Abstract
Live cells of Campylobacter jejuni and Campylobacter coli can induce release of interleukin-8 (IL-8) from INT407 cells. Additionally, membrane fractions of C. jejuni 81-176, but not membrane fractions of C. coli strains, can also induce release of IL-8. Membrane preparations from 81-176 mutants defective in any of the three membrane-associated protein subunits of cytolethal distending toxin (CDT) were unable to induce IL-8. The presence of the three cdt genes on a shuttle plasmid in trans restored both CDT activity and the ability to release IL-8 to membrane fractions. However, CDT mutations did not affect the ability of 81-176 to induce IL-8 during adherence to or invasion of INT407 cells. When C. jejuni cdt genes were transferred on a shuttle plasmid into a C. coli strain lacking CDT, membrane preparations became positive in both CDT and IL-8 assays. Growth of C. jejuni in physiological levels of sodium deoxycholate released all three CDT proteins, as well as CDT activity and IL-8 activity, from membranes into supernatants. Antibodies against recombinant forms of each of the three CDT subunit proteins neutralized both CDT activity and the activity responsible for IL-8 release. The data suggest that C. jejuni can induce IL-8 release from INT407 cells by two independent mechanisms, one of which requires adherence and/or invasion and the second of which requires CDT.
Campylobacter spp. are among the most common causes of human bacterial diarrhea worldwide. While campylobacter infections are quite common and often severe, relatively little is known about mechanisms of pathogenesis. Campylobacters are generally considered invasive, and invasiveness appears to be associated with disease in the ferret diarrheal disease model (3, 42). In addition, numerous cytotoxins in campylobacters have been described (39), but only cytolethal distending toxin (CDT) has been well characterized (29, 30, 40). CDT has been found in a variety of other bacteria including Escherichia coli (17, 33), Shigella spp. (26), Haemophilus ducreyi (12), Actinobacillus actinomycetemcomitans (25, 35, 36), and Helicobacter hepaticus (43). Although CDT has been shown to block eukaryotic cells in the G2 phase of the cell cycle (6, 28, 40, 43), its role in disease caused by such a diverse group of pathogens remains unclear. However, there is some evidence suggesting a role for CDT in diarrheal disease. An epidemiological study in Bangladesh showed a trend towards increased numbers of CDT-positive E. coli cells in diarrheal cases compared to asymptomatic controls, but the difference did not reach statistical significance (1). When fed to suckling mice, partially purified CDT from Shigella dysenteriae produced watery diarrhea and tissue damage in the descending colon (26).
Campylobacter entercolitis is typically associated with a local acute inflammatory response and involves intestinal tissue damage. It is thought that the host inflammatory response may mediate many of the clinical symptoms (20), and inflammatory cytokine responses are recognized components of enteric infections. Interleukin-8 (IL-8) is a proinflammatory cytokine, a potent chemotactic factor for many immune effector cells, and a mediator of localized inflammatory responses. Helicobacter pylori, a primary cause of gastritis in humans, is known to induce IL-8 release from epithelial cells (15, 34). Salmonella enterica serovar Typhimurium, Listeria monocytogenes, and Shigella spp. have also been shown to elicit IL-8 secretion from intestinal epithelial cells in vitro during invasion (9, 19). We have previously shown that Campylobacter jejuni also induces IL-8 secretion from intestinal epithelial cells by a process which correlated with adherence and/or bacterial invasion (14). In this report we demonstrate that C. jejuni mediates IL-8 secretion from intestinal epithelial cells by multiple mechanisms. One of these mechanisms, as previously described, involves adherence and/or invasion (14), while a second mechanism is mediated directly by CDT.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Campylobacters were routinely grown on Mueller-Hinton (MH) agar (Difco, Detroit, Mich.) under microaerobic conditions or in biphasic MH cultures. MH medium was supplemented with kanamycin to a final concentration of 50 μg/ml or chloramphenicol at a final concentration of 20 μg/ml in some cases. For counterselection of E. coli DH5α (RK212.1) donors (11, 21) in complementation experiments, trimethoprim was added to MH medium at a final concentration of 20 μg/ml. In some experiments MH medium was supplemented with 25 mM (0.1%) sodium deoxycholate (Sigma, St. Louis, Mo.) (8). Campylobacters were routinely grown for 18 to 20 h at 37°C. E. coli were grown in Luria-Bertani medium with appropriate antibiotics.
