Conspectus

Even in the gas phase single atoms possess catalytic properties, which can be crucially enhanced and modulated by the chemical interaction with a solid support. This effect, known as electronic metal–support interaction, encompasses charge transfer, orbital overlap, coordination structure, etc., in other words, all the crucial features of the chemical bond. These very features are the object of this Account, with specific reference to open-shell (paramagnetic) single metal atoms or ions on oxide supports. Such atomically dispersed species are part of the emerging class of heterogeneous catalysts known as single-atom catalysts (SACs). In these materials, atomic dispersion ensures maximum atom utilization and uniform active sites, whereby the nature of the chemical interaction between the metal and the oxide surface modulates the catalytic activity of the metal active site by tuning the energy of the frontier orbitals. A comprehensive set of examples includes fourth period metal atoms and ions in zeolites on insulating (e.g., MgO) or reducible (e.g., TiO2) oxides and are among the most relevant catalysts for a wealth of key processes of industrial and environmental relevance, from the abatement of NOx to the selective oxidation of hydrocarbons and the conversion of methane to methanol.
There exist several spectroscopic techniques able to inform on the geometric and electronic structure of isolated single metal ion sites, but either they yield information averaged over the bulk or they lack description of the intimate features of chemical bonding, which include covalency, ionicity, electron and spin delocalization. All of these can be recovered at once by measuring the magnetic interactions between open-shell metals and the surrounding nuclei with Electron Paramagnetic Resonance (EPR) spectroscopy. In the case of oxides, this entails the synthesis of 17O isotopically enriched materials. We have established 17O EPR as a unique source of information about the local binding environment around oxygen of magnetic atoms or ions on different oxidic supports to rationalize structure–property relationships. Here, we will describe strategies for 17O surface enrichments and approaches to monitor the state of charge and spin delocalization of atoms or ions from K to Zn dispersed on oxide surfaces characterized by different chemical properties (i.e., basicity or reducibility). Emphasis is placed on chemical insight at the atomic-scale level achieved by 17O EPR, which is a crucial step in understanding the structure–property relationships of single metal atom catalysts and in enabling efficient design of future materials for a range of end uses.
Key References
Chiesa M.; Giamello E.; Di Valentin C.; Pacchioni G.; Sojka Z.; Van Doorslaer S.. Nature of the Chemical Bond between Metal Atoms and Oxide Surfaces: New Evidences from Spin Density Studies of K Atoms on Alkaline Earth Oxides. J. Am. Chem. Soc. 2005, 127, 16935–16944.1Here by means of 17O EPR and DFT, we showed that single K atoms are relatively strongly bound to oxide anions at particular morphological irregularities of the surface and provide clear insights into the nature of the metal–support interaction.
Livraghi S.; Chiesa M.; Paganini M. C.; Giamello E.. On the Nature of Reduced States in Titanium Dioxide As Monitored by Electron Paramagnetic Resonance. I: The Anatase Case. J. Phys. Chem. C 2011, 115, 25413–25421.2The introduction of the 17O magnetic isotope at the surface and in the bulk of TiO2 allows us to elucidate the nature of TiO2 reduced states originated in various ways, providing evidence for the presence of delocalized states in the anatase polymorph.
Morra E.; Signorile M.; Salvadori E.; Bordiga S.; Giamello E.; Chiesa M.. Nature and Topology of Metal–Oxygen Binding Sites in Zeolite Materials: 17O High-Resolution EPR Spectroscopy of Metal-Loaded ZSM-5. Angew. Chem., Int. Ed. 2019, 58, 12398–12403.3In this work through a combination of selective 17O isotopic enrichment and the unique properties of open-shell s-state monovalent group 12 cations, we derive a site-specific topological description of active sites in an MFI zeolite.
Bruzzese P. C.; Salvadori E.; Jäger S.; Hartmann M.; Civalleri B.; Pöppl A.; Chiesa M.. 17O-EPR Determination of the Structure and Dynamics of Copper Single-Metal Sites in Zeolites. Nat. Commun. 2021, 12, 4638..4Here, 17O EPR is combined with DFT modeling to determine the local structure of single site CuII species, quantify the covalency of the metal–framework bond and assess how this scenario is modified by the presence of solvating water molecules.
1. Introduction
“Adsorbed, chemisorbed, embedded, anchored, grafted” are all different words used to describe the variety of bonding interactions of an atom with the surface of a support.5 Spatially isolated metal atoms on surfaces represent a relevant class of heterogeneous catalysts, referred to as “single atom catalysts” (SACs). Here “single atom” denotes well isolated and atomically dispersed species at specific surface sites. The basic rules of chemistry apply to this interfacial situation, which define the interaction between the atom and the surface as dispersive, covalent or ionic depending on the degree of orbital overlap and energy difference of the interacting orbitals. The understanding of such interactions grew up in parallel with the development of methods for the controlled deposition of metal particles and, in general, with the improvement of the performances of advanced surface science6,7 and computational techniques.8,9 Evolving from early models,10−12 the evidence of an enhancement of the catalytic activity induced by electronic interactions between a metal and an oxide13 led to the definition in 2012 of the electronic metal–support interaction (EMSI).14 EMSI encompasses the metal–oxide interactions based on charge transfer, orbital overlap etc. In other words, the crucial features of the chemical bond, whose formation is critically dependent on the spin state of the electrons.
For open-shell metals, the spin, and its delocalization, is central in dictating design principles for the development of new sustainable catalytic pathways15 and plays a key role in the evolution of new materials16 and quantum information technologies.17
A powerful yet underexploited experimental technique is Electron Paramagnetic Resonance (EPR) because of its intrinsic ability to monitor the electron spin density in paramagnetic systems.18,19 EPR has been successfully applied to study ensembles of single atoms dispersed on polycrystalline surfaces–the object of this Account–and on thin oxide films.20 Noteworthy, the recent combination of EPR with scanning tunneling microscopy (STM) enabled addressing the EPR signature of an individual atom on the surface.21−23 In the case of metals on oxide surfaces, the coupling between the electron and 17O nuclear spins is a unique source of information about the local binding environment around the open-shell metal center that allows one to rationalize structure–property relationships in the most diverse systems.24−31 In our laboratory 17O EPR has been used over the past 15 years1−4,32−38 to investigate the interaction of single paramagnetic metal atoms or ions with different oxidic supports in order to monitor the redistribution of the electron spin density and to understand, in this way, the specific features of the metal–oxide chemical interaction. Here we will focus on single atoms and ions with complementary valence electron configurations across the fourth period of the periodic table, from K to Zn, and their interactions with oxide substrates featuring complementary chemical properties (Figure 1). For simplicity, regardless of whether they are neutral or charged, we will address them as single atoms. Emphasis will therefore be on studies from our group focused on oxide surfaces. For details on other supports, synthetic procedures and catalytic performances of SACs, the interested reader is referred to the literature.5,39−43
Figure 1.
Single metal atoms and relative oxide supports described in this Account. For each element, including oxygen, the relevant magnetic properties are listed: isotope (A), nuclear spin (I), nuclear g factor (gn), and natural abundance (%Ab).
