Abstract
Context:
The recent discovery of the existence of a human genitourinary microbiome has led to the investigation of its role in mediating the pathogenesis of genitourinary malignancies, including bladder, kidney, and prostate cancers. Furthermore, although it is largely recognized that members of the gastrointestinal microbiota are actively involved in drug metabolism, new studies demonstrate additional roles and the potential necessity of the gastrointestinal microbiota in dictating cancer treatment response.
Objective:
To summarize the current evidence of a mechanistic role for the genitourinary and gastrointestinal microbiome in genitourinary cancer initiation and treatment response.
Evidence acquisition:
We conducted a literature search up to October 2018. Search terms included microbiome, microbiota, urinary microbiome, bladder cancer, urothelial carcinoma, renal cell carcinoma, kidney cancer, testicular cancer, and prostate cancer.
Evidence synthesis:
There is preliminary evidence to implicate the members of the genitourinary microbiota as causative factors or cofactors in genitourinary malignancy. Likewise, the current evidence for gastrointestinal microbes in dictating cancer treatment response is mainly correlative; however, we provide examples where therapeutic agents used for the treatment of genitourinary cancers are affected by the human-associated microbiota, or vice versa. Clinical trials, such as fecal microbiota transplant to increase the efficacy of immunotherapy, are currently underway.
Conclusions:
The role of the microbiome in genitourinary cancer is an emerging field that merits further studies. Translating microbiome research into clinical action will require incorporation of microbiome surveillance into ongoing and future clinical trials as well as expansion of studies to include metagenomic sequencing and metabolomics.
Patient summary:
This review covers recent evidence that microbial populations that reside in the genitourinary tract—and were previously not known to exist—may influence the development of genitourinary malignancies including bladder, kidney, and prostate cancers. Furthermore, microbial populations that exist at sites outside of the genitourinary tract, such as those that reside in our gut, may influence cancer development and/or treatment response.
Keywords: Microbiome, Prostate cancer, Bladder cancer, Kidney cancer, Immunotherapy
1. Introduction
1.1. The human microbiome—what and where are the microbes?
We are roughly 10% human. The number of microbes that inhabit our bodies greatly outnumbers the human cells, and these commensal relationships exist at all body sites that are exposed to the outside world, including the gastrointestinal tract, skin, oral cavity, and genitourinary tract. Each body site that harbors microbial ecosystems differs in microbial composition as well as metabolic functions [1,2]. Each person’s microbiome is unique, rapidly developing throughout early childhood and then with differing rates of variability in adulthood [2,3]. Variations in microbial composition can be influenced by both genetic and environmental factors, including diet, geographic location, toxin/carcinogen exposure, and hormones.
The recent discovery of microbial populations that reside in the urinary tract, termed the “urinary microbiome,” is of particular relevance to genitourinary cancers. Whereas urine was traditionally thought to be sterile, recent evidence has challenged this dogma with studies showing both culture- and molecular-based detection of microbes in the urinary tract and the bladder [4–7]. As the majority of these studies have been performed with clean catch and/or catheterized urine, the lingering questions are how much of the urinary tract is colonized and which species are coming from where. Urine samples may contain microbes (or microbial fragments and DNA) that originated from the urethra, bladder, ureter, and/or kidney [8]. Clean catch urine samples may also be contaminated by microbes residing in or on the vagina or on the penis. In situ localization of bacteria at any of these anatomic sites is limited. In addition, most studies have focused exclusively on bacteria, with the contribution of other microbial populations such as fungus and viruses to the urinary microbiome yet to be elucidated.
The urinary microbiome may change with aging, as age-specific genera are reported in the urinary microbiome community structure [9]. It is not known if these age-related changes in the urinary microbiota contribute to the risk of genitourinary cancer later in life. Sex-related disparities have been better studied, and distinct differences in the composition of the urinary microbiome by sex have been identified. The female urinary microbiota is largely predominated by the genera Lactobacillus and Gardnerella, whereas Corynebacterium, Staphylococcus, and Streptococcus are the majority genera of the urinary microbiota in males [10,11]. As a result, it may be postulated that the sex disparity in the commensal genitourinary flora might contribute to the well-known sex disparity of genitourinary cancer incidence in men.
This review summarizes the current evidence of a mechanistic role for the genitourinary and gastrointestinal microbiome in genitourinary cancer initiation and treatment response.
2. Evidence acquisition
We conducted a literature search for research studies and review articles related to the microbiome and genitourinary cancers (bladder, kidney, and prostate) up to October 2018. Our search included peer-reviewed publications available through PubMed as well as preprints available through bioRxiv. Keywords in our search included microbiome, microbiota, urinary microbiome, bladder cancer, urothelial carcinoma (UC), renal cell carcinoma (RCC), kidney cancer, testicular cancer, and prostate cancer. We did not find reported evidence linking the urinary or gastrointestinal microbiome to testicular cancer risk or biology; therefore, testicular cancer is not covered in this review.
3. Evidence synthesis
3.1. Mechanisms by which the microbiome impacts human cancer etiology/pathology
3.1.1. Infections and the urinary microbiome in genitourinary cancer etiology/pathology
Pathogenic infections are not synonymous with the presence of a “microbiome,” as the latter implies a commensal relationship between microbes and human cells. However, a history of genitourinary tract infections is a risk factor for the development of certain malignancies, such as squamous cell bladder cancer following parasitic infection [12,13]. Many infectious agents elicit a chronic inflammatory response upon resolution of the acute infection, and this chronic inflammatory response may be a cofactor in driving carcinogenesis [12,14,15]. Certain commensal strains of bacteria in the urinary microbiome may control pathogenic outgrowth of bacteria in the genitourinary tract similar to the beneficial effect of vaginal Lactobacillus species in controlling vaginal infections in women [16]. Thus, further study of the genitourinary microbiome is warranted to fully elucidate its role in regulating pathogenic infections and mediating cancer development.
3.1.2. Bladder cancer
The association between bladder schistosomiasis infections, inflammation, and bladder cancer risk has been well established [12]. The archetypical example of this is in the areas of the world where schistosomiasis is endemic. There is a long-recognized association of chronic Schistosoma haematobium bladder infection and the subsequent development of bladder squamous cell carcinoma [17]. Interestingly, there is evidence that members of the urinary microbiome may influence schistosomiasis-induced bladder cancer. Specifically, Adebayo and colleagues [18] studied urine samples from 70 participants from southwestern Nigeria and reported that several urinary taxa such as Fusobacterium, Sphingobacterium, and Enterococcus distinguished urogenital schistosomiasis-induced bladder pathologies from urogenital schistosomiasis infection alone and from healthy persons. Strains of bacteria that can mediate the formation of N-nitrosamines have been proposed to contribute to schistosomiasis-induced bladder cancer as well (Fig. 1) [13]. Finally, given that epidemiological evidence is inconsistent, the idea that chronic urinary tract infections (UTIs) may be a proposed risk factor for bladder cancer development deserves additional investigation [19].
Fig. 1 –

Examples of potential direct interactions between the urinary microbiota and the development of genitourinary cancer. There is a long-recognized association between chronic bladder infection with S. haematobium and the subsequent development of bladder squamous cell carcinoma in both men and women. However, not everyone afflicted with schistosomiasis develops bladder cancer. The presence of species of bacteria such as Staphylococcus albus, Proteus mirabilis, Escherichia coli, and Klebsiella spp. that can mediate the formation of carcinogenic N-nitrosamines as part of the urinary microbiota may contribute to schistosomiasis-induced bladder cancer. Likewise, in prostate cancer, infection of the prostate with proinflammatory bacteria originating from the urinary tract is proposed to contribute to the development of a prostate cancer risk factor lesion termed proliferative inflammatory atrophy.
Copyright 2018, Johns Hopkins University, by Lydia Gregg.
Whether the urinary microbiome influences the development or progression of bladder cancer or alternatively whether bladder cancer impacts the composition, diversity, or abundance of bladder-associated microorganisms remains to be determined. One hypothesis is that the bladder microbiome may alter the extracellular matrix, which may promote or inhibit urothelial carcinogenesis [20]. Likewise, as the intestinal microbiome has been shown to influence the development of cancer and the response to therapeutics [21,22], it might not be unexpected to find similar links in the context of the urinary microbiome and bladder cancer. Preliminary studies have identified differences in the bladder urinary microbiome of UC patients compared with healthy cancer-free individuals, with Streptococcus, Pseudomonas, or Anaerococcus genera appearing more frequently in UC patients [23]. In another small series, no differences in microbial alpha diversity (interindividual diversity) or composition of the urinary microbiota were observed in 12 patients with bladder cancer and 11 age- and sex-matched healthy donors [24]. The only differences found were in the relative abundance of specific taxa between bladder cancer patients and healthy donors, in which a subset of cancer patients had an enrichment of taxa belonging to the genera Fusobacterium, Actinobaculum, Facklamia, and Campylobacter, whereas Veillonella, Streptococcus, and Corynebacterium species were more prevalent in healthy human urine [24]. These findings are at odds with other studies of gastrointestinal microbiota that have reported a decrease in diversity with disease state in the gut [25].
Another study, in which urine samples of 31 bladder cancer patients and 18 non-neoplastic patients were sequenced, determined that bacterial richness was increased in cancer patients compared with the control group, whereas diversity was not [26]. The relative abundance of organisms at taxonomic levels from phylum to genus was not different between bladder cancer patients and the control group, with the exception of the families of Thermoactinomycetaceae and Sphingobacteriaceae, which were increased in cancer patients or control patients, respectively [26]. Additional significant differences were observed in the abundance of several organisms at the genus level using linear discriminant analysis effect size, an analysis tool based on relative abundance between two or more populations, which in this case were those facilitating identification of microbial populations associated with cancer [26]. Of note, bacterial richness was increased in groups classified at a higher risk of recurrence or progression [26], suggesting that microbial composition may provide clues to patient outcome. Nevertheless, studies involving larger numbers of patients, with various stages of disease, will be needed to properly evaluate whether urinary microbiota differences exist between bladder cancer patients and healthy individuals. Indeed, as our understanding of molecular subtypes in bladder cancer deepens [27], it is conceivable that correlations in microbiome composition or diversity and specific disease manifestation may arise.
Notably, all the bladder cancer patients enrolled in the studies of Popović et al. [24] and Wu et al. [26] were male. However, as the composition of the urinary microbiome has been shown to differ between men and women, it may be that the microbiome in women—in particular the composition, diversity, or abundance of microorganisms—provides a level of protection against or facilitates the development of bladder cancer. As such, further studies in female bladder cancer patients and healthy controls are needed as well.
3.1.3. Kidney cancer
The epidemiology of RCC has been extensively studied and described [28]. However, the contribution of prior UTIs to the incidence of RCC is of unclear significance. UTIs have been described as a modifiable risk factor for the development of RCC [14]. In one retrospective study, a self-reported history of a physician-diagnosed kidney or bladder infection conferred a significantly higher risk of RCC (odds ratio 1.9 [95% confidence interval: 1.5–2.5]) compared with no prior infection [29]. This risk was further increased in males (vs females) and smokers (vs nonsmokers) [29]. Moreover, the recent discovery of a distinct microbiome of the urinary tract [4] sheds new light on the possibility that there is an underappreciated degree of direct interaction between microbiota and the kidney, although this interaction has not yet been studied in relation to RCC pathogenesis. As the technology to identify specific bacteria on a species level is now available, this may prompt further investigation of the urine microbiome with respect to RCC incidence.
