Abstract
Repeated excessive alcohol consumption increases the risk of developing cognitive decline and dementia. Hazardous drinking among older adults further increases such vulnerabilities. To investigate whether alcohol induces cognitive deficits in older adults, we performed a chronic intermittent ethanol exposure paradigm (ethanol or water gavage every other day 10 times) in 8-week-old young adult and 70-week-old aged rats. While spatial memory retrieval ascertained by probe trials in the Morris water maze was not significantly different between ethanol-treated and water-treated rats in both age groups after the fifth and tenth gavages, behavioral flexibility was impaired in ethanol-treated rats compared to water-treated rats in the aged group but not in the young adult group. We then examined ethanol-treatment-associated hippocampal proteomic and phosphoproteomic differences distinct in the aged rats. We identified several ethanol-treatment-related proteins, including the upregulations of the Prkcd protein level, several of its phosphosites, and its kinase activity and downregulation in the Camk2a protein level. Our bioinformatic analysis revealed notable changes in pathways involved in neurotransmission regulation, synaptic plasticity, neuronal apoptosis, and insulin receptor signaling. In conclusion, our behavioral and proteomic results identified several candidate proteins and pathways potentially associated with alcohol-induced cognitive decline in aged adults.
1. Introduction
Worldwide, alcohol is the most commonly misused drug with recent estimates of over 2.3 billion people being classified as current alcohol drinkers.1,2 Alcohol use has risen significantly during the worldwide COVID pandemic across demographics,3 resulting in more alcohol-related deaths.4 These surges in alcohol consumption and corresponding deaths highlight the need to better understand the negative impacts and potential health risks of excessive alcohol consumption.
With the average population ages of most countries on the rise, an estimated 2.1 billion people over 60 years old by 2050,5 health care systems are threatened to be overwhelmed by a “silver tsunami” of older adults needing geriatric care,6 thus studying the health impacts and risks specific to older adults is an imperative public health issue. Alcohol use has been high or rising in most demographics including adolescents,7 young adults, and older females without children.8 However, compared to younger drinkers, the percentage of older adult drinkers has had a greater escalation in recent years,9 and older adults often consume alcohol in a hazardous binge drinking pattern.10−14 Recent preclinical research has demonstrated that aged rodents are more sensitive to the effects of acute ethanol exposure compared to younger adult rodents.15−20 Furthermore, chronic intermittent ethanol exposure (CIEE), a model of binge-like ethanol consumption, may produce tolerance to a high-dose ethanol challenge in aged animals faster than in younger adult animals21 and impair cognition late in life.15,22 Given the increases in the percentages of drinkers and the engagement of more hazardous consumption patterns in the aged population, understanding the potential negative impacts of alcohol on the aged is critical.
Ethanol impairs cognition through a variety of mechanisms that alter the function of specific brain regions including the hippocampus as determined by both behavioral studies and electrophysiological recordings of hippocampal place cells.23−26 One such cognitive function impacted by CIEE is behavioral flexibility, i.e., the learning of new response strategies in the face of previously learned stimuli. Impaired behavioral flexibility has been found in both young adult rats27−31 and aged rats15,22 following chronic ethanol exposure in adolescence. Due to well-known cognitive deficits in aged animals,32 it seems likely that CIEE in aged animals will also produce deficits in behavioral flexibility in aged animals. Behavioral flexibility has been argued to be a form of episodic memory in rats32 and provides a behavioral tool to assess the impact of chronic ethanol and aging on cognitive function. Recently, it has been shown that chronic ethanol consumption is a predictive factor in the development of dementia including Alzheimer’s disease (AD).33 Patients with AD have various cognitive deficits including impairments in declarative memory and executive function. Most, but not all, studies reported that patients with AD exhibit deficits in the Wisconsin Card Sorting Task, a procedure that tests behavioral flexibility (see review by Guarino et al.34). Given that CIEE and AD impair behavioral flexibility, it is possible to use rodent models of CIEE in aged animals to investigate molecular pathways underlying alcohol-induced cognitive deficits associated with AD.
Behavioral flexibility can be measured using several tasks in rodent models including reversal learning in the Morris water maze. During a reversal learning task, subjects are first trained to swim to a submerged platform that is in a constant location. After learning, the platform location is rotated 180°, and the animal is given a series of trails to locate the platform in the new location. This task requires the animal to suppress previous learning and develop a new learning strategy. A variety of brain regions are important for successful behavioral flexibility performance, including reversal learning tasks in the Morris water maze. One critical brain region for reversal learning is the hippocampus, as it has been demonstrated that blocking hippocampal LTD impairs reversal learning performance in the Morris water maze35 and that reversal learning increases the functional connectivity between the hippocampus and other brain regions in mice.36 Furthermore, lesions of either the perforant path or the fimbria/fornix (prominent hippocampal afferents) impair reversal learning in rodents.37,38 Given the involvement of the hippocampus in behavioral flexibility and the well-documented impairments in hippocampal function produced by ethanol23 and normal biological aging,32 it is important to understand how CIEE in aged animals alters the hippocampal proteome.
Understanding how ethanol exposure interacts with molecular factors to impact a complex trait, such as dementia, requires the triangulation of multiple research techniques and the use of established databases. Proteomics and phosphoproteomics have proven valuable in identifying potential molecular factors for several complex traits, including serial reaction times following chronic ethanol exposure38 and hippocampal protein network dysfunction in mouse models of AD.39 Consequently, it is likely that leveraging the use of proteomic and phosphoproteomic tools in the hippocampus to understand the interaction of chronic ethanol exposure with cognition and biological age will reveal important molecular targets for in-depth investigations.40
This work is focused on identifying cognitive impairments and protein pathways correlated with AD in aged rats. We have undertaken a multilevel strategy, first by investigating if CIEE impaired behavioral flexibility in young adult rats (8 weeks old) and aged rats (70 weeks old) differentially, followed by conducting extensive proteomic and phosphoproteomic analyses of the hippocampus from the same animals to identify proteins, phosphosites, and pathways that might impact the observed differential cognitive deficits as a result of CIEE. We report that CIEE selectively impairs behavioral flexibility in aged but not young adult rats. Furthermore, we identified several candidate proteins and molecular pathways affected by CIEE in the hippocampus of aged rats that have been previously implicated in cognitive deficits and AD. These results suggest that chronic binge-like alcohol exposure in the older population is a risk factor for the development of cognitive deficits associated with protein pathways that may underly the development of dementia.
2. Experimental Section
2.1. Animals
Two groups of 16 male Sprague–Dawley rats were used in this study (Envigo, Indianapolis, IN): 8-week-old young adults and 70-week-old retired breeders. Rats were housed under standard housing conditions in pairs on a 12:12 light–dark cycle (lights on at 6 AM). Wood chips were used as bedding with chow (Teklad food pellets, Envigo), and tap water was provided ad libitum throughout the experiment. Animals were allowed to acclimate for a month during which they were handled 5 days/week for approximately 5 min to minimize handling stress in subsequent experiments. Animal care and handling procedures were approved by the University of Wisconsin—Eau Claire IACUC.
2.2. Spatial Learning Training
A month after arrival, all rats were trained in the standard submerged platform task in a Morris water maze.41 The water maze was 6 feet in diameter and was placed in a room dedicated to behavioral experiments. The tank was painted white to mask proximal cues, leaving several distal cues in the room (wall posters, door, computer rack, and experimenter). The water was made opaque by white water-soluble paint (Tempera) and was kept at room temperature (∼21 °C). A video camera was mounted above the center of the water tank connected with the ANYMAZE tracking system, version 5.3 (Stoelting, Wood Dale, IL), for data collection. Rats were trained four trials/day, starting from the compass points to a slightly submerged platform (∼0.75 in. below the water surface) located in a constant spatial location. The order of the start locations was counterbalanced in a Latin square design. To ensure that animals could learn the spatial memory task prior to chronic intermittent ethanol administration, rats were trained in the Morris water maze until they reached a mean escape latency of approximately 10 s for two to three consecutive days.42 In addition, aged animals were impaired in the initial spatial learning (see below), and therefore all analyses were separated by age of the animal.
2.3. Chronic Intermittent Ethanol Exposure (CIEE) Paradigm
CIEE treatment began on the day after the rats achieved the aforementioned performance. Rats were randomly assigned by cage to receive either 5 g/kg ethanol (37% v/v; nyoung adult = 6 and naged = 8) or water (nyoung adult = 7 and naged = 7) via gavage every other day for 20 days, thereby resulting in 10 intoxication and 10 withdrawal episodes. Rats were weighed, and their general health was assessed before every gavage. In a subset of the ethanol-treated rats, tail vein blood was sampled an hour after the first gavage to ascertain blood ethanol levels on an Analox AM-1 Analyzer (Analox Instruments, Stourbridge, UK). The median blood ethanol levels after the first ethanol gavage were 156 mg/dL (range = 114.6–169.8 mg/dL) for the young adults (n = 4) and 186 mg/dL (range = 97.4–237.5 mg/dL) for the aged rats (n = 5).
2.4. Spatial Memory Test
To investigate the impact of CIEE on hippocampal-dependent spatial memory, rats underwent a 60 s probe trial in the Morris water maze on the fifth and tenth withdrawal days (i.e., the days after the fifth and tenth ethanol gavages). The platform was removed from the water tank in the probe trials. The rats started at the point farthest away from the original goal location and were allowed to swim for 60 s. Afterward, rats were dried and returned to their home cages. The total time spent in the initial escape quadrant of the maze was monitored via the ANYMAZE system and used as a measure of spatial memory.
2.5. Behavioral Flexibility
To investigate the impact of CIEE on behavioral flexibility, the rats underwent reversal training (i.e., relearning to a new spatial location in the same environmental context) 2 days after the last gavage. The escape platform was relocated 180° to the initial training location in the water maze. The rats were trained for 2 days, four trials per day, using the initial starting locations. As during initial spatial learning, latency to the platform and swim path length were measured by the ANYMAZE system. For rats that could not find the platform within 45 s, they were gently guided to the platform and allowed to rest on it for ∼5 s. The first two trials were deemed “learning trials” for the new platform location, while the subsequent six trials were considered behavioral flexibility trials.
2.6. Tissue Preparation, Protein Extraction, and Trypsin Digestion
The day after the last behavioral flexibility test (i.e., 4 days after the last gavage), rats were sacrificed by rapid decapitation, and brains were extracted. Bilateral ventral and dorsal hippocampuses were microdissected on wet ice, frozen on dry ice, and then stored at −80 °C until use. All rats’ hippocampal tissues were planned to be used for both proteomics and phosphoproteomics analyses. However, a few samples were excluded from the proteomics analysis due to technical issues. Therefore, proteomics data of 13 young adult rats (7 controls vs 6 ethanol-treated) and 12 aged rats (6 controls vs 6 ethanol-treated) were analyzed, while phosphoproteomics data of 13 young adult rats (7 controls vs 6 ethanol-treated) and 15 aged rats (7 controls vs 8 ethanol-treated) were evaluated.
