruyama T., Yoshida S., Nishimori K., Kato T., Numan M., Kuroda K.O. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J. Comp. Neurol. 2012;521:1633–1663. doi: 10.1002/cne.23251. [DOI] [PubMed] [Google Scholar]
- 99.Lee A., Clancy S., Fleming A.S. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 1999;100:15–31. doi: 10.1016/S0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 100.Lee A., Li M., Watchus J., Fleming A.S. Neuroanatomical basis of maternal memory in postpartum rats: Selective role for the nucleus accumbens. Behav. Neurosci. 1999;113:523–538. doi: 10.1037/0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- 101.Gaffori O., Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol. Behav. 1979;23:317–323. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- 102.Shahrokh D.K., Zhang T.-Y., Diorio J., Gratton A., Meaney M.J. Oxytocin-Dopamine Interactions Mediate Variations in Maternal Behavior in the Rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tsuneoka Y., Funato H. Cellular Composition of the Preoptic Area Regulating Sleep, Parental, and Sexual Behavior. Front. Neurosci. 2021;15:649159. doi: 10.3389/fnins.2021.649159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohl J., Babayan B.M., Rubinstein N.D., Autry A.E., Marin-Rodriguez B., Kapoor V., Miyamishi K., Zweifel L.S., Luo L., Uchida N., et al. Functional circuit architecture underlying parental behaviour. Nature. 2018;556:326–331. doi: 10.1038/s41586-018-0027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsuneoka Y., Yoshida S., Takase K., Oda S., Kuroda M., Funato H. Neurotransmitters and neuropeptides in gonadal steroid receptor-expressing cells in medial preoptic area subregions of the male mouse. Sci. Rep. 2017;7:9809. doi: 10.1038/s41598-017-10213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rivas M., Torterolo P., Ferreira A., Benedetto L. Hypocretinergic system in the medial preoptic area promotes maternal behavior in lactating rats. Peptides. 2016;81:9–14. doi: 10.1016/j.peptides.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 107.Li X.-Y., Han Y., Zhang W., Wang S.-R., Wei Y.-C., Li S.-S., Lin J.-K., Yan J.-J., Chen A.-X., Zhang X., et al. AGRP Neurons Project to the Medial Preoptic Area and Modulate Maternal Nest-Building. J. Neurosci. 2018;39:456–471. doi: 10.1523/JNEUROSCI.0958-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Insel T.R., E Shapiro L. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc. Natl. Acad. Sci. USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu Y., Wang Z. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/S0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- 110.Aragona B.J., Liu Y., Curtis J.T., Stephan F.K., Wang Z. A Critical Role for Nucleus Accumbens Dopamine in Partner-Preference Formation in Male Prairie Voles. J. Neurosci. 2003;23:3483–3490. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gingrich B., Liu Y., Cascio C., Wang Z., Insel T.R. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behav. Neurosci. 2000;114:173–183. doi: 10.1037/0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- 112.Hansen S., Bergvall A.H., Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: A microdialysis study. Pharmacol. Biochem. Behav. 1993;45:673–676. doi: 10.1016/0091-3057(93)90523-V. [DOI] [PubMed] [Google Scholar]
- 113.Champagne F.A., Chretien P., Stevenson C.W., Zhang T.Y., Gratton A., Meaney M.J. Variations in Nucleus Accumbens Dopamine Associated with Individual Differences in Maternal Behavior in the Rat. J. Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keer S., Stern J. Dopamine Receptor Blockade in the Nucleus Accumbens Inhibits Maternal Retrieval and Licking, but Enhances Nursing Behavior in Lactating Rats. Physiol. Behav. 1999;67:659–669. doi: 10.1016/S0031-9384(99)00116-X. [DOI] [PubMed] [Google Scholar]
- 115.Numan M., Numan M.J., Pliakou N., Stolzenberg D.S., Mullins O.J., Murphy J.M., Smith C.D. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- 116.Romero-Morales L., García-Saucedo B., Martínez-Torres M., Cárdenas M., Cárdenas-Vázquez R., Luis J. Neural activation associated with maternal and aversive interactions with pups in the mongolian gerbil (Meriones unguiculatus) Behav. Brain Res. 2022;437:114153. doi: 10.1016/j.bbr.2022.114153. [DOI] [PubMed] [Google Scholar]
- 117.Fleming A.S., Korsmit M. Plasticity in the maternal circuit: Effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behav. Neurosci. 1996;110:567–582. doi: 10.1037/0735-7044.110.3.567. [DOI] [PubMed] [Google Scholar]
- 118.Fleming A.S., Rosenblatt J.S. Maternal behavior in the virgin and lactating rat. J. Comp. Physiol. Psychol. 1974;86:957–972. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- 119.Fleming A.S., Rosenblatt J.S. Olfactory regulation of maternal behavior in rats: I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J. Comp. Physiol. Psychol. 1974;86:221–232. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- 120.Fleming A.S., Rosenblatt J.S. Olfactory regulation of maternal behavior in rats: II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J. Comp. Physiol. Psychol. 1974;86:233–246. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- 121.Inoue S., Kamiyama H., Matsumoto M., Yanagawa Y., Hiraide S., Saito Y., Shimamura K.-I., Togashi H. Synaptic Modulation via Basolateral Amygdala on the Rat Hippocampus–Medial Prefrontal Cortex Pathway in Fear Extinction. J. Pharmacol. Sci. 2013;123:267–278. doi: 10.1254/jphs.13123FP. [DOI] [PubMed] [Google Scholar]
- 122.Lahoud N., Maroun M. Oxytocinergic manipulations in corticolimbic circuit differentially affect fear acquisition and extinction. Psychoneuroendocrinology. 2013;38:2184–2195. doi: 10.1016/j.psyneuen.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 123.Deji C., Yan P., Ji Y., Yan X., Feng Y., Liu J., Liu Y., Wei S., Zhu Y., Lai J. The Basolateral Amygdala to Ventral Hippocampus Circuit Controls Anxiety-Like Behaviors Induced by Morphine Withdrawal. Front. Cell. Neurosci. 2022;16:894886. doi: 10.3389/fncel.2022.894886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wardlaw S.L., Frantz A.G. Brainβ-Endorphin during Pregnancy, Parturition, and the Postpartum Period. Endocrinology. 1983;113:1664–1668. doi: 10.1210/endo-113-5-1664. [DOI] [PubMed] [Google Scholar]
- 125.Neumann I., Russell J., Landgraf R. Chapter 8: Endogenous opioids regulate intracerebral oxytocin release during parturition in a region-specific manner. Prog. Brain Res. 1992;91:55–58. doi: 10.1016/s0079-6123(08)62315-8. [DOI] [PubMed] [Google Scholar]
- 126.Kalivas P.W., Abhold R. Enkephalin release into the ventral tegmental area in response to stress: Modulation of mesocorticolimbic dopamine. Brain Res. 1987;414:339–348. doi: 10.1016/0006-8993(87)90015-1. [DOI] [PubMed] [Google Scholar]
- 127.Beauregard M., Leroux J.-M., Bergman S., Arzoumanian Y., Beaudoin G., Bourgouin P., Stip E. The functional neuroanatomy of major depression: An fMRI study using an emotional activation paradigm. Neuroreport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- 128.Broad K., Hinton M., Keverne E., Kendrick K. Involvement of the medial prefrontal cortex in mediating behavioural responses to odour cues rather than olfactory recognition memory. Neuroscience. 2002;114:715–729. doi: 10.1016/S0306-4522(02)00231-2. [DOI] [PubMed] [Google Scholar]
- 129.Stamatakis A., Kalpachidou T., Raftogianni A., Zografou E., Tzanou A., Pondiki S., Stylianopoulou F. Rat dams exposed repeatedly to a daily brief separation from the pups exhibit increased maternal behavior, decreased anxiety and altered levels of receptors for estrogens (ERα, ERβ), oxytocin and serotonin (5-HT1A) in their brain. Psychoneuroendocrinology. 2015;52:212–228. doi: 10.1016/j.psyneuen.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 130.Wu Z., Autry A.E., Bergan J.F., Watabe-Uchida M., Dulac C.G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Osterhout J.A., Kapoor V., Eichhorn S.W., Vaughn E., Moore J.D., Liu D., Lee D., DeNardo L.A., Luo L., Zhuang X., et al. A preoptic neuronal population controls fever and appetite during sickness. Nature. 2022;606:937–944. doi: 10.1038/s41586-022-04793-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paxinos G., Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press, Inc.; San Diego, CA, USA: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.