Abstract
Lipopolysaccharides (LPS), either in the free form or complexed to CD14, a LPS receptor, are elicitors of the immune system. Lactoferrin (Lf), a LPS-chelating glycoprotein, protects animals against septic shock. Since optimal protection requires administration of Lf prior to lethal doses of LPS, we hypothesized that interactions between Lf and soluble CD14 (sCD14) exist. In a first step, human sCD14 and human Lf (hLf) were used to determine the kinetic binding parameters of hLf to free sCD14 in an optical biosensor. The results demonstrated that hLf bound specifically and with a high affinity (Kd = 16 ± 7 nM) to sCD14. Affinity chromatography studies showed that hLf interacted not only with free sCD14 but also, though with different binding properties, with sCD14 complexed to LPS or lipid A–2-keto-3-deoxyoctonic acid–heptose. In a second step, we have investigated whether the capacity of hLf to interact with sCD14 could modulate the expression of endothelial-leukocyte adhesion molecule 1 (E-selectin) or intercellular adhesion molecule 1 (ICAM-1) induced by the sCD14-LPS complex on human umbilical vein endothelial cells (HUVEC). Our experiments show that hLf significantly inhibited both E-selectin and ICAM-1 expressions at the surface of HUVEC. In conclusion, these observations suggest that the anti-inflammatory effects of hLf are due not only to the ability of the molecule to chelate LPS but also to its ability to interact with sCD14 and with the sCD14 complexed to LPS, thus modifying the activation of endothelial cells.
One of the central proinflammatory functions of endothelial cells is the recruitment of circulating leukocytes at inflammatory tissue sites. Lipopolysaccharides (LPS) derived from Gram-negative bacteria are potent stimulators of inflammation (34, 43) that induce either directly or through the intermediary of cytokines (11), the expression of adhesion molecules such as endothelial-leukocyte adhesion molecule 1 (E-selectin) and intercellular adhesion molecule 1 (ICAM-1) (7, 36). Endotoxin stimulation of endothelial cells is mediated by soluble CD14 (sCD14), a specific LPS receptor (3, 18, 19, 36). CD14 is a 55-kDa glycoprotein that exists both as a soluble protein found in serum at concentrations of 2 to 6 μg/ml (16) and as a glycosylphosphatidylinositol-anchored protein (mCD14) on the surface of monocytes-macrophages (5, 50, 52). At low endotoxin levels, a serum acute protein called the LPS-binding protein (LBP), which catalyzes the transfer of LPS monomers from aggregates to CD14, enhances the sensitivity of cells to LPS (19, 36, 44). Nevertheless, at high LPS concentrations, LBP is not essential to the activation of endothelial cells and LPS may directly bind to CD14 to form an sCD14-LPS complex (18, 19, 42). Thus, the activation of endothelial cells by the sCD14-LPS complex promotes leukocyte infiltration and microvascular thrombosis and contributes, during septic shock, to the pathogenesis of disseminated intravascular inflammation. This phenomenon leads to severe damage of endothelium (8). Various LPS-binding proteins modulate the activation of cells (45), among which is lactoferrin (Lf), an iron-binding glycoprotein found in exocrine secretions of mammals and released from granules of neutrophils during inflammation (31). Following infection, Lf concentrations higher than 20 μg/ml can be detected in blood (6). Interactions between Lf and LPS have been thoroughly investigated. Human Lf (hLf) binds to the lipid A region of LPS with a high affinity (2). Experiments using hLf variants and mutants demonstrated that amino acid residues 1 to 5 and 28 to 34 of hLf interact with Escherichia coli LPS (14, 17). In vitro, Lf prevents the LBP-mediated binding of LPS to mCD14 (13) and decreases the release of cytokines such as interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha from LPS-stimulated monocytes (10, 33). Lf might also modulate the inflammatory process in vivo. Indeed, studies reported the protective function of Lf against sublethal doses of LPS in mice (29, 51). Recently, the protective effect of Lf feeding against endotoxin lethal shock in germfree piglets has been described (25). These observations indicate that Lf is one of the key molecules which modulates the inflammatory responses (4).
The ability of Lf to bind free LPS may account, in part, for the anti-inflammatory activities of the protein. However, since optimal protection of animals against the septic shock requires a 12- to 24-h preinjection of Lf, it may be assumed that other mechanisms are involved. We hypothesized that interactions between Lf and LPS receptors such as sCD14 exist.
In this study, we analyzed the potential protective effect of Lf under LBP-independent septic shock conditions. We first studied the binding of various concentrations of sCD14 to hLf with an optical biosensor. Affinity chromatography was then used to study the binding of sCD14 to hLf in the presence of E. coli 055:B5 LPS and LPS moieties. Lastly, we investigated whether hLf modifies the activating properties of the sCD14-LPS complex on endothelial cells. For this purpose, the effect of hLf on the expression of E-selectin and ICAM-1 induced by the sCD14-LPS complex on human umbilical vein endothelial cells (HUVEC) was determined.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium was obtained from Gibco-BRL (Eragny, France) and endothelial cell growth medium SFM supplemented with fetal calf serum, endothelial cell growth supplement, heparin, epidermal growth factor, bovine fibroblast growth factor, hydrocortisone, gentamicin, and amphotericin B was from PromoCell (Heidelberg, Germany). Both biotin hydrazide and Ultralink hydrazide were from Pierce Chemicals Co. (Rockford, Ill.). SP-Sepharose fast flow column and PD10 G-25 column were from Pharmacia (Uppsala, Sweden). The apyrogen water was from Cooper (Melun, France), the Centricon-30 ultrafiltration units were from Amicon (Danvers, Mass.), and the nitrocellulose was from Schleicher & Schuell (Dassel, Germany). Dulbecco's phosphate-buffered saline (PBS), human serum transferrin (hTF), bovine serum albumin, collagenase, gelatin, diaminobenzidine peroxidase substrate tablet set, o-phenylenediamine-dihydrochloride, and LPS O55:B5 from E. coli were purchased from Sigma Chemical Co. (St. Louis, Mo.). Lipid A–2-keto-3-deoxyoctonic acid (KDO)–heptose and lipid A were purified from a rough mutant (395MR10, Rd chemotype) of Salmonella enterica serovar Typhimurium as previously described (32), and the purity of preparations was checked by mass spectral analysis. These LPS fractions were generous gifts from I. Mattsby-Baltzer (Department of Clinical Bacteriology, Göteborg, Sweden). Recombinant human sCD14 was purchased from Biometec (Greifswald, Germany). It was obtained from serum-free culture supernatant of CHO cells transfected with human CD14 cDNA cloned into pPOL-DHFR expression vector (41). Rabbit anti-CD14 polyclonal antibodies were purchased from Biometec, goat peroxidase-labeled anti rabbit immunoglobulin G (IgG) from Biosys (Compiègne, France), mouse monoclonal anti-ICAM-1 antibodies (clone 15.1) from Tebu (Le Perray-en-Yvelines, France), mouse monoclonal anti-E-selectin antibodies (clone 1.2B6) from Immunotech (Marseille, France), peroxidase-conjugated sheep anti mouse IgG from Sanofi (Marnes-la-Coquette, France) and isotype control IgG1 from Sigma Chemical Co.
Endothelial cell culture.
Endothelial cells (HUVEC) were derived from human umbilical vein, according to the method previously described (21). Briefly, after treatment of umbilical vein with 0.2% (wt/vol) collagenase in 37°C-prewarmed RPMI for 30 min, HUVEC were collected by centrifugation (600 × g for 15 min). Cells were resuspended in endothelial cell growth medium SFM and cultured in gelatin-coated 35-mm-diameter tissue culture wells at 37°C and 5% CO2. They were collected after trypsinization and then cultured in gelatin-coated 96-well flat-bottomed culture plates until confluency. Only cells of the third and the fourth passages were used. Viability was over 96% as determined by trypan blue dye exclusion.
Preparation of LPS-free hLf.
Native hLf was purified from fresh human milk by cation-exchange chromatography and iron saturated, as previously reported (35, 39). Homogeneity of the protein was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Since the interaction between LPS and Lf was abrogated by NaCl concentrations higher than 0.4 M (46), 50 mg of purified hLf was injected on a 7-by-1-cm SP-Sepharose fast flow column equilibrated in 0.1 M NaCl and then washed with 70 ml of 0.5 M NaCl. hLf was eluted with 2 M NaCl and desalted on a PD10 G-25 column equilibrated in 0.1 M NaCl. All buffers were prepared with pyrogen-free water. The LPS contamination of these hLf fractions was less than 50 pg of endotoxin/mg of protein, as estimated by the Limulus amoebocyte lysate assay (QCL1000; BioWhittaker, Walkersville, Md.).
Preparation of biotin-labeled hLf.
To avoid possible steric hindrance of the interactions of the hLf polypeptide with sCD14, we labeled hLf through its glycan moiety after mild periodate oxidation of N-acetylneuraminic acid residues. This method has been successfully carried out to label Lf without affecting its biological activity (26, 28). All solutions were prepared with pyrogen-free water. The glycan moiety of hLf was biotinylated by coupling biotin hydrazide to aldehyde groups produced by mild periodate oxidation of N-acetylneuraminic acid residues. Briefly, hLf (5 mg) dissolved in 230 μl of 0.1 M sodium acetate–0.15 M NaCl (pH 5.6) was mixed with 100 μl of 0.018 M sodium periodate and incubated for 10 min at 4°C. The reaction was stopped by adding 10 μl of ethylene glycol and desalted on a Sephadex G-25 PD10 column in PBS. Oxidized hLf was then incubated with biotin hydrazide (5 mg in 3 ml of PBS) for 2 h at room temperature with gentle mixing. Free biotin hydrazide was removed through Centricon-30 filters. After concentration of labeled protein to a final volume of 500 μl, biotinylated hLf was passed through a Sephadex PD10 G-25 column in PBS. LPS contamination of the labeled hLf was controlled. Biotinylated hLf was used for the biosensor studies.
Analysis of sCD14 binding to hLf in an optical biosensor.
Binding reactions were carried out in an IAsys two-channel resonant mirror biosensor at 20°C (Affinity Sensors, Saxon Hill, Cambridge, United Kingdom) (37, 38) with minor modifications. Planar biotin surfaces, with which a signal of 600 arc s corresponds to 1 ng of bound protein/mm2, were derivatized with streptavidin according to the manufacturer's instructions. Controls showed that sCD14 did not bind to streptavidin-derivatized biotin surfaces (result not shown). Biotinylated hLf was immobilized on planar streptavidin-derivatized surfaces, which were then washed with PBS. The distribution of the immobilized hLf and of the bound sCD14 on the surface of the biosensor cuvette was inspected by the resonance scan, which showed that at all times these molecules were distributed uniformly on the sensor surface and therefore were not microaggregated.
Binding assays were conducted in a final volume of 30 μl of PBS at 20 ± 0.1°C. The ligate was added at a known concentration in 1 μl to 5 μl of PBS to the cuvette to give a final concentration of sCD14 ranging from 14 to 73 nM. To remove residual bound ligate after the dissociation phase, and thus regenerate the immobilized ligand, the cuvette was washed three times with 50 μl of 2 M NaCl–10 mM Na2HPO4, pH 7.2, and three times with 50 μl of 20 mM HCl. Data were pooled from experiments carried out with different amounts of immobilized hLf (0.2, 0.6, and 1.2 ng/mm2). For the calculation of kon, low concentrations of ligate (sCD14) were used, whereas for the measurement of koff, higher concentrations of ligate were employed (1 μM) to avoid any rebinding artifacts. The binding parameters kon and koff were calculated from the association and dissociation phases of the binding reactions, respectively, using the nonlinear curve-fitting FastFit software (Affinity Sensors) provided with the instrument. The dissociation constant (Kd) was calculated from the association and dissociation rate constants and from the extent of binding observed near equilibrium.
Affinity chromatography studies.
Purified hLf and human serum transferrin were immobilized on Ultralink hydrazide gel according to manufacturer's instructions and used to study the binding of human recombinant sCD14. Two milligrams of protein was bound per ml of Ultralink hydrazide gel.