3.1.4. Prostate cancer
Although an infectious etiology to prostate cancer has long been discussed, the evidence for such a potential causal relationship has been conflicting [15,30]. For example, while several bacterial species are known to infect the prostate and cause acute and/or chronic bacterial prostatitis, studies to identify prostatitis as a risk factor for prostate cancer development are contradictory and may be flawed by potential biases such as detection bias (reviewed by Sfanos et al. [15]). Likewise, epidemiological studies on single infectious agents such as those associated with sexually transmitted infections (STIs) are inconclusive, and almost all have focused on one infectious agent or a few specific infectious agents and typically on known pathogens. It has been proposed that one potential reason why it has been so difficult to settle on a single infectious origin for prostate cancer development is that there may be multiple different types of microorganisms that contribute to prostate cancer risk via eliciting persistent chronic inflammation [15]. In support of this concept, Shrestha et al. [11] reported that the urinary microbiome of men with biopsy-proven prostate cancer may be enriched for proinflammatory bacterial species that are associated with genitourinary disease such as prostatitis, UTIs, bacterial vaginosis, and STIs. The composition and prevalence of each of these species differed among individuals, and several were associated with the degree of acute or chronic inflammation in the prostate biopsy [11]. A potential hypothesis is that these proinflammatory species, if introduced into the prostate, could elicit chronic inflammation that mediates the development of proliferative inflammatory atrophy, a suspected risk factor lesion for prostate cancer development (Fig. 1) [15]. One major limitation to this study is that it was not longitudinal, and therefore it was hard to determine a causative role of the species versus their presence as a consequence of prostate cancer.
Extensive studies have also been performed to detect pathogens in prostate cancer tissues, as was recently reviewed by Porter et al. [31]. Although microbial DNA is detectable in prostate cancer tissues [32–34], similar to studies of the urinary microbiome, few studies have visualized or localized the detected bacteria in the respective tissue samples from which they were identified. It is unlikely that the prostate contains a ubiquitous commensal microbiota or that there is a “prostate microbiome” per se (reviewed by Porter et al. [31]), but instead microbial presence is likely pathogenic in nature and may present in focal regions, and perhaps in regions associated with foci of acute or chronic inflammation or “fossilized” in prostatic corpora amylacea that are thought to be remnants of past infections [32,35]. Thus, in the absence of epidemiological studies linking infections to prostate cancer risk or studies that have visualized bacteria or other microorganisms in prostate tissues, the contributions of prostate infections to prostate cancer risk remains unknown.
3.1.5. Impact of the microbiome on genitourinary cancer treatment
Many members of the gastrointestinal microbiota are known to influence the metabolism, pharmacokinetics, and toxicity of drugs and xenobiotics (Fig. 2) [36]. Specifically with regard to cancer therapies, examples of this relationship include the finding that intratumoral Mycoplasma hyorhinis, as well as species of Proteobacteria, may metabolize and inactivate the chemotherapy drug gemcitabine, resulting in drug resistance [37]. Likewise, reactivation of the excreted, inactive metabolite of the topoisomerase I inhibitor irinotecan can occur with β-glucuronidases produced by bacterial species in the gastrointestinal tract, causing adverse drug toxicities, including severe diarrhea [38]. Finally, although the mechanism is not fully understood, there is emerging evidence that the gastrointestinal microbiota can influence the efficacy of immunotherapy [39–45].
Fig. 2 –
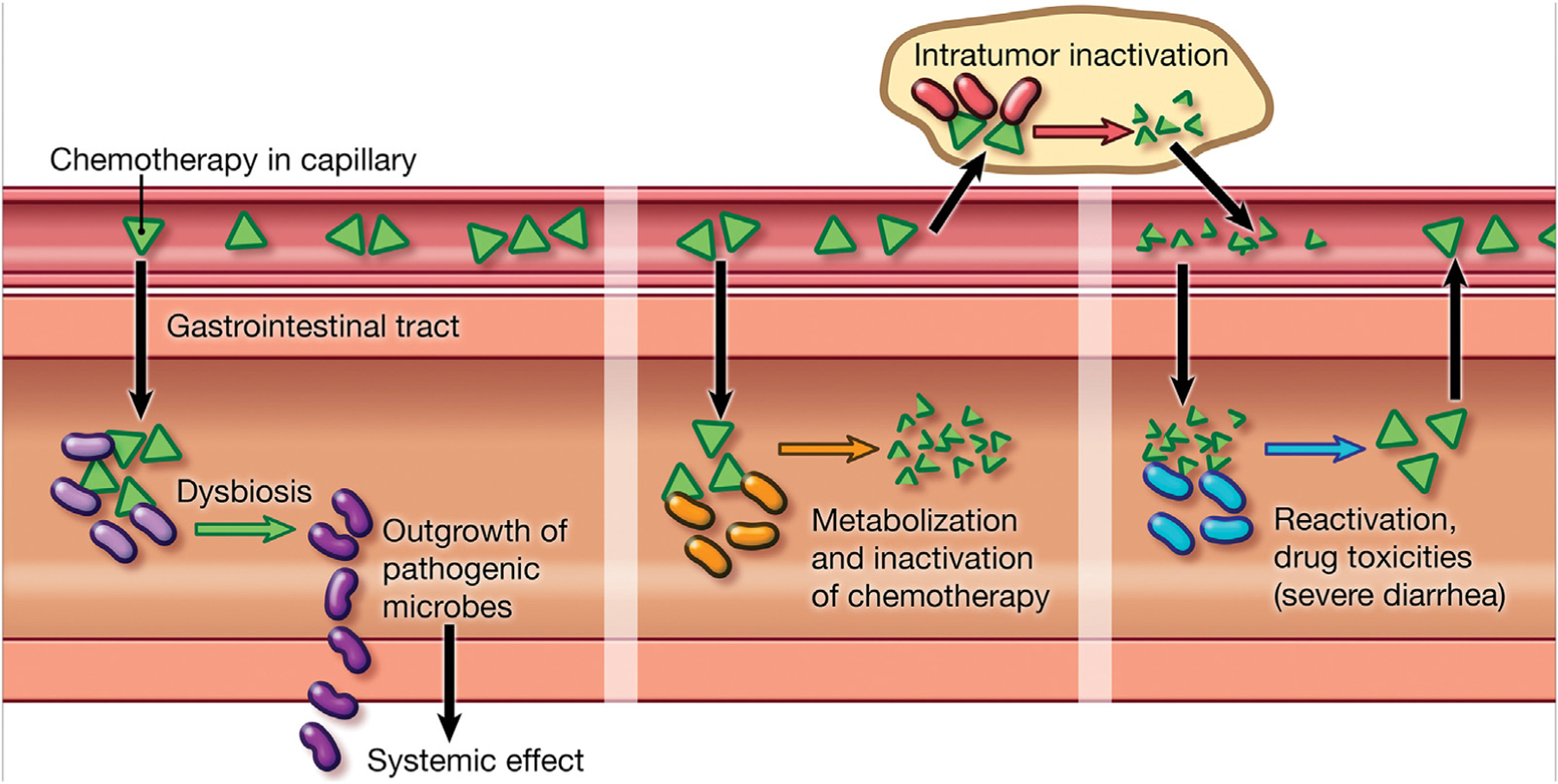
Examples of interactions between the gastrointestinal (GI) microbiota and cancer therapies. Cancer therapies, such as chemotherapy, can have a bacteriostatic effect on the GI microbiota, leading to GI microbial dysbiosis and the outgrowth of pathogenic species. This pathogenic outgrowth can lead to systemic effects, such as the production of proinflammatory cytokines that influence tumor growth. The GI microbiota is likewise implicated in the metabolism and inactivation of cancer therapies both in the GI tract and locally within the tumor if there is a bacterial presence. Finally, reactivation of therapies by the GI microbiota locally within the GI tract can lead to treatment-related drug toxicities, including severe diarrhea.
Copyright 2018, Johns Hopkins University, by Lydia Gregg.
There is also a reciprocal relationship between cancer therapies and the gastrointestinal microbiome, whereby cancer therapies may alter the gastrointestinal microbial composition (see Table 1). Such alterations could conceivably impact factors such as the local inflammatory environment in the intestinal tract, systemic inflammatory effects, and/or the efficacy of any subsequently administered cancer therapies. A screen of >1000 marketed nonantibiotic drugs against 40 representative gastrointestinal bacterial strains found that nearly a quarter of the drugs with human targets exhibited bacteriostatic properties [46]. Chemotherapy and immunotherapy induce dysbiosis of the gastrointestinal microbiota in rodent models [41,47], and bone marrow transplant conditioning chemotherapy in non-Hodgkin’s lymphoma patients induces pathogenic shifts in the gastrointestinal microbiota that are associated with treatment toxicities [48,49]. Finally, chemotherapy given to pediatric acute myeloid leukemia patients demonstrated direct bacteriostatic effects on the gastrointestinal microbiota, as well as outgrowth of pathogenic enterococci that could not be explained fully by concurrent use of antibiotics [50].
Table 1 –
Examples of interactions between human-associated bacteria and therapeutic agents used in the treatment of genitourinary cancers
| Therapeutic agents altered by bacteria | Therapeutic influence | Reference(s) |
|---|---|---|
| Gemcitabine | Efficacy decreased by select bacteria | PMID: 26416623, PMID: 28912244 |
| Doxorubicin | Efficacy decreased by select bacteria | PMID: 26416623 |
| Etoposide phosphate | Efficacy decreased by select bacteria | PMID: 26416623 |
| Mitoxantrone | Efficacy decreased by select bacteria | PMID: 26416623 |
| Floxuridine | Efficacy increased by select bacteria | PMID: 28431244 |
| α-PD-1 immunotherapy | Efficacy increased by select bacteria | PMID: 29097493, PMID: 29302014, PMID: 29097494 |
| Therapeutic agents that inhibit bacteria | Relationship | Reference(s) |
|
| ||
| Gemcitabine | Bacteriostatic | PMID: 29555994, PMID: 27575730 |
| Methotrexate | Bacteriostatic | PMID: 29555994, PMID: 6704313 |
| Doxorubicin hydrochloride | Bacteriostatic | PMID: 29555994, PMID: 27575730 |
| Paclitaxel | Bacteriostatic | PMID: 29555994 |
| 5-Fluorouracil | Bacteriostatic | PMID: 29555994, PMID: 2416271, PMID: 6704313, PMID: 6704313, PMID: 27575730 |
| Gefitinib | Bacteriostatic | PMID: 29555994 |
| Etoposide | Bacteriostatic | PMID: 29555994, PMID: 2416271, PMID: 3196009 |
| Floxuridine | Bacteriostatic | PMID: 29555994, PMID: 27025633 |
| Cisplatin | Bacteriostatic, cytotoxic | PMID: 14287410, PMID: 8637503 |
| Carboplatin | Cytotoxic | PMID: 8637503 |
| Mitomycin | Bacteriostatic | PMID: 2416271, PMID: 6704313 |
Indirect effects of the microbiome on tumorigenesis are not well understood, and could include modulation of antitumor immune responses and effects on host metabolism [51]. Exciting evidence is emerging to indicate that the gastrointestinal microbiota can exert a profound influence on the efficacy of certain cancer treatments, including chemotherapy and immunotherapy [39–42,44,45]. Recent studies in animal models have demonstrated that members of the intestinal microbiota are essential for therapeutic efficacy of agents such as cyclophosphamide [41], platinum chemotherapy (oxaliplatin) [40], and both CTLA-4 blockade [39] and anti-PD-L1 [42] immunotherapies. In these studies, eradication of the commensal intestinal microbiota by antibiotic treatment or via deriving specific pathogen-free and/or germ-free animals eliminated the therapeutic efficacy of these agents in multiple different tumor models. Intriguingly, a study using a melanoma model showed that anti-PD-L1 immunotherapy could be bolstered by feeding animals a strain of Bifidobacterium—a species commonly used in probiotic supplements—prior to initiating therapy [42].
In addition, three separate recent studies, including one in patients with RCC, demonstrated that transplanting fecal material from patients who responded to immune checkpoint inhibitors into germ-free mice recapitulated the phenotype of anti-PD-1 response in the animal model [43–45]. This response was attenuated with antibiotics, suggesting an influence of the microbiome—in the gut or at other sites—on the response to anti-PD-1 treatment. Furthermore, Routy et al. [44] studied a cohort of patients that included 67 patients with RCC and 42 patients with UC, in addition to 140 patients with advanced non-small cell lung cancer (NSCLC), 28% of whom were prescribed antibiotics (β-lactam ± inhibitors, fluoroquinolones, or macrolides) within 2 mo before or 1 mo after the first administration of anti-PD-1/PD-L1 immunotherapy. These investigators reported that progression-free survival (PFS) and overall survival (OS) were significantly shorter in the antibiotic-treated group. Antibiotic treatment predicted resistance to anti-PD-1 immunotherapy in univariate and multivariate Cox regression analyses, independently of classical prognostic markers in both NSCLC and RCC. This study was recently expanded to include additional RCC patients and fully reanalyzed for multivariate analysis. Antibiotics use within 1 mo before the first dose of anti-PD-L1 antibody was associated with shorter PFS and shorter OS in patients with advanced RCC and NSCLC, even after multivariate analyses adjusted for known risk factors in each tumor type [52].
With regard to the specific gastrointestinal species that might mediate the relationship between immunotherapy and treatment response, Routy et al. [44] identified Akkermansia muciniphila as a relevant bacterium. This species restored efficacy of PD-l blockade when provided as a probiotic supplement to murine models where immunotherapy efficacy had been abrogated via antibiotic treatment. In a similar study, the composition of the gut microbiome was examined in melanoma patients, with the finding that gastrointestinal alpha diversity and relative abundance of Ruminococcaceae species correlated with clinical responses to checkpoint inhibition [45].
In addition to immunotherapy, other advanced RCC treatments, such as oral anti-vascular endothelial growth factor (anti-VEGF) therapy, may be influenced by the gastrointestinal microbiota. A common toxicity of anti-VEGF therapy is diarrhea, which can be dose limiting. In a pilot study of RCC patients on anti-VEGF therapy, stool microbiota profiling studies identified high levels of Bacteroides spp. and low levels of Prevotella spp. in patients experiencing diarrhea [53]. Interestingly, in patients with poor-risk RCC, the use of antibiotics with Bacteroides spp. coverage resulted in improved PFS, suggesting that alterations of the gut microbiome can impact outcome following non-immune-based treatments [54]. With multiple clinical trials investigating the effect of the combination of anti-VEGF and immunotherapies, understanding how the microbiome can be altered and how it may influence treatment responses is of paramount importance.
Prostate cancer is the least mature in terms of data assessing the potential impact of the gastrointestinal microbiome on cancer treatment response. Sfanos et al. [55] recently profiled the fecal microbiota in a cross-sectional study of patients that included healthy male volunteers and men with different clinical states of prostate cancer (ie, localized, biochemically recurrent, and metastatic disease). They reported compositional differences in the gastrointestinal microbiota of men taking oral androgen receptor axis-targeted therapies (such as enzalutamide and abiraterone acetate), including a greater abundance of species previously linked to the response to anti-PD-1 immunotherapy, such as A. muciniphila and Ruminococcaceae spp. Of interest, these authors reported an enriched representation of bacteria predicted to contain gene pathways involved in steroid hormone biosynthesis in the fecal microbiota of men taking oral androgen receptor axis-targeted therapies. This finding led to the speculation that alterations to the gastrointestinal microbiome driven by androgen axis-targeted therapies might represent a mechanism for potential alternative pathways of production of steroid metabolites that could, in turn, influence treatment response to these drugs [55].
3.2. Translating microbiome research to clinical action and relevance
Not only can microbes be present within cancer tissue, they also densely populate the intestinal tract where we deliver many therapeutic drugs for host absorption. The presence of bacteria at both oncological and drug delivery sites should drive us to have a better understanding of their broad functional roles in disease, diagnosis, and therapy (Table 2).
Table 2 –
Proposed mechanisms of interaction between the microbiome and genitourinary cancer
| Direct Interactions |
| Urinary microbiome |
| Control of pathogen outgrowth in the urinary tract |
| Cofactor for cancer development (eg, inciting chronic inflammation, production of N-nitrosamines and schistosomiasis, etc.) |
| Intratumoral cancer drug metabolism |
| Intratumoral cancer drug efficacy (eg, competition for fibronectin binding with BCG therapy) |
| Indirect Interactions |
| Gastrointestinal microbiome |
| Cancer drug metabolism |
| Cancer drug efficacy (eg, dictating response to immunotherapy) |
| Xenobiotic metabolism (dietary carcinogens, hormones, pro- or anti-inflammatory compounds, etc.) |
| Control of systemic immunity (chronic inflammation, immunotherapy response, etc.) |
BCG = Bacillus Calmette-Guerin.
3.2.1. The microbiome and screening/risk identification
The etiological role of microbiota in some cancers has been determined, such as malignancies in the stomach and colon, and as discussed above, similar interactions are starting to be uncovered in the urinary and reproductive systems [26,31,56,57]. Although the ability to relate specific microbes with tumor pathogenesis and treatment response will be crucial, challenges remain as microorganism composition depends upon how biological fluids such as urine are collected. Indeed, urine may have microorganisms originating from various sites in the urinary (urethra, bladder, and kidneys) and reproductive systems (vagina and prostate). Even microbes from urine obtained directly from the bladder may not be entirely representative of the focal site where the tumor may be present unless a considerable field change has occurred [8]. While there are technical hurdles to overcome to evaluate this technology for “liquid biopsy,” noninvasive diagnostic and prognostic tools would be of great clinical value.
Utilizing the urinary microbiome to predict cancer risk and response to treatment is an intriguing possibility with technological advancements. Bacterial 16S rDNA gene sequencing in particular has advanced our understanding of the compositional characterization of the bacterial populations occupying urinary and reproductive sites. However, future broad metagenomic sequencing and other nucleic acid technologies, coupled with metabolomic studies examining the small molecules produced through metabolism may provide extensive additional information about the functional abilities of these microbes, and might in time yield useful biomarkers, both prognostic and predictive. For instance, Liss et al. [57] discovered 10 gastrointestinal microbiome-derived metabolic pathways that were found to be differentially present in men with prostate cancer versus those without (ie, folate, arginine) in addition to enrichments in bacterial species. Likewise, multiple studies have examined metabolomic biomarkers in the urine in relation to genitourinary cancer risk [58–61], and future studies linking the presence of these metabolites to the urinary microbiome structure are warranted. Ultimately, it may be that specific metabolites, rather than specific species of microbes, drive pathological changes, tumor development, or therapy response.
3.2.2. Therapeutic possibilities
If microorganisms are responsible for cancer initiation and/or progression, or if they impact cancer treatment, then we need to consider how to modulate the microbiota for potential benefit in the urinary tract. Researchers in the “pre-microbiome” era understood this concept, which led them to use probiotic bacteria such as the intestinal Lactobacillus casei Shirota strain, among others, to potentially reduce recurrence of bladder cancer. Nitrosamine-induced bladder carcinogenesis was inhibited in animal models [62–68]. Probiotics prevented secondary tumor growth and modulated cytokine production, and lower grades of disease were observed in the probiotic-treated animals. This naturally led to studies in humans where results initially looked promising, but confounding factors such as high discontinuation rates and study design have stalled further clinical investment [69–73]. With the invigoration of the microbiome field and new tools for its investigation, these lactobacilli-based studies should be reinvestigated.
Perhaps the most exciting area at present time is how the microbiome, in both the urinary system and the intestinal tract, may alter drug therapies and influence urological oncological outcomes (see Tables 1 and 2, and Fig. 2). For example, Bacillus Calmette-Guerin (BCG), the Mycobacterium bovis-derived vaccine strain for tuberculosis, is widely used to prevent recurrence of bladder cancer by direct bladder instillation. BCG induces an immune response, in part through the potential binding of fibronectin in the bladder, which increases in intensity over the treatment period to induce tumor-specific immunity [74]. However, we now understand that commensal microorganisms are also present in the bladder and may potentially interact with BCG, influencing the development of immunity to bladder cancer. At other mucosal sites, the microbiome plays a role in barrier homeostasis and moderates inflammatory events induced by potentially opportunistic microorganisms and other insults. Different species of Lactobacillus have various abilities to bind fibronectin [75,76]. McMillan et al. [77] demonstrated that urinary-specific lactobacilli, such as Lactobacillus iners, are potent binders of fibronectin, and this species is also present in the bladder [78]. This finding may have consequences for BCG therapy if BCG is competing for fibronectin-binding positions. Therefore, it is imperative that we assess the microbiome at the target site, before, during, and following therapy, and with subsequent outcome following BCG therapy in future clinical trials.
The microbiome may also have direct influences on treatments used for urological cancers. For example, abiraterone acetate, an androgen deprivation (CYP17A inhibitor) agent used for the treatment of advanced prostate cancer, is poorly absorbed and spends considerable time in the intestinal tract. Abiraterone and other agents may potentially undergo xenobiotic metabolism and also alter the microbiome, subsequently affecting the drug’s biological availability and activity [55]. In addition, the microbiome may influence systemic treatments that never actually come into direct contact with it, such as chemotherapy or immunotherapy. One of the most rapidly emerging areas is that of the role of the intestinal microbiome on a patient’s response to immunotherapy, especially to immune checkpoint inhibitors. These agents potentially have application in prostate cancer and are approved for use in RCC and bladder cancer. We are rapidly becoming aware that the host’s microbial factors may significantly influence the outcome of these agents, as observed for other cancers as described in previous sections. Notably, as mentioned above, antibiotic treatment of patients with RCC, NSCLC, and UC for common indications (dental, pulmonary, and urinary infections) significantly reduced the PFS and OS of immunotherapy-treated patients [44]. Therefore, it may be possible to alter the immune response by modifying the patient’s microbiome using fecal microbiota transplant (FMT) for a beneficial immunotherapeutic outcome. This concept is currently being tested in clinical studies (NCT03341143 and NCT03353402), with additional studies likely following. FMT is not the only way to alter the microbiome response or composition through the intestinal tract, but it has shown to dramatically shift bacterial populations in patients with severe intestinal infectious disease such as Clostridium difficile infection or ulcerative colitis.
4. Conclusions
We are only now beginning to appreciate the potential influence of the microbiome at sites in the urinary tract on the pathobiology of genitourinary cancer. We have much to learn regarding its composition and function during homeostasis and in disease states. Perhaps with further study, we can develop the means to manipulate the urinary and/or gastrointestinal microbiome to improve patient outcome. Translating microbiome research into clinical practice requires the joint efforts of multidisciplinary teams of microbiome researchers, infectious disease experts, tumor immunologists, and medical oncologists to successfully translate laboratory-based research to relevant patient care.
Acknowledgments:
K.S.S. and M.C.M. would like to acknowledge the Prostate Cancer Foundation for ongoing research support.
Footnotes
Financial disclosures: Karen S. Sfanos certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
References
- [1].Huttenhower C, Gevers D, Knight R, et al. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207–14 http://www.nature.com/nature/journal/v486/n7402/abs/nature11234.html#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lloyd-Price J, Mahurkar A, Rahnavard G, et al. Strains, functions and dynamics in the expanded Human Microbiome Project. Nature 2017;550:61–6 https://www.nature.com/articles/nature23889#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chong C, Bloomfield F, O’Sullivan J. Factors affecting gastrointestinal microbiome development in neonates. Nutrients 2018;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Whiteside SA, Razvi H, Dave S, Reid G, Burton JP. The microbiome of the urinary tract—a role beyond infection. Nat Rev Urol 2015;12:81–90. [DOI] [PubMed] [Google Scholar]
- [5].Hilt EE, McKinley K, Pearce MM, et al. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol 2014;52:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anderson M, Bollinger D, Hagler A, et al. Viable but nonculturable bacteria are present in mouse and human urine specimens. J Clin Microbiol 2004;42:753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolfe AJ, Toh E, Shibata N, et al. Evidence of uncultivated bacteria in the adult female bladder. J Clin Microbiol 2012;50:1376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bao Y, Al KF, Chanyi RM, et al. Questions and challenges associated with studying the microbiome of the urinary tract. Ann Transl Med 2017;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lewis DA, Brown R, Williams J, et al. The human urinary microbiome; bacterial DNA in voided urine of asymptomatic adults. Front Cell Infect Microbiol 2013;3:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pearce MM, Zilliox MJ, Rosenfeld AB, et al. The female urinary microbiome in urgency urinary incontinence. Am J Obstet Gynecol 2015;213:347.e1–347.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shrestha E, White JR, Yu SH, et al. Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J Urol 2018;199:161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 2013;63:234–41. [DOI] [PubMed] [Google Scholar]
- [13].Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev 1999;12: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dhote R, Thiounn N, Debre B, Vidal-Trecan G. Risk factors for adult renal cell carcinoma. Urol Clin North Am 2004;31:237–47. [DOI] [PubMed] [Google Scholar]
- [15].Sfanos KS, Yegnasubramanian S, Nelson WG, De Marzo AM. The inflammatory microenvironment and microbiome in prostate cancer development. Nat Rev Urol 2018;15:11–24. [DOI] [PubMed] [Google Scholar]
- [16].Spiegel CA. Bacterial vaginosis. Clin Microbiol Rev 1991;4:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zaghloul MS. Bladder cancer and schistosomiasis. J Egypt Natl Cancer Inst 2012;24:151–9. [DOI] [PubMed] [Google Scholar]
- [18].Adebayo AS, Suryavanshi MV, Bhute S, et al. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLOS Negl Trop Dis 2017;11:e0005826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anderson-Otunu O, Akhtar S. Chronic infections of the urinary tract and bladder cancer risk: a systematic review. Asian Pac J Cancer Prev 2016;17:3805–7. [PubMed] [Google Scholar]
- [20].Alfano M, Canducci F, Nebuloni M, Clementi M, Montorsi F, Salonia A. The interplay of extracellular matrix and microbiome in urothelial bladder cancer. Nat Rev Urol 2016;13:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer 2017;17:271–85. [DOI] [PubMed] [Google Scholar]
- [22].Garrett WS. Cancer and the microbiota. Science 2015;348:80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Xu W, Yang L, Lee P, et al. Mini-review: perspective of the microbiome in the pathogenesis of urothelial carcinoma. Am J Clin Exp Urol 2014;2:57–61. [PMC free article] [PubMed] [Google Scholar]
- [24].Popović VB, Šitum M, Chow CT, Chan LS, Roje B, Terzić J. The urinary microbiome associated with bladder cancer. Sci Rep 2018;8:12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: Should we reintroduce key predators in our ecosystem? Front Microbiol 2016;7:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wu P, Zhang G, Zhao J, et al. Profiling the urinary microbiota in male patients with bladder cancer in China. Front Cell Infect Microbiol 2018;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McConkey DJ, Choi W, Dinney CP. Genetic subtypes of invasive bladder cancer. Curr Opin Urol 2015;25:449–58. [DOI] [PubMed] [Google Scholar]
- [28].Lipworth L, Tarone RE, McLaughlin JK. The epidemiology of renal cell carcinoma. J Urol 2006;176:2353–8. [DOI] [PubMed] [Google Scholar]
- [29].Parker AS, Cerhan JR, Lynch CF, Leibovich BC, Cantor KP. History of urinary tract infection and risk of renal cell carcinoma. Am J Epidemiol 2004;159:42–8. [DOI] [PubMed] [Google Scholar]
- [30].Sfanos KS, Isaacs WB, De Marzo AM. Infections and inflammation in prostate cancer. Am J Clin Exp Urol 2013;1:3–11. [PMC free article] [PubMed] [Google Scholar]
- [31].Porter CM, Shrestha E, Peiffer LB, Sfanos KS. The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis 2018;21:345–54. [DOI] [PubMed] [Google Scholar]
- [32].Sfanos KS, Sauvageot J, Fedor HL, Dick JD, De Marzo AM, Isaacs WB. A molecular analysis of prokaryotic and viral DNA sequences in prostate tissue from patients with prostate cancer indicates the presence of multiple and diverse microorganisms. Prostate 2008;68:306–20. [DOI] [PubMed] [Google Scholar]
- [33].Yow MA, Tabrizi SN, Severi G, et al. Characterisation of microbial communities within aggressive prostate cancer tissues. Infect Agents Cancer 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cavarretta I, Ferrarese R, Cazzaniga W, et al. The microbiome of the prostate tumor microenvironment. Eur Urol 2017;72:625–31. [DOI] [PubMed] [Google Scholar]
- [35].Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB. Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA 2009;106:3443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 2016;14:273–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Geller LT, Barzily-Rokni M, Danino T, et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017;357:1156–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wallace BD, Wang H, Lane KT, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010;330:831–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015;350:1079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013;342:967–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013;342:971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015;350:1084–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018;359:104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018;359:91–7. [DOI] [PubMed] [Google Scholar]
- [45].Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maier L, Pruteanu M, Kuhn M, et al. Extensive impact of nonantibiotic drugs on human gut bacteria. Nature 2018;555:623–8 https://www.nature.com/articles/nature25979#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Fijlstra M, Ferdous M, Koning AM, Rings EH, Harmsen HJ, Tissing WJ. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support Care Cancer 2015;23:1513–22. [DOI] [PubMed] [Google Scholar]
- [48].Montassier E, Batard E, Massart S, et al. 16S rRNA gene pyrosequencing reveals shift in patient faecal microbiota during high-dose chemotherapy as conditioning regimen for bone marrow transplantation. Microbial Ecol 2014;67:690–9. [DOI] [PubMed] [Google Scholar]
- [49].Montassier E, Gastinne T, Vangay P, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther 2015;42:515–28. [DOI] [PubMed] [Google Scholar]
- [50].van Vliet MJ, Tissing WJ, Dun CA, et al. Chemotherapy treatment in pediatric patients with acute myeloid leukemia receiving antimicrobial prophylaxis leads to a relative increase of colonization with potentially pathogenic bacteria in the gut. Clin Infect Dis 2009;49:262–70. [DOI] [PubMed] [Google Scholar]
- [51].Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013;13:800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018;29:1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pal SK, Li SM, Wu X, et al. Stool bacteriomic profiling in patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor-tyrosine kinase inhibitors. Clin Cancer Res 2015;21:5286–93. [DOI] [PubMed] [Google Scholar]
- [54].Hahn AW, Froerer C, VanAlstine S, et al. Targeting bacteroides in stool microbiome and response to treatment with first-line VEGF tyrosine kinase inhibitors in metastatic renal-cell carcinoma. Clin Genitourin Cancer 2018;16:365–8. [DOI] [PubMed] [Google Scholar]
- [55].Sfanos KS, Markowski MC, Peiffer LB, et al. Compositional differences in gastrointestinal microbiota in prostate cancer patients treated with androgen axis-targeted therapies. Prostate Cancer Prostatic Dis 2018;21:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Arthur JC, Perez-Chanona E, Mühlbauer M, et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012;338:120–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Liss MA, White JR, Goros M, et al. Metabolic biosynthesis pathways identified from fecal microbiome associated with prostate cancer. Eur Urol 2018;74:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kelly RS, Vander Heiden MG, Giovannucci E, Mucci LA. Metabolomic biomarkers of prostate cancer: prediction, diagnosis, progression, prognosis, and recurrence. Cancer Epidemiol Biomarkers Prev 2016;25:887–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Loras A, Trassierra M, Sanjuan-Herráez D, et al. Bladder cancer recurrence surveillance by urine metabolomicsanalysis. SciRep 2018;9172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shen C, Sun Z, Chen D, et al. Developing urinary metabolomic signatures as early bladder cancer diagnostic markers. Omics 2015;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ganti S, Weiss RH. Urine metabolomics for kidney cancer detection and biomarker discovery. Urol Oncol 2011;29:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tomita K, Akaza H, Nomoto K, et al. Influence of Lactobacillus casei on rat bladder carcinogenesis. Nihon Hinyokika Gakkai zasshi 1994;85:655–63. [DOI] [PubMed] [Google Scholar]
- [63].Takahashi T, Kushiro A, Nomoto K, et al. Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT-2. J Urol 2011;166:2506–11. [PubMed] [Google Scholar]
- [64].Seow SW, Cai S, Rahmat JN, et al. Lactobacillus rhamnosus GG induces tumor regression in mice bearing orthotopic bladder tumors. Cancer Sci 2010;101:751–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Ohashi Y, Nakai S, Tsukamoto T, et al. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int 2002;68:273–80. [DOI] [PubMed] [Google Scholar]
- [66].Davis CP, Cohen MS, Hackett RL, Anderson MD, Warren MM. Urothelial hyperplasia and neoplasia III. Detection of nitrosamine production with different bacterial genera in chronic urinary tract infections of rats. J Urol 1991;145:875–80. [DOI] [PubMed] [Google Scholar]
- [67].Brandau S, Bohle A. Re: Antitumor effects of the intravesical instillation of heat killed cells of the Lactobacillus casei strain Shirota on the murine orthotopic bladder tumor MBT-2. J Urol 2002;168:199–200, [author reply 200]. [PubMed] [Google Scholar]
- [68].Asano M, Karasawa E, Takayama T. Antitumor activity of Lactobacillus casei (LC 9018) against experimental mouse bladder tumor (MBT-2). J Urol 1986;136:719–21. [DOI] [PubMed] [Google Scholar]
- [69].O’Donnell MA. Does the probiotic L. casei help prevent recurrence after transurethral resection for superficial bladder cancer? Nat Clin Pract Urol 2008;5:526–7. [DOI] [PubMed] [Google Scholar]
- [70].Naito S, Koga H, Yamaguchi A, et al. Prevention of recurrence with epirubicin and Lactobacillus casei after transurethral resection of bladder cancer. J Urol 2008;179:485–90. [DOI] [PubMed] [Google Scholar]
- [71].Naito S Comment on “Does the probiotic L. casei help prevent recurrence after transurethral resection for superficial bladder cancer?”. Nat Clin Pract Urol 2009;6:E5. [DOI] [PubMed] [Google Scholar]
- [72].Aso Y, Akazan H. Prophylactic effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer. BLP Study Group. Urol Int 1992;49:125–9. [DOI] [PubMed] [Google Scholar]
- [73].Aso Y, Akaza H, Kotake T, Tsukamoto T, Imai K, Naito S. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double-blind trial. The BLP Study Group. Eur Urol 1995;27:104–9. [DOI] [PubMed] [Google Scholar]
- [74].Pettenati C, Ingersoll MA. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat Rev Urol 2018;15:615–25. [DOI] [PubMed] [Google Scholar]
- [75].Munoz-Provencio D, Perez-Martinez G, Monedero V. Characterization of a fibronectin-binding protein from Lactobacillus casei BL23. J Appl Microbiol 2010;108:1050–9. [DOI] [PubMed] [Google Scholar]
- [76].Lindgren SE, Swaisgood HE, Janolino VG, et al. Binding of Lactobacillus reuteri to fibronectin immobilized on glass beads. Zentralbl Bakteriol 1992;277:519–28. [DOI] [PubMed] [Google Scholar]
- [77].McMillan A, Macklaim JM, Burton JP, Reid G. Adhesion of Lactobacillus iners AB-1 to human fibronectin: a key mediator for persistence in the vagina? Reprod Sci 2013;20:791–6. [DOI] [PubMed] [Google Scholar]
- [78].Thomas-White K, Forster SC, Kumar N, et al. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat Commun 2018;9:1557. [DOI] [PMC free article] [PubMed] [Google Scholar]


