Abstract
In men with biochemically-recurrent prostate cancer, prostate-specific antigen doubling time ≤ 7.5 months, prostate-specific antigen ≥ 0.5 ng/mL, and Gleason score are independent predictors of metastasis-free survival on multivariable analysis. These data can assist physicians during discussions with patients regarding the risk of developing metastatic disease and for clinical trial planning.
Introduction:
The aim of this study was to investigate the association of prostate-specific antigen (PSA) values on metastasis-free survival (MFS) in men with biochemically recurrent prostate cancer (BRPC) and PSA doubling time (PSADT) < 12 months. This dataset also reflects an update with longer follow-up of our prior publications on the natural history of BRPC in the absence of treatment.
Materials and Methods:
In this report, we combined databases from the Center for Prostate Disease Research and Johns Hopkins University (CPDR/JHU). In the CPDR/JHU radical prostatectomy database (30,936 total patients), 656 men with BRPC (> 0.2 ng/mL) after prostatectomy and PSADT < 12 months, who received no adjuvant/salvage androgen deprivation and/or radiation therapy, were prospectively followed until radiologic evidence of metastasis and are included in this analysis.
Results:
Metastasis occurred in 250 of 656 patients with BRPC (median follow-up, 5 years). PSADT < 7.5 months and Gleason score were independent risk factors for distant metastasis in multivariable analysis. Risk of metastasis increased for PSADT 6.01 to 7.50, 4.51 to 6.0, 3.01 to 4.50, and ≤ 3.0 months, after adjusting for Gleason score. A PSA value ≥ 0.5 ng/mL significantly and independently increased risk of metastasis in patients with PSADT < 12 months (hazard ratio, 2.79; 95% confidence interval, 1.47–5.29; P = .001).
Conclusions:
In men with PSADT < 12 months, PSADT ≤ 7.5 months, PSA ≥ 0.5 ng/mL, and Gleason score are independent predictors of MFS on multivariable analysis.
Keywords: Biochemical recurrence, Deferred ADT, Natural history, Post-prostatectomy, PSA cut-point
Introduction
Prostate cancer is a common malignancy in men with over 164,000 estimated new cases diagnosed in 2018.1 Following radical prostatectomy (RP) for organ-confined disease, approximately one-third of men will develop a biochemical relapse (BCR) with a detectable PSA level.2–4 The appropriate management of patients with biochemically relapsed disease still requires further study. Prospective randomized studies suggest a role for radiation with or without androgen deprivation.5,6 However, patient selection algorithms for salvage radiation are largely based on post hoc/subset analyses of large prospective studies or nomograms based on large databases retrospectively analyzed.7 Despite salvage therapy, many patients will still experience a continued rise in PSA levels.
The institution of systemic treatment is a subject of unresolved controversy. Although it has been shown that early androgen deprivation therapy (ADT) for patients with biochemically recurrent prostate cancer (BRPC) will delay the onset of metastatic disease,8 it is unclear if this is associated with improvements in survival and quality of life. The early clinical benefit of ADT before the development of metastatic disease may be offset by toxicities of prolonged treatment, development of castration resistance, and consequently reduced efficacy of androgen receptor-targeted treatment approaches. Following BCR, metastasis-free survival (MFS) predicts for overall survival, suggesting further study is warranted of risk factors for the development of metastatic disease in BRPC.9 In our previous reports on the natural history of patients with BRPC after RP, we identified independent variables for the development of metastatic disease, including Gleason score, time to BRPC, and PSA doubling time (PSADT).10–12 Estimates of risk for metastasis and overall survival in patients with BRPC were developed to facilitate discussions regarding patient counseling on outcomes and the design of clinical trials.
Currently, it is clear that BRPC is a heterogeneous disease with variable clinical outcomes. Although MFS is a strong surrogate for overall survival,13 decisions about BRPC treatment can be improved with better stratification of risk of metastatic disease and subsequent death from prostate cancer. To this end, we combined 2 large prostate cancer databases to further refine the prognostic value of clinical and pathologic variables for estimating the risk of metastatic disease in men with BRPC. The primary objective of this analysis is to update and refine our original observations and describe outcomes in a larger population of men with BRPC with longer follow-up time.10–12 In particular, we define a PSA cut-point, prior to clinically evident metastasis, that heralded the imminent emergence of metastasis as a potential trigger for clinical decision-making.