2. 17O Hyperfine Spectroscopy
Single metal species interact with oxide surfaces forming new bonds at the interface, which can range from weak interactions, dominated by dispersive forces and polarization effects, to covalent bonds, involving the mixing of metal and oxide orbitals, up to net electron transfer interactions resulting in ionic bonds. All these situations, which crucially depend on the nature of the oxide support, can be monitored for paramagnetic atoms by assessing the total spin density distribution, i.e., the total electron density of electrons of one spin (α) minus the total electron density of the electrons of the opposite spin (β). The spin density is a property directly related to the hyperfine interaction and associated coupling constants, which are experimental observables in a EPR measurement.44 The hyperfine interaction splits the EPR resonance transition in 2nI + 1 hyperfine lines, where n is the number of equivalent nuclei and I is the nuclear spin quantum number. Therefore, the number of hyperfine lines in the EPR spectrum directly identifies the presence of a single metal species (n = 1) through the detection of 2I + 1 transitions. The integrated intensity of a Continuous Wave (CW) EPR spectrum is directly proportional to the concentration of spin centers in the sample. In our case, if the total metal loading is known (e.g., via ICP analysis) then the fraction of EPR active centers can be determined.4 The splitting between the 2I + 1 transitions–referred to as the hyperfine coupling (hfc) constant–directly reflects the perturbation of the metal wave function induced by the substrate, i.e., the nature of the chemical bond. Magnetic interactions are very sensitive to the chemical properties of the binding site and are able to probe the full range of chemical interactions. However, to draw meaningful conclusions and avoid being misled by hasty interpretations, hfc to all nuclei (the single atom and the ligands) encompassed by the unpaired electron wave function must be measured. Notice that hfc in general consists of a dipolar (orientationally dependent) and an isotropic term; therefore, in the case of polycrystalline materials, where a “powder” average of all orientations with respect to the magnetic field is observed, the EPR spectra can be rather complex and simulation analysis is needed to reliably extract the relevant parameters. For details on the analysis of powder EPR spectra, the interested Reader is referred to the many books and review articles available in the literature.18,19,44 Indeed, it is the analysis of all the hyperfine data that allows the determination of the atomistic and electronic structure of coordinated metal atoms. In more detail, the isotropic hfc, aiso, is a direct measure of the s electron spin density, ρNα–β at a given nucleus N, whereas the dipolar hfc, T, yields the contribution of orbitals with higher angular momentum (p, d, etc.).44 The isotropic hyperfine coupling at a certain nucleus N is given in energy units by
| 1 |
where μ0 is the vacuum permeability, ge the electron g-factor, μB the Bohr magneton, gn the nuclear g factor, and μN the nuclear magneton. With reference to 17O, knowing aiso and T for the atomic species, and assuming that the hfc at a given nucleus is proportional to the electron spin density at that nucleus, it is possible to estimate the spin population in s-type orbitals (ρs) and p-type orbitals (ρp). For an unpaired electron (free electron, ge = 2.0023) on a 17O nucleus with a unitary spin population (ρs = 1) in an s-type orbital, one would observe an isotropic hyperfine coupling constant of a0 = −4622.83 MHz. If the electron resides in a p-type orbital, one would observe a uniaxial hyperfine constant of b0 = 130.5 MHz. Including a correction for the difference in the g values, the spin populations in s-type and p-type orbitals can thus be estimated as
| 2 |
For instance, for the case relevant to this Account–a single metal atom on an oxide surface–the ratio of the measured isotropic and dipolar hfc to the corresponding values for the free atomic state quantifies the contribution of the metal atomic orbitals to the molecular orbital containing the unpaired electron. On the other hand, the hfc with 17O nuclei reflects the electron spin density distribution over the coordinating ligands, which provide a clear-cut answer to the thorny question on how metals bind on oxide surfaces.
Experimentally, hfc can be directly detected in a standard CW-EPR experiment if large enough. If small, they can be recovered by means of hyperfine techniques such as Electron Nuclear Double Resonance (ENDOR), Electron Spin Echo Envelope Modulation (ESEEM) and Hyperfine Sublevel Correlation (HYSCORE) spectroscopies,45,46 which exploit the interaction between electron and nuclear magnetic moments to measure the NMR spectrum associated with the paramagnetic center. ENDOR experiments (in both CW or pulse variants) are based on a combination of micro- and radiowaves to measure the response of the electron spin as an incident radio frequency (rf) sweeps through different nuclear transitions.45 ESEEM and HYSCORE are based on a sequence of microwave (mw) pulses to generate an electron spin echo, whose intensity is monitored as a function of the variation of one or more time intervals between the pulses. The resulting time-domain signal is modulated by the nuclear frequencies, and after Fourier transformation a frequency-domain spectrum reproducing the nuclear frequencies is obtained.46,47
In general, the two classes of hyperfine techniques give complementary information. ESEEM techniques are mainly used to detect small hfc (<5 MHz), while ENDOR techniques are usually preferred to observe larger nuclear frequencies, i.e., larger hfc.
3. 17O Surface Doping of Metal Oxides
Hyperfine techniques require the presence of magnetic nuclei (i.e., nuclei having a spin). In the case of oxides this implies magnetic oxygen ions. Oxygen has only one magnetic isotope, 17O, characterized by a high spin quantum number (I = 5/2) and a natural abundance (0.038%), far too low to detect any hyperfine structure in naturally occurring samples. The exploitation of hyperfine techniques to assess the metal–oxygen bond requires therefore isotopic enrichment of the oxide matrix. This involves cost and effort but can be very rewarding. In the case of polycrystalline materials, the high cost of 17O isotopically enriched reagents makes bulk synthesis very inconvenient. Moreover, opposite to 17O NMR studies,48 where uniform substitution of 17O throughout the lattice is often sought to allow for quantitative measurements, selective isotopic enrichment can be advantageous in the case of EPR studies of single atoms on the surface. 17O surface enrichment can be performed following specific procedures that depend on the nature of the oxide. For nonreducible oxides (such as alkali-earth metal oxides, alumina, zeolites, etc.) the preferred isotope carrier is isotopically labeled water (H217O).1,33−35 In this case hydration/dehydration cycles using H217O vapors provide an effective and atom-efficient method to incorporate 17O isotopes at the surface. By adjustment of contact time and temperature, the process can be selective, limiting the isotopic exchange to the most reactive surface sites, which are the very sites involved in the stabilization of surface single atoms.
In the case of rock-salt structures such as MgO (Figure 2a), the exchangeable and chemically relevant sites correspond to three- and four-coordinated oxygen ions at corners and edges, whereas five-coordinated sites at dominant (100) faces are less involved in the exchange process.33 Similarly, zeolite (Figure 2b) can readily exchange their Si–O–Al and Si–O–Si framework sites with the oxygen of H217O, while keeping their highly crystalline frameworks.3,4,37,48,49 The reactivity order is Si–O–Al > Si–O–Si, where Si–O–Al are the privileged metal binding sites because of charge compensation. The reported isotopic enrichment protocols were carefully scrutinized against structural or morphological alteration of the pristine oxide materials. Such careful structural characterization should always be performed when water is employed as isotopic carrier in light of possible oxide sintering or Al leaching from the zeolite framework.3
Figure 2.
