Abstract
Cerebral ischemic stroke is characterized by acute ischemia in a certain part of the brain, which leads to brain cells necrosis, apoptosis, ferroptosis, pyroptosis, etc. At present, there are limited effective clinical treatments for cerebral ischemic stroke, and the recovery of cerebral blood circulation will lead to cerebral ischemia-reperfusion injury (CIRI). Cerebral ischemic stroke involves many pathological processes such as oxidative stress, inflammation, and mitochondrial dysfunction. Nuclear factor erythroid 2-related factor 2 (Nrf2), as one of the most critical antioxidant transcription factors in cells, can coordinate various cytoprotective factors to inhibit oxidative stress. Targeting Nrf2 is considered as a potential strategy to prevent and treat cerebral ischemia injury. During cerebral ischemia, Nrf2 participates in signaling pathways such as Keap1, PI3K/AKT, MAPK, NF-κB, and HO-1, and then alleviates cerebral ischemia injury or CIRI by inhibiting oxidative stress, anti-inflammation, maintaining mitochondrial homeostasis, protecting the blood–brain barrier, and inhibiting ferroptosis. In this review, we have discussed the structure of Nrf2, the mechanisms of Nrf2 in cerebral ischemic stroke, the related research on the treatment of cerebral ischemia through the Nrf2 signaling pathway in recent years, and expounded the important role and future potential of the Nrf2 pathway in cerebral ischemic stroke.
Keywords: Nrf2, cerebral ischemic stroke, oxidative stress, inflammation, mitochondrial function, blood–brain barrier, ferroptosis
1. Introduction
1.1. Cerebral Ischemic Stroke
Stroke refers to the obstruction or bleeding of arteries in a certain part of the brain tissue, which leads to focal neurological impairment in the corresponding part, including ischemic stroke and hemorrhagic stroke. Investigation showed that the incidence and prevalence of stroke increased with years, and stroke caused high rates of disability and mortality, which seriously endangered human life and health [1]. Among them, cerebral ischemic stroke makes up the vast majority, accounting for about 70% of all stroke events [2]. Cerebral ischemic stroke is featured with cerebral vascular congestion and insufficient blood supply, leading to insufficient supply of oxygen and nutrients in brain tissue, and then leads to a series of pathophysiological changes or injuries, and the death of brain cells, eventually leading to neurological dysfunction. At present, it is known that the pathophysiological changes caused by ischemic stroke include energy metabolism disorder of brain tissue, oxidative stress injury, excitatory amino acid poisoning, inflammatory reaction, etc. [3]. The ischemic parts of the brain are segmented into the ischemic core area and the penumbra area. The supply of glucose and oxygen in the ischemic core almost completely stops, resulting in irreversible damage to all cells and death due to necrosis. The penumbra can still acquire a small amount of glucose and oxygen supply from the blood flow, and prevent the cells from dying immediately [4]. If the blood flow can be restored in a timely manner, the function of brain cells may be restored. However, during the onset of acute stroke, partial opening of collateral blood circulation, or unobstructed blood circulation again after embolectomy, will cause ischemia-reperfusion injury, which will lead to the release of free radicals, which will lead to the destruction of brain cell structure and further aggravate brain tissue damage [5].
1.2. Nrf2
Transcription factor NF E2-related factor 2 (Nrf2) is a member of the transcription factor family, widely exists in various cells, and has redox sensitivity [6,7]. When cells are stimulated by oxidative stress, Nrf2 can regulate the antioxidant stress by combining with the antioxidant response element (ARE) in nucleus [8]. The cytoplasmic kelch-like epichlorohydrin-associated protein 1 (Keap1) can sense the oxidative insults, and Nrf2 will serve for the counter responses [9]. More than 600 genes, among them more than 200 encoding proteins linked to inflammation, cancer, neurological disorders, aging, cardiovascular disease, and other serious illnesses, are regulated by the Nrf2 signaling pathway [10]. Nrf2 can regulate most downstream factors, and it can play plenty of roles, including antiapoptosis, anti-inflammatory injury, reducing calcium overload, antioxidative stress, etc., and help the body maintain the redox reaction of brain tissue and brain cells. Nrf2 signaling is increasingly complex as Nrf2 is sited at the center of a vast regulatory network now. Thus, it is vital to stabilize Nrf2 activity to maintain the redox balance and brain homeostasis. As the brain is vulnerable to oxidative stress [11], Nrf2 activation has now become a promising target in the setting of cerebrovascular accidents such as ischemia. Nrf2 plays a crucial role in the management of excessive oxidative stress after stroke [12]. Hydrogen peroxide, which increases after ischemia-reperfusion, is the main stimulator of Nrf2 activation [13]. Nrf2−/− rats have greater cerebral damage after ischemic stroke modeling due to the lack of Nrf2 protection [14]. The above results indicate that Nrf2 is an important molecule to regulate oxidative stress. Moreover, the expression level of Nrf2 can affect the recovery and prognosis of ischemic stroke injury.
1.3. Oxidative Stress
Free radicals of oxygen and nitrogen are necessary for all aerobic organisms. Reactive oxygen species (ROS) participate in the basic biochemical process of the organism, which can maintain the redox steady state of tissues and cells. The physiological health of the organism is very important. However, excessive oxidative stress may lead to lipid, protein, and DNA damage, which is harmful to the body. Oxidative stress means that the balance between the oxidative system and antioxidant system in the body is broken, which leads to the destruction of intracellular biological macromolecules such as sugars, lipids, protein, and nucleic acids, and further leads to the damage of organs, tissues, and functions, which in turn leads to the further accumulation of oxidation products, thus aggravating the damage and forming a vicious circle [15]. Oxidative stress includes exogenous and endogenous mechanisms. Endogenous stress usually comes from intracellular signaling pathways, metabolism, and inflammatory processes [16]. Different types of ROS will be produced by metabolism, including superoxide anion radical (O2•−), peroxy radical (ROO•), hydroxyl radical (HO•), as well as the non-free radical compounds such as hydrogen peroxide (H2O2) and singlet oxygen (1O2) [17]. At present, it is known that the action of free radicals is concentration-dependent; that is, ROS is beneficial to the body at normal physiological concentration, but harmful to the body when it exceeds a certain concentration [18]. If the content of ROS exceeds the cell tolerance level, it will oxidize DNA and induce DNA breakage and damage, which is common during the occurrence of tumors [19]. Oxidative stress participates in cerebral ischemia injury through many ways, which damages the normal function of cells, causes irreversible damage to cells, and causes cell necrosis and apoptosis [20]. After cerebral ischemia, oxidative DNA leads to cell damage, activates several death-related signal pathways, and then leads to programmed cell death, leading to neurological dysfunction [21]. The antioxidant system is the body’s response to all kinds of oxidative damage in order to reduce oxidative damage. After ischemic stroke, cells start the antioxidant defense function, and activate enzymes such as superoxide dismutase or heme oxygenase [22]. Superoxide dismutase (SOD) and glutathione (GSH) play an antioxidant role by maintaining the redox balance of tissues and cells [23]. However, brain tissue contains many unsaturated fatty acids and high concentrations of lipids, and its antioxidant capacity is weak, which makes brain tissue vulnerable to the damage of ROS [24]. Therefore, it is of great significance to study the mechanism of oxidative stress after cerebral ischemia.
1.4. The Role of Nrf2 in Cerebral Ischemic Stroke
Study has shown that cells significantly elevate the Nrf2 expression during the acute phase of stroke [25]. In the models of middle cerebral artery occlusion (MCAO), the expression of Nrf2 was upregulated from 3 h after occlusion, and reached its zenith at 24 h [26]. Other studies have shown that Nrf2 participates in the process of CIRI and has endogenous neuroprotective effects [27,28]. The activation of Nrf2 is caused by excessive ROS generation after cerebral ischemia. After Nrf2 activation, transcription of a series of antioxidant genes was initiated. Some studies confirmed that heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 (NQO1) expression increased after cerebral ischemia [29]. Nrf2 activator reversibly reduced the expression of SODs, and the overexpression of SOD1 or SOD2 significantly reduced the brain damage caused by cerebral ischemic stroke [30,31]. The mechanism underlying the protective effect of Nrf2 in cerebral ischemic stroke is not yet completely understood. It was shown that Nrf2 alleviates CIRI by activating the expression of antioxidant genes, defending against the damage caused by ROS, alleviating blood–brain barrier disruption and attenuating inflammation [32]. The study showed that following transient middle cerebral artery embolism in Nrf2 knockout mice, the basic and induced activation of antioxidant enzymes and detoxification enzymes were inhibited, the cerebral infarction area was larger, and the behavioral performance was worse than that in the control group [33]. All the above studies show that Nrf2 plays a vital role in alleviating ischemic stroke and CIRI, and most studies show that this role is a beneficial protective role.
2. The Structure and Function of Nrf2
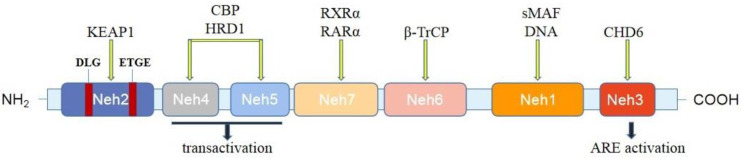
The relative molecular weight of Nrf2 protein was 6.6 × 104 Da. It is a transcription factor with a highly conserved basic leucine zipper structure, and is regulated by Keap1 at a low level and has a short half-life in non-activated cells [34]. Nrf2 mainly has seven highly conserved epichlorohydrin-related protein homologous domains (Nrf2 epichlorohydrin homology, Neh) [10,35] (Figure 1): The Neh1 region is a leucine zipper motif associated with intranuclear small musculoaponeurotic fibrosarcoma (sMaf), forms a heterodimer, recognizes and binds related sequences on the ARE, and can also interact with E2 ubiquitin-binding enzyme to adjust Nrf2 stability. The Neh2 region is a Keap1-dependent regulatory region, which contains two binding units, DLG and ETGE, to negatively regulate the Nrf2 transcriptional activity and promote its degradation [36]. The DLG-binding motif of Nrf2 functions as a “latch” separating from Keap1, while the ETGE-binding motif acts like a ‘hinge’ that remains connected to Keap1 [37]. Neh3 is located at the carboxyl terminus of Nrf2 and acts as a transactivation domain to activate transcription of related genes downstream [38]. Neh4 and Neh5 are two independent activation regions, both of which are rich in acidic amino acid residues and participate in the regulation of Nrf2 transcription by binding to cyclic AMP response element-binding protein (CREB) [39]. The Neh6 region is a Keap1 independent regulatory region and participates in the Keap1 alternative degradation pathway. Neh7 can specifically interact with retinoic acid X receptor α (RXRα) [40]. Brain microglia, macrophages, and astrocytes are the main producers of Nrf2; they produce a large amount of Nrf2 during the first 24 h after the induced middle cerebral artery occlusion [41,42].
Figure 1.
Domain structures of Nrf2. The Nrf2 protein contains seven domains: Neh1–Neh7. Neh2 is the Keap1-binding domain. Neh4, 5, and 3 domains are critical for transactivation. Neh3 can activate transcription of downstream-related genes. The Neh6 region participates in the Keap1 alternative degradation pathway. The Neh1 region binds to small musculoaponeurotic fibrosarcoma (sMaf) and forms a heterodimer. Neh7 can bind to RXRα and RaRα. KEAP1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; CBP, cyclic AMP response element-binding protein; HRD1, HMG-CoA reductase degradation protein 1; RXRα, retinoid X receptor α; RARα, retinoic acid receptor α; β-TrCP, β-transducin repeat-containing protein; sMAF, small musculoaponeurotic fibrosarcoma; CHD6, chromodomain helicase DNA-binding protein 6; ARE, antioxidant response element.
Normally, Nrf2 forms a complex with Keap1, a negative regulator of Nrf2, at a low expression level. Nrf2 will disconnect from Keap1 under oxidative stress, and then Nrf2 will help to transfer Keap1 into the nucleus. Nrf2 possesses protective effects against oxidative stress and inflammation [43]. Nrf2 can adjust the expression of antioxidant enzymes, including catalase (CAT), glutathione peroxidase (GSH-Px), and SOD, and it can regulate the expression of some phase II detoxification enzymes, such asNQO1, HO-1, and glutathione S-transferase (GST) [44,45]. Many Nrf2-driven genes become involved in glutathione synthesis or action. The de novo synthesis of glutathione starts with γ-glutamylcysteine synthetase (γ-GCS), which binds cysteine with glutamate to produce γ-glutamylcysteine. HO-1 is one of the crucial and well-studied antioxidant genes adjusted by Nrf2 and is of particular importance in endothelial homeostasis. HO-1 is usually upregulated along with ferritin. HO-1 combines with biliverdin reductase to release bilirubin, which ranks as one of the most robust endogenous antioxidants by clearing reactive oxygen species and reactive nitrogen species ROS/RNS [46].
In addition, other downstream classes of genes involved in protein transport, ubiquitination, phosphorylation, proliferation, and apoptosis, have also been shown to be potentially Nrf2-regulating endogenous encoding genes [47]. Nrf2 has also been shown to regulate genes in response to air pollution or increased reactive oxygen species [48]. Nrf2-related signaling pathways play an important role in maintaining cellular homeostasis under stress, inflammation, carcinogenesis and proapoptotic conditions [49,50]. This shows that Nrf2 is widely involved in the physiological and pathological processes of the body, and it is an important cytokine.
In addition to regulating cellular oxidative stress response, Nrf2 is also involved in the regulatory process of maintaining intracellular redox homeostasis. By activating the expression of various kinds of antioxidant proteins, Nrf2 can reduce ROS-induced cellular damage and electrophilics, and maintain a redox dynamic balance in the human body. Under oxidative stress, the content of ROS in vivo increases and the Nrf2 system is activated, the cells express more antioxidant enzymes and proteases of synthetic antioxidants, the antioxidant mechanism is enhanced, and the content of ROS decreases, thus achieving the dynamic redox balance [51]. Therefore, Nrf2 is an important factor to maintain cell homeostasis and organism homeostasis.
3. Oxidative Stress and Cerebral Ischemic Stroke
Oxidative stress refers to the excessive production of ROS and reactive nitrogen species (RNS) when the body is under many external or internal stimuli, and the imbalance of oxidation system and antioxidant system leads to cell damage. The main mechanism of oxidative stress injury is the increase of free radical sources and the decrease of antioxidant capacity. ROS are the oxygenated molecules with high activity properties, including O2•−, H2O2, NO, and ONOO−, etc. In living cells such as neurons, ROS can be produced in response to hypoxia, serum deprivation, or cytokine stimulation [52]. Mitochondria are the main organelles that generate ROS in cells [53]. RNS refers to the redox molecule derived from nitric oxide (NO). NO is generated by NOS catalyzing the L-arginine guanidine-based terminal nitrogen oxidation reaction. Low concentrations of NO can resist oxidative damage, while high concentrations of NO can aggravate the oxidative damage of the body [54,55]. Oxidative stress is known to be involved in several pathophysiological processes [56]. Therefore, the regulation of oxidative stress is an important factor to maintain the balance of the body.
After CIRI, the blood–brain barrier of brain tissue is destroyed, and oxidative stress is one of the factors [57]. It has been found that oxidative stress is a significant mechanism leading to brain tissue injury after cerebral ischemic stroke [58,59]. In CIRI, when the generation of oxidants surpasses the body’s antioxidant capacity, the accumulated ROS will trigger the oxidation reaction of DNA, lipid, and protein, leading to the death of tissue cells, and then cause neurological dysfunction [60]. However, during reperfusion, a great deal of reactive oxygen species, such as hydroxyl radicals and superoxide radicals, and an excess increase in hydrogen peroxide, are produced [61]. ROS tend to affect cell membranes lipids, and lipid peroxidation will generate a vast number of lipid peroxides (4-HNE and other toxic aldehydes), which increase the cell membranes’ permeability and destroy their structural integrity [62]. ROS and RNS interact to produce reactive oxygen and nitrogen molecules, such as strong oxidant peroxynitrite. It has been found that NADPH oxidase 2 (NOX2) plays a key role in CI/R-induced oxidative stress injury and is a key enzyme causing ROS [63]. In addition, several studies showed that decreasing NOX2 expression and reducing NOX activity are two effective ways to attenuate CIRI [64]. When studying the protective mechanism of ischemic preconditioning, some researchers found that ischemic preconditioning (IPC) can induce cerebral ischemic tolerance, which is initiated by oxidative stress. This mechanism is related to the opening up of the adenosine triphosphate (ATP)-sensitive potassium channel (mito K+ ATP), which is necessary for IPC to play a protective role. Moreover, it was found that there is a delicate balance in ROS generation; for instance, a high level of ROS produced during IR is cytotoxic, while a low level of IPC-produced ROS is neuroprotective [65].
The SOD family includes SOD1, SOD2, and SOD3 proteins that neutralize the first oxygen-sourced ROS. SOD belongs to the body of free radical scavenging agents, and protect cells against oxidative stress, especially in the removal of superoxide produced during metabolism [66]. In addition, SODs can inhibit the damage of oxygen free radicals, and also reduce the number of peroxidation products generated by brain tissue under ischemia and reperfusion, and increase the ability of the cerebral cortex to withstand hypoxia [67]. This shows that SODs have a protective effect on ischemic brain injury. Moreover, the balance between SODs and ROS is very important for the homeostasis of the body. If the balance between them cannot be maintained, the cell homeostasis will be destroyed.
Mitochondria are the energy factories of cells. Excessive ROS has a highly toxic effect on key cellular macromolecules, and ROS can damage the mitochondria, leading to disorder of the cellular energy metabolism and ultimately cell death [68,69]. After stroke, the blood supply of local brain tissue is insufficient, mitochondrial dysfunction occurs, ATP cannot be generated, and calcium homeostasis is disrupted. After cerebral blood flow reperfusion, oxidative stress further aggravates mitochondrial damage [70]. This is followed by apoptosis and, in turn, mitochondrial dysfunction produces excess ROS [71]. The mechanism may be that under the condition of cell hypoxia, the function of mitochondria to produce ATP is inhibited, thus affecting the normal function of the Na(+)/K(+)-ATPase and Ca2+/H+-ATPase plasma pump, the increase of intracellular Na+, Ca2+, and adenosine diphosphate (ADP) content, the disruption of cell ion homeostasis, and the depolarization of the cell membrane, leading to the change of intracellular mitochondrial membrane potential and the release of a large amount of ROS. SOD and GSH-Px are endogenous antioxidant enzymes that can clear free radicals and protect cells from oxidative injury. After cerebral ischemic stroke, the activities of SOD and GSH-Px were significantly decreased, resulting in serious damage to the structure and function of mitochondria, and inducing brain tissue damage and neurologic function deficits [72]. The above results demonstrate that ROS and mitochondrial damage interact and promote each other during stroke.
More and more researches have shown that oxidative stress is involved in stroke and CIRI; therefore, many drugs take effect by regulating oxidative stress, especially some traditional Chinese medicines or compounds. This paper summarizes that these drugs play a protective role in stroke and CIRI by regulating oxidative stress in the past five years. Dihydrocapsaicin (DHC) is the main active substance of capsaicin in chili peppers. The study showed that DHC can protect the brain and blood–brain barrier from I/R damage by inhibiting oxidative stress and inflammation [73]. Acteoside (ACT) has neuroprotective and antioxidant effects in neurodegenerative diseases. The results showed that ACT could reduce oxidative stress in the middle cerebral artery occlusion/reperfusion (MCAO/R) models [74]. Schizandrin A (Sch A) is isolated from Schisandra chinesnesis and it has significant antioxidant activities. It has been shown that Sch A has a protective role against cerebral stroke [75]. One of the major active ingredients of Herba Epimedii, Icariside II, can attenuate CIRI through inhibiting oxidative stress [76]. Rutaecarpine (Rut) is an alkaloid isolated from Evodia officinalis and has many biological activities. Rut can inhibit inflammation, oxidative stress, and apoptosis, and it can attenuate CIRI [77]. Biochanin A is a natural phytoestrogen. The results showed that biochanin A had protective effects on cerebral ischemic injury by antioxidation in rats [78]. Studies have proven that Theaflavin possesses strong antioxidative capacity. Theaflavin can reduce oxidative stress, thereby attenuating cerebral ischemia-reperfusion injury [79]. Carvacryl acetate (CA) is a semi-synthetic monoterpenic ester extracted from essential oils, which has antioxidant properties. The study has shown that CA can alleviate oxidative stress injury induced by cerebral ischemia-reperfusion through the Nrf2 signaling pathway [80]. Convolvulus pluricaulis Choisy is traditionally prescribed for nerve debility. The study showed that Convolvulus pluricaulis Choisy has a neuroprotective effect on the oxidative stress model of CIRI in rats [81]. Cepharanthine (CEP) has anti-inflammatory and antioxidative properties. It has been shown that CEP attenuates cerebral I/R injury by inhibiting nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome-induced inflammation [82]. Studies have shown that Sanggenon C (SC) has antioxidant effect. The results showed that SC can inhibit inflammation and oxidative stress in CIRI [83]. Coicis Semen has antioxidative roles. Coicis Semen can alleviate ischemic brain injury, which may be related to the inhibition of oxidative stress [84]. It has been shown that resveratrol has an anti-inflammatory effect. Pic can reduce oxidative stress and apoptosis caused by CIRI, and the effect is realized by adjusting the Sirtuin1 (SIRT1)/fork-head box O1(FoxO1) signaling pathway [85]. Garcinol is a polyisoprenylated benzophenone derivative. Study has shown that Garcinol can inhibit oxidative stress and improve cerebral ischemia injury [86]. Fisetin has an antioxidant and anti-inflammatory effect. Fisetin protected CIRI injury, perhaps due to suppression of oxidative stress and inflammatory reaction [87]. Geraniin is a polyphenol isolated from Phyllanthus amarus. Study has shown that Geraniol can protect against CIRI by inhibiting oxidative stress [88]. Cucurbitacin B (CuB) has been demonstrated to possess antioxidative properties. The study showed that CuB can decrease lactate dehydrogenase (LDH) release and ROS production; that is, it can reduce cerebral I/R injury by reducing the level of oxidative stress [89]. Fraxin, one of the primary active ingredients of Cortex Fraxini, may have potent anti-inflammatory activity. A recent study showed that Fraxin significantly improved CIRI by inhibiting oxidative stress and inflammatory response [90]. Scutellarin can protect against cerebral ischemia injury, and study has shown that this is related to its antioxidant effect [91]. These drugs play a protective role in cerebral ischemic stroke or CIRI by regulating oxidative stress, and their specific regulatory mechanisms are different. We summarized these studies, and showed them in the table (Table 1) below.
Table 1.
Several traditional Chinese medicine/compounds affect cerebral ischemic stroke by regulating Nrf2 signaling pathways.
| Medicine | Model (s) | Related Signaling Pathway | Related Mechanism | Effect | Reference |
|---|---|---|---|---|---|
| Dihydrocapsaicin (DHC) | MCAO/R | Activate SOD and GPx, downregulate ROS, NOX2, NOX4, NF-ĸB, NO, and MMP-9 | Attenuate oxidative stress and inflammation | Protective | [73] |
| Acteoside (ACT) | MCAO/R | Decrease ROS and MDA, increase SOD and CAT | Attenuate oxidative stress and neuronal apoptosis | Protective | [74] |
| Schizandrin A (Sch A) | MCAO/R and OGD/R | Downregulate iNOS, COX-2, IL-1β, IL-6, and TNF-α, increase SOD, CAT, HO-1 and NQO-1 | Suppress inflammation and oxidative stress | Protective | [75] |
| Icariside II | MCAO/R | Decrease ROS and MDA, increase SOD, GSH-Px, catalase, Nrf2, and HO-1 | Attenuate oxidative stress | Protective | [76] |
| Rutaecarpine (Rut) | MCAO/R | Alleviate IL-6, IL-1β, LDH, MDA, and ROS, increase IL-4, IL-10, SOD, HO-1, and NQO1 | Alleviate inflammatory response and oxidative stress | Protective | [77] |
| Biochanin A | MCAO/R | Activate SOD, GSH-Px, and HO-1, suppress MDA, NF-kB | Antioxidative and anti-inflammatory actions | Protective | [78] |
| Theaflavin | MCAO/R and OGD/R | Decrease ROS and MDA, increase SOD and GSH-Px | Restore the impaired antioxidant defense system | Protective | [79] |
| Carvacryl acetate (CA) | MCAO/R | Decrease ROS and MDA, increase SOD | Antioxidant stress | Protective | [80] |
| Convolvulus pluricaulis Choisy | BCCA | Increase SOD, catalase, glutathione, and total thiol | Protect against oxidative damage | Protective | [81] |
| Cepharanthine (CEP) | MCAO/R and OGD/R | Decrease ROS and MDA, NLRP3, ASC, and cleaved caspase-1, increase SOD | Inhibit microglia activation, inflammation, and reduce oxidative stress | Protective | [82] |
| Sanggenon C (SC) | MCAO/R and OGD/R | Decrease TNF-α, IL-1β, IL-6, ROS, and MDA, increase SOD | Inhibit inflammation and oxidative stress | Protective | [83] |
| Coicis Semen | MCAO/R and OGD/R | Decrease ROS and MDA, increase SOD, GSH-Px, ZO-1 and occludin, CD31, and VEGF | Inhibit oxidative stress and promote angiogenesis | Protective | [84] |
| Piceatannol (Pic) | MCAO/R | Decrease ROS, increase SOD, CAT, and GSH-Px | Suppress oxidative stress | Protective | [85] |
| Garcinol | MCAO/R and OGD/R | Inhibit IL-1β, IL-6, TNF-α, NF-κB, MDA, and nitric oxide (NO), increase SOD | Attenuate inflammation and oxidative stress | Protective | [86] |
| Fisetin | MCAO/R and OGD/R | Decrease IL-1, TNF-α, iNOS, IL-1β, COX-2, IL-6, and PGE2 | Inhibit inflammation and oxidative stress | Protective | [87] |
| Geraniin | MCAO/R and OGD/R | Decrease LDH, NO, nNOS and MDA, increase SOD | Decrease oxidative stress and neuronal apoptosis | Protective | [88] |
| Cucurbitacin B (CuB) | MCAO/R and OGD/R | Decrease LDH, ROS, and NLRP3 | Inhibit oxidative stress and inflammation | Protective | [89] |
| Fraxin | MCAO/R and OGD/R | Decrease ROS, NF-κB, IKK-β, p38 MAPK, ERK1/2, and Keap1 | Inhibit oxidative stress, inflammatory response, and cell apoptosis | Protective | [90] |
| Scutellarin | tMCAO | Decrease ROS, 4-HNE, 8-OHDG, NT-3, PARP1, NOX1, NOX2, and NOX4 | Suppress oxidative stress | Protective | [91] |
4. Activation of Nrf2 Signaling Pathway in Cerebral Ischemic Stroke
4.1. Keap1/Nrf2/ARE Signaling Pathway
Keap1 (kelch-like ECH-associated protein 1) is an important regulatory protein. It forms a complex with ubiquitin protein kinase Cul3/rbx1 through specific binding with the target protein, and mediates the ubiquitination and degradation of the target protein. The primary structure of Keap1 contains 624 amino acids, including five domains: DGR, NTR, BTB/POZ, IVR, and CTR [92]. Among them, the binding site of Keap1 and Neh2 is located in the DGR region of the C-terminus, which is a repetitive fragment with six double-chain glycines. It is an Nrf2 inhibitory polypeptide and plays a role in inhibiting the translocation of Nrf2 into the nucleus as a negative regulatory protein. BTB/POZ is involved in the dimerization process of Keap1 protein and can enhance the binding force of Nrf2-Keap1. The core site of the BTB/POZ region is Ser-104, and its mutation will affect the dimerization of Keap1 and, thus, interfere with the ability of Keap1 to bind Nrf2 [93]. IVR contains 25 cysteine residues and is a region that regulates Keap1 activity. It can trigger intracellular redox reaction and contribute to the maintenance of intracellular redox balance [94].
Under physiological condition, Nrf2 and Keap1 exist in the cytoplasm, and the inactive Nrf2 is ubiquitinated and then degraded. When oxidative stress occurs in the body, Nrf2 and Keap1 are dissociated and activated, transferred to the nucleus, and combined with the corresponding sites of the ARE in the nucleus, thereby activating the transcription of antioxidant enzymes and detoxification enzymes downstream of the pathway (Figure 2), and working with related factors to improve the antioxidant capacity of cells [95]. Keap1 contains an NES in the IVR domain. Oxidative stress can inhibit NES activity [96]. DJ-1 promotes Nrf2 transport to the nucleus by inhibiting Nrf2 ubiquitination and preventing its binding to Keap1 [97]. Nrf2 mediates the transcriptional activation of antioxidant enzyme genes such as HO-1, NQO1, and those belonging to the GST family [98]. Studies have shown that the Keap1-Nrf2 signaling pathway is one of the main defense mechanisms against oxidative stress through the regulation of cell protective gene expression [7]. The following research shows that some Nrf2 activators will not affect the binding of Keap1 and Nrf2 [37]. In parallel with the experimental approaches focusing on the oxidative stress-induced regulation of Nrf2, a cDNA expression screen for activators of Nrf2-dependent gene expression identified the autophagy adaptor protein p62 as a novel regulator of ARE gene expression [99]. P62 plays a role in many cellular functions, and p62 can activate Nrf2. The phosphorylation of p62 can increase the binding affinity of Keap1 [100]. Recent study has shown that p62 can not only competitively bind to Keap1, but also directly promote the degradation of Keap1 through selective autophagy [101].
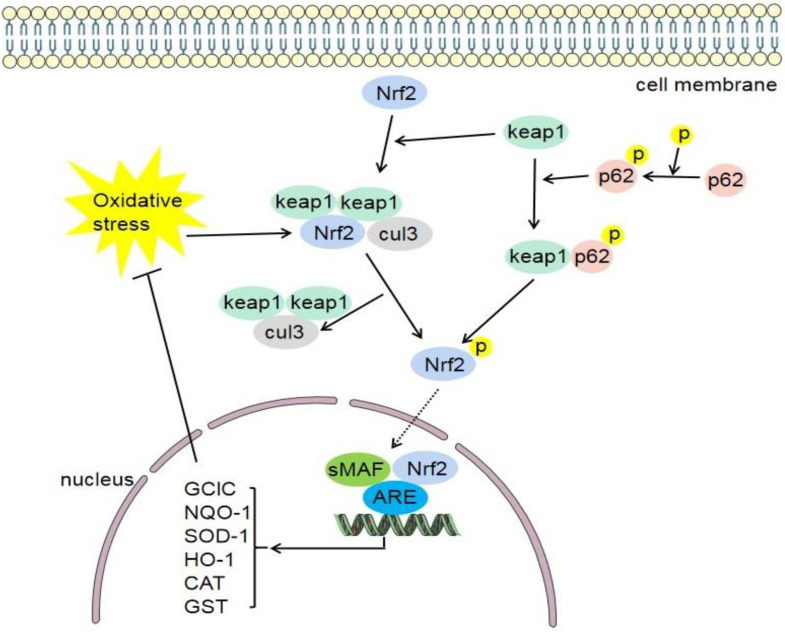
Figure 2.
Keap1-Nrf2-ARE signaling pathway. Under normal physiological conditions, Keap1 binds to Nrf2 and makes it inactive. When oxidative stress occurs in the body, Nrf2 and Keap1 are dissociated and activated, transferred to the nucleus, and combined with the ARE in the nucleus. Furthermore, they can promote the activation of various target genes (GCLC, NQO-1, SOD-1, HO-1, CAT, GST, etc.) and inhibit oxidative stress in feedback. Keap1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; cul3, cullin-3; GCLC, glutamate–cysteine ligase catalytic; NQO1, NADPH quinone oxidoreductase enzyme; SOD-1, superoxide dismutase-1; HO-1, heme oxygenase-1; CAT, catalase; GST, glutathione S-transferase.
The Keap1/Nrf2 signaling pathway is the most important antioxidant defense pathway found at present, which can resist cellular oxidative stress injury and play an important role in neurological diseases [102]. Trilobatin (TLB) is a naturally occurring SIRT3 agonist, and TLB can regulate neuroinflammation and oxidative reaction by regulating the TLR4/nuclear factor-kappa B and Nrf2/Keap1 signal pathway, reducing neuroinflammation and oxidative damage induced by CIRI, and playing a neuroprotective role [103]. In a cerebral ischemia model, the researchers observed the downregulation of MicroRNA-139-5p (miR-139-5p) and the pyroptosis induced by the activation of NLRP3. In addition, it was found that Ginsenoside Rd (Rd) played a protective role by regulating the ROS/TXNIP/NLRP3 inflammasome axis. These findings indicate that Rd can protect against ischemic stroke by regulating the FoxO1/Keap1/Nrf2 pathway [104]. Polyphenol-rich fraction (PRF) has an obvious protective effect on cerebral ischemia injury, which is manifested in PRF’s ability to improve the neurological deficit after transient middle cerebral artery occlusion (tMCAO) and to reduce the infarction rate and improve the cell morphology of the hippocampal CA1 area. PRF regulated oxidative stress by regulating the Keap1/Nrf2/HO-1 pathway and exhibited a good protective effect against CIRI [105]. To summarize, Keap1/Nrf2 signaling pathway is an effective mechanism to regulate brain injury.
4.2. Phosphatidylinositol-4,5-Bisphosphate 3-Kinase (PI3K)/Protein Kinase B (Akt)-Nrf2 Signaling Pathway
The PI3Ks are a family of lipid kinases. The PI3K/Akt pathway is associated with proliferation, cancer, and longevity. Akt is a serine/threonine kinase, which serves an important role in apoptosis, cell proliferation, transcription, and cell migration. There are three Akt subtypes: PKBα (Akt1), PKBβ (Akt2), and PKBγ (Akt3) [106]. In general, PI3K is mainly activated directly and indirectly through the focal adhesion kinase (FAK) pathway. Akt is the downstream signal molecule of PI3K [107].
Studies have shown that the PI3K/Akt pathway plays a key role in ischemic damage of other organs. Studies have shown that hyperbaric oxygen preconditioning protects myocardial ischemia-reperfusion injury, and its effect is related to the regulating of the PI3K/Akt/Nrf2 pathway [108]. PI3K/Akt signaling pathway is involved in Nrf2 translocation, and upregulation of PI3K and Akt can activate Nrf2 [109,110]. Studies on ischemic stroke have found that the PI3K/Akt signaling pathway can promote cell survival and inhibit cell apoptosis, and play an important role in neuroprotection during cerebral ischemia-reperfusion [111]. Compared with a model group, the cognitive impairment and neurological deficits of a Rehmannioside A group were significantly improved, and the protective role was related to the inhibition of ferroptosis and activation of the PI3K/AKT/Nrf2 pathway [112]. Another study showed that diterpene ginkgolides meglumine injection (DGMI) could improve CIRI by activating Nrf2 mediated by PI3K/Akt [113]. A study showed that 6′-O-galloylpaeoniflorin (GPF) could improve the neurological deficit of CIRI rats. Further research shows that this process can be inhibited by PI3K inhibitor Ly294002. In conclusion, GPF possesses neuroprotective effects against oxidative stress after CIRI by activation of the PI3K/Akt/Nrf2 pathway [114]. A study showed that hydrogen sulfide (H2S) preconditioning could protect mice against CIRI by regulating the PI3K/Akt/Nrf2 pathway [115]. The role of the PI3K/Akt-Nrf2 signaling pathway in cerebral ischemia stroke is shown in Figure 3.
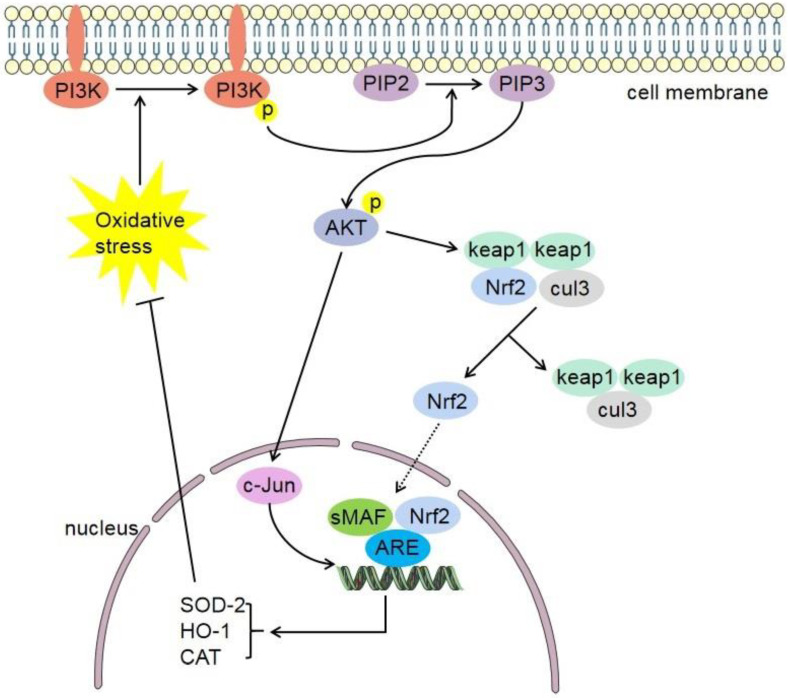
Figure 3.
PI3K/Akt-Nrf2 signaling pathway. Under oxidative stress conditions, the phosphorylation expression of PI3K increases. Akt is a downstream target of PI3K. Akt promotes the dissociation of Nrf2 and Keap1 and transfers them to the nucleus. Nrf2 translocates into the nucleus, where in it heterodimerizes with small Maf proteins (sMaf) and binds to an enhancer sequence termed ARE. Furthermore, Nrf2 can promote the activation of various target molecules (HO-1, CAT, SOD-2, etc.) and inhibit oxidative stress in feedback. AKT can also promote the expression of C-Jun and the combination of Nrf2 with sMaf and ARE. Keap1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; PI3K, phosphatidylinositol 3-kinase; PIP2, Lipid phosphatidylinositol 4,5-bisphophate; PIP3, Phosphatidylinositol 3,4,5-triphosphate; cul3, cullin-3; HO-1, heme oxygenase-1; SOD-2, superoxide dismutase-2; CAT, catalase.
4.3. MAPK/Nrf2 Signaling Pathway
Mitogen-activated protein kinase (MAPK) can transmit extracellular signals from cell surface to nucleus. It is widely involved in a variety of signal transmission processes in the cell and plays a role in the activation and expression regulation of the Nrf2/HO-1 pathway [116]. MAPKs respond to a variety of stimuli, including a variety of endogenous and exogenous stress signals. Thus, they are traditionally classified in mitogen and stress activated MAPKs, with classic representatives being extracellular signal-regulated kinase (ERK) as mitogen responsive and C-Jun N-terminal kinase (JNK) and p38 as stress responsive MAPKs (Figure 4). p phosphorylates Nrf2 to separate it from Keap1 in the cytoplasm, thereby promoting Nrf2 activation [117]. p38-specific inhibitors prevent degradation of Keap1 and nuclear translocation of Nrf2 and subsequent expression of HO-1 by inhibiting phosphorylation of p38 [118].
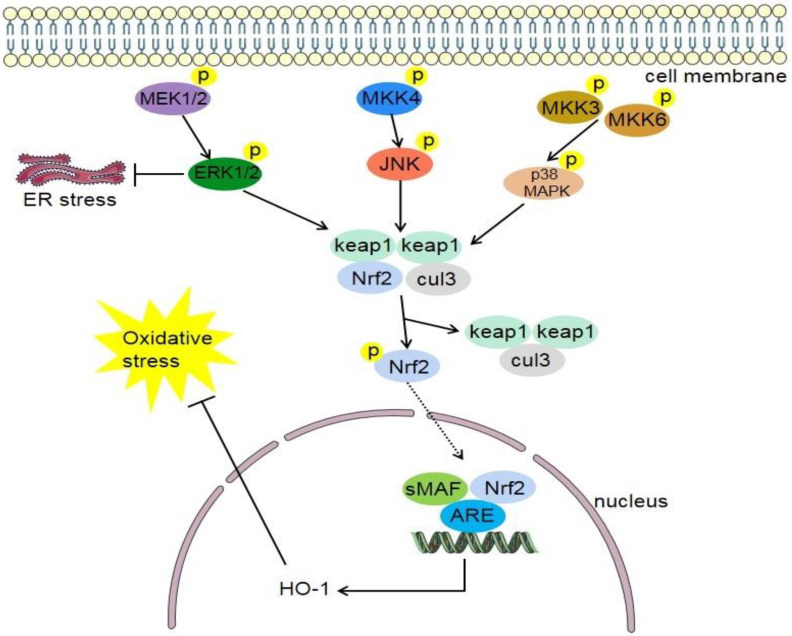
Figure 4.
Mitogen-activated protein kinase (MAPK) pathway. It consists of three pathways which involve extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 MAPK signaling pathways. Phosphorylated MEK1 and MEK2 promote the expression of ERK1 and ERK2, and then promote the dissociation of Nrf2 and Keap1. Phosphorylated MKK4 promotes the expression of JNK, and then promotes the dissociation of Nrf2 and Keap1. Phosphorylated MKK3 and MKK6 promote the expression of p38 MAPK, and then promote the dissociation of Nrf2 and Keap1. Nrf2 translocates into the nucleus, wherein it heterodimerizes with small Maf proteins (sMaf) and binds to an enhancer sequence termed ARE. This, in turn, promotes the expression of HO-1 and Oxygenase-1, and inhibits oxidative stress in feedback. MEK, mitogen-activated protein kinase; MKK4, MAPK kinase 4; MKK3, MAPK kinase 3; MKK6, MAPK kinase 6; ERK1/2, extracellular signal-regulated kinase 1 and 2; JNK, c-Jun N-terminal kinase; ER stress, endoplasmic reticulum stress; Keap1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; cul3, cullin-3; HO-1, heme oxygenase-1.
Phosphorylated ERK is the activated form of ERK. Studies have shown that downregulating the expression levels of phosphorylated ERK and Nrf2 can prevent diseases related to oxidative stress and inflammatory response [119]. ERK 1/2 inhibitor can significantly inhibit quercetin-induced expression of Nrf2 and HO-1, indicating that ERK 1/2 activation is necessary for Nrf2 stabilization and HO-1 transcription [120]. The JNK family is encoded by three genes: JNK1, JNK2, and JNK3 [121]. Under the stimulation of oxidative stress or other injury effects, the body produces cytokines such as IL, which can activate MAPK molecules [122], promote the transfer of Nrf2 [123], and thus upregulate the expression of HO-1 [124]. Carbon monoxide (CO), the product of HO-1 degradation of heme, may regulate many MAPKs [125]. Through this series of reactions, the balance of oxidation and antioxidation, proinflammatory, and anti-inflammatory reactions is finally achieved in the body.
Studies have shown that some Chinese herbs or compounds play a protective role in ischemic lesions by regulating the MAPK/Nrf2 signaling pathway. Shengmai formula (SMF) is a traditional Chinese medicine with antioxidant properties. Pretreatment of a myocardial ischemia model with SMF significantly relieved heart injury, which was related to the regulation of the PI3K/AKT/p38 MAPK/Nrf2 signal pathway [126]. Studies have shown that during ischemic stroke, p38 MAPK is transferred from cytoplasm to nucleus, thus promoting apoptosis [127]. Another study showed that artesunate has a protective effect on cerebral ischemia injury, which may be regulated by activating the Nrf2-dependent p38 MAPK signaling pathway. Study showed that artesunate could inhibit the occurrence of CIRI by inhibiting oxidative and inflammatory processes, and the protective effect of artesunate is related to the activation of the p38 MAPK pathway [128]. The study showed that in the MCAO model, lupeol activated Nrf2, inhibited caspase-3 activity, inhibited phosphorylation of p38 MAPK, and played a protective role against cerebral ischemia [129].
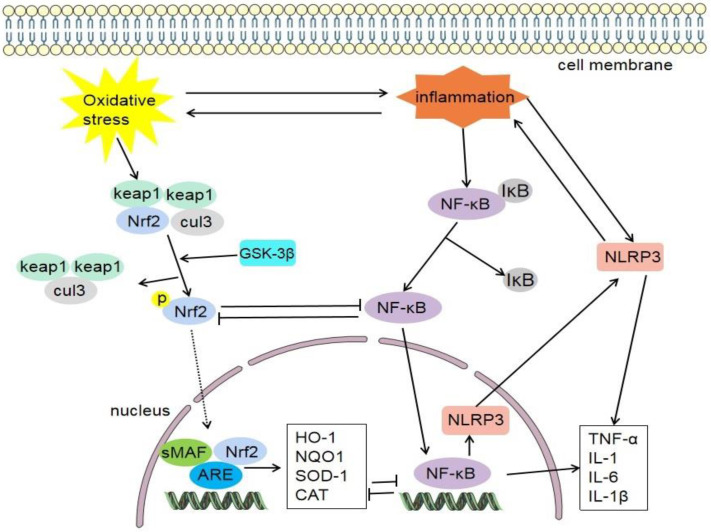
4.4. Nrf2/Nuclear Transcription Factor-Kappa B (NF-κB) Signaling Pathway
NF-κB is widely involved in cell proliferation and differentiation [130]. It is a very classical signaling pathway in vivo and plays an important role in the processes of inflammation [131,132], cell proliferation [130], and oxidative stress [133]. The NF-κB family is mainly composed of five members: p65 (Rel A), Rel B, C-Rel, p52/P100, and p50/P105. Under normal conditions, the NF-κB family is usually associated with inhibitor κB(IκB) in the form of homo- or heterodimer binding. Members of the family then form stable NF-κB/IκB complex and exist in the cytoplasm in an inactive form. The main role of IκB protein is to shield the nuclear localization site of NF-κB dimer, and prevent it from entering the nucleus. On the other hand, the IκB in the nucleus can also dissociate NF-κB from the DNA binding site, bringing it back to the cytoplasm [134].
The transcription factor NF-κB is a key regulator involved in inflammation [132]. NF-κB participates in the pathophysiological process of ischemic stroke [135]. Protein arginine methyltransferase 5 (PRMT5) is a type of methyltransferase enzyme. In cerebral ischaemia/reperfusion (I/R) injury, PRMT5 activates the NF-κB/NLRP3 axis to play a pro-inflammatory and pro-pyroptotic role. The administration of the PRMT5 inhibitor LLY-283 alleviated the neurological deficit [136]. Fingolimod (FTY720) FTY720 has protective effect on rats with ischemic stroke injury, which is related to the inhibition of the p38 MAPK/NF-κB signal pathway [137]. The study showed that Luteoloside reduced neurological deficit and brain edema in MCAO rats. Its protective effect is related to the inhibition of the PPARγ/Nrf2/NF-κB pathway [138]. In the model of CIRI, Eriocitrin attenuated oxidative stress and inflammatory response in CIRI rats by regulating the Nrf2/HO-1/NF-κB pathway [139]. Another study showed that the protective effect of Dl-3-n-butylphthalide (NBP) on neuroinflammation in mice with CIRI was related to Nrf2. Nrf2 regulates the TLR4/MyD88/NF-κB pathway to participate in this neuroprotective effect [140].
Nrf2 can negatively regulate the NF-κB signaling pathway (Figure 5). The expression of toll-like receptor 4 (TLR-4) and NF-κB in Nrf2 knockout mice was higher than that of wild-type mice treated with tMCAO, and there were more severe neurological defects, infarct size, and inflammatory damage. Deletion of Nrf2 may lead to enhanced NF-κB activity and thus promote cytokine production, while NF-κB can also regulate the expression of Nrf2 in turn, and they crosstalk with each other. Keap1 plays a key role in the two signaling pathways [141].
Figure 5.
NF-κB is usually associated with IκB and forms stable NF-κB/IκB complex. Inflammatory reaction promotes the dissociation of NF-κB/IκB complex and separates active NF-κB. NF-κB can promote the expression of NLRP3, and then promote the inflammatory response. NF-κB can also promote the release of inflammatory factors (TNF-α, IL-1, IL-6, and IL-1β). Nrf2 can negatively regulate the NF-κB signaling pathway. Keap1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; cul3, cullin-3; NLRP3, nod-like receptor family pyrin domain-containing 3; HO-1, heme oxygenase-1; NQO1, quinine oxidoreductase 1; SOD-1, superoxide dismutase-1; CAT, catalase; NF-κB, Nuclear factor-kappa B; IκB, inhibitor κB; IL-1β, interleukin-1 β; TNFα, tumour necrosis factor-alpha.
4.5. Nrf2/HO-1 Signaling Pathway
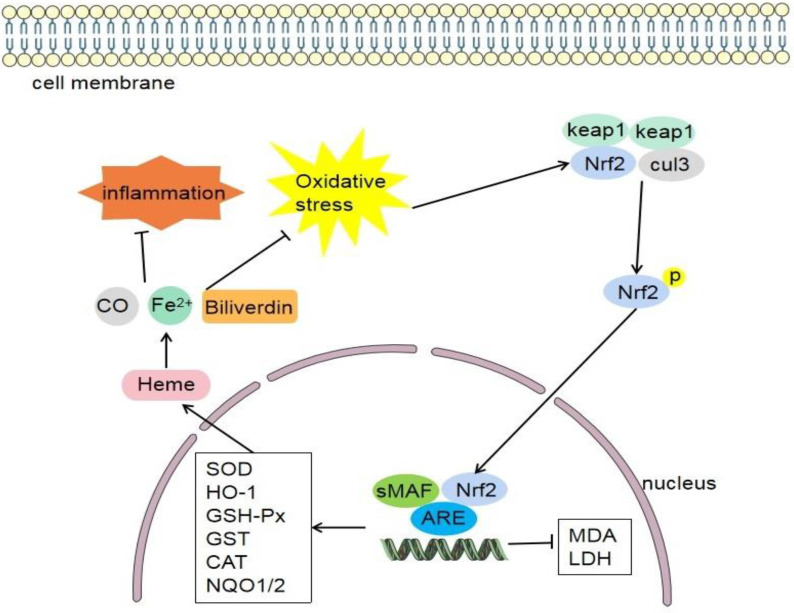
HO-1 is the rate limiting enzyme in the process of heme catabolism. It can degrade heme into CO, biliverdin, and iron ion (Fe2+) (Figure 6). These enzymatic products generally have anti-inflammatory and antioxidant effects [142], which are important mediators for Nrf2 to exert anti-inflammatory and antioxidant effects [143]. The Nrf2/HO-1 signaling pathway is an important mechanism for the body to defend against oxidative stress. Nrf2 can help maintain the physiological function of mitochondria, cellular redox reaction, and the normal function of proteins [144]. The expression of HO-1 gene is regulated by Nrf2. When Nrf2 is activated, it can promote the expression of HO-1. The upregulation of HO-1 expression can regulate these enzymes, such as SOD, GSH-Px, and CAT. Antioxidant enzymes can decompose free radicals in the body into water and molecular oxygen, reduce oxidative stress damage, and reduce the production of oxidation products, thus playing an anti-inflammatory and antioxidant role [143].
Figure 6.
When oxidative stress occurs in the body, Nrf2 and Keap1 are dissociated and activated, transferred to the nucleus, Thereby promoting the activation of various target molecules (HO-1, SOD-1, GSH-Px, GST, CAT, NQO-1, NQO-2, etc.). Then, heme is further degraded into carbon monoxide (CO), biliverdin and iron ions (Fe2+), and the oxidative stress and inflammatory reaction are feedback inhibited. Keap1, cytoplasmic kelch-like epichlorohydrin-associated protein 1; Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; cul3, cullin-3; HO-1, heme-oxygenase-1; NQO, quinine oxidoreductase; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; GST, glutathione S-transferase; CAT, catalase; MDA, malondialdehyde; LDH, lactate dehydrogenase.
In recent years, more and more studies demonstrate that the Nrf2/HO-1 pathway can enhance the tolerance of brain tissue to ischemic oxidative damage [145,146]. As a target gene of Nrf2, HO-1 has a protective effect on brain injury. Studies have shown that pelargonidin can reduce cerebral ischemic volume, and improve memory and learning ability in MCAO rats. And the protective effect of pelargonidin is related to the activation of Nrf2/HO-1 signaling pathway [147]. Studies have shown that Rutaecarpine (Rut) can inhibit apoptosis, inflammation and oxidative stress, and then reduce CI/R-induced neuronal damage. And the protective effect is achieved by activating the expression of Nrf2/HO-1 pathway [77]. The activation of Nrf2/HO-1 signaling pathway can inhibit the expression of reactive oxygen species and pro-inflammatory factors, and significantly reduce the oxidative damage of ischemic brain tissue [148,149]. Wang. et al. demonstrated that when HO-1 decomposes heme, the produced CO can treat CIRI and permanent ischemic stroke stroke [150]. Geraniin is a kind of polyphenol isolated from Phyllanthus amarus. A study has shown that Geraniin can activate Nrf2/HO-1 signaling pathway, inhibit oxidative stress and neuronal apoptosis, and thus play a protective role in cerebral ischemia injury [88]. β-Caryophyllene (BCP) is a natural bicyclic sesquiterpene. Study has proved that BCP can improve the neurological function score, infarct volume after CIRI. The mechanism is related to the activation of Nrf2/HO-1 axis [151].
5. The Role and Mechanism of Nrf2 in Cerebral Ischemic Stroke
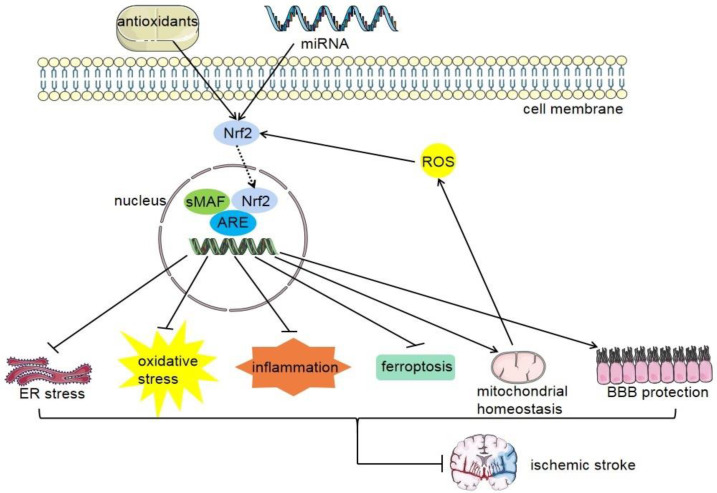
Many studies have shown that Nrf2 is significantly overexpressed in the acute phase of stroke, and the content of Nrf2 is higher in the peri-infarct area than in the central area [152]. Compared with wild-type mice, after transient middle cerebral artery occlusion was established in Nrf2 knockout mice, the basic and induced activities of antioxidant enzymes and detoxification enzymes were decreased, the infarct area was larger, and the neurological function score was lower [153]. The mechanism of action of Nrf2 in cerebral ischemic stroke (Figure 7) may include the following points:
Figure 7.
The beneficial impacts of Nrf2 activation on cerebral ischemia stroke. Antioxidants and miRNA can upregulate the expression of Nrf2 and then promote the expression of related target genes, which can inhibit ER stress, oxidative stress, inflammation, iron death, BBB, mitochondrial homeostasis, and brain protection.
Nrf2, nuclear factor erythroid 2-related factor 2; sMAF, small musculoaponeurotic fibrosarcoma; ARE, antioxidant response element; ROS, reactive oxygen species; ER stress, endoplasmic reticulum stress; BBB, blood–brain barrier.
5.1. Nrf2 Regulates Oxidative Stress and Antioxidant Effect
Previous studies have confirmed that oxidative stress is a key mechanism for brain tissue injury during cerebral ischemic stroke [58,59]. Oxidative stress is induced by elevated production of ROS and RNS, which cause damage to all components of the cell, including proteins, lipids, and DNA [154]. The Nrf2 pathway is one of the important mechanisms involved in antioxidant stress. When oxidative stress occurs in cells, the Nrf2 signaling pathway is activated first, and then a large number of antioxidants and related enzymes are induced to reduce ROS production and resist cell damage caused by oxidative stress [155]. It has been confirmed that Nrf2 expression increases after cerebral ischemia and reperfusion (I/R), which induces the production of many endogenous antioxidant enzymes, such as NQO1, HO-1, SOD, GST, GSH-Px, etc., thus reducing or eliminating oxygen free radicals and improving the antioxidant capacity of cells and tissues [156].
Thioredoxin-1 (Trx1) is a redox regulatory protein that widely exists in organisms. The expression of Trx1 decreased during ischemic stroke. Adiponectin peptide (APNp) increased the expression of Trx1 and suppressed the activation of the NLRP3 inflammasome. Study has shown that APNp can reduce the volume of cerebral infarction, improve neurological function, and have antioxidant and antiapoptosis effects in a CIRI model [157]. Previous studies have shown that in the CIRI model, the transcription of Nrf2 and its downstream gene NQO1 in the treatment group increased, the activities of SOD and cat increased, and CIRI decreased. However, these protective effects were inhibited after knockdown of Nrf2. It indicates that the activation of Nrf2 can upregulate the activities of SOD, cat, and NQO1, thereby inhibiting oxidative stress and alleviating CIRI [75].
A study showed that after CIRI, the activities of SOD, GSH, and GSH-Px were significantly decreased and the level of MDA was significantly increased. However, phloretin pretreatment significantly inhibited these oxidative stress processes, reduced infarct volume, and improved neurological score. This indicates that phloretin exhibits neuroprotective effects in CIRI, and its mechanism is related to the inhibition of oxidative stress and the activation of the Nrf2 defense pathway [158]. Carvacryl acetate (CA) has an antioxidant effect. Studies have shown that CA can reduce CIRI in MCAO rats. The mechanism may be to increase the expression of Nrf2 and decrease the expression of ROS and MDA [80]. Biochanin A is a natural phytoestrogen. Biochanin A can enhance the activities of SOD and GSH-Px and inhibit the production of MDA. Biochanin A promotes nuclear translocation of Nrf2, promotes the expression of HO-1, and inhibits the activation of NF-kappaB. Pretreatment with Biochanin A can significantly alleviate brain injury and reduce infarct size and cerebral edema. Biochanin A protects the brain against ischemic injury through antioxidant and anti-inflammatory effects [78]. Geraniin may play a protective role against brain ischemia-reperfusion injury by regulating the Nrf2/HO-1 pathway and inhibiting oxidative stress and neuronal apoptosis [88]. Studies have shown that in cell models, diosmetin increased cell viability, decreased lactate dehydrogenase (LDH) release and ROS levels, and inhibited oxidative stress. In addition, diosmetin increased the protein expression of Nrf2, NQO1, and HO-1. This indicates that diosmetin can inhibit oxidative stress and alleviate CIRI through the SIRT1/Nrf2 signaling pathway [159].
5.2. Nrf2 Regulates Inflammation and Anti-Inflammatory Effects
Inflammation is a host defense mechanism triggered by injury and plays an important role in ischemic stroke [160]. In addition, the inflammatory process is mainly aseptic inflammatory reaction. Thus, endogenous damage-associated molecular patterns (DAMPs) are the exclusive trigger activating the innate immune system in ischemia [161]. The local excessive inflammatory reaction is accompanied by the process of cerebral ischemia, and it is also one of the main causes of brain tissue damage caused by cerebral ischemia [162]. A variety of inflammatory cells and inflammatory factors are involved in it. The main inflammatory cells involved are leukocytes, astrocytes, and microglia [161]. After cerebral ischemia, the blood–brain barrier (BBB) is destroyed, resulting in a large number of neutrophils infiltrating and attacking the brain [163,164]. In the acute phase of cerebral ischemia, inflammatory cells are infiltrated to produce different kinds of inflammatory factors, and participate in the main inflammatory mediators including cytokines, chemokines, and adhesion molecules. Cerebral ischemic injury and blood flow reperfusion can cause inflammatory cascades, including oxidative stress, excitotoxicity, and inflammatory cell infiltration, as well as the production of cytokines and chemokines, which further lead to nerve tissue injury and apoptosis [165]. The regulation of NF-κB activity by Nrf2 is manifested in inhibiting inflammatory responses. Studies have shown that Nrf2 can regulate the TLR4/MyD88/NF-κB pathway in CIRI models and play a neuroprotective role [140]. Another target gene of Nrf2, p62, can regulate antioxidant and inflammatory activities. P62, as a protein scaffold, enhances the activity of Nrf2 by mediating the autophagic degradation of Keap1. P62 has oligomerization and can promote the ubiquitination and activation of TNF receptor-related factor 6 (TRAF6), enhance nerve growth factor NGF, and mediate the NF-κB signaling pathway [166].
The NOD-like receptor protein 3 (NLRP3) inflammasome is a protein complex located in the cells. Its main function is to activate the production of caspase-1, IL-1, and IL-18, thus promoting inflammation and apoptosis, and causing neuronal damage [167]. The NLRP3 inflammasome plays an important role in ischemia-induced inflammatory injury, and thioredoxin interacting protein (TXNIP) is closely related to the activation of NLRP3. Various factors such as ROS, toll-like receptor (TLR) agonists, and pro-inflammatory cytokines can promote NLRP3 expression [168]. It has been shown that knockdown of the NADPH oxidase subunit or free radical scavenger can inhibit ROS production and thus reduce NLRP3-induced IL-1 β, which reveals that ROS plays a key role in inflammasome activation [169]. Bruton’s tyrosine kinase (BTK) is a class of tyrosine kinases involved in the activation of the NLRP3 inflammatory complex in brain ischemia and reperfusion, resulting in the expression of mature caspase-1, as well as increased levels of IL-1. An inhibitor of BTK, ibrutinib, was found to inhibit the activation of the NLRP3 inflammatory complex and reduce IL-1 expression in the brain I/R model [170]. The study showed that Pleckstrin homology-like domain, family A, member 1 (PHLDA1) effectively alleviates CIRI by inhibiting the activation of NLRP3 [171]. Study has shown that Nrf2 downregulates NLRP3 inflammasome activity by acting on thioredoxin-1 (Trx1)/thioredoxin interacting protein (TXNIP) complex, thereby inhibiting inflammatory response and playing a protective role in CIRI [172]. Increasing evidence suggests an interaction between Nrf2 and the inflammasome. Administration of the Nrf2 activator tert-butyl hydroquinol (t BHQ) significantly reduced the expression of TXNIP and NLRP3 inflammasome, as well as the downstream factors caspase-1, IL-1 β,and IL-18 after t MCAO [172].
Evidence suggests that ischemic stroke triggers a range of cellular responses, including the accumulation of inflammatory cells in systemic circulation. The progress is linked to stroke-related secondary brain injury and can lead to further aggravation of infarction. It is currently known that the expression of Nrf2/HO-1 inhibits cellular inflammation and thus plays a neuroprotective role in the course of stroke [173]. Local inflammatory processes in the ischemic brain tissue can cause damage to the brain tissue. In addition, recent studies showed that peripheral inflammation also plays an important role in stroke injury. For instance, in rats suffering from MCAO, the spleen size was decreased after a stroke, mainly due to the upregulation of catecholamines causing the release of monocytes, and neutrophils [174]. Although most of the damage is caused by inflammation itself, the ensuing immunosuppression also poses non-negligible challenges. Immunosuppression is a response to inflammation after stroke, and it can trigger a variety of infections, such as pneumonia and urinary tract infections [175]. The role of Nrf2 in peripheral inflammation after stroke is still an exploratory topic, and further research is needed to understand its interaction and mechanism in the peripheral inflammatory organs of the body.
5.3. Nrf2 and Regulation of Mitochondrial Function
Mitochondria are the sites of oxidative metabolism in eukaryotes, and the sites where sugars, fats, and amino acids are finally oxidized to release energy. Study has shown that mitochondrial damage is one of the mechanisms of cell death [176]. Studies have shown that acute ischemia and hypoxia can cause mitochondrial dysfunction [177]. The physiological function of mitochondria is important for adult neurogenesis, which helps to repair neuronal cells damaged by cerebral ischemia. Neurogenesis requires sufficient ATP to provide energy. As the main source of cellular ATP, healthy mitochondrial function is an important prerequisite for effective neurogenesis [178]. The balance of mitochondrial fusion and division plays a crucial role in maintaining the normal function of neurons. CIRI can disrupt the balance of mitochondrial fusion and division by regulating the expression and modification of fusion- and division-related proteins, thereby disrupting the homeostasis of the intracellular environment and leading to neuronal death [179]. Many studies have shown that mitochondrial dysfunction is involved in the process of ischemic injury, so we can speculate that ischemic injury can be alleviated by reducing mitochondrial dysfunction. In the process of ischemic stroke, the lack of nutrition and oxygen promotes cell death [180]. After cell death, it promoted the production of ROS, which in turn aggravates mitochondrial dysfunction in feedback [181]. At the same time, mitochondrial dysfunction can lead to severe energy deficiency, increase ROS production in neurons, and ultimately lead to cell death. Most intracellular ROS are produced in mitochondria, mainly by complexes I and III of the mitochondrial electron transport chain. Mitochondria stress is accompanied by the accumulation of unfolded proteins, oxidative phosphorylation, fatty acid oxidation, and other pathological processes [182].
Activation of Nrf2 leads to mitochondrial biogenesis and the enhancement of mitochondrial antioxidant reaction [183,184]. Although the activation of Nrf2 is usually related to the induction of mitochondrial-localized antioxidant enzymes, its regulatory mechanism is not fully understood. Nrf2 is able to directly bind nuclear respiratory factor 1 (Nrf1) and the promoter region of PTEN-induced putative kinase 1 (PINK1) [185], which are mainly responsible for mitochondrial biogenesis and quality control, respectively. Some studies have shown that ROS induced the activation of Nrf2 in some way [186]. In one study, researchers found a novel biscoumarin compound, called COM3, which has substantial antioxidant effects in neurons. Study showed that COM 3 can improve neuronal mitochondrial energy metabolism after experiencing oxidative stress caused by oxygen-glucose deprivation (OGD) or MCAO. The mechanism may be that COM 3 activates nuclear transcription of Nrf2 by interfering with Keap1, thereby balancing endogenous redox activity and restoring mitochondrial function [187]. Notoginsenoside R1 (NGR1) is a novel phytoestrogen that is isolated from Panax notoginseng. The researchers found that NGR1 showed a protective effect on cerebral I/R injury in vivo and in vitro. The neuroprotective mechanism of NGR1 may be to inhibit mitochondrial dysfunction by regulating the ER-dependent Akt/Nrf2 pathway. In addition, Nrf2 activation can increase the expression of antiapoptotic protein Bcl-2 and inhibit the translocation of Bcl-2-related x (Bax) protein to mitochondria, thus alleviating the release of mitochondrial cytochrome c and the activation of downstream apoptotic proteases [188]. In conclusion, Nrf2 is a potent mediator to inhibiting mitochondrial dysfunction of cerebral ischemia through multiple mechanisms.
5.4. Nrf2 and Protection of Blood–Brain Barrier Function
The endothelial blood–brain barrier (BBB) represents a barrier between the circulation and the CNS compartment [189]. The normal structure and function of the BBB is the key to maintain the stability of the central nervous system. BBB injury occurs soon after cerebral ischemia [190]. Cerebral ischemia-reperfusion can damage the integrity of the blood–brain barrier and increase the permeability, further aggravating the damage of ischemic brain tissue [191]. The destruction of the BBB after cerebral ischemia leads to the infiltration of inflammatory factors and cells, which in turn leads to brain edema [192]. Ischemic preconditioning (IPC) can protect the blood–brain barrier, and the mechanisms underlying IPC-mediated protection of the BBB involve VEGF, ERK, or inflammatory pathways [193]. Tight junctions (TJs) and adherens junctions (AJs) play a critical role in maintaining BBB integrity. In a study, IPC directly upregulated the TJ protein claudin 5 and the AJ protein CDH5 [194]. The content of ROS increased after cerebral ischemic stroke; related research has shown that ROS production increased BBB permeability [195]. MMPs could destroy tight junctions of the BBB [196].
In in vivo experiments, the Nrf2 activator dimethyl fumarate (DMF) can prevent the destruction of tight junctions between endothelium and reduce the activity of matrix metalloproteinase in brain tissue. In in vitro experiments, DMF helps to maintain endothelial tight junctions, inhibit the expression of inflammatory cytokines, and weaken leukocyte migration. However, knockdown of Nrf2 expression aggravated the delocalization of tight junction protein ZO-1 under ischemia and weakened the protective effect of DMF, indicating the protective role of Nrf2 in BBB integrity [197]. Another study showed that in response to H/R stress, the expression of Abcc1, Abcc2, and Abcc4 mRNA in the BBB increased, and the expression of Abcc gene was regulated by the Nrf2 pathway [198].
Sulforaphane treatment before stroke activates the Nrf2 pathway. BBB disruption and brain damage were reduced by sulforaphane treatment. The authors believe that if the Nrf2 defense pathway in the brain microvascular system can be activated, it will help to prevent BBB disruption and neurological dysfunction in ischemic stroke [199]. Other Nrf2 activators, such as sulforaphane (SFN), have also shown protective effects on the blood–brain barrier [200]. Nomilin (NOM) is a limonoid compound obtained from the extracts of citrus fruits. NOM protects against cerebral I/R-induced neurological deficits and BBB disruption by regulating the Nrf2 pathway [201]. Astragaloside IV (ASIV) is isolated from Astragalus membranaceus. A study showed that ASIV protected the integrity of the BBB, and the Nrf2 pathway is involved in this process [202]. The destruction of the BBB after ischemic stroke permits peripheral inflammatory factors or bacteria to easily enter brain tissue, leading to brain injury. Therefore, focusing on the protection of the BBB through Nrf2 should be further studied.
5.5. Nrf2 Regulates Ferroptosis
Ferroptosis is a recently identified nonapoptotic-programmed cell death process. Ferroptosis is a form of cell death that depends on iron and oxidative stress [203]. It is characterized by increased lipid peroxidation, which leads to cell death by destroying the integrity of the cell membrane. The pathological process of ferroptosis can be regulated by glutathione peroxidase 4 (GPX4). Study showed that oxidative stress and iron metabolism are both closely related to ferroptosis initiation [204]. Further studies showed that ferroptosis can be inhibited by iron chelators, iron intake inhibitors, and lipophilic antioxidants [205]. Now, scholars believe that the main cause of cell death caused by ferroptosis is the inactivation of the cellular antioxidant system, the decrease of cellular antioxidant capacity, and the accumulation of intracellular lipid ROS leading to cell death [206].
Studies have shown that ferroptosis plays a key role in cerebral I/R injury. Moreover, inhibition of ferroptosis can play a neuroprotective role [207]. Furthermore, studies have shown that ferroptosis was detected in a mouse model of cerebral ischemia. The application of ferroptosis inhibitors significantly reduced brain injury [208]. Chen et al. [209] analyzed and identified ferroptosis-related differentially expressed genes (DEGs) in ischemic stroke by bioinformatics. A study showed that acute cerebral ischemia induces neuronal ferroptosis. Administration of drug therapy can alleviate cerebral ischemia injury, and its mechanism includes inhibition of ferroptosis through the transferrin receptor 1/divalent metal transporter 1(TFR1/DMT1) and solute carrier family 7 member 11/glutathione peroxidase 4(SCL7A11/GPX4) pathways [210].
A previous study showed that proper activation of Nrf2 can promote the reduction of cerebral ischemia injury [211]. Numerous enzymes and proteins involved in lipid peroxidation are Nrf2 target genes, including those involved in iron metabolism [212], such as FPN and heme-oxygenase 1 (HMOX-1) [213]. Furthermore, Nrf2 is strongly associated with the ferroptosis oxidative stress pathway. Studies have shown that ferroptosis is involved in the process of injury induced by ischemia-reperfusion. The protective effect of BCP on ischemic brain injury is related to the regulation of ferroptosis, and its mechanism is related to the activation of the Nrf2/HO-1 pathway [151]. Another study showed that rehmannioside A can improve cognitive impairment after ischemic stroke, which may be related to inhibition of neuroptosis and activation of the PI3K/AKT/Nrf2 pathway [112].
6. Drugs or Compounds Affect Cerebral Ischemic Stroke by Regulating Nrf2 Signaling Pathways
In recent years, more and more people have found that natural compounds extracted from plants can protect against cerebral ischemia. These compounds have anti-inflammatory, antioxidation, and antiapoptosis effects [214]. We speculate that it is possible to screen out drugs for treating or preventing cerebral ischemia injury from these compounds. There have been many studies on the role of Nrf2 in cerebral ischemic stroke. It has been found that regulating Nrf2 and related signaling pathways or mechanisms can alleviate cerebral ischemic stroke injury. In some studies (Table 2), cell survival was improved by targeting Nrf2-related signaling pathways.
Table 2.
Several traditional Chinese medicine/compounds affect cerebral ischemic stroke by regulating Nrf2 signaling pathways.
| Medicine | Model (s) | Related Signaling Pathway | Related Mechanism | Effect | Reference |
|---|---|---|---|---|---|
| β-Caryophyllene (BCP) | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Protect against ferroptosis | Protective | [151] |
| Diosmetin | MCAO/R and OGD/R | Activate Keap1/Nrf2/ARE pathway | Attenuate oxidative stress and inflammation | Protective | [215] |
| Dl-3-n-butylphthalide (NBP) | Repeated CIRI | Nrf2-modulated TLR4/MyD88/NF-κB pathway | Antioxidant, antineuroinflammatory | Protective | [140] |
| Edaravone dexborneol | Repeated CIRI | Activate Nrf2/HO-1 pathway | Antioxidant, antineuroinflammatory | Protective | [216] |
| Rhodiola sacra | tGCI | Activate AMPK/Nrf2 pathway | prevent oxidant stress | Protective | [217] |
| Geraniin | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Suppress oxidative stress and neuronal apoptosis. | Protective | [88] |
| Cajaninstilbene acid (CSA) | MCAO/R and OGD/R | Activate AMPK/Nrf2 pathway | Reduce oxidative stress and mitochondrial disfunction | Protective | [218] |
| Thymus quinquecostatus Celak | tMCAO | Activate Keap1/Nrf2/HO-1 pathway | Antioxidant stress | Protective | [105] |
| Isorhapontigenin (ISO) | MCAO/R and OGD/R | Activate PKCε/Nrf2/HO-1 pathway | Protect against oxidative damage | Protective | [219] |
| Palmatine (PAL) | tMCAO | Activate AMPK/Nrf2 pathway | Reduce oxidative stress and inflammatory response | Protective | [220] |
| Pelargonidin | MCAO/R | Activate Nrf2/HO-1 pathway | Reduce oxidative stress and inflammatory response | Protective | [147] |
| Eriocitrin | MCAO/R | Activate Nrf2/HO-1/NQO1/NF-κB pathway | Attenuate oxidative injury and inflammatory response | Protective | [139] |
| Lupeol | MCAO/R | Involve Nrf2 and P38 MAPK modulation | Suppress oxidative stress and inflammatory response | Protective | [129] |
| A biscoumarin compound COM 3 | MCAO/R and OGD/R | Modulate Nrf2/Keap1/ARE pathway | Antioxidant stress | Protective | [187] |
| Chlorogenic acid (CGA) | CI/R model | Activate Nrf2/HO-1 pathway | Regulate oxidative stress | Protective | [221] |
| Lyciumamide A (LyA) | MCAO/R and OGD/R | Activate PKCε/Nrf2/HO-1 pathway | Ameliorate oxidative damage and neuronal apoptosis | Protective | [222] |
| Nomilin | MCAO/R and OGD/R | Activate Nrf2/NQO1 pathway | Mitigate oxidative stress | Protective | [201] |
| Rutaecarpine (Rut) | MCAO/R | Activate Nrf2/HO-1 pathway | Inhibit apoptosis, inflammation, and oxidative stress | Protective | [77] |
| Swertiamarin (Swe) | MCAO/R and OGD/R | Activate Nrf2/HO-1 pathway | Suppress oxidative stress | Protective | [223] |
| Ginkgolides and bilobalide | MCAO/R and OGD/R | Mediate Akt/Nrf2 pathway | Inhibit oxidative stress | Protective | [109] |
| Icariside II | MCAO/R | Activate Nrf2/HO-1 pathway | Inhibit oxidative stress | Protective | [76] |
| Schizandrin A (Sch A) | MCAO/R and OGD/R | Regulate AMPK/Nrf2 pathway | Suppress inflammation and oxidative stress | Protective | [75] |
| Luteoloside | MCAO/R | Regulate PPARγ/Nrf2/NF-κB pathway | Inhibit neuroinflammation | Protective | [138] |
| Total Flavonoids from A. esculentus | TCI-RI model | Activate Nrf2-ARE pathway | Inhibit oxidative stress | Protective | [224] |
| Diterpene ginkgolides | MCAO/R and OGD/R | Activate PI3K/Akt/Nrf2 pathway | Inhibit oxidative stress | Protective | [113] |
| Artesunate | CI/RI model | Activate MAPK/Nrf2 pathway | Suppress oxidative stress and inflammatory process | Protective | [128] |
| 6’-O-galloylpaeoniflorin (GPF) | MCAO/R and OGD/R | Activate PI3K/Akt/Nrf2 pathway | Prevent oxidative stress, inflammation, and apoptosis | Protective | [114] |
| Liraglutide | MCAO/R | Activate Nrf2/HO-1 pathway | Antioxidant stress | Protective | [225] |
tGCI: transient global cerebral ischemia; PKC-ε: protein kinase C ε; TCI-RI: transient cerebral ischemia-reperfusion injury.
7. Concluding Remarks
After cerebral ischemic stroke, the degree of functional damage of nerve cells depends on the degree of insufficient tissue perfusion. The purpose of cerebral ischemic stroke treatment is to preserve the neuronal function in ischemic penumbra as much as possible. Nrf2 plays an important role in maintaining the redox homeostasis of cells. In this review, we introduced the signaling pathway for regulating Nrf2 in the process of cerebral ischemic stroke. In addition, the moderate activation of Nrf2 is beneficial to reduce brain tissue damage after cerebral ischemia, involving mechanisms such as antioxidative stress, anti-inflammation, regulation of mitochondrial function, protection of the blood–brain barrier, regulation of iron death, etc., which can reduce neurological deficit. To summarize, we maintain that Nrf2 is a valuable therapeutic target.
In the future, we need to clarify the specific mechanism behind these phenomena through more in-depth research. For example, does the specific role of Nrf2 in ischemic stroke protect or exacerbate ischemic stroke? What is the role of Nrf2 in different cell types? What is the role and regulatory mechanism of Nrf2 in neuronal cells and microglia? The role and mechanism of Nrf2 in peripheral inflammation or immune organs deserve further study. Recently, there are many studies on the role of ferroptosis in ischemic stroke. What is the relationship between Nrf2 and ferroptosis in the process of ischemic stroke and reperfusion injury? What are the specific regulatory mechanisms? Further researches are needed. How does ferroptosis lead to cell death after ischemic stroke? Is it involved in the process of neuronal cell death? Further research is needed. In addition, what is the relationship between ferroptosis and oxidative stress? None of this is known yet. In addition, there are many studies on the mechanism of oxidative stress, but few studies on the interaction between oxidative stress and endoplasmic reticulum stress. We believe that the interaction between these processes deserves further study. Solving these problems will promote the treatment of ischemic stroke. More researches are needed to transform this therapy from laboratory to clinical.
More and more evidence shows that the activation of Nrf2 by some Chinese herbs and may be a promising therapeutic method for ischemic stroke. However, the specific activation pathway of Nrf2 and the specific mechanism of alleviating cerebral ischemia and its substrates still need to be further studied. In addition, the time window in which these drugs function requires more careful study. In conclusion, the treatment targeting Nrf2 in cerebral ischemic stroke is a promising field, and the development of drugs targeting Nrf2 has important clinical significance for the treatment of cerebral ischemic stroke.
Author Contributions
Conceptualization, H.Z. and R.C.; writing—original draft preparation, L.W., X.Z. and X.X.; writing—review and editing, S.Z., G.C. and Z.J.; supervision, G.C. and Z.J. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that there are no conflict of interest.
Funding Statement
This work was supported by the Health Commission of Hubei Province Scientific Research Project (No. WJ2021M148), the National Natural Science Foundation of China (Nos. 82171336 and 81870939), and the Hubei Province Key Laboratory Open Project (No. 2021KFY044).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu S., Wu B., Liu M., Chen Z., Wang W., Anderson C., Sandercock P., Wang Y., Huang Y., Cui L., et al. Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18:394–405. doi: 10.1016/S1474-4422(18)30500-3. [DOI] [PubMed] [Google Scholar]
- 2.Hankey G. Stroke. Lancet. 2017;389:641–654. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 3.Hitomi E., Simpkins A., Luby M., Latour L., Leigh R., Leigh R. Blood-ocular barrier disruption in patients with acute stroke. Neurology. 2018;90:e915–e923. doi: 10.1212/WNL.0000000000005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lassen N., Fieschi C., Lenzi G. Ischemic penumbra and neuronal death: Comments on the therapeutic window in acute stroke with particular reference to thrombolytic therapy. Cerebrovasc. Dis. 1991;1:32–35. doi: 10.1159/000108878. [DOI] [Google Scholar]
- 5.Amer A., Oorschot D. Xenon Combined With Hypothermia in Perinatal Hypoxic-Ischemic Encephalopathy: A Noble Gas, a Noble Mission. Pediatr. Neurol. 2018;84:5–10. doi: 10.1016/j.pediatrneurol.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli C., Chio I., Tuveson D. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagan S., Khurana N., Chandra S., Abdel-Mageed A., Mondal D., Hellstrom W., Sikka S. Differential expression of novel biomarkers (TLR-2, TLR-4, COX-2, and Nrf-2) of inflammation and oxidative stress in semen of leukocytospermia patients. Andrology. 2015;3:848–855. doi: 10.1111/andr.12074. [DOI] [PubMed] [Google Scholar]
- 8.Thanas C., Ziros P., Chartoumpekis D., Renaud C., Sykiotis G. The Keap1/Nrf2 Signaling Pathway in the Thyroid-2020 Update. Antioxidants. 2020;9:1082. doi: 10.3390/antiox9111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto M., Kensler T., Motohashi H. The Keap1-Nrf2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khodakarami A., Adibfar S., Karpisheh V., Abolhasani S., Jalali P., Mohammadi H., Navashenaq J.G., Mohammad Hojjat-Farsangi M., Jadidi-Niaragh F. The molecular biology and therapeutic potential of Nrf2 in leukemia. Cancer Cell Int. 2022;22:241. doi: 10.1186/s12935-022-02660-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed S., Shad K., Saleem T., Javed F., Khan M. Some new prospects in the understanding of the molecular basis of the pathogenesis of stroke. Exp. Brain Res. 2007;182:1–10. doi: 10.1007/s00221-007-1050-9. [DOI] [PubMed] [Google Scholar]
- 12.Mazur A., Fangman M., Ashouri R., Arcenas A., Dore S. Nrf2 as a therapeutic target in ischemic stroke. Expert Opin. Ther. Targets. 2021;25:163–166. doi: 10.1080/14728222.2021.1890716. [DOI] [PubMed] [Google Scholar]
- 13.Farina M., Vieira L., Buttari B., Profumo E., Saso L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules. 2021;26:5001. doi: 10.3390/molecules26165001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L., Vollmer M., Fernandez V., Dweik Y., Kim H., Dore S. Korean Red Ginseng Pretreatment Protects Against Long-Term Sensorimotor Deficits After Ischemic Stroke Likely Through Nrf2. Front. Cell. Neurosci. 2018;12:74. doi: 10.3389/fncel.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee C., Yu H. Role of mitochondria, ROS, and DNA damage in arsenic induced carcinogenesis. Front. Biosci. 2016;8:312–320. doi: 10.2741/s465. [DOI] [PubMed] [Google Scholar]
- 16.De Bont R., Van Larebeke N. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis. 2004;19:169–185. doi: 10.1093/mutage/geh025. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 18.Bano T., Kumar N., Dudhe R. Free radical scavenging properties of pyrimidine derivatives. Org. Med. Chem. Lett. 2012;2:34. doi: 10.1186/2191-2858-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caputo F., Vegliante R., Ghibelli L. Redox modulation of the DNA damage response. Biochem. Pharmacol. 2012;84:1292–1306. doi: 10.1016/j.bcp.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 20.Yao Y., Miao W., Liu Z., Han W., Shi K., Shen Y., Li H., Liu Q., Fu Y., Huang D., et al. Dimethyl Fumarate and Monomethyl Fumarate Promote Post-Ischemic Recovery in Mice. Transl. Stroke Res. 2016;7:535–547. doi: 10.1007/s12975-016-0496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H., Lee H., Lee J., Yim N., Gu M., Ma J. Protective Effects of Spatholobi Caulis Extract on Neuronal Damage and Focal Ischemic Stroke/Reperfusion Injury. Mol. Neurobiol. 2018;55:4650–4666. doi: 10.1007/s12035-017-0652-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao H., Wang R., Tao Z., Yan F., Gao L., Liu X., Wang N., Min L., Jia Y., Zhao Y., et al. Activation of T-LAK-cell-originated protein kinase-mediated antioxidation protects against focal cerebral ischemia-reperfusion injury. FEBS J. 2014;281:4411–4420. doi: 10.1111/febs.12948. [DOI] [PubMed] [Google Scholar]
- 23.Singh L., Singh A., Bhatti R. Mechanistic interplay of various mediators involved in mediating the neuroprotective effect of daphnetin. Pharmacol. Rep. 2021;73:1220–1229. doi: 10.1007/s43440-021-00261-z. [DOI] [PubMed] [Google Scholar]
- 24.Lee C., Yu L., Wang J. Nitroxide antioxidant as a potential strategy to attenuate the oxidative/nitrosative stress induced by hydrogen peroxide plus nitric oxide in cultured neurons. Nitric Oxide. 2016;54:38–50. doi: 10.1016/j.niox.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Chang C., Kao T., Chen W., Ou Y., Li J., Liao S., Raung S., Chen C. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem. Biophys. Res. Commun. 2015;463:421–427. doi: 10.1016/j.bbrc.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 26.Michalickova D., Hrncir T., Canova N., Slanar O. Targeting Keap1/Nrf2/ARE signaling pathway in multiple sclerosis. Eur. J. Pharmacol. 2020;873:172973. doi: 10.1016/j.ejphar.2020.172973. [DOI] [PubMed] [Google Scholar]
- 27.Park Y., Park H., Kim E., Lee H., Hwang J., Jeon Y., Lim Y. The antioxidant effect of preischemic dexmedetomidine in a rat model: Increased expression of Nrf2/HO-1 via the PKC pathway. Braz. J. Anesthesiol. 2021 doi: 10.1016/j.bjane.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H., Wu Z., Wang X., Gao C., Liu R., Kang F., Dai M. Protective effects of combined treatment with mild hypothermia and edaravone against cerebral ischemia/reperfusion injury via oxidative stress and Nrf2 pathway regulation. Int. J. Oncol. 2020;57:500–508. doi: 10.3892/ijo.2020.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H., Jing X., Wei X., Perez R., Ren M., Zhang X., Lou H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J. Neurochem. 2015;133:298–308. doi: 10.1111/jnc.12986. [DOI] [PubMed] [Google Scholar]
- 30.Ashabi G., Khalaj L., Khodagholi F., Goudarzvand M., Sarkaki A. Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab. Brain Dis. 2015;30:747–754. doi: 10.1007/s11011-014-9632-2. [DOI] [PubMed] [Google Scholar]
- 31.Ding Y., Chen M., Wang M., Li Y., Wen A. Posttreatment with 11-Keto-beta-Boswellic Acid Ameliorates Cerebral Ischemia-Reperfusion Injury: Nrf2/HO-1 Pathway as a Potential Mechanism. Mol. Neurobiol. 2015;52:1430–1439. doi: 10.1007/s12035-014-8929-9. [DOI] [PubMed] [Google Scholar]
- 32.Sandberg M., Patil J., D’Angelo B., Weber S., Mallard C. Nrf2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology. 2014;79:298–306. doi: 10.1016/j.neuropharm.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W., Wei R., Zhang L., Tan Y., Qian C. Sirtuin 6 protects the brain from cerebral ischemia/reperfusion injury through Nrf2 activation. Neuroscience. 2017;366:95–104. doi: 10.1016/j.neuroscience.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J., Wang H., Ji X., Zhu L., Sun Q., Cong Z., Zhou Y., Liu H., Zhou M. Differential Nrf2 expression between glioma stem cells and non-stem-like cells in glioblastoma. Oncol. Lett. 2014;7:693–698. doi: 10.3892/ol.2013.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kryszczuk M., Kowalczuk O. Significance of NRF2 in physiological and pathological conditions an comprehensive review. Arch. Biochem. Biophys. 2022;730:109417. doi: 10.1016/j.abb.2022.109417. [DOI] [PubMed] [Google Scholar]
- 36.Hayes J., Dinkova-Kostova A. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Horie Y., Suzuki T., Inoue J., Iso T., Wells G., Moore T., Mizushima T., Dinkova-Kostova A., Kasai T., Kamei T., et al. Molecular basis for the disruption of Keap1-Nrf2 interaction via Hinge & Latch mechanism. Commun. Biol. 2021;4:576. doi: 10.1038/s42003-021-02100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T., Liang X., Abeysekera I., Iqbal U., Duan Q., Naha G., Lin L., Yao X. Activation of the Nrf2-Keap 1 Pathway in Short-Term Iodide Excess in Thyroid in Rats. Oxid. Med. Cell. Longev. 2017;2017:4383652. doi: 10.1155/2017/4383652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krajka-Kuzniak V., Paluszczak J., Baer-Dubowska W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017;69:393–402. doi: 10.1016/j.pharep.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J., et al. RXRalpha inhibits the Nrf2-ARE signaling pathway through a direct interaction with the Neh7 domain of Nrf2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 41.Takagi T., Kitashoji A., Iwawaki T., Tsuruma K., Shimazawa M., Yoshimura S., Iwama T., Hara H. Temporal activation of Nrf2 in the penumbra and Nrf2 activator-mediated neuroprotection in ischemia-reperfusion injury. Free Radic. Biol. Med. 2014;72:124–133. doi: 10.1016/j.freeradbiomed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Christopher E., Loan J., Samarasekera N., McDade K., Rose J., Barrington J., Hughes J., Smith C., Al-Shahi S.R. Nrf2 activation in the human brain after stroke due to supratentorial intracerebral haemorrhage: A case-control study. BMJ Neurol. Open. 2022;4:e000238. doi: 10.1136/bmjno-2021-000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuadrado A., Rojo A., Wells G., Hayes J., Cousin S., Rumsey W., Attucks O., Franklin S., Levonen A., Kensler T., et al. Therapeutic targeting of the Nrf2 and Keap1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldi T.M., Bocanegra V., Manucha W., Gil L.A., Valles P. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chaperones. 2011;16:57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., Wu S., Shi N., Lian S., Lin W. Nrf2/HO-1 pathway activation by manganese is associated with reactive oxygen species and ubiquitin-proteasome pathway, not MAPKs signaling. J. Appl. Toxicol. 2011;31:690–697. doi: 10.1002/jat.1654. [DOI] [PubMed] [Google Scholar]
- 46.Jansen T., Hortmann M., Oelze M., Opitz B., Steven S., Schell R., Knorr M., Karbach S., Schuhmacher S., Wenzel P., et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J. Mol. Cell. Cardiol. 2010;49:186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Fuse Y., Kobayashi M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules. 2017;22:436. doi: 10.3390/molecules22030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardo M., Qiu X., Zimmermann R., Rudich Y. Particulate Matter Toxicity Is Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2020;33:1110–1120. doi: 10.1021/acs.chemrestox.0c00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh S., Nagalakshmi D., Sharma K., Ravichandiran V. Natural antioxidants for neuroinflammatory disorders and possible involvement of Nrf2 pathway: A review. Heliyon. 2021;7:e06216. doi: 10.1016/j.heliyon.2021.e06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tu W., Wang H., Li S., Liu Q., Sha H. The Anti-Inflammatory and Anti-Oxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019;10:637–651. doi: 10.14336/AD.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cederbaum A. Nrf2 and antioxidant defense against CYP2E1 toxicity. Subcell Biochem. 2013;67:105–130. doi: 10.1007/978-94-007-5881-0_2. [DOI] [PubMed] [Google Scholar]
- 52.Novo E., Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2008;1:5. doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Niizuma K., Yoshioka H., Chen H., Kim G., Jung J., Katsu M., Okami N., Chan P. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim. Biophys. Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer M., Cinar R., Katz A., Gao M., Erdelyi K., Jourdan T., Coffey N., Pacher P., Kunos G. Design, Synthesis, and Biological Evaluation of Novel, Non-Brain-Penetrant, Hybrid Cannabinoid CB1R Inverse Agonist/Inducible Nitric Oxide Synthase (iNOS) Inhibitors for the Treatment of Liver Fibrosis. J. Med. Chem. 2017;60:1126–1141. doi: 10.1021/acs.jmedchem.6b01504. [DOI] [PubMed] [Google Scholar]
- 55.Cerkezkayabekir A., Sanal F., Bakar E., Ulucam E., Inan M. Naringin protects viscera from ischemia/reperfusion injury by regulating the nitric oxide level in a rat model. Biotech. Histochem. 2017;92:252–263. doi: 10.1080/10520295.2017.1305499. [DOI] [PubMed] [Google Scholar]
- 56.Yao X., Carlson D., Sun Y., Ma L., Wolf S., Minei J., Zang Q. Mitochondrial ROS Induces Cardiac Inflammation via a Pathway through mtDNA Damage in a Pneumonia-Related Sepsis Model. PLoS ONE. 2015;10:e0139416. doi: 10.1371/journal.pone.0139416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Enzmann G., Kargaran S., Engelhardt B. Ischemia-reperfusion injury in stroke: Impact of the brain barriers and brain immune privilege on neutrophil function. Ther. Adv. Neurol. Disord. 2018;11:1756286418794184. doi: 10.1177/1756286418794184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Su X., Wang L., Ma S., Cao Y., Yang N., Lin L., Fisher M., Yang J., Liu C. Mechanisms of Acupuncture in the Regulation of Oxidative Stress in Treating Ischemic Stroke. Oxid. Med. Cell. Longev. 2020;2020:7875396. doi: 10.1155/2020/7875396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malko P., Jiang L. TRPM2 channel-mediated cell death: An important mechanism linking oxidative stress-inducing pathological factors to associated pathological conditions. Redox Biol. 2020;37:101755. doi: 10.1016/j.redox.2020.101755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Radak D., Resanovic I., Isenovic E. Link between oxidative stress and acute brain ischemia. Angiology. 2014;65:667–676. doi: 10.1177/0003319713506516. [DOI] [PubMed] [Google Scholar]
- 61.Chen S., Yang J., Lin T., Yang D. Emerging Roles of Sestrins in Neurodegenerative Diseases: Counteracting Oxidative Stress and Beyond. J. Clin. Med. 2019;8:1001. doi: 10.3390/jcm8071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo J., Ma L., Shi H., Zhu J., Wu J., Ding Z., An Y., Zou Y., Ge J. Alginate Oligosaccharide Prevents Acute Doxorubicin Cardiotoxicity by Suppressing Oxidative Stress and Endoplasmic Reticulum-Mediated Apoptosis. Mar. Drugs. 2016;14:231. doi: 10.3390/md14120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuo M., Wang A., Song G., Yang Z. miR-652 protects rats from cerebral ischemia/reperfusion oxidative stress injury by directly targeting NOX2. Biomed. Pharmacother. 2020;124:109860. doi: 10.1016/j.biopha.2020.109860. [DOI] [PubMed] [Google Scholar]
- 64.Yang Z., Xiang Y., Zuo M., Mao L., Hu G., Song G., Sheikh M., Tan L. lncRNA PINK1-AS Aggravates Cerebral Ischemia/Reperfusion Oxidative Stress Injury through Regulating ATF2 by Sponging miR-203. Oxid. Med. Cell. Longev. 2022;2022:1296816. doi: 10.1155/2022/1296816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raval A., Dave K., DeFazio R., Perez-Pinzon M. epsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cadenas E., Davies K. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 67.Masiero S., Celia A., Rosati G., Armani M. Robotic-assisted rehabilitation of the upper limb after acute stroke. Arch. Phys. Med. Rehabil. 2007;88:142–149. doi: 10.1016/j.apmr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 68.Orrenius S., Gogvadze V., Zhivotovsky B. Mitochondrial oxidative stress: Implications for cell death. Annu. Rev. Pharmacol. Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 69.Sanderson T., Reynolds C., Kumar R., Przyklenk K., Huttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: Pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol. Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halestrap A. Calcium, mitochondria and reperfusion injury: A pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST0340232. [DOI] [PubMed] [Google Scholar]
- 71.Cai Z., Zeng W., Tao K., Lu F., Gao G., Yang Q. Myricitrin alleviates MPP(+)-induced mitochondrial dysfunction in a DJ-1-dependent manner in SN4741 cells. Biochem. Biophys. Res. Commun. 2015;458:227–233. doi: 10.1016/j.bbrc.2015.01.060. [DOI] [PubMed] [Google Scholar]
- 72.Qu Y., Zhang H., Zhang X., Jiang H. Arachidonic acid attenuates brain damage in a rat model of ischemia/reperfusion by inhibiting inflammatory response and oxidative stress. Hum. Exp. Toxicol. 2018;37:135–141. doi: 10.1177/0960327117692134. [DOI] [PubMed] [Google Scholar]
- 73.Janyou A., Wicha P., Jittiwat J., Suksamrarn A., Tocharus C., Tocharus J. Dihydrocapsaicin Attenuates Blood Brain Barrier and Cerebral Damage in Focal Cerebral Ischemia/Reperfusion via Oxidative Stress and Inflammatory. Sci. Rep. 2017;7:10556. doi: 10.1038/s41598-017-11181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xia D., Zhang Z., Zhao Y. Acteoside Attenuates Oxidative Stress and Neuronal Apoptosis in Rats with Focal Cerebral Ischemia-Reperfusion Injury. Biol. Pharm. Bull. 2018;41:1645–1651. doi: 10.1248/bpb.b18-00210. [DOI] [PubMed] [Google Scholar]
- 75.Zhou F., Wang M., Ju J., Wang Y., Liu Z., Zhao X., Yan Y., Yan S., Luo X., Fang Y. Schizandrin A protects against cerebral ischemia-reperfusion injury by suppressing inflammation and oxidative stress and regulating the AMPK/Nrf2 pathway regulation. Am. J. Transl. Res. 2019;11:199–209. [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Meng F. Effects of icariside II on brain tissue oxidative stress and Nrf2/HO-1 expression in rats with cerebral ischemia-reperfusion injury1. Acta Cir. Bras. 2019;34:e201900208. doi: 10.1590/s0102-8650201900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han M., Hu L., Chen Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 2019;13:2923–2931. doi: 10.2147/DDDT.S216156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo M., Lu H., Qin J., Qu S., Wang W., Guo Y., Liao W., Song M., Chen J., Wang Y. Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury by Nrf2-Mediated Inhibition of Oxidative Stress and Inflammation Signaling Pathway in Rats. Med. Sci. Monit. 2019;25:8975–8983. doi: 10.12659/MSM.918665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li R., Li X., Wu H., Yang Z., Fei L., Zhu J. Theaflavin attenuates cerebral ischemia/reperfusion injury by abolishing miRNA1283pmediated Nrf2 inhibition and reducing oxidative stress. Mol. Med. Rep. 2019;20:4893–4904. doi: 10.3892/mmr.2019.10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song Y., Wang L., Bei Y., Qin D., Ai L., Ma Q., Lin P. Carvacryl acetate, a semisynthetic monoterpenic ester obtained from essential oils, provides neuroprotection against cerebral ischemia reperfusion-induced oxidative stress injury via the Nrf2 signalling pathway. Food Funct. 2020;11:1754–1763. doi: 10.1039/C9FO02037C. [DOI] [PubMed] [Google Scholar]
- 81.Shalavadi M., Chandrashekhar V., Muchchandi I. Neuroprotective effect of Convolvulus pluricaulis Choisy in oxidative stress model of cerebral ischemia reperfusion injury and assessment of MAP2 in rats. J. Ethnopharmacol. 2020;249:112393. doi: 10.1016/j.jep.2019.112393. [DOI] [PubMed] [Google Scholar]
- 82.Zhao J., Piao X., Wu Y., Liang S., Han F., Liang Q., Shao S., Zhao D. Cepharanthine attenuates cerebral ischemia/reperfusion injury by reducing NLRP3 inflammasome-induced inflammation and oxidative stress via inhibiting 12/15-LOX signaling. Biomed. Pharmacother. 2020;127:110151. doi: 10.1016/j.biopha.2020.110151. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Y., Xu J. Sanggenon C Ameliorates Cerebral Ischemia-Reperfusion Injury by Inhibiting Inflammation and Oxidative Stress through Regulating RhoA-ROCK Signaling. Inflammation. 2020;43:1476–1487. doi: 10.1007/s10753-020-01225-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Du J., Yin G., Hu Y., Shi S., Jiang J., Song X., Zhang Z., Wei Z., Tang C., Lyu H. Coicis semen protects against focal cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting angiogenesis via the TGFbeta/ALK1/Smad1/5 signaling pathway. Aging. 2020;13:877–893. doi: 10.18632/aging.202194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang K., Zhang W., Liu J., Cui Y., Cui J. Piceatannol protects against cerebral ischemia/reperfusioninduced apoptosis and oxidative stress via the Sirt1/FoxO1 signaling pathway. Mol. Med. Rep. 2020;22:5399–5411. doi: 10.3892/mmr.2020.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kang Y., Sun Y., Li T., Ren Z. Garcinol protects against cerebral ischemia-reperfusion injury in vivo and in vitro by inhibiting inflammation and oxidative stress. Mol. Cell. Probes. 2020;54:101672. doi: 10.1016/j.mcp.2020.101672. [DOI] [PubMed] [Google Scholar]
- 87.Zhang P., Cui J. Neuroprotective Effect of Fisetin Against the Cerebral Ischemia-Reperfusion Damage via Suppression of Oxidative Stress and Inflammatory Parameters. Inflammation. 2021;44:1490–1506. doi: 10.1007/s10753-021-01434-x. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y., He B., Zhang X., Yang R., Xia X., Chen L., Li R., Shen Z., Chen P. Geraniin Protects against Cerebral Ischemia/Reperfusion Injury by Suppressing Oxidative Stress and Neuronal Apoptosis via Regulation of the Nrf2/HO-1 Pathway. Oxid. Med. Cell. Longev. 2022;2022:2152746. doi: 10.1155/2022/2152746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chu X., Zhang L., Zhou Y., Fang Q. Cucurbitacin B alleviates cerebral ischemia/reperfusion injury by inhibiting NLRP3 inflammasome-mediated inflammation and reducing oxidative stress. Biosci. Biotechnol. Biochem. 2022;86:846–854. doi: 10.1093/bbb/zbac065. [DOI] [PubMed] [Google Scholar]
- 90.Yao H., Zhao J., Song X. Protective effects of fraxin on cerebral ischemia-reperfusion injury by mediating neuroinflammation and oxidative stress through PPAR-gamma/NF-kappaB pathway. Brain Res. Bull. 2022;187:49–62. doi: 10.1016/j.brainresbull.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Deng M., Sun J., Peng L., Huang Y., Jiang W., Wu S., Zhou L., Chung S., Cheng X. Scutellarin acts on the AR-NOX axis to remediate oxidative stress injury in a mouse model of cerebral ischemia/reperfusion injury. Phytomedicine. 2022;103:154214. doi: 10.1016/j.phymed.2022.154214. [DOI] [PubMed] [Google Scholar]
- 92.Uruno A., Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 93.Baird L., Dinkova-Kostova A. The cytoprotective role of the Keap1-Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 94.Zhang M., An C., Gao Y., Leak R., Chen J., Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zborowski V., Heck S., Vencato M., Pinton S., Marques L., Nogueira C. Keap1/Nrf2/HO-1 signaling pathway contributes to p-chlorodiphenyl diselenide antidepressant-like action in diabetic mice. Psychopharmacology. 2020;237:363–374. doi: 10.1007/s00213-019-05372-3. [DOI] [PubMed] [Google Scholar]