Two micrograms of sCD14 was preincubated in the absence or in the presence of an excess of E. coli O55:B5 LPS (10 μg in 200 μl of PBS) for 1 h at 37°C and further incubated for 3 h at 37°C with 50 μg of hLf immobilized on the Ultralink hydrazide gel. As reported elsewhere (17), such experimental conditions led to full complexation of sCD14 to LPS in the absence of LBP. Prior to use, LPS suspensions were sonicated and diluted in Dulbecco's PBS without Ca2+ and Mg2+ to avoid aggregation of LPS molecules. Similar experiments were also performed with lipid A and lipid A-KDO-heptose from serovar Typhimurium. Nonspecific binding of sCD14 was estimated on uncoupled Ultralink Hydrazide gel. A control was performed with immobilized hTf. The gel was collected by centrifugation at 600 × g for 5 min and washed with 10 ml of PBS. The sCD14 bound to the gel was sequentially eluted with 3 volumes of 200 μl of 0.5 M NaCl in 20 mM sodium phosphate buffer, pH 7.4, 3 volumes of 200 μl of 1 M NaCl in this buffer, 2 volumes of 200 μl of 0.2 M glycine–HCl (pH 2.3) containing 0.5% (vol/vol) Triton X-100, and 300 μl of SDS (10%, wt/vol). Polypeptides in 100 μl of each fraction were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) in 7.5% (wt/vol) acrylamide gels and then transferred to a 0.45-μm-pore-size nitrocellulose membrane. The membranes were soaked in PBS containing 2% (wt/vol) gelatin for 90 min and then incubated for 2 h with an antiserum to human CD14 (1:1,500 dilution in PBS containing 0.05% [vol/vol] Tween 20). The membranes were washed three times with PBS containing 0.1% (vol/vol) Tween 20 and then incubated for 1 h with goat peroxidase-labeled anti-rabbit IgG (1:1,000 dilution in PBS containing 0.05% [vol/vol] Tween 20). Immunoreactive sCD14 was detected with the diaminobenzidine peroxidase substrate tablet set. All immunochemical staining steps were performed at room temperature.
Expression of ICAM-1 and E-selectin on HUVEC.
Endothelial ICAM-1 and E-selectin expressions were measured by enzyme immunoassay, as previously described (20, 36, 49). Cells were plated into gelatin-coated 96-well tissue culture plates and grown to confluence.
To study the ICAM-1 expression, cells were washed twice with RPMI and incubated for 24 h at 37°C in 5% CO2, either with E. coli O55:B5 LPS (100 ng/ml) or with a mixture of LPS (100 ng/ml) and sCD14 (2 μg/ml) preincubated for 30 min at room temperature. Controls were performed without LPS and without sCD14, with sCD14 and without LPS, with hLf alone, and with hLf and sCD14. As previously described (24) the maximal ICAM-1 expression was obtained after 24 h of incubation with cells. The effect of hLf on the expression of ICAM-1 was investigated under conditions similar to those described above. Before incubation with cells, hLf (50 μg/ml) was preincubated for 30 min at room temperature with LPS in the presence of sCD14. In some experiments, the hLf-sCD14, LPS-sCD14, or LPS-hLf mixtures were incubated for 30 min at room temperature before the addition of LPS, hLf, or sCD14, respectively, and then added for 24 h with cells at 37°C. The medium was removed, and the cells were washed twice with PBS. HUVEC monolayers were fixed at room temperature for 15 min with 2% paraformaldehyde. The fixative was removed and replaced with 100 mM glycine to block reactive aldehyde groups. After two washes with PBS, plates were incubated for 30 min at 37°C with 2% (wt/vol) bovine serum albumin. Then, anti-mouse ICAM-1 monoclonal antibody (4 μg/ml) was added and incubated at 37°C for 1 h. After washing, peroxidase-conjugated sheep anti-mouse IgG diluted 1:2,000 in PBS was added for 30 min at 37°C. An isotype control (IgG1) was used to evaluate the nonspecific binding of the monoclonal antibody. Staining was achieved by adding 150 μl of o-phenylenediamine-dihydrochloride per well for 20 min at room temperature, according to manufacturer's instructions. The reaction was stopped with 50 μl of 2 M H2SO4 per well, and the absorbance at 490 nm was measured on an enzyme-linked immunosorbent assay plate reader.
To study the endothelial E-selectin expression, the protocol used was the same as that described above for ICAM-1, but the incubation with the cells was only 5 h at 37°C and the concentration of LPS used was 1 μg/ml. Moreover, mouse monoclonal anti-ICAM-1 antibody was replaced by a mouse monoclonal anti-E-selectin antibody at a concentration of 4 μg/ml.
Statistical analysis.
Data are presented as the mean ± standard error (SE) for the indicated number of independent experiments. Statistical significance was analyzed by Student's t test for unpaired data. Values of P < 0.05 were considered to be significant.
RESULTS
Analysis of sCD14 binding to hLf in an optical biosensor.
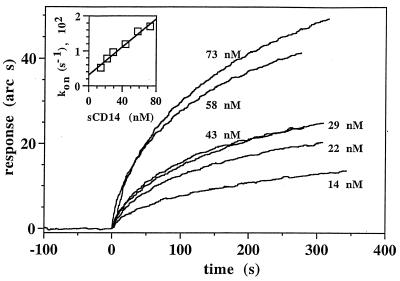
The association phase of the binding reaction between sCD14 and hLf was fairly rapid (Fig. 1). Analysis of the binding curves from four experiments indicated that the binding of sCD14 to hLf was saturable and monophasic (Fig. 1 and Table 1). The association rate constant (kass) and dissociation rate constant (kdiss) for the sCD14-hLf interaction were 360,000 ± 110,000 M−1 s−1 and 0.0058 ± 0.0017 s−1, respectively (Table 1). The equilibrium dissociation constant (Kd) calculated from the ratio of the kinetic rate constants (kdiss/kass) was 16 ± 7 nM. The Kd calculated from the extent of binding observed near equilibrium was 45 ± 30 nM, a value which was similar to that calculated from kinetic parameters. These results demonstrate that sCD14 binds to hLf with a high affinity and with fairly fast kinetics.
FIG. 1.
Analysis of sCD14 binding to hLf in an optical biosensor. Biotinylated hLf was immobilized on a streptavidin-derivatized biotin surface as described in Materials and Methods. The binding of different concentrations of sCD14 (14 to 73 nM) to immobilized hLf was monitored in real time for about 300 s. Four independent sets of binding reactions were performed, of which one is presented. The inset shows that a plot of kon against ligand concentration yields a straight line (r = 0.989), the slope of which corresponds to kass. The kon of sCD14 for hLf at each concentration of sCD14 was determined using the FastFit software as described in Materials and Methods.
TABLE 1.
