Abstract

Liquid crystals are considered to be the fourth state of matter with an intermediate order and fluidity in comparison to solids and liquids. Calixarenes are among one of the most versatile families of building blocks for supramolecular chemistry due to their unique vaselike structure that can be chemically engineered to have different shapes and sizes. During the last few decades, calixarenes have drawn much attention in the field of supramolecular chemistry due to their diverse applications in the fields of ion and molecular recognition, ion-selective electrodes for catalysis, drug delivery, gelation, organic electronics and sensors, etc. Imbuing liquid crystallinity to the calixarene framework leads to functionalized calixarene derivatives with fluidity and order. Columnar self-assembly of such derivatives in particular enhance the charge migration along the column due to the 1D stacking due to the enhanced π–π overlap. Considering limited reports and reviews on this new class of calixarene based liquid crystals, a comprehensive account of the synthesis of calixarene liquid crystals along with their mesomorphic behavior and potential applications are presented in this review.
1. Introduction
Liquid crystal (LC) phases represent a unique state of matter that possesses mobility and order on different macroscopic, supramolecular, and molecular levels.1−3 The hierarchical molecular arrangement or self-assembly in liquid crystals provides a platform for developing various nanostructured materials.4,5 Moreover, microsegregated molecular fragments, molecular shapes with various interactions, and self-organization are the essential key factors that have led to the stabilization of various LC phases.6 The distinguishing feature of having order and mobility in LC phases provides multiresponsive self-healing supramolecular systems, which have given rise to many devices that impact modern day life and also have become the subject of intense investigation.7−11 Their application in low power consuming display devices has revolutionized industry and enhanced the human standard of living.12 Further, liquid crystal based biosensors are also widely utilized as diagnostic tools.13
The liquid crystalline compounds are broadly categorized into two different classes, namely, thermotropic mesogens in which the mesophase formation is generally temperature dependent and lyotropic LCs in which the mesophase formation is concentration and temperature dependent.14−16 Thermotropic mesogens are mainly categorized into conventional and nonconventional liquid crystals. Rodlike (calamitic) and disklike (discotic) mesogens are the most widely known conventional LCs. Banana-shaped, star-shaped, shuttlecock-shaped, polycatenar (dumbbell-shaped), and hockey-stick-shaped mesogens are some examples of nonconventional LCs (Figure 1).17−20 Calamitic LCs usually stabilize nematic (N) phase and smectic (Sm) phases, which are formed by the parallel alignment of the molecules along the director without any positional order or by the presence of both positional and orientational orders (layered structure) respectively. Smectic phases are classified into many based on intralayer ordering. Equivalent mesophase structures are found in the case of discotic molecules, where they organize to form nematic and columnar phases.21 Columnar (Col) phases are classified again based on the organization of these columns into different lattices, namely, hexagonal (Colh), rectangular (Colr), oblique (Colob), helical (H), and tetragonal (Coltet), respectively (Figure 2).22,23 In recent years, more attention has been focused on discotic LCs, especially the Col phase due to its potential in organic electronics.24−30
Figure 1.
Schematic diagram showing conventional and nonconventional molecular shapes used for the stabilization of LC phases.
Figure 2.

Schematic diagram showing mesophases stabilized by conventional liquid crystals: (a) calamitic LC phases and (b) discotic LC phases.
Calixarenes are an important class of supramolecules that can be chemically prepared by the simple reaction of p-tert-butylphenol and formaldehyde.31−34 By the judicious choice of base, reaction temperature, and reaction time, calixarenes with various shapes and ring sizes can be synthesized with good yield and purity.35,36 The framework of calixarene entails the presence of a wide hydrocarbon rim that contains methylene bridges along with a narrow rim having phenolic groups to give rise to three-dimensional supramolecules (Figure 3). Resorcinarenes are a type of calixarenes formed by the condensation of resorcinol with aldehyde.
Figure 3.
Basic molecular structures of calixarene and resorcinarene cores and their self-assembly on functionalization.
The calixarenes can be readily functionalized at the lower and upper rims with a wide variety of functional groups to make versatile receptors for cations, anions, and neutral species and thus can be utilized for the sensing of the same. However, the development of such materials possessing LC properties has not been explored much.37 Importantly, LC material based on a calixarene core possesses some interesting properties such as good stability across a wide thermal range and a reduced tendency for crystallization. In addition, the functionalization of calixarene with flexible alkyl side chains can be carried out by linking the core with different functional units. The various possibilities to form calixarene based LCs by chemical functionalization on a calixarene core are represented in Figure 3. It has also been noted that some of the calixarene based functional LCs exhibit excellent mesomorphic properties with emissive behavior, columnar self-assembly, electrochemical behavior, electroluminescence, molecular switch properties, emissive gelation properties, and ion-selective behavior, respectively. Their application in ion and molecular sensing, as fluorescent probes, and as drug delivery agents has been discussed in earlier elaborate reviews.38−40
However, there are few reports on the preparation and applications of calix[4]arene and resorcin[4]arene core based functionalized mesogens. This review provides a brief account of the synthesis of calixarene based LCs, their mesomorphic behavior, and their potential in diverse applications.
2. CALIXARENE BASED LIQUID CRYSTALS
2.1. Calixarene Ethers and Esters
Initial functionalization of calixarenes to self-assembling structures started with the etherification and esterification of the hydroxyl groups. In subsequent years the flexible chain was introduced through the aldehyde component that takes part in the formation of the calixarene unit to improve the solubility. In 1992, Dalcanale and co-workers reported LC calixarenes for the first time.41 The acid catalyzed condensation of pyrogallol with 1,1-diethoxyethane in ethanol yielded a bowl-shaped macrocyclic precursor in a good yield. The free hydroxyl groups were esterified later with long chain carboxylic acids of different lengths to get the final compounds. They have also prepared calixarene derivatives by treating pyrogallol and paraformaldehyde in the presence of HCl at 85 °C. The crude compound was treated with butyryl chloride to give pure 1b in a yield of 53%. Further hydrolysis of the same yielded compound 1a in pure form, which was treated with appropriate alkanoyl chlorides to get the corresponding dodecaacylated compounds 1c–1k (Scheme 1). The compounds bearing chains with 12 carbon units onward (1d–1g) exhibited the Colh phase (Figure 4),42 while the branched chain esters did not show any LC phase due to the increased steric bulk preventing 1D packing. Compounds 1b, 1c, 1h, 1i, and 1j were crystalline, and 1k turned out to be liquid. The bowl shape of the core in these compounds leads to the net dipole moment of the compounds directed along their C4 symmetry axis. Thus, the orientation of both molecular and columnar dipoles can lead to a potential ferroelectric or antiferroelectric switchable Col phase in an appropriate molecular design.
Scheme 1. Bowl-Shaped Liquid Crystalline Derivatives of Calixarene.
Figure 4.

Graphical representation of the thermal behavior of bowl-shaped liquid crystalline derivatives (1d–1g, 3e–3h) (considered the cooling scan).
The same group also developed columnar mesogens with bowl-like structures formed from conformationally mobile macrocyclic cores (Scheme 2). Compounds with methyl groups in the upper rims and eight chains in the lower rims (2a–2m) were non liquid crystalline.43 However, the compounds with methyl groups in the upper rims and 12 chains in the lower rims (3a–3k) exhibited improved liquid crystalline behavior. Out of the 10 derivatives, four compounds (3e–3h) exhibited a Colr mesophase.42 The aromatic esters (2j–2m, 3k) were non-LC due to the out-of-plane orientation of phenyl groups hindering the 1D packing of calixarene cores. Deuterium NMR spectroscopy of deuterated benzene added as a probe to compound 3f showed the formation of the uniaxial Col phase, with domain directors perpendicular to the magnetic field.44 This also showed that the mesogen forms a cavity with strong host properties toward the guest. The CPK model of compound 3f showed the conelike conformation of the macromolecule (Figure 5). Calixarene derivatives with ethyl groups in the upper rims and 12 peripheral chains in the lower rims (4b–4e) turned out to be crystalline.43 These studies provided the effect of structural and conformational alterations by the length of the chains in the upper rim and lower rim of the macrocyclic core and the effect on the liquid crystallinity by the number and type of chains in calixarene derivatives.
Scheme 2. Calixarene Based Liquid Crystalline Derivatives.
Figure 5.

CPK model of compound 3f showing the hollow side view. Reproduced with permission from ref (43). Copyright 1990 Taylor & Francis.
Gajjar et al.45 synthesized calix[4]resorcinarene derivatives (5a–5c) bearing alkoxy chains, which were prepared by the reaction of cinnamaldehyde and resorcinol (Scheme 3). These compounds exhibited a Colh phase, with the lower chain homologues exhibiting a wider mesophase range while the higher alkyl chain derivatives exhibited a lower mesophase width with lowered melting and clearing temperatures (Figure 6).
Scheme 3. Synthesis of Calix[4]resorcinarene Derived from Cinnamaldehyde (5a–5c).
Figure 6.

Graphical representation of the thermal behavior of octasubstituted resorcinarene alkyl arm functionalized derivatives (5a–5c) (considered the cooling scan).
A series of lower rim functionalized octasubstituted resorcin[4]arene based compounds with variable alkoxy side chains (−OC4H9, −OC6H13, −OC8H17, −OC10H21) have been reported by Sharma et al.46 The final compounds were prepared by a simple two-step procedure (Scheme 4). All four compounds displayed Colh phases over a wide thermal range (Figure 7). Compound 6d with a decyloxy side chain was further tested for the window layer application in solar cells, especially as an alternative to the commercially available toxic Cd-based compounds which are used in solar cells. Herein, we used compound 6d as an optical window layer to achieve maximum transmittance of incident radiation toward the absorber layer and further studied it as a function of temperature. The current–voltage relation shows ohmic-type behavior. The optical properties display high transmittance in the visible and near IR regions at room temperature and 200 °C. The derivative showed three optical band gaps (1.61, 4.01, and 2.27 eV) at different thin film states and variable temperature which are more suitable for an eco-friendly, light absorbing window layer in solar cell applications.
Scheme 4. Synthesis of Liquid Crystalline Resorcinarene Derivatives (6a–6d).
Figure 7.

Graphical representation of the thermal behavior of octasubstituted resorcinarene alkyl arm functionalized derivatives 6a–6d (considered the cooling scan).
2.2. Calixarene Derivatives with Azobenzene Moieties
Azobenzenes have the ability to photoisomerize in the presence of UV light of a particular wavelength from the thermodynamically stable trans form (E-form) to the metastable cis form (Z-form). The reverse isomerization can occur by thermal back relaxation or by the irradiation of visible light. This reversible isomerization brings about a large change in the molecular shape and dipole moment, and when incorporated as a part of the mesogen, it will lead to the light controlled switchability of the mesophase, thus adding a new functionality to the system. Thus, the combination of photoswitchable azobenzene with mesogens is an active area of research.47
Swager et al. for the first time reported the synthesis of bowl-like tungsten oxo calix[4]arenes functionalized with azobenzene moieties (7a, 7b) which differ by the number of alkoxy chains on the peripheral phenyl ring (Scheme 5).48 These compounds exhibit an ordered Colh phase (Figure 8a). They also form host–guest complexes with DMF and pyridine solvents. The host–guest complexes do not show any mesophase, while the removal of guests allows the molecules to pack in a column by the incorporation of tungsten oxo groups inside the cavity leading to the stabilization of ordered Colh phase as shown in Figure 8b.
Scheme 5. Synthesis of Tungsten Oxo Calix[4]arene Derivatives (7a, 7b).
Figure 8.
(a) Graphical representation of the thermal behavior of tungsten oxo calix[4]arene derivatives 7a and 7b. (b) Rearrangement of bowl-shaped calixarene linked tungsten core into columnar structure (7a, 7b).48 Reproduced from ref (48). Copyright 1993 American Chemical Society.
Swager et al. also reported a bowl-shaped conformationally stable calixarene derivative (8) stabilizing the Colh phase by host–guest interactions (Figure 9).49 They found that these conformationally flexible materials displayed an unusual transient mesophase which was mainly detected during initial heating. Further, heating up to 163 °C showed a normal liquid type isotropic phase and, upon cooling, produced a solid crystalline compound with no birefringence. However, X-ray diffraction (XRD) analysis pointed toward an ordered arrangement. This ordered phase was confirmed to be a cubic lattice, which is reliable due to the absence of any birefringence.
Figure 9.

Structure of the azobenzene based calix[4]arene (8) and N-n-butylformamide (a). Schematic representation of the stacking calix[4]arenes by forming a 2:1 complex with N-n-butylformamide in the mesophase (b). Optical texture of the Bh phase stabilized by the host–guest complex in 2:1 ratio (c).49 Reproduced from ref (49). Copyright 1995 American Chemical Society.
After recrystallization, the initial crystal phase was found in methanol/tetrahydrofuran solvent. Upon melting of the crystallized bowl-shaped calix[4]arene, the molecules organized into a Bh phase with a head-to-tail order type arrangement and dipole–dipole interactions. They found that this mesophase can be stabilized by forming a complex (9) with a guest containing two formamide units separated by a butyl spacer. Here, the one amide binds in the cavity and enforces the cone formation while the other one forms hydrogen bonds with the nearest neighbor, thereby leading to head-to-tail organizations (Figure 9b). It is interesting to note that only a small amount of guest is required to stabilize a Bh phase, suggesting the cooperative nature of the guest induced transition (Figure 9c).
Along similar lines, Oh et al.50 reported the perpendicular orientation of a nematic LC azobenzene (C6Azo, 4,4′-dihexyloxy azobenzene) on a water surface when mixed with O-octacarboxymethylated calix[4]resorcinarene (10) as a host. A host–guest mixed monolayer of C6Azo with O-octacarboxymethylated calix[4]resorcinarene exhibited the formation of a double-layered structure by further compression. Hence compound 10 acts as an excellent host for the C6Azo LC compound (Figure 10).
Figure 10.
Structures of calix[4]arenes reported by Oh et al.50 (a). Presentation of molecular organization of compound 10 and C6Azo in the form of a mixed monolayer on a water surface (b).50 Reproduced with permission from ref (50). Copyright 2001 The Royal Society of Chemistry.
Besides host–guest interactions, Matsuzawa et al.51 investigated the relationship between nematic orientation and the wetting qualities of mixed monolayers generated by the coadsorption of two O-octa substituted carboxymethylated calix[4]resorcinarenes (11a, 11b) with perfluorooctyl or octyl linked azobenzene groups (Figure 11). In addition, they also looked at photoirradiation of 11a and 11b monolayers, which caused a reversible trans–cis photoisomerization of the surface azobenzenes, which causes changes in the LC alignment and wettability of the monolayers. The photogenerated LC alignments are influenced by the surface compositions of the mixed monolayers. They also discovered that when LC cells produced using single-component monolayers of compounds 11a and 11b containing p-octylazobenzenes are exposed to oblique nonpolarized UV light, the orientation of the LC molecules switches from homeotropic to homogeneous, tilting toward the direction of light propagation. In contrast, single-component and mixed monolayers of the above calix[4]resorcinarenes with p-perfluorooctylazobenzenes cause homeotropic alignments and tilted homeotropic alignments with high pretilt angles, respectively.
Figure 11.
Structures of perfluorooctylazobenzene (11a) or octylazobenzene (11b) functionalized O-octacarboxymethylated calix[4]arene derivatives.
In 2013, Sutariya et al. reported basket-shaped supramolecules constructed by introducing two azo-ether groups on the bottom side of calix[4]arene as shown in Scheme 6.52 These compounds (12a–12d) exhibited enantiotropic mesomorphic properties with good thermal stability. Azo materials 12a and 12b showed smectic C and nematic phases, while oligomers 12c and 12d exhibited smectic C phase exclusively (Figures 12 and 13). Further, these supramolecules exhibited promising results in a dielectric study, which was carried out between 100 Hz and 2 MHz in the temperature range 50–110 °C. Figure 12 represents the thermal behavior of all four calixarene–azobenzene oligomers.
Scheme 6. Synthesis of Lower Rim Azocalix[4]arene Derivatives (12a–12d).
Figure 12.

Graphical representation of the thermal behavior of azocalix[4]arene derivatives (12a–12d) (considered the heating scan).
Figure 13.

Polarized optical textures: (A) focal conic for compound 12a at 85 °C; (B) schlieren type for compound 12a at 175 °C; (C) rodlike domains obtained for SmC for compound 12c at 97 °C; (D) rodlike domains obtained for SmC for compound 12b; (E) nematic droplets for compound 12b at 200 °C; (F) needlelike pattern of SmC obtained for compound 12d at 92 °C. Reproduced with permission from ref (52). Copyright 2013 The Royal Society of Chemistry.
The same group prepared two homologous series based on azo-ether (13a–13e) and azo-ester (14a–14e) linked calixarene LC oligomers (Scheme 7).53 Among all the derivatives, 13d, 13e, 14d, and 14e with decyloxy and dodecyloxy terminal chains showed smectic C and nematic phases with characteristic optical textures (Figure 14). Further, dielectric studies were reported for the synthesized materials. The lamellar type molecular packing of smectic C phase was confirmed by XRD studies.
Scheme 7. Azo-Ester Linked Lower Rim Substituted Calixarene Derivatives (13a–13e and 14a–14e).
Figure 14.

Graphical representation of the thermal behavior of azo-ester linked lower rim substituted calixarene derivatives (13d, 13e and 14d, 14e) (considered the cooling scan).
2.3. Calixarene Derivatives with Schiff Base Moeities
Schiff bases formed by the condensation of aldehyde and amine functionalized molecules provide an easy access to variety of mesogens in a rapid way, though they have moderate hydrolytic stability. Utilizing this approach, Shinkai et al.54 reported O-methylated calix[n]arenes (n = 4, 6, and 8) coupled to 4-alkylbenzene through a Schiff base linkage, where the alkyl chain length was varied with octyl, dodecyl, tetradecyl, and hexadecyl (Scheme 8). All compounds exhibited flow birefringence with broad melting points. The nature of the mesophase is not clearly illustrated; however, the broad diffused peak in the wide angle for compound 158C16 suggested the liquidlike packing of alkyl chains that is characteristic of a liquid crystalline phase. This phase existed from 20 to 55 °C.
Scheme 8. Structures of LC Calixarenes Reported by Shinkai and Co-workers.
In another interesting report, Komari and Shinkai reported columnar liquid crystals (16a–16e) based on conformationally rigid calix[4]arenes were coupled to C6H4-p-CmH2m+1 (m = 3, 8, 12, 14, and 16) via a Schiff base linkage. It was found that compounds with octyl and higher chain lengths exhibited a Col phase (Figure 16).55 As shown in Figure 15, the crystal to mesophase transition involves the melting of aliphatic chains, while the mesophase to isotropic transition involves the unsettling of calixarene cores.
Figure 16.

Graphical representation of the thermal behavior of cone-shaped calix[4]arenes derivatives (16a–16e) (considered the heating scan).
Figure 15.
Schematic representation of the phase transitions of cone-shaped calix[4]arenes. In K, the molecular motion of the aliphatic chains is frozen, whereas in M it is allowed.55 Reproduced with permission from ref (55). Copyright 1993 CSJ Publisher.
Menon and co-workers prepared six mesogens based on upper/lower rim functionalization on a calix[4]arene core through a Schiff base unit by treating four different types of aldehydes (2-vanillin, 4-vanillin, 4-hydroxy benzaldehyde, and 2-hydroxy naphthaldehyde) with a tetraaminocalix[4[arene core. Further, these compounds were also tested for their dielectric behavior.56 The synthetic route of Schiff base molded calixarene derivatives (17a–17c and 18a–18c) from calix[4]arene (1) is shown in Scheme 9. All six calix[4]arenes exhibited smectic A, smectic C, and nematic mesophases with a wide thermal range (Figure 18). From polarized optical microscopic (POM) texture images, a broken focal conic texture was observed for smectic C, a needle/lancet or fanlike texture was observed for smectic A, and schlieren type textures were observed for nematic phases of these compounds (Figure 17). Subsequently, the same group reported the dielectric properties of calixarene based Schiff base LCs (17a, 17b, 18a, and 18b).57
Scheme 9. Synthesis of Calixarene Schiff Base Derivatives (17a–17e and 18a–18e).
Figure 18.

Graphical representation of the thermal behavior of Schiff base calixarene derivatives (17a–17e and 18a–18e) (considered the heating scan).
Figure 17.

Compound 17c nematic phase at 185 °C (a). SmA phase at 139 °C (b). SmC phase at 88 °C (c). Compound 18c nematic phase at 176 °C (d). SmA phase at 130 °C. (e) SmC phase at 90 °C (f). Reproduced with permission from ref (56). Copyright 2010 Springer.
Romero et al.58 prepared calixarene Schiff base derivatives by the reaction of three different promesogenic aldehydes with tetraaminocalix[4]arene as the rigid core. In this study, all three compounds (19–21) displayed an SmA or nematic mesophase depending on the number of terminal alkyl chains (Figure 19). The synthetic route to prepare calixarene linked Schiff base derivatives is given in Scheme 10. Furthermore, the compounds displayed Zn2+ binding and selectivity. Complexation with the Zn2+ ion increased the ligand’s emission intensity, and a mass study confirmed the presence of 1:2 and 1:1 complexes, respectively. The possible arrangement to form smectic and nematic mesophases is shown in Figure 20.
Figure 19.

Graphical representation of the thermal behavior of Schiff base calixarene derivatives (19, 20, 21, and Zn/20) (considered the heating scan).
Scheme 10. Synthetic Route of Schiff Base Molded Calix[4]arene Derivatives (19–21).
Figure 20.

Proposed arrangements of compounds 19 and 20 in SmA phase (a) and compound 21 in nematic phase (b). Reproduced with permission from ref (58). Copyright 2017 Wiley.
Sharma et al.59 reported two series of lower rim functionalized calixarene Schiff base derivatives. These two homologous series were synthesized from the Schiff base derivatives of 4-amino benzoic acid and 4-n-alkoxy benzaldehyde at the bottom side of the p-tert-butylcalix[4]arene rigid core (Scheme 11). In this work, they noted that compounds 22g–22m in series 1 and 23d–23m in series 2 showed both smectic and nematic phases over a wide temperature range in heating and cooling conditions. Compounds 22f and 23c showed nematic phases exclusively. Lower homologues in both series turned out to be crystalline (Figure 21). Compounds 22c, 22g, 23f, 23g, 23i, and 23j were tested in vitro against two Gram-positive bacteria (Staphylococcus aureus, Streptococcus pyogenes), two Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa), and three fungal strains (Candida albicans, Aspergillus niger, Aspergillus clavatus). These compounds exhibited good antibacterial and antifungal activity.59
Scheme 11. Synthetic Route of Schiff Base Ester Calixarene Derivatives (22a–22m and 23a–23m).

Figure 21.

Graphical representation of the thermal behavior of Schiff base ester calixarene derivatives (a) 22a–22m and (b) 23a–23m (considered the heating scan).
Blue light emitting materials have received considerable attention as they find potential use in the production of white organic light emitting diodes (WOLEDs) utilized in display systems. Calixarene attached with Schiff base units based on 4-n-alkoxy aniline in the lower rim with varying terminal alkyl chain lengths were prepared as shown in Scheme 12.60 All seven bowl-shaped compounds exhibited columnar hexagonal phases with good thermal stability. In comparison to higher alkyl chain analogues, the compounds with shorter chains showed a wider temperature range of the mesophase (Figure 22). All the materials displayed cyan colored fluorescence on exposure to long wavelength UV light, which is promising for their application in OLEDs.
Scheme 12. Synthetic Route of Lower Rim Substituted Calixarene Derivatives (24a–24g).
Figure 22.

Graphical representation of the thermal behavior of Schiff base derivatives (24a–24g) (considered the cooling scan).
Sharma et al.61 reported a calixarene linked with Schiff bases that stabilize the enantiotropic Colh phase with good photophysical and electrochemical properties. The synthetic route of calixarene derivatives (25a–25d) is shown in Scheme 13. In Figure 23, bar graphs represent the thermal behaviors of supramolecular mesogens. An increase in the terminal chain witnessed a lowering in the melting and clearing points of these mesogens. A characteristic mosaic texture was observed for the Colh phases of these compounds (Figure 24). In addition, they exhibited sky-blue fluorescence both in solution and in the thin film state with good quantum yields.
Scheme 13. Preparation of Lower Rim Substituted Schiff Base Ester Derivatives (25a–25d).
Figure 23.

Graphical representation of the thermal behavior of Schiff base ester derivatives (25a–25d) (considered the cooling scan).
Figure 24.

Photomicrographs of 25a at 93.1 °C (a), 25b at 84.2 °C (b), 25c at 76.4 °C (c), and 25d at 66.6 °C (d) on heating from the solid crystalline state as seen under cross polarizers.61 Reproduced with permission from ref (61). Copyright 2020 The Royal Society of Chemistry.
Rathod et al. reported a cone-shaped thiacalix[4]arene appended with quinoline moieties that were stabilizing liquid crystallinity (Scheme 14).62 The thiacalixarene core bearing bisubstituted quinoline units stabilized the Colh phase, when the other two hydroxyl groups were free (26c and 26d) and where the terminal chains were n-octyloxy and n-decyloxy units. In the case of tetrasubstituted thiacalix[4]arene derivatives, lower chain homologues exhibited Colh phases while the higher chain substituted compounds showed nematic phases (Figure 25). Thus, in the class of cone-shaped supramolecules, the number of substitution and the length of the peripheral alkoxy chains affect the phase transition temperature including self-organization of the molecules, which is contrary to traditional disk-shaped mesogens. These materials showed blue fluorescence in solution and in the thin film state under a long UV irradiation. The introduction of quinoline substituted arms on the lower rim of the thiacalix core enhances the intermolecular interactions by enhancing the mesophase width. These compounds can be used for bioimaging applications, which was evidenced by the translocation of the fluorescent molecules in nematodes (Figure 26).
Scheme 14. Preparation of Lower Rim Substituted Schiff Base Ester Derivatives (26a–26d and 27a–27d).
Figure 25.

Graphical representation of the thermal behavior of quinoline armed thiacalix[4]arene derivatives (26a–26d and 27a–27d) (considered the cooling scan).
Figure 26.

Distribution of parent calixarene linked quinoline derivative (27d) in nematodes (Caenorhabditis elegans) (5 μM). (a) Nematode exposed with aqueous THF solution in the blue filter; (b) nematode exposed with aqueous THF solution in green filter; (c, d) nematode exposed with compound 27d in THF solvent.62 Reproduced with permission from ref (62). Copyright 2022 Elsevier.
2.4. Calixarene Derivatives with Stilbene Moieties
Stilbene is a widely investigated fluorophore with linear geometry and photoswitchability, thus finding applications in many organic materials and liquid crystals. Shinkai et al.63 prepared thermotropic liquid crystals (28) stabilized by intermolecular hydrogen bonds formed between a pyridyl linked calix[4]arene core and four p-alkoxy benzoic acids of different chain lengths (CnH2n+1, n = 10, 12, 14, 16, 18) (Figure 27). Except for the complex with 4-n-decyloxybenzoic acid, all the complexes showed Col phases. The increase in the chain length led to an increase in the melting and clearing points (Figure 28). The proposed columnar stacking model for these calixarene based compounds is shown in Figure 27.63
Figure 27.
Structures of calix[4]arenes and 4-n-alkoxybenzoic acids and schematic representation of stacking calix[4]arenes to form Col phase (for chain length 12 and above).63 Reproduced with permission from ref (63). Copyright 1995 Elsevier.
Figure 28.

Graphical representation of the thermal behavior of calix[4]arene derivatives (M10, M12, M14, M16, and M18) (considered the heating scan).
Nierengarten et al.64 developed π-conjugated liquid crystalline derivatives where a calix[4]arene core is connected to four oligophenylenevinylene (OPV) moieties (Scheme 15). Photophysical investigations also revealed strong electronic coupling between the four OPV fragments of the calix[4]OPV derivative. From the XRD studies it was shown that the compound exhibits a liquid crystalline phase with a bilayered arrangement spanning from 140 to 180 °C. However, the corresponding oligophenylacetylene derivative was non liquid crystalline. Such compounds are useful in optoelectronic applications and in the field of host–guest chemistry.
Scheme 15. Synthetic Route for Oligophenylenevinylene Functionalized Calixarene Derivatives (29).
2.5. Calixarene Derivatives with Cinnamic Ester Moieties
Sharma et al.65 reported bowl-shaped molecules with a calix[4]arene connected with 4-n-alkoxy cinnamic acids at four sides (30a–30f) or two sides (31a–31f) of the calix[4]arenes (Scheme 16). All the compounds turned out to be enantiotropic LCs. The disubstituted derivatives showed higher thermal stability of the mesophase as compared to the tetrasubstituted derivatives. The lower (n = 4) and middle member (n = 5, 8, 10, and 12) derivatives from both series showed smectic C and smectic A phases, while the higher member derivatives (n = 14) displayed only the nematic phase without displaying any smectic properties (Figure 29).65
Scheme 16. Synthesis of Bowl-Shaped Calixarene Derivatives Bearing trans-Cinnamic Acid Derivatives (30a–30f and 31a–31f).
Figure 29.
Graphical representation of the thermal behavior of bowl-shaped calixarene derivatives bearing trans-cinnamic acid: 30a–30f (a) and 31a–31f (b) (considered the cooling scan).
2.6. Calixarene Derivatives with Oligophenylene Moities
Calixarene units were directly functionalized with aromatics or through spacers to stabilize macromolecules stabilizing liquid crystallinity. Wong et al. reported two series of tetraoligophenylene substituted luminescent calix[4]arene derivatives (32a–32d and 33a–33f) prepared by Suzuki reaction of arylboronic acid and tetraiodocalixarene and investigated their liquid crystalline properties (Scheme 17).66 Thus, in contrast to the conventional rodlike 4-cyano-4′-alkoxybiphenyl mesogens, which exhibit nematic and/or smectic A phases, cyano-phenyl-calix[4]arene and methyl-biphenyl-calix[4]arene derivatives exhibit highly ordered smectic phases with interdigitated antiparallel packing (Figure 30). The observed POM images of synthesized compounds are shown in Figure 31. These studies proved that the presence of strong dipoles in the calixarene framework is important to stabilize the formation of highly ordered layered structures.
Scheme 17. Synthesis of Tetraoligophenylene Substituted Luminescent Calix[4]arene Derivatives (32/8–32/11, 33a–33e, and 34/8–34/16).
Figure 30.
(a) Graphical representation of the thermal behavior of tetraoligophenylene substituted luminescent calix[4]arene derivatives (32/9, 32/10, and 34/8–34/16) (considered the cooling scan). (b) Phase diagram showing the smectic phase in the 34/n compounds series versus the tail alkoxy carbon number n = 9–16 measured from the second heating cycle. Reproduced from ref (66). Copyright 2006 American Chemical Society.
Figure 31.

Some representative polarized optical microscope textures (magnification 200×): (a) 34/11 at 168 °C, mosaic and lancet texture with some homeotropic area; (b) 34/14 at 148 °C, mosaic texture; (c) 34/15 at 153 °C, grasslike and fanlike texture; (d) 31/9 at 150 °C, mosaic texture. Reproduced from ref (66). Copyright 2006 American Chemical Society.
Yonetake et al.67 developed new calixarene LCs (35 and 36), which were synthesized by reaction of calixarene, tert-butylcalix[8]arene and C-methyloctakis(2-hydroxyethyl)calix[4]resorcinarene, and 11-[(4′-cyano-1,1′-biphenyl-4-yl)oxy]decanoyl chloride in the presence of triethylamine as a base (Scheme 18). Both compounds exhibited smectic mesophases. Compound 35 with tert-butylcalix[8]arene exhibited a highly ordered SmA phase. Compound 36 was found to adopt a specific molecular structure due to the rigid bowlic calix[4]resorcinarene core, i.e., a conelike structure with mesogenic units aligned within the molecule. In addition to SmA phase, this compound also exhibited a nematic phase. The supercooling observed for this compound during the phase transition was very small due to the rigid molecular structure. Such compounds usefully have a memory effect due to their high glass transition temperatures as in the case of side chain polymers.
Scheme 18. Synthetic Route of Calix[4]resorcinarene and Calix[8]arene Based Derivatives (35, 36).
Yonetake et al. introduced calix[4]resorcinarene based mesogenic compounds and their composites with dendrimers (Figure 32) for magneto processing.68 They used photocurable liquid crystals and fixed homogeneous, homeotropic, and bend oriented structures. The LC monomers were irradiated by UV light through a photomask under the influence of a magnetic field. Molecular orientation was used to record the pattern in the film successfully. Using this technique, low viscous branched LCs based on calix[4]resorcinarene 35 and dendrimer (LCD2 decorated with CN-C6) could be aligned under the magnetic field. Through this finding they could align the vapor grown carbon fiber and polycarbonate nanocrystal under the magnetic field.
Figure 32.
Structural formula of calix[4]resorcinarene derivative (35) and LC dendrimer.
2.7. Octahomotetraoxacalix[4]arene Based Derivatives
Octahomotetraoxacalix[4]arene derivatives are less studied but provide a wider rim and enhanced flexibility. Kohmoto et al.69 prepared octahomotetraoxacalix[4]arene based derivatives (37a–37c and 38a–38c) containing long alkyl chains on the lower rim and studied their mesomorphic properties. Scheme 19 depicts the synthesis of target compounds. Among them, compound 38c with an octadecyloxy group showed a smectic A phase (Figure 33). It was also shown that homocalixarenes (38a–38c) formed LC phases with longer layer distances when 2 equiv of 1,2-ethylenediamine was added as a linker. It was interesting to note that, in these cases, 1,2-ethylenediamine played a catalytic role to form the smectic LC phase. An addition of 1,2-ethylenediamine assisted the conformational fixation of cone conformation via the salt formation during equilibrium (38a/EN, 38b/EN, 38c/EN). The cone conformation thus formed was stable and did not revert to other conformations. These are schematically represented in Figure 34.
Scheme 19. Synthesis of Octahomotetracalix[4]arene Derivatives (37a–37c and 38a–38c).
Figure 33.

Graphical representation of the thermal behavior of octahomotetracalix[4]arene derivatives (38a–38c and 38a/EN–38c/EN) (considered the cooling scan).
Figure 34.

Schematic representation of the proposed conformational change of 38 with 1,2-ethylenediamine.69 Reproduced from ref (69). Copyright 2006 American Chemical Society.
2.8. Amphiphilic Calix[4]arene Derivatives
Calixarenes provide the option to functionalize the upper and lower rims with different types of flexible chains (of different miscibilities) to facilitate nanosegregation of incompatible molecular units driving to different kinds of thermotropic and lyotropic self-assemblies. Along similar lines, amphiphilic calix[4]arene compounds with carboxylic acid (39a, 39b) and trimethylammonium moieties (40a, 40b) and variable alkyl chains were prepared by Strobel et al.70 They have investigated the self-assembly of these compounds in an aqueous solution. The carboxylated calixarene mesogens formed vesicles in dilute solutions and stable monolayers on water. On the other hand, calixarene derivatives with ammonium head groups had high water solubilities without showing any aggregation. All calixarene amphiphiles formed lyotropic LCs at high concentrations. This proves that the nature of the ionic head group of amphiphilic calixarenes is a key parameter to control and tune the self-assembly in aqueous solution. It was also shown that the symmetry of the assemblies was independent of the concentration of the calixarene amphiphiles, i.e., carboxylated derivatives exhibited vesicles and lamellar LCs, whereas trimethylammonium derivatives formed micelles below 50 Å diameter that aggregate at higher concentrations into rectangular lyotropic LCs. The synthetic route of calixarene linked derivatives is illustrated in Scheme 20.
Scheme 20. Synthetic Route of Amphiphilic Calixarene Derivatives (39a, 39b; 40a, 40b).
Zakharova et al.71 prepared amphiphilic calix[4]arene derivatives (Figure 35) which are oxyethylated at the lower rim and studied their behavior in water and aqueous–organic solutions (41a, 41b). From POM and XRD studies they established the liquid crystalline organization of these compounds. Analysis of the phase diagram obtained reveals that the parameters of the existence of LC phases are significantly influenced by the structure of substituents at both the upper and lower rims, as well as by the nature of the solvent. It is noteworthy that the LC systems are formed within a wide concentration range of lanthanum ions. This is of high importance from the viewpoint of the template design of novel supramolecular materials with magnetic, luminescent, and catalytic activities.
Figure 35.

