Abstract
Biochar (BC) is a carbon-rich material that can be obtained by thermal decomposition of agricultural solid waste under oxygen-limited conditions. It has received increasing attention as a cost-effective sorbent to treat metal-contaminated water due to attributes such as high porosity and the presence of various functional groups. The heavy metal (HM) sorption and removal capacity of BC can be enhanced by developing novel biochar nanohybrids (BNHs) that can be produced via surface modification of BC with nanomaterials. Loading of nanomaterials on the biochar surface can improve its physicochemical properties through alterations in the functional group profile, porosity, and availability of active sites on the BC surface which can enhance the HM adsorption ability. This manuscript provides information on preparation of nano-based biochar hybrids emanating from the type of modifying agent for the removal of different HM ions from wastewaters, and the underlying mechanisms have been discussed. Further, this compilation discusses published literature depicting the influence of different processes of preparation on the physicochemical properties and adsorption capacity of nanobiochar hybrids. The potential risks of BNHs have been reviewed to effectively avoid the possible harmful impacts on the environment, and future research directions have been proposed.
1. Introduction
Increased pollution due to industrial wastewaters derived from anthropogenic and geogenic activities has become a global concern. Elimination of heavy metals (HM) (metals having a density greater than 5 g/cm3 with biological toxicity) from contaminated streams is of prime concern due to their negative impact on the growth and metabolic activities of the flora and fauna. Therefore, the HM decontamination of the wastewaters must be carried out. HM removal from industrial wastewaters could be performed through chemical precipitation, adsorption, and electrochemical and biological treatments.1,2 Among these techniques, the ease of operation and cost-effectiveness associated with the adsorption technique makes it an effective technique for the removal of HM ions.3,4 A variety of adsorbents have been evaluated for adsorption of heavy metal contaminants. However, utilization of agri-wastes for HM adsorption can be a win–win concept.
Agricultural wastes have been reported as economic and environmentally friendly substrates for the adsorption of HMs.5 About 2 billion tonnes of agricultural waste is produced every year worldwide.6 Agricultural wastes are associated with all leftover and residual products of the agricultural activities which need disposal as this waste has no direct economic value for the farmers. However, agricultural wastes can be used as soil amendment because these are rich in organic matter. Biochar, a carbon-rich solid agricultural waste, is usually produced via a thermochemical process including pyrolysis and flash carbonization of biomass.7 Applications of biochar as a soil amendment and its ability to enhance crop yield besides reduction in emission of greenhouse gases, decrease in nutrient leaching, and reduction in fertilizer requirement has caught the interest of researchers for further exploration.8−14 Highly porous structure of biochar and the presence of various functional groups on it increases its biosorption capacity. Biochar produced from sewage sludge can be used for the immobilization of Pb and Cd in contaminated calcareous soil.15 The use of biochar for the removal of HM ions from wastewaters is a useful technology for waste management, water pollution control, and carbon sequestration.16−23 Several researchers have explored biochar produced from various feedstocks such as rice husk, pinewood, wood bark, cottonwood, and sugar cane bagasse using different pyrolytic conditions for removal of HMs including cadmium (Cd), lead (Pb), nickel (Ni), arsenic (As), mercury (Hg), and chromium (Cr).3,24−27
The HM adsorption from wastewaters by biochar involves a variety of mechanisms such as electrostatic interactions between metal and the functional surface of the biochar, cation exchange between metals and alkaline metals on biochar surface, metal precipitation, and metal reduction followed by sorption, and metal complexation with functional groups and π electron-rich domains of biochar.28 The sorption mechanisms vary considerably with feedstock and the method used for biochar production and target metal. The pyrolytic methods, conditions, and feedstock type also affect the physical and chemical properties of biochar which thereby affect its metal removal capacity.23,29 Generally, high temperature pyrolysis causes the breakdown of active functional groups present on the biochar surface which leads to decrease in its sorption capacity.
Studies have shown that the adsorption capacity of pristine biochar is limited, and it is necessary to modify it to improve the adsorption performance. Enhancement of the HM adsorption capacity from wastewaters is a key factor which can affect the utilization of carbonaceous adsorbents and thermo-disposal of biomass. Improving the functionalities (surface area, pore volume, functional groups, etc.) of biochar can improve its adsorption capacity. One of the strategies to improve functional moieties involves crushing biochar into smaller particle size to yield nanobiochar.30 However, the particle size of the biochar in the published studies was reported to be much larger than 50 mm. Further, the researchers have observed that the particle size had very little effect on the adsorption of heavy metals from wastewaters and contaminated soils.31,32 Additionally, low yields of nanobiochars during production are considered as a fundamental bottleneck for their potential use as HM adsorbents.
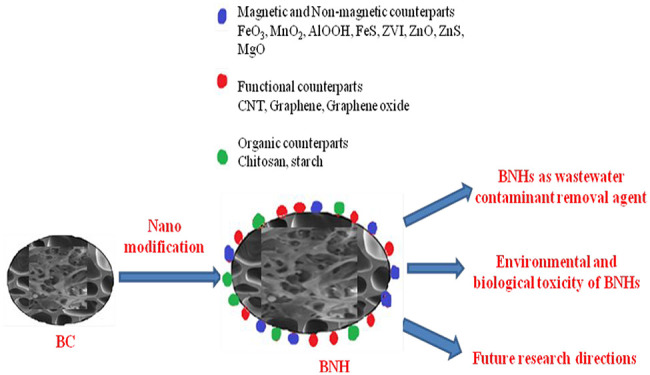
The alternative strategies that have been developed to enhance the functionalities of biochar involved BC modification. Biochar can be modified by loading it with organic functional groups, minerals such as hematite (γ-Fe2O3) and reductants; and by activating it with an alkali solution to enhance its sorption capacity. Chemically modified biochar usually exhibits enhanced adsorption capacity as compared to the raw biochar.3,33 Surface modification of biochar with nanomaterials is a promising strategy affecting its sorption capacity for contaminant removal.34,35 Nanomaterials exhibit higher surface-to-volume ratio compared to the bulk materials which help in improving functional groups, surface active sites, pore size, and catalytic degradation ability of biochar. Therefore, nanomaterials have properties that endow these materials to exhibit both chemical reduction and catalysis reactions that can help to mitigate the pollutants or contaminants, i.e., nanoremediation. A biochar-ZnS nanocomposite study involved synthesis of the composite by deposition of the ZnS nanocrystals onto biochar that led to improvement in the sorption capacity up to 368 mg/g, about 10 times higher than that of unmodified biochar.36 Manganese dioxide-loaded biochar was investigated by Zhang et al. for enhanced removal of HM ions from the aqueous solution.37
Nano-based modification of biochar represents a promising technology for expanding the environmental applications of biochar and nanomaterials to achieve integrated goals including waste management, HM removal, and carbon sequestration. The remediation of contaminated sites is not only the main target, but sustainable remediation is the ultimate desirable entity.38 Therefore, nanoremediation may be considered as a promising strategy for control of HM pollution and management using agri-waste-based nanomaterials.38
Engineered or modified biochars have already been investigated in a couple of reviews with the aim of wastewater treatment. For example, Chen et al. emphasized physical activation of BC (using nitrogen, carbon dioxide, and steam) and chemical activation methods (using acid, alkali along with oxidizing reagents) for HM removal from wastewaters.39 The concept of metal and heteroatom modified codoped BC was given by Liu et al. for organic and inorganic decontamination.40 Similarly, Weidner et al. and Zhao et al. elaborated the modification methods of BC with metal oxides for removal of organic and inorganic pollutants.41,42 No other modifying agent was discussed in their reviews. In another review article, metal sorption mechanisms based on various surface functional groups of BC and functionalization of BC via oxidation, nitrogenation, and sulfuration have been presented.43 Considering the advantages of nanotechnology in the modern era, researchers have reviewed biochar-based nanocomposites for HM decontamination of wastewaters. Ho et al. focused on coprecipitation, carbothermal reduction, and pyrolytic methods for the production of nanoscale metal-assisted BC.44 Li et al. focused on possible mechanisms of metal sorption by BC with a brief note on nanomodification of BC to enhance metal sorption.28 The preparation of nanometal oxide/hydroxide-BC, magnetic BC, functional nanoparticles coated BC for removal of HMs, other inorganic metals, and organic contamination from wastewaters has been reviewed by Tan et al.29 However, asystematic review outlining the strategies being used for the preparation of BNHs using various organic/inorganic functional components is lacking in the literature (Supplementary Table 1). Hence, this review paper aims to fill the gap of knowledge giving comprehensive information on recently published methods of preparation of biochar-assisted nanomaterials based on the type of modifying components added to BC for removal of different HM ions from the wastewaters. Various modifying agents such as metal precursors, nanoparticles, and organic and inorganic polymers have also been discussed for modification of biomass feedstock and pristine BC followed by their underlying mechanisms. The influence of different components added to biochar either during pre-treatment or post-treatment on the physicochemical properties and adsorption capacity of biochar have been identified, and the potential knowledge gaps in the existing basic and advanced technologies for contaminant removal have also been summarized. Furthermore, potential environmental risks of biochar and its nano amended forms have been illustrated.
2. Methods of Preparation of Biochar Nanohybrids (BNHs)
Considerable research efforts have been done to combine the advantages of porous biochar and unique physical properties of nanomaterials resulting in an augmented organic and inorganic contaminant removal capacity of biochar. There are two major methods to produce nano-modified biochar possessing superior functionalities equipping them to have wide range of applications; viz., (i) the first method involves the modification of biomass to be used in the preparation of BC followed by pyrolysis/calcination/coprecipitation; (ii) the second method involves the direct modification of the prepared BC (Figure 1). During these modifications, BC/biomass could undergo surface treatment with various metal precursors (metal oxides, chlorides, sulfides, sulfates, nitrates), nanoparticles and organic–inorganic polymers producing BNHs as discussed below.
Figure 1.
Different modification techniques of biomass and BC.
2.1. Modification of Biomass
Pretreatment of biomass involves modification of raw material to be used for generation of BC and then it can be subjected to pyrolysis/calcination. This method is considered to be energy efficient as it involves simultaneous pyrolysis of biomass and metal precursors.
2.1.1. Modification with Metal Oxides/Sulfides/Chlorides
The catalytic pyrolysis of biomass where biomass is impregnated by metal salts, is an interesting way to produce BNHs with improved sorption capacity. Impregnation is usually carried out with the use of aqueous solution of metal salts such as ZnSO4, MgCl2, Mg(Ac)2, AlCl3, and FeCl3.45−48 The metal ion-rich biomass when processed at high temperature during pyrolysis treatment converts these ions to form BNHs or nanocomposites. These nanocomposites exhibit improved adsorption performance because of increased surface area and high porosity features of the nano counterparts. For instance, Li et al. have reported the fabrication of nano ZnO/ZnS modified biochar by carrying out pyrolysis of zinc contaminated corn stover biomass.45 Modified biochar, evenly covered by nano ZnO/ZnS, exhibited better porous structure (BET = 397.4 m2/g and TPV = 0.43 cm3/g) compared to original biochar (BET = 102.9 m2/g and TPV = 0.20 cm3/g). Similarly, Li et al. prepared five different Mg-loaded biochars from banana straw, cassava straw, corn straw, camellia nutshells, and taro straw by mixing the biomass with MgCl2 solution followed by pyrolysis.46 The SEM analysis of the final product revealed occurrence of spherical to irregular shaped particles of Mg in the forms of Mg2(OH)3Cl·4H2O and MgO on the biochar surface providing the possibility of ion exchange and other reactions. Similarly, impregnation of MnCl2·H2O onto Loblolly pine (Pinus taeda) biochar followed by pyrolysis at 600 °C resulted in the formation of MnO-loaded biochar containing 6.7% less carbon than the pristine biochar.49 However, BET analysis revealed a doubling of the surface area due to increase in pore volume (approximately 7 times). The same technique was utilized for preparation of MgO hybrid carbonaceous composite derived from sugar cane leaf waste.50 The presence of Mg compounds on the surface of the composite was confirmed by the SEM-EDX analysis. Diffraction peaks at 2θ around 37.1° (111), 43.1° (200), 62.5° (220), 74.7° (311), and 78.6° (222), observed in XRD analysis, corresponded to MgO confirming the formation of MgO flakes. MgO hybridization improved the formation of the nanotube-like carbon sponge composite.
In addition to Zn, Mg, and Mn, some other nanometals and their precursors also play a crucial role in providing more functional biochar for a wide range of applications. For example, AlCl3 pretreated biomass of cottonwood on slow pyrolysis produced well-crystallized AlOOH/biochar possessing rough surface morphology.47 By this method the Fe2O3–biochar nanocomposite was also prepared from FeCl3-impregnated pulp and paper sludge by pyrolysis at 750 °C.51 BET surface area and porosity were lower for Fe2O3–BC than unmodified biochar which could be attributed to the blocking of pores in the biochar matrix by Fe2O3 and other metal compounds inherent in the biosorbent. By pyrolyzing the FeCl3-treated biochar, magnetic iron oxide rice husk and wheat husk hybrids were prepared.52 Rice husk biochar hybrid exhibited a higher percent carbon (38.4 wt %) than that of wheat (36.8 wt %). Both biochars had large ash contents (rice: 53.5 wt % and wheat: 50.5 wt %).
The methods discussed above involved high temperature treatment of metal salt impregnated biomass using either pyrolysis or calcination. The effect of high temperature applied during pyrolysis or calcination greatly affects the physicochemical properties and the yield of produced biochar composites. High temperature improves the physicochemical properties of the product providing high porosity, large surface area, and more active sites for enhanced metal adsorption. However, decreased char yield with an increase in temperature has been reported by Hanif et al.53 They observed that a relatively lower temperature of 400 °C was optimum for maximum (91%) production of char of combined biomass (cotton gin trash, cow manure, and microalgae: Nannochloropsis oculata). High cost and low yield of the produced biochar due to high temperature are considered the potential obscurities of pyrolysis and calcination methods. Coprecipitation is a facile and convenient approach whereby impurities precipitate out from a solution via an agent at less than 100 °C. Coprecipitation of biomass and metal salts is considered as a low-cost alternative to pyrolysis. This process is widely used by researchers for the preparation of nanoparticles because it is simple, economic, and eco-sustainable; exhibits high yield and purity; is easily reproducible; and involves no use of solvents.54 This method also ensures the formation of uniform-sized nanoparticles.55 Therefore, it can be generalized that the morphological properties of the obtained char particles and composition are highly dependent on temperature and pH.56
Coprecipitation of biomass and metal salts is a low temperature technique for the modification of the char. Gupta et al. developed a method for successful surface modification of the orange peel powder (OPP) by coprecipitating it with FeCl3·6H2O and FeSO4·7H2O resulting in the fabrication of a novel magnetic nano adsorbent Fe3O4–OPP (MNP–OPP).57 Carboxyl groups of OPP played a role in forming a covalent bond with hydroxyl groups of MNP. The MNP–OPP had morphology and particle size similar to those of MNP but exhibited a much smoother surface topography. Shifting of characteristic peaks of MNP at 3434 cm–1 (−OH stretch) and 575 cm–1 (Fe–O group) to 3413 and 578 cm–1, respectively, in MNP–OPP spectra indicated the interaction of the hydroxyl groups and metal oxide during the synthesis process.
2.1.2. Modification with Nanoparticles
Functionalized nanoparticles such as CNT because of their fine-grained nature and large surface area are finding their ways in improving adsorption characteristics of BC. Inyang et al. utilized CNT suspension prepared via ultrasonication for the preparation of CNT–biochar nanocomposite.27 Milled hickory chips and sugar cane bagasse biomass were stirred separately, in CNT suspension followed by pyrolysis to obtain CNT–biochar nanocomposites.
2.2. Modification of BC
Biochar is the residue of incomplete organic pyrolysis. Pyrolysis involves thermal decomposition of organic materials at high temperatures in an inert atmosphere. Based on the operating thermal conditions, pyrolysis can be differentiated as conventional, fast, and flash pyrolysis.54 Biochar, produced by high temperature treatment, has high porosity and large surface area which can load functional materials or metals on its surface in an efficient, accurate, and effective manner to produce BNHs. Hybridization of BC and loaded functional components provide in BNHs provides new and unique characteristics to the synthesized BNH showing advantages of both the components.
2.2.1. Modification with Metal Oxides/Sulfate/Nitrates
Improving the surface chemistry of BC by modification with metal precursors is the most common strategy to obtain BNHs. Incorporation of metallic species to carbon matrix of BC enhances surface properties of BC and facilitates its catalytic and magnetic activity to recover the adsorbent material.58 Generally, the inorganic counterpart is allowed to adhere to BC surface in aqueous medium. Loading of various inorganic oxides on biochar obtained from different feedstocks, using a coprecipitation technique, has been reported by many researchers. Enhanced porosity of MnO2-biochar nanocomposite with pore volume about 3 times smaller than that of the BC and 1.5 times smaller than that of nano MnO2 could be achieved by ethanol mediated reduction of KMnO4 in a biochar suspension through the coprecipitation process.59 Results indicated that the surface of the NMBCs was covered with nanospheres of MnO2 (size: 30 nm). Similarly, loading of MnO2 as tangled MnO2 nanosheets covering the whole surface of the water-hyacinth BC was reported in the literature.37 In another study, FeSO4 was used to produce magnetic nanobiochar coated with well dispersed zerovalent iron through coprecipitation technique in the presence of NaBH4 solution as reducing agent.60 The FTIR peaks exhibiting absorption bands at about 680 cm–1 confirmed the formation of Fe-biochar bonds.
In recent years, researchers have focused on the calcination of biomass to produce biochar and its composites. Calcination refers to the heating of inorganic materials which increases crystallinity of the material and removes any impurities and volatile components present on the surface. Preparation of sugar cane bagasse-Fe3O4 composite was successfully carried out by coprecipitation of bagasse residue with FeSO4·7H2O and FeCl3·6H2O in the presence of ammonia followed by calcination.61 The TEM analysis of the product revealed occurrence of spherical or rod-shaped structures of the modified bagasse. The interaction of bagasse with Fe–OH bond in their hybrid was confirmed by shifting the peak at 897 cm–1 in the bagasse to 874.6 cm–1. Fe–O vibration bands also appeared in the IR spectrum of bagasse-Fe3O4 composite. Metal nitrates ((Fe(NO3)3·9H2O, Zn(NO3)2·6H2O, and Ni(NO3)2·6H2O)) and calcined ash of Acacia nilotica seed shells in aqueous solution produced a sol–gel of magnetic nanoparticles of Ni0.5Zn0.5Fe2O4 which underwent calcination to yield BNHs.52
2.2.2. Modification with Organic–Inorganic Polymers
Development of BNHs involves another modification method by impregnating the biochar with some organic or inorganic polymers which impregnate the BC into an efficient composite by improving its porosity and interface chemistry with heavy metal pollutants. However, information on organic–inorganic modification of BC is lacking in the literature. Arabyarmohammadi et al. reported the synthesis of chitosan-clay modified biochar nanobiocomposite of bark chips by stirring aqueous solution of biochar and chitosan-clay containing acetic acid (2% v/v).62 The researchers chose chitosan as it is potentially biocompatible, environmentally friendly, and suitable for preparation of biocomposite.63 The nanoclay, due to the presence of exchangeable hydrated cations in a layered structure, is widely used as additive materials to improve various physical and adsorption properties of polymers like chitosan.64 The presence of acetamido and amino groups enabled chitosan to chelate the metal ions.65
2.2.3. Multiple Cation Modification of Prepared BNHs
Though BNHs exhibited enhanced adsorption characteristics, yet it is expected that a multiple cation salt can cause adsorption enhancement by providing dual adsorption mechanism for biochar. Biochar supported Ag/Fe nanoparticles had been reported by Wu et al.66 Nano zerovalent iron (nZVI)-biochar obtained from biochar and FeSO4·7H2O in the presence of NaBH4 was treated with AgNO3 under severe stirring to produce a discontinuous layer of Ag on the surface of nZVI/biochar. FTIR peaks at 565 and 616 cm–1 referred to the Fe–O stretching and vibration, respectively, for Fe2O3. Similarly, Fe0@NH2-biochar could be fabricated from Fe0-biochar produced by coprecipitation of FeCl3 solution and biochar in the presence of NaBH4.67
3. HM Sorption by BNHs from Wastewaters
Removal of heavy metals from wastewaters for its decontamination by BNHs is one of the primary research interests. The recent development of BNHs and their adsorption characteristics are represented in Table 1. Different adsorption mechanisms of heavy metals on the surface of BNHs are given in Figure 2. Various agricultural waste/residues have been explored as excellent nano hybrid adsorbents for HMs which is discussed below:
Table 1. Modification and Adsorption Characteristics of BNHs.
| Feedstock Type | Modification method | Feedstock | Modification | Particle size of BNH | Heavy metal removed | Sorption capacity | Adsorption model | Mechanism | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Food crops | Modification of biomass | Cottonwood | AlOOH | thicknesses = 30 nm lengths and widths = 100 nm | As (V) | 17410 mg/kg | Freundlich first and second order | Not available | (47) |
| Corn stover | ZnO/ZnS | 21.25–34.3 nm | to Pb (II), Cu (II), and Cr (VI) | 135.8, 91.2, and 24.5 mg/g (2.14, 3.37, and 1.60 times than BC) | Freundlich pseudo-second order | Cation exchange | (59) | ||
| Sugar cane leafy trash | MgO | 8.63 nm | As(V) and Pb(II) | 157 and 103 mg/g | Langmuir pseudo second order | C π–π* transition | (50) | ||
| Straws of banana Cassava, corn, camellia nut shells and taro | Mg2+ | 7.05–23.89 nm | Cd(II), Cu(II), and Pb(II) | 333.33, 370.37, and 302.58 mg g–1 | Langmuir pseudo second order | Complex precipitation, cation exchange, and cation-π play | (46) | ||
| Rice and wheat husk | Fe3O4 | 2–20 nm | Arsenite [As(III)] | 111 μg/g | Radke and Prausnitz pseudo second order | Complex formation | (53) | ||
| Modification of BC | Not available | zerovalent iron | Not available | Cd(II), Co(II), Zn(II), and Pb(II) ions | Not available | Langmuir Pseudo second order | Not available | (75) | |
| Wheat straw | FeS-CMC | <100 nm | Cr(VI) | 130.57 mg/g, 38.6 mg/g for FeS and 25.4 mg/g for biochar | Redlich-Peterson pseudo second order | Complex formation | (69) | ||
| Corn stalk | Nano-MnO2 | 30 nm | Cu(II) | 142.02 mg/g | Langmuir, Freundlich second order | metal–ligand complexes formation | (37) | ||
| Sugar cane bagasse | Fe3O4 | 50–200 nm | Cr6+ | 47.62 mg Cr 61/mg M-NIOB | Langmuir pseudo first and second | Pore diffusion | (61) | ||
| Other crops | Modification of biomass | Orange peel | Fe3O4 nanoparticles | Not available | Cd2+ | 82% | Langmuir second order | Complexation and ion exchange | (57) |
| Acacia Nilotica seed shell | Ni0.5Zn0.5Fe2O4 Nanoparticles | <100 nm | Pb(II) | 94.8% | Langmuir second order | Not available | (52) | ||
| Modification of BC | Invasive water hyacinth | MnO2 | 2–4 nm | Cd(II), Cu(II), Zn(II), and Pb(II) | 232.5, 248.9, 239.4, and 249.2 mg/g | Langmuir-Pseudo second | inner-sphere complexation of heavy metal ions with MnO2 | (37) |
Figure 2.
Different adsorption mechanisms of heavy metals on the surface of BNHs.
3.1. HM Sorption by BNHs Prepared from Food Crop Residues
Nano-modified BC produced from food crop wastes exhibited great potential to remove HM ions from wastewater bodies. Zhou et al. studied the adsorption of Cu2+ ions from wastewater using the nano MnO2-biochar hybrid produced from corn stalk powder.59 Compared to adsorption rates of biochar (26.88 mg/g) and nano MnO2 (93.91 mg/g), higher adsorption (142.02 mg/g) was recorded for their hybrid indicating the significance of loading nano MnO2 onto the biochar surface. It was also reported that by increasing pH value from 3 to 6, adsorption capacity increased. When the pH of the solution was lower (3–6) than pHZPC, high concentrations of H+ ions competed with Cu2+ ions for the adsorbent surface. Also, high positive charge on the adsorbent system caused electrostatic repulsion between the cations, thereby reducing the adsorption capacity for both H+ and Cu2+ ions.68 The XPS spectrum exhibited binding energies at 934.7 eV corresponding to Cu 2p3/2. Most of the Cu2+ ions were observed to exist on the surface of adsorbent in the forms of CuO (47.06%), Cu(C2H3O2)2 (28.87%), and Cu(OH)2 (24.07%). The adsorption mechanism involved the formation of metal–ligand complexes as indicated by the FTIR spectrum of metal adsorbed adsorbent which showed peaks at lower wavenumbers than that present in the FTIR spectrum of the composite before adsorption, indicating the interaction between Cu2+ and O-containing groups after Cu2+ adsorption. The formation of COO–Cu was confirmed by the alteration in peak at 1504 cm–1, while peaks at 1385 and 511 cm–1 wavenumbers can be attributed to −OH deformation vibrations of hydrated MnO2 on the surface of NMBCs forming Mn–O–Cu.
Sorption mechanisms of magnetic nano Fe2O3 modified bagasse from sugar cane had been successfully discussed for the removal of Cr6+ ions from the wastewater.61 High percentage removal was observed at low concentrations of Cr6+ with the optimum time of adsorption to be 180 min. The adsorption of Cr6+ increased when the adsorbent concentration varied from 200 to 800 mg for 150 mL of metal solution (1 ppm). Further, an increase in adsorbent concentration did not considerably alter the adsorption capacity. The Cr6+ ion adsorption increased from pH 3 to 5 and did not alter at pH > 5.0. At all pH lower than pHpzc (5.8) the high positive charge on the adsorbent surface attracted more Cr6+ ions, present in the form of CrO42– ions. At pH > 5.8 the adsorption capacity of negatively charged surface decreased because of the repulsive electrostatic effect. An increase in the HM percent removal from 15.31% to 71.29% was observed as the pH of the solution varied from 3.0 to 5.0. Metal–ligand complexation can be considered the prominent mechanism behind the successful adsorption of Cr6+ ions. Another mechanistic theory involved the transportation of Cr6+ ions from the solution to the adsorbent surface where ions can diffuse into pores of the adsorbent surface. In another study, it was observed that negatively charged groups on zerovalent iron modified biochar surface attracted positively charged metal ions Cd(II), Co(II), Zn(II), and Pb(II) ions from the wastewater.60 The adsorption process was found to be exothermic physical adsorption which was favored at low temperatures. With an increase in the contact time, adsorption first increased due to the presence of a large number of active sites on the surface, and then moved down gradually as the state of equilibrium was reached. The adsorption capacity of all the metal ions was low at a pH of 2 due to the presence of H+ ions that occupied the active sites on the sorbent surface and hindered metal ion binding. However, at higher pH, an increase in adsorption was observed with the highest value obtained at pH 5.
The pH-dependent adsorption of heavy metals by Mg-loaded biochars prepared from six different straw feedstocks was studied by Li et al.48 The maximum adsorption capacities for Cd(II), Cu(II), and Pb(II) were 333.33, 370.37, and 302.58 mg/g, respectively, at a pH of 6.5. When the pH was low, poor adsorption was observed. With the increase in the pH, the adsorption of all the biochars for Cd(II), Cu(II), and Pb(II) increased quickly. After adsorption of the metals, the pH values of solutions increased due to an increase in the number of hydroxyl ions in the solution. At higher pH, the biochar surface became negatively charged enhancing the adsorption of positively charged metal ions. A large number of functional groups in biochar load a large number of Mg2+ ions forming complexes with metal ions and perform strong ion exchange which was confirmed by shifting of FTIR peaks of various groups. The increased metal content in the form of their compounds (Pb3(CO3)2(OH)2, CdCO3, Cu(OH)2·NO3, and Cu2Cl(OH)3, etc.) and decreased Mg content in the XRD pattern and SEM-EDS images confirmed the adsorption of three metal ions via ion exchange mechanisms.
Hybridization of AlOOH with biochar obtained from cottonwood resulted in better adsorption of As(V) in aqueous solution than Al2O3 adsorbent following the Langmuir model, but it was comparable to activated Al2O3.47 In another study, MgO hybridized biochar exhibited an excellent adsorption capacity of 157 mg/g and 103 mg/g for As(V) and Pb(II), respectively, obtained from the Langmuir model.50 The As(V) adsorption capacity decreased, while that of Pb(II) increased with increasing the solution pH. Formation of MgHAsO4 and Mg(H2AsO4)2 crystals on the nanocomposite confirmed the As(II) adsorption as indicated by FT-IR, XRD, and XPS analysis. The C π–π* transition involving the Pb–carboxylate interaction participated in Pb(II) adsorption. The typical peaks of H2AsO4–1 and HAsO42–, AsO43–, and Pb 4f in XPS spectra of hybrid after adsorption of metals confirmed the adsorption of two metals on a hybrid surface. The positively charged surface showed a strong affinity for As(V) ions existing in the form of negatively charged H2AsO4–1 and HAsO42– at pH 3–7. The alteration of the FT-IR peaks corresponding to O-containing groups on the adsorbent surface advocated the formation of Mg–O and Mg–OH groups. Iron also formed similar complexes with heavy metals on the adsorbent surface enhancing the adsorption capacity of the adsorbent.50 Monodentate (≡Fe–OAs(OH)2) and bidentate ((≡Fe–O)2AsOH) complexes were formed by the Fe–OH group on the biochar hybrid surface of Fe2O3 modified rice/wheat husk thereby removing arsenite As(III) from groundwater. Excellent adsorption was observed at substantially high pH range (values ranging from 3 to 10) with a maximum adsorption capacity of 111 μg/g. Further, biochar hybrid was also regenerated with an insignificant decrease in As(III) removal capacity for four consecutive rounds.
The well developed porous structure of corn stover BC during the pyrolysis process and the hydroxyl groups on the surface of nano ZnO/ZnS particles enhanced the HM adsorption capacity of hybrid nano ZnO/ZnS-BC multiple times over the unmodified BC.45 Increased numbers of adsorption sites for Pb(II), Cu(II), and Cr(VI) provided by Zn nanocomposites resulted in maximum adsorption capacities of 135.8, 91.2, and 24.5 mg/g, respectively, which were higher than the unmodified biochar (63.29, 27.05, and 15.23 mg/g, respectively). Iron(II) sulfide, an efficient, economical, and environmentally friendly reducing agent, produces quite unstable and agglomerated nanoparticles in an aqueous solution. Carboxymethyl cellulose (CMC)-stabilized FeS nanoparticles coated on the biochar surface exhibited enhanced removal efficiency of Cr(VI).69 The researchers reported chemical sorption of Cr(VI) onto adsorbent surfaces through surface pores and oxygen containing functional groups forming Cr(III)–Fe(III) complexes through ion exchange.
3.2. HM Sorption by BNHs Prepared from Other Crop Residue
Nano-modified orange peel powder was successfully utilized for the removal of toxic cadmium ions from wastewater released by the electroplating industry using Fe2O3 nanoparticles.57 Results revealed a pseudo second-order kinetics for the adsorption of the cadmium ions which was well explained by the Langmuir isotherm (R2 = 0.9). Metal complex formation and ion exchange were considered as the principal mechanisms involved in the HM sorption process. The improved adsorption could be credited to nanosized Fe3O4 particles providing large surface area as well as the presence of hydroxyl groups on the surface of modified peel powder which acted as in enhanced adsorption sites for metal binding. These hydroxyl groups resulted in the formation of Fe–OH and Fe–O–(CO)–(OH)n bonds at pH > pHpzc which exhibited electrostatic adsorption of Cd2+ and formed metal–ligand magnetic composite complexes. Further studies of desorption trials showed 98% desorption of the adsorbed Cd2+ ions inflicting reusability of the prepared BNH as an advanced adsorbent. Results revealed that adsorption capacity increased by increasing the pH with maximum adsorption capacity to range from 42.4% to 96.0% for the pH range of 2–7.
Omidvar-Hosseini and Moeinpour have reported adsorption of Pb2+ from wastewater by Acacia nilotica seed shell ash supported Ni0.5Zn0.5Fe2O4 nanoparticles.52 They have observed that the adsorption increased when pH value increased from 2 to 6 with maximum adsorption being exhibited at pH 5. The researchers have argued that this may have occurred due to the presence of positively charged Pb2+ and Pb(OH)+ ions at pH < 5.5, and neutral or negatively charged (Pb(OH)2 and Pb(OH)42–) ions at pH > 5.5.70 Adsorption capacity also exhibited contact time dependence. For a minimum of 5 min of contact, adsorption was 94.9%, while the maximum adsorption of 98.0% was observed for 20 min of contact time. The adsorption process obeyed pseudo-second-order kinetics, and data fitted well in the Langmuir isotherm model (R2 = 0.999) giving a maximum adsorption capacity of 37.6 mg/g. Though the adsorption capacity increased with the contact time, the removal percentage of Pb2+ ions decreased with increasing concentration of Pb2+ ions from 50 to 800 mg/L and maximum at 50 mg/L at pH 5. This may probably have occurred due to availability of Pb2+ ions at increased concentration, while the number of active sites on the adsorbent surface remained constant resulting in a decrease in the percent removal.
Enhanced surface area and porosity of unmodified biochar of invasive water hyacinth by the nano MnO2 counterpart improved the pH-dependent adsorption of four heavy metals (Cd(II), Cu(II), Zn(II), and Pb(II)) from the wastewater.37 HM adsorption increased with increasing MnO2 load up to 26.6% and remained almost constant from 26.6% to 30.2%. At pH > 6.5, the maximum adsorption for BC@MnO2–26.6 was 232.5, 248.9, 239.4, and 249.2 mg/g for Cd(II), Cu(II), Zn(II), and Pb(II), respectively, which decreased by decreasing pH. Heavy metal ions struggle for negatively charged adsorption sites on biochar surfaces due to the presence of natural coexisting ions (K(I), Mg(II), Na(I), and Ca(II)) in the wastewater leading to the reduced adsorption capacity of modified biochar. The higher adsorption capacity of the modified biochar was due to inner-sphere complexation of heavy metal ions with MnO2 which was justified from the extended X-ray absorption fine spectroscopy (XAFS) analysis.71 The presence of PbO 4f7/2 peaks in the XPS spectra at a binding energy of 137.4 eV demonstrated Pb(II) adsorption via O-containing groups on the biochar surface. Other peaks corresponding to CuMn2O4, CdO, and ZnO confirmed the successful adsorption of Cu(II), Cd(II), and Zn(II), respectively.
4. Environmental and Biological Toxicity of BNHs
The BC produced by thermochemical treatment of biomass in limited oxygen supply has been used for improving soil and plant health, remediation of contaminated waters and soils, reducing greenhouse gases and fertilizer demands, and as soil amendment and industrial catalyst in the past decade.72,10−14 However, the toxic effects of BC on the environment and human health remain a critically less investigated area of research and must be focused on two potential risks. Primarily, the aspects to be probed include increasing use of toxic chemicals during the production process, and the other aspect is the release of toxins from biochar based adsorbents. Incomplete or partial pyrolysis or pyrolysis performed under oxidized conditions leads to the production of a large number of toxic compounds like dioxins, polycyclic aromatic hydrocarbons (PAHs), and furans.73 However, studies have shown that pyrolysis of completely dried biomass may reduce the release of PAHs due to partial combustion of organic compounds formed during pyrolysis by moisture present in biomass.74 Several materials used in the production process or alkali/acids used for activation of adsorbent serve as corrosive pollutants causing environmental degradation. Moreover, BCs exhibit phyto- and cytotoxic properties.75−77
The potential risk of nanotoxicity of BNHs (though reduced toxicity as compared to nanoparticles alone is expected) arises due to unintended release of nano counterparts in the environment during both the production and application of BNHs which cannot be ignored. Regarding the synthesis of nanoparticles, an important element of BNHs that are being directly used for BC modification, an important aspect of green, eco-friendly, and safer chemistry has now been considered and explored. Green synthesis of nano adsorbents using several crops and their waste make a promise of a good environment to avoid the use of toxic and costly chemicals during the chemical production process. The stability of BNHs to avoid nanotoxicity is required for a safe ecosystem. Therefore, long-term studies on the physicochemical interactions of BNHs with the environment are mandatory.
Expanding BC and its nanohybrid research calls for the development of strategies to reduce the potential harm caused by them. Investigatory studies of their health and environmental risks are required for the development of safer BNHs. Gelardi et al. reported biochar-related dust emissions and potentially toxic properties and reported that the low density and high porosity of BC renders its spread in the atmosphere.78 No survey has been carried out to compare the strategies to minimize biochar-associated risks. It is necessary to critically analyze parameters for BNHs production and management strategies that safeguard human health and the environment.
5. Conclusions
BC-based nano-adsorbents (BNHs) with greater surface area and active sites, improved surface functionalities, and enhanced adsorption capacity had received great attention in the past half a decade. Considering the advantages of BNHs, various preparation methods, performance of BNHs for enhanced adsorption properties, and the corresponding adsorption mechanisms have been discussed in this review. Different surface morphology, size, adsorption capacity, and underlying mechanisms revealed that the type of biomass feedstock, doped metal precursor, and method of preparation used play a crucial role. Although pristine BC and modified BC are greatly being used for wastewater treatment, there are still some research gaps to be filled with advance research in the field.
6. Future Research Directions
The studies discussed in different sections present convincing improved HM adsorption potentials of BNHs. However, their use is still limited due to certain unresolved eco-safety issues that restrict their dissemination in open, dynamic niches and ecosystems. Further, the effects of feedstock type on morphology, chemical composition, and surface functional properties of BC are still unknown. Selection of cheaper feedstock, optimum fabrication techniques, and specific production conditions are few other critical factors that affect the properties of generated BC. Recently, researchers have started using machine learning and artificial intelligence for the optimization of adsorption variables and BC feedstock properties.79 The stability of BC, nanoparticles, and their hybrids should also be identified before the development of effective formulations. The functional properties of BC must be improved by modification with nanometal/metal oxides/metal hydroxides. As the size of the nano counterpart used for the modification of BC plays a crucial role in improving the functional attributes of biochar, it may affect the adsorption of heavy metal ions. However, the nanoparticle size optimization has to be performed which can help reduce the nanotoxicity besides exhibiting effective heavy metal removal, but the precise control of surface properties and functionalities of nano-modified BC have not been achieved yet.
There are plenty of lab-scale research reports on adsorption of individual metal ions/elements by BC/BNHs from spiked aqueous solutions. Though these reports demonstrate the potential of the BC/BNHs for HM removal, their feasible use for actual wastewater treatment is yet to be realized. The occurrence of various contaminating metals in the wastewaters may lead to reduced adsorption of one metal element relative to others due to competition between various metal ions for the adsorption sites, in actual contaminated waters. Therefore, the studies involving competitive adsorption of multiple metals must be considered to evaluate the actual adsorption behavior of the nanoadsorbents. Further, the insights from the pilot scale studies on the application of the BC nanohybrids must be obtained to identify the actual remediation efficacy of the screened formulations.
It is also desirous to consolidate the comparative and true quantitative HM adsorption potentials of the biochar nanohybrids with respect to their bulk counterparts. Therefore, the conventional techniques for the elucidation of the adsorbed or sequestered HM appear to fall short of the attributes desirous for noninvasive analysis, as the sample is consumed during the preparation protocols. All these techniques do not allow reanalysis of the samples. Contrary to the conventional techniques, the use of noninvasive and powerful 2-D, 3-D, or comprehensive multiscale simultaneous/cascade-driven separation and detection analysis techniques will allow for the reanalysis of the adsorbed contaminants and the adsorbent substrate.80 The potential noninvasive techniques can involve conjugate spectroscopy-microscopy or augmented multiple spectroscopy techniques such as hyper-spectral imaging/spectroscopy (HSI/S), excitation–emission matrix fluorescence spectroscopy (EEMFS), and 2D correlation spectroscopy (2D-COS).
Hyper-spectral spectroscopy/imaging methods include an array of remote-sensing enabled quantitative spectroscopic-microscopy analysis to obtain quick and sensitive on-site estimation of a variety of attributes such as occurrence of heavy metal in land, tailing pond water, groundwater, river water, wastewater, soil, and plant tissue.81−87 However, now HSI has transcended from remote sensing to up-close spectroscopy imaging analysis of samples in situ or ex situ in research laboratories for heavy metal contaminated soil samples.88,89 The fundamental benefit of the technique lies in its ability to perform analysis in a nondestructive manner at high sensitivity and with higher throughput which are critical to obtain accurate inferences regarding the concentration and spatial distribution of the heavy metal elements in soil matrix and water samples.85
The EEMFS has the potential to compare and identify the dynamics and transformations of a chromophore in three dimensions with high sensitivity and without degradation of the test sample.80 It can be utilized to discern the effect of heavy metal contamination in the soil and/or water sample. The organic matter components such as humic and fulvic acids, short peptides (amino acids-tryptophan, tyrosine containing peptides), and other compounds exhibit chromogenic signals which can help in identification of the heavy metal transformation events by monitoring the quenching of signals from these moieties.90
The 2D-COS is a multimodular analytical technique that can be conjugated with a variety of spectroscopy (vibrational—IR, NIR, and Raman; optical—UV–vis, fluorescence, X-ray, and NMR), chromatography and microscopy techniques.80,90−96 It can be well utilized to identify the relative effect of external perturbations such as temperature, pH, and interfering ion concentration of a heavy metal absorption/transformation study in a systematic manner of any analytical signal by obtaining complex cross correlation analysis.97
Also, partially noninvasive techniques that can help in identification of relative heavy metal elemental speciation, and high-resolution spatial distribution of the metal element among the various carbon fractions in the sample at high sensitivity includes a range of synchrotron radiation-based methods, viz., SR-micro-X-ray fluorescence spectroscopy (SR-μXRFS), sub-μm-synchrotron X-ray computed tomography (SR-XCT), synchrotron X-ray diffraction spectroscopy (SR-XRDS), and synchrotron near-edge X-ray absorption fine structure (SR-NEXAFS).98 Diverse environmental samples can be analyzed by these techniques.99 Therefore, several studies report the effects of contamination of the heavy metals and metalloids as examined through synchrotron based X-ray techniques in groundwater, in dissolved organic matter derived from groundwater, in soil, in human remains, and also in the soil–plant system to assess their negative impacts.100−104 These techniques can provide high-resolution information on complex environmental samples especially soil matrices in the form of the chemical environments of metal elements besides the microbial habitats which are presented as multidimensional maps.
Acknowledgments
Anu Kalia thanks the Director of Research, Punjab Agricultural University, Ludhiana, Punjab, India for allocation of funds in the Rashtriya Krishi Vikas Yojna (RKVY) scheme (RKVY-17) to carry out the research work.
Glossary
Abbreviations, Notation, Nomenclature, and Symbols Used
- HM
Heavy metal
- BC
Biochar
- BNHs
Biochar nanohybrids
- BET
Brunauer–Emmett–Teller
- CNT
Carbon nanotube
- SEM
Scanning Electron Microscopy
- XRD
X-ray powder diffraction
- XPS
X-ray Photoelectron Spectroscopy
- TEM
Transmission Electron Microscopy
- SEM-EDX
Scanning Electron Microscopy with Energy Dispersive X-ray
- FT-IR
Fourier transform infrared
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05117.
Table – Originality and novelty of present study (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Demirbas A. Heavy metal adsorption onto agro-based waste materials: a review. J. Hazard. Mater. 2008, 157 (2–3), 220–229. 10.1016/j.jhazmat.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Sud D.; Mahajan G.; Kaur M. P. Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solutions–A review. Biores. Techn. 2008, 99 (14), 6017–6027. 10.1016/j.biortech.2007.11.064. [DOI] [PubMed] [Google Scholar]
- Karunanayake A. G.; Todd O. A.; Crowley M.; Ricchetti L.; Pittman C. U. Jr; Anderson R.; Mlsna T. Lead and cadmium remediation using magnetized and nonmagnetized biochar from Douglas fir. Chem. Engin. J. 2018, 331, 480–491. 10.1016/j.cej.2017.08.124. [DOI] [Google Scholar]
- Kaliannan D.; Palaninaicker S.; Palanivel V.; Mahadeo M. A.; Ravindra B. N.; Jae-Jin S. A novel approach to preparation of nano-adsorbent from agricultural wastes (Saccharum officinarum leaves) and its environmental application. Environ. Sci. Pollu. Res. 2019, 26 (6), 5305–5314. 10.1007/s11356-018-3734-z. [DOI] [PubMed] [Google Scholar]