Kinetics of sCD14 binding to human Lf
| Parameter | Value |
|---|---|
| kass (M−1 s−1)a | 360,000 ± 110,000 |
| rb | 0.963 |
| Kd (nM)c | 16 ± 7 |
| kdiss (s−1)d | 0.0058 ± 0.0017 |
| Kd (nM)e | 45 ± 30 |
Result given as mean ± SE. The SE is derived from the deviation of the data from a one-site model and was calculated by matrix inversion using the FastFit software provided with the instrument. Each set of values of kon (e.g., Fig. 1) produced a value for kass and an associated SE. Data are pooled values from four experiments on three planar biotin surfaces with different amounts of immobilized hLf as described under “Materials and Methods”. The likehood of a one-site or a two-site model describing the association phase of each binding reaction was determined by four parameters of goodness of fit, as described previously (15). Firstly, if a one-site model could not fit the data, a two-site model was favored. Secondly, a direct statistical comparison of goodness of fit of a one-site and a two-site model was made for each binding curve using the F statistic; a two-site model was favored at a probability below 0.005. Thirdly, the randomness of the distribution of the data about the model was examined; a two-site model was favored over a one-site model when the data were nonrandomly distributed about the latter. Fourthly, the model had to yield realistic values, e.g., a positive value of kon at all concentrations of sCD14. In all experiments, at the lowest concentrations of sCD14 (<40 nM) a two-site model did not fit the data, whereas at higher concentrations of sCD14 a one-site model provided at least as good a fit as a two-site model.
The correlation coefficient of the linear regression through the values of kon for the binding model.
Kd was calculated from the kdiss/kass ratio, and the SE is the combined SE of the two kinetic parameters.
The kdiss is the mean ± SE of 10 values, obtained at high concentrations (1 μM) of sCD14.
Kd was calculated from the extent of binding observed near equilibrium at five or more different concentrations of ligate in four independent experiments. The SE is the combined error of the experiments.
Similar binding experiments were performed with the complex of sCD14 and E. coli O55:B5 LPS. However, the addition of free LPS in PBS to the cuvette induced a negative bulk shift (10 arc s), suggesting that the refractive index of a solution containing LPS in PBS is lower than that of PBS alone, in contrast to the positive bulk shift observed with proteins (37, 38). Since excess LPS attenuated the response of the optical biosensor, the binding parameters of sCD14 to hLf in the presence of LPS could not be determined. Therefore, the binding of sCD14 to hLf in the presence of LPS and LPS moieties was assessed by affinity chromatography on Ultralink hydrazide gel-immobilized hLf.
Binding of sCD14 to immobilized hLf in the presence of LPS and LPS moieties.
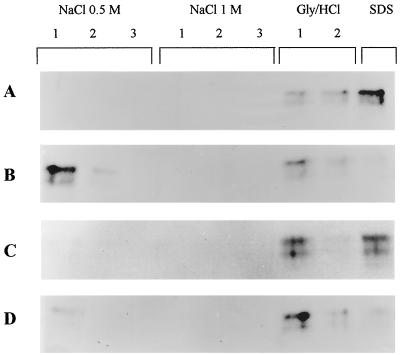
Human sCD14 alone or in the presence of E. coli O55:B5 LPS was incubated with Ultralink hydrazide gel-immobilized hLf and then sequentially eluted by solutions containing 0.5 and 1 M NaCl, glycine–HCl (pH 2.3), 0.5% (vol/vol) Triton X-100, and 10% (wt/vol) SDS. Figure 2A shows that free sCD14 only dissociated from hLf under stringent conditions such as pH 2.3, Triton X-100, and especially SDS treatment. No sCD14 was detected in the NaCl fractions. This result ties well with the high-affinity interactions detected by the biosensor technique. When sCD14 was incubated with excess LPS prior to affinity chromatography, comparable amounts of sCD14 bound to hLf, but dissociation occurred under milder conditions (Fig. 2B). As a matter of fact, sCD14 mainly eluted from the hLf gel in the first 0.5 M NaCl fraction. Only traces of sCD14 eluted in the pH 2.3 fraction. This result suggests that the sCD14-LPS complex did bind to hLf but through labile interactions. No nonspecific binding of sCD14 to the uncoupled Ultralink hydrazide gel was detected (data not shown). Likewise in a control performed with hTf immobilized in a manner identical to that employed for hLf, CD14 eluted in the PBS wash steps and not in the NaCl, acidic, or detergent fractions (data not shown).
FIG. 2.
Affinity chromatography of sCD14 to immobilized hLf in the absence of LPS (A) or in the presence of LPS (B), lipid A (C), or lipid A-KDO-heptose (D). sCD14 was preincubated 1 h with or without the different LPS moieties, and affinity chromatography was performed on hLf bound to Ultralink hydrazide gel as described in Materials and Methods. After 3 h of incubation, the gels were washed three times with 20 mM sodium phosphate, pH 7.4, buffers containing 0.5 M and 1 M NaCl, twice with a 0.2 M glycine–HCl, pH 2.3, buffer containing 0.5% (vol/vol) Triton X-100 (Gly/HCl), and once with 300 μl of SDS (10%, wt/vol). Identical volumes of the corresponding washing solutions were subjected to SDS-PAGE (7.5% polyacrylamide) and transferred to nitrocellulose. Immunostaining was performed with specific anti-CD14 polyclonal antibodies. Lanes labeled 1, 2, and 3 correspond to the successive washes with each buffer.
In order to investigate the region of LPS responsible for weaker saline-labile interactions between the sCD14-LPS complex and hLf, experiments were performed with both lipid A and lipid A-KDO-heptose (LPS inner core) moieties. Figure 2C shows that the presence of lipid A on sCD14 only slightly altered its binding to hLf, since sCD14 was detected in equal amounts in the first pH 2.3 and SDS fractions but not in the NaCl fractions. In the presence of the lipid A-KDO-heptose core, intermediate elution features were observed (Fig. 2D). As a matter of fact, sCD14 was mainly found in the first pH 2.3 fraction, but substantial amounts were also detected in the first NaCl 0.5 M, the second pH 2.3, and the SDS fractions. Our results indicate that the size (and nature) of the LPS moiety influenced the binding characteristics of sCD14 to hLf.
Effect of hLf on expression of endothelial ICAM-1 and E-selectin induced by the sCD14-LPS complex.