C. jejuni strains 81-176 and CH5 (also known as 4483) and Campylobacter coli strain VC167 have been described previously (4, 13, 16, 24). C. coli 12498 was a gift from A. O'Brien. The campylobacter shuttle plasmid pRY111 (41) was used in complementation experiments.
Cloning and DNA sequence analysis of cdt genes from C. jejuni CH5.
A 1.4-kb AccI fragment of pDS7.96 containing the last 450 bp of cdtA, all of cdtB, and the first 100 bp of cdtC from E. coli 6468/62 (33) was used as a probe to identify the homologous CDT genes from C. jejuni CH5. A 4.5-kb NcoI fragment of the CH5 chromosome which hybridized to the E. coli probe was cloned into the NcoI site of pACYC184 to generate pRAM6. The insert from pRAM6 was subsequently transferred into the high-copy-number vector pLITMUS28 (New England Biolabs, Beverly, Mass.) to generate pRAM12. The insert in pRAM12 was sequenced using Amplitaq DNA polymerase and FS Dyedeoxy terminator cycle sequencing kits (Perkin-Elmer–Applied Biosystems, Foster City, Calif.). The sequencing reactions were done according to the manufacturer's instructions, and analysis was performed on an Applied Biosystems model 373 automated sequencer. Sequences were assembled using AssemblyLIGN and analyzed with MacVector (Oxford Molecular, Oxford, United Kingdom).
Cell cultures.
Human embryo intestinal epithelial (INT407) and HeLa cells were maintained in minimal essential medium–Earle's salts supplemented with 10% fetal bovine serum and 2 mM l-glutamine (Gibco-BRL, Gaithersburg, Md.).
CDT assays.
The CDT assay was done as described by Johnson and Lior (18), with minor modifications. Cells were harvested with trypsin, and the number of viable cells was determined by trypan blue exclusion. The HeLa cell suspension was then diluted to obtain a concentration of 2 × 104 to 4 × 104 cells/ml, and 150 μl of this suspension was added to each well of a 96-well polystyrene plate (Costar, Corning, N.Y.). The plate was incubated for at least 1 h at 37°C in 5% CO2 prior to addition of either filter-sterilized culture supernatants or cell fractions. When activities in different samples were compared, equal amounts of total protein from each were serially diluted and the end point titer was determined. The plates were incubated for 5 days at 37°C in 5% CO2. The medium was then removed, and the cells were fixed with 95% ethanol and stained with Giemsa. Wells in which greater than 50% of the HeLa cells were enlarged were considered positive.
Assay for IL-8 secretion.
IL-8 assays were done as previously described using INT407 cells (14). Briefly, bacteria, membranes, or supernatants were added to the INT407 monolayers, shaken at 2,500 rpm for 1 min, and then centrifuged at 1,000 rpm in a Sorvall RT6000D centrifuge for 10 min and incubated for 24 h at 37°C in 5% CO2. Culture medium was harvested and stored at −70°C until analyzed for IL-8 protein by enzyme-linked immunosorbent assay. Nunc Maxi-sorp plates were coated with 3 ng of rabbit anti-human IL-8 (Endogen, Cambridge, Mass.) per well. Culture supernatants were diluted 1:1 in phosphate-buffered saline–0.1% Tween 20 and 3 mg of bovine serum albumin/ml. Bound IL-8 was detected with a biotin-coupled detection antibody (0.5 μg/ml). The assay was developed with avidin-peroxidase (500 μg/ml) (Gibco-BRL) and TMB (3,3′,5,5′-tetramethylbenzidine; Sigma).
Synthesis of oligonucleotides.
Oligonucleotides for use as PCR primers and as primers for DNA sequencing were synthesized on an Applied Biosystems model 392 automated DNA synthesizer.
RT-PCR assays.
Total RNA was isolated from C. jejuni 81-176 and CDT mutants using RNAeasy kits (Qiagen, Chatsworth, Calif.). Initial reverse transcriptase PCR (RT-PCR) analyses were performed using kits from Stratagene (La Jolla, Calif.), and later analyses were done with the MasterAmp kit from Epicentre Technologies (Madison, Wis.). The positions of the primer sets used are shown in Fig. 1. Primers were as follows: primer set 1 (between cdtA and cdtB), DS18 (5′-CCTTGTGATGCAAGCAATC-3′) and DS15 (5′-ACACTCCATTTGCTTTCTG-3′); primer set 2 (between cdtB and cdtC), DS21 (5′-GGGATTTTAACCGTGATCCTTC-3′) and DS14 (5′-AGATTTTGCTCCAAAGGