Most of the terminology used to define the host-microbe interaction has been in use for nearly a century. Early in this period, microbes were thought to be primary aggressors that governed the host-pathogen interaction, resulting in disease. Later, new information about the attributes of microbes and their hosts resulted in the understanding that the host-pathogen interaction does not always result in disease. This recognition, in turn, led to the introduction of terms to explain states in which microbes exist within hosts without causing overt disease and why some microbes only cause disease in certain hosts. Commensal, carrier state, and opportunist were terms put forth to account for microbes and conditions that were sometimes associated with disease but for which Koch's postulates could not be fulfilled for one reason or another. Most of these terms were originally proposed to describe the behavior of particular microbes, rather than to define a more general host-microbe relationship.
Recently, we reviewed the concepts of virulence and pathogenicity and described how the definitions for these terms changed over the years as microbiologists tried to find ways to convey that microbial pathogenesis reflects an interaction between two entities, host and pathogen (7). Based on the concept that host damage was the most relevant outcome of the host-pathogen interaction, we proposed revisions to the definitions of the terms pathogen, pathogenicity, and virulence (7). However, the proposed framework suggested a need to reexamine the terms used to define the outcomes of host-microbe interactions. Here, we critically review the origin and historical evolution of key concepts used to describe the outcome of host-microbe interactions, namely, infection, commensalism, colonization, persistence, infection, and disease. We propose that the meaning of these terms can be clarified by placing them in the context of the damage framework put forth previously (7).
LEXICON OF MICROBIAL PATHOGENESIS
Once the germ theory of disease was accepted, microbes were considered to be pathogens if they met the stipulations of Koch's postulate. However, it rapidly became apparent that (i) although there are many microbes, most human infections were caused by only a few; (ii) some microbes were classified as pathogens although they did not cause disease in every host; and (iii) some microbes were classified as nonpathogens, although they did cause disease in certain hosts (for an early review, see reference 56). In addition, it became evident that normal individuals harbored, in their mouth, gut, and skin, large numbers of microbes that did not cause disease. New ideas and terminology, heretofore referred to collectively as a lexicon, were devised to accommodate this information.
By the early twentieth century, it was apparent that pathogenicity was neither an invariant nor a stable characteristic of most microbes and that the acquisition of pathogenic microbes was not necessarily synonymous with disease. In the laboratory, the successful attenuation of pathogens revealed that virulence could be increased or decreased by animal passage and/or in vitro culture (for a review of early experimentation, see reference 4). This scientific advance eventually led to the development of vaccines that controlled many of the major childhood diseases of the past. In the clinical realm, it was recognized that a microbe responsible for an epidemic disease could be isolated from both symptomatic and asymptomatic individuals in the midst of an epidemic. For example, Henrici noted that only a small number of individuals developed disease when epidemics of cerebrospinal meningitis (Neisseria meningiditis) occurred in a community, whereas others carried the bacteria but remained healthy (i.e., carriers); however, the majority were neither sick nor carriers (18). Based on the ability to culture staphylococci and streptococci from most people despite the absence of any manifestation of disease, Kolmer proposed a condition called subinfection (26). This concept blurred the distinction between pathogens and nonpathogens and challenged a prevailing concept of microbial pathogenesis that was based on Koch's postulate. The recognition of the carrier state was problematic vis a vis the terminology of the time, because a necessary condition for associating certain pathogens with particular diseases was that the converse of Koch's postulate should be fulfilled; the causative microbe should not be found in unaffected individuals (55). However, the carrier state had not been accounted for in the formulation of Koch's postulate, and the recovery of pathogens from healthy hosts challenged the second part of the postulate that a parasite “occurs in no other disease as a fortuitous and nonpathogenic parasite” (postulate wording as suggested by Evans [12]).
The description of the carrier state confused existing definitions of pathogens. To both explain the carrier state and preserve the distinction between pathogenic and nonpathogenic microbes, the concept that host and pathogen could adapt to one another was put forth. Karsner and Ecker described this adaptation as involving changes to both host and microbe, such that the host suffered no damage and the microbe was resistant to the immune system (25). Similarly, Park and Williams described the carrier state as a commensal development by the pathogenic microorganism (36). Later, the view emerged that the carrier state was transient and true pathogens elicited immune responses that eliminated the pathogens, whereas commensals did not (54). Asymptomatic carriage of pathogens that elicited immunity was considered to be a benefit to the host that was tolerated at the risk of serious disease (54). Conversely, the recognition that a carrier state could follow resolution of clinical disease, e.g., for Salmonella enterica serovar Typhi, illustrated that in certain hosts there was a persistent risk of transmission, and possibly reacquisition, of infection. Another variation of the carrier state with great clinical importance is “microbial persistence,” which refers to the situation in which susceptible pathogens are not eradicated despite antimicrobial therapy (31). The carrier state remains poorly understood, and, as noted by Smith, this area of study is relatively underrepresented in microbial pathogenesis research (41). Although the concept of the carrier state may have undermined the pathogen-centered view of microbial pathogenesis, it invoked a mutability that paved the way for defining host-microbe interaction as a regulated relationship.