(a) Chemical structure of amphiphilic oxyethylated calix[4]arene. (b) Molecular model of 9CO16; gas phase energy minimized structure optimized by MM method with MM2 force field; Chem3D program.71 Reproduced with permission from ref (71). Copyright 2010 Elsevier.
2.9. Calix[4]arene Derivatives with Amide Moieties
Amides are formed by the condensation of acids and amines, which provide hydrogen bonding units to assist the self-assembly. In 2015, Fang et al.72 synthesized gallic-calixarene based derivatives with amide units (42–44) in yields ranging from 57 to 76% (Scheme 21). Gallic-calix[4]arene derivative and gallic-thiacalix[4]arene derivatives possess stable cone conformations, but gallic-calix[6]arene derivatives did not show any stable conformation. Thus, the first two derivatives exhibited a Col LC phase while the last one did not show any LC behavior.
Scheme 21. Synthesis Route of Supramolecular Amide Linked Calixarene Derivatives (42–44).
Lower rim substitution on the three calixarene cores, namely calix[4]arene, calix[6]arene, and thiacalix[4]arene, yielded three supramolecular derivatives. Compounds 42 and 44 showed a stable cone shape with mesomorphic characteristics. On the other hand compound 43 did not show the expected liquid crystalline property due to the lack of a stable structure. The supramolecular derivatives (42 and 44) showed a Colh phase, which was confirmed through POM and differential scanning calorimetry (DSC) studies (Figures 36 and 37).
Figure 36.

Graphical representation of the thermal behavior of gallic-calixarene derivatives (42–44) (considered the cooling scan).
Figure 37.

Schematic representations of the columnar self-assembly of compounds 42 and 43 (a). Textures of compounds 42 and 43 under POM on cooling at 70 °C (b, c). Reproduced with permission from ref (72). Copyright 2015 Elsevier.
In another study, six resorcinarene derivatives (45a–45f) were synthesized with excellent yields using microwave-assisted methodology (Scheme 22).73 All the compounds displayed enantiotropic nematic mesophases (Figures 38 and 39). Further, these Schiff base containing resorcinarene derivatives showed good antimicrobial potency.
Scheme 22. Synthesis of Calix[4]resorcinarene Molded Schiff Base Amide Derivatives (45a–45f).
Figure 38.
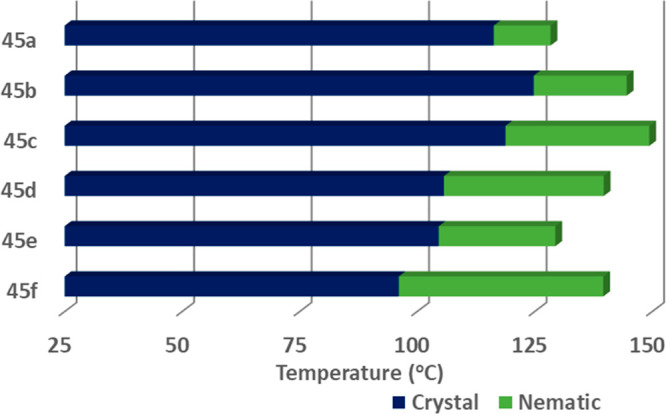
Graphical representation of the thermal behavior of Schiff base functionalized resorcin[4]arene derivatives (45a–45f) (considered the cooling scan).
Figure 39.

Nematic textures of compound 45e at 50 °C (a) and compound 45c at 70 °C (b). Reproduced with permission from ref (73). Copyright 2021 Taylor & Francis.
2.10. Calix[4]arene Derivatives Containing Heterocycles
The introduction of heterocycles in liquid crystals is a rewarding exercise often because of the extra properties they bring to the materials. Heterocycles offer lateral and/or longitudinal dipoles, a particular shape to the molecule, which can be changed based on the number and nature of the heteroatom in the ring, and optical and electronic properties they impart to the molecule. Thus, many heterocycles containing N, O, and S are utilized in the design of liquid crystals with new functional behaviors.74 Thus many research groups combined heterocyclic moieties with calixarene moieties to stabilize liquid crystalline phases.
Budig et al.75 have prepared pyrogallol substituted exo-calix[4]arenes (46a, 46b, and 47a–47e) with 12 3-oxaalkanoyloxy chains and exo-calix[4]arenes in which 8 or 12 rodlike phenylthiadiazole and phenylpyramidine units were connected through spacers with the central calix[4]arene core (Scheme 23). Compound 46a showed a smectic phase, while compound 46b turned out to be crystalline. This could be due to the increase in the length of chain in the upper rim from methyl to propyl. One of the 3-oxaalkanoates (C12 chain, 47b) forms a Colh mesophase, whereas compounds 47c and 47d incorporating heterocyclic calamitic units in the lateral chains give liquid crystalline materials with a smectic A phase. Compound 47e again turned out to be crystalline (Figure 40). Some of the selected compounds form condensed films at the air–water interface as studied with the Langmuir technique, whereby the molecular areas at the collapse points were determined by the densely packed peripheral chains.
Scheme 23. Structures of exo-Calix[4]arene Derivatives.
Figure 40.

Graphical representation of the thermal behavior of exo-calix[4]arene derivatives (46a, 46b, and 47a–47e) (considered the cooling scan).
Recently, Sharma et al. reported calixarene based LCs with upper rims having the azo group and lower rims connected with Schiff bases through ester linking groups.76 In this study, six derivatives (48a–48f) with propyloxy (−OC3H7) and octyloxy (−OC8H17) terminal chains and trisubstituted phenyl thiadiazole unit connected through an ester linkage. The synthetic route of target thiadiazole based calixarene derivatives is shown in Scheme 24. All six derivatives showed a Colh mesophase (Figure 41). Increase in the length of the terminal chain led to a decrease in the melting and clearing temperatures of the compounds. The optical textures observed for columnar self-assembly for these azo based supramolecules are shown in Figure 42. Further, these materials showed blue light emission and good electrochemical properties.
Scheme 24. Preparation of Thiadiazole Linked Azo Calixarene Based Supramolecular Compounds (48a–48f).
Figure 41.

Graphical representation of the thermal behavior of supramolecular calix[4]arene substituted with 1,3,4-thiadiazole derivatives (48a–48f) (considered the cooling scan).
Figure 42.

POM texture images of compound 48a at 142.6 °C (a), compound 48f at 112.9 °C (b), compound 48e at 121.2 °C (c), and compound 48c at 133.8 °C (d), obtained on heating from the solid crystalline state.76 Reproduced with permission from ref (76). Copyright 2019 The Royal Society of Chemistry.
Sharma et al. synthesized 1,3,4-oxadiazole linked calixarene molecules to assess the effect on mesomorphism and thermal stability.77 Six blue light emitting mesogenic compounds (49a–49f) were varied from each other based on the length of the alkyl chain on the phenyl group.
All the compounds exhibited a Colh phase with a wide thermal range. Increase in the peripheral chain length reduced the melting and clearing temperatures (Figures 43 and 44) These compounds exhibited blue light emission in solution and in the thin film state. The synthetic scheme is given in Scheme 25.
Figure 43.

Graphical representation of the thermal behavior of supramolecular calix[4]arene LCs based on oxadiazole derivatives (49a–49f) (considered the cooling scan).
Figure 44.

POM texture image of Colh phase in compound 49c at 131.3 °C (a), compound 49d at 136.6 °C (b), compound 49e at 124.8 °C (c), and compound 49f at 104.2 °C (d), on heating condition as seen under cross polarizers. Reproduced with permission from ref (77). Copyright 2018 Elsevier.
Scheme 25. Synthetic Route of Oxadiazole Linked Schiff Base Calixarene Derivatives (49a–49f).
In a continuation of our work on heterocyclic ring based calixarene LCs, we designed four supramolecular cone-shaped calixarene derivatives by linking them to 1,3,4-oxadiazole (50a, 50b) and 1,3,4-thiadiazole (51a, 51b) units.78 The yields of the desired materials were in the range 71–76% (Scheme 26). All the compounds exhibited a Colh phase (Figure 45). Moreover, these materials showed excellent photophysical behavior with the exhibition of blue light emitting properties. In comparison to oxadiazole linked mesogens, thiadiazole mesogens were found to be efficient with wide range Colh phases. Furthermore, when compared to thiadiazole compounds in solution and thin films, oxadiazole compounds emit a deep blue fluorescence under long wavelength UV radiation. These compounds were also tested for their capability to aggregate in polar and nonpolar solvents. Compounds 50b and 51b were able to form gels in a nonpolar solvent. The development of organogels was confirmed by analyzing the solution’s emission spectra as a function of time and temperature (Figure 46).
Scheme 26. Synthetic Route of Oxadiazole and Thiadiazole Linked Calixarene Derivatives (50a, 50b; 51a, 51b).
Figure 45.
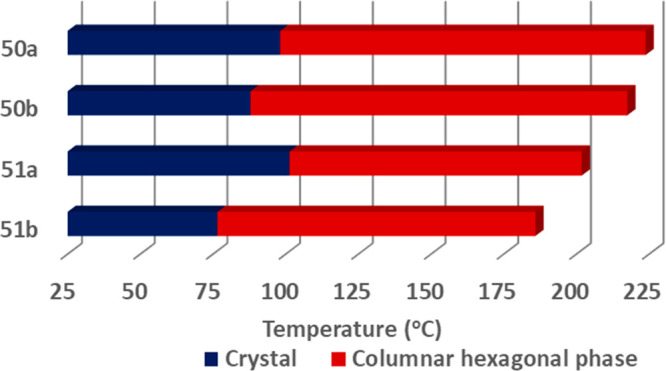
Graphical representation of the thermal behavior of supramolecular calix[4]arene LCs based on oxadiazole and thiadiazole derivatives (50a, 50b; and 51a, 51b) (considered the cooling scan).
Figure 46.

AFM images of the aggregates of compound 50b (a) and compound 51b (b) in pure dodecane (scale bar 1 mm). Images showing solutions of compound 50b (c1) and compound 10d (d1) in daylight. Images showing the formation of gels in daylight and UV light of compound 50b (c2 and c3) and compound 51b (d2 and d3). Reproduced with permission from ref (78). Copyright 2019 The Royal Society of Chemistry.
Recently, we reported a new class of thiacalixarenes decorated with 1,3,4-thiadiazole derivatives and further compared them with results obtained from calixarene linked mesogens.79 Four compounds (52a–52d) were obtained in good yield (69–76%) by reaction of thiacalixarene scaffolds and Schiff base thiadiazole derivatives. The synthetic route for these materials is represented in Scheme 27. All derivatives showed a stable Colh phase with a wide thermal range (Figure 47). Further, these compounds also exhibited blue fluorescence under long UV radiation. One of the compounds was used in the fabrication of a blue OLED; by doping in a CBP host it exhibited an external quantum efficiency of 0.8%. These compounds also showed the ability to form organogels in nonpolar solvents like decane and dodecane (Figure 48). The xerogel of compound 52d exhibited a columnar self-assembly, as was evident from the XRD studies.
Scheme 27. Synthetic Route of Thiadiazole Linked Thiacalixarene Derivatives (52a–52d).
Figure 47.