- Vu T. M.; Trinh V. T.; Doan D. P.; Van H. T.; Nguyen T. V.; Vigneswaran S.; Ngo H. H. Removing ammonium from water using modified corncob-biochar. Sci. Total Environ. 2017, 579, 612–619. 10.1016/j.scitotenv.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Millati R.; Cahyono R. B.; Ariyanto T.; Azzahrani I. N.; Putri R. U.; Taherzadeh M. J. Agricultural, industrial, municipal, and forest wastes: An Overview. Sustainable Resource Recovery and Zero Waste Approaches 2019, 1–22. 10.1016/B978-0-444-64200-4.00001-3. [DOI] [Google Scholar]
- Meyer S.; Glaser B.; Quicker P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45 (22), 9473–9483. 10.1021/es201792c. [DOI] [PubMed] [Google Scholar]
- Lehmann J.; Pereira da Silva J. Jr.; Steiner C.; Nehls T.; Zech W.; Glaser B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: fertilizer, manure and charcoal amendments. Plant. Soil. 2003, 249 (2), 343–357. 10.1023/A:1022833116184. [DOI] [Google Scholar]
- Bird M. I.; Ascough P. L.; Young I. M.; Wood C. V.; Scott A. C. X-ray microtomographic imaging of charcoal. J. Archaeol. Sci. 2008, 35 (10), 2698–2706. 10.1016/j.jas.2008.04.018. [DOI] [Google Scholar]
- Nguyen B. T.; Lehmann J.; Kinyangi J.; Smernik R.; Riha S. J.; Engelhard M. H. Long-term black carbon dynamics in cultivated soil. Biogeochem. 2009, 92 (1), 163–176. 10.1007/s10533-008-9248-x. [DOI] [Google Scholar]
- Ding Y.; Liu Y.; Liu S.; Li Z.; Tan X.; Huang X.; Zeng G.; Zhou L.; Zheng B. Biochar to improve soil fertility. A review. Agro. Sustain. Develop. 2016, 36 (2), 1–18. [Google Scholar]
- Rawat J.; Saxena J.; Sanwal P. Biochar.: A sustainable approach for improving plant growth and soil properties. Biochar-an imperative amendment for soil and the environment 2019, 12, 1–17. 10.5772/intechopen.82151. [DOI] [Google Scholar]
- Das S.; Mohanty S.; Sahu G.; Rana M.; Pilli K. Biochar: A Sustainable Approach for Improving Soil Health and Environment. Soil Erosion-Current Challenges and Future Perspectives in a Changing World 2021, 1. 10.5772/intechopen.97136. [DOI] [Google Scholar]
- Torabian S.; Qin R.; Noulas C.; Lu Y.; Wang G. Biochar: an organic amendment to crops and an environmental solution. AIMS Agriculture and Food 2021, 6 (1), 401–415. 10.3934/agrfood.2021024. [DOI] [Google Scholar]
- Karimi F.; Rahimi G.; Kolahchi Z.; Nezhad A. K. J. Using industrial sewage sludge-derived biochar to immobilize selected heavy metals in a contaminated calcareous soil. Waste. Bio. Valor. 2020, 11 (6), 2825–2836. 10.1007/s12649-018-00563-z. [DOI] [Google Scholar]
- Mansoor S.; Kour N.; Manhas S.; Zahid S.; Wani O. A.; Sharma V.; Wijaya L.; Alyemeni M. N.; Alsahli A. A.; El-Serehy H. A.; Paray B. A. Biochar as a tool for effective management of drought and heavy metal toxicity. Chemosphere 2021, 271, 129458. 10.1016/j.chemosphere.2020.129458. [DOI] [PubMed] [Google Scholar]
- Qiu B.; Tao X.; Wang H.; Li W.; Ding X.; Chu H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Analy. Appl. Pyrol. 2021, 155, 105081. 10.1016/j.jaap.2021.105081. [DOI] [Google Scholar]
- Li Y.; Yu H.; Liu L.; Yu H. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates. J. Hazard. Mater. 2021, 420, 126655. 10.1016/j.jhazmat.2021.126655. [DOI] [PubMed] [Google Scholar]
- Kumar P. S.; Gayathri R.; Rathi B. S. A review on adsorptive separation of toxic metals from aquatic system using biochar produced from agro-waste. Chemosphere 2021, 285, 131438. 10.1016/j.chemosphere.2021.131438. [DOI] [PubMed] [Google Scholar]
- Boraah N.; Chakma S.; Kaushal P. Attributes of wood biochar as an efficient adsorbent for remediating heavy metals and emerging contaminants from water: a critical review and bibliometric analysis. J. Environ. Chem. Engin. 2022, 10, 107825. 10.1016/j.jece.2022.107825. [DOI] [Google Scholar]
- Liu M.; Almatrafi E.; Zhang Y.; Xu P.; Song B.; Zhou C.; Zeng G.; Zhu Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806, 150531. 10.1016/j.scitotenv.2021.150531. [DOI] [PubMed] [Google Scholar]
- Ahmad M.; Rajapaksha A. U.; Lim J. E.; Zhang M.; Bolan N.; Mohan D.; Vithanage M.; Lee S. S.; Ok Y. S. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere 2014, 99, 19–33. 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- Tan X.; Liu Y.; Zeng G.; Wang X.; Hu X.; Gu Y.; Yang Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. 10.1016/j.chemosphere.2014.12.058. [DOI] [PubMed] [Google Scholar]
- Brar J. S.; Sikka R; Singh D; Kalia A. Effect of nanoparticles of rice husk ash on the bioavailability of lead to Indian mustard (Brassica juncea) in lead spiked soil. Agricul. Res. J. 2018, 55 (3), 478–484. 10.5958/2395-146X.2018.00086.8. [DOI] [Google Scholar]
- Qian L.; Chen M.; Chen B. Competitive adsorption of cadmium and aluminum onto fresh and oxidized biochars during aging processes. J. Soils Sedi. 2015, 15 (5), 1130–1138. 10.1007/s11368-015-1073-y. [DOI] [Google Scholar]
- Gan C.; Liu Y.; Tan X.; Wang S.; Zeng G.; Zheng B.; Li T.; Jiang Z.; Liu W. Effect of porous zinc–biochar nanocomposites on Cr (VI) adsorption from aqueous solution. RSC Adv. 2015, 5 (44), 35107–35115. 10.1039/C5RA04416B. [DOI] [Google Scholar]
- Inyang M.; Gao B.; Zimmerman A.; Zhang M.; Chen H. Synthesis, characterization, and dye sorption ability of carbon nanotube–biochar nanocomposites. Chem. Engin. J. 2014, 236, 39–46. 10.1016/j.cej.2013.09.074. [DOI] [Google Scholar]
- Li H.; Dong X.; da Silva E. B.; de Oliveira L. M.; Chen Y.; Ma L. Q. Mechanisms of metal sorption by biochars: biochar characteristics and modifications. Chemosphere. 2017, 178, 466–478. 10.1016/j.chemosphere.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Tan X. F.; Liu Y. G.; Gu Y. L.; Xu Y.; Zeng G. M.; Hu X. J.; Liu S. B.; Wang X.; Liu S. M.; Li J. Biochar-based nano-composites for the decontamination of wastewater: a review. Biores. Techn. 2016, 212, 318–333. 10.1016/j.biortech.2016.04.093. [DOI] [PubMed] [Google Scholar]
- Ramanayaka S.; Vithanage M.; Alessi D. S.; Liu W. J.; Jayasundera A. C.; Ok Y. S. Nanobiochar: production, properties, and multifunctional applications. Environ. Sci. Nano. 2020, 7 (11), 3279–3302. 10.1039/D0EN00486C. [DOI] [Google Scholar]
- Shen Z.; Jin F.; Wang F.; McMillan O.; Al-Tabbaa A. Sorption of lead by Salisbury biochar produced from British broadleaf hardwood. Biores. Techn. 2015, 193, 553–556. 10.1016/j.biortech.2015.06.111. [DOI] [PubMed] [Google Scholar]
- Shen Z.; McMillan O.; Jin F.; Al-Tabbaa A. Salisbury biochar did not affect the mobility or speciation of lead in kaolin in a short-term laboratory study. J. Hazard. Mater. 2016, 316, 214–220. 10.1016/j.jhazmat.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Wan Ngah W. S.; Hanafiah M. A. K. M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Biores. Techn. 2008, 99 (10), 3935–3948. 10.1016/j.biortech.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Awang N. A.; Wan Salleh W. N.; Aziz F.; Yusof N.; Ismail A. F. A review on preparation, surface enhancement and adsorption mechanism of biochar-supported nano zero-valent iron adsorbent for hazardous heavy metals. J. Chem. Technol. Biotechnol. 2022, 253, 256–345. [Google Scholar]
- Chin J. F.; Heng Z. W.; Teoh H. C.; Chong W. C.; Pang Y. L. Recent development of magnetic biochar crosslinked chitosan on heavy metal removal from wastewater–Modification, application and mechanism. Chemosphere 2022, 291, 133035. 10.1016/j.chemosphere.2021.133035. [DOI] [PubMed] [Google Scholar]
- Yan J.; Han L.; Gao W.; Xue S.; Chen M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Biores. Techn. 2015, 175, 269–274. 10.1016/j.biortech.2014.10.103. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Xu F.; Xue J.; Chen S.; Wang J.; Yang Y. Enhanced removal of heavy metal ions from aqueous solution using manganese dioxide-loaded biochar: Behavior and mechanism. Sci. Rep. 2020, 10 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ramady H.; Alshaal T.; Abowaly M.; Abdalla N.; Taha H. S.; Al-Saeedi A. H.; Shalaby T.; Amer M.; Fári M.; Domokos-Szabolcsy É.; Sztrik A. Nanoremediation for sustainable crop production. Nanoscience in Food and Agriculture 2017, 26, 335–363. 10.1007/978-3-319-58496-6_12. [DOI] [Google Scholar]
- Chen W. H.; Hoang A. T.; Nižetić S.; Pandey A.; Cheng C. K.; Luque R.; Ong H. C.; Thomas S.; Nguyen X. P. Biomass-derived biochar: From production to application in removing heavy metal-contaminated water. Process Safety and Environmental Protection 2022, 160, 704–733. 10.1016/j.psep.2022.02.061. [DOI] [Google Scholar]
- Liu Y.; Chen Y.; Li Y.; Chen L.; Jiang H.; Li H.; Luo X.; Tang P.; Yan H.; Zhao M.; Yuan Y. Fabrication, application, and mechanism of metal and heteroatom co-doped biochar composites (MHBCs) for the removal of contaminants in water: A review. J. Hazard. Mater. 2022, 431, 128584. 10.1016/j.jhazmat.2022.128584. [DOI] [PubMed] [Google Scholar]