Intrinsic to the terms commensalism, colonization, and the carrier state was the concept that some microbes had the capacity to persist in their hosts. According to a model proposed by Blaser (3), ongoing interactions between host and microbe have different outcomes that depend upon regulation of the host-microbe relationship and coevolution of host and microbe favors an outcome in which the cost of eliminating the microbe is high. In the late twentieth century, studies of bacterial pathogenesis led to the identification of molecular differences between pathogenic and nonpathogenic microbes, and this engendered the belief that pathogens and nonpathogens were intrinsically different (14, 15, 17, 41). In this regard, historical definitions of terms used in the field of microbial pathogenesis have focused on distinctions between pathogens and nonpathogens rather than on the frequently divergent outcomes that often characterize different host-microbe relationships. The existence of the latter underscores the need for terminology that describes host-microbe interaction, rather than pathogen-specific characteristics.
Table 1 lists historical definitions for terms commonly used in the field of microbial pathogenesis. These definitions reveal a conceptual evolution that paralleled emerging concepts in microbial pathogenesis and clinical infectious diseases, albeit in some conflict with current concepts of infection and immunity. For example, the implication that states of colonization or commensalism do not invoke an immune response are inconsistent with evidence that normal microflora can elicit specific antibody responses (reviewed in reference 46). In fact, antibodies to Staphylococcus aureus and Candida albicans are frequent in healthy individuals that harbor these microbes without disease (16, 38, 39), and carriers of group A Streptococcus often have titers of antibody to streptococcal antigens which are attributed to prior infection (47). Moreover, the presence of antibodies can represent outcomes as diverse as ongoing viral replication (e.g., in human immunodeficiency virus [HIV] natural or vaccine-elicited immunity, latency, carriage, and cross-reactivity with antigens of another microbe or an unknown antigen. However, if the presence of antibodies implies present or past infection, the view that certain states (e.g., colonization and the carrier state) are precursors to infection requires modification. Thus, rather than providing definitions based on the outcome of host-microbe interaction, the current lexicon puts forth terms that are primarily intended to define a pathogen and whether or not it causes infection and/or disease.
TABLE 1.
Some historical definitions in the field of microbial pathogenesisa
| Term | Definitionb | Reference |
|---|---|---|
| Carrier state | The retained invader (e.g., microbe)—under the influence of the immune environment—gradually dissociates into a saprophytic state | 57 |
| A state of animal adaptation whereby the microbe and its products cause no damage; in this state the “organisms themselves have probably developed a state of resistance against substances which ordinarily would destroy the organisms and neutralize their products” | 25 | |
| When an organism of relatively high pathogenicity may appear in the normal flora without causing disease | 44 | |
| Certain individuals may continue to harbor a pathogen after clinical recovery from an infectious disease and may serve as carriers of infection | 35 | |
| Colonization | An agent is considered to colonize a host when its presence in that host does not cause a specific immune response or infection | 34 |
| Microorganisms which do not belong to the normal flora of the host but do not inflict local damage to the host | 51 | |
| The appearance or increase in numbers of a particular invasive bacterial species in the resident microflora | 52 | |
| Implantation of a microbe at a site, such as multiplication of staphylococci in the anterior nares | 24 | |
| Multiplication of an organism on a body surface without evoking an immune response | 13 | |
| Commensal | A harmless parasite | 36 |
| The organisms of the normal flora | 44 | |
| Microbes that can establish themselves in the throat, nose, or intestines without damage to the host | 53 | |
| Nonpathogenic organisms present in varying numbers at sites of the normal host's body that are in contact with the environment | 1 | |
| An organism which “eats at the same table” as another of a different species but which confers on the latter neither benefit nor harm | 35 | |
| Pertaining to or characterized by commensalism; an organism participating in commensalism | 28 | |
| Commensalism | The mutual but almost inconsequential association between bacteria and higher organisms | 57 |
| The presence of microorganisms on skin and mucous membranes | 33 | |
| A symbiotic association between host and microorganism in which the microorganism is benefitted but the host is neither helped nor harmed | 32 | |
| An organism that lives in close association with another of a different species without either harming or benefitting it | 23 | |
| A form of parasitism in which no injury is dealt to either participant by the other | 19 | |
| The ability (of a microorganism) to live on the external or internal surfaces of the body without causing disease | 48 | |
| A symbiotic relationship in which one species derives benefit and the other is unharmed | 28 | |
| Germ carrier or carrier | A person who harbors and releases pathogenic organisms without manifesting symptoms of the disease associated with the pathogen | 24 |
| A host that harbors a pathogenic organism in a commensal state | 36 | |
| Healthy individuals who harbor in their body parasitic organisms which are harmful to others | 18 | |
| Referred to as subinfection, the state whereby a microbe is intimately associated with and has its normal habitat in a certain part of the body and does no harm until special conditions arise, when it may rapidly invade the tissues and produce infection | 26 | |
| A carrier is a person, animal, or arthropod who harbors a specific infectious agent in the absence of clinical illness with or without a detectable immune response | 13 | |
| Infection | The invasion of the body tissues by microorganisms resulting in disease | 18 |
| When microparasites have passed the normal barriers of the skin or mucous membranes and have invaded and proliferated in the deeper tissues | 26 | |
| A process in which an organism enters, establishes itself, and multiplies in the host (not in others) | 32 | |
| Invasion of the body by harmful organisms (pathogens), such as bacteria, fungi, protozoa, rickettsiae, or viruses | 23 | |
| The process whereby pathogenic organisms become established and multiply in or on the body of the host | 24 | |
| The deposition, colonization, and multiplication of a microorganism in a host; usually accompanied by a host response | 13 | |
| Invasion of the body with organisms that have the potential to cause disease | 28 | |
| Infestation | Distinct from infection in that it applies specifically to animal parasites of macroscopic size, such as intestinal worms | 26 |
| Infectious disease | The result of parasitism in which no mutual adaptation has taken place and in which the invasion of the host by the parasite is marked by a struggle, the local and systemic manifestations of which constitute the disease | 56 |
| The abnormal state resulting from the deleterious local and general interaction between a host and an invading parasite, with consequent tissue changes and symptoms | 26 | |
| The manifestations of the fight between the disease-producing or pathogenic organisms and the host with all its defense mechanisms | 33 | |
| Infection that becomes apparent | 32 | |
| Mutualism | A relation between two dissimilar organisms in which both are benefitted | 32 |
| Commensalism in which the relationship is mutually beneficial | 19 | |
| Opportunist or opportunistic | Microbes which cause no overt clinical or pathological conditions in the normal state but can become invasive when the defenses are disturbed | 37 |
| Pathogens which attack persons with compromised immune function | 35 | |
| These infections represent the colonization of normally sterile tissues by bacteria from tissues that always support autochthonous populations, and because these autochthonous organisms are well adapted for survival on other tissue surfaces of the same animal, their control and clearance poses a whole spectrum of unique problems | 8 | |
| Pathogen not able to cause disease in healthy hosts but only in those with impaired defense mechanisms | 1 | |
| Normally harmless organisms which take the opportunity afforded by lowered host resistance to act as pathogens | 48 |
Not a complete list. Definitions are representative of the variable definitions used for these terms encountered in the literature.