- 96.Velichkova M., Hasson T. Keap1 regulates the oxidation-sensitive shuttling of Nrf2 into and out of the nucleus via a Crm1-dependent nuclear export mechanism. Mol. Cell. Biol. 2005;25:4501–4513. doi: 10.1128/MCB.25.11.4501-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cuevas S., Yang Y., Konkalmatt P., Asico L., Feranil J., Jones J., Villar V., Armando I., Jose P. Role of nuclear factor erythroid 2-related factor 2 in the oxidative stress-dependent hypertension associated with the depletion of DJ-1. Hypertension. 2015;65:1251–1257. doi: 10.1161/HYPERTENSIONAHA.114.04525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Waz S., Heeba G., Hassanin S., Abdel-Latif R. Nephroprotective effect of exogenous hydrogen sulfide donor against cyclophosphamide-induced toxicity is mediated by Nrf2/HO-1/NF-kappaB signaling pathway. Life Sci. 2021;264:118630. doi: 10.1016/j.lfs.2020.118630. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y., Kern J., Walker J., Johnson J., Schultz P., Luesch H. A genomic screen for activators of the antioxidant response element. Proc. Natl. Acad. Sci. USA. 2007;104:5205–5210. doi: 10.1073/pnas.0700898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon J., Han E., Bui C., Shin W., Lee J., Lee S., Choi Y., Lee A., Lee K., Park C., et al. Assurance of mitochondrial integrity and mammalian longevity by the p62-Keap1-Nrf2-Nqo1 cascade. EMBO Rep. 2012;13:150–156. doi: 10.1038/embor.2011.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bae S., Sung S., Oh S., Lim J., Lee S., Park Y., Lee H., Kang D., Rhee S. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell Metab. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 102.Sun Y., He L., Wang T., Hua W., Qin H., Wang J., Wang L., Gu W., Li T., Li N., et al. Activation of p62-Keap1-Nrf2 Pathway Protects 6-Hydroxydopamine-Induced Ferroptosis in Dopaminergic Cells. Mol. Neurobiol. 2020;57:4628–4641. doi: 10.1007/s12035-020-02049-3. [DOI] [PubMed] [Google Scholar]
- 103.Gao J., Chen N., Li N., Xu F., Wang W., Lei Y., Shi J., Gong Q. Neuroprotective Effects of Trilobatin, a Novel Naturally Occurring Sirt3 Agonist from Lithocarpus polystachyus Rehd.; Mitigate Cerebral Ischemia/Reperfusion Injury: Involvement of TLR4/NF-kappaB and Nrf2/Keap-1 Signaling. Antioxid. Redox Signal. 2020;33:117–143. doi: 10.1089/ars.2019.7825. [DOI] [PubMed] [Google Scholar]
- 104.Yao Y., Hu S., Zhang C., Zhou Q., Wang H., Yang Y., Liu C., Ding H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 2022;105:108582. doi: 10.1016/j.intimp.2022.108582. [DOI] [PubMed] [Google Scholar]
- 105.Fan S., Liu X., Wang Y., Ren X., Liu Y., Dong Y., Fan Q., Wei J., Ma J., Yu A., et al. Thymus quinquecostatus Celak. ameliorates cerebral ischemia-reperfusion injury via dual antioxidant actions: Activating Keap1/Nrf2/HO-1 signaling pathway and directly scavenging ROS. Phytomedicine. 2021;91:153673. doi: 10.1016/j.phymed.2021.153673. [DOI] [PubMed] [Google Scholar]
- 106.Xie Y., Shi X., Sheng K., Han G., Li W., Zhao Q., Jiang B., Feng J., Li J., Gu Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol. Med. Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang L., Cao J., Cao L., Gao L., Yang Y., Xu L. Puerarin induces cell apoptosis in human chondrosarcoma cell line SW1353 via inhibition of the PI3K/Akt signaling pathway. Oncol. Lett. 2017;14:5585–5590. doi: 10.3892/ol.2017.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yin X., Wang X., Fan Z., Peng C., Ren Z., Huang L., Liu Z., Zhao K. Hyperbaric Oxygen Preconditioning Attenuates Myocardium Ischemia-Reperfusion Injury Through Upregulation of Heme Oxygenase 1 Expression: PI3K/Akt/Nrf2 Pathway Involved. J. Cardiovasc. Pharmacol. Ther. 2015;20:428–438. doi: 10.1177/1074248414568196. [DOI] [PubMed] [Google Scholar]
- 109.Liu Q., Jin Z., Xu Z., Yang H., Li L., Li G., Li F., Gu S., Zong S., Zhou J., et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones. 2019;24:441–452. doi: 10.1007/s12192-019-00977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu Y., Han F., Tan J. Edaravone protects the retina against ischemia/reperfusioninduced oxidative injury through the PI3K/Akt/Nrf2 pathway. Mol. Med. Rep. 2017;16:9210–9216. doi: 10.3892/mmr.2017.7739. [DOI] [PubMed] [Google Scholar]
- 111.Wang J., Wang A., He H., She X., He Y., Li S., Liu L., Luo T., Huang N., Luo H., et al. Trametenolic acid B protects against cerebral ischemia and reperfusion injury through modulation of microRNA-10a and PI3K/Akt/mTOR signaling pathways. Biomed. Pharmacother. 2019;112:108692. doi: 10.1016/j.biopha.2019.108692. [DOI] [PubMed] [Google Scholar]
- 112.Fu C., Wu Y., Liu S., Luo C., Lu Y., Liu M., Wang L., Zhang Y., Liu X. Rehmannioside A improves cognitive impairment and alleviates ferroptosis via activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signaling pathway after ischemia. J. Ethnopharmacol. 2022;289:115021. doi: 10.1016/j.jep.2022.115021. [DOI] [PubMed] [Google Scholar]
- 113.Zhang W., Song J., Yan R., Li L., Xiao Z., Zhou W., Wang Z., Xiao W., Du G. Diterpene ginkgolides protect against cerebral ischemia/reperfusion damage in rats by activating Nrf2 and CREB through PI3K/Akt signaling. Acta Pharmacol. Sin. 2018;39:1259–1272. doi: 10.1038/aps.2017.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wen Z., Hou W., Wu W., Zhao Y., Dong X., Bai X., Peng L., Song L. 6’-O-Galloylpaeoniflorin Attenuates Cerebral Ischemia Reperfusion-Induced Neuroinflammation and Oxidative Stress via PI3K/Akt/Nrf2 Activation. Oxid. Med. Cell. Longev. 2018;2018:8678267. doi: 10.1155/2018/8678267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ji K., Xue L., Cheng J., Bai Y. Preconditioning of H2S inhalation protects against cerebral ischemia/reperfusion injury by induction of HSP70 through PI3K/Akt/Nrf2 pathway. Brain Res. Bull. 2016;121:68–74. doi: 10.1016/j.brainresbull.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 116.Kim E., Choi E. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 117.Zhang H., Yuan B., Huang H., Qu S., Yang S., Zeng Z. Gastrodin induced HO-1 and Nrf2 up-regulation to alleviate H2O2-induced oxidative stress in mouse liver sinusoidal endothelial cells through p38 MAPK phosphorylation. Braz. J. Med. Biol. Res. 2018;51:e7439. doi: 10.1590/1414-431x20187439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Z., Ka S., Lee Y., Park B., Bae E. Butein induction of HO-1 by p38 MAPK/Nrf2 pathway in adipocytes attenuates high-fat diet induced adipose hypertrophy in mice. Eur. J. Pharmacol. 2017;799:201–210. doi: 10.1016/j.ejphar.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 119.Mahli A., Saugspier M., Koch A., Sommer J., Dietrich P., Lee S., Thasler R., Schulze-Luehrmann J., Luehrmann A., Thasler W., et al. ERK activation and autophagy impairment are central mediators of irinotecan-induced steatohepatitis. Gut. 2018;67:746–756. doi: 10.1136/gutjnl-2016-312485. [DOI] [PubMed] [Google Scholar]
- 120.Sun G., Chen Z., Jasmer K., Chuang D., Gu Z., Hannink M., Simonyi A. Quercetin Attenuates Inflammatory Responses in BV-2 Microglial Cells: Role of MAPKs on the Nrf2 Pathway and Induction of Heme Oxygenase-1. PLoS ONE. 2015;10:e0141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar A., Singh U., Kini S., Garg V., Agrawal S., Tomar P., Pathak P., Chaudhary A., Gupta P., Malik A. JNK pathway signaling: A novel and smarter therapeutic targets for various biological diseases. Future Med. Chem. 2015;7:2065–2086. doi: 10.4155/fmc.15.132. [DOI] [PubMed] [Google Scholar]
- 122.Han M., Barrett T., Brehm M., Davis R. Inflammation Mediated by JNK in Myeloid Cells Promotes the Development of Hepatitis and Hepatocellular Carcinoma. Cell Rep. 2016;15:19–26. doi: 10.1016/j.celrep.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao M., Zhu P., Fujino M., Nishio Y., Chen J., Ito H., Takahashi K., Nakajima M., Tanaka T., Zhao L. 5-Aminolevulinic acid with sodium ferrous citrate induces autophagy and protects cardiomyocytes from hypoxia-induced cellular injury through MAPK-Nrf-2-HO-1 signaling cascade. Biochem. Biophys. Res. Commun. 2016;28:663–669. doi: 10.1016/j.bbrc.2016.09.156. [DOI] [PubMed] [Google Scholar]
- 124.Chi P., Lin C., Chen Y., Hsiao L., Yang C. CO Induces Nrf2-Dependent Heme Oxygenase-1 Transcription by Cooperating with Sp1 and c-Jun in Rat Brain Astrocytes. Mol. Neurobiol. 2015;52:277–292. doi: 10.1007/s12035-014-8869-4. [DOI] [PubMed] [Google Scholar]
- 125.Serizawa F., Patterson E., Potter R., Fraser D., Cepinskas G. Pretreatment of human cerebrovascular endothelial cells with CO-releasing molecule-3 interferes with JNK/AP-1 signaling and suppresses LPS-induced proadhesive phenotype. Microcirculation. 2015;22:28–36. doi: 10.1111/micc.12161. [DOI] [PubMed] [Google Scholar]
- 126.Chen L., Guo Y., Qu S., Li K., Yang T., Yang Y., Zheng Z., Liu H., Wang X., Deng S., et al. The Protective Effects of Shengmai Formula Against Myocardial Injury Induced by Ultrafine Particulate Matter Exposure and Myocardial Ischemia are Mediated by the PI3K/AKT/p38 MAPK/Nrf2 Pathway. Front. Pharmacol. 2021;12:619311. doi: 10.3389/fphar.2021.619311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jie P., Hong Z., Tian Y., Li Y., Lin L., Zhou L., Du Y., Chen L., Chen L. Activation of transient receptor potential vanilloid 4 induces apoptosis in hippocampus through downregulating PI3K/Akt and upregulating p38 MAPK signaling pathways. Cell Death Dis. 2015;6:e1775. doi: 10.1038/cddis.2015.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lu H., Wang B., Cui N., Zhang Y. Artesunate suppresses oxidative and inflammatory processes by activating Nrf2 and ROS dependent p38 MAPK and protects against cerebral ischemiareperfusion injury. Mol. Med. Rep. 2018;17:6639–6646. doi: 10.3892/mmr.2018.8666. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Z., Xu C., Hao J., Zhang M., Wang Z., Yin T., Lin K., Liu W., Jiang Q., Li Z., et al. Beneficial consequences of Lupeol on middle cerebral artery-induced cerebral ischemia in the rat involves Nrf2 and P38 MAPK modulation. Metab. Brain Dis. 2020;35:841–848. doi: 10.1007/s11011-020-00565-8. [DOI] [PubMed] [Google Scholar]
- 130.Ma H., Wang L., Lv W., Lv Z. Effects of miR-7 on Hcy-induced rat cerebral arterial vascular smooth muscle cell proliferation, migration and inflammatory factor expression by targeting MMP-14 to regulate TLR4/NF-κB signaling pathway. Cell Mol. Biol. 2020;66:12–17. doi: 10.14715/cmb/2020.66.7.3. [DOI] [PubMed] [Google Scholar]
- 131.Capece D., Veraella D., Flati I., Arboretto P., Cornice J., Franzoso G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022;43:757–775. doi: 10.1016/j.it.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Liu D., Zhong Z., Karin M. NF-κB: A Double-Edged Sword Controlling Inflammation. Biomedicines. 2022;10:1250. doi: 10.3390/biomedicines10061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roberti A., Chaffey L., Greaves D. NF-κB Signaling and Inflammation-Drug Repurposing to Treat Inflammatory Disorders? Biology. 2022;11:372. doi: 10.3390/biology11030372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Medunjanin S., Schleithoff L., Fiegehenn C., Weinert S., Zuschratter W., Braun-Dullaeus R. GSK-3β controls NF-kappaB activity via IKKγ/NEMO. Sci. Rep. 2016;6:38553. doi: 10.1038/srep38553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Howell J., Bidwell G. Targeting the NF-κB pathway for therapy of ischemic stroke. Ther. Deliv. 2020;11:113–123. doi: 10.4155/tde-2019-0075. [DOI] [PubMed] [Google Scholar]
- 136.Wu X., Wang B., Li J., Yang Z., Zhou Y., Ma X., Kou Z., Jiang L., Song J. Inhibition of PRMT5 attenuates cerebral ischemia/reperfusion-Induced inflammation and pyroptosis through suppression of NF-kappaB/NLRP3 axis. Neurosci. Lett. 2022;776:136576. doi: 10.1016/j.neulet.2022.136576. [DOI] [PubMed] [Google Scholar]
- 137.Zhang L., Sui R., Zhang L. Fingolimod protects against cerebral ischemia reperfusion injury in rats by reducing inflammatory cytokines and inhibiting the activation of p38 MAPK and NF-kappaB signaling pathways. Neurosci. Lett. 2022;771:136413. doi: 10.1016/j.neulet.2021.136413. [DOI] [PubMed] [Google Scholar]
- 138.Li Q., Tian Z., Wang M., Kou J., Wang C., Rong X., Li J., Xie X., Pang X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARgamma/Nrf2/NF-kappaB signaling pathway. Int. Immunopharmacol. 2019;66:309–316. doi: 10.1016/j.intimp.2018.11.044. [DOI] [PubMed] [Google Scholar]
- 139.He J., Zhou D., Yan B. Eriocitrin alleviates oxidative stress and inflammatory response in cerebral ischemia reperfusion rats by regulating phosphorylation levels of Nrf2/NQO-1/HO-1/NF-kappaB p65 proteins. Ann. Transl. Med. 2020;8:757. doi: 10.21037/atm-20-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao Y., Hu M., Niu X., Li M., Xu L., Xiao Y., Zhang J., Wang H., Li L., Chu B., et al. Dl-3-n-Butylphthalide Improves Neuroinflammation in Mice with Repeated Cerebral Ischemia-Reperfusion Injury through the Nrf2-Mediated Antioxidant Response and TLR4/MyD88/NF-kappaB Signaling Pathway. Oxid. Med. Cell. Longev. 2022;2022:8652741. doi: 10.1155/2022/8652741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li L., Zhang X., Cui L., Wang L., Liu H., Ji H., Du Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013;1497:32–39. doi: 10.1016/j.brainres.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 142.Vijayan V., Wagener F., Immenschuh S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018;153:159–167. doi: 10.1016/j.bcp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 143.Loboda A., Damulewicz M., Pyza E., Jozkowicz A., Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016;73:3221–3247. doi: 10.1007/s00018-016-2223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paladino S., Conte A., Caggiano R., Pierantoni G., Faraonio R. Nrf2 Pathway in Age-Related Neurological Disorders: Insights into MicroRNAs. Cell. Physiol. Biochem. 2018;47:1951–1976. doi: 10.1159/000491465. [DOI] [PubMed] [Google Scholar]
- 145.Yang Z., Weian C., Susu H., Hanmin W. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur. J. Pharmacol. 2016;771:145–151. doi: 10.1016/j.ejphar.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 146.Lou J., Cao G., Li R., Liu J., Dong Z., Xu L. beta-Caryophyllene Attenuates Focal Cerebral Ischemia-Reperfusion Injury by Nrf2/HO-1 Pathway in Rats. Neurochem. Res. 2016;41:1291–1304. doi: 10.1007/s11064-016-1826-z. [DOI] [PubMed] [Google Scholar]
- 147.Fu K., Chen M., Zheng H., Li C., Yang F., Niu Q. Pelargonidin ameliorates MCAO-induced cerebral ischemia/reperfusion injury in rats by the action on the Nrf2/HO-1 pathway. Transl. Neurosci. 2021;12:20–31. doi: 10.1515/tnsci-2021-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lv C., Maharjan S., Wang Q., Sun Y., Han X., Wang S., Mao Z., Xin Y., Zhang B. alpha-Lipoic Acid Promotes Neurological Recovery After Ischemic Stroke by Activating the Nrf2/HO-1 Pathway to Attenuate Oxidative Damage. Cell. Physiol. Biochem. 2017;43:1273–1287. doi: 10.1159/000481840. [DOI] [PubMed] [Google Scholar]
- 149.Lu J., Huang Q., Zhang D., Lan T., Zhang Y., Tang X., Xu P., Zhao D., Cong D., Zhao D., et al. The Protective Effect of DiDang Tang Against AlCl3-Induced Oxidative Stress and Apoptosis in PC12 Cells Through the Activation of SIRT1-Mediated Akt/Nrf2/HO-1 Pathway. Front. Pharmacol. 2020;11:466. doi: 10.3389/fphar.2020.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang B., Cao W., Biswal S., Dore S. Carbon monoxide-activated Nrf2 pathway leads to protection against permanent focal cerebral ischemia. Stroke. 2011;42:2605–2610. doi: 10.1161/STROKEAHA.110.607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu Q., Zuo T., Deng L., Chen S., Yu W., Liu S., Liu J., Wang X., Fan X., Dong Z. beta-Caryophyllene suppresses ferroptosis induced by cerebral ischemia reperfusion via activation of the Nrf2/HO-1 signaling pathway in MCAO/R rats. Phytomedicine. 2022;102:154112. doi: 10.1016/j.phymed.2022.154112. [DOI] [PubMed] [Google Scholar]
- 152.Srivastava S., Alfieri A., Siow R., Mann G., Fraser P. Temporal and spatial distribution of Nrf2 in rat brain following stroke: Quantification of nuclear to cytoplasmic Nrf2 content using a novel immunohistochemical technique. J. Physiol. 2013;591:3525–3538. doi: 10.1113/jphysiol.2013.257964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shih A., Li P., Murphy T. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao H., Han Z., Ji X., Luo Y. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis. 2016;7:295–306. doi: 10.14336/AD.2015.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dinkova-Kostova A., Kostov R., Canning P. Keap1, the cysteine-based mammalian intracellular sensor for electrophiles and oxidants. Arch. Biochem. Biophys. 2017;617:84–93. doi: 10.1016/j.abb.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang R., Xu M., Wang Y., Xie F., Zhang G., Qin X. Nrf2-a Promising Therapeutic Target for Defensing Against Oxidative Stress in Stroke. Mol. Neurobiol. 2017;54:6006–6017. doi: 10.1007/s12035-016-0111-0. [DOI] [PubMed] [Google Scholar]
- 157.Liu H., Wu X., Luo J., Zhao L., Li X., Guo H., Bai H., Cui W., Guo W., Feng D., et al. Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3beta. Exp. Neurol. 2020;329:113302. doi: 10.1016/j.expneurol.2020.113302. [DOI] [PubMed] [Google Scholar]