Hippocampal tissue was homogenized with homogenizing buffer (20 mM HEPES/8 M urea, pH 8.2) and 200 mg of disruptor beads in Bioruptor Plus TPX tubes (Diagenode, Denville, NJ, USA) using a Bioruptor Plus sonication device (Diagenode; 30 cycles of 30 s on and 30 s off). The homogenates were then transferred to LoBind tubes (Eppendorf, Hamburg, Germany) and centrifuged at 14 000 rpm for 15 min at 4 °C. Protein levels in the supernatant were measured by a BCA protein assay, and the volume corresponding to 200 μg of protein was brought up to 100 μL by adding homogenizing buffer. The samples were then reduced and alkylated with 10 mM TCEP and 9 mM iodoacetamide before dilution with 850 μL of 25 mM Tris, pH 8.2, and digestion with 10 μg of trypsin (Promega, Madison, WI, USA) for 24 h at 32 °C.
2.7. TMT Labeling and Basic HPLC Fractionation
The trypsin-digested samples were desalted using C18 MacroSpin tips (Nest Group, Ipswich, MA, USA) and were then lyophilized. The lyophilized samples were solubilized in 100 μL of 100 mM TEAB, pH 8.5, and then labeled with 200 μg/10 μL acetonitrile of TMTpro 16plex tandem mass tag reagents (ThermoFisher Scientific, Waltham, MA, USA) for 60 min. The reaction was quenched by 5 μL of 5% hydroxylamine. Each sample was confirmed to have >98% labeling efficiency. Samples were then pooled by age groups (i.e., samples from young and old rats were pooled separately) at volumes normalized by the reporter ion intensities for each channel. The pooled samples were desalted using Sep-Pak C18 Plus Long cartridges (Waters, Milford, MA, USA), then lyophilized.
The samples were then fractionated using basic pH reverse-phase HPLC on an Ultimate 3000 Rapid Separation Dual System (ThermoFisher Scientific) with an XBridge Peptide BEH C18 column (3.5 μm, 4.6 mm × 250 mm; Waters) running a 60 min gradient of 5% to 60% of 5 mM ammonium formate/90% acetonitrile solvents. There were 96 fractions collected over the 80 min method for each pooled sample, which were then concentrated into 12 fractions.
2.8. Phosphopeptide Enrichment
For phosphoproteomic experiments, phosphopeptides were enriched by the use of Fe-NTA cartridges on an AssayMAP Bravo Protein Sample Prep Platform (Agilent Technologies, Santa Clara, CA, USA).
2.9. Nano-LC-MS/MS Acquisition
An Orbitrap Exploris 480 mass spectrometer (Thermo Scientific) coupled to a Thermo Ultimate 3000 RSLC nano-HPLC system was used for data-dependent acquisition tandem mass spectrometry (MS/MS) analysis of TMT-labeled proteomic and phosphoproteomic samples, with nanoliquid chromatography separation and electrospray ionization of eluting peptides. The digested peptide mixture was loaded onto a HALO C18 2.7 μm EXP Stem Trap (Optimize Technologies, Oregon City, OR, USA). For proteomic analysis, nano-LC was performed using 0.2% formic acid in both the solvent A (98% water/2% acetonitrile) and solvent B (80% acetonitrile/10% isopropanol/10% water) with a solvent B gradient from 5% to 40% over 120 min at 300 nL/min with an EASY-Spray C18, 75 μL × 50 cm column (ThermoFisher Scientific). For phosphoproteomic analysis, nano-LC was performed using solvent A (0.1% formic acid) and solvent B (80% acetonitrile/0.1% formic acid) over 120 min at 300 nL/min with an EASY-Spray C18, 75 μL × 50 cm column (ThermoFisher Scientific).
The mass spectrometer data-dependent acquisition method used for both proteomic and phosphoproteomic analyses involved a 3 s cycle time with the MS1 survey scan from 340 to 1600 m/z at a resolution of 120 000 (at 200 m/z), followed by HCD MS/MS scans at a resolution of 45 000 with an NCE setting of 32, and the isolation width was set to 0.7 m/z. Ions with charge states of 2 to 5 for proteomics or 2 to 4 for phosphoproteomics were allowed for MS/MS and, if selected, were placed on an exclusion list for 30 s. The normalized AGC target settings were 100% for MS1 and 200% for MS2 scans with maximum ion inject times of 50 and 120 ms, respectively.
2.10. Database Search
Both proteomic and phosphoproteomic mass spectrometry raw files were analyzed by MaxQuant software (version 1.6.17). MS/MS spectra were searched against the Swiss-Port Rattus norvegicus database (8135 entries; UniPort UP000002494; downloaded February 2021; setup for MS2 reporter ion quantification with TMTpro 16plex isobaric labels) with the Andromeda peptide search engine embedded in MaxQuant.
2.11. Proteomic Parameters and Analysis
Mass tolerances were set at 4.5 ppm for MS1 and 20 ppm for MS2. Cysteine carbamidomethylation (+57.02 Da) was set as a fixed modification, and methionine oxidation (+15.99 Da) was set as a variable modification. Protein identifications were filtered at a 1% false discovery rate (FDR) with a minimum of one peptide. Reporter ion channel correction factors were applied to PSMs which were then filtered to exclude peptides exceeding the threshold maximum of 50% isolation interference. Sample groups missing 50% of values were removed from comparisons with no imputation applied. Samples were normalized by median subtraction.
Normalized protein levels were compared between water- and ethanol-treated groups by a t-test in each age group separately. Proteins were considered significant and differentially expressed if they had a p-value < 0.05 and were defined as downregulated when Log2 fold change (Log2FC) < 0 or upregulated when Log2FC > 0. Comparisons between age groups were not performed due to the lack of a reference sample across separate protein profiling runs.
2.12. Phosphoproteomic Parameters and Analysis
Static modifications set for TMTpro 16plex adducts were assigned to lysine and N-terminus acetylation (+43.01 Da) and cysteine carbamidomethyl (+57.02 Da). The variable modifications allowed were phosphorylation at serine, threonine, and tyrosine (+79.97 Da) and methionine oxidation (+15.99 Da). TMTpro 16plex labeled peptide identifications were filtered at a maximum isolation interference of 50%, a maximum FDR of 1%, and a phosphorylation site (phosphosite) localization probability of 75% or higher. Samples were normalized with median subtraction.
Normalized phosphosite levels were compared between water and ethanol treatment groups by a t-test in each age group separately. Phosphosites were considered significantly and differentially regulated between treatment groups if they had a p-value < 0.05 and were defined as downregulated when Log2FC < −0.263 (i.e., fold change ratio <0.833) or upregulated when Log2FC > 0.263 (i.e., fold change ratio >1.2).
2.13. Functional Annotation
Two lists of proteins were subjected to overrepresentation analysis: one with proteins that were differentially expressed in the proteomic analysis and the other with proteins that were mapped to the differentially regulated phosphosites. The clusterprofiler and ReactomePA packages in R v4.1.1 were used to detect pathway enrichment in Kyoto Encyclopedia of Genes and Genome (KEGG) and reactome pathways and Gene Ontology (GO) terms for molecular functions, cellular components, and biological processes. Significant overrepresented pathways and GO terms were considered at an FDR-adjusted p < 0.05.
2.14. Kinase Activity Profiling Analysis (KAPA)
To identify the upstream regulatory kinase pathways altered based on the changes in phosphorylation associated with the chronic intermittent ethanol treatment, we performed the kinase activity profiling analysis (KAPA), which was implemented in R v4.1.1 as described previously.43 The kinase-substrate annotation database was downloaded from PhosphoSitePlus44 and species filtered for Rattus norvegicus to create a rat kinase–substrate annotation profile. To assess the kinase activity, we extracted the site-specific phosphorylation Log2 ratios of identified kinase substrates based on the rat kinase–substrate annotation profile and calculated the average Log2 ratio of the kinase substrate targets. Bootstrapping-based random sampling was then performed. For k substrate targets of a specific kinase identified from the significantly differentially regulated phosphoproteomic data set, a random selection of k phosphorylation sites from the phosphorylation data set was selected, and the average Log2 ratio was calculated. The random selection process was repeated 10 000 times. The Z-score for the average Log2 ratio of the specific kinase substrate targets was then calculated and termed the kinase activity score.
S represents the average Log2 ratio of the extracted site-specific phosphorylation Log2 ratio of a specific kinase; Ave and Std represent the average and standard deviation of the normal distribution obtained from the averages of 10 000 random samplings of k phosphorylation sites; and k is the number of kinase substrate sites identified for a specific kinase from the data sets.
3. Results and Discussion
3.1. Spatial Learning Was Different in Young Adult and Aged Rats
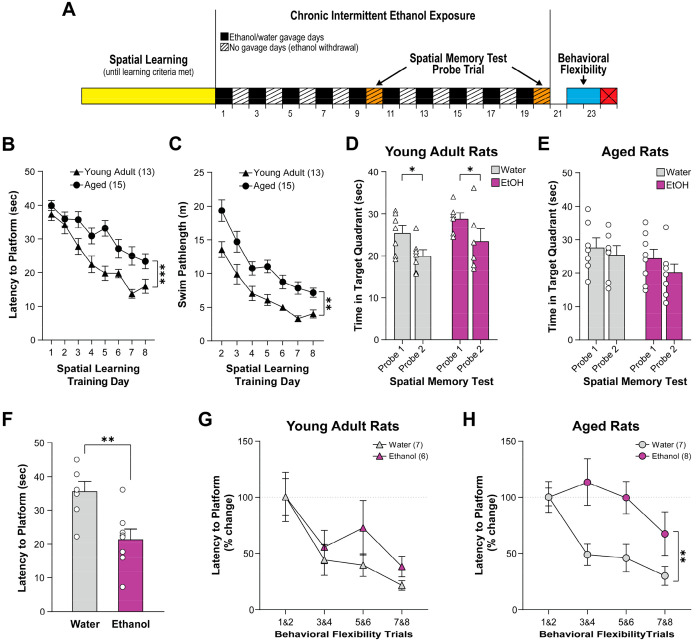
We first checked if spatial learning performance was different between young adult (8 weeks old) and aged rats (70 weeks old) by analyzing the rats’ Morris water maze performance in the first 8 days before any subjects had reached the learning criteria (see Figure 1A for an experimental timeline). Young adult rats performed better than aged rats in initial spatial learning in the water maze as evidenced by shorter latency (two-way ANOVA with repeated measures; age effect: F1,26 = 21.97, p < 0.001; training days effect: F5,132 = 23.36, p < 0.001; Figure 1B) and shorter swim path length (two-way ANOVA with repeated measures; age effect: F1,26 = 27.79, p < 0.001; training days effect: F7,182 = 34.58, p < 0.001; Figure 1C) to reach the escape platform. Given these differences in baseline spatial learning capacity, subsequent cognitive test data analysis was performed in each age group independently.
Figure 1.