In most cases the definition was taken verbatim from the source stated. However, the wording of some sources was modified to construct a definition based on the meaning implied as understood by the authors.
In summary, practically all past treatises on the subject of host-microbe interaction made some distinction between colonization, infection, and disease. However, the basis for the definitions of these terms was embedded in examples of specific microbes rather than in a general framework. The lack of such a framework has led to some ambiguity in the meaning of these terms.
IMPACT OF CHANGING SPECTRUM OF INFECTIOUS DISEASES ON LEXICON
Though many historical definitions (Table 1) are adequate when considered in the context of specific microbes and diseases, most definitions do not account for the varied outcomes of the host-microbe interaction. Early definitions were formulated following the rapid acquisition of new knowledge in the late nineteenth century. At that time, the distinction between pathogenic, nonpathogenic, and commensal organisms may have been more clear-cut, since the majority of human hosts probably had what would be considered normal immunity today, as those with immune impairment were unlikely to survive childhood. The adjective classical has been applied to pathogens that were major causes of infectious disease in the past (54). However, the introduction of sanitation, serum therapy, vaccination, and then effective antimicrobial therapy reduced the prevalence of and mortality from classical pathogens, though such microbes remain a major health problem in underdeveloped regions. By the 1950s, neoplasia and inflammatory and degenerative diseases, rather than infectious diseases, were thought to be the major medical problems in industrialized nations. However, the development of corticosteroid and cytotoxic therapies, organ transplantation, invasive surgeries, and ultimately the catastrophe of the HIV epidemic, produced a new population of human hosts with impaired immune systems (27, 51) that were vulnerable to infections with various microbes previously thought to be nonpathogenic (2).
A major change in the prevalence of certain pathogens occurred in the twentieth century. This was exemplified by the shift in the etiologic agents of bloodstream infections from gram-positive to gram-negative microbes in the early part of the century, and then back again to gram-positive and fungal microbes toward the end of the century (11, 14). The primary cause of these shifts was antibiotic selection. Since multiple microbes may be present in the hospitalized setting, infection represents an outcome of selective pressures in the context of the host-microbe relationship. Unexpected outcomes of the host-microbe relationship are best illustrated by the increased prevalence of unusual infections in individuals with advanced HIV infection. For example, by the middle of the first decade of the HIV epidemic in the early 1990s, Cryptococcus neoformans was the most frequent cause of meningitis in New York City, when the more than 1,000 cases of cryptococcal meningitis (9) outnumbered the 285 cases of meningitis caused by all bacterial pathogens (29). This reflects the influence of the HIV epidemic upon the spectrum of infectious diseases in a population as well as the impact of the introduction of an effective vaccine against an important pathogen, namely, Haemophilus influenzae type b.
The recognition that microbes thought to be nonpathogens caused disease in certain hosts challenged the definitions of saprophyte and commensal. As a result, additional terms were added to the lexicon in an attempt to find terminology that could accommodate the new medical and scientific findings (Table 1). In addition to the terms listed in Table 1, other terms and adjectives used to describe microbes and their interactions with the host in the literature include pure saprophytes (57), pure parasite (57), half parasite (57), classical (54), persister (31), nosocomial (1), iatrogenic (1), convalescent carrier (48), precocious carrier (48), chronic carrier (48), contact carrier (48), symptomless carrier (48), and emerging and reemerging (43). For example, an opportunistic microorganism has been defined as “one that utilizes the opportunity offered by weakened defense mechanisms to inflict damage to the host” but does not exclude pathogenicity for a normal host when a large inoculum or specific virulence factors can overcome normal defenses (51). This definition was so broad that, depending on the clinical situation, it could also include Streptococcus pneumoniae, S. aureus, and Streptococcus pyogenes, which also cause disease in normal individuals (51). In this regard, it has been noted that if invasion and disease require a breakdown in normal defenses, then all infectious agents can actually be considered opportunistic (27). These definitions illustrate that although the concept of opportunism has been extremely important for our understanding of the host-microbe relationship in the setting of immune impairment, the term opportunistic does not convey a universal meaning and its use should probably be abandoned.