The capacity of hLf to modulate the expression of E-selectin and ICAM-1 induced by the sCD14-LPS complex on HUVEC was assessed in enzyme immunoassays using E-selectin and ICAM-1 antibodies (20, 36, 49). LPS concentrations ranging from 10 ng/ml to 10 μg/ml were used to trigger the expression of ICAM-1 and of E-selectin. Since maximal expressions were gained with 100 ng/ml and 1 μg/ml LPS for ICAM-1 and E-selectin, respectively (data not shown), these concentrations were used in further experiments.
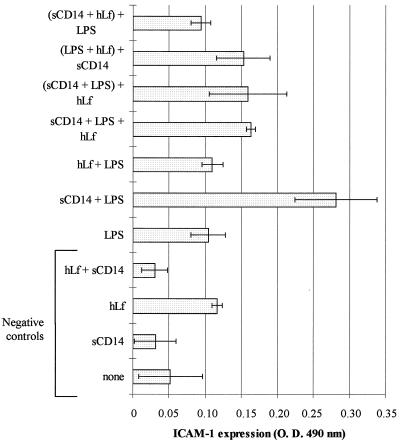
As shown in Fig. 3, HUVEC exhibited a low basal expression of ICAM-1, which was not significantly increased in the presence of sCD14, hLf, or both proteins (negative controls) or LPS. When compared to unstimulated cells, a fivefold-higher level of ICAM-1 was detected in the presence of sCD14-LPS (P < 0.05). This demonstrated that the sCD14-LPS complex induced the expression of ICAM-1 on endothelial cells. Interestingly, the ICAM-1 expression induced by the sCD14-LPS complex on HUVEC was significantly decreased in the presence of hLf (50 μg/ml). Taking the expression level induced by sCD14-LPS as a reference, a 51% ± 2% inhibition of the ICAM-1 expression was calculated when hLf was incubated with sCD14 and LPS at the same time (P < 0.05). This inhibition was similar to that detected when hLf was mixed with LPS 30 min prior to sCD14 (56% ± 14%) or when an sCD14-LPS complex was preformed before the addition to hLf (53% ± 19%) (P < 0.05). A higher inhibition (81% ± 5%) was measured when LPS was added after the preincubation of sCD14 with hLf.
FIG. 3.
Effect of hLf on the LPS-induced ICAM-1 expression on HUVEC. Various mixtures were preincubated for 30 min at room temperature before 24 h of incubation at 37°C with cells (per milliliter) as follows: 2 μg of sCD14 (sCD14); 50 μg of hLf alone (hLf) or with 2 μg of sCD14 (hLf + sCD14); 100 ng of LPS alone (LPS) or with 2 μg of sCD14 (sCD14 + LPS); 50 μg of hLf with 100 ng of LPS (hLf + LPS); or 2 μg of sCD14, 100 ng of LPS, and 50 μg of hLf (sCD14 + LPS + hLf). In some cases, the sCD14-LPS, LPS-hLf, and sCD14-hLf mixtures were preincubated for 30 min at room temperature prior to addition of hLf [(sCD14 + LPS) + hLf], sCD14 [(LPS + hLf) + sCD14], and LPS [(sCD14 + hLf) + LPS], respectively, and further incubation with cells. A control was performed with cells in the absence of hLf, sCD14, and LPS (none). The expression of ICAM-1 on HUVEC was estimated by enzyme immunoassay as described in Materials and Methods. Results are expressed as mean values of optical density at 490 nm (O. D. 490 nm) ± SE (error bars) from quadruplicates, after subtracting nonspecific binding of antibodies, and are representative of at least two separate experiments conducted with HUVEC isolated from human umbilical veins from different donors.
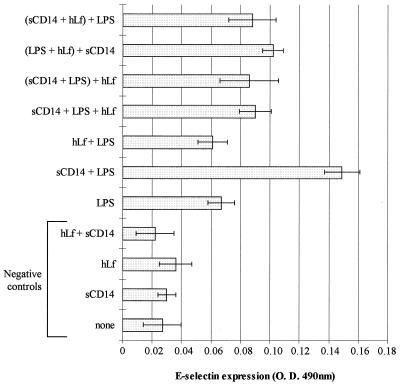
As shown in Fig. 4, similar results were obtained with the E-selectin expression on HUVEC. Indeed, two- and fivefold-higher levels of E-selectin were detected in the presence of LPS and sCD14-LPS, respectively. hLf had no effect on the low activation level of cells obtained with LPS in the absence of sCD14. In contrast, the inhibition of the expression induced by sCD14-LPS in the presence of hLf was 48% ± 9% when hLf was incubated with sCD14 and LPS at the same time (P < 0.05), 50% ± 13% when hLf was mixed with sCD14 30 min prior to LPS (P < 0.05), 52% ± 17% when an sCD14-LPS complex was preformed before the addition of hLf, and 38% ± 6% when sCD14 was added after the preincubation of hLf with LPS.
FIG. 4.
Effect of hLf on the LPS-induced E-selectin expression on HUVEC. Various mixtures were preincubated for 30 min at room temperature before 5 h of incubation at 37°C with cells (per milliliter) as follows: 2 μg of sCD14 (sCD14); 50 μg of hLf alone (hLf) or with 2 μg of sCD14 (hLf + sCD14); 1 μg of LPS alone (LPS) or with 2 μg of sCD14 (sCD14 + LPS); 50 μg of hLf with 1 μg of LPS (hLf + LPS); or 2 μg of sCD14, 1 μg of LPS, and 50 μg of hLf (sCD14 + LPS + hLf). In some cases, the sCD14-LPS, LPS-hLf, and sCD14-hLf mixtures were preincubated for 30 min at room temperature prior to addition of hLf [(sCD14 + LPS) + hLf], sCD14 [(LPS + hLf) + sCD14], and LPS [(sCD14 + hLf) + LPS], respectively, and further incubation with cells. A control was performed with cells in the absence of hLf, sCD14, and LPS (none). The expression of E-selectin on HUVEC was estimated by enzyme immunoassay as described in Materials and Methods. Results are expressed as mean values of optical density at 490 nm (O. D. 490 nm) ± SE (error bars) from quadruplicates, after subtracting nonspecific binding of antibodies, and are representative of at least four separate experiments conducted with HUVEC isolated from human umbilical veins from different donors.
These findings suggest that hLf modulates the expressions of endothelial ICAM-1 and E-selectin through its interaction with sCD14 and the sCD14-LPS complex.
DISCUSSION
The protective effect of Lf against endotoxin lethal shock in mice or in germfree piglets was reported (25, 29, 51). The endotoxin-chelating properties of Lf and its ability to compete with LBP for LPS binding (13) explain in part the role of the protein in the modulation of inflammation (2, 13, 14). Indeed, Lf prevents the release of cytokines induced by LPS from monocytes in vitro (10, 33) and in vivo (29, 51). However, the optimal protection of animals against induced septicemia requires a 12- to 24-h preinjection of Lf (51), which suggests that this protein may act by mechanisms in addition to simple LPS scavenging. We hypothesized that interactions between hLf and LPS receptors such as sCD14 exist and thus interfere with the activation of target cells. The aim of this study was to investigate the potential protective effect of lactoferrin under septic shock conditions. Since LBP is not essential at high LPS concentrations and in order to focus on potential interactions between hLf and sCD14, LBP was not included in the experiments.
In the present report, we provide evidence that sCD14 binds specifically to hLf with a high affinity (Kd = 16 ± 7 nM). Basic sequences 2RRRR5 and 28RKVRGPP34, which are close and accessible at the surface of hLf (1), interact with various anionic molecules such as heparin (9), proteoglycans (27), and LPS (23, 48) (Fig. 5). In sCD14, acidic residues 35AVEVE39 and 53RVDADADPRQY63 are involved in the interactions with LPS (23, 48), while amino acids 7ELDDEDF13 are essential for cell activation (22, 40) (Fig. 5). We postulate that all or part of the basic or acidic amphipatic stretches present in hLf and sCD14 are responsible for the high-affinity interactions between these two glycoproteins, resulting in the formation of a stable sCD14-hLf complex. The use of mutated recombinant hLf and sCD14 will gain further insight into the importance of these stretches in the interactions.
FIG. 5.
N-terminal sequences of human sCD14 and hLf. The underlined amino acids in the sCD14 sequence represent the cell signaling site (22, 40), and the residues in boldface type are those implicated in the LPS binding (23, 48). The amino acids in boldface type in the hLf sequence are the two N-terminal basic stretches involved in the interactions of hLf with LPS (2, 13, 14, 47).
Since Biosensor technology failed in determining the binding parameters of sCD14 to hLf in the presence of E. coli O55:B5 LPS, comparative affinity chromatography studies were undertaken. Our results show that the sCD14-LPS complex bound to hLf but through more-labile interactions than that between sCD14 and hLf. As a matter of fact, while sCD14 alone mainly dissociated from hLf under drastic conditions (SDS), its binding to hLf in the presence of LPS was mainly disrupted in the first NaCl 0.5 M wash. This phenomenon may be relevant to the way that hLf binds to the sCD14-LPS complex. In fact, it is not known whether hLf binds to the sCD14 or to the LPS counterpart of the complex. If hLf binds to the sCD14 moiety, it is likely that the presence of LPS impedes further interactions of hLf with the LPS-binding residues 35 to 39 and 53 to 63 (23, 48) of sCD14. Nevertheless, the basic region of hLf could still bind to the acidic stretch 7ELDDEDF13 of sCD14, thus generating saline-labile interactions. The ability of LPS moieties to weaken sCD14-hLf interactions was also observed but to a lower extent and in an LPS moiety size-dependent way. Lipid A, the smallest moiety, only slightly interfered in the binding of hLf to sCD14. The binding properties of LPS moieties to sCD14, which are not as well defined as those of LPS, are probably responsible for this difference. In particular, it is not known whether the LPS moieties bind to one, two, or none of the sCD14 LPS-binding stretches, residues 35 to 39 and 53 to 63, which could thus remain partially accessible to hLf. Furthermore, it may be assumed that the affinities of sCD14 for either lipid A or lipid A-KDO-heptose are lower than that for LPS (34 nM [46]) or hLf (16 ± 7 nM). Therefore, hLf could displace the LPS moieties from sCD14.
Another possibility is the binding of hLf to the LPS moiety of the sCD14-LPS complex. It was recently reported that sCD14 possesses lectin-like properties and recognizes the inner core of LPS and the peptidoglycan (9, 12, 17). Within LPS, the major carbohydrate determinants of the interaction are the KDO sugars (9), but the N-acylated glucosamine residues of lipid A also contribute to this recognition (12). It may be hypothesized that the interactions of sCD14 with the sugar moiety of LPS still allow the recognition of the lipid A by hLf. This hypothesis is supported by previous data showing that NaCl concentrations above 0.4 M inhibit the binding of LPS to hLf (47), thus explaining the saline-labile interactions between hLf and sCD14-LPS.
The evidence for interactions between hLf and sCD14 led us to investigate whether hLf interferes in the biological activity of the sCD14-LPS complex. ICAM-1 and E-selectin are adhesion molecules whose expression is induced by LPS in the presence of sCD14 on endothelial cells (18, 19, 36). The results show that the expression of ICAM-1 and E-selectin on HUVEC is strongly induced by O55:B5 E. coli LPS in the presence of sCD14 but that these levels of expression are inhibited by hLf, whatever the order of presentation of hLf to sCD14 and LPS. Thus, the binding of hLf to sCD14 in the presence of LPS leads to complexes, which are then unable to activate endothelial cells. One could speculate whether the sCD14-hLf complexes are then still able to bind LPS or not, but in either case, a loss of the cell-activating properties of LPS will occur. These phenomena could significantly lower the availability of sCD14 in serum and/or disable sCD14-LPS complexes, thus further decreasing the responsiveness of the organism to LPS. In light of these results, it can be assumed that the septic shock-preventive effect of hLf administered to animals 12 to 24 h before lethal doses of LPS (51) is related, at least in part, to the ability of hLf to bind sCD14. The delay in the protective effect of hLf may be a requisite in vivo for the optimal neutralization of free sCD14 by hLf. It may also be relevant to some more complex phenomena.
In conclusion, our findings provide evidence that the role of hLf in the modulation of the inflammatory process cannot be attributed solely to its LPS-chelating properties (2, 14, 47). We have previously shown that hLf may compete with LBP for the binding of LPS to mCD14 (13). We demonstrate here the ability of hLf to bind to sCD14 and to inhibit at least one of its cell activation functions, the expression of ICAM-1 and E-selectin, two molecules essential to the recruitment process of leukocytes. Since CD14 plays an essential role in the endotoxin-mediated inflammatory response, it is possible that hLf interferes with the expression and/or activation of other molecules involved in the leucocyte recruitment process such as VCAM-1, integrins, and chemokines. The interactions of hLf with CD14 may also account for its previously reported effects on the expression of proinflammatory cytokines tumor necrosis factor alpha, IL-1, and IL-6 (10, 29). Therefore, hLf may modulate the recruitment of immune cells on inflammatory sites and hLf, either released from neutrophils during inflammation or used as a therapeutic agent, may have such a modulating effect. These results open the way to investigate potential interactions between hLf and other LPS receptors and proinflammatory molecules.
ACKNOWLEDGMENTS
This work was supported in part by the Ministère de l'Enseignement et de la Recherche Scientifique, the Centre National de la Recherche Scientifique, the North West Cancer Research Fund, the Mizutani Foundation for Glycoscience, and the Royal Society. D. G. Fernig was a Université des Sciences et Technologies de Lille-supported visiting professor.
We are grateful to A. Clermont and M. Masson for their skillful technical assistance. We are indebted to I. Mattsby-Baltzer, who provided us the different LPS derivatives.
REFERENCES
- 1.Anderson B F, Baker H M, Norris G E, Rice D W, Baker E N. Structure of human lactoferrin: crystallographic structure analysis and refinement at 2.8 A resolution. J Mol Biol. 1989;209:711–734. doi: 10.1016/0022-2836(89)90602-5. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk B J, An Y Q, Geerts M, Thijs B G, de Boer H A, MacLaren D M, de Graaff J, Nuijens J H. Lactoferrin is a lipid A-binding protein. Infect Immun. 1994;62:2628–2632. doi: 10.1128/iai.62.6.2628-2632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arditi M, Zhou J, Dorio R, Rong G W, Goyert S M, Kim K S. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect Immun. 1993;61:3149–3156. doi: 10.1128/iai.61.8.3149-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281–286. doi: 10.1515/CCLM.1999.049. [DOI] [PubMed] [Google Scholar]
- 5.Bazil V, Baudys M, Hilgert I, Stefanova I, Low M G, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 6.Bennett R M, Mohla C. A solid-phase radioimmunoassay for the measurement of lactoferrin in human plasma: variations with age, sex, and disease. J Lab Clin Med. 1976;88:156–166. [PubMed] [Google Scholar]
- 7.Bevilacqua M P, Pober J S, Mendrick D L, Cotran R S, Gimbrone M A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 9.Cavaillon J M, Marie C, Ledur A, Godard I, Poulain D, Fitting C, Haeffner-Cavaillon N. CD14/LPS receptor exhibits lectin-like properties. J Endotoxin Res. 1996;3:471–480. [Google Scholar]
- 10.Crouch S P, Slater K J, Fletcher J. Regulation of cytokine release from mononuclear cells by the iron-binding protein lactoferrin. Blood. 1992;80:235–240. [PubMed] [Google Scholar]
- 11.Dentener M A, Bazil V, Von Asmuth E J, Ceska M, Buurman W A. Involvement of CD14 in lipopolysaccharide-induced tumor necrosis factor-alpha, IL-6 and IL-8 release by human monocytes and alveolar macrophages. J Immunol. 1993;150:2885–2891. [PubMed] [Google Scholar]
- 12.Dziarski R, Tapping R I, Tobias P S. Binding of bacterial peptidoglycan to CD14. J Biol Chem. 1998;273:8680–8690. doi: 10.1074/jbc.273.15.8680. [DOI] [PubMed] [Google Scholar]
- 13.Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias P S, Mazurier J, Spik G. Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun. 1998;66:486–491. doi: 10.1128/iai.66.2.486-491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elass-Rochard E, Roseanu A, Legrand D, Trif M, Salmon V, Motas C, Montreuil J, Spik G. Lactoferrin-lipopolysaccharide interaction: involvement of the 28-34 loop region of human lactoferrin in the high-affinity binding to Escherichia coli O55B5 lipopolysaccharide. Biochem J. 1995;312:839–845. doi: 10.1042/bj3120839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernig D G, Rudland P S, Smith J A. Rat mammary myoepithelial-like cells in culture possess kinetically distinct low-affinity receptors for fibroblast growth factor that modulate growth stimulatory responses. Growth Factors. 1992;7:27–39. doi: 10.3109/08977199209023935. [DOI] [PubMed] [Google Scholar]
- 16.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta D, Kirkland T N, Viriyakosol S, Dziarski R. CD14 is a cell-activating receptor for bacterial peptidoglycan. J Biol Chem. 1996;271:23310–23316. doi: 10.1074/jbc.271.38.23310. [DOI] [PubMed] [Google Scholar]
- 18.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haziot A, Rong G W, Silver J, Goyert S M. Recombinant soluble CD14 mediates the activation of endothelial cells by lipopolysaccharide. J Immunol. 1993;151:1500–1507. [PubMed] [Google Scholar]
- 20.Hess D C, Thompson Y, Sprinkle A, Carroll J, Smith J. E-selectin expression on human brain microvascular endothelial cells. Neurosci Lett. 1996;213:37–40. doi: 10.1016/0304-3940(96)12837-8. [DOI] [PubMed] [Google Scholar]
- 21.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juan T S, Hailman E, Kelley M J, Wright S D, Lichenstein H S. Identification of a domain in soluble CD14 essential for lipopolysaccharide (LPS) signaling but not LPS binding. J Biol Chem. 1995;270:17237–17242. doi: 10.1074/jbc.270.29.17237. [DOI] [PubMed] [Google Scholar]
- 23.Juan T S, Hailman E, Kelley M J, Busse L A, Davy E, Empig C J, Narhi L O, Wright S D, Lichenstein H S. Identification of a lipopolysaccharide binding domain in CD14 between amino acids 57 and 64. J Biol Chem. 1995;270:5219–5224. doi: 10.1074/jbc.270.10.5219. [DOI] [PubMed] [Google Scholar]
- 24.Lee C H, Reid Y A, Jong J S, Kang Y H. Lipopolysaccharide-induced differential cell surface expression of intercellular adhesion molecule-1 in cultured human umbilical cord vein endothelial cells. Shock. 1995;3:96–101. [PubMed] [Google Scholar]
- 25.Lee W J, Farmer J L, Hilty M, Kim Y B. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect Immun. 1998;66:1421–1426. doi: 10.1128/iai.66.4.1421-1426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Legrand D, Mazurier J, Maes P, Rochard E, Montreuil J, Spik G. Inhibition of the specific binding of human lactotransferrin to human peripheral-blood phytohaemagglutinin-stimulated lymphocytes by fluorescein labelling and location of the binding site. Biochem J. 1991;276:733–738. doi: 10.1042/bj2760733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Legrand D, Van Berkel P H, Salmon V, Van Veen H A, Slomianny M C, Nuijens J H, Spik G. The N-terminal Arg2, Arg3 and Arg4 of human lactoferrin interact with sulphated molecules but not with the receptor present on Jurkat human lymphoblastic T-cells. Biochem J. 1997;327:841–846. doi: 10.1042/bj3270841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leveugle B, Mazurier J, Legrand D, Mazurier C, Montreuil J, Spik G. Lactotransferrin binding to its platelet receptor inhibits platelet aggregation. Eur J Biochem. 1993;213:1205–1211. doi: 10.1111/j.1432-1033.1993.tb17871.x. [DOI] [PubMed] [Google Scholar]
- 29.Machnicki M, Zimecki M, Zagulski T. Lactoferrin regulates the release of tumour necrosis factor alpha and interleukin 6 in vivo. Int J Exp Pathol. 1993;74:433–439. [PMC free article] [PubMed] [Google Scholar]
- 30.Mann D M, Romm E, Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 31.Masson P L, Heremans J F, Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969;130:643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattsby-Baltzer I, Gemski P, Alving C R. Heterogeneity of lipid A: comparison of lipid A types from different gram-negative bacteria. J Bacteriol. 1984;159:900–904. doi: 10.1128/jb.159.3.900-904.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattsby-Baltzer I, Roseanu A, Motas C, Elverfors J, Engberg I, Hanson L A. Lactoferrin or a fragment thereof inhibits the endotoxin-induced interleukin-6 response in human monocytic cells. Pediatr Res. 1996;40:257–262. doi: 10.1203/00006450-199608000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Mayeux P R. Pathobiology of lipopolysaccharide. J Toxicol Environ Health. 1997;51:415–435. doi: 10.1080/00984109708984034. [DOI] [PubMed] [Google Scholar]
- 35.Mazurier J, Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta. 1980;629:399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- 36.Pugin J, Schurer-Maly C C, Leturcq D, Moriarty A, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahmoune H, Rudland P S, Gallagher J T, Fernig D G. Hepatocyte growth factor/scatter factor has distinct classes of binding site in heparan sulfate from mammary cells. Biochemistry. 1998;37:6003–6008. doi: 10.1021/bi972468t. [DOI] [PubMed] [Google Scholar]
- 38.Rahmoune H, Chen H L, Gallagher J T, Rudland P S, Fernig D G. Interaction of heparan sulfate from mammary cells with acidic fibroblast growth factor (FGF) and basic FGF. Regulation of the activity of basic FGF by high and low affinity binding sites in heparan sulfate. J Biol Chem. 1998;273:7303–7310. doi: 10.1074/jbc.273.13.7303. [DOI] [PubMed] [Google Scholar]
- 39.Spik G, Strecker G, Fournet B, Bouquelet S, Montreuil J, Dorland L, Van Halbeek H, Vliegenthart J F. Primary structure of the glycans from human lactotransferrin. Eur J Biochem. 1982;121:413–419. doi: 10.1111/j.1432-1033.1982.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 40.Stelter F, Loppnow H, Menzel R, Grunwald U, Bernheiden M, Jack R S, Ulmer A J, Schutt C. Differential impact of substitution of amino acids 9-13 and 91-101 of human CD14 on soluble CD14-dependent activation of cells by lipopolysaccharide. J Immunol. 1999;163:6035–6044. [PubMed] [Google Scholar]
- 41.Stelter F, Pfister M, Bernheiden M, Jack R S, Bufler P, Engelmann H, Schutt C. The myeloid differentiation antigen CD14 is N- and O-glycosylated. Contribution of N-linked glycosylation to different soluble CD14 isoforms. Eur J Biochem. 1996;236:457–464. doi: 10.1111/j.1432-1033.1996.00457.x. [DOI] [PubMed] [Google Scholar]
- 42.Tapping R I, Tobias P S. Cellular binding of soluble CD14 requires lipopolysaccharide (LPS) and LPS-binding protein. J Biol Chem. 1997;272:23157–23164. doi: 10.1074/jbc.272.37.23157. [DOI] [PubMed] [Google Scholar]
- 43.Tobias P S, Tapping R I, Gegner J A. Endotoxin interactions with lipopolysaccharide-responsive cells. Clin Infect Dis. 1999;28:476–481. doi: 10.1086/515163. [DOI] [PubMed] [Google Scholar]
- 44.Tobias P S, Soldau K, Ulevitch R J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989;264:10867–10871. [PubMed] [Google Scholar]
- 45.Tobias P S, Mathison J C, Ulevitch R J. A family of lipopolysaccharide binding proteins involved in responses to gram-negative sepsis. J Biol Chem. 1988;263:13479–13481. [PubMed] [Google Scholar]
- 46.Tobias P S, Soldau K, Gegner J A, Mintz D, Ulevitch R J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 47.Van Berkel P H, Geerts M E, Van Veen H A, Mericskay M, de Boer H A, Nuijens J H. N-terminal stretch Arg2, Arg3, Arg4 and Arg5 of human lactoferrin is essential for binding to heparin, bacterial lipopolysaccharide, human lysozyme and DNA. Biochem J. 1997;328:145–151. doi: 10.1042/bj3280145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viriyakosol S, Kirkland T N. A region of human CD14 required for lipopolysaccharide binding. J Biol Chem. 1995;270:361–368. doi: 10.1074/jbc.270.1.361. [DOI] [PubMed] [Google Scholar]
- 49.Wellicome S M, Thornhill M H, Pitzalis C, Thomas D S, Lanchbury J S, Panayi G S, Haskard D O. A monoclonal antibody that detects a novel antigen on endothelial cells that is induced by tumor necrosis factor, IL-1, or lipopolysaccharide. J Immunol. 1990;144:2558–2565. [PubMed] [Google Scholar]
- 50.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 51.Zagulski T, Lipinski P, Zagulska A, Broniek S, Jarzabek Z. Lactoferrin can protect mice against a lethal dose of Escherichia coli in experimental infection in vivo. Br J Exp Pathol. 1989;70:697–704. [PMC free article] [PubMed] [Google Scholar]
- 52.Ziegler-Heitbrock H W, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]