(A) Timeline of the behavioral experimental procedures. Rats were sacrificed on Day 24. The spatial learning performances of young adult rats during training were better than aged rats as they demonstrated reduced latency (B) and shorter swim path length (C) to reach the platform (***page < 0.001). Spatial memory did not significantly differ between ethanol- and water-treated groups on the fifth (probe 1) and tenth (probe 2) no-gavage days in both young adult rats (D) and aged rats (E). Young adult rats improved in probe 2 as they showed significantly reduced time spent in the target quadrant in probe 2 compared to probe 1 (D; *pprobe = 0.013), but only a trended reduction was found in aged rats (E). In aged rats, the latency to the platform was lower in ethanol-treated rats than water-treated rats overall in the first two behavioral flexibility training sessions (F; **p = 0.004). Young adult rats improved dramatically (i.e., reduced latency to platform) across behavioral flexibility testing sessions without treatment group difference (G; ptrial = 0.002). Aged rats treated with water showed very similar behavioral flexibility improvement as young rats, but those treated with ethanol did not show improvement until the last two testing sessions (H; **ptreatment = 0.009, ptrial = 0.008). Graphs are plotted in means with standard error of mean as error bars.
3.2. Effects of CIEE on Spatial Memory
In young adult rats, CIEE did not alter spatial memory as measured by probe trials. Specifically, there was no significant difference between ethanol- and water-treated groups in the time spent in the initial escape quadrant in the absence of the platform as a function of probe trial, although subjects, regardless of drug condition, spent less time in the initial target quadrant in the second probe trial than the first probe trial (two-way ANOVA with repeated measures; treatment effect: F1,11 = 2.66, p = 0.131; trials effect: F1,11 = 8.82, p = 0.013; Figure 1D). In aged rats, neither CIEE nor probe trials significantly affected the time spent in the initial escape quadrant (p > 0.05; Figure 1E).
3.3. CIEE Altered Behavioral Flexibility in Aged Rats But Not in Young Adult Rats
During the first block of two trials on reversal day 1 (i.e., the relearning trials), ethanol-treated aged rats demonstrated faster escape latencies than water-treated aged rats (t-test; t13 = 3.44; p = 0.004; Figure 1F), while no significant treatment group effect was found in adult rats (p > 0.05). The significant effect found in aged animals was not due to differential swimming speed between conditions on the first reversal day (two-way ANOVA, age by ethanol dose: F1,24 = 0.79, p > 0.05). It is likely this effect in older animals treated with ethanol occurred due to subjects in this condition searching outside the original platform quadrant earlier in the trial and swimming into the platform in the new location. Additional research is needed to determine if this is an accurate conclusion. Due to these unexpected differences in baseline relearning in the reversal session for aged animals, we analyzed the subsequent behavioral flexibility using two different strategies to gain insights into the effect of CIEE on cognition in aged animals. First, we determined the effect of CIEE on behavioral flexibility by using average raw latency data for blocks of two trails for both the aged and adult animals. In aged animals, CIEE significantly interacted with the behavioral flexibility session to alter performance (two-way ANOVA with repeated measures, ethanol treatment × trial block interaction effect: F3,39 = 4.161, p = 0.012; Figure S1). Post hoc analysis revealed that for the control aged animals performance was significantly better in the second trial block (Sidak’s multiple comparisons p = 0.049) and the last trial block (Sidak’s multiple comparisons p = 0.001). However, post hoc analysis also demonstrated that CIEE-treated aged animals did not improve across the trial blocks (all p-values > 0.05). For adult animals, no effect of CIEE was found (two-way ANOVA with repeated measures, trials effect, F3,33 = 10.11, p < 0.001; Figure S1). Given the unexpected difference in the first block of behavioral flexibility trials in aged animals administered either water or ethanol, we also analyzed the effect of CIEE on behavioral flexibility by analyzing the percentage change in performance in the subsequent three blocks of two trials of reversal learning. In young adult rats, CIEE did not impact behavioral flexibility performance as measured by percent change in escape latencies (two-way ANOVA; trials effect: F3,33 = 9.07, p = 0.002; treatment group effect: F1,11 = 1.40, p = 0.262; Figure 1G). In aged rats, CIEE significantly impaired behavioral flexibility performance as measured by percent change in escape latencies as evidenced by ethanol-treated rats displaying significantly less improvement in escape latencies over trials compared to water-treated rats (two-way ANOVA; trials effect: F3,39 = 5.04, p = 0.008; treatment group effect: F1,13 = 9.55, p = 0.009; Figure 1H). Given the finding that ethanol-treated aged rats had faster latencies in the relearning trials (the first two trials following reversal location of the platform), it is possible that a floor effect inhibits ethanol-treated aged animals from performing better over the subsequent behavioral flexibility trials. We do not believe this is the case for three reasons. First, subjects did not progress through the study procedure unless they previously learned the spatial task at a threshold of approximately 10 s for more than 1 day. As such, all animals tested had previously demonstrated better performance than what was found in the behavioral flexibility test. Second, as previously reported, no significant difference in swim speed by condition was found in the first day of reversal learning. Finally, ethanol-treated aged animals performed better, on average, in the last block of two trials in the behavioral flexibility test than they did in the first two blocks of trials, demonstrating a floor effect likely did not exist. However, due to the unexpected difference in the first two relearning trials, future research should use additional procedures such as serial position reaction times to investigate the effect of CIEE on behavioral flexibility in aged animals.
Acute ethanol administration has been shown to impair a variety of cognitive functions that are dependent on the hippocampus (reviewed by Van Skike et al.23). Such deficits in cognition produced by acute ethanol occur in both adult animals and aged animals that demonstrate successful baseline cognition functions.38 However, little is known about the effects of chronic ethanol exposure on cognition in aged animals. Behavioral flexibility is a measure of cognition where subjects have to learn a new response strategy in the face of previously learned stimuli and therefore requires a measure of cognitive flexibility. The current results demonstrate a selective impairment in behavioral flexibility in aged animals undergoing CIEE.
Previous research has demonstrated that CIEE during adolescence can impair behavioral flexibility during adulthood27−31,45,46 and late in life.22 In addition to producing behavioral impairments, CIEE during adolescence also resulted in alterations in the hippocampus. Specifically, such an ethanol exposure pattern was found to decrease the hippocampal volume,42 impair NMDA-dependent long-term depression (LTD), and alter both AMPA and NMDA receptor levels.45 However, not all studies found significant changes in hippocampal volume or BDNF levels following CIEE in adolescent animals.28,29
3.4. Hippocampal Proteomic and Phosphoproteomic Differences in Ethanol-Treated Rats Were Largely Distinct in Young Adult and Aged Rats
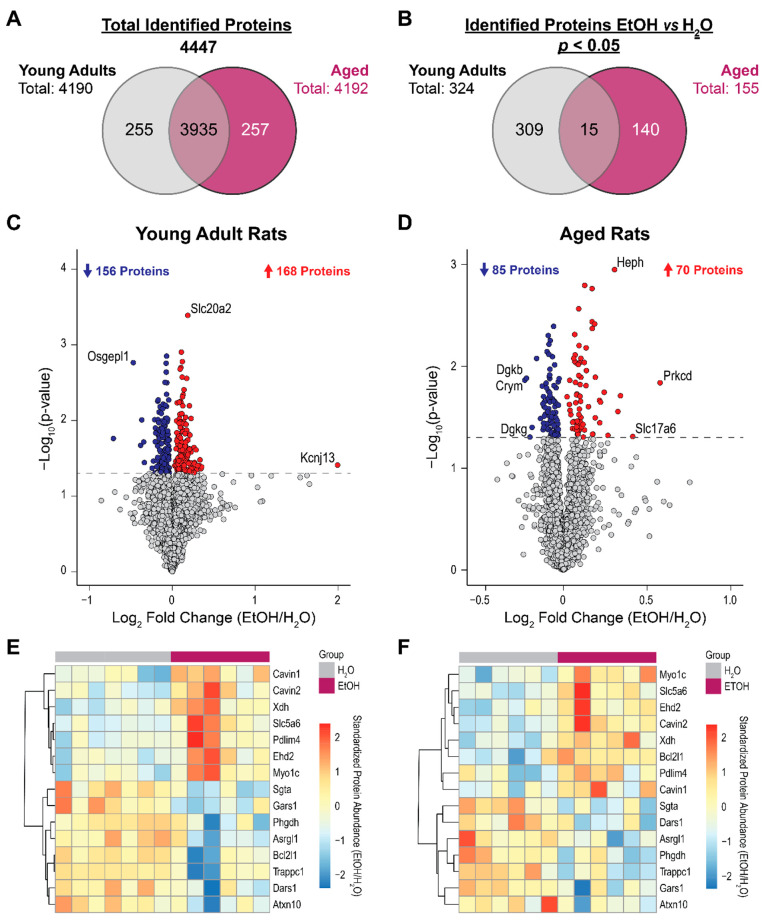
Figure 2A shows the number of proteins detected and identified in each age group, and Figure 2B shows the number of identified proteins that differed between ethanol-treated and water-treated groups at p < 0.05. The number of differentially expressed proteins in young adult rats (n = 324) was about double that of aged rats (n = 155). In young adult rats, 324 proteins were differentially expressed (p < 0.05) between treatment groups with absolute Log2FCs (ethanol/water) ranging from 0.029 to 1.994 (Table S1). These proteins include Kcnj13 (Log2FC = 1.994), Slc20a2 (Log2FC = 0.187), Osgepl1 (Log2FC = −0.470), and Fuctinin-3 (Log2FC = −0.711; Figure 2C). In aged rats, 155 proteins were differentially expressed between treatment groups with absolute Log2FCs ranging from 0.022 to 0.578 (Table S2). These proteins include Prkcd (Log2FC = 0.578), Slc17a6 (Log2FC = 0.414), and Heph (Log2FC = 0.306; Figure 2D). Fifteen proteins were differentially expressed between treatment groups in both young adult and aged groups, which might be associated with general proteomic changes due to chronic ethanol treatment (Figure 2E and Figure 2F). All proteins were altered in the same directions in both age groups apart from one protein (Bcl2I1).
Figure 2.
Overview of the proteomic differences in the hippocampus of ethanol (EtOH)-exposed rats and water (H2O)-exposed controls. (A) Total numbers of proteins identified in each and both age groups. (B) The numbers of identified proteins differed between EtOH and H2O treatment groups at p < 0.05 in each and both age groups. Volcano plots of proteins differed between treatment groups in (C) young adult rats and (D) aged rats. The x-axis represents the Log2 fold change (FC) ratio (EtOH group/water group), while the y-axis represents the −Log10p-value of the between-treatment-group comparison. The gray line indicates the p < 0.05 threshold. Proteins that are upregulated in the EtOH group (Log2FC > 0) are labeled red, while those that are downregulated (Log2FC < 0) are labeled blue. Heatmaps of relative abundance of the 15 proteins that differed at p < 0.05 between treatment groups in both age groups are shown in (E) for young adult rats and in (F) for aged rats.
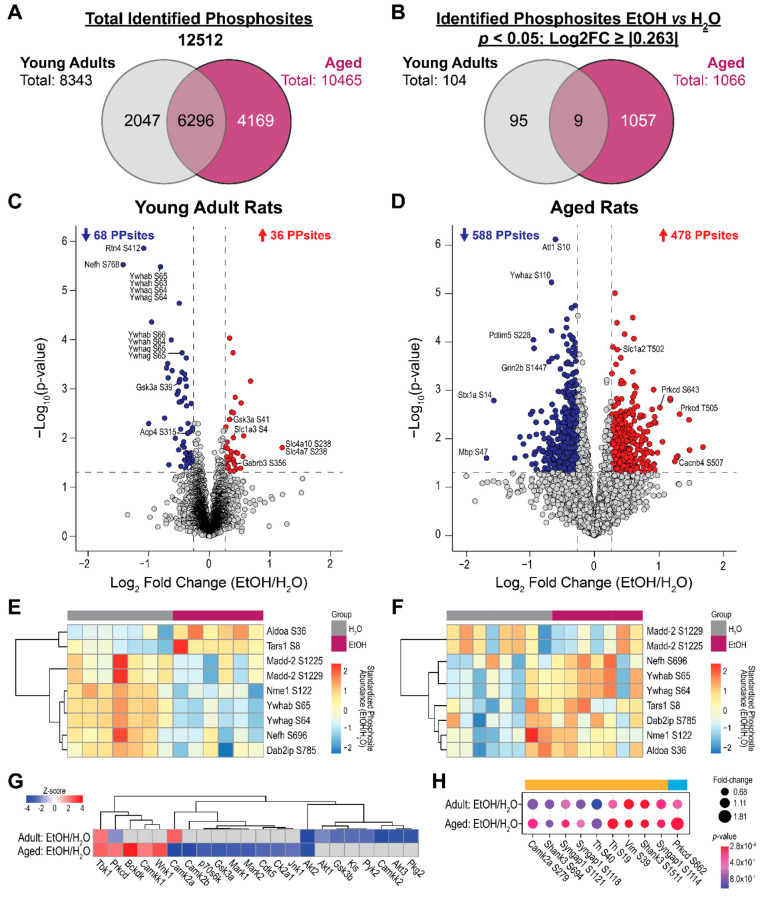
Figure 3A shows the number of phosphosites detected and identified in each age group, and Figure 3B shows the number of identified phosphosites that differed between ethanol-treated and water-treated groups at p < 0.05 and Log2FC ≥ |0.263|. Phosphosites that were compared between treatment groups are listed in Table S3 and Table S4 for young adults and aged rats, respectively. Contrasting to the proteomic comparison in which more proteins were differentially expressed in the young adult group, the number of phosphosites differentially regulated in aged rats (n = 1066) was about 10 times more than that in young adult rats (n = 104).
Figure 3.
Overview of the phosphoproteomic differences in the hippocampus of ethanol (EtOH)-exposed rats and water (H2O)-exposed controls. (A) Total numbers of phosphosites detected and identified in each and both age groups. (B) The numbers of identified phosphosites that are different in relative abundance between EtOH and H2O treatment groups at p < 0.05 and Log2 fold change (FC) ≥ |0.263| in each and both age groups. Volcano plots of phosphosites differed between treatment groups in (C) young adult rats and (D) aged rats. The x-axis represents the Log2FC (EtOH group/water group), while the y-axis represents the −Log10p-value of the between-treatment-group comparison. The gray horizontal line indicates the p < 0.05 threshold, while the gray vertical lines indicate the Log2FC threshold of ±0.263. Phosphosites with upregulated abundance in the EtOH group (Log2FC > 0) are labeled red, while those that are downregulated (Log2FC < 0) are labeled blue. Heatmaps of relative abundance of the 9 phosphosites that are different between treatment groups in both age groups at the aforementioned statistical thresholds are shown in (E) for young adult rats and in (F) for aged rats. Kinase activity profiling analysis results are shown (G). Kinases with significant activity scores (p < 0.05) in young adult and aged rats are compared between EtOH- and water-treated rats. (H) Identified substrate phosphosites of Camk2a (orange bar) and Prkcd (blue bar) in both age groups. Only phosphosites with Log2FC ≥ |0.263| in at least one of the age groups are shown.
In young adult rats, upregulated phosphosites included Slc4a10 S238/Slc4a7 S238 (Log2FC = 1.202), Slc1a3 S4 (Log2FC = 0.397), and Gabrb3 S356 (Log2FC = 0.407), and top downregulated phosphosites included Rtn4 S412 (Log2FC = −1.081), Nefh S768 (Log2FC = −1.422), and sites in the 14–3–3 family of proteins (Log2FC = −0.805 and −0.450; Figure 3C). In aged rats, top upregulated phosphosites included Slc1a2 T502 (Log2FC = 0.352) and Prkcd T643/T505/S682 (Log2FCs = 1.012/1.317/0.855), and top downregulated phosphosites included Atl1 S10 (Log2FC = −0.604), Pdlim5 S228 (Log2FC = −0.945), Grin2b S1477 (Log2FC = −0.428), Stx1a S14 (Log2FC = −1.559), and Mbp S47 (Log2FC = −1.672; Figure 3D). Nine ethanol-treatment-associated phosphosites were found in both age groups, of which seven phosphosites were regulated in opposite directions between age groups, and only Aldoa S36 and Tars1 S8 were upregulated in both age groups (Figure 3E and Figure 3F).
Our proteomic and phosphoproteomic analyses showed that CIEE caused notable molecular impacts in the hippocampus of both young adult and aged rats. While more proteins were differentially expressed in the young adult rats than the aged rats, there were far more differentially regulated phosphosites in the aged rats than in the young adult rats. Moreover, only several proteomic and phosphoproteomic differences were shared between age groups. These results suggest that (1) the hippocampal proteomic and phosphoproteomic changes in response to CIEE were unique in the two age groups and/or (2) the two age groups were moving at different speeds and/or taking different routes of molecular recovery.
Ethanol-treatment-associated proteins that overlapped between age groups are involved in caveolae-mediated endocytosis (Cavin1, Cavin2, and Ehd2; all upregulated), translation (Gars1 and Dars1; both downregulated), biotin transport (Slc5a6; upregulated), neuron survival, neuron differentiation and neuritogenesis (Atxn10; downregulated), and purine metabolism (Xdh; upregulated). Only Bcl2l1, which is an apoptosis regulator, was found to be downregulated in young adult rats but upregulated in aged rats. A few of these proteins had been found to be affected by ethanol exposure. For example, Cavin1 was found to be upregulated in the serum and liver of ethanol-fed mice and protected hepatocytes from ethanol-mediated apoptosis by inhibiting reactive nitrogen species.47 Caveolae-dependent endocytosis was shown to be important in regulating ethanol-induced toll-like receptor 4 (TLR4) internalization in cortical astrocytes which triggered inflammation response.48 Another example, Slc5a6, a major contributor of biotin and pantothenic acid at the human blood–brain barrier,49 was observed to have its gene and protein levels downregulated by chronic ethanol exposure in enteroid and colonoid cells and inhibited biotin uptake.50 Xdh is a key purine catabolism enzyme with interconvertible forms: xanthine dehydrogenase can be converted into xanthine oxidase, leading to reactive oxygen species production. As the ethanol metabolite acetaldehyde can induce such conversion, xanthine oxidase has been proposed to contribute to organ damage by reactive oxygen species.51 Injection of high-dose ammonia was found to deplete ATP and convert xanthine dehydrogenase to xanthine oxidase in rat brains via the activation of NMDA receptors.52 Although most of these studies were conducted in non-neural tissues, the general effects of ethanol on these proteins might be applied to brain tissues and cell types. Finally, additional brain regions other than the hippocampus, such as the prefrontal cortex and orbitofrontal cortex, are critical for accurate behavioral flexibility, and further research needs to conduct omics studies on these brain regions.53,54
3.5. Kinase Activities of Prkcd and Camk2a Were Regulated Differentially by CIEE in Young Adult and Aged Rats
Cluster analysis using Euclidean distance revealed the activity profiles of kinases identified in our experiment as well as distant kinases that might be altered in the ethanol-treated rats compared to water-treated rats in each age group (Figure 3G). Three clusters resulted: the first cluster contained Tbk1, Prkcd, Bckdk, Camkk1, and Wnk1, which were generally activated in ethanol-treated aged rats; the second cluster contained Camk2a, Gsk3a, Jnk1, etc., which were generally deactivated in ethanol-treated aged rats; and the third cluster consisted of Akt3, Camkk2, Gsk3b, etc., which were generally activated in ethanol-treated young adult rats. Tbk1 was activated, and Akt2 was deactivated in ethanol-treated rats of both age groups; however, Prkcd and Camk2a showed the opposite activation patterns in ethanol-treated rats between age groups. Prkcd and Camk2a were activated in ethanol-treated aged and young adult rats, respectively, but were deactivated in the other age group correspondingly.
Nine Camk2a-substrate phosphosites and one Prkcd-substrate phosphosite were differentially regulated in ethanol-treated rats of either age group (p < 0.05 and Log2FC ≥ |0.263|; Figure 3H). These phosphosites represented potential phosphorylation changes by these kinases that were associated with CIEE in a specific age group. Note that the kinase activity changes reported could be due to the changes in the abundances and/or posttranslational modifications of the kinase substrates.
3.6. Different Pathways Were Enriched in CIEE-Associated Proteins and Phosphosites in Young Adult and Aged Rats
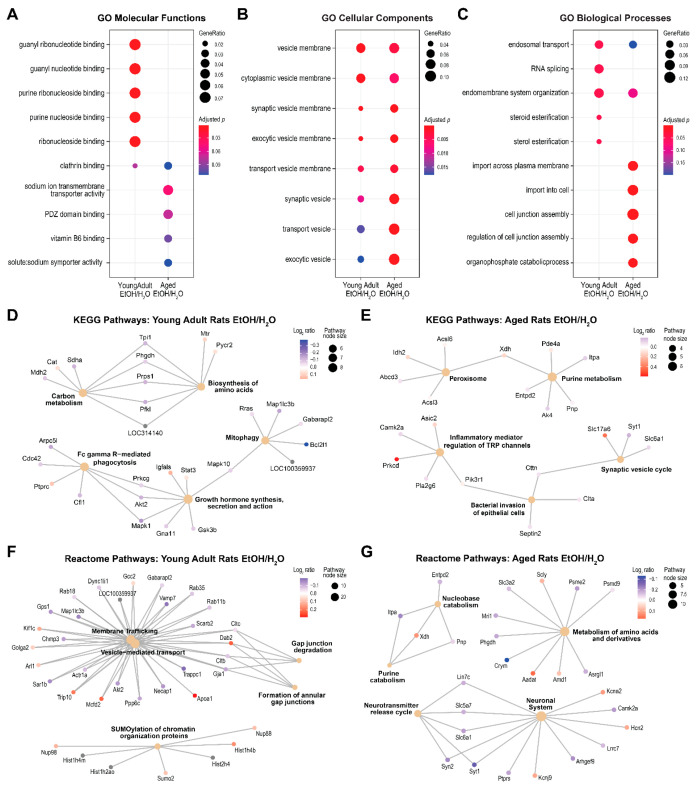
Differentially expressed proteins between treatment groups were enriched in various functional pathways distinctly in each age group (Figure 4). In young adult rats, significantly enriched pathways included GO purine (ribo)nucleoside and guanyl (ribo)nucleotide binding, RNA splicing, and endosomal transport; KEGG “biosynthesis of amino acid”, “mitophagy”, and “growth hormone synthesis secretion and action”; and reactome “vesicle-mediated transport”, “gap junction degradation”, and “SUMOylation of chromatin organization proteins”. In aged rats, significantly enriched pathways included GO sodium transmembrane transporter activity, cell junction assembly, and import into cells; KEGG “purine metabolism”, “peroxisome”, and “synaptic vesicle”; and reactome “amino acid and derivative metabolism”, “purine catabolism”, “neurotransmitter release cycle”, and “neuronal system”. Table S5 and Table S6 list the details of pathway analyses for young adult and aged rats, respectively.
Figure 4.
Pathway enrichment analysis results of the proteins differed between ethanol (EtOH)-exposed rats and water (H2O)-exposed controls. (A–C) Results of gene ontology (GO) enrichment analysis. The y-axes list the top enriched pathways by either age group, and columns represent the enrichment results of the young adult and aged rat groups. (D, E) Excerpt results of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses and (F, G) reactome pathway analysis separated by age groups. Each small node represents a protein; each large node represents a pathway; and a path represents a protein that is mapped to a pathway.
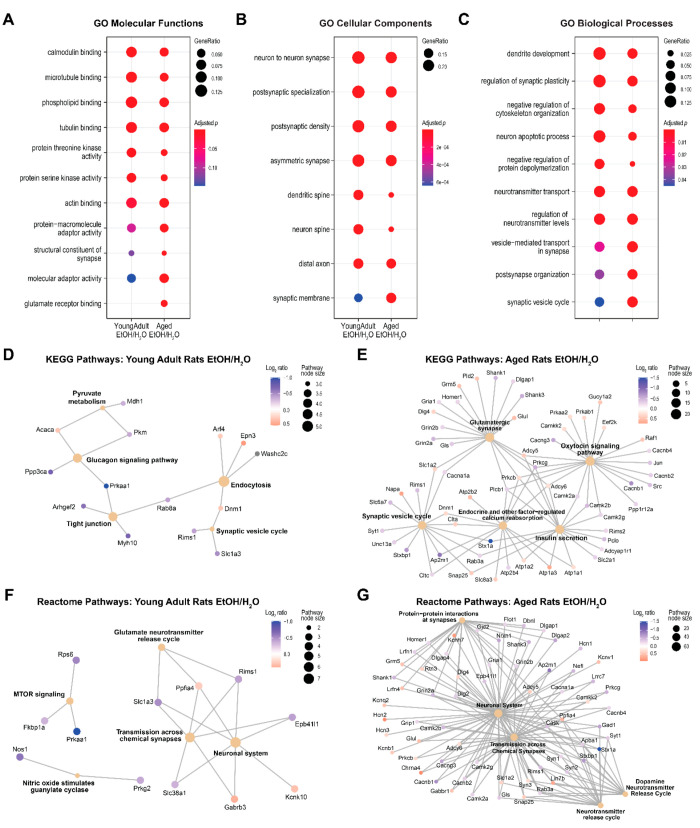
Top GO terms enriched in the proteins mapped to the differential phosphosites were similar between age groups (Figure 5A to Figure 5C), and these terms encompassed neuronal processes (axons and dendrites), synaptic function (postsynaptic membrane organization, vesicle-mediated transport), neurotransmitter level regulation, the binding of calmodulin, phospholipids, microtubules and actin, and protein kinase activities. The aged rat group was distinctly enriched in “glutamate receptor binding” and had fewer proteins enriched in “dendritic/neuron spine” as well as “negative regulation of protein depolymerization”. In young adult rats, additional significantly enriched pathways included KEGG “endocytosis”, “glucagon signaling pathway”, and “tight junction” and reactome “transmission across chemical synapse”, “glutamate neurotransmitter release cycle”, and “MTOR signaling” (Figure 5D and Figure 5F). In aged rats, other significantly enriched pathways included KEGG “glutamatergic synapse”, “oxytocin signaling pathway”, “insulin secretion”, and “endocrine and other factor-regulated calcium reabsorption” and reactome “dopamine neurotransmitter release”, “neurotransmitter release cycle”, and “protein–protein interactions at synapse” (Figure 5E and Figure 5G). Note that a few pathways were enriched in both age groups (KEGG “synaptic vesicle cycle”, reactome “neuronal system” and “transmission across chemical synapses”), but many more proteins hit these pathways in the aged group than the young adult group. Table S7 and Table S8 list the details of pathway analyses for young adult and aged rats, respectively.
Figure 5.
Pathway enrichment analysis results of the proteins mapped to the phosphosites that differed between ethanol (EtOH)-exposed rats and water (H2O)-exposed controls. (A–C) Results of gene ontology (GO) enrichment analysis. The y-axes list the top enriched pathways in either age group, and columns represent the enrichment results of the young adult and aged rat groups. (D, E) Excerpt results of Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses and (F, G) reactome pathway analysis separated by age groups. Each small node represents a protein; each large node represents a pathway; and a path represents a protein that is mapped to a pathway.
3.7. Notable Proteomic and Phosphoproteomic Changes Associated with CIEE in Aged Rats Only
Ethanol-treatment-associated proteins that were unique in each age group might provide insights into the molecular attributes of the differences in behavioral flexibility after CIEE that were observed between age groups. One of such proteins is Prkcd (protein kinase C delta) which was found to be upregulated in ethanol-treated aged rats compared to their water-treated counterparts but not in young adult rats. Previous reports showed that chronic ethanol exposure increased the expression of protein kinase C isoforms, including Prkcd, in PC-12 and NG108-15 cell lines.55,56 Single acute ethanol exposure could also increase Prkcd expression in cultured rat hippocampal neurons, in turn decreasing the surface level of the GABAA receptor δ subunit and reducing extrasynaptic GABAA receptor-mediated tonic inhibition.57 Given that Prkcd is expressed in the hippocampus and dentate gyrus58 and can regulate ethanol’s effect on extrasynaptic GABAA receptors, CIEE might have upregulated Prkcd expression in the hippocampus of the aged rats, thereby altering extrasynaptic tonic GABAergic currents and in turn contributing to the impaired behavioral flexibility. Moreover, Prkcd had been implicated in AD.59 Increased PRKCD levels were found in brain tissues of patients with AD compared to those of individuals without the disease, and the inhibition of PRKCD expression was shown to reduce BACE1 expression and amyloid-β levels.60 These reports support the hypothesis that an increased Prkcd level may be a key step in the development of AD and related cognitive impairments. A few Prkcd phosphosites (S643, T505, and S662; Table S4) and the kinase activity of Prkcd were also upregulated in the ethanol-treated aged rats compared to their water-treated counterpart (Figure 3G), suggesting the pathways that involve Prkcd might be affected by the change in its protein levels, activation via phosphorylation, and kinase activity. Contrastingly in the young adult group, Prkcd kinase activity was downregulated without any Prkcd phosphosites showing significant differential regulation, thus inferring that the changes in Prkcd associated with the ethanol treatment were age dependent. Pathway analyses in aged rats revealed pathways potentially affected by the alterations in Prkcd protein level and phosphorylation, including pathways involved in insulin signaling and response, negative regulation of phosphorylation, positive regulation of dephosphorylation, molecular import into cells (e.g., glucose, lipid, protein, ion), protein import into the nucleus, and actin filament organization, which were all predicted to be upregulated (Table S6 and Table S8).
Other top treatment-associated proteins that showed distinct proteomic differences in the aged group are Heph and Slc17a6. Heph (hephaestin) is a transmembrane copper-dependent ferroxidase expressed mainly in the intestines but is also found in the brain, including the hippocampus, and participates in iron transport and homeostasis. Brain iron overload has been recently proposed to be a potential pathogenic process associated with the accelerated cognitive decline in alcohol use disorder (AUD), which is supported by increased iron absorption in individuals with AUD as a result of alcohol-induced elevation in intestinal permeability, iron accumulation in the brains of individuals with AUD, increased iron levels found in the brain tissues of AD patients, neuron metabolism, and amyloid-β plaque deposition caused by iron dyshomeostasis.61 Here we found upregulated Heph levels in aged ethanol-treated rats compared to their water-treated counterparts. Although brain and blood iron levels were not measured in this study, it would be intriguing to investigate in the future whether chronic excessive ethanol exposure or consumption elevates intestinal iron absorption and iron influx into the brain in aged animals and humans.
Slc17a6 (vesicular glutamate transporter 2) is a glutamate transporter that loads glutamate into presynaptic vesicles and is expressed during the early postnatal stage and mostly in the thalamus and brainstem of mature rodents but is also found in the hippocampus, cortex, and cerebellum.62 Little is known about the involvement of Slc17a6 in memory, cognition, and the effect of ethanol exposure. Mice exposed to ethanol during gestation were found to have increased Slc7a6 gene expression but decreased protein levels in the hippocampus via microRNA regulation.63 In contrast to this report, we found upregulation of Slc17a6 in aged ethanol-treated rats compared to their water-treated counterparts, which might be attributed to the differences in the ethanol-exposure paradigm and the age of exposure. Slc17a6 changes in the brain across age, particularly toward more advanced years, need to be explored.
Camk2a (calcium/calmodulin-dependent protein kinase II alpha) is a key player in calcium signaling and is required for hippocampal long-term potentiation (LTP) and hippocampus-dependent cognitive tasks such as spatial learning. It is found in several regions of the hippocampus, primarily localized in pyramidal neuron dendrites and glutamate synapses,64,65 but is also found in pyramidal neuron cell bodies (reviewed by Ghosh and Anshua66). Camk2a knockout mice showed decreased LTP in the CA1 region of the hippocampus67 and exhibited selective impairments in hippocampus-dependent spatial tasks.68,69 Reduced Camk2a in the hippocampus, in particular the CA3 region, had been associated with normal aging,70 and age-related alteration in Camk2a activity resulted in impaired cognition later in life.71 Phosphorylated CAMK2A was found to be reduced, and autophosphorylated CAMK2A at T286 was found to be redistributed in the hippocampus tissues of patients with AD.66,72,73 Here, we found that Camk2a protein levels, three Camk2a phosphosites (S257, S333, and T334; Table S4), and Camk2a kinase activity were downregulated in ethanol-treated aged rats compared to their water-treated counterparts, which were consistent with the behavioral flexibility observed in the aged rats. The reduction of Camk2a kinase activity in turn differentially regulated phosphorylation in proteins associated with cognitive functions such as Shank3 (SH3 and multiple ankyrin repeat domains 3; at S694 and S1511), Syngap1 (synaptic Ras GTPase activating protein 1; at S1118 and S1121), and Th (tyrosine hydroxylase; at S19 and S40; Figure 3H). Interestingly, Camk2a kinase activity was upregulated in ethanol-treated young adult rats, suggesting that Camk2a was differentially regulated after CIEE and/or in the subsequent withdrawal in the two age groups.
Proteomic pathway analysis identified pathways potentially affected by CIEE in aged rats, including those involved in (synaptic) the vesicle membrane, and exocytosis, the synaptic vesicle cycle, sodium transporter activities, and neurotransmitter release cycle indicated a dysregulation of neurotransmission. Similar pathways were found in the phosphoproteomic pathway analysis with additional emphasis on glutamatergic and dopaminergic neurotransmissions which were both important in hippocampus-dependent cognitive functions. Pathways related to synaptic structure, dendrite development, synaptic plasticity, and neuronal apoptosis were also found to be enriched by between-treatment proteomic and phosphoproteomic differences. Interestingly, the insulin receptor signaling pathway and insulin resistance were revealed to be altered in the phosphoproteomic analysis. Insulin and its receptor function are critical for hippocampal-dependent cognitive tasks,74 and insulin resistance also impaired performance in the Morris water maze.75,76 Insulin function had also been implicated in AD.77−79
3.8. Notable Proteomic and Phosphoproteomic Changes Associated with CIEE in Young Adult Rats Only
Proteomic and phosphoproteomic changes distinct in the young adult rats may reflect protective mechanisms against the effects of ethanol on memory and cognition. Kcnj13 (potassium inwardly rectifying channel subfamily J member 13) was the most upregulated protein in young adult ethanol-treated rats compared to the water-treated counterparts. It is a member of the inwardly rectifying potassium channel family and was found to be widely expressed in neurons and glial cells of the adult mouse brain, including the hippocampus.80 While no direct research has investigated Kcnj13 in terms of hippocampal function and interaction with ethanol, inwardly rectifying potassium channels are known to be sites of action for ethanol.81 It is important to investigate in the future the role of Kcnj13 in hippocampal function, interaction with ethanol, and cognitive functions.
Another top treatment-associated protein in young adult rats was Slc20a2 (solute carrier family 20 member 2), a type III sodium-dependent phosphate transporter that plays a key role in phosphate homeostasis in the brain by importing phosphate into cells.82 Mutations in SLC20A2 are associated with idiopathic basal ganglia calcification which can lead to various neuropsychiatric, cognitive, and motor symptoms.83 Parallel with the symptoms in humans, Slc20a2 homozygous knockout mice exhibited spatial learning memory impairments and sensorimotor gating deficits which might be independent of the hippocampus and dorsal striatum since calcification seldom occurred in these regions at day 80.84Slc20a2 was among the list of genes with gene expression levels altered in astrocytes isolated from the mouse medial prefrontal cortex at peak withdrawal after chronic ethanol intoxication.85 Although the roles of hippocampal Slc20a2 in response to ethanol and behavioral flexibility are yet to be uncovered, here we demonstrated that an upregulation of its protein level might confer a protective effect on behavioral flexibility against CIEE.
Among the pathways that were dysregulated only in young adult rats based on between-treatment-group proteomics differences (Table S5) were those related to mitochondrial respiratory function, which concurred with previous reports that involved chronic ethanol exposure.86,87 Ethanol was found to induce mitochondrial reactive oxygen species production,88 and we found pathways related to the cellular response to reactive oxygen species (hydrogen peroxide in particular) were downregulated. Pathways related to phosphatidylinositol phosphate (in particular phosphatidylinositol-4,5 biphosphate; PIP2), GDP, and GTP binding as well as GTPase activity were estimated to be downregulated, suggesting the signaling transduction pathways and other regulatory pathways—such as ion channel regulation and exocytosis89,90—using these major signaling molecules might also be downregulated. Pathways related to mRNA splicing and processing were upregulated, but those related to translation regulation (particularly in translation initiation and RNA binding) were downregulated, which agreed with the reported effects of chronic ethanol exposure on genome-wide splicing changes91 and synaptosome transcriptomic changes92 and on protein synthesis impairment and translation initiation.93,94
3.9. Limitations
This study is limited by the lack of female rats and further functional studies of candidate proteins. Furthermore, we utilized retired male breeders which may impact our results. Future research with female rodents is required to explore sex-specific ethanol-induced hippocampal proteomic and phosphoproteomic changes as well as to elucidate the functions of candidate proteins and related pathways in ethanol-induced behavioral inflexibility. In addition, an unexpected difference in the first two trials of the behavioral flexibility test highlights the need to use additional procedures to further investigate this finding. Moreover, rats were sacrificed 4 days after the last gavage, and the molecular changes reported might not reflect the effects immediately upon the end of the ethanol exposure paradigm or earlier withdrawal. Lastly, our findings in the proteomic and phosphoproteomic analyses need to be interpreted cautiously, and empirical validations are needed to ascertain whether these differences and the proposed mechanisms are true.
4. Conclusions
This study demonstrated that CIEE led to selective behavioral flexibility impairments in aged male Sprague–Dawley rats but not in young adult rats, suggesting that late-in-life AUD may confer an increased risk of cognitive impairments and/or a faster cognitive decline. Potential age-group-dependent differences in hippocampal proteins, phosphosites, and functional pathways associated with CIEE were identified, and some have been implicated in cognitive deficits and AD. Future research needs to validate these candidate proteins and pathways and further explore the molecular impact of CIEE in aged animals.
Acknowledgments
We acknowledge the assistance of the Mayo Clinic Proteomics Core, which is a shared resource of the Mayo Clinic Cancer Center (NCI P30 CA15083), and Dr. Yue Chen for providing guidance on the KAPA algorithm.
Glossary
ABBREVIATIONS
- AD
Alzheimer’s disease
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AGC
automatic gain control
- ATP
adenosine triphosphate
- AUD
alcohol use disorder
- CIEE
chronic intermittent ethanol exposure
- GABA
γ-aminobutyric acid
- GO
gene ontology
- KAPA
kinase activity profiling analysis
- KEGG
Kyoto Encyclopedia of Genes and Genome
- LC
liquid chromatography
- MS
mass spectrometry
- NCE
normalized collision energy
- NMDA
N-methyl-d-aspartate
- TMT
tandem mass tag
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE95 partner repository with the data set identifier PXD034443 and 10.6019/PXD034443 (Reviewer account details: Username = reviewer_pxd034443@ebi.ac.uk; Password = hrJ6EgcE).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c04528.
Author Present Address
# Behavioral Neuroscience Program, Department of Psychology, Binghamton University—SUNY, Binghamton, New York 13902, U.S.A
Author Present Address
¶ Mayo Clinic, Rochester, Minnesota 55905, U.S.A.
Author Contributions
● These authors contributed equally to this work. The study was conceived and designed by D.B.M. and D.S.C. Animal experiments were conducted by D.B.M., S.J.S., S.T., and A.S., and data collected were analyzed by D.B.M. Proteomic and phosphoproteomic data acquisition and bioinformatic analyses were performed by B.J.M. and M.P.P. respectively, with guidance by D.S.C. Manuscript and visualization were prepared by A.M.C.H. and M.P.P. with data interpretation by D.B.M. and A.M.C.H. D.B.M. and D.S.C. contributed equally. All authors critically reviewed and have given approval to the final version of the manuscript.
This study was funded by Mayo Clinic — UWEC Research Innovation Council. This study was partially supported by the Samuel C. Johnson Genomics of Addiction Program at the Mayo Clinic, the Ulm Foundation, the National Institute on Alcohol Abuse and Alcoholism (AA028968 and AA029258), and the National Institute on Aging (AG072898).
The authors declare the following competing financial interest(s): Dr. Doo-Sup Choi is a scientific advisory board member to Peptron Inc. and Peptron had no role in the preparation, review, or approval of the manuscript nor the decision to submit the manuscript for publication. The remaining authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
References
- Ferreira M. P.; Willoughby D. Alcohol consumption: the good, the bad, and the indifferent. Appl. Physiol. Nutr. Metab. 2008, 33 (1), 12–20. 10.1139/H07-175. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Global status report on alcohol and health 2018; World Health Organization: Geneva, 2018. [Google Scholar]
- Pollard M. S.; Tucker J. S.; Green H. D. Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw. Open 2020, 3 (9), e2022942 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A. M.; Castle I. P.; Powell P. A.; Hingson R. W.; Koob G. F. Alcohol-related deaths during the COVID-19 pandemic. JAMA 2022, 327 (17), 1704–1706. 10.1001/jama.2022.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations, Department of Economic and Social Affairs, Population Division. The World Population Prospects: 2015 Revision, Key Findings and Advance Tables; New York, 2015. [Google Scholar]
- Tampi R. R.; Tampi D. J.; Ghori A. K. Substance use disorders in late life: The Next Silver Tsunami. J. Addict. Res. Ther. 2015, 06 (6), e127 10.4172/2155-6105.1000e127. [DOI] [Google Scholar]
- Witt E. D. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol 2010, 44 (1), 119–24. 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- McKetta S.; Keyes K. M. Heavy and binge alcohol drinking and parenting status in the United States from 2006 to 2018: An analysis of nationally representative cross-sectional surveys. PLoS Med. 2019, 16 (11), e1002954 10.1371/journal.pmed.1002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K. M.; Gary D.; O’Malley P. M.; Hamilton A.; Schulenberg J. Recent increases in depressive symptoms among US adolescents: trends from 1991 to 2018. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54 (8), 987–996. 10.1007/s00127-019-01697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D. G.; Wu L. T. The epidemiology of at-risk and binge drinking among middle-aged and elderly community adults: National survey on drug use and health. Am. J. Psychiatry 2009, 166 (10), 1162–9. 10.1176/appi.ajp.2009.09010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laberge S.; Bigelow P.; Lagarde E.; Crizzle A. M. Examining the association between alcohol consumption and health conditions in community dwelling older adults. J. Community Health 2021, 46 (1), 51–63. 10.1007/s10900-020-00842-8. [DOI] [PubMed] [Google Scholar]