INADEQUACY OF LEXICON IN CLINICAL PRACTICE
Microbes capable of causing disease are routinely cultured from patients, and the decision to administer antimicrobial therapy often depends on whether the microbe is judged to be a pathogen or colonizer. The medical literature contains different conclusions regarding the implications of recovering certain microorganisms from patients. For example, some bacterial commensals of the vagina have been associated with neonatal pneumonia, demonstrating that the same organism can be a commensal in one host and a pathogen in another (10). The recovery of C. albicans from multiple sites presents the vexing question of whether it is a reflection of colonization or infection. Similarly, it is often very difficult to distinguish between colonization and infection when gram-negative microbes, such as Pseudomonas spp., are recovered from individuals on ventilator support in intensive care units. In patients with chronic obstructive lung disease, a variety of well-recognized bacterial pathogens can be continuously isolated from the lower airways, even between disease exacerbations (50). Concern that colonization can lead to higher rates of clinical disease and transmission to others underlies the practice of administering antimicrobial prophylaxes to contacts of individuals with N. meningitidis and S. pyogenes infections (47).
In clinical medicine, the presence of an infection generally constitutes a requirement for therapy. In the absence of objective data indicative of infection, treatment of colonization is usually avoided due to its cost and the risk of adverse reactions and of induction of microbial resistance. The standard definitions of infection and colonization are not helpful clinically in determining the significance of the isolation of certain microbes from wounds and body sites that are normally sterile (49). At present, efforts to establish guidelines for the treatment of states of microbial colonization in the hospital setting are limited by an inadequate understanding of the pathogenesis of colonization and the aspects of the host-microbe relationship that influence the development of infection after colonization. This has probably fostered empiricism in the management of infectious diseases that may have significant deleterious consequences for patient care and increase the emergence of resistant strains (6).
USE OF DAMAGE FRAMEWORK TO CLARIFY LEXICON
Changes in the epidemiology of infectious diseases, including an increased prevalence of emerging and nosocomial infections, increased numbers of hosts with immune impairment, and new basic scientific information about microbial pathogenesis, have rendered parts of the lexicon inadequate and in need of revision. The absence of a unified framework to serve as a theoretical foundation for the lexicon has resulted in the persistent use of terms that emphasize differences between microbes and specific microbial attributes at the expense of common themes. A major consequence has been fragmentation in the field, such that the disciplines of bacteriology, mycology, parasitology, and virology are increasingly insular, despite the fact that all study similar questions. In our view, this problem may be ameliorated by an integrated theory of microbial pathogenesis that considers the contributions of both host and pathogen in this process. Our first approach to this conundrum was to introduce the concept that host damage is the relevant outcome in host-microbe interactions and to propose a pathogen classification scheme based on the ability of a microbe to cause damage as a function of the host's immune response (7). That framework grouped microbes based on their ability to inflict damage as a function of the host response, irrespective of their phylogenetic derivation. By focusing on damage instead of pathogen or host, common themes in microbial pathogenesis became more apparent. The same framework can be used to clarify the lexicon of microbial pathogenesis.
INTEGRATED LEXICON TO DESCRIBE HOST-PATHOGEN INTERACTION
We propose that the outcome of host-pathogen interaction is determined by the nature of the damage that results from the host-microbe relationship (Fig. 1). This concept is based upon three assumptions. First, the acquisition of a microbe by a host can result in some form of damage to host tissues that elicits a microbe-specific immune response (7). Second, except for congenitally acquired microbes, mammalian hosts are born without a significant microbial burden and the initial acquisition of microbial flora therefore results from infection. Third, the amount and type of damage that occurs during the initial and/or ongoing host-microbe relationship determines the outcome of the host-microbe relationship. This view is consistent with the danger hypothesis proposed by Matzinger (30) and others (22) that immune responses arise from the detection of danger signals produced by microbes or infected tissues. Thus, we propose that host damage is often a requirement for the induction of a pathogen-specific immune response and that the constancy, type, and magnitude of damage that ensues should form the basis of the lexicon of microbial pathogenesis.
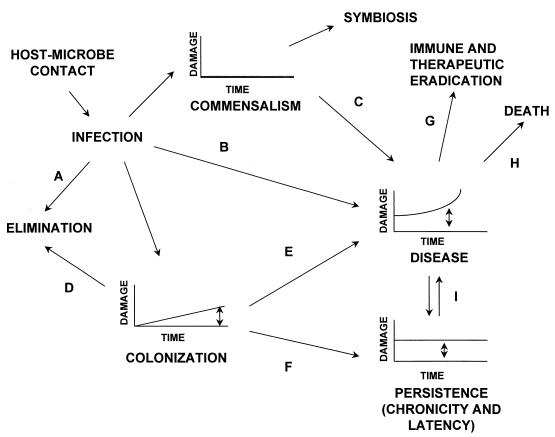
FIG. 1.