- 158.Liu Y., Zhang L., Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015;351:88–92. doi: 10.1016/j.jns.2015.02.045. [DOI] [PubMed] [Google Scholar]
- 159.Mei Z., Du L., Liu X., Chen X., Tian H., Deng Y., Zhang W. Diosmetin alleviated cerebral ischemia/reperfusion injury in vivo and in vitro by inhibiting oxidative stress via the SIRT1/Nrf2 signaling pathway. Food Funct. 2022;13:198–212. doi: 10.1039/D1FO02579A. [DOI] [PubMed] [Google Scholar]
- 160.Tobin K., Bonds J., Minshall R., Pelligrino D., Testai F., Lazarov O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J. Cereb. Blood Flow Metab. 2014;34:1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koyama R., Shichita T. Glial roles in sterile inflammation after ischemic stroke. Neurosci. Res. 2022 doi: 10.1016/j.neures.2022.10.002. [DOI] [PubMed] [Google Scholar]
- 162.Przykaza Ł. Understanding the Connection Between Common Stroke Comorbidities, Their Associated Inflammation, and the Course of the Cerebral Ischemia/Reperfusion Cascade. Front. Immunol. 2021;12:782569. doi: 10.3389/fimmu.2021.782569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Maestrini I., Tagzirt M., Gautier S., Dupont A., Mendyk A., Susen S., Tailleux A., Vallez E., Staels B., Cordonnier C., et al. Analysis of the association of MPO and MMP-9 with stroke severity and outcome: Cohort study. Neurology. 2020;95:e97–e108. doi: 10.1212/WNL.0000000000009179. [DOI] [PubMed] [Google Scholar]
- 164.Kang L., Yu H., Yang X., Zhu Y., Bai X., Wang R., Cao Y., Xu H., Luo H., Lu L., et al. Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke. Nat. Commun. 2020;11:2488. doi: 10.1038/s41467-020-16191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sakai S., Shichita T. Inflammation and neural repair after ischemic brain injury. Neurochem. Int. 2019;130:104316. doi: 10.1016/j.neuint.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 166.Geetha T., Zheng C., McGregor W., Douglas White B., Diaz-Meco M.T., Moscat J., Babu J. TRAF6 and p62 inhibit amyloid β-induced neuronal death through p75 neurotrophin receptor. Neurochem. Int. 2012;61:1289–1293. doi: 10.1016/j.neuint.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ghafouri-Fard S., Shoorei H., Poornajaf Y., Hussen B., Hajiesmaeili Y., Abak A., Taheri M., Eghbali A. NLRP3: Role in ischemia/reperfusion injuries. Front. Immunol. 2022;13:926895. doi: 10.3389/fimmu.2022.926895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bai R., Lang Y., Shao J., Deng Y., Refuhati R., Cui L. The Role of NLRP3 Inflammasome in Cerebrovascular Diseases Pathology and Possible Therapeutic Targets. ASN Neuro. 2021;13:17590914211018100. doi: 10.1177/17590914211018100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang K., Yao Y., Zhu X., Zhang K., Zhou F., Zhu L. Amyloid beta induces NLRP3 inflammasome activation in retinal pigment epithelial cells via NADPH oxidase- and mitochondria-dependent ROS production. J. Biochem. Mol. Toxicol. 2017;31:e21887. doi: 10.1002/jbt.21887. [DOI] [PubMed] [Google Scholar]
- 170.Ito M., Shichita T., Okada M., Komine R., Noguchi Y., Yoshimura A., Morita R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015;6:7360. doi: 10.1038/ncomms8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao H., Liu Y., Chen N., Yu H., Liu S., Qian M., Zhang Z. PHLDA1 Blockade Alleviates Cerebral Ischemia/Reperfusion Injury by Affecting Microglial M1/M2 Polarization and NLRP3 Inflammasome Activation. Neuroscience. 2022;487:66–77. doi: 10.1016/j.neuroscience.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 172.Hou Y., Wang Y., He Q., Li L., Xie H., Zhao Y., Zhao J. Nrf2 inhibits NLRP3 inflammasome activation through regulating Trx1/TXNIP complex in cerebral ischemia reperfusion injury. Behav. Brain Res. 2018;336:32–39. doi: 10.1016/j.bbr.2017.06.027. [DOI] [PubMed] [Google Scholar]
- 173.Jiang L., Zhang S., Li C., Tang J., Che F., Lu Y. Roles of the Nrf2/HO-1 pathway in the anti-oxidative stress response to ischemia-reperfusion brain injury in rats. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1532–1540. [PubMed] [Google Scholar]
- 174.Dotson A., Chen Y., Zhu W., Libal N., Alkayed N., Offner H. Partial MHC Constructs Treat Thromboembolic Ischemic Stroke Characterized by Early Immune Expansion. Transl. Stroke Res. 2016;7:70–78. doi: 10.1007/s12975-015-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Miller C., Behrouz R. Impact of Infection on Stroke Morbidity and Outcomes. Curr. Neurol. Neurosci. Rep. 2016;16:83. doi: 10.1007/s11910-016-0679-9. [DOI] [PubMed] [Google Scholar]
- 176.Narne P., Pandey V., Phanithi P. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: An epigenetic connection. Mol. Cell. Neurosci. 2017;82:176–194. doi: 10.1016/j.mcn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 177.Anne S.R., Leak R., Gao Y., Chen J. The dynamics of the mitochondrial organelle as a potential therapeutic target. J. Cereb. Blood Flow Metab. 2013;33:22–32. doi: 10.1038/jcbfm.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xavier J., Rodrigues C., Sola S. Mitochondria: Major Regulators of Neural Development. Neuroscientist. 2016;22:346–358. doi: 10.1177/1073858415585472. [DOI] [PubMed] [Google Scholar]
- 179.Chen Y., Guo S., Tang Y., Mou C., Hu X., Shao F., Yan W., Wu Q. Mitochondrial Fusion and Fission in Neuronal Death Induced by Cerebral Ischemia-Reperfusion and Its Clinical Application: A Mini-Review. Med. Sci. Monit. 2020;26:e928651. doi: 10.12659/MSM.928651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sekerdag E., Solaroglu I., Gursoy-Ozdemir Y. Cell Death Mechanisms in Stroke and Novel Molecular and Cellular Treatment Options. Curr. Neuropharmacol. 2018;16:1396–1415. doi: 10.2174/1570159X16666180302115544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Anrather J., Iadecola C. Inflammation and Stroke: An Overview. Neurotherapeutics. 2016;13:661–670. doi: 10.1007/s13311-016-0483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barcena C., Mayoral P., Quiros P. Mitohormesis, an Antiaging Paradigm. Int. Rev. Cell Mol. Biol. 2018;340:35–77. doi: 10.1016/bs.ircmb.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 183.Tsushima M., Liu J., Hirao W., Yamazaki H., Tomita H., Itoh K. Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease. Arch. Pharm. Res. 2020;43:286–296. doi: 10.1007/s12272-019-01188-z. [DOI] [PubMed] [Google Scholar]
- 184.Kasai S., Yamazaki H., Tanji K., Engler M., Matsumiya T., Itoh K. Role of the ISR-ATF4 pathway and its cross talk with Nrf2 in mitochondrial quality control. J. Clin. Biochem. Nutr. 2019;64:1–12. doi: 10.3164/jcbn.18-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Murata H., Takamatsu H., Liu S., Kataoka K., Huh N., Sakaguchi M. Nrf2 Regulates PINK1 Expression under Oxidative Stress Conditions. PLoS ONE. 2015;10:e0142438. doi: 10.1371/journal.pone.0142438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Imhoff B., Hansen J. Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem. J. 2009;424:491–500. doi: 10.1042/BJ20091286. [DOI] [PubMed] [Google Scholar]
- 187.Wang J., Zhang W., Lv C., Wang Y., Ma B., Zhang H., Fan Z., Li M., Li X. A novel biscoumarin compound ameliorates cerebral ischemia reperfusion-induced mitochondrial oxidative injury via Nrf2/Keap1/ARE signaling. Neuropharmacology. 2020;167:107918. doi: 10.1016/j.neuropharm.2019.107918. [DOI] [PubMed] [Google Scholar]
- 188.Bahar E., Kim J., Yoon H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-kappaB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017;18:1989. doi: 10.3390/ijms18091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Armulik A., Genove G., Mae M., Nisancioglu M., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 190.Shi Y., Zhang L., Pu H., Mao L., Hu X., Jiang X., Xu N., Stetler R., Zhang F., Liu X., et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chen H., He Y., Chen S., Qi S., Shen J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020;158:104877. doi: 10.1016/j.phrs.2020.104877. [DOI] [PubMed] [Google Scholar]
- 192.Moskowitz M., Lo E., Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shin J., Kim Y., Jeong S., Lee K., Kim H., Park E. Extracellular signal-regulated kinase1/2-dependent changes in tight junctions after ischemic preconditioning contributes to tolerance induction after ischemic stroke. Brain Struct. Funct. 2015;220:13–26. doi: 10.1007/s00429-013-0632-5. [DOI] [PubMed] [Google Scholar]
- 194.Yang T., Sun Y., Mao L., Zhang M., Li Q., Zhang L., Shi Y., Leak R., Chen J., Zhang F. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018;17:323–337. doi: 10.1016/j.redox.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Haorah J., Ramirez S., Schall K., Smith D., Pandya R., Persidsky Y. Oxidative stress activates protein tyrosine kinase and matrix metalloproteinases leading to blood-brain barrier dysfunction. J. Neurochem. 2007;101:566–576. doi: 10.1111/j.1471-4159.2006.04393.x. [DOI] [PubMed] [Google Scholar]
- 196.Gu Y., Dee C., Shen J. Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Front. Biosci. 2011;3:1216–1231. doi: 10.2741/222. [DOI] [PubMed] [Google Scholar]
- 197.Kunze R., Urrutia A., Hoffmann A., Liu H., Helluy X., Pham M., Reischl S., Korff T., Marti H. Dimethyl fumarate attenuates cerebral edema formation by protecting the blood-brain barrier integrity. Exp. Neurol. 2015;266:99–111. doi: 10.1016/j.expneurol.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 198.Ibbotson K., Yell J., Ronaldson P. Nrf2 signaling increases expression of ATP-binding cassette subfamily C mRNA transcripts at the blood-brain barrier following hypoxia-reoxygenation stress. Fluids Barriers CNS. 2017;14:6. doi: 10.1186/s12987-017-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Alfieri A., Srivastava S., Siow R., Cash D., Modo M., Duchen M., Fraser P., Williams S., Mann G. Sulforaphane preconditioning of the Nrf2/HO-1 defense pathway protects the cerebral vasculature against blood-brain barrier disruption and neurological deficits in stroke. Free Radic. Biol. Med. 2013;65:1012–1022. doi: 10.1016/j.freeradbiomed.2013.08.190. [DOI] [PubMed] [Google Scholar]
- 200.Mao L., Yang T., Li X., Lei X., Sun Y., Zhao Y., Zhang W., Gao Y., Sun B., Zhang F. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J. Cereb. Blood Flow Metab. 2019;39:352–366. doi: 10.1177/0271678X18764083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shi Y., Zhang Y., Liu B., Li C., Wu J., Li Y. Nomilin protects against cerebral ischemia-reperfusion induced neurological deficits and blood-brain barrier disruption via the Nrf2 pathway. Food Funct. 2019;10:5323–5332. doi: 10.1039/C9FO01481K. [DOI] [PubMed] [Google Scholar]
- 202.Li H., Wang P., Huang F., Jin J., Wu H., Zhang B., Wang Z., Shi H., Wu X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018;340:58–66. doi: 10.1016/j.taap.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 203.Dixon S., Lemberg K., Lamprecht M., Skouta R., Zaitsev E., Gleason C., Patel D., Bauer A., Cantley A., Yang W., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xu T., Ding W., Ji X., Ao X., Liu Y., Yu W., Wang J. Molecular mechanisms of ferroptosis and its role in cancer therapy. J. Cell. Mol. Med. 2019;23:4900–4912. doi: 10.1111/jcmm.14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu Y., Yan Y., Niu F., Wang Y., Chen X., Su G., Liu Y., Zhao X., Qian L., Liu P., et al. Ferroptosis: A cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 2021;7:193. doi: 10.1038/s41420-021-00579-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li J., Cao F., Yin H., Huang Z., Lin Z., Mao N., Sun B., Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.DeGregorio-Rocasolano N., Marti-Sistac O., Gasull T. Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front. Neurosci. 2019;13:85. doi: 10.3389/fnins.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tuo Q., Lei P., Jackman K., Li X., Xiong H., Li X., Liuyang Z., Roisman L., Zhang S., Ayton S., et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol. Psychiatry. 2017;22:1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- 209.Chen G., Li L., Tao H. Bioinformatics Identification of Ferroptosis-Related Biomarkers and Therapeutic Compounds in Ischemic Stroke. Front. Neurol. 2021;12:745240. doi: 10.3389/fneur.2021.745240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lan B., Ge J., Cheng S., Zheng X., Liao J., He C., Rao Z., Wang G. Extract of Naotaifang, a compound Chinese herbal medicine, protects neuron ferroptosis induced by acute cerebral ischemia in rats. J. Integr. Med. 2020;18:344–350. doi: 10.1016/j.joim.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 211.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kerins M., Ooi A. The Roles of Nrf2 in Modulating Cellular Iron Homeostasis. Antioxid. Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sarutipaiboon I., Settasatian N., Komanasin N., Kukongwiriyapan U., Sawanyawisuth K., Intharaphet P., Senthong V., Settasatian C. Association of Genetic Variations in Nrf2, NQO1, HMOX1, and MT with Severity of Coronary Artery Disease and Related Risk Factors. Cardiovasc. Toxicol. 2020;20:176–189. doi: 10.1007/s12012-019-09544-7. [DOI] [PubMed] [Google Scholar]
- 214.Mohsenpour H., Pesce M., Patruno A., Bahrami A., Pour P., Farzaei M. A Review of Plant Extracts and Plant-Derived Natural Compounds in the Prevention/Treatment of Neonatal Hypoxic-Ischemic Brain Injury. Int. J. Mol. Sci. 2021;22:833. doi: 10.3390/ijms22020833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shi M., Wang J., Bi F., Bai Z. Diosmetin alleviates cerebral ischemia-reperfusion injury through Keap1-mediated Nrf2/ARE signaling pathway activation and NLRP3 inflammasome inhibition. Environ. Toxicol. 2022;37:1529–1542. doi: 10.1002/tox.23504. [DOI] [PubMed] [Google Scholar]
- 216.Xu L., Gao Y., Hu M., Dong Y., Xu J., Zhang J., Lv P. Edaravone dexborneol protects cerebral ischemia reperfusion injury through activating Nrf2/HO-1 signaling pathway in mice. Fundam. Clin. Pharmacol. 2022;36:790–800. doi: 10.1111/fcp.12782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li K., Jiang J., Shi Z., Zhan L., Peng L., Sun W., Tang Y., Zuo X., Xu E. Neuroprotective Effects of Rhodiola Sacra on Transient Global Cerebral Ischemia Through Activating AMPK/Nrf2 Pathway in Rats. Antioxid. Redox Signal. 2022;36:567–591. doi: 10.1089/ars.2020.8224. [DOI] [PubMed] [Google Scholar]
- 218.Xu H., Shen J., Xiao J., Chen F., Wang M. Neuroprotective effect of cajaninstilbene acid against cerebral ischemia and reperfusion damages by activating AMPK/Nrf2 pathway. J. Adv. Res. 2021;34:199–210. doi: 10.1016/j.jare.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xue Z., Zhao K., Sun Z., Wu C., Yu B., Kong D., Xu B. Isorhapontigenin ameliorates cerebral ischemia/reperfusion injury via modulating Kinase Cepsilon/Nrf2/HO-1 signaling pathway. Brain Behav. 2021;11:e02143. doi: 10.1002/brb3.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Tang C., Hong J., Hu C., Huang C., Gao J., Huang J., Wang D., Geng Q., Dong Y. Palmatine Protects against Cerebral Ischemia/Reperfusion Injury by Activation of the AMPK/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2021;2021:6660193. doi: 10.1155/2021/6660193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu D., Wang H., Zhang Y., Zhang Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020;14:51–60. doi: 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 222.Gao K., Liu M., Ding Y., Yao M., Zhu Y., Zhao J., Cheng L., Bai J., Wang F., Cao J., et al. A phenolic amide (LyA) isolated from the fruits of Lycium barbarum protects against cerebral ischemia-reperfusion injury via PKCepsilon/Nrf2/HO-1 pathway. Aging. 2019;11:12361–12374. doi: 10.18632/aging.102578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang H., Wei W., Lan X., Liu N., Li Y., Ma H., Sun T., Peng X., Zhuang C., Yu J. Neuroprotective Effect of Swertiamain on Cerebral Ischemia/Reperfusion Injury by Inducing the Nrf2 Protective Pathway. ACS Chem. Neurosci. 2019;10:2276–2286. doi: 10.1021/acschemneuro.8b00605. [DOI] [PubMed] [Google Scholar]
- 224.Luo Y., Cui H., Jia A., Jia S., Yuan K. The Protective Effect of the Total Flavonoids of Abelmoschus esculentus L. Flowers on Transient Cerebral Ischemia-Reperfusion Injury Is due to Activation of the Nrf2-ARE Pathway. Oxid. Med. Cell. Longev. 2018;2018:8987173. doi: 10.1155/2018/8987173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Deng C., Cao J., Han J., Li J., Li Z., Shi N., He J. Liraglutide Activates the Nrf2/HO-1 Antioxidant Pathway and Protects Brain Nerve Cells against Cerebral Ischemia in Diabetic Rats. Comput. Intell. Neurosci. 2018;2018:3094504. doi: 10.1155/2018/3094504. [DOI] [PMC free article] [PubMed] [Google Scholar]