- Breslow R. A.; Castle I. P.; Chen C. M.; Graubard B. I. Trends in alcohol consumption among older Americans: National health interview surveys, 1997 to 2014. Alcohol.: Clin. Exp. Res. 2017, 41 (5), 976–986. 10.1111/acer.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B. H.; Moore A. A.; Ferris R.; Palamar J. J. Binge drinking among older adults in the United States, 2015 to 2017. J. Am. Geriatr. Soc. 2019, 67 (10), 2139–2144. 10.1111/jgs.16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E.; Allel K.; Staudinger U. M.; Castillo-Carniglia A.; Medina J. T.; Keyes K. M. Cross-country differences in age trends in alcohol consumption among older adults: a cross-sectional study of individuals aged 50 years and older in 22 countries. Addiction 2021, 116 (6), 1399–1412. 10.1111/add.15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. B.; Mittleman G. Age-dependent effects of chronic intermittent ethanol treatment: Gross motor behavior and body weight in aged, adult and adolescent rats. Neurosci. Lett. 2017, 657, 146–150. 10.1016/j.neulet.2017.08.012. [DOI] [PubMed] [Google Scholar]
- Novier A.; Van Skike C.; E; Diaz-Granados J. L.; Mittleman G.; Matthews D. B. Acute alcohol produces ataxia and cognitive impairments in aged animals: a comparison between young adult and aged rats. Alcohol.: Clin. Exp. Res. 2013, 37 (8), 1317–24. 10.1111/acer.12110. [DOI] [PubMed] [Google Scholar]
- Watson M. R.; James K.; Mittleman G.; Matthews D. B. Impact of acute ethanol exposure on body temperatures in aged, adult and adolescent male rats. Alcohol 2020, 82, 81–89. 10.1016/j.alcohol.2019.08.001. [DOI] [PubMed] [Google Scholar]
- Novier A.; Ornelas L. C.; Diaz-Granados J. L.; Matthews D. B. Differences in behavioral responding in adult and aged rats following chronic ethanol exposure. Alcohol.: Clin. Exp. Res. 2016, 40 (7), 1462–72. 10.1111/acer.13098. [DOI] [PubMed] [Google Scholar]
- Ornelas L. C.; Novier A.; Van Skike C.; E; Diaz-Granados J. L.; Matthews D. B. The effects of acute alcohol on motor impairments in adolescent, adult, and aged rats. Alcohol 2015, 49 (2), 121–126. 10.1016/j.alcohol.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Perkins A. E.; Vore A. S.; Lovelock D.; Varlinskaya E.; Deak T. Late aging alters behavioral sensitivity to ethanol in a sex-specific manner in Fischer 344 rats. Pharmacol., Biochem. Behav. 2018, 175, 1–9. 10.1016/j.pbb.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. B.; Watson M. R.; James K.; Kastner A.; Schneider A.; Mittleman G. The impact of low to moderate chronic intermittent ethanol exposure on behavioral endpoints in aged, adult, and adolescent rats. Alcohol 2019, 78, 33–42. 10.1016/j.alcohol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- Matthews D. B.; Scaletty S.; Trapp S.; Kastner A.; Schneider A. M.; Schreiber A.; Rossmann G. Chronic intermittent ethanol administration during adolescence produces sex dependent impairments in behavioral flexibility and survivability. Brain Sci. 2022, 12 (5), 606. 10.3390/brainsci12050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Skike C.; E; Goodlett C.; Matthews D. B. Acute alcohol and cognition: Remembering what it causes us to forget. Alcohol 2019, 79, 105–125. 10.1016/j.alcohol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- Matthews D. B.; Simson P. E.; Best P. J. Ethanol alters spatial processing of hippocampal place cells: a mechanism for impaired navigation when intoxicated. Alcohol.: Clin. Exp. Res. 1996, 20 (2), 404–7. 10.1111/j.1530-0277.1996.tb01660.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann S. E.; Matthews D. B. Ethanol-induced impairments in spatial working memory are not due to deficits in learning. Alcohol.: Clin. Exp. Res. 2001, 25 (6), 856–61. 10.1111/j.1530-0277.2001.tb02291.x. [DOI] [PubMed] [Google Scholar]
- White A. M.; Matthews D. B.; Best P. J. Ethanol, memory, and hippocampal function: a review of recent findings. Hippocampus 2000, 10 (1), 88–93. . [DOI] [PubMed] [Google Scholar]
- Barker J. M.; Bryant K. G.; Osborne J. I.; Chandler L. J. Age and sex interact to mediate the effects of intermittent, high-dose ethanol exposure on behavioral flexibility. Front. Pharmacol. 2017, 8, 450. 10.3389/fphar.2017.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman L. G. Jr.; Liu W.; Oguz I.; Styner M.; Crews F. T. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol., Biochem. Behav. 2014, 116, 142–51. 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G. M.; Savage L. M. Adolescent binge ethanol exposure alters specific forebrain cholinergic cell populations and leads to selective functional deficits in the prefrontal cortex. Neuroscience 2017, 361, 129–143. 10.1016/j.neuroscience.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sey N. Y. A.; Gómez A. A.; Madayag A. C.; Boettiger C. A.; Robinson D. L. Adolescent intermittent ethanol impairs behavioral flexibility in a rat foraging task in adulthood. Behav. Brain Res. 2019, 373, 112085. 10.1016/j.bbr.2019.112085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macht V.; Elchert N.; Crews F. Adolescent alcohol exposure produces protracted cognitive-behavioral impairments in adult male and female rats. Brain Sci. 2020, 10 (11), 785. 10.3390/brainsci10110785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. C. Animal models for studies of alcohol effects on the trajectory of age-related cognitive decline. Alcohol 2022, 10.1016/j.alcohol.2022.04.005. [DOI] [PubMed] [Google Scholar]
- Schwarzinger M.; Pollock B. G.; Hasan O. S. M.; Dufouil C.; Rehm J.; et al. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health 2018, 3, e124 10.1016/S2468-2667(18)30022-7. [DOI] [PubMed] [Google Scholar]
- Guarino A.; Favieri F.; Boncompagni I.; Agostini F.; Cantone M.; Casagrande M. Executive functions in Alzheimer disease: A systematic review. Front. Aging Neurosci. 2019, 10.3389/fnagi.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z.; Bai Y.; Wu X.; Li H.; Gong B.; Howland J. G.; Huang Y.; He W.; Li T.; Wang Y. T. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropsychopharmacology 2013, 64, 65–73. 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- Shah D.; Verhoye M.; Van der Linden A.; D’Hooge R. Acquisition of spatial search strategies and reversal learning in the Morris water maze depend on disparate brain functional connectivity in mice. Cereb. Cortex 2019, 29 (11), 4519–4529. 10.1093/cercor/bhy329. [DOI] [PubMed] [Google Scholar]
- de Bruin J. P.C.; Moita M. P.; de Brabander H. M.; Joosten R. N.J.M.A. Place and response learning of rats in a Morris water maze: differential effects of fimbria fornix and prefrontal cortex leisons. Neurobiol. Learn Mem. 2001, 75 (2), 164–178. 10.1006/nlme.2000.3962. [DOI] [PubMed] [Google Scholar]
- Skelton R. W.; McNamara R. K. Bilateral knife cuts to the perforant path disrupt spatial learning in the Morris water maze. Hippocampus 1992, 2 (1), 73–80. 10.1002/hipo.450020110. [DOI] [PubMed] [Google Scholar]
- Starski P.; Peyton L.; Oliveros A.; Heppelmann C. J.; Dasari S.; Choi D. S. Proteomic profile of a chronic binge ethanol exposure model. J. Proteome Res. 2019, 18 (9), 3492–3502. 10.1021/acs.jproteome.9b00394. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Xiao W.; Su Z.; Cheng J.; Zheng C.; Zhang Z.; Wang Y.; Wang L.; Xu B.; Li S.; Yang X.; Pui Man Hoi M. Hippocampal proteomic alteration in triple transgenic mouse model of Alzheimer’s disease and implication of PINK 1 Regulation in Donepezil treatment. J. Proteome Res. 2019, 18 (4), 1542–1552. 10.1021/acs.jproteome.8b00818. [DOI] [PubMed] [Google Scholar]
- Morris R. G. M. Spatial localization does not require the presence of local cues. Learn Motiv. 1981, 12 (2), 239–260. 10.1016/0023-9690(81)90020-5. [DOI] [Google Scholar]
- Matthews D. B.; Scaletty S.; Schreiber A.; Trapp S. Acute ethanol administration produces larger spatial and nonspatial memory impairments in 29–33 month old rats compared to adult and 18–24 month old rats. Pharmacol., Biochem. Behav. 2020, 199, 173074. 10.1016/j.pbb.2020.173074. [DOI] [PubMed] [Google Scholar]
- Erber L. N.; Luo A.; Gong Y.; Beeson M.; Tu M.; Tran P.; Chen Y. Iron deficiency reprograms phosphorylation signaling and reduces O-GlcNAc pathways in neuronal cells. Nutrients 2021, 13 (1), 179. 10.3390/nu13010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck P. V.; Zhang B.; Murray B.; Kornhauser J. M.; Latham V.; Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras A.; Polín E.; Miguéns M.; Pérez-García C.; Pérez V.; Ruiz-Gayo M.; Morales L.; Del Olmo N. Intermittent-excessive and chronic-moderate ethanol intake during adolescence impair spatial learning, memory and cognitive flexibility in the adulthood. Neuroscience 2019, 418, 205–217. 10.1016/j.neuroscience.2019.08.051. [DOI] [PubMed] [Google Scholar]
- Gass J. T.; Glen W. B. Jr.; McGonigal J. T.; Trantham-Davidson H.; Lopez M. F.; Randall P. K.; Yaxley R.; Floresco S. B.; Chandler L. J. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology 2014, 39 (11), 2570–83. 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.; Zhou Y.; Zhong W.; Zhao X.; Chen C.; Chen X.; Gu Y.; Chen J.; Lv Z.; Shen J. Caveolin-1 is essential for protecting against binge drinking-induced liver damage through inhibiting reactive nitrogen species. Hepatology 2014, 60 (2), 687–99. 10.1002/hep.27162. [DOI] [PubMed] [Google Scholar]
- Pascual-Lucas M.; Fernandez-Lizarbe S.; Montesinos J.; Guerri C. LPS or ethanol triggers clathrin- and rafts/caveolae-dependent endocytosis of TLR4 in cortical astrocytes. J. Neurochem. 2014, 129 (3), 448–62. 10.1111/jnc.12639. [DOI] [PubMed] [Google Scholar]
- Uchida Y.; Ito K.; Ohtsuki S.; Kubo Y.; Suzuki T.; Terasaki T. Major involvement of Na(+) -dependent multivitamin transporter (SLC5A6/SMVT) in uptake of biotin and pantothenic acid by human brain capillary endothelial cells. J. Neurochem. 2015, 134 (1), 97–112. 10.1111/jnc.13092. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy K.; Sabui S.; Srinivasan P.; Al-Juburi S.; Pham Q.; Chu B. D.; Simoes R. D.; Fleckenstein J. M.; Said H. M. Effect of chronic alcohol exposure on gut vitamin B7 uptake: involvement of epigenetic mechanisms and effect of alcohol metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321 (2), G123–G133. 10.1152/ajpgi.00144.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultatos L. G. Effects of acute ethanol administration on the hepatic xanthine dehydrogenase/oxidase system in the rat. J. Pharmacol. Exp. Ther. 1988, 246 (3), 946–9. [PubMed] [Google Scholar]
- Kaminsky Y.; Kosenko E. Brain purine metabolism and xanthine dehydrogenase/oxidase conversion in hyperammonemia are under control of NMDA receptors and nitric oxide. Brain Res. 2009, 1294, 193–201. 10.1016/j.brainres.2009.07.082. [DOI] [PubMed] [Google Scholar]