Outcome of the host-microbe interaction in the context of the damage framework proposed in reference 7. The various states shown in this figure are defined in Table 2. Double-headed arrows indicate conditions where there may be variable amounts of damage. Single-headed arrows indicate the following. A, acquisition of a microbe can be followed by elimination through physical defenses or immune mechanisms. B, acquisition of certain microbes results in damage and disease in certain hosts. C, certain commensal microbes can cause disease if the state of commensalism is disturbed by immune impairment or alterations of the host microbial flora (e.g., C. albicans can cause pharyngeal candidiasis and vaginal candidiasis in the settings of immune suppression and antibiotic use, respectively). D, the state of colonization may be terminated by an immune response (e.g., transient nasopharyngeal carriage of N. meningiditis or S. pneumoniae). The trigger for the immune response is not well understood, but may occur after the damage threshold is reached. E, the state of colonization may lead to disease if sufficient damage ensues from the interaction. The damage may be host-mediated, pathogen-mediated, or both. F, the state of colonization may lead to a state of persistence (chronicity and latency), whereby the immune response is unable to eradicate the infection despite continued damage (e.g., latent M. tuberculosis or Histoplasma capsulatum infection in tissue granuloma). G, an immune response or therapy may eradicate the infection but this does not always terminate disease, because the damage may be irreversible (e.g., poliomyelitis) or continue through immunological mechanisms (e.g., reactive arthropathies). H, if sufficient damage is incurred as a result of the host-microbe interaction, death ensues. I, persistent infections may reactivate and cause overt disease (e.g., reactivation tuberculosis).
Infection.
The word infection is from the Latin word infiere, which means to dye, stain, corrupt, or spoil and has been associated with disease since antiquity (17, 40). Ancient usage of this term includes the idea that disease was caused by invisible agents that entered the body (17). However, whereas some authors consider an infection to be the outcome of a tissue invasion (26, 49), others have used the word to include initial contact between parasite and host (42, 55, 56) and others have defined the term to include disease causation (4, 15).
We propose to define infection as the acquisition of a microbe by a host (Table 2; Fig. 1). Eradication of the microbe by the host can occur at first contact, thus bypassing infection, by nonimmune (e.g., mechanical) mechanisms and subsequently by immunologic mechanisms. Successful immune responses and/or antimicrobial therapy reduces the amount of continuing damage caused by the microbe to a level that is insignificant. Notably, eradication of a microbe may not eliminate clinical disease, since immunological damage to the host may persist following a successful antipathogen response, e.g., rheumatic heart disease following streptococcal pharyngitis and reactive arthritis following gastrointestinal infection with certain bacteria. The definition of infection proposed here avoids the confusion surrounding the synonymous use of infection and disease and is consistent with several of the definitions listed in Table 1 and the ancient origins of the word (see above). Furthermore, the concept that clinical disease is associated with damage and the induction of an immune response is consistent with the historical concept of immunologic proof of causation and applies to different types of microbes (12) and to the currently accepted paradigm that immune cells must interact directly with microbes or their antigens to produce an antigen-specific response.
TABLE 2.
Proposed revisions to terminology of microbial pathogenesis in the context of the damage framework
| Term | Revised definition |
|---|---|
| Carrier state | Synonymous with colonization |
| Chronicity | Synonymous with persistence |
| Colonization | A state of infection that results in a continuum of damage from none to great, with the latter leading to the induction of host responses that could eliminate or retain the microbe, or progress to chronicity or disease; for organisms that induce no damage during infection this state is indistinguishable from commensalism |
| Commensal | Microbe that induces either no damage or clinically inapparent damage after primary infection; a state that is thought to be established early in life |
| Commensalism | A state of infection that results in either no damage or clinically inapparent damage to the host, though it can elicit an immune response |
| Damage | The interruption of normal tissue structure and/or function of the host that applies at the cellular, tissue, and organ levels (necrosis, apoptosis, mutation, synaptic blockage, and malignant transformation are examples of damage at the cellular level; granulomatous inflammation, fibrosis, tumor are examples of damage at the tissue level; Ductal obstruction is an example of damage at the organ level); the presence of a microbe-specific immune response may be indicative of a heretofore unrecognized manifestation of damage |
| Elimination | Removal of the microbe from the boundaries of the host by either physical factors, interference by host flora, an immune response, or therapy |
| Infection | Acquisition of a microbe by host; most infections are followed by multiplication of the microbe in the host, but this is not universal because some helminth infections can involve a single organism that does not replicate in the host |
| Infectious disease | The clinical manifestation of damage that results from a host-microbe interaction |
| Latency | Synonymous with persistence, this term is often used to describe infections that are asymptomatic over long periods of time but can evolve into overt disease |
| Persistence | A state of infection in which the host response does not eliminate the microbe, resulting in continued damage over time; persistence may evolve into overt disease, depending on the balance of the host-microbe interaction (Fig. 1) |
| Pathogen | A microbe capable of causing host damage (as defined in reference 7) |
| Symbiosis and mutualism | A state of infection whereby both the host and the microbe benefit as a consequence of infection |
Commensalism.
The word commensal (from the Latin roots com meaning with, mensa meaning table, and al meaning pertaining to) can be translated “eating at the same table” (21). Our suggested revision to the definition of commensal is in the spirit of the original meaning of the word. Commensalism is defined as a host-microbial interaction that does not result in perceptible, ongoing, and/or persistent host damage (Fig. 1; Table 2). However, it is notable that this may not be absolute, since the initial acquisition of commensal organisms may elicit damage in some hosts. For example, Escherichia coli is acquired shortly after birth and this encounter places some infants at risk for E. coli meningitis during the first month of life (reviewed in reference 46). Similarly, symptomatic infections with C. albicans can occur in the first year of life. Microbes that establish themselves early in life encounter an immature immune system that may be unable to mount effective responses. The latter raises the possibility that immunologic immaturity may facilitate the establishment of a commensal state, but more work is needed to examine the validity of this concept. Nevertheless, the microbes that comprise the normal microflora of the oral, respiratory, and gastrointestinal tracts provide important stimuli for the development of immunity. Commensal microbes are antigenic and can elicit antibody responses (reviewed in reference 46). It is not known whether these immune responses reflect the occurrence of an unidentified form of damage to the host. Commensals also synthesize metabolites that are essential nutrients for the host (reviewed in reference 46) and protect the host by physically and metabolically preventing the acquisition and establishment of more pathogenic microorganisms. In view of the fact that the endogenous microbial flora plays a protective role against more-pathogenic microbes, conditions that compromise the viability of this flora may damage the host, as evidenced by the observation that disruption of endogenous microflora by antimicrobials predisposes to certain infectious diseases. Hence, the endogenous microflora, collectively called commensals, is acquired by infection, stimulates the immune system upon acquisition, and plays a beneficial role throughout life. Interestingly, it may be difficult to distinguish host and microbe, as illustrated by the fact that the DNA of endogenous retroviruses comprises 1% of the human genome (45). In fact, upon noting that there are 1013 cells in the human body and 1014 to 1015 individual microbes, Isenberg profoundly asked who parasitizes whom (20).
Colonization, persistence, and disease.
The word disease derives from Old French and originally implied a departure from normal or easy living (40). Here, we define disease as a clinical manifestation of damage that results from host-microbe interaction. Colonization is defined as a state in which the microbe may be present in the host for a variable duration of time. In a setting in which the level of damage is insignificant, there may be no distinction between commensalism and colonization. However, in keeping with historical definitions, the establishment of the commensal state generally occurs early in life. When the damage associated with prolonged states of colonization induces a new state in the host, e.g., a granuloma following infection with Mycobacterium tuberculosis, the outcome is persistence (Fig. 1). Thus, commensalism, colonization, and persistence are separate outcomes of infection, but they are potentially linked and continuous based upon the induction of damage. Therefore, the damage experienced by the host during colonization is part of a continuum which spans from none, as with that induced by a commensal, to significant, as with that induced by a pathogen (Fig. 1). Colonization is a state characterized by microbial replication that may induce host damage and trigger a microbe-specific immune response which in turn could eradicate or contain the microbe. If the host immune response, antimicrobial therapy, and/or vaccination succeed in eradicating the microbe, the state of colonization is eliminated. If the microbe is not eliminated, a state of persistence may ensue. Progressive damage that results from this state may lead to disease and death. Thus, colonization and its modified versions, namely, persistence and disease, represent the continued presence of a microbe(s) in the host with a variable degree of, but continued, host damage.
SYNTHESIS OF REVISED LEXICON
We propose that the outcomes of infection represent a continuum and that the occurrence of one versus another is the result of an interplay between host and microbial factors for a particular microbe in a particular host. This interplay permits some microbes to be commensals in some hosts but to cause disease in others. Therefore, as discussed in our previous review, the attribute of microbial virulence and the distinction between pathogens and nonpathogens are critically dependent on host factors (7). Here, we reason that the outcome of an infection with an organism is also a function of host-microbe interaction. For example, C. albicans is a commensal in normal individuals with intact endogenous flora but a pathogen in some immunosuppressed patients (e.g., those with an immune disturbance), those receiving antibiotics (e.g., those with a microflora disturbance), and neonates who have immature gastrointestinal tracts and have not yet established their endogenous microbial flora (e.g., immature hosts). Consistent with this view, it has been proposed that immunologic function determines whether contact with C. albicans results in clearance, colonization, or candidiasis (5).
Notably, the proposed definitions in Table 2 are consistent with most, if not all, of the many definitions already found in the literature (Table 1), e.g., our definition of commensal is similar to that of White and Timbury (53), and the word damage is already part of many definitions (Table 1). This review represents an effort to integrate these terms into a unified framework based on the damage-response hypothesis put forth previously (7) to produce a simplified lexicon centered upon the outcome of host-microbe interaction rather than on distinctions between pathogens and nonpathogens. This approach avoids the current problem of definitions that are dependent upon specific microbial or host characteristics and often require qualification because of the inherent variability among the participants in the host-microbe interaction. Thus, an advantage of the proposed lexicon may be reduced ambiguity.
CONCLUSIONS
This and our previous article (7) arose directly from our experiences teaching microbial pathogenesis to graduate and medical students. We have found it difficult to teach basic concepts of microbial pathogenesis because the terminology is not based on an integrated framework that can be used to organize the available information or easily accommodate new information. We hope that the proposed modifications to the lexicon will stimulate discussion and experimentation to support or refute existing concepts. Experimental validation for the damage framework may require the development of more sensitive assays to measure host damage. Current measures of damage that rely on mortality, tissue destruction, or clinical disease may be too insensitive to characterize host-pathogen interactions that lead to colonization or chronicity. Given the daunting complexity of the host-microbe relationship, our suggestions should be considered part of a work in progress that will undoubtedly require additional modifications as more information becomes available. Our proposal that damage be used to characterize host-microbe interaction provides a flexible framework to bring order and predictability to the lexicon, since damage is a relevant and potentially quantifiable outcome that can serve as the common denominator for analysis of the outcome of host-pathogen interactions.
ACKNOWLEDGMENTS
A.C. is supported by NIH awards AI33774, AI3342, and HL-59842-01 and is a recipient of a Burroughs Wellcome Development Therapeutics Award. L.P. is supported by NIH grant AI35370.
We are grateful to Stephanie Tucker, Bettina Fries, Marta Feldmesser, and Joshua Nosanchuk for critical reading of this manuscript and many helpful suggestions.
Both authors contributed equally to this work.