Graphical representation of the thermal behavior of thiadiazole linked calixarene derivatives (52a–52d) (considered the cooling scan).
Figure 48.

(a, b) Images showing solutions of compound 52c (a1) and compound 52d (b1) in daylight. Gels in daylight and UV light of compound 52c (a1–a3) and compound 52d (b1–b3). Reproduced from ref (79). Copyright 2019 American Chemical Society.
Very recently, our group prepared four calixarene based upper/lower rim functionalized supramolecular derivatives (53a–53d) by Sonogashira coupling reaction of dibromo calixarene scaffold with 4-n-pentyloxy phenyl acetylene (Scheme 28).80 The synthesis involved nine reaction steps to obtain the desired target supramolecules in good yields of 64–71%. All the calixarene based compounds showed an enantiotropic Colh phase with a wide thermal range and with higher thermal stability (Figure 49).
Scheme 28. Synthetic Route of Thiadiazole Linked Calixarene Derivatives (53a–53d).
Figure 49.

Graphical representation of the thermal behavior of thiadiazole linked calixarene derivatives (53a–53d) (considered the cooling scan).
2.11. Calix[4]arene Derivatives Containing Cholesterol Moieties
Cholesterol has been used extensively in the construction of supramolecular materials, liquid crystals,81 and anchors/tethers for model biological membranes82 due to the commercial availability, ease of functionalization, and rigid structure with high chiral purity which is conducive for the various types of self-assembly. In fact, the first reported liquid crystal was cholesteroyl benzoate.83 Thus, there are many reports on the oligomers formed by linking cholesterol units to calix[4]arene.
In 2015, Yang and co-workers reported the first calix[4]arene–cholesterol oligomeric LCs (54a, 54b, 55a, 55b).84 These di- and tetrasubstituted cholesterol linked supramolecules were prepared in good yields by treating calix[4]arene and cholesteryl esters in acetonitrile solvent (Scheme 29). All two side and four side linked cholesterol materials exhibited Col phases. The authors observed that having long spacers and more cholesterol units is more conducive to enhancing the mesophase width (Figure 50). All four derivatives showed the typical focal-conic texture of a Col LC phase (Figure 51).
Scheme 29. Synthetic Routes of Cholesterol Linked Calix[4]arene Derivatives (54a, 54b; 55a, 55b).
Figure 50.

Graphical representation of the thermal behavior of calix[4]arene–cholesterol oligomeric LCs (54a, 54b; and 55a, 55b) (considered the cooling scan).
Figure 51.

POM images obtained for the Col phase of compounds 54a (a), 54b (b), 55a (c), and 55b (d) obtained at 80 °C on cooling the isotropic melt. Reproduced with permission from ref (84). Copyright 2015 Elsevier.
Additionally, Yang et al. also prepared two oligomers bearing cholesterol and calix[4]arene skeletons (56a, 56b) having an imine bridge.85 The complete synthesis of cholesterol–calixarene oligomers is shown in Scheme 30. They investigated the mesogenic properties of the calixarene bowl-shaped column with imine linked cholesterol unit as an ancillary lateral column. These compounds exhibited Col phases. The increase in the spacer length showed a decrease in the melting and clearing points (Figure 52). Further, they also made AgClO4 complexes of these oligomers (56a, 56b); however, they were not mesogenic implying that the ion complexation process may be used to alter the mesogenic behavior of these supramolecular LCs. Figure 53 depicts a schematic illustration of columnar self-assemblies and their behavior on ion complexation.
Scheme 30. Preparation of Cholesterol Linked Calix[4]arene Derivatives (56a, 56b).
Figure 52.

Graphical representation of the thermal behavior of calix[4]arene–cholesterol derivatives with Schiff base bridges (56a, 56b) (considered the cooling scan).
Figure 53.

Schematic representation of (a) columnar layered molecular arrangements, (b) their dimensions, and (c) the complexation models. Reproduced with permission from ref (85). Copyright 2016 Elsevier.
In another study, Guo et al.86 linked four cholesterol units on calix[4]resorcinarene and investigated the liquid crystalline properties. The synthetic route for the preparation of these compounds (57a, 57b) is given in Scheme 31. These compounds exhibited a Colh phase. The mesogenic properties were dependent on the structures of the spacers. Incorporation of rigid Schiff base spacers led to the increase of phase transition temperatures and mesophase thermal ranges, while soft alkyl spacers reversed the trend by having broad transition and increased viscosity (Figure 54). The typical focal-conic and band-type textures obtained for the Colh phase of resorcinarene based compounds 57a, 57b, 58a, and 58b are presented in Figure 55.
Scheme 31. Synthetic Route of Calix[4]resorcinarene Linked Cholesterol Derivatives (57a, 57b; 58a, 58b).
Figure 54.

Graphical representation of the thermal behavior of calix[4]resorcinarene–cholesterol LCs (57a, 57b; 58a, 58b) (considered the cooling scan).
Figure 55.

Mesomorphic texture of Colh phase obtained under POM on cooling for compounds 57a, 57b, 58a, and 58b at 75, 90, 170, and 160 °C (500×) (a–d). Reproduced with permission from ref (86). Copyright 2017 Elsevier.
2.12. Calix[4]arene Derivatives Containing Triphenylene Moieties
Hexaalkoxy triphenylene is considered as a workhorse in the field of discotic liquid crystals. The structure can be easily modified to attain different mesomorphic behaviors. There are several oligomeric and polymeric liquid crystals reported, where the triphenylene acts as a major component.87−89 Many research groups tried to connect a calixarene core with triphenylene units with spacers of different lengths and natures. In 2011, Yang’s group90 synthesized the first macrocyclic compounds based on a calixarene core connected with a discotic triphenylene moiety in good yields by varying the spacer length (hexyl and decyl) of the triphenylene unit (59a, 59b). The liquid crystalline behavior was seen in a symmetrical calix[4]arene linked triphenylene dimer with a decyl spacer (59b) (Figure 56). The length of the peripheral side chain, length of the spacer, molecular planarity, and stability of the calixarene geometry played a crucial role in the mesomorphic features of calixarene linked triphenylenes. The synthetic route to these mesogens is given in Scheme 32.90 To study the influence of the conformation on the properties of the materials, thiacalix[4]arene, which was bridged by S groups with more flexible conformation reversal, was tried (Scheme 33). Here only monosubstituted thiacalix[4]triphenylene derivatives were obtained, but they were crystalline. Thus, the atoms on the rim also affect the reactivity and conformational stability of the resultant molecules.
Figure 56.

Graphical representation of the thermal behavior of calixarene linked discotic triphenylene derivatives (59a, 59b; 60a, 60b) (considered the heating scan).
Scheme 32. Synthesis Route of Calixarene Linked Discotic Triphenylene Derivatives (59a, 59b).
Scheme 33. Synthesis Route of Thiacalix[4]triphenylene Derivatives (60a, 60b).
In continuation with their work on triphenylene–calix[4]arene LCs, Yang and co-workers reported calixarene linked symmetrical triphenylene dimers (61, 62) in good yields and investigated their mesomorphic properties.91 The nature of the spacer was varied either by having an aromatic amido spacer or with a hydrazine spacer.
The results obtained were more promising compared to their previous work on calix[4]arene linked triphenylene mesogens. The systematic routes of syntheses of compounds 61 and 62 are shown in Schemes 34 and 35, respectively. The optical textures obtained for both compounds are presented in Figure 57. The synthesized compounds showed enantiotropic Col phases with low clearing temperatures. Further, the columnar stacking behavior of compound 61 was also confirmed by atomic force microscopy (AFM) by the tapping method. Due to the presence of amide units, compound 61 exhibited higher melting and clearing temperatures than compound 62 (Figure 58).
Scheme 34. Synthetic Route of Triphenylene Linked Amide Based Calixarene Derivatives (61).
Scheme 35. Synthetic Route of Triphenylene Linked Hydrazone Based Calixarene Derivatives (62).
Figure 57.

Textures of columnar phase of triads 61 and 62 under POM on cooling at 45 °C (400×) (a, b). Reproduced with permission from ref (91). Copyright 2012 Elsevier.
Figure 58.

Graphical representation of the thermal behavior of symmetrical triads of triphenylene–calix[4]arene–triphenylene columnar LCs (61, 62) (considered the cooling scan).
Yang et al.92 reported another set of symmetrical triphenylene dimers bridged by a central calixarene unit (63a, 63b) connected with hydrazone groups. In this study, they discovered an interesting mesomorphic change between two columnar phases caused by ion complexation. Further, they showed that the silver complexes of materials 63a and 63b with triphenylene moieties also displayed enantiotropic Col phases with wide thermal ranges which were higher than those of the uncomplexed compounds (Figure 59). The route of chemical synthesis of triphenylene based mesogens (63a, 63b) is shown in Scheme 36. A schematic representation of the self-assembly of the neat compound and the silver complex of the same to form a columnar layered phase is represented in Figure 60.
Figure 59.

Graphical representation of the thermal behavior of symmetrical triads of triphenylene–calix[4]arene–triphenylene columnar LCs (63a, 63b) and their complexes (63a+Ag+, 63b+Ag+) (considered the cooling scan).
Scheme 36. Synthetic Route of Novel Triads of Triphenylene–Calix[4]arene–Triphenylenes (63a, 63b).
Figure 60.

Two kinds of schematic representation of the columnar layered molecular arrangement for triads of triphenylene–calixarene–triphenylenes 63a and 63b before and after complexation.92 Reproduced with permission from ref (92). Copyright 2013 Elsevier.
In a similar work, Hong et al.93 synthesized triphenylene substituted calixarene derivatives (64a, 64b) via the click reaction and investigated their mesogenic properties. In this study, they studied the liquid crystalline behaviors of compounds before and after complexation with metal salts like NaSCN, AgSCN, and NaCl. The synthetic route for the triazole based mesogens is shown in Scheme 37. It was observed that the compounds (64a, 64b) exhibited a mesomorphic state, but their complexes did not display any LC properties. Figure 61 shows the POM images at specific temperatures, with both derivatives having fanlike textures. These texture patterns were similar to the well-known reported POM images of triphenylene substituted mesogens. Both compounds exhibited an enantiotropic Col phase with a wide thermal range (Figure 62).
Scheme 37. Synthetic Route of Calixarene Linked Azide–Triphenylene Derivatives (64a, 64b).
Figure 61.

Fanlike textures of columnar phase in compounds 64a and 64b (a, b) were obtained with polarized optical microscopy on cooling at 90 °C (400×). Reproduced with permission from ref (93). Copyright 2014 Elsevier.
Figure 62.