17O enrichment of different oxide systems: (a) alkaline earth oxides, (b) zeolites, (c) TiO2.
For reducible oxides such as TiO2 (Figure 2c), the strategy exploits the easy lattice oxygen depletion at the surface achieved by thermal treatments under vacuum.2 This generates oxygen vacancies, which can be replenished by heating in 17O2 atmosphere.50 Due to the surface nature of oxygen vacancies, this method ensures a selective isotopic enrichment of the surface.51
4. The Effect of Acid–Base Properties of the Support: Single Metal Atoms and Ions with 4s1 Electron Configuration
Central to the problem of the metal atom–oxide interaction is the ability to monitor the electron spin density distribution over the atoms of the support. While the number of EPR lines and their relative intensity provides direct compelling evidence for the presence of single metal atoms on the surface, the hfc with ligated atoms of the support encodes important information on the electronic structure and binding geometry. A paradigmatic example is that of neutral alkali metal atoms stabilized on the surface of alkaline earth oxides,1,52,53 which represent an excellent test-bed to interpret the bonding mechanisms of neutral metals on nonreducible oxides.20 In the following we will discuss the case of K on MgO (K/MgO, Figure 3a).
Figure 3.
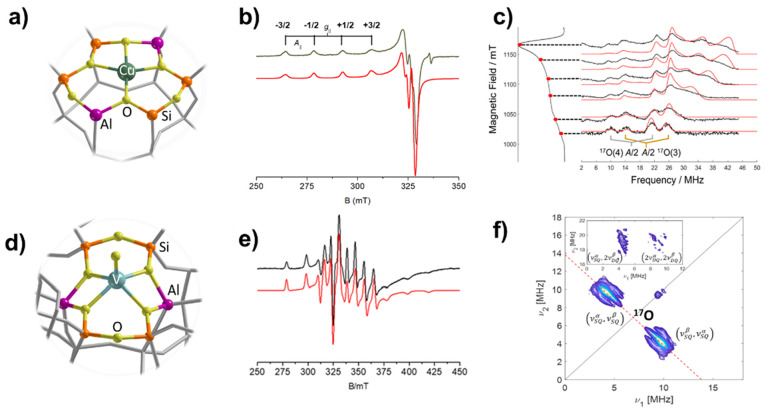
(a) Model for single K atoms adsorbed on MgO. (b) CW-EPR spectrum of single K atoms on Mg16O and (c) on surface enriched Mg17O. (d) Model for single Zn+ ions adsorbed on H-ZSM-5. (e) Pulsed EPR spectrum of single Zn+ ions on H-ZSM-5. (f) CW-EPR spectrum of Zn+ ions on 17O enriched H-ZSM-5. Black lines, experimental; red lines, simulations. Panels a, b, and c reproduced with permission from ref (1). Copyright 2005 American Chemical Society. Panels e and f adapted with permission from ref (3). Copyright 2019 Wiley.
The four hyperfine lines observed in a standard CW-EPR spectrum (Figure 3b) are due to the interaction of the unpaired electron with the I = 3/2 nuclear spin of K and firmly demonstrate the presence of single potassium atoms on the surface of MgO.1 The EPR spectrum of Figure 3b proves that surface K species retain the recognizable parentage of alkali atoms in the gas phase, but are subject to large perturbations arising from strong atom–surface interactions. In fact, the isotropic hfc of the metal is reduced by about 50% as compared to that of gas phase K atoms.54 A naive interpretation may point to a partial charge transfer from the metal to the surface. However, analysis of the complementary 17O hfc in an enriched Mg17O sample showed that K atoms bind strongly to two surface oxygen ions (O1 and O2 in Figure 3a) and weakly to a third (O3 in Figure 3a), but the measured maximum 17O hfc of ≈9 MHz is far too small to account for a significant spin delocalization over the matrix ions.1 This evidence thus firmly excludes that the origin of the lowered hfc observed at the K nucleus is due to a spin (charge) delocalization over the oxide support. In fact, the mechanism responsible for the reduction of the spin density at the K nucleus1 is principally a polarization of the singly occupied 4s orbital of neutral K atoms induced by Pauli repulsion effects brought about by the lone pairs of surface oxygens. The net result of this interaction is to lift in energy the 4s K orbital, favoring some degree of sp hybridization. This “expanded atom” (or polarized) state explains the reduced hfc with respect to the free atom without invoking any significant metal to surface electron transfer. This is a general bonding scheme for neutral metals on the surface of basic oxides20 and depends on the degree of interaction between the oxide ion lone pairs and the ns orbital or, in other words, on the basicity of the oxide. A systematic study of alkaline earth oxides demonstrated that the reduction of the metal hfc linearly correlates with the well-known trend of basic strength MgO < CaO < SrO < BaO.52
To fully appreciate the role of the substrate, it is instructive to compare the data on MgO to a different oxide substrate featuring opposite characteristics, i.e., a covalent and acidic nature, namely, zeolite (Figure 3d). Zeolites are composed of corner sharing SiO4 and AlO4 tetrahedra, arranged into three-dimensional frameworks in such a manner that they contain regular channels and cavities of molecular dimensions. The substitution of aluminum (formally Al3+), in place of silicon (Si4+), produces a net negative charge, which is balanced by acidic (Brønsted) protons (or other cations) resident in the cavities. When incursive atoms from the vapor of, for example, alkali metals enter a dehydrated zeolite, they are spontaneously ionized reducing the acidic protons and forming a variety of unusual ions, clusters, and filamentary structures.55 Particularly interesting to our discussion is the case of zinc. The exposure of a protonated zeolite to Zn vapors leads to the reduction of H+ Brønsted sites and the formation of the unusual monovalent Zn+ species, characterized by a 4s1 electronic structure, which are isoelectronic with K.56 The EPR spectrum of sublimated Zn in zeolites is characterized by a nearly isotropic signal flanked by six evenly spaced satellite transitions due to the hfc with 67Zn (I = 5/2, 4.1% natural abundance).
While the six satellite transitions (Figure 3e) provide evidence for the presence of single metal ions, analysis of the 67Zn+ hfc points to 80% electron spin density localization in the 4s Zn orbital, proving the formation of a genuine Zn+ ion. The 20% missing spin density is shared between two equivalent coordinating oxygen atoms as testified by the 11 hyperfine lines (hfc ≈ 60 MHz) of the 17O EPR spectrum (Figure 3f). While the local metal coordination is similar for K/MgO and Zn/ZSM-5, the electronic structure is dramatically different. The coordinating oxygen ions of the ionic and basic MgO strongly polarize the K atom wave function with a minute spin delocalization to the surface (17O hfc ≈ 9 MHz); on the other hand the more covalent and acidic zeolite yields a bond mostly ionic, with a non-negligible degree of covalency (17O hfc ≈ 60 MHz). This comparison illustrates the unique level of details provided by 17O hfc in the description of the metal coordination environment in disordered systems. This knowledge allows us to pinpoint the structure of the metal binding sites with atomistic precision and discriminate among different potential binding sites and it is the prerequisite to map the spatial distribution of single metal atoms in the nanometer range.57 When the 17O hfc is large enough to be resolved, the CW-EPR spectrum provides a handle to assess the level of isotopic enrichment at the metal site. In the case of K/MgO and Zn/ZSM-5, both characterized by a digonal coordination, this is done by comparing the relative intensity of the 17O hfc pattern and assuming a binomial distribution. In this way, we estimated a 10% 17O enrichment for K/MgO1 and a 70% enrichment for Zn/ZSM-5.3 Considering the procedure described in section 3, we note that the isotopic enrichment is metal independent but strictly related to the specific experimental conditions (17O water enrichment, temperature, and contact time).
5. Nature and Topology of Single Metal Binding Sites in Zeolites: The Role of 3d Orbitals
As outlined in the previous section, zeolites provide an ideal platform for the stabilization of single (transition) metal ions at sites, whose nature depends on the zeolite structure, Si/Al and metal/Al ratios. By controlling these parameters, single metal sites can be engineered prompting metal-loaded zeolites toward a number of important catalytic transformations.43 Copper- and vanadium-exchanged zeolites are relevant examples. In this case, the metal–oxide interaction involves the metal 3d orbitals, and 17O hfc measures the σ- and π-contribution to the chemical bond. During catalysis, Cu and V cycle through paramagnetic states with complementary electronic configurations, namely, Cu2+ (3d9) and V4+ (3d1). The siting of both elements over the large internal surface of the zeolite is primarily driven by electrostatic interactions brought about by the Al3+ distribution in the framework, while the fine details of the electronic structure arise from the favorable interactions between the metal 3d orbitals and the oxide frontier orbitals.
Cu2+ species in dehydrated zeolites adopt an unsaturated square planar coordination in the proximity of two framework aluminum ions, that act as charge compensating agents (Figure 4a,b).4,38 Quantitative analysis indicate Cu2+/Cu ratio of 0.60 in the dehydrated material. The fine details of the metal coordination structure and the location of the Al in the framework play a crucial role in modulating the catalytic activity but are notoriously difficult to determine. We demonstrated that 17O EPR provides a robust handle for their quantification. ENDOR experiments performed on Cu-exchanged zeolites with Chabazite (CHA)4 and ZSM-5 (MFI)38 topology enriched in 17O show 17O coupling on the order of 60 MHz, similar to those observed in the case of Zn+ (Figure 4c) and characteristic of σ-bonding. Quantum chemical analysis of the spin density distribution shows that the 17O hfc is very sensitive to the local structural deformations induced by nearby framework Al ions, whose location can be pinpointed with accuracy.4
Figure 4.
(a) Periodic model for single Cu atoms docked on CHA. (b) CW-EPR spectrum of single Cu ions on CHA. (c) 17O ENDOR spectra of single Cu ions on 17O enriched CHA. (d) Periodic model for single VO2+ ions docked on H-ZSM-5. (e) CW-EPR spectrum of single VO2+ ions on H-ZSM-5. (f) 17O HYSCORE spectrum of VO2+ ions on 17O enriched H-ZSM-5. The level of 17O enrichment at the metal site is estimated to be ≈70% in analogy with Zn/ZSM-5.3 Panels a, b, and c adapted with permission from ref (4). Copyright 2021 the Authors. Published by Springer Nature under the terms of the Creative Commons CC BY license (CC BY 4.0). Panels e and f reproduced with permission from ref (37). Copyright 2020 Elsevier.
Similar siting sites stabilize V4+ species in ZSM-5 (Figure 4d), in the form of isolated vanadyl ions as revealed by CW-EPR (Figure 4e).37 However, 17O HYSCORE spectra (Figure 4f) detected only a minute (≈ 7 MHz) 17O hfc with framework oxide ions, which is nearly 1 order of magnitude smaller than the one recorded for Zn+ and Cu2+ in the same system. In this case the small 17O hfc is not related to a reduced covalent character with respect to Cu2+ and Zn+ but reflects the fact that the unpaired electron is localized in a nonbonding σ orbital and the measured 17O hfc weighs the degree of metal–oxygen π-bonding.
6. Single Metal Centers with 3d1 Electronic Configurations on the Surface of Reducible Oxides
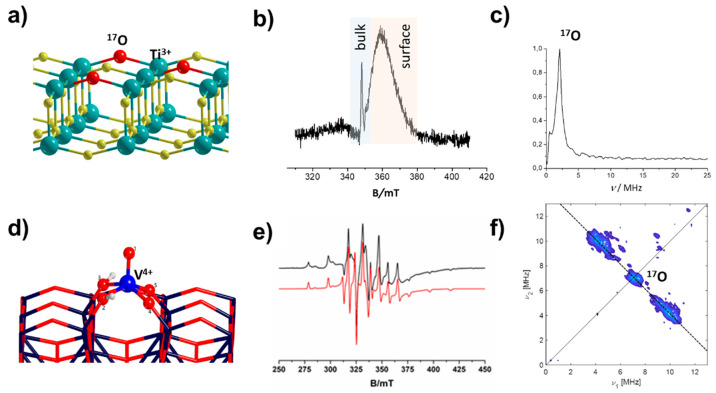
While alkali metal atoms bind on the surface of insulating oxides (MgO) through a polarization interaction (see section 4), on the surface of reducible oxides such as TiO2 they spontaneously ionize and forfeit any parentage in the electronic states of the gas-phase alkali atoms.2 The released “excess electrons” reduce Ti4+ forming paramagnetic Ti3+ ions, which can be stabilized either in the bulk or at the surface (Figure 5a). 17O EPR of TiO2 anatase selectively enriched at the surface provided evidence for surface localization of the Ti3+ species (Figure 5b,c).2,51 The small width of the 17O ESEEM signal is compatible with a maximum hfc of ≈3 MHz (Figure 5c). When dealing with solid-state semiconductors, it is instructive to benchmark the magnitude of the measured hfc against corresponding molecular complexes to gauge the extent of the wave function delocalization. For instance, the fully localized [Ti(H217O)6]3+ yields a 17O hfc of 8 MHz,58 which is also similar to that measured for the strongly localized Ti3+ in the bulk of Ti17O2 rutile.36 Thus, comparison of the 17O hfc of oxygen atoms coordinated to Ti3+ species in different TiO2 polymorphs (anatase vs. rutile) together with the use of molecular complexes as a benchmark demonstrates that the electron wave function of Ti3+ at the anatase surface is characterized by a larger degree of delocalization. That excess electrons localize in rutile to form what in the physics’ trade is called a small polaron, while in anatase they prefer delocalized (free-carrier) states was independently confirmed by scanning tunneling microscopy and spectroscopy.59
Figure 5.
(a) Model of the (100) surface of TiO2 anatase. (b) Pulsed EPR spectrum and (c) 17O ESEEM spectrum of Ti3+ at the surface of anatase. (d) Model for single VO2+ ions adsorbed on the (100) surface of anatase. (e) CW-EPR spectrum surface (black experimental and red simulated spectrum) and (f) 17O HYSCORE spectrum of single VO2+ ions on 17O enriched on anatase (100). Panels a, b, and c adapted with permission from ref (2). Copyright 2011 American Chemical Society. Panels d, e, and f reproduced with permission from ref (32). Copyright 2022 Elsevier.
The scenario is dramatically different when a transition metal ion with the same 3d1 electronic configuration such as V4+ is deposited on the surface of TiO2 (Figure 5d,e).32 In this case the observed 17O HYSCORE spectrum (Figure 5f) is characterized by cross-peaks separated by approximately 7 MHz corresponding to a hfc comparable to those observed in VO/ZSM537 and reported for [VO(H217O)5]2+ molecular complexes25 when the unpaired electron is fully localized. This comparison illustrates the profound difference in the electronic structure of an atom at the surface, i.e., part of the lattice (Ti3+, Figure 5a) versus an atom deposited on the surface (V4+, Figure 5d). The intrinsic differences between these two cases should always be considered carefully, when discussing the electronic structure of metals on oxides and surfaces in general.
7. Interfacial Coordination Chemistry of Single Metal Atoms and Ions
The examples presented in the previous sections highlight the role of 17O as a particularly interesting target nucleus to study the metal–oxide interaction. To extract meaningful results, the measured hfc parameters need to be interpreted in the light of some knowledge of the electron wave function as obtained at different levels of accuracy in the frame of electronic structure theory.8 The use of simple interpretative models such as ligand field theory provides, however, a great deal of insight allowing us to establish sound correlations between classical coordination chemistry and the bonding of transition metal ions at surfaces. The properties of single paramagnetic atoms on oxide surfaces may be interpreted in a general way, considering that the unpaired valence electron does not materially affect the binding process but rather represents a convenient probe to measure the degree of interaction between the metal and the oxide adsorption site. A simplified orbital correlation diagram for the cases discussed in this Account, i.e., 3d1, 3d9 and 4s1 valence electron configurations, captures the essence of the problem (Figure 6). In the case of surface vanadyl ions (sections 5 and 6), the local geometry can be described in terms of a square pyramid with ideal C4v symmetry (Figure 6a). Under these circumstances, the unpaired electron of the V4+ ion (S = 1/2, 3d1) is allocated in a vanadium 3dxy orbital (singly occupied molecular orbital, SOMO) with nonbonding σ character and the weak π-interactions with framework O ligands are responsible for the small (≈7 MHz) 17O hfc. In the case of Cu2+ (S = 1/2, 3d9) the SOMO is the σ-antibonding orbital contained in the CuO4 square plane (Figure 6b), in which the Cu 3dx2–y2 orbital is combined out-of-phase with the 2p orbitals of O framework ligands. In this case the 17O hfc (≈60 MHz) is a direct measure of the degree of covalency in the Cu–O bond and crucially depends on the local structure distortion (bond lengths and angles).
Figure 6.
Simplified orbital correlation diagrams illustrating the sigma bonding of (a) VO2+ with ZSM-5 (diagram assumes C4v local symmetry), (b) Cu2+ with ZSM-5 zeolite, assuming D4h local symmetry, and (c) Zn+ on ZSM-5 assuming C2v local symmetry. For each case, the SOMO was calculated on a cluster model at PBE060/CP(PPP)61 level of theory (isovalue of 0.03).
In a similar fashion for the 4s1 elements (K+ and Zn+), the unpaired electron is allocated in a SOMO consisting of the out-of-phase combination of the metal 4s orbital and the 2p orbitals of coordinating O framework ligands (Figure 4c). In general, regardless of the atom considered, there is little electron transfer at the boundary between the metal and the surface of nonreducible oxides like MgO or zeolites (i.e., the unpaired electron is largely localized in the metal orbitals), but the fine details of the bonding interaction, crucially depend on the chemical nature of the substrates. In the case of the basic and ionic MgO, the metal–oxide interaction is dominated by strong polarization, while in the case of the isoelectronic Zn+ bound to the acidic and covalent ZSM-5 in a structurally similar surface site, the bond has a non-negligible covalent character, as revealed by the spin density sharing between the metal and the support. A completely different situation occurs for a reducible oxide such as TiO2. In this case neutral metal atoms (K) are fully ionized and the released electrons localize in the empty 3d orbitals of Ti4+ ions. The use of surface selective 17O enrichment allows us to monitor the fraction of electrons migrating to the bulk and those remaining at the surface. Moreover, the small 17O hfc indicates a significant delocalized character of the wave function of surface Ti3+ ions in anatase, a situation remarkably different from the rutile polymorph.
8. Concluding Remarks
Numerous experimental and theoretical studies aimed at a better understanding of the unique properties of single metal atoms and ions on oxide surfaces are currently being performed. Our studies demonstrate that 17O EPR can be a unique source of information on the structure and bonding interactions of open-shell single metal atom species. This is particularly important in cases of structural disorder often encountered in heterogeneous catalysts where the selective information from 17O EPR can be vital since other, more established structural techniques are not able to provide the same atomic-scale insight. We illustrated some of the guiding principles for a selective and atom-economic 17O isotopic enrichment of oxide surfaces and the application of EPR techniques to unravel fine details of the metal–oxide interaction. The chosen examples exemplify the role of the surface as a solid solvent whereby, depending on the oxide characteristics (acidity, basicity, reducibility), metal atoms or ions can be described in term of polarized atoms maintaining a recognizable parentage to gas phase species (alkali metals on MgO), coordination complexes (e.g., transition metal ions in zeolites), or ionized fragments (alkali metals on TiO2). The role of the metal support in modifying the electronic structure of single atoms on surfaces is highly system-dependent. The oxide support can affect the oxidation state of the adatom, stabilize unusual oxidation states and coordination geometries, or promote rearrangements of the orbital energy levels. Under all circumstances, the detection of 17O hfc is key to the understanding of the substrate-dependent changes in the electronic structure of supported single metal atoms on oxides, which are ultimately responsible for their unique catalytic properties. The level of structural information that can be obtained from 17O hfc and hyperfine techniques in general provides a powerful tool to help quantify and ultimately tune the multiple features required to design efficient single-metal catalysts, such as well-tailored electronic and geometric structure, high stability to sintering or leaching, and sufficient and uniform single site distributions. We expect this approach to be extended beyond the study of metal-oxide based catalysts to other substrates such as two-dimensional carbon-based hosts and other layered materials, where the most favorable coordination sites are usually provided by N, O, S, or P. Also, for this emerging class of materials, selective isotopic enrichment in conjunction with magnetic resonance techniques can provide unique opportunities to develop an atomistic understanding of the highly synergistic interactions between metals and supports that are the basis of unique chemical reactivity and catalytic performances of single-atom catalysts.
Acknowledgments
This work is part of a project that has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant agreement no. 813209.
Biographies
Enrico Salvadori is an Associate Professor of Inorganic Chemistry at the University of Torino. His current research efforts include the study of photoexcited paramagnetic states and the spatial distribution of defective species in solid-state inorganic materials.
Paolo Cleto Bruzzese is a Ph.D. student at the University of Leipzig, Germany, working under the joint supervision of Profs. Andreas Pöppl and Mario Chiesa within the frame of the MSCA-ITN project Paracat.
Elio Giamello is Emeritus Professor at the University of Torino. His scientific interests revolve around the surface chemistry and photochemistry of metal oxides investigated, in particular, by Electron Paramagnetic Resonance spectroscopy.
Mario Chiesa is Full Professor of Inorganic Chemistry at the University of Torino. His research interests are broadly based in the fundamental understanding of open-shell electronic structure, with applications ranging from catalysis to quantum information science.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. CRediT: Enrico Salvadori data curation (equal), formal analysis (equal), investigation (equal), resources (equal), writing-review & editing (lead); Paolo Cleto Bruzzese data curation (equal), software (lead), writing-review & editing (equal); Elio Giamello validation (equal), writing-review & editing (equal); Mario Chiesa conceptualization (lead), funding acquisition (lead), methodology (lead), resources (equal), supervision (lead), writing-original draft (lead).
The authors declare no competing financial interest.
References
- Chiesa M.; Giamello E.; Di Valentin C.; Pacchioni G.; Sojka Z.; Van Doorslaer S. Nature of the Chemical Bond between Metal Atoms and Oxide Surfaces: New Evidences from Spin Density Studies of K Atoms on Alkaline Earth Oxides. J. Am. Chem. Soc. 2005, 127 (48), 16935–16944. 10.1021/ja0542901. [DOI] [PubMed] [Google Scholar]
- Livraghi S.; Chiesa M.; Paganini M. C.; Giamello E. On the Nature of Reduced States in Titanium Dioxide As Monitored by Electron Paramagnetic Resonance. I: The Anatase Case. J. Phys. Chem. C 2011, 115 (51), 25413–25421. 10.1021/jp209075m. [DOI] [Google Scholar]
- Morra E.; Signorile M.; Salvadori E.; Bordiga S.; Giamello E.; Chiesa M. Nature and Topology of Metal–Oxygen Binding Sites in Zeolite Materials: 17O High-Resolution EPR Spectroscopy of Metal-Loaded ZSM-5. Angew. Chemie Int. Ed. 2019, 58 (36), 12398–12403. 10.1002/anie.201906488. [DOI] [PubMed] [Google Scholar]
- Bruzzese P. C.; Salvadori E.; Jäger S.; Hartmann M.; Civalleri B.; Pöppl A.; Chiesa M. 17O-EPR Determination of the Structure and Dynamics of Copper Single-Metal Sites in Zeolites. Nat. Commun. 2021, 12 (1), 4638. 10.1038/s41467-021-24935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S. K.; Chen Z.; Faust Akl D.; Mitchell S.; Pérez-Ramírez J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120 (21), 11703–11809. 10.1021/acs.chemrev.0c00576. [DOI] [PubMed] [Google Scholar]
- Risse T.; Shaikhutdinov S.; Nilius N.; Sterrer M.; Freund H.-J. Gold Supported on Thin Oxide Films: From Single Atoms to Nanoparticles. Acc. Chem. Res. 2008, 41 (8), 949–956. 10.1021/ar800078m. [DOI] [PubMed] [Google Scholar]
- Hurdax P.; Hollerer M.; Puschnig P.; Lüftner D.; Egger L.; Ramsey M. G.; Sterrer M. Controlling the Charge Transfer across Thin Dielectric Interlayers. Adv. Mater. Interfaces 2020, 7 (14), 2000592. 10.1002/admi.202000592. [DOI] [Google Scholar]
- Pacchioni G. Electronic Interactions and Charge Transfers of Metal Atoms and Clusters on Oxide Surfaces. Phys. Chem. Chem. Phys. 2013, 15 (6), 1737–1757. 10.1039/c2cp43731g. [DOI] [PubMed] [Google Scholar]
- Tosoni S.; Pacchioni G. Magnetic Nature and Hyperfine Interactions of Transition Metal Atoms Adsorbed on Ultrathin Insulating Films: A Challenge for DFT. Phys. Chem. Chem. Phys. 2022, 24 (26), 15891–15903. 10.1039/D2CP01224C. [DOI] [PubMed] [Google Scholar]
- Schwab G.-M.; Schlütes H. Die Wirkungsweise von Mischkatalysatoren Beim Zerfall Des Stickoxyduls 1930, 9B (1), 265–288. 10.1515/zpch-1930-0920. [DOI] [Google Scholar]
- Schwab G.-M.Electronics of Supported Catalysts; In Advances in Catalysis; Eley D. D., Pines H., Weisz P. B., Eds.; Academic Press, 1979; Vol. 27, pp 1–22. [Google Scholar]
- Tauster S. J.; Fung S. C.; Garten R. L. Strong Metal-Support Interactions. Group 8 Noble Metals Supported on Titanium Dioxide. J. Am. Chem. Soc. 1978, 100 (1), 170–175. 10.1021/ja00469a029. [DOI] [Google Scholar]
- Bruix A.; Rodriguez J. A.; Ramírez P. J.; Senanayake S. D.; Evans J.; Park J. B.; Stacchiola D.; Liu P.; Hrbek J.; Illas F. A New Type of Strong Metal–Support Interaction and the Production of H2 through the Transformation of Water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) Catalysts. J. Am. Chem. Soc. 2012, 134 (21), 8968–8974. 10.1021/ja302070k. [DOI] [PubMed] [Google Scholar]
- Campbell C. T. Electronic Perturbations. Nat. Chem. 2012, 4 (8), 597–598. 10.1038/nchem.1412. [DOI] [PubMed] [Google Scholar]
- Nandy A.; Kulik H. J. Why Conventional Design Rules for C–H Activation Fail for Open-Shell Transition-Metal Catalysts. ACS Catal. 2020, 10 (24), 15033–15047. 10.1021/acscatal.0c04300. [DOI] [Google Scholar]
- Wolf S. A.; Awschalom D. D.; Buhrman R. A.; Daughton J. M.; von Molnár S.; Roukes M. L.; Chtchelkanova A. Y.; Treger D. M. Spintronics: A Spin-Based Electronics Vision for the Future. Science 2001, 294 (5546), 1488–1495. 10.1126/science.1065389. [DOI] [PubMed] [Google Scholar]
- Heinrich A. J.; Oliver W. D.; Vandersypen L. M. K.; Ardavan A.; Sessoli R.; Loss D.; Jayich A. B.; Fernandez-Rossier J.; Laucht A.; Morello A. Quantum-Coherent Nanoscience. Nat. Nanotechnol. 2021, 16 (12), 1318–1329. 10.1038/s41565-021-00994-1. [DOI] [PubMed] [Google Scholar]
- Roessler M. M.; Salvadori E. Principles and Applications of EPR Spectroscopy in the Chemical Sciences. Chem. Soc. Rev. 2018, 47 (8), 2534–2553. 10.1039/C6CS00565A. [DOI] [PubMed] [Google Scholar]
- Chiesa M.; Giamello E.; Che M. EPR Characterization and Reactivity of Surface-Localized Inorganic Radicals and Radical Ions. Chem. Rev. 2010, 110 (3), 1320–1347. 10.1021/cr800366v. [DOI] [PubMed] [Google Scholar]
- Yulikov M.; Sterrer M.; Heyde M.; Rust H.-P.; Risse T.; Freund H.-J.; Pacchioni G.; Scagnelli A. Binding of Single Gold Atoms on Thin MgO(001) Films. Phys. Rev. Lett. 2006, 96 (14), 146804. 10.1103/PhysRevLett.96.146804. [DOI] [PubMed] [Google Scholar]
- Baumann S.; Paul W.; Choi T.; Lutz C. P.; Ardavan A.; Heinrich A. J. Electron Paramagnetic Resonance of Individual Atoms on a Surface. Science 2015, 350 (6259), 417–420. 10.1126/science.aac8703. [DOI] [PubMed] [Google Scholar]
- Willke P.; Bae Y.; Yang K.; Lado J. L.; Ferrón A.; Choi T.; Ardavan A.; Fernández-Rossier J.; Heinrich A. J.; Lutz C. P. Hyperfine Interaction of Individual Atoms on a Surface. Science 2018, 362 (6412), 336–339. 10.1126/science.aat7047. [DOI] [PubMed] [Google Scholar]
- Kovarik S.; Robles R.; Schlitz R.; Seifert T. S.; Lorente N.; Gambardella P.; Stepanow S. Electron Paramagnetic Resonance of Alkali Metal Atoms and Dimers on Ultrathin MgO. Nano Lett. 2022, 22 (10), 4176–4181. 10.1021/acs.nanolett.2c00980. [DOI] [PubMed] [Google Scholar]
- Burdi D.; Willems J.-P.; Riggs-Gelasco P.; Antholine W. E.; Stubbe J.; Hoffman B. M. The Core Structure of X Generated in the Assembly of the Diiron Cluster of Ribonucleotide Reductase: 17O2 and H217O ENDOR. J. Am. Chem. Soc. 1998, 120 (49), 12910–12919. 10.1021/ja9824270. [DOI] [Google Scholar]
- Baute D.; Goldfarb D. The 17O Hyperfine Interaction in V17O(H217O)52+ and Mn(H217O)62+ Determined by High Field ENDOR Aided by DFT Calculations. J. Phys. Chem. A 2005, 109 (35), 7865–7871. 10.1021/jp052132q. [DOI] [PubMed] [Google Scholar]
- Wang F.; Büchel R.; Savitsky A.; Zalibera M.; Widmann D.; Pratsinis S. E.; Lubitz W.; Schüth F. In Situ EPR Study of the Redox Properties of CuO–CeO2 Catalysts for Preferential CO Oxidation (PROX). ACS Catal. 2016, 6 (6), 3520–3530. 10.1021/acscatal.6b00589. [DOI] [Google Scholar]
- Brezová V.; Barbieriková Z.; Zukalová M.; Dvoranová D.; Kavan L. EPR Study of 17O-Enriched Titania Nanopowders under UV Irradiation. Catal. Today 2014, 230, 112–118. 10.1016/j.cattod.2013.11.026. [DOI] [Google Scholar]
- Raitsimring A. M.; Astashkin A. V.; Baute D.; Goldfarb D.; Caravan P. W-Band 17O Pulsed Electron Nuclear Double Resonance Study of Gadolinium Complexes with Water. J. Phys. Chem. A 2004, 108 (35), 7318–7323. 10.1021/jp040306i. [DOI] [Google Scholar]
- Rapatskiy L.; Cox N.; Savitsky A.; Ames W. M.; Sander J.; Nowaczyk M. M.; Rögner M.; Boussac A.; Neese F.; Messinger J.; Lubitz W. Detection of the Water-Binding Sites of the Oxygen-Evolving Complex of Photosystem II Using W-Band 17O Electron–Electron Double Resonance-Detected NMR Spectroscopy. J. Am. Chem. Soc. 2012, 134 (40), 16619–16634. 10.1021/ja3053267. [DOI] [PubMed] [Google Scholar]
- Hecker F.; Stubbe J.; Bennati M. Detection of Water Molecules on the Radical Transfer Pathway of Ribonucleotide Reductase by 17O Electron–Nuclear Double Resonance Spectroscopy. J. Am. Chem. Soc. 2021, 143 (19), 7237–7241. 10.1021/jacs.1c01359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakshe A.; Astashkin A. V.; Klein E. L.; Reichmann D.; Mendel R. R.; Bittner F.; Enemark J. H. Structural Studies of the Molybdenum Center of Mitochondrial Amidoxime Reducing Component (MARC) by Pulsed EPR Spectroscopy and 17O-Labeling. Biochemistry 2011, 50 (41), 8813–8822. 10.1021/bi2005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagostina V.; Romeo E.; Maria Ferrari A.; Maurino V.; Chiesa M. Monomeric (VO2+) and Dimeric Mixed Valence (V2O33+) Vanadium Species at the Surface of Shape Controlled TiO2 Anatase Nano Crystals. J. Catal. 2022, 406, 28–38. 10.1016/j.jcat.2021.12.029. [DOI] [Google Scholar]
- Chiesa M.; Martino P.; Giamello E.; Di Valentin C.; del Vitto A.; Pacchioni G. Local Environment of Electrons Trapped at the MgO Surface: Spin Density on the Oxygen Ions from 17O Hyperfine Coupling Constants. J. Phys. Chem. B 2004, 108 (31), 11529–11534. 10.1021/jp0497357. [DOI] [Google Scholar]
- Chiesa M.; Giamello E.; Valentin C. Di; Pacchioni G. The 17O Hyperfine Structure of Trapped Holes Photo Generated at the Surface of Polycrystalline MgO. Chem. Phys. Lett. 2005, 403 (1), 124–128. 10.1016/j.cplett.2004.12.110. [DOI] [Google Scholar]
- Chiesa M.; Paganini M. C.; Giamello E.; Di Valentin C.; Pacchioni G. Electron Traps on Oxide Surfaces: (H+)(e–) Pairs Stabilized on the Surface of 17O Enriched CaO. ChemPhysChem 2006, 7 (3), 728–734. 10.1002/cphc.200500564. [DOI] [PubMed] [Google Scholar]
- Livraghi S.; Maurelli S.; Paganini M. C.; Chiesa M.; Giamello E. Probing the Local Environment of Ti3+ Ions in TiO2 (Rutile) by 17O HYSCORE. Angew. Chemie Int. Ed. 2011, 50 (35), 8038–8040. 10.1002/anie.201100531. [DOI] [PubMed] [Google Scholar]
- Lagostina V.; Salvadori E.; Chiesa M.; Giamello E. Electron Paramagnetic Resonance Study of Vanadium Exchanged H-ZSM5 Prepared by Vapor Reaction of VCl4. The Role of 17O Isotope Labelling in the Characterisation of the Metal Oxide Interaction. J. Catal. 2020, 391, 397–403. 10.1016/j.jcat.2020.06.029. [DOI] [Google Scholar]
- Actis A.; Salvadori E.; Chiesa M. Framework Coordination of Single-Ion Cu2+ Sites in Hydrated 17O-ZSM-5 Zeolite. Catal. Sci. Technol. 2021, 11 (15), 5191–5199. 10.1039/D1CY00838B. [DOI] [Google Scholar]
- Mitchell S.; Vorobyeva E.; Pérez-Ramírez J. The Multifaceted Reactivity of Single-Atom Heterogeneous Catalysts. Angew. Chemie Int. Ed. 2018, 57 (47), 15316–15329. 10.1002/anie.201806936. [DOI] [PubMed] [Google Scholar]
- Qiao B.; Wang A.; Yang X.; Allard L. F.; Jiang Z.; Cui Y.; Liu J.; Li J.; Zhang T. Single-Atom Catalysis of CO Oxidation Using Pt1/FeOx. Nat. Chem. 2011, 3 (8), 634–641. 10.1038/nchem.1095. [DOI] [PubMed] [Google Scholar]
- Wang A.; Li J.; Zhang T. Heterogeneous Single-Atom Catalysis. Nat. Rev. Chem. 2018, 2 (6), 65–81. 10.1038/s41570-018-0010-1. [DOI] [Google Scholar]
- Single Atom Catalysts Push the Boundaries of Heterogeneous Catalysis. Nat. Commun. 2021, 12 ( (1), ), 5884. [DOI] [PMC free article] [PubMed]
- Liu L.; Corma A. Confining Isolated Atoms and Clusters in Crystalline Porous Materials for Catalysis. Nat. Rev. Mater. 2021, 6 (3), 244–263. 10.1038/s41578-020-00250-3. [DOI] [Google Scholar]
- Bennati M.EPR Interactions–Hyperfine Couplings. In eMagRes; Harris R. K., Wasylishen R. L., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2017; Vol. 6, pp 271–282. [Google Scholar]
- Harmer J. R.Hyperfine Spectroscopy–ENDOR. In eMagRes; Harris R. K., Wasylishen R. L., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2016; Vol. 5, pp 1493–1514. [Google Scholar]
- Van Doorslaer S.Hyperfine Spectroscopy: ESEEM. In eMagRes; Harris R. K., Wasylishen R. L., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2017; Vol. 6, pp 51–70. [Google Scholar]
- Stoll S.; Goldfarb D.. EPR Interactions–Nuclear Quadrupole Couplings. In eMagRes; Harris R. K., Wasylishen R. L., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2017; Vol. 6, pp 495–510. [Google Scholar]
- Ashbrook S. E.; Davis Z. H.; Morris R. E.; Rice C. M. 17O NMR Spectroscopy of Crystalline Microporous Materials. Chem. Sci. 2021, 12 (14), 5016–5036. 10.1039/D1SC00552A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H.; Mineo K.; Mizuno N.; Iwamoto M. Active Sites for Isotopic Exchange of Oxygen between Proton-Exchanged ZSM-5 Zeolites and Water. J. Chem. Soc. Chem. Commun. 1994, (8), 975–976. 10.1039/c39940000975. [DOI] [Google Scholar]
- Bikondoa O.; Pang C. L.; Ithnin R.; Muryn C. A.; Onishi H.; Thornton G. Direct Visualization of Defect-Mediated Dissociation of Water on TiO2(110). Nat. Mater. 2006, 5 (3), 189–192. 10.1038/nmat1592. [DOI] [Google Scholar]
- Chiesa M.; Paganini M. C.; Livraghi S.; Giamello E. Charge Trapping in TiO2 Polymorphs as Seen by Electron Paramagnetic Resonance Spectroscopy. Phys. Chem. Chem. Phys. 2013, 15 (24), 9435–9447. 10.1039/c3cp50658d. [DOI] [PubMed] [Google Scholar]
- Chiesa M.; Napoli F.; Giamello E. The Interaction of Na Atoms with the Surface of Alkaline-Earth Oxides. Possible Implications for a “Magnetic Basicity” Scale. J. Phys. Chem. C 2007, 111 (14), 5481–5485. 10.1021/jp068350g. [DOI] [Google Scholar]
- Finazzi E.; Di Valentin C.; Pacchioni G.; Chiesa M.; Giamello E.; Gao H.; Lian J.; Risse T.; Freund H.-J. Properties of Alkali Metal Atoms Deposited on a MgO Surface: A Systematic Experimental and Theoretical Study. Chem. Eur. J. 2008, 14 (14), 4404–4414. 10.1002/chem.200702012. [DOI] [PubMed] [Google Scholar]
- Knight W. D. Electromagnetic Experiments on Metal Clusters in Beams. Surf. Sci. 1981, 106 (1), 172–177. 10.1016/0039-6028(81)90197-7. [DOI] [Google Scholar]
- Edwards P. P.; Anderson P. A.; Thomas J. M. Dissolved Alkali Metals in Zeolites. Acc. Chem. Res. 1996, 29 (1), 23–29. 10.1021/ar950152c. [DOI] [PubMed] [Google Scholar]
- Morra E.; Berlier G.; Borfecchia E.; Bordiga S.; Beato P.; Chiesa M. Electronic and Geometrical Structure of Zn+ Ions Stabilized in the Porous Structure of Zn-Loaded Zeolite H-ZSM-5: A Multifrequency CW and Pulse EPR Study. J. Phys. Chem. C 2017, 121 (26), 14238–14245. 10.1021/acs.jpcc.7b04289. [DOI] [Google Scholar]
- Salvadori E.; Fusco E.; Chiesa M. Long-Range Spatial Distribution of Single Aluminum Sites in Zeolites. J. Phys. Chem. Lett. 2022, 13 (5), 1283–1289. 10.1021/acs.jpclett.1c03554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli S.; Livraghi S.; Chiesa M.; Giamello E.; Van Doorslaer S.; Di Valentin C.; Pacchioni G. Hydration Structure of the Ti(III) Cation as Revealed by Pulse EPR and DFT Studies: New Insights into a Textbook Case. Inorg. Chem. 2011, 50 (6), 2385–2394. 10.1021/ic1021802. [DOI] [PubMed] [Google Scholar]
- Franchini C.; Reticcioli M.; Setvin M.; Diebold U. Polarons in Materials. Nat. Rev. Mater. 2021, 6 (7), 560–586. 10.1038/s41578-021-00289-w. [DOI] [Google Scholar]
- Adamo C.; Barone V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0Model. J. Chem. Phys. 1999, 110 (13), 6158–6170. 10.1063/1.478522. [DOI] [Google Scholar]
- Sinnecker S.; Slep L. D.; Bill E.; Neese F. Performance of Nonrelativistic and Quasi-Relativistic Hybrid DFT for the Prediction of Electric and Magnetic Hyperfine Parameters in 57Fe Mössbauer Spectra. Inorg. Chem. 2005, 44 (7), 2245–2254. 10.1021/ic048609e. [DOI] [PubMed] [Google Scholar]