Materials and Methods
Study Cohort, Design, and Period
The study cohort was comprised of men enrolled in the institutional review board-approved Center for Prostate Disease Research (CPDR) multicenter national database and John Hopkins University (JHU) prostate cancer database. Databases for both the CPDR cohort and the JHU cohort, and their related data collection activities, have been described previously.14,15 In this study, data for men who were diagnosed with prostate cancer and had a RP between 1983 and 2014 were analyzed. The inclusion criteria included: BRPC after RP without neo-adjuvant, adjuvant, or salvage hormonal therapy (HT) or radiotherapy (RT). BCR was defined as a single PSA ≥ 0.2 ng/mL more than 8 weeks following RP; neoadjuvant treatment was defined as any treatments initiated within 9 months before surgery; adjuvant therapy was defined as any treatment initiated within 6 months after surgery and before BRPC; salvage therapy was defined as any treatment initiated either after 6 months post-RP or after BRPC.
There were 2 calculated variables included in the study: PSADT and PSA. PSADT was calculated using the natural log of 2 (= 0.693) divided by the slope of the linear regression of the natural log of PSA versus time (in months). This determination was made using all PSA values obtained within 24 months after PSA rising (but before metastasis). A minimum of 2 PSA values were required for calculating PSADT. If the slope of the linear regression was 0 (elevated but constant PSA level) or negative (decreasing PSA level after initial increase), the PSADT was arbitrarily set to 100 months, as previously described.5 The most recent PSA value within a window of 6 to 18 months prior to diagnosis of first metastasis or censoring was included for this analysis.
Dependent Study Outcomes
The primary outcome of this study was distant metastasis. Presence of metastases was ascertained by review of each patient’s complete imaging history, including bone scan, computed tomography scan, and magnetic resonance imaging, as well as bone biopsy results. Independent radiology review was not conducted. Time to distant metastasis was calculated as the number of years elapsed from BCR until the first metastasis. Subjects who had no evidence of metastases were censored at the last clinical follow-up.
Statistical Analysis
Analysis of variance and the Kruskal-Wallis test were used to compare the distribution of continuous variables (age, PSA, etc) across PSADT subgroups, whereas the χ2 test was used to compare categorical variables across PSADT subgroups. Unadjusted Kaplan-Meier survival curves and log-rank test were used to compare MFS probabilities across categorical variables, such as PSADT subgroups and PSA subgroups. A multivariable Cox proportional hazards model was used to evaluate the association of MFS with PSADT and PSA, adjusting for other prognostic factors. In patients who had a PSA available and a PSADT < 12 months, the optimal PSA cut-point was determined as the PSA value that maximized Youden’s index.16 Youden’s index was defined as Sensitivity + Specificity − 1 by evaluating overall PSA values; it is represented graphically as the height above the chance line, and it is also equivalent to the area under the curve subtended by a single operating point. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and statistical significance was set at P < .05.
Results
In the combined CPDR and JHU database, nearly 31,000 men underwent RP for localized prostate cancer from 1983 to 2014 (Figure 1). Within this cohort, there were 656 men who experienced BRPC following surgery with PSADT < 12 months, and no neoadjuvant or postoperative RT or HT (Figure 1 – Step 8). After a median follow-up of 5 years after BCR, 250 (38.1%) of 656 patients developed metastatic disease at the time of analysis. The median follow-up times for the metastatic and non-metastatic groups were 6.4 and 4.0 years, respectively.
Figure 1. Combined Cohort of Patients With Prostate Cancer Who Underwent RP at the Center for Prostate Disease Research and Johns Hopkins University Between 1983 and 2014. Flow Diagram of Steps in Cohort Selection With Study Sample Size (N). A Total of 656 Patients Were Identified Who Experienced a Biochemical Recurrence of Prostate Cancer With PSADT < 12 Months and had Not Received Adjuvant/Salvage RT and/or HT.
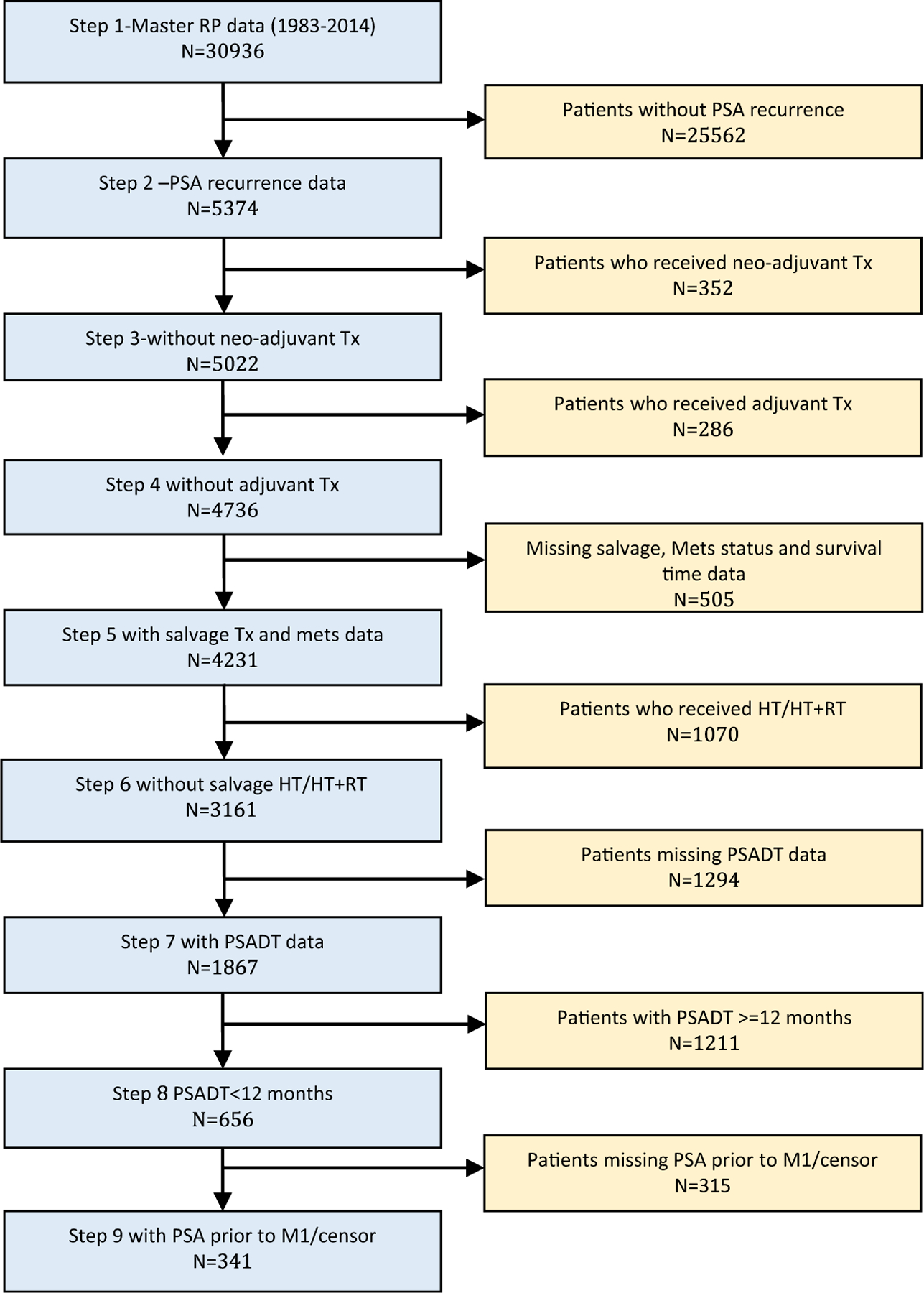
Abbreviations: BCR = biochemical recurrence; HT = hormonal therapy; Mets = metastasis; PSADT = prostate-specific antigen doubling time; RP = radical prostatectomy; RT = radiotherapy; Tx = treatment.