Graphical representation of the thermal behavior of calixarene linked discotic triphenylene columnar LCs (64a, 64b) (considered the cooling scan).
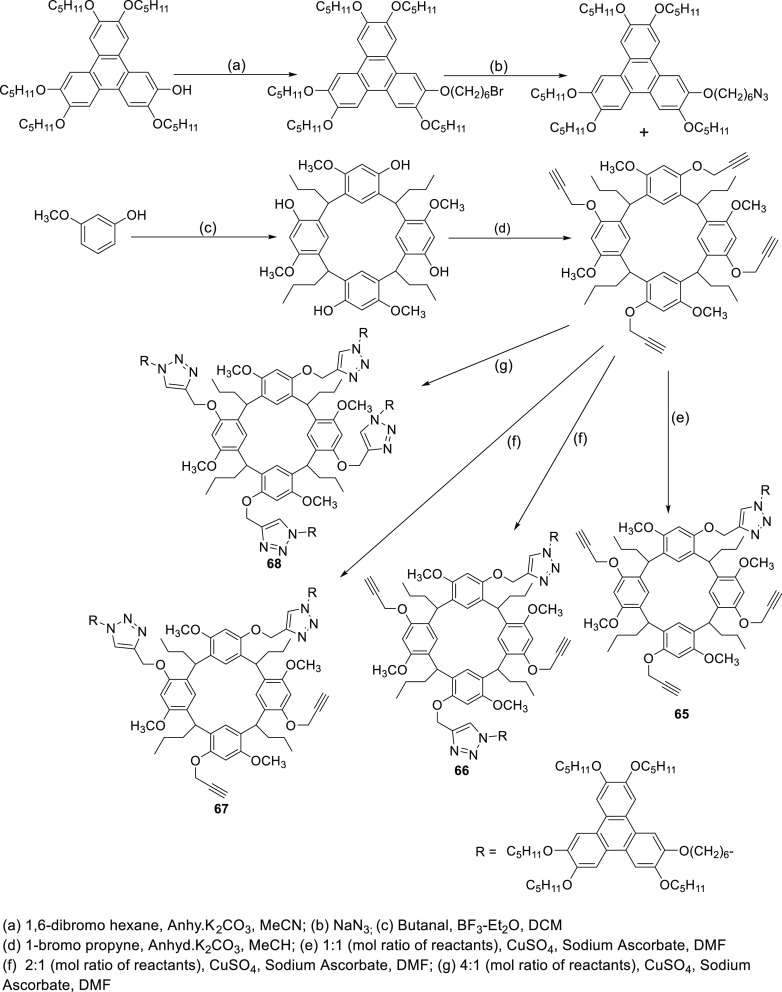
Yang and co-workers, by controlling the molar ratios of reactants, prepared calix[4]resorcinarene–triphenylene monomer, dimer, and tetramers via click chemistry and studied their thermal behaviors (Scheme 38).94 They summarized that an increase in the number of triphenylene moieties on the resorcinarene core provides a higher mesophase temperature range and stability. The typical focal-conic texture images of compounds 65–67 observed in the cooling condition is shown in Figure 63. X-ray studies confirmed that the monomer (65) and dimers (66, 67) had mixed columnar mesophases with the Colh structure and the disordered lamellar type columnar structure (ColL), whereas compound 68 exhibited only a disordered lamellar columnar structure (Figure 64). Figure 65 represents the organization of tetramer 65 into a lamellar columnar structure.
Scheme 38. Synthetic Route of Calix[4]resorcinarene–Triphenylene Oligomers (65–68).
Figure 63.
Mesomorphic columnar hexagonal textures of compounds 65 (a) and 66/67 (b) and distorted lamellar type columnar phase of compound 68 (c) obtained under POM on cooling at 70 °C (500×). Reproduced with permission from ref (94). Copyright 2017 Taylor & Francis.
Figure 64.

Graphical representation of the thermal behavior of calix[4]resorcinarene–triphenylene oligomer columnar LCs (65–68) (considered the cooling scan).
Figure 65.

Two possible schematic representations of the hexagonal columnar phase and lamellar columnar structure for compound 65.94 Reproduced with permission from ref (94). Copyright 2017 Taylor & Francis.
Patel et al.95 recently reported similar triphenylene derivatives with central thiacalixarene spacers (Scheme 39). These molecules displayed blue light emitting properties in solution as well as thin films with high good quantum yields. All the compounds exhibited a room temperature Colh phase (Figure 66). With the increase in the spacer length, a lowering of melting and clearing temperatures was noticed. These compounds displayed excellent aggregation induced emission (AIE) behavior in the THF–H2O system with addition to the intramolecular charge transfer (ICT) effect. They have also shown good electrochemical behavior as confirmed by cyclic voltammetry study and further compared with computational study results to correlate the electronic properties and reactivity.95 These compounds were further tested for their dielectric behavior in the frequency range from 20 Hz to 2 MHz and showed good dielectric behavior in the thermal range of 20–50 °C.
Scheme 39. Synthetic Route of Thiacalixarene Linked Triphenylene Derivatives (69a–69d).
Figure 66.

Graphical representation of the thermal behavior of thiacalixarene linked triphenylene based room temperature columnar LCs (69a–69d) (considered the cooling scan).
2.13. Calix[4]arene Derivatives Containing Chalcone Moieties
Chalcones are prepared by the Claisen–Schmidt base catalyzed condensation of aromatic ketones and aldehydes. These compounds have extended conjugation and hence are colored. Apart from biological activity, they are also known as materials for photoalignment, photo-cross-linkers, and fluorescent dyes and as nonlinear optically (NLO) active materials. Thus, many liquid crystals were prepared by using chalcone derivatives.96,97
Our research group prepared four calixarene derivatives (70a–70d) substituted by peripheral chains containing biphenyl amine chalcone moieties (Scheme 40).98 In this report, we described seven reaction steps to obtain target compounds in good yield (64–73%) with cone confirmation as depicted in Scheme 33. All synthesized materials showed high thermal stability and a wide range enantiotropic Colh phase (Figure 67). The compounds displayed blue light emission with good quantum yields. One of the compounds (70d) was used to fabricate blue OLEDs, as either neat or doped conditions in polyvinyl carbazole host. Compound 70d showed the best electroluminescence results in the series, with a 0.71% external quantum efficiency. Here, with the increase in the peripheral chain length, a gradual drop in the melting and clearing temperatures was noted.
Scheme 40. Synthetic Route of Chalcone Linked Calixarene Derivatives (70a–70d).
Figure 67.