- Uddin L. Q. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. 10.1038/s41583-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J.; Noonan M. P.; Thomas A.; O’Reilly J. X.; Anderson J.; Papageorgiou G. K.; Neubert F. X.; Ahmed B.; Smith J.; Bell A. H.; Buckley M. J.; Roumazeilles L.; Cuell S.; Walton M. E.; Krug K.; Mars R. B.; Rushworth M. F. S. Behavioral flexibility is associated with changes in structure and function distributed across a frontal cortical network in macaques. PloS Biol. 2020, 18 (5), e3000605 10.1371/journal.pbio.3000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing R. O.; Petersen P. J.; Henrich C. J. Chronic ethanol exposure increases levels of protein kinase C delta and epsilon and protein kinase C-mediated phosphorylation in cultured neural cells. J. Biol. Chem. 1991, 266 (34), 23428–32. 10.1016/S0021-9258(18)54514-2. [DOI] [PubMed] [Google Scholar]
- Coe I. R.; Yao L.; Diamond I.; Gordon A. S. The role of protein kinase C in cellular tolerance to ethanol. J. Biol. Chem. 1996, 271 (46), 29468–72. 10.1074/jbc.271.46.29468. [DOI] [PubMed] [Google Scholar]
- Chen J.; He Y.; Wu Y.; Zhou H.; Su L. D.; Li W. N.; Olsen R. W.; Liang J.; Zhou Y. D.; Shen Y. Single ethanol withdrawal regulates extrasynaptic δ-GABA(A) receptors via PKCδ activation. Front. Mol. Neurosci. 2018, 11, 141. 10.3389/fnmol.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. S.; Wei W.; Deitchman J. K.; Kharazia V. N.; Lesscher H. M.; McMahon T.; Wang D.; Qi Z. H.; Sieghart W.; Zhang C.; Shokat K. M.; Mody I.; Messing R. O. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J. Neurosci. 2008, 28 (46), 11890–9. 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore D.; Pacifici F.; Dave K. R.; Palmirotta R.; Bellia A.; Pasquantonio G.; Guadagni F.; Donadel G.; Di Daniele N.; Abete P.; Lauro D.; Rundek T.; Perez-Pinzon M. A.; Della-Morte D. Age-dependent levels of protein kinase Cs in brain: Reduction of endogenous mechanisms of neuroprotection. Int. J. Mol. Sci. 2019, 20 (14), 3544. 10.3390/ijms20143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y.; Zhao Y.; Li C.; Zheng Q.; Tian J.; Li Z.; Huang T. Y.; Zhang W.; Xu H. Inhibition of PKCδ reduces amyloid-β levels and reverses Alzheimer disease phenotypes. J. Exp Med. 2018, 215 (6), 1665–1677. 10.1084/jem.20171193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listabarth S.; Konig D.; Vyssoki B.; Hametner S. Does thiamine protect the brain from iron overload and alcohol-related dementia?. Alzheimers Dement. 2020, 16 (11), 1591–1595. 10.1002/alz.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D.; Moechars D.; Callaerts-Vegh Z.; Vermaercke B.; Van Acker N.; Andries L.; D’Hooge R. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cerebral Cortex 2010, 20 (3), 684–693. 10.1093/cercor/bhp133. [DOI] [PubMed] [Google Scholar]
- Zhang C. R.; Ho M. F.; Vega M. C.; Burne T. H.; Chong S. Prenatal ethanol exposure alters adult hippocampal VGLUT2 expression with concomitant changes in promoter DNA methylation, H3K4 trimethylation and miR-467b-5p levels. Epigenetics Chromatin. 2015, 8, 40. 10.1186/s13072-015-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone M. E.; Pollock J. A.; Jones Y. Z.; Ellisman M. H. Ultrastructural localization of dendritic messenger RNA in adult rat hippocampus. J. Neurosci. 1996, 16 (23), 7437–46. 10.1523/JNEUROSCI.16-23-07437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-B.; Murray K. D. Neuronal excitability and calcium/calmodulin-dependent protein kinase type II: Location, location, location. Epilepsia 2012, 53 (s1), 45–52. 10.1111/j.1528-1167.2012.03474.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A.; Giese K. P. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol. Brain 2015, 8 (1), 78. 10.1186/s13041-015-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. J.; Stevens C. F.; Tonegawa S.; Wang Y. Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 1992, 257 (5067), 201–6. 10.1126/science.1378648. [DOI] [PubMed] [Google Scholar]
- Silva A. J.; Paylor R.; Wehner J. M.; Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 1992, 257 (5067), 206–11. 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Ohno M.; Sametsky E. A.; Silva A. J.; Disterhoft J. F. Differential effects of alphaCaMKII mutation on hippocampal learning and changes in intrinsic neuronal excitability. Eur. J. Neurosci. 2006, 23 (8), 2235–40. 10.1111/j.1460-9568.2006.04746.x. [DOI] [PubMed] [Google Scholar]
- Nyffeler M.; Zhang W. N.; Feldon J.; Knuesel I. Differential expression of PSD proteins in age-related spatial learning impairments. Neurobiol. Aging 2007, 28 (1), 143–55. 10.1016/j.neurobiolaging.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K.; Kumar A.; Foster T. C. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J. Neurosci. 2010, 30 (5), 1914–24. 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese L. C.; Laezza F.; Woltjer R.; Taglialatela G. Dysregulated phosphorylation of Ca(2+) /calmodulin-dependent protein kinase II-α in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease. J. Neurochem. 2011, 119 (4), 791–804. 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amada N.; Aihara K.; Ravid R.; Horie M. Reduction of NR1 and phosphorylated Ca2+/calmodulin-dependent protein kinase II levels in Alzheimer’s disease. Neuroreport 2005, 16 (16), 1809–13. 10.1097/01.wnr.0000185015.44563.5d. [DOI] [PubMed] [Google Scholar]
- Goodman E. K.; Mitchell C. S.; Teo J. D.; Gladding J. M.; Abbott K. N.; Rafiei N.; Zhang L.; Herzog H.; Begg D. P. The effect of insulin receptor deletion in neuropeptide Y neurons on hippocampal dependent cognitive function in aging mice. J. Integr. Neurosci. 2022, 21 (1), 6. 10.31083/j.jin2101006. [DOI] [PubMed] [Google Scholar]
- Stranahan A. M.; Norman E. D.; Lee K.; Cutler R. G.; Telljohann R. S.; Egan J. M.; Mattson M. P. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008, 18 (11), 1085–8. 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R.; Barnard R. J.; Ying Z.; Roberts C. K.; Gómez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112 (4), 803–14. 10.1016/S0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- Convit A. Links between cognitive impairment in insulin resistance: An explanatory model. Neurobiology of Aging 2005, 26 (1), 31–35. 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Burns J. M.; Honea R. A.; Vidoni E. D.; Hutfles L. J.; Brooks W. M.; Swerdlow R. H. Insulin is differentially related to cognitive decline and atrophy in Alzheimer’s disease and aging. Biochim. Biophys. Acta - Mol. Basis Dis. 2012, 1822 (3), 333–339. 10.1016/j.bbadis.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S.; Stennis Watson G Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004, 3 (3), 169–178. 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- Papanikolaou M.; Lewis A.; Butt A. M. Glial and neuronal expression of the inward rectifying potassium channel Kir7.1 in the adult mouse brain. J. Anat. 2019, 235 (5), 984–996. 10.1111/joa.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J.; Blednov Y. A.; Harris R. A. Behavioral and genetic evidence for GIRK channels in the CNS: Role in physiology, pathophysiology, and drug addiction. Int. Rev. Neurobiol. 2015, 123, 279–313. 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inden M.; Kurita H.; Hozumi I. Characteristics and therapeutic potential of sodium-dependent phosphate cotransporters in relation to idiopathic basal ganglia calcification. J. Pharmacol. Sci. 2022, 148 (1), 152–155. 10.1016/j.jphs.2021.11.004. [DOI] [PubMed] [Google Scholar]
- de Oliveira D. F.; de Lemos R. R.; de Oliveira J. R. M. Mutations at the SLC20A2 gene and brain resilience in families with idiopathic basal ganglia calcification (“Fahr’s disease”). Front. Hum. Neurosci. 2013, 7, 420. 10.3389/fnhum.2013.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y.; Shen Y.; Si N.; Fan S.; Zhang Y.; Xu W.; Shi L.; Zhang X. Slc20a2-Deficient mice exhibit multisystem abnormalities and impaired spatial learning memory and sensorimotor gating but normal motor coordination abilities. Front. Genet. 2021, 10.3389/fgene.2021.639935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm C. J.; Hashimoto J. G.; Roberts M. L.; Bloom S. H.; Andrew M. R.; Wiren K. M. Astrocyte dysfunction induced by alcohol in females but not males. Brain Pathol. 2016, 26 (4), 433–51. 10.1111/bpa.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T. E.; Nunes M. E. M.; Rodrigues N. R.; Fontana B. D.; Hartmann D. D.; Franco J. L.; Rosemberg D. B. Neurochemical mechanisms underlying acute and chronic ethanol-mediated responses in zebrafish: The role of mitochondrial bioenergetics. Neurochem. Int. 2019, 131, 104584. 10.1016/j.neuint.2019.104584. [DOI] [PubMed] [Google Scholar]
- Shang P.; Lindberg D.; Starski P.; Peyton L.; Hong S. I.; Choi S.; Choi D. S. Chronic alcohol exposure induces aberrant mitochondrial morphology and inhibits respiratory capacity in the medial prefrontal cortex of mice. Front. Neurosci. 2020, 14, 561173. 10.3389/fnins.2020.561173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet-Ponce L.; Saez-Atienzar S.; da Casa C.; Flores-Bellver M.; Barcia J. M.; Sancho-Pelluz J.; Romero F. J.; Jordan J.; Galindo M. F. On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta 2015, 1852 (7), 1400–9. 10.1016/j.bbadis.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Katan M.; Cockcroft S. Phosphatidylinositol(4,5)bisphosphate: diverse functions at the plasma membrane. Essays Biochem. 2020, 64 (3), 513–531. 10.1042/EBC20200041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Thathiah A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015, 589 (14), 1607–1619. 10.1016/j.febslet.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Van Booven D.; Mengying L.; Sunil Rao J.; Blokhin I. O.; Dayne Mayfield R.; Barbier E.; Heilig M.; Wahlestedt C. Alcohol use disorder causes global changes in splicing in the human brain. Transl. Psychiatry 2021, 11 (1), 2. 10.1038/s41398-020-01163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most D.; Ferguson L.; Blednov Y.; Mayfield R. D.; Harris R. A. The synaptoneurosome transcriptome: a model for profiling the emolecular effects of alcohol. Pharmacogenomics J. 2015, 15 (2), 177–88. 10.1038/tpj.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang C. H.; Kimball S. R.; Frost R. A.; Vary T. C. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int. J. Biochem. Cell Biol. 2001, 33 (5), 457–73. 10.1016/S1357-2725(00)00081-9. [DOI] [PubMed] [Google Scholar]
- Lang C. H.; Wu D.; Frost R. A.; Jefferson L. S.; Vary T. C.; Kimball S. R. Chronic alcohol feeding impairs hepatic translation initiation by modulating eIF2 and eIF4E. Am. J. Physiol. 1999, 277 (5), e805 10.1152/ajpendo.1999.277.5.E805. [DOI] [PubMed] [Google Scholar]
- Perez-Riverol Y.; Bai J.; Bandla C.; Hewapathirana S.; García-Seisdedos D.; Kamatchinathan S.; Kundu D.; Prakash A.; Frericks-Zipper A.; Eisenacher M.; Walzer M.; Wang S.; Brazma A.; Vizcaíno J. A. The PRIDE database resources in 2022: A Hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50 (D1), D543–D552. 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE95 partner repository with the data set identifier PXD034443 and 10.6019/PXD034443 (Reviewer account details: Username = reviewer_pxd034443@ebi.ac.uk; Password = hrJ6EgcE).