REFERENCES
- 1.Albini B, van Oss C J. Microbial pathogenicity and host-parasite relationships. In: Milgrom F, Flanagan T D, editors. Medical microbiology. Ann Arbor, Mich: Books on Demand; 1982. pp. 81–106. [Google Scholar]
- 2.Armstrong D. History of opportunistic infection in the immunocompromised host. Clin Infect Dis. 1993;17(Suppl. 2):S318–S321. doi: 10.1093/clinids/17.supplement_2.s318. [DOI] [PubMed] [Google Scholar]
- 3.Blaser M J. Ecology of Helicobacter pylori in the human stomach. J Clin Investig. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnet E. Microbes & toxins. New York, N.Y: G.P. Putnam's Sons; 1912. Pathogenic microbes—infection; pp. 107–129. [Google Scholar]
- 5.Cannon R D, Holmes A R, Mason A B, Monk B C. Oral Candida: clearance, colonization, or candidiasis? J Dent Res. 1995;74:1152–1161. doi: 10.1177/00220345950740050301. [DOI] [PubMed] [Google Scholar]
- 6.Casadevall A. Crisis in infectious diseases: time for a new paradigm? Clin Infect Dis. 1996;23:790–794. doi: 10.1093/clinids/23.4.790. [DOI] [PubMed] [Google Scholar]
- 7.Casadevall A, Pirofski L. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect Immun. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng K-J, Irvin R T, Costerton J W. Autochthonous and pathogenic colonization of animal tissues by bacteria. Can J Microbiol. 1981;27:461–490. doi: 10.1139/m81-071. [DOI] [PubMed] [Google Scholar]
- 9.Currie B P, Casadevall A. Estimation of the prevalence of cryptococcal infection among HIV infected individuals in New York City. Clin Infect Dis. 1994;19:1029–1033. doi: 10.1093/clinids/19.6.1029. [DOI] [PubMed] [Google Scholar]
- 10.Davies P A. Pathogen or commensal? Arch Dis Child. 1980;55:169–170. doi: 10.1136/adc.55.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edmond M B, Wallace S E, McClish D K, Pfaller M A, Jones R N, Wenzel R P. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–244. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 12.Evans A S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Sci. 1976;49:175–195. [PMC free article] [PubMed] [Google Scholar]
- 13.Evans A S. Epidemiological concepts. In: Evans A S, Brachman P S, editors. Bacterial infections of humans. New York, N.Y: Plenum Medical Book Company; 1998. p. 3-64. [Google Scholar]
- 14.Finland M. Changing ecology of bacterial infections as related to antimicrobial therapy. J Infect Dis. 1970;122:419–431. doi: 10.1093/infdis/122.5.419. [DOI] [PubMed] [Google Scholar]
- 15.Ford W W. Text-book of bacteriology. W. B. Philadelphia, Pa: Saunders Company; 1927. Principles of infection. General properties of infectious agents; pp. 826–844. [Google Scholar]
- 16.Gentle T A, Warnock D W, Eden O B. Prevalence of oral colonization with Candida albicans and anti-C. albicans IgA in the saliva of normal children and children with acute lymphoblastic leukaemia. Mycopathologia. 1984;87:111–114. doi: 10.1007/BF00436638. [DOI] [PubMed] [Google Scholar]
- 17.Haubrich W S. Medical meanings. A glossary of word origins. San Diego, Calif: Harcourt Brace Jovanovich; 1984. [Google Scholar]
- 18.Henrici A T. The biology of bacteria. D.C. Boston, Mass: Heath and Co.; 1934. Infection; pp. 230–241. [Google Scholar]
- 19.Hoeprich P D. Host-parasite relationships and the pathogenesis of infectious disease. In: Hoeprich P D, editor. Infectious diseases. Philadelphia, Pa: Harper & Row; 1983. pp. 45–56. [Google Scholar]
- 20.Isenberg H D. Pathogenicity and virulence: another view. Clin Microbiol Rev. 1988;1:40–53. doi: 10.1128/cmr.1.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaeger E C, Page I H. A source-book of medical terms. Springfield, Ill: Charles C. Thomas; 1953. [Google Scholar]
- 22.Janeway C A, Goodnow C C, Medzhitov R. Immunological tolerance: danger—pathogen on the premises! Curr Biol. 1996;6:519–522. doi: 10.1016/s0960-9822(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 23.John Wiley & Sons. Urdang dictionary of current medical terms. New York, N.Y: John Wiley & Sons; 1981. [Google Scholar]
- 24.John Wiley & Sons. International dictionary of medicine and biology. New York, N.Y: John Wiley & Sons; 1986. [Google Scholar]
- 25.Karsner H T, Echer E E. The principles of immunology. J.B. Philadelphia, Pa: Lippincott Co.; 1921. Virulence of organisms; pp. 1–10. [Google Scholar]
- 26.Kolmer J A. Infection, immunity and biologic therapy. W. B. Philadelphia, Pa: Saunders Co.; 1924. Infection; pp. 58–83. [Google Scholar]
- 27.Lauter C B. Opportunistic infections. Heart Lung. 1975;5:601–606. [PubMed] [Google Scholar]
- 28.Lippincott Williams & Wilkins. Stedman's medical dictionary. Baltimore, Md: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 29.Lowe C E. Summary of reportable diseases and conditions, 1995. City Health Inf. 1995;15:1–24. [Google Scholar]
- 30.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 31.McDermott W. Microbial persistence. Yale J Biol Med. 1958;30:257–291. [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer E A. Microorganisms and human disease. New York, N.Y: Appleton-Century-Crofts; 1974. How microorganisms cause disease; pp. 53–57. [Google Scholar]
- 33.Neter E. Medical microbiology. F.A. Philadelphia, Pa: Davis Co.; 1969. [Google Scholar]
- 34.Osterholm M T, Hedberg C W, Moore K A. Epidemiologic principles. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. Philadelphia, Pa: Churchill Livingstone; 2000. pp. 156–167. [Google Scholar]
- 35.Oxford University Press. The Oxford companion to medicine. Oxford, United Kingdom: Oxford University Press; 1986. [Google Scholar]
- 36.Park W H, Williams A W. Pathogenic microorganisms. New York, N.Y: Lea & Febiger; 1917. The relationship of microorganisms to disease; pp. 137–150. [Google Scholar]
- 37.Poindexter H A, Washington T D. Microbial opportunism in clinical medicine. J Natl Med Assoc. 1974;66:284–291. [PMC free article] [PubMed] [Google Scholar]
- 38.Ritz H L, Kirkland J J, Bond G G, Warner E K, Petty G P. Association of high levels of serum antibody to staphylococcal toxic shock antigen with nasal carriage of toxic shock antigen-producing strains of Staphylococcus aureus. Infect Immun. 1984;43:954–958. doi: 10.1128/iai.43.3.954-958.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savolainen J, Kortekangas-Savolainen O, Nermes M, Viander M, Koivikko A, Kalimo K, Terho E O. IgE, IgA, and IgG responses to common yeasts in atopic patients. Allergy. 1998;53:506–512. doi: 10.1111/j.1398-9995.1998.tb04088.x. [DOI] [PubMed] [Google Scholar]
- 40.Skinner H A. The origin of medical terms. Baltimore, Md: The Williams & Wilkins Co.; 1961. [Google Scholar]
- 41.Smith H. The revival of interest in mechanisms of bacterial pathogenicity. Biol Rev. 1995;70:277–316. doi: 10.1111/j.1469-185x.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 42.Sparling P F. Bacterial virulence and pathogenesis: an overview. Rev Infect Dis. 1983;5(Suppl. 4):S637–S646. doi: 10.1093/clinids/5.supplement_4.s637. [DOI] [PubMed] [Google Scholar]
- 43.Stephens D S, Moxon E R, Adams J, Altizer S, Antonovics J, Aral S, Berkelman R, Bond E, Bull J, Cauthen G, Farley M M, Glasgow A, Glasser J W, Katner H P, Kelley S, Mittler J, Nahmias A J, Nichol S, Perrot V, Pinner R W, Schrag S, Small P, Thrall P H. Emerging and reemerging infectious diseases: a multidisciplinary perspective. Am J Med Sci. 1998;315:64–75. doi: 10.1097/00000441-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Stewart F S. Bacteriology and immunology for students of medicine. Baltimore, Md: The Williams & Wilkins Co.; 1968. Bacteria in health and disease; pp. 72–91. [Google Scholar]
- 45.Sverdlov E D. Retroviruses and primate evolution. Bioessays. 2000;22:161–171. doi: 10.1002/(SICI)1521-1878(200002)22:2<161::AID-BIES7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 46.Tannock G W. The normal microflora: an introduction. In: Tannock G W, editor. Medical importance of the normal microflora. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1999. pp. 1–23. [Google Scholar]
- 47.Tanz R R, Shulman S. Streptococcal pharyngitis: the carrier state, definition, and management. Pediatr Ann. 1998;27:281–285. doi: 10.3928/0090-4481-19980501-07. [DOI] [PubMed] [Google Scholar]
- 48.Thomas C G A. Medical microbiology. 6th ed. London, United Kingdom: Bailliere-Tindall; 1988. [Google Scholar]
- 49.Thomson P D, Smith D J. What is infection? Am J Surg. 1994;167:7S–11S. doi: 10.1016/0002-9610(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 50.van Alphen L, Jansen H M, Dankert J. Virulence factors in the colonization and persistence of bacteria in the airways. Am J Crit Care Med. 1995;151:2094–2100. doi: 10.1164/ajrccm.151.6.7767563. [DOI] [PubMed] [Google Scholar]
- 51.von Graevenitz A. The role of opportunistic bacteria in human disease. Annu Rev Microbiol. 1977;31:447–471. doi: 10.1146/annurev.mi.31.100177.002311. [DOI] [PubMed] [Google Scholar]
- 52.Weinstein L, Musher D M. Antibiotic-induced suprainfection. J Infect Dis. 1969;119:662–665. doi: 10.1093/infdis/119.6.662. [DOI] [PubMed] [Google Scholar]
- 53.White R G, Timbury M C. Parasitism: the mechanisms of pathogenicity. Philadelphia, Pa: J.B. Lippincott Co.; 1973. pp. 35–47. [Google Scholar]
- 54.Williams R E O. Benefit and mischief from commensal bacteria. J Clin Pathol. 1973;26:811–818. doi: 10.1136/jcp.26.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson G S, Miles A. Topley and Wilson's principles of bacteriology, virology and immunity. Baltimore, Md: The Williams & Wilkins Co.; 1975. The mechanisms of bacterial invasion; pp. 1273–1299. [Google Scholar]
- 56.Zinsser H. Infection and resistance. New York, N.Y: The Macmillan Company; 1914. Infection and the problem of virulence; pp. 1–27. [Google Scholar]
- 57.Zinsser H, Bayne-Jones S. A textbook of bacteriology. New York, N.Y: D. Appleton-Century Company Inc.; 1939. pp. 313–347. [Google Scholar]