Graphical representation of the thermal behavior of chalcone–biphenyl amine functionalized supramolecular columnar LCs (70a–70d) (considered the cooling scan).
3. CONCLUSION AND FUTURE PERSPECTIVES
In the past decade, calixarene based materials have garnered huge interest for various applications. Calixarene based materials are extensively used as supramolecular hosts for many guests, and also as motifs for molecular self-assembly. The substituted calixarene LC compounds can be easily prepared in good yield by the upper/lower rim functionalization of the calixarene core. These compounds stabilized the mesophases that appear for calamitic and discotic LCs over a wide thermal range with low melting and clearing temperatures. They have also shown the capability to form organogels with ordered molecular arrangements.
An understanding of the thorough structure–property relationships is required to realize their full potential and successful application in the fields of electronics and optics. Host–guest complexation is a good option to realize new modes of self-assembly and functions; further, it may unravel a new area of supramolecular polymers. Creating porous organic polymers and porous membranes is another area of application that can be investigated. Introduction of the amphiphilic nature in calixarene opens a new avenue for the utilization of this class of molecules in chemical biology and biomembrane studies. Incorporation of fluorophores in the molecular structure also helps in bioimaging, sensing, and organic light emitting applications. Introduction of electroactive molecular units will help in the realization of self-assembling organic materials that will be useful in organic electronics. In this regard, we have summarized some of the recent developments in the synthesis of calixarene based materials and their liquid crystalline behaviors in this review. We believe that this review article will be useful for researchers working in this area of supramolecular chemistry, liquid crystals, and self-assembly.
Acknowledgments
The authors gratefully acknowledge the Department of Chemistry, School of Science, Gujarat University, Ahmedabad, for their support. INFLIBNET Gandhinagar is acknowledged for providing e-source facilities. V.S.S. gratefully acknowledges the Human Resource Development Group, Council of Scientific & Industrial Research (CSIR), New Delhi, for a research associate fellowship (File No. 09/070(0075)/2020 EMR-I).
Author Contributions
∥ V.S.S. and V.K.V. made equal contributions.
A.S.A. sincerely thanks the Science and Engineering Research Board (SERB) DST, Government of India, and BRNS-DAE for funding this work through Project CRG/2018/000362 and No. 2012/34/31/BRNS/1039, respectively. We thank the Ministry of Human Resource and Development for the Centre of Excellence in FAST (F. No. 5-7/2014-TS-VII).
The authors declare no competing financial interest.
References
- Collings P. J.Liquid Crystals: Nature’s Delicate Phase of Matter; Princeton University Press: 2002. [Google Scholar]
- Pal S. K.; Kumar S.. Liquid Crystal Dimers; Cambridge University Press: 2017. [Google Scholar]
- Kumar S.Chemistry of Discotic Liquid Crystals: From Monomers to Polymers; CRC Press: Boca Raton, FL, 2011. [Google Scholar]
- Faul C. F. J. Ionic Self-Assembly for Functional Hierarchical Nanostructured Materials. Acc. Chem. Res. 2014, 47, 3428–3438. 10.1021/ar500162a. [DOI] [PubMed] [Google Scholar]
- Zhai J.; Fong C.; Tran N.; Drummond C. J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. 10.1021/acsnano.8b07961. [DOI] [PubMed] [Google Scholar]
- Lagerwall J. P.; Scalia G. A new era for liquid crystal research: Applications of liquid crystals in soft matter nano-, bio- and microtechnology. Curr. Appl. Phys. 2012, 12, 1387–1412. 10.1016/j.cap.2012.03.019. [DOI] [Google Scholar]
- Woon K. L.; Aldred M. P.; Vlachos P.; Mehl G. H.; Stirner T.; Kelly S. M.; O’Neill M. Electronic Charge Transport in Extended Nematic Liquid Crystals. Chem. Mater. 2006, 18, 2311–2317. 10.1021/cm0601335. [DOI] [Google Scholar]
- Dmochowska E.; Herman J.; Czerwiński M.; Stulov S.; Bubnov A.; Kula P. Self-Assembling Behaviour of Chiral Calamitic Monoacrylates Targeted for Polymer Stabilisation of Polar Smectic Phases in Chiral Liquid Crystals. J. Mol. Liq. 2021, 331, 115723. 10.1016/j.molliq.2021.115723. [DOI] [Google Scholar]
- Panja S.; Adams D. J. Stimuli-responsive dynamic transformations in supramolecular gels. Chem. Soc. Rev. 2021, 50, 5165–5200. 10.1039/D0CS01166E. [DOI] [PubMed] [Google Scholar]
- Zola R. S.; Bisoyi H. K.; Wang H.; Urbas A. M.; Bunning T. J.; Li Q. Dynamic Control of Light Direction Enabled by Stimuli-Responsive Liquid Crystal Gratings. Adv. Mater. 2019, 31, 1806172. 10.1002/adma.201806172. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wang J.; Yang Y.; Yang F.; Wu D. Fabrication of Multi-Stimuli Responsive Supramolecular Hydrogels Based on Host-Guest Inclusion Complexation of a Tadpole-Shaped Cyclodextrin Derivative with the Azobenzene Dimer. Polym. Chem. 2017, 8, 3901–3909. 10.1039/C7PY00698E. [DOI] [Google Scholar]
- Haas W. E. Liquid crystal adiSplay research: the first fifteen years. Mol. Cryst. Liq. Cryst. 1983, 94, 1–31. 10.1080/00268948308084244. [DOI] [Google Scholar]
- Prakash J.; Parveen A.; Mishra Y. K.; Kaushik A. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 168, 112562. 10.1016/j.bios.2020.112562. [DOI] [PubMed] [Google Scholar]
- Etxebarria J.; Blanca Ros M. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926. 10.1039/b803507e. [DOI] [Google Scholar]
- Reddy R. A.; Tschierske C. Bent-Core Liquid Crystals: Polar Order, Superstructural Chirality and Spontaneous Desymmetrisation in Soft Matter Systems. J. Mater. Chem. 2006, 16, 907–961. 10.1039/B504400F. [DOI] [Google Scholar]
- Shanker G.; Yelamaggad C. V. Synthesis and Phase Transitional Behavior of Dimer-Like Optically Active Liquid Crystals. J. Phys. Chem. B 2011, 115, 10849–10859. 10.1021/jp206224k. [DOI] [PubMed] [Google Scholar]
- Goodby J. W. Twist grain boundary and frustrated liquid crystal phases. Curr. Opin. Colloid Interface Sci. 2002, 7, 326. 10.1016/S1359-0294(02)00090-0. [DOI] [Google Scholar]
- Hird M. Fluorinated liquid crystals–properties and applications. Chem. Soc. Rev. 2007, 36, 2070. 10.1039/b610738a. [DOI] [PubMed] [Google Scholar]
- Bisoyi H. K.; Kumar S. Liquid-crystal nanoscience: an emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306. 10.1039/B901793N. [DOI] [PubMed] [Google Scholar]
- Devadiga D.; Ahipa T. N. Recent synthetic advances in pyridine-based thermotropic mesogens. RSC Adv. 2019, 9, 23161. 10.1039/C9RA04389F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoyi H. K.; Kumar S. Discotic nematic liquid crystals: science and technology. Chem. Soc. Rev. 2010, 39, 264. 10.1039/B901792P. [DOI] [PubMed] [Google Scholar]
- Sergeyev S.; Pisula W.; Geerts Y. H. Discotic liquid crystals: a new generation of organic semiconductors. Chem. Soc. Rev. 2007, 36, 1902. 10.1039/b417320c. [DOI] [PubMed] [Google Scholar]
- Vishwakarma V. K.; Sudhakar A. S. Structure-property relationships of quinoxaline-based liquid crystals. Soft Matter 2021, 17, 8221. 10.1039/D1SM00932J. [DOI] [PubMed] [Google Scholar]
- Garbovskiy Y.; Emelyanenko A. V.; Glushchenko A. Inverse “guest–host” effect: ferroelectric nanoparticles mediated switching of nematic liquid crystals. Nanoscale. 2020, 12, 16438. 10.1039/D0NR05301E. [DOI] [PubMed] [Google Scholar]
- Steck K.; Dieterich S.; Stubenrauch C.; Giesselmann F. Surfactant-based lyotropic liquid crystal gels-the interplay between anisotropic order and gel formation. Journal of Materials Chemistry C 2020, 8, 5335–5348. 10.1039/D0TC00561D. [DOI] [Google Scholar]
- Pathak S. K.; Nath S.; De J.; Pal S. K.; Achalkumar A. S. The Effect of Regioisomerism on the Mesomorphic and Photophysical Behavior of Oxadiazole-Based Tris(N-salicylideneaniline)s: Synthesis and Characterization. New J. Chem. 2017, 41, 9908–9917. 10.1039/C7NJ01766A. [DOI] [Google Scholar]
- Gupta R. K.; Das D.; Gupta M.; Pal S. K.; Iyer P. K.; Achalkumar A. S. Electroluminescent room temperature columnar liquid crystals based on bay-annulated perylene tetraesters. J. Mater. Chem. C 2017, 5, 1767–1781. 10.1039/C6TC04166C. [DOI] [Google Scholar]
- Bala I.; De J.; Gupta S. P.; Singh H.; Pandey U. K.; Pal S. K. High Hole Mobility in Room Temperature Discotic Liquid Crystalline Tetrathienoanthracenes. Chem. Commun. 2020, 56, 5629–5632. 10.1039/D0CC01226B. [DOI] [PubMed] [Google Scholar]
- Paladugu S.; Kaur S.; Mohiuddin G.; Pujala R. K.; Pal S. K.; Dhara S. Microrheology to probe smectic clusters in bent-core nematic liquid crystals. Soft Matter 2020, 16, 7556–7561. 10.1039/D0SM00796J. [DOI] [PubMed] [Google Scholar]
- De J.; M. M. A. H.; Yadav R. A. K.; Gupta S. P.; Bala I.; Chawla P.; Kesavan K. K.; Jou J.-H.; Pal S. K. AIE-Active Mechanoluminescent Discotic Liquid Crystals for Applications in OLEDs and Bio-Imaging. Chem. Commun. 2020, 56, 14279–14282. 10.1039/D0CC05813K. [DOI] [PubMed] [Google Scholar]
- Gutsche C. D.; Muthukrishnan R. Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem. 1978, 43, 4905–4906. 10.1021/jo00419a052. [DOI] [Google Scholar]
- Sameni S.; Jeunesse C.; Matt D.; Harrowfield J. Calix[4]arene daisychains. Chem. Soc. Rev. 2009, 38, 2117–2146. 10.1039/b900183b. [DOI] [PubMed] [Google Scholar]
- McIldowie M. J.; Mocerino M.; Ogden M. I. A Brief Review of Cn-Symmetric Calixarenes and Resorcinarenes. Supramol. Chem. 2010, 22, 13–39. 10.1080/10610270902980663. [DOI] [Google Scholar]
- Ryu E. H.; Zhao Y. Efficient Synthesis of Water Soluble Calixarenes using Click Chemistry. Org. Lett. 2005, 7, 1035–1037. 10.1021/ol047468h. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Sun Y.; Tian D.; Shin W. S.; Kim J. S.; Li H. Selective Molecular Recognition on Calixarene Functionalized 3D Surfaces. Chem. Commun. 2016, 52, 12685–12693. 10.1039/C6CC05876K. [DOI] [PubMed] [Google Scholar]
- Gorbunov A.; Kuznetsova J.; Deltsov I.; Molokanova A.; Cheshkov D.; Bezzubov S.; Kovalev V.; Vatsouro I. Selective azide-alkyne cycloaddition reactions of azidoalkylated calixarenes. Org. Chem. Front. 2020, 7, 2432–2441. 10.1039/D0QO00650E. [DOI] [Google Scholar]
- Yang F.; Guo H.; Vicens J. Mini-Review: Calixarene Liquid Crystals. J. Inclusion Phenom. Macrocyclic Chem. 2014, 80, 177–186. 10.1007/s10847-014-0394-6. [DOI] [Google Scholar]
- Joseph R.; Rao C. P. Ion and Molecular Recognition by Lower Rim 1,3-Di-conjugates of Calix[4]arene as Receptors. Chem. Rev. 2011, 111, 4658–4702. 10.1021/cr1004524. [DOI] [PubMed] [Google Scholar]
- Kim J. S.; Quang D. T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. 10.1021/cr068046j. [DOI] [PubMed] [Google Scholar]
- Galindres D. M.; Cifuentes D.; Tinoco L. E.; Murillo-Acevedo Y.; Rodrigo M. M.; Ribeiro A. C. F.; Esteso M. A. A Review of the Application of Resorcinarenes and SBA-15 in Drug Delivery. Processes 2022, 10, 684. 10.3390/pr10040684. [DOI] [Google Scholar]
- Cometti G.; Dalcanale E.; Vosel A. D.; Levelut A.-M. A new, conformationally mobile macrocyclic core for bowl-shaped columnar liquid crystals. Liq. Cryst. 1992, 11, 93–100. 10.1080/02678299208028973. [DOI] [Google Scholar]
- Cometti G.; Dalcanale E.; Du Vosel A.; Levelut A. M. New bowl-shaped columnar liquid crystals. J. Chem. Soc., Chem.Commun. 1990, 2, 163–165. 10.1039/c39900000163. [DOI] [Google Scholar]
- Bonsignore S.; Cometti G.; Dalcanale E.; Du Vosel A. New columnar liquid crystals Correlation between molecular structure and mesomorphic behaviour. Liq. Cryst. 1990, 8, 639–649. 10.1080/02678299008047377. [DOI] [Google Scholar]
- Abis L.; Arrighi G.; Cometti E.; Dalcanale E.; Du Vosel A. Deuterium NMR investigation of a new class of macrocyclic columnar liquid crystal. Liq. Cryst. 1991, 9, 277–284. 10.1080/02678299108035505. [DOI] [Google Scholar]
- Gajjar J.; Vekariya R. H.; Sharma V. S.; Parekh H. Synthesis, design and characterization of supramolecular self-assembly of calix[4]resorcinare substituted LCs. Mol. Cryst. Liq. Cryst. 2018, 668, 48–58. 10.1080/15421406.2018.1562616. [DOI] [Google Scholar]
- Sharma V. K.; Rathod S. L.; Suthar D.; Sharma A.; Ganga V. S. R.; Desai V.; Shrivastav P. Resorcinarene appended octa-substituted alkyl arms: a new strategy to fabricate supramolecular material for liquid crystal and solar-cell application. New J. Chem. 2022, 10.1039/D2NJ03792K. [DOI] [Google Scholar]
- Li Q.Photoactive Functional Soft Materials: Preparation, Properties, and Applications; Wiley-VCH: Weinheim, 2019. [Google Scholar]
- Xu B.; Swager T. M. Rigid bowlic liquid crystals based on tungsten-oxo calix[4]arenes: host-guest effects and head-to-tail organization. J. Am. Chem. Soc. 1993, 115, 1159–1160. 10.1021/ja00056a056. [DOI] [Google Scholar]
- Xu B.; Swager T. M. Host-Guest Mesomorphism: Cooperative Stabilization of a Bowlic Columnar Phase. J. Am. Chem. Soc. 1995, 117, 5011–5012. 10.1021/ja00122a039. [DOI] [Google Scholar]
- Oh S. K.; Nakagawa M.; Ichimura K. J. Relationship between the ability to control liquid crystal alignment and wetting properties of calix[4]resorcinarene monolayers. J. Mater. Chem. 2001, 11, 1563–1569. 10.1039/b007739i. [DOI] [Google Scholar]
- Matsuzawa Y.; Seki T.; Ichimura K. Mixed Monolayers of a Calix[4]resorcinarene with a Nematic Liquid Crystal and Their Bilayered Structuring. Chem. Lett. 1998, 27, 411–412. 10.1246/cl.1998.411. [DOI] [Google Scholar]
- Sutariya P. G.; Modi N. R.; Pandya A.; Rana V. A.; Menon S. K. Synthesis, mesomorphism and dielectric behaviour of novel basket shaped scaffolds constructed on lower rim azocalix[4]arenes. RSC Advances. 2013, 3, 4176–4180. 10.1039/c3ra22422h. [DOI] [Google Scholar]
- Sutariya P. G.; Pandya A.; Rana V. A.; Menon S. K. The influence of linking group in exterior point on mesogenic properties of the basket moulded molecules: calix[4]arene. Liq. Cryst. 2013, 40, 374–383. 10.1080/02678292.2012.749305. [DOI] [Google Scholar]
- Komori T.; Shinkai S. A New Class of Mesomorphic Materials Designed from Calix[n]arenes. Chem. Lett. 1992, 21, 901–904. 10.1246/cl.1992.901. [DOI] [Google Scholar]
- Komori T.; Shinkai S. Novel Columnar Liquid Crystals Designed from Cone-shaped Calix[4]arenes. The Rigid Bowl Is Essential for the Formation of the Liquid Crystal phase. Chem. Lett. 1993, 22, 1455–1458. 10.1246/cl.1993.1455. [DOI] [Google Scholar]
- Patel R. V.; Panchal J. G.; Rana V. A.; Menon S. K. Liquid crystals based on calix[4]arene Schiff bases. J. Incl Phenom. Macrocycl. Chem. 2010, 66, 285–295. 10.1007/s10847-009-9620-z. [DOI] [Google Scholar]
- Menon S. K.; Patel R. V.; Panchal J. G.; Mistry B. R.; Rana V. A. Dielectric study of novel liquid crystals based on calix[4]arene Schiff bases. Liq. Cryst. 2011, 38, 123–134. 10.1080/02678292.2010.524943. [DOI] [Google Scholar]
- Romero J.; Barbera J.; Blesa M. J.; Concellon A.; Romero P.; Serrano S. L.; Marcos M. Liquid Crystal Organization of Calix[4]arene-Appended Schiff Bases and Recognition towards Zn2+. Chemistry Select. 2017, 2, 101–109. 10.1002/slct.201601826. [DOI] [Google Scholar]
- Sharma V. S.; Singh H. K.; Vekariya R. H.; Sharma A. S.; Patel R. B. Mesomorphic Properties of Novel Supramolecular Calix[4]arene Schiff Base Ester Derivatives: Design and Biological Investigation. Chemistry Select. 2017, 2, 8596–8606. 10.1002/slct.201701306. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Vekariya R. H. Columnar self-assembly of bowl shaped fluorescent liquid crystals based on calix[4]arene with Schiff base units. New J. Chem. 2018, 42, 15044–15051. 10.1039/C8NJ01721B. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Worthington S. J. B.; Shah P.; Shrivastav P. Columnar self-assembly, electrochemical and luminescence properties of basket-shaped liquid crystalline derivatives of Schiff-base-moulded p-tert-butyl-calix[4]arene. New J. Chem. 2020, 44, 20610–20619. 10.1039/D0NJ04148C. [DOI] [Google Scholar]
- Rathod S. L.; Sharma V. S.; Sharma A. S.; Athar M.; Shrivastav P. S.; Parekh H. M. Blue light-emitting Quinoline armed Thiacalix [4]arene 3D-scaffold: A systematic platform to construct fluorescent liquid crystals with bio-imaging applications. J. Mol. Struct. 2022, 1270, 133830. 10.1016/j.molstruc.2022.133830. [DOI] [Google Scholar]
- Koh K. N.; Araki K.; Komori T.; Shinkai S. Thermotropic liquid crystal construction through the hydrogen-bonds between a stilbazole calix[4]arene and carboxylic acids. Tetrahedron Lett. 1995, 36, 5191–5194. 10.1016/0040-4039(95)00918-3. [DOI] [Google Scholar]
- Gu T.; Accorsi G.; Armaroli N.; Guillon D.; Nierengarten J. F. Calix[4]oligophenylenevinylene: a new rigid core for the design of π-conjugated liquid crystalline derivatives. Tetrahedron Lett. 2001, 42, 2309–2312. 10.1016/S0040-4039(01)00169-1. [DOI] [Google Scholar]
- Sharma V. S.; Singh H. K.; Sharma A. S.; Shah A. P.; Shah P. A. Bowl-shaped fluorescent liquid crystals derived from 4-tert butyl calix[4]arene and trans cinnamic acid derivatives. New J. Chem. 2019, 43, 15575–15584. 10.1039/C9NJ03097B. [DOI] [Google Scholar]
- Lo P. K.; Chen D.; Meng Q.; Wong M. S. Highly Ordered Smectic Phases from Polar Calix[4]arene Derivatives. Chem. Mater. 2006, 18, 3924–3930. 10.1021/cm0607076. [DOI] [Google Scholar]
- Yonetake K.; Nakayama T.; Ueda M. New liquid crystals based on calixarenes. J. Mater. Chem. 2001, 11, 761–767. 10.1039/b005461p. [DOI] [Google Scholar]
- Yonetake K.; Takahashi T. New material design for liquid crystals and composites by magneto-processing. Sci. Technol. Adv. Mater. 2006, 7, 332–336. 10.1016/j.stam.2006.02.008. [DOI] [Google Scholar]
- Kohmoto S.; Someya Y.; Masu H.; Yamaguchi K.; Kishikawa K. Liquid Crystal and Crystal Structure of Octahomotetraoxacalix[4]arenes. J. Org. Chem. 2006, 71, 4509–4515. 10.1021/jo060355m. [DOI] [PubMed] [Google Scholar]
- Strobel M.; Kita-Tokarczyk K.; Taubert A.; Vebert C.; Heiney P.; Chami M.; Meier W. Self-Assembly of Amphiphilic Calix[4]arenes in Aqueous Solution. Adv. Funct. Mater. 2006, 16, 252–259. 10.1002/adfm.200500212. [DOI] [Google Scholar]
- Zakharova L. Y.; Kudryashova Y. R.; Selivanova N. M.; Voronin M. A.; Ibragimova A. R.; et al. Novel membrane mimetic systems based on amphiphilic oxyethylated calix[4]arene: Aggregative and liquid crystalline behavior. J. Membr. Sci. 2010, 364, 90–101. 10.1016/j.memsci.2010.08.005. [DOI] [Google Scholar]
- Fang X.; Guo H.; Yang F.; Wu Y. Novel gallic-calixarene liquid crystals: syntheses and conformation influences on mesomorphism. Tetrahedron Lett. 2015, 56, 6128–6131. 10.1016/j.tetlet.2015.09.093. [DOI] [Google Scholar]
- Gajjar J. A.; Vekariya R. H.; Sharma V. S.; Kher S.; Rajani D. P.; Parekh H. Mesomorphic properties, microwave-assisted synthesis, and antimicrobial evaluation of novel Schiff base functionalized resorcin[4]arene derivatives. Mol. Cryst. Liq. Cryst. 2021, 715, 37–55. 10.1080/15421406.2020.1856615. [DOI] [Google Scholar]
- Roy B.; De N.; Majumdar K. C. Advances in Metal-Free Heterocycle-Based Columnar Liquid Crystals. Chem.—Eur. J. 2012, 18, 14560–14588. 10.1002/chem.201200483. [DOI] [PubMed] [Google Scholar]
- Budig H.; Diele S.; Paschke R.; Tschierske C.; Strohl D. Mesomorphic properties and monolayer behaviour of novel liquid crystalline exo-calix[4]arene derivatives. J. Chem. Soc., Perkin Trans. 2 1996, 1901–1906. 10.1039/P29960001901. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Shah A. P. A new class of supramolecular liquid crystals derived from azo calix[4]arene functionalized 1,3,4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. 10.1039/C8NJ04997A. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Vekariya R. H. Columnar self-assembly of bowl-shaped luminescent oxadiazole calix[4]arene derivatives. J. Mol. Liq. 2018, 271, 319–327. 10.1016/j.molliq.2018.09.011. [DOI] [Google Scholar]
- Sharma V. S.; Shah A. P.; Athar M.; Sharma A. S. Columnar self-assembly, gelation and electrochemical behavior of cone-shaped luminescent supramolecular calix[4]arene LCs based on oxadiazole and thiadiazole derivatives. New J. Chem. 2019, 43, 1910–1925. 10.1039/C8NJ04922J. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Shah A. P.; Shah P. A.; Shrivastav P. S.; Athar M. New Class of Supramolecular Bowl-Shaped Columnar Mesogens Derived from Thiacalix[4]arene Exhibiting Gelation and Organic Light-Emitting Diodes Applications. ACS Omega. 2019, 4, 15862–15872. 10.1021/acsomega.9b01776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V. S.; Shah P. A.; Sharma A. S.; Ganga V. S. R.; Shrivastav P. S.; Prajapat V. Upper/lower rim functionalized calixarene based AIE-active liquid crystals with self-assembly behavior: Photophysical and electrochemical studies. J. Mol. Liq. 2022, 348, 118047. 10.1016/j.molliq.2021.118047. [DOI] [Google Scholar]
- Yelamaggad C. V.; Shanker G.; Hiremath U. S.; Krishna Prasad S. Cholesterol-Based Nonsymmetric Liquid Crystal Dimers: An Overview. J. Mater. Chem. 2008, 18 (25), 2927–2949. 10.1039/b804579h. [DOI] [Google Scholar]
- Achalkumar A. S.; Bushby R. J.; Evans S. D. Cholesterol-Based Anchors and Tethers for Phospholipid Bilayers and for Model Biological Membranes. Soft Matter. 2010, 6, 6036–6051. 10.1039/c0sm00030b. [DOI] [Google Scholar]
- Reinitzer F. Beiträge zur kenntniss des cholesterins. Monatsh. Chem. 1888, 9, 421–44. 10.1007/BF01516710. [DOI] [Google Scholar]
- Guo H.; Yang F.; Liu W.; Lai J. Novel supramolecular liquid crystals: synthesis and mesomorphic properties of calix[4]arene-cholesterol derivatives. Tetrahedron Lett. 2015, 56, 866–870. 10.1016/j.tetlet.2014.12.137. [DOI] [Google Scholar]
- Zhang X.; Guo H.; Yang F.; Yuan J. Ion complexation-controlled columnar mesophase of calix[4]arene–cholesterol derivatives with Schiff-base bridges. Tetrahedron Lett. 2016, 57, 905–909. 10.1016/j.tetlet.2016.01.047. [DOI] [Google Scholar]
- Han C.; Guo H.; Lai J.; Yang F. Calix[4]resorcinarene-cholesterol columnar liquid crystals: Synthesis, mesomorphism and the influence of spacers on liquid crystalline behaviors. J. Mol. Liq. 2017, 231, 220–224. 10.1016/j.molliq.2017.01.111. [DOI] [Google Scholar]
- Kumar S. Recent developments in the chemistry of triphenylene-based discotic liquid crystals. Liq. Cryst. 2004, 31, 1037–1059. 10.1080/02678290410001724746. [DOI] [Google Scholar]
- Kumar S. Triphenylene-based discotic liquid crystal dimers, oligomers and polymers. Liq. Cryst. 2005, 32, 1089–1113. 10.1080/02678290500117415. [DOI] [Google Scholar]
- Pal S. K.; Setia S.; Avinash B. S.; Kumar S. Triphenylene-Based Discotic Liquid Crystals: Recent Advances. Liq. Cryst. 2013, 40, 1769–1816. 10.1080/02678292.2013.854418. [DOI] [Google Scholar]
- Yang F.; Guo H.; Xie J.; Lin J. Synthesis of Calixarene-Linked Discotic Triphenylene. Eur. J. Org. Chem. 2011, 2011, 5141–5145. 10.1002/ejoc.201100685. [DOI] [Google Scholar]
- Yang F.; Xu B.; Guo H.; Xie J. Novel symmetrical triads of triphenylene-calix[4]arene-triphenylene: synthesis and mesomorphism. Tetrahedron Lett. 2012, 53, 1598–1602. 10.1016/j.tetlet.2012.01.067. [DOI] [Google Scholar]
- Yang F.; Bai X.; Guo H.; Li C. Ion complexation-induced mesomorphic conversion between two columnar phases of novel symmetrical triads of triphenylene-calix[4]arene-triphenylenes. Tetrahedron Lett. 2013, 54, 409–413. 10.1016/j.tetlet.2012.11.029. [DOI] [Google Scholar]
- Hong B.; Yang F.; Guo H.; Jiao Z. Synthesis, complexation, and mesomorphism of novel calixarene-linked discotic triphenylene based on click chemistry. Tetrahedron Lett. 2014, 55, 252–255. 10.1016/j.tetlet.2013.11.008. [DOI] [Google Scholar]
- Tang H.; Guo H.; Yang F.; Zhu S. Synthesis and mesomorphic properties of calix[4]resorcinarene–triphenylene oligomers. Liq. Cryst. 2017, 44, 1566–1574. 10.1080/02678292.2017.1305459. [DOI] [Google Scholar]
- Patel A.; Sharma V. S.; Rao V. G.; Rana V.; Sharma A. S.; Shrivastav P. S. Design of thiacalixarene linked triphenylene based room temperature columnar LCs with self-assembly and dielectric property: Impact of flexibility on molecular systems. J. Mol. Liq. 2022, 366, 120261. 10.1016/j.molliq.2022.120261. [DOI] [Google Scholar]
- Yelamaggad C. V.; Achalkumar A. S.; Bonde N. L.; Prajapati A. K. Liquid Crystal Abrikosov Flux Phase: The Exclusive Wide Thermal Range Enantiotropic Occurrence. Chem. Mater. 2006, 18, 1076–1078. 10.1021/cm052570+. [DOI] [Google Scholar]
- Yelamaggad C. V.; Bonde N. L.; Achalkumar A. S.; Shankar Rao D. S.; Prasad S. K.; Prajapati A. K. Frustrated Liquid Crystals: Synthesis and Mesomorphic Behavior of Unsymmetrical Dimers Possessing Chiral and Fluorescent Entities. Chem. Mater. 2007, 19, 2463–2472. 10.1021/cm0625880. [DOI] [Google Scholar]
- Sharma V. S.; Sharma A. S.; Agarwal N. K.; Shah P. A.; Shrivastav P. S. Self-assembled blue-light emitting materials for their liquid crystalline and OLED applications: from a simple molecular design to supramolecular materials. Mol. Syst. Des. Eng. 2020, 5, 1691–1705. 10.1039/D0ME00117A. [DOI] [Google Scholar]