Table 1 describes the clinical and pathologic characteristics of the 656 patients with BRPC with PSADT < 12 months. The JHU and CPDR databases differed significantly with respect to distributions of prognostic variables. Initially, PSADT was categorized into 7 discrete subgroups. Demographic and clinicopathologic characteristics of patients were compared across PSADT subgroups. (see Supplemental Table 1 in the online version). Shorter PSADTs were significantly associated with higher Gleason scores; other prognostic variables did not differ significantly by PSADT subgroup. Additionally, the percentage of patients with metastatic disease increased with shorter PSADTs. The majority of patients with PSADT less than 6 months had developed radiographic metastases at the time of analysis.
Table 1.
Descriptive Statistics for PSADT < 12 Months Cohort, Stratified by Site
| Variable | All, n (%) | CPDR, n (%) | JHU, n (%) | P Value |
|---|---|---|---|---|
| N | 656 | 246 | 410 | |
| Age at RP, y | ||||
| Median (IQR) | 61.0 (56.0–65.1) | 62.9 (57.1–67.1) | 60.0 (55.0–64.0) | <.001 |
| Follow-up from RP, y | ||||
| Median (IQR) | 8.0 (4.9–13.0) | 8.5 (5.4–12.9) | 8.0 (4.0–13.0) | .098 |
| Race | ||||
| Caucasian | 544 (85.0) | 183 (77.2) | 361 (89.6) | |
| African American | 96 (15.0) | 54 (22.8) | 42 (10.4) | <.001 |
| Pathologic T stage | ||||
| T2 | 179 (27.6) | 104 (43.0) | 75 (18.5) | |
| T3–4 | 469 (72.4) | 138 (57.0) | 331 (81.5) | <.001 |
| Gleason sum | ||||
| ≤6 | 153 (23.5) | 98 (40.3) | 55 (13.4) | |
| 7 | 320 (49.1) | 102 (42.0) | 218 (53.0) | |
| 8–10 | 179 (27.4) | 43 (17.7) | 136 (33.2) | <.001 |
| Surgical margin | ||||
| Negative | 394 (61.4) | 119 (50.2) | 275 (67.9) | |
| Positive | 248 (38.6) | 118 (49.8) | 130 (32.1) | <.001 |
| PSADT, mos | ||||
| 10.51–11.99 | 80 (12.0) | 31 (12.6) | 49 (12.0) | |
| 9.01–10.50 | 86 (13.1) | 42 (17.1) | 44 (10.7) | |
| 7.51–9.00 | 80 (12.0) | 39 (15.8) | 41 (10.0) | |
| 6.01–7.50 | 93 (14.2) | 38 (15.4) | 55 (13.4) | |
| 4.51–6.00 | 99 (15.1) | 31 (12.6) | 68 (16.6) | |
| 3.01–4.50 | 97 (14.8) | 25 (10.2) | 72 (17.6) | |
| ≤3 | 121 (18.4) | 40 (16.3) | 81 (19.8) | .006 |
| Metastasis | ||||
| No | 406 (61.9) | 194 (78.9) | 212 (51.7) | |
| Yes | 250 (38.1) | 52 (21.1) | 198 (48.3) |
Abbreviations: CPDR = Center for Prostate Disease Research; IQR = interquartile range; JHU = Johns Hopkins University; PSADT = PSA doubling time; RP = radical prostatectomy.
The effect of PSADT subgroups < 12 months, patient demographics, and pathologic characteristics on MFS was examined in a multivariable analysis (Table 2). Gleason score was an independent predictor of MFS. The risk of developing metastatic disease was significantly associated with higher Gleason scores (Gleason 7 vs. ≤ 6: hazard ratio [HR], 1.76; 95% confidence interval [CI], 1.14–2.73; P = .011; Gleason 8–10 vs. ≤ 6: HR, 3.44; 95% CI, 2.18–5.43; P ≤ .001). Surgical margin status, pathologic T stage, and race were not significantly associated with metastasis. The risk of metastasis was increased for shorter PSADT subgroups versus groups with PSADT of 10.51 to 11.99 months (PSADT = 6.01–7.5 months: HR, 2.42; 95% CI, 1.31–4.50; P = .004; PSADT = 4.51–6.0 months: HR, 2.96; 95% CI, 1.60–5.47; P = .001; PSADT = 3.01–4.5 months: HR, 4.24; 95% CI, 2.38–7.56; P ≤ .001; PSADT < 3 months: HR, 5.24; 95% CI, 2.96–9.26; P ≤ .001). All HR values were adjusted for age, surgical year, and institution.
Table 2.
Multivariable Cox Proportional Hazards Analysis Predicting Distant Metastasis-free Survival
| Variable | HR | 95% CI of HR | P Value | |
|---|---|---|---|---|
| Race | ||||
| AA vs. CA | 0.82 | 0.48 | 1.37 | .438 |
| Pathologic T stage | ||||
| pT3 vs. pT2 | 1.44 | 0.97 | 2.16 | .071 |
| Gleason sum | ||||
| 7 vs. ≤6 | 1.76 | 1.14 | 2.73 | .011 |
| 8–10 vs. ≤6 | 3.44 | 2.18 | 5.43 | <.001 |
| Surgical margin | ||||
| Positive vs. negative | 0.91 | 0.69 | 1.20 | .501 |
| PSADT, mos | ||||
| 10.50–11.99 | ref | |||
| 9.01–10.50 | 1.71 | 0.87 | 3.35 | .119 |
| 7.51–9.00 | 1.75 | 0.91 | 3.36 | .091 |
| 6.01–7.50 | 2.42 | 1.31 | 4.50 | .004 |
| 4.51–6.00 | 2.96 | 1.60 | 5.47 | .001 |
| 3.01–4.51 | 4.24 | 2.38 | 7.56 | <.001 |
| ≤3 | 5.24 | 2.96 | 9.26 | <.001 |
All models were adjusted by surgery year, site, and age.
Abbreviations: AA = African-American; CA = Caucasian; CI = confidence interval; HR = hazard ratio; PSADT = prostate-specific antigen doubling time; Ref = reference value.
We next examined MFS in patients with PSADT < 12 months stratified by an optimal PSA value obtained between 6 and 18 months prior to metastatic disease or censor (N = 342) (Figure 1, Step 9). A PSA of 0.5 ng/mL generated the maximum Youden’s Index and was used for further analysis. In multivariable analysis adjusted by surgical year, site, age, race, pathologic T stage, Gleason score, surgical margin status, and PSADT; a PSA value ≥ 0.5 ng/mL independently predicted for the development of metastatic disease (HR, 2.79; 95% CI, 1.47–5.29; P = .001) (Figure 2, inset). Patients with a PSA < 0.5 ng/mL versus ≥ 0.5 ng/mL had significantly longer median MFS (not reached vs. 3 years; P < .001) (Figure 2).
Figure 2. Kaplan-Meier Estimates of MFS in Patients With BRPC With PSADT < 12 Months, Stratified by PSA Value. A Total of 341 Patients With BRPC had PSADT < 12 Months and PSA Values Between 6 and 18 Months Prior to M1 Disease or Censor (Median Follow-up, 5 Years). Patients Were Stratified by PSA < 0.5 ng/mL (Blue) and ≥ 0.5 ng/mL (Red). The Median MFS was 3 Years at PSA ≥ 0.5 ng/mL Versus Not Reached at PSA < 0.5 ng/mL (Log-rank P ≤ .001). Multivariable Cox Proportional HR provided for a PSA of < 0.5 Versus ≥ 0.5 ng/mL (Inset). The Multivariable Model Was Adjusted by Surgical Year, Site, Age, Race, Pathologic T Stage, Gleason Score, Surgical Margin Status, and PSADT.
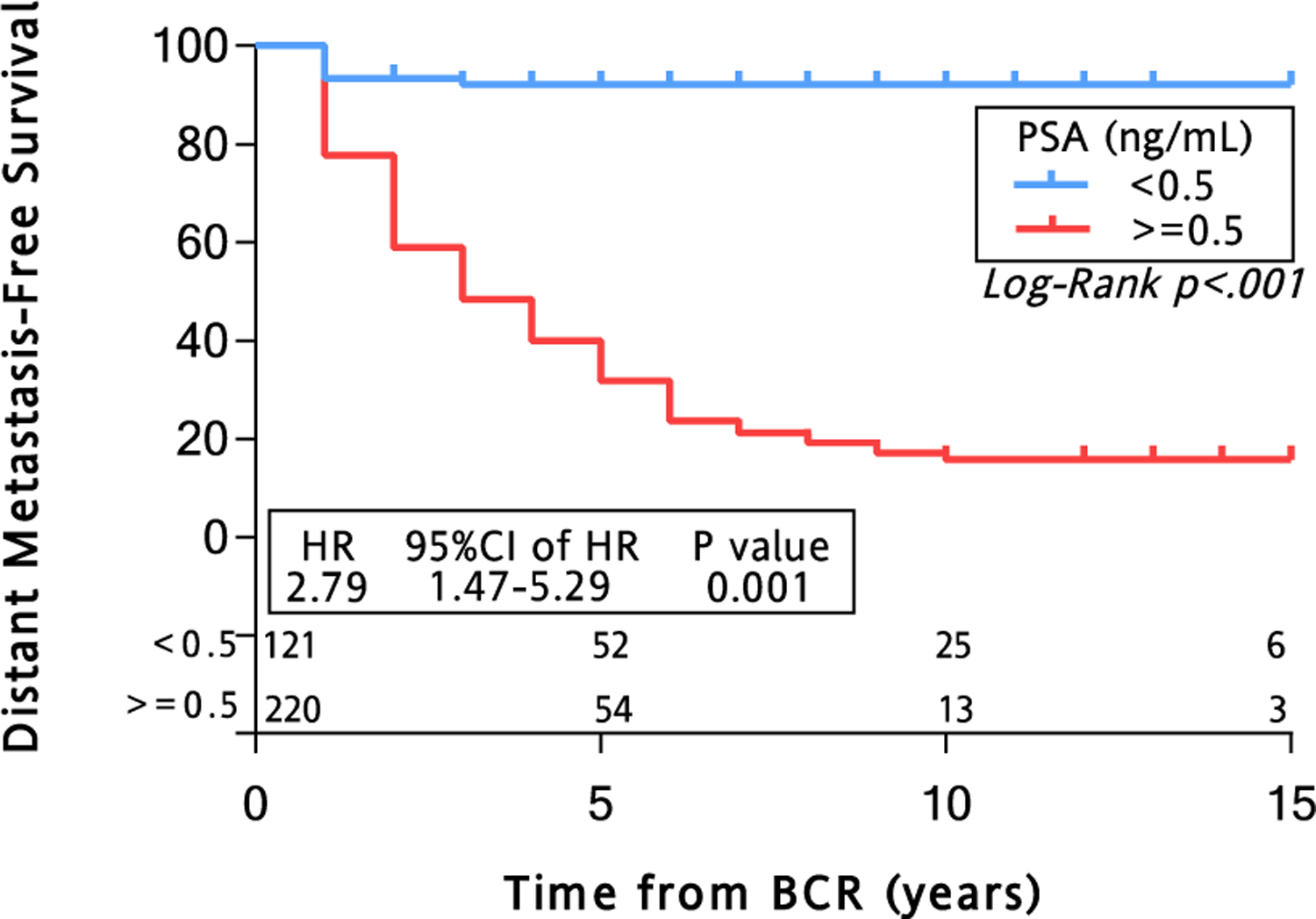
Abbreviations: BCR = biochemical recurrence; BRPC = biochemically recurrent prostate cancer; CI = confidence interval; HR = hazard ratio; MFS = metastasis-free survival; PSA = prostate-specific antigen; PSADT = PSA doubling time.
Discussion
The optimal management of BRPC has been subject to a longstanding clinical debate, without clear guidelines, making it difficult to counsel patients and design clinical trials for these patients. In a prior study, in the JHU cohort of patients with BRPC, we found PSADT < 9 months to be the strongest predictor of metastatic disease and prostate cancer-specific mortality.12 However, the optimal cut-point for PSADT to define a high-risk population remains unclear, with several studies suggesting values ranging from < 3 months to < 12 months.10,17–19 In our current analyses, we stratified men with BRPC and PSADT < 12 months into multiple discrete PSADT subgroups. In multivariable analysis, the risk of developing metastatic disease increased significantly in subgroups with successively shorter PSADT. Although not unexpected, this confirmed and further refined our findings from prior studies.10–12
We observed that a PSA cut-point of 0.5 ng/mL maximized sensitivity and specificity for MFS independently of Gleason score and PSADT. We acknowledge that a PSA of 0.5 ng/mL is low and that many patients with BCR will go on to have a higher PSA value without M1 disease. However, our model uses a PSA that was obtained 6 to 18 months prior to the development of metastatic disease, which allows clinicians to counsel on future metastatic risk. Identification of an at-risk patient population using a low PSA cut-point is not without precedent. Prior studies in patients with BRPC with low PSA values suggested a clinical benefit from salvage radiotherapy. Salvage radiotherapy without ADT in patients with BRPC and PSA < 0.5 ng/mL resulted in a 48% disease-free survival (DFS) at 6 years (vs. 18% in the PSA > 1.5 ng/mL subgroup).7 This DFS benefit was similar for the subset of patients with PSA < 0.5 ng/mL and PSADT < 10 months (41% DFS at 6 years). A retrospective study by Stephenson and colleagues observed a 53% 4-year progression-free survival benefit and 52 month median progression-free survival following salvage radiotherapy in patients with BRPC with PSA <1.0 ng/mL.20 In our study, we observed a robust and durable MFS benefit for those patients with BRPC with PSADT < 12 months and PSA < 0.5 ng/mL in the absence of hormonal or radiation-based therapies. Our findings also support the argument that the optimal timing for salvage radiotherapy for certain patients with BRPC may be when their PSA values remain < 0.5 ng/mL, because metastatic risk is lowest in these men. In addition, given the favorable outcomes in patients with a PSADT ≥ 7.5 months in our series, it may be argued that older patients with a low PSA and slow PSA dynamics can be safely observed and may potentially never need therapy. Patients with significant comorbidities or those with contraindications to salvage radiotherapy may also have favorable outcomes with observation. Irrespective of PSADT, absolute PSA values provides additional insight into the natural history of prostate cancer and also serve as valuable information for counseling patients with a rising PSA after RP.
Our analysis has a number of strengths. It combines 2 large RP well-characterized populations with consistent data collection and long-term follow-up but very different patient clinical profiles, suggesting that our results are not only relevant to academic centers of excellence. We excluded patients who had undergone neoadjuvant, adjuvant, and salvage therapy because we wanted to determine the prognostic impact of PSADT and PSA in patients with BCR for whom a decision to administer salvage therapy does not have definitive guidelines. The sample size is large enough that we have sufficient representation within each of the PSADT subgroups.
There are also limitations in the study. First, the chosen cut-point of 0.5 ng/mL for PSA was based on the optimal sensitivity/specificity, an approach that may be associated with increased type I error.21 However, given the small P-value observed (log-rank P ≤ .001, multivariable Cox proportional hazards model P = .001) for our chosen PSA cut-point, the result would be statistically significant even with a conservative Bonferroni adjustment. Furthermore, the HR associated with the optimally selected cut-point is likely to be overestimated by no more than 10%.22 Second, we included only those PSA values obtained between 6 and 18 months from the development of metastatic disease. The rationale is that we wanted to focus on a time window that still represented occult systemic disease, but was not so early that it would be difficult to determine if it was actionable. This resulted in exclusion of patients who did not have a recorded PSA value within that time window. However, the clinical and pathologic characteristics of those patients who were excluded versus those who were included were not significantly different. Third, we excluded patients that had prior adjuvant, neoadjuvant, or salvage therapies, which may have biased our findings. For instance, by excluding these patients who potentially had more aggressive disease, we may have overestimated the MFS in this study. Lastly, as the use of prostate-specific membrane antigen positron emission tomography imaging becomes more commonplace, it is unclear how these newer, more sensitive imaging modalities will be applied in clinical practice. We speculate that certain radiographic characteristics identified on prostate-specific membrane antigen-targeted imaging will be used in concert with PSADT to better guide therapies for patients with prostate cancer with detectable PSA levels but without metastatic disease on conventional imaging. Similarly, novel markers, such as p2PSA, in peripheral blood may be more sensitive than PSA for the detection of BCR prostate cancer and could supplant traditional PSA testing in the future.23
Conclusion
Our study suggests that Gleason score, PSADT, and a PSA ≥ 0.5 ng/mL are each independently associated with the development of metastatic disease patients with in BRPC. Clinical providers should consider the addition of absolute PSA values along with PSADT when counseling patients and designing clinical trials.
Clinical Practice Points
Men with BRPC can have a variable clinical course. Prior studies have demonstrated that a PSADT of less than 12 months portends a worse prognosis and may have better clinical outcomes with early androgen deprivation therapy.
This study has better defined an “at risk” population of patients with BRPC who have a rapid PSADT. We observed that a PSADT ≤ 7.5 months, Gleason score, and a PSA value ≥ 0.5 ng/mL are independent predictors of metastatic disease.
These data may allow clinicians to better counsel patients with BRPC on observation versus early hormonal therapy. Moreover, these variables also define and identify a subgroup of patients with BRPC that may benefit from ongoing clinical trials.
Supplementary Material
Acknowledgments
The project described was supported by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins with National Institutes of Health grants P30 CA006973 and a PCF Young Investigator Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Supplemental Data
Supplemental table accompanying this article can be found in the online version at https://doi.org/10.1016/j.clgc.2019.08.002.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am 1997; 24:395–406. [DOI] [PubMed] [Google Scholar]
- 3.Zincke H, Bergstralh EJ, Blute ML, et al. Radical prostatectomy for clinically localized prostate cancer: long-term results of 1,143 patients from a single institution. J Clin Oncol 1994; 12:2254–63. [DOI] [PubMed] [Google Scholar]
- 4.Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994; 152:1821–5. [DOI] [PubMed] [Google Scholar]
- 5.Trock BJ, Han M, Freedland SJ, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008; 299:2760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipley WU, Seiferheld W, Lukka HR, et al. NRG Oncology RTOG. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017; 376:417–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007; 25:2035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol 2004; 171:1141–7. [DOI] [PubMed] [Google Scholar]
- 9.Schweizer MT, Zhou XC, Wang H, et al. Metastasis-free survival is associated with overall survival in men with PSA-recurrent prostate cancer treated with deferred androgen deprivation therapy. Ann Oncol 2013; 24:2881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281:1591–7. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294:433–9. [DOI] [PubMed] [Google Scholar]
- 12.Antonarakis ES, Feng Z, Trock BJ, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int 2012; 109:32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie W, Regan MM, Buyse M, et al. ICECaP Working Group. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017; 35:3097–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Gancarczyk K, Paquette E, et al. Introduction to Department of Defense Center for Prostate Disease Research Multicenter National Prostate Cancer Database, and analysis of changes in the PSA-era. Urol Oncol 2001; 6:203–9. [Google Scholar]
- 15.Ludwig WW, Feng Z, Trock BJ, Humphreys E, Walsh PC. Prostate specific antigen testing after radical prostatectomy-can we stop at 20 years? J Urol 2018; 199:114–9. [DOI] [PubMed] [Google Scholar]
- 16.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005; 16:73–81. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen MH. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 2003; 95:1376–83. [DOI] [PubMed] [Google Scholar]
- 18.Okotie OT, Aronson WJ, Wieder JA, et al. Predictors of metastatic disease in men with biochemical failure following radical prostatectomy. J Urol 2004; 171:2260–4. [DOI] [PubMed] [Google Scholar]
- 19.D’Amico AV, Cote K, Loffredo M, Renshaw AA, Schultz D. Determinants of prostate cancer-specific survival after radiation therapy for patients with clinically localized prostate cancer. J Clin Oncol 2002; 20:4567–73. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004; 291:1325–32. [DOI] [PubMed] [Google Scholar]
- 21.Keegan PE, Matthews JN, Lunec J, Neal DE. Statistical problems with ‘optimal’ thresholds in studies of new prognostic factors in urology. BJU Int 2000; 85:392–7. [DOI] [PubMed] [Google Scholar]
- 22.Faraggi D, Simon R. A simulation study of cross-validation for selecting an optimal cutpoint in univariate survival analysis. Stat Med 1996; 15:2203–13. [DOI] [PubMed] [Google Scholar]
- 23.Casale P, Saita A, Lazzeri M, et al. p2PSA for predicting biochemical recurrence of prostate cancer earlier than total prostate-specific antigen after radical prostatectomy: an observational prospective cohort study. Minerva Urol Nefrol 2019; 71:273–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