- Weidner E.; Karbassiyazdi E.; Altaee A.; Jesionowski T.; Ciesielczyk F. Hybrid Metal Oxide/Biochar Materials for Wastewater Treatment Technology: A Review. ACS omega 2022, 7 (31), 27062–27078. 10.1021/acsomega.2c02909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C.; Wang B.; Theng B. K.; Wu P.; Liu F.; Wang S.; Lee X.; Chen M.; Li L.; Zhang X. Formation and mechanisms of nano-metal oxide-biochar composites for pollutants removal: A review. Sci. Total Environ. 2021, 767, 145305. 10.1016/j.scitotenv.2021.145305. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wan Y.; Zheng Y.; He F.; Yu Z.; Huang J.; Wang H.; Ok Y. S.; Jiang Y.; Gao B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem. Engin. J. 2019, 366, 608–621. 10.1016/j.cej.2019.02.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. H.; Zhu S.; Chang J. S. Recent advances in nanoscale-metal assisted biochar derived from waste biomass used for heavy metals removal. Biores. Techn. 2017, 246, 123–134. 10.1016/j.biortech.2017.08.061. [DOI] [PubMed] [Google Scholar]
- Li C.; Zhang L.; Gao Y.; Li A. Facile synthesis of nano ZnO/ZnS modified biochar by directly pyrolyzing of zinc contaminated corn stover for Pb (II), Cu (II) and Cr (VI) removals. Waste Management 2018, 79, 625–637. 10.1016/j.wasman.2018.08.035. [DOI] [PubMed] [Google Scholar]
- Li A. Y.; Deng H.; Jiang Y. H.; Ye C. H.; Yu B. G.; Zhou X. L.; Ma A. Y. Superefficient removal of heavy metals from wastewater by Mg-Loaded biochars: Adsorption characteristics and removal mechanisms. Langmuir 2020, 36 (31), 9160–9174. 10.1021/acs.langmuir.0c01454. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Gao B. Removal of arsenic, methylene blue, and phosphate by biochar/AlOOH nanocomposite. Chem. Engin. J. 2013, 226, 286–292. 10.1016/j.cej.2013.04.077. [DOI] [Google Scholar]
- Singh P.; Sarswat A.; Pittman C. U. Jr; Mlsna T.; Mohan D. Sustainable low-concentration arsenite [As (III)] removal in single and multicomponent systems using hybrid Iron oxide–biochar nanocomposite adsorbents—a mechanistic study. ACS Omega 2020, 5 (6), 2575–2593. 10.1021/acsomega.9b02842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Gao B.; Li Y.; Mosa A.; Zimmerman A. R.; Ma L. Q.; Harris W. G.; Migliaccio K. W. Manganese oxide-modified biochars: preparation, characterization, and sorption of arsenate and lead. Biores. Techn. 2015, 181, 13–17. 10.1016/j.biortech.2015.01.044. [DOI] [PubMed] [Google Scholar]
- Li R.; Liang W.; Wang J. J.; Gaston L. A.; Huang D.; Huang H.; Lei S.; Awasthi M. K.; Zhou B.; Xiao R.; Zhang Z. 2018. Facilitative capture of As (V), Pb (II) and methylene blue from aqueous solutions with MgO hybrid sponge-like carbonaceous composite sugarcane leafy trash. J. Environ. Manag. 2018, 212, 77–87. 10.1016/j.jenvman.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Chaukura N.; Murimba E. C.; Gwenzi W. Synthesis, characterisation and methyl orange adsorption capacity of ferric oxide–biochar nano-composites derived from pulp and paper sludge. Applied Water Science 2017, 7 (5), 2175–2186. 10.1007/s13201-016-0392-5. [DOI] [Google Scholar]
- Omidvar-Hosseini F.; Moeinpour F. Removal of Pb (II) from aqueous solutions using Acacia Nilotica seed shell ash supported Ni0.5Zn0. 5Fe2O4 magnetic nanoparticles. J. Wat. Reuse. Desalin. 2016, 6 (4), 562–573. 10.2166/wrd.2016.073. [DOI] [Google Scholar]
- Hanif M. U.; Capareda S. C.; Iqbal H.; Arazo R. O.; Baig M. A. Effects of pyrolysis temperature on product yields and energy recovery from co-feeding of cotton gin trash, cow manure, and microalgae: a simulation study. PloS ONE 2016, 11 (4), 0152230. 10.1371/journal.pone.0152230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines K. R.; Abdullah E. C.; Mubarak N. M.; Ruthiraan M. Synthesis of magnetic biochar from agricultural waste biomass to enhancing route for waste water and polymer application: A review. Renew. Sustain. Energy Rev. 2017, 67, 257–276. 10.1016/j.rser.2016.09.057. [DOI] [Google Scholar]
- Peternele W. S.; Monge Fuentes V.; Fascineli M. L.; Rodrigues da Silva J.; Silva R. C.; Lucci C. M.; Bentes de Azevedo R. Experimental investigation of the coprecipitation method: An approach to obtain magnetite and maghemite nanoparticles with improved properties. J. Nanomater. 2014, 15, 256–68. 10.1155/2014/682985. [DOI] [Google Scholar]
- Liu J.; Ye X.; Wang H.; Zhu M.; Wang B.; Yan H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram. Internat. 2003, 29 (6), 629–633. 10.1016/S0272-8842(02)00210-9. [DOI] [Google Scholar]
- Gupta V. K.; Nayak A. Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chem. Engin. J. 2012, 180, 81–90. 10.1016/j.cej.2011.11.006. [DOI] [Google Scholar]
- Mian M. M.; Liu G. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 2019, 215, 101–114. 10.1016/j.chemosphere.2018.10.027. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Huang Y.; Qiu W.; Sun Z.; Liu Z.; Song Z. Adsorption properties of nano-MnO2–biochar composites for copper in aqueous solution. Molecules 2017, 22 (1), 173. 10.3390/molecules22010173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołodyńska D.; Ba̧k J.; Kozioł M.; Pylychuk L. V. Investigations of heavy metal ion sorption using nanocomposites of iron-modified biochar. Nano. Res. Lett. 2017, 12 (1), 1–13. 10.1186/s11671-017-2201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabasingh S. A.; Belachew H.; Yimam Iron oxide induced bagasse nanoparticles for the sequestration of Cr6+ ions from tannery effluent using a modified batch reactor. J. Appl. Polym. Sci. 2018, 135 (36), 46683. 10.1002/app.46683. [DOI] [Google Scholar]
- Arabyarmohammadi H.; Darban A. K.; Abdollahy M.; Yong R.; Ayati B.; Zirakjou A.; van der Zee S. E. Utilization of a novel chitosan/clay/biochar nanobiocomposite for immobilization of heavy metals in acid soil environment. J. Polym. Environ. 2018, 26 (5), 2107–2119. 10.1007/s10924-017-1102-6. [DOI] [Google Scholar]
- Daraei P.; Madaeni S. S.; Salehi E.; Ghaemi N.; Ghari H. S.; Khadivi M. A.; Rostami E. Novel thin film composite membrane fabricated by mixed matrix nanoclay/chitosan on PVDF microfiltration support: Preparation, characterization and performance in dye removal. J. Membr. Sci. 2013, 436, 97–108. 10.1016/j.memsci.2013.02.031. [DOI] [Google Scholar]
- Yu-shan X. I. E.; Shao-zao T. A. N.; Ma-hua L.; Ren-fu L. I. U. Structure and antibacterial activity of modified montmorillonite. Chemical Research in Chinese Universitie 2010, 26 (4), 509–513. [Google Scholar]
- Krajewska B. Diffusion of metal ions through gel chitosan membranes. Reactive and Functional Polymers. 2001, 47 (1), 37–47. 10.1016/S1381-5148(00)00068-7. [DOI] [Google Scholar]
- Wu H.; Feng Q.; Yang H.; Alam E.; Gao B.; Gu D. Modified biochar supported Ag/Fe nanoparticles used for removal of cephalexin in solution: Characterization, kinetics and mechanisms. Colloids Surf., A 2017, 517, 63–71. 10.1016/j.colsurfa.2017.01.005. [DOI] [Google Scholar]
- Kumar A.; Kumar A.; Sharma G.; Naushad M.; Stadler F. J.; Ghfar A. A.; Dhiman P.; Saini R. V. Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants-Synergism of adsorption, photocatalysis & photo-ozonation. J. Clean. Prod. 2017, 165, 431–451. 10.1016/j.jclepro.2017.07.117. [DOI] [Google Scholar]
- Wang S. G.; Gong W. X.; Liu X. W.; Yao Y. W.; Gao B. Y.; Yue Q. Y. Removal of lead (II) from aqueous solution by adsorption onto manganese oxide-coated carbon nanotubes. Sep. Puri. Technol. 2007, 58 (1), 17–23. 10.1016/j.seppur.2007.07.006. [DOI] [Google Scholar]
- Lyu H.; Tang J.; Huang Y.; Gai L.; Zeng E. Y.; Liber K.; Gong Y. Removal of hexavalent chromium from aqueous solutions by a novel biochar supported nanoscale iron sulfide composite. Chem. Engin. J. 2017, 322, 516–524. 10.1016/j.cej.2017.04.058. [DOI] [Google Scholar]
- Ucun H.; Bayhana Y. K.; Kaya Y.; Cakici A.; Algur O. F. Biosorption of lead (II) from aqueous solution by cone biomass of Pinus sylvestris. Desalin. 2003, 154 (3), 233–238. 10.1016/S0011-9164(03)80038-3. [DOI] [PubMed] [Google Scholar]
- Li X.; Pan G.; Qin Y.; Hu T.; Wu Z.; Xie Y. EXAFS studies on adsorption–desorption reversibility at manganese oxide–water interfaces: II. Reversible adsorption of zinc on δ-MnO2. J. Colloid Interface Sci. 2004, 271 (1), 35–40. 10.1016/j.jcis.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Park C. S.; Roy P. S.; Kim S. H. Current developments in thermochemical conversion of biomass to fuels and chemicals 2018, 2, 19–41. [Google Scholar]
- Buss W.; Graham M. C.; MacKinnon G.; Mašek O. Strategies for producing biochars with minimum PAH contamination. J. Analyt. Appl. Pyrol. 2016, 119, 24–30. 10.1016/j.jaap.2016.04.001. [DOI] [Google Scholar]
- Dunnigan L.; Morton B. J.; Hall P. A.; Kwong C. W. Production of biochar and bioenergy from rice husk: Influence of feedstock drying on particulate matter and the associated polycyclic aromatic hydrocarbon emissions. Atmos. Environ. 2018, 190, 218–225. 10.1016/j.atmosenv.2018.07.028. [DOI] [Google Scholar]
- Visioli G.; Conti F. D.; Menta C.; Bandiera M.; Malcevschi A.; Jones D. L.; Vamerali T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188 (3), 166. 10.1007/s10661-016-5173-y. [DOI] [PubMed] [Google Scholar]
- Yang X.; Ng W.; Wong B. S. E.; Baeg G. H.; Wang C. H.; Ok Y. S. Characterization and ecotoxicological investigation of biochar produced via slow pyrolysis: Effect of feedstock composition and pyrolysis conditions. J. Hazard. Mater. 2019, 365, 178–185. 10.1016/j.jhazmat.2018.10.047. [DOI] [PubMed] [Google Scholar]
- Wang Y. Y.; Jing X. R.; Li L. L.; Liu W. J.; Tong Z. H.; Jiang H. Biotoxicity evaluations of three typical biochars using a simulated system of fast pyrolytic biochar extracts on organisms of three kingdoms. ACS Sustain. Chem. Engin. 2017, 5 (1), 481–488. 10.1021/acssuschemeng.6b01859. [DOI] [Google Scholar]
- Gelardi D. L.; Li C.; Parikh S. J. An emerging environmental concern: Biochar-induced dust emissions and their potentially toxic properties. Sci. Tot. Environt. 2019, 678, 813–820. 10.1016/j.scitotenv.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Lakshmi D.; Akhil D.; Kartik A.; Gopinath K. P.; Arun J.; Bhatnagar A.; Rinklebe J.; Kim W.; Muthusamy G. Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci. Tot. Environt. 2021, 801, 149623. 10.1016/j.scitotenv.2021.149623. [DOI] [PubMed] [Google Scholar]
- Duarte R. M.; Duarte A. C. Multidimensional analytical techniques in environmental research: Evolution of concepts. Multidimen. Analyt. Technol. Environ. Res. 2020, 6, 153–161. 10.1016/B978-0-12-818896-5.00001-6. [DOI] [Google Scholar]
- Vigneshkumar M.; Yarrakula K. Titanium metal identification in southern region of Tamil Nadu using hyperspectral imagery. Indian J. Geo-Marine Sci. 2018, 47, 2100–2105. [Google Scholar]
- Rui W.; Shuang W.; Kan W.; Shiqiao H.; Ruijie W.; Bo L.; Min L.; Liang L.; Dawei Z.; Xinpeng D. Estimation and Spatial Analysis of Heavy Metals in Metal Tailing Pond Based on Improved PLS With Multiple Factors. IEEE Access 2021, 9, 64880–64894. 10.1109/ACCESS.2021.3073933. [DOI] [Google Scholar]
- Patil G. K.; Bhere S.; Gaikwad T.; Joshi K.; Sawant S.; Rathod R. Water Quality of Inland Water Bodies of Mumbai using Hyperspectral Remote Sensing Hyperion EO1. Roorkee Water Conclave 2020, 1–10. [Google Scholar]
- Kisevic M.; Morovic M.; Andricevic R. The use of hyperspectral data for evaluation of water quality parameters in the River Sava. Fresenius Environ. Bull. 2016, 25, 4814–4822. [Google Scholar]
- Abdlaty R.; Gobara M.; Naiem I.; Mokhtar M. Innovative technique for analysis of wastewater contaminants using hyperspectral imaging. J. Spectral. Imag. 2020, 9, a12. 10.1255/jsi.2020.a12. [DOI] [Google Scholar]
- Wang F.; Gao J.; Zha Y. Hyperspectral sensing of heavy metals in soil and vegetation: Feasibility and challenges. ISPRS Journal of Photogrammetry and Remote Sensing 2018, 136, 73–84. 10.1016/j.isprsjprs.2017.12.003. [DOI] [Google Scholar]
- Wei L.; Zhang Y.; Lu Q.; Yuan Z.; Li H.; Huang Q. Estimating the spatial distribution of soil total arsenic in the suspected contaminated area using UAV-Borne hyperspectral imagery and deep learning. Ecolog. Indicat. 2021, 133, 108384. 10.1016/j.ecolind.2021.108384. [DOI] [Google Scholar]
- Liu Y.; Li W.; Wu G.; Xu X. Feasibility of estimating heavy metal contaminations in floodplain soils using laboratory-based hyperspectral data-A case study along Le’an River, China. Geo-Spatial. Inf. Sci. 2011, 14, 10–16. 10.1007/s11806-011-0424-0. [DOI] [Google Scholar]
- Jian J.; Fang Y.; Li W. L.; Chen Q. Y.; Tian H.-Y.; You S. L. Estimate of Heavy Metals in Soil with Non-Soil Removed. J. Data Anal. Inf. Process. 2017, 5, 140–155. 10.4236/jdaip.2017.54011. [DOI] [Google Scholar]
- Wei D.; Ngo H. H.; Guo W.; Xu W.; Zhang Y.; Du B.; Wei Q. Biosorption of effluent organic matter onto magnetic biochar composite: Behavior of fluorescent components and their binding properties. Biores. Techn. 2016, 214, 259–265. 10.1016/j.biortech.2016.04.109. [DOI] [PubMed] [Google Scholar]
- Schwille P.; Haustein E. Fluorescence Correlation Spectroscopy An Introduction to its Concepts and Applications Petra Schwille and Elke Haustein Experimental Biophysics Group Max-Planck-Institute for Biophysical Chemistry Am. Fassberg, Göttingen 2001, 11, 37077. [Google Scholar]
- Haustein E.; Schwille P. Fluorescence correlation spectroscopy: Novel variations of an established technique. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 151–169. 10.1146/annurev.biophys.36.040306.132612. [DOI] [PubMed] [Google Scholar]
- Hur J.; Lee B. M. Comparing the heterogeneity of copper-binding characteristics for two different-sized soil humic acid fractions using fluorescence quenching combined with 2D-COS. ScientificWorld Journal 2011, 11, 1865–1876. 10.1100/2011/640598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Ozaki Y.; Noda I.; Jung Y. M.. 2D Correlation Spectroscopy and Its Application in Vibrational and Optical Spectroscopy; Elsevier Inc, 2018. [Google Scholar]
- Mecozzi M.: Two-dimensional correlation spectroscopy to assess the dynamics of complex environmental mixtures. Elsevier Inc; (2020). [Google Scholar]
- Mohammad Alwi M. A.; Normaya E.; Ismail H.; Iqbal A.; Mat Piah B.; Abu Samah M. A.; Ahmad M. N. Two-Dimensional Infrared Correlation Spectroscopy, Conductor-like Screening Model for Real Solvents, and Density Functional Theory Study on the Adsorption Mechanism of Polyvinylpolypyrrolidone for Effective Phenol Removal in an Aqueous Medium. ACS Omega 2021, 6, 25179–25192. 10.1021/acsomega.1c02699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Habibul N.; Liu X. Y.; Sheng G. P.; Yu H. Q. FTIR and synchronous fluorescence heterospectral two-dimensional correlation analyses on the binding characteristics of copper onto dissolved organic matter. Environ. Sci. Technol. 2015, 49 (4), 2052–2058. 10.1021/es5049495. [DOI] [PubMed] [Google Scholar]
- Lehmann J.; Solomon D. Organic carbon chemistry in soils observed by synchrotron-based spectroscopy. Developments in soil science 2010, 34, 289–312. 10.1016/S0166-2481(10)34010-4. [DOI] [Google Scholar]
- Hu H.; Zhao J.; Wang L.; Shang L.; Cui L.; Gao Y.; Li B.; Li Y. F.. Synchrotron-based techniques for studying the environmental health effects of heavy metals: Current status and future perspectives; Elsevier B.V., 2020. [Google Scholar]
- Moreira S.; Ficaris M.; Vives A. E. S.; Filho V. F. N.; Zucchi O. L.; Barroso R. C.; De Jesus E. F. Heavy Metals in Groundwater using Synchrotron Radiation Total Reflection X-Ray Analysis. Instrum. Sci. Technol. 2006, 34, 567–585. 10.1080/10739140600811682. [DOI] [Google Scholar]
- Sun F.; Li Y.; Wang X.; Chi Z.; Yu G. Using new hetero-spectral two-dimensional correlation analyses and synchrotron-radiation-based spectromicroscopy to characterize binding of Cu to soil dissolved organic matter. Environ. Pollut. 2017, 223, 457–465. 10.1016/j.envpol.2017.01.046. [DOI] [PubMed] [Google Scholar]
- Sun F.; Polizzotto M. L.; Guan D.; Wu J.; Shen Q.; Ran W.; Wang B.; Yu G. Exploring the interactions and binding sites between Cd and functional groups in soil using two-dimensional correlation spectroscopy and synchrotron radiation based spectromicroscopies. J. Hazard. Mater. 2017, 326, 18–25. 10.1016/j.jhazmat.2016.12.019. [DOI] [PubMed] [Google Scholar]
- Lanzirotti A.; Bianucci R.; LeGeros R.; Bromage T. G.; Giuffra V.; Ferroglio E.; Fornaciari G.; Appenzeller O. Assessing heavy metal exposure in Renaissance Europe using synchrotron microbeam techniques. J. Archaeol. Sci. 2014, 52, 204–217. 10.1016/j.jas.2014.08.019. [DOI] [Google Scholar]
- Kopittke P. M.; Wang P.; Lombi E.; Donner E. Synchrotron-based X-Ray Approaches for Examining Toxic Trace Metal(loid)s in Soil-Plant Systems. J. Environ. Qual. 2017, 46, 1175–1189. 10.2134/jeq2016.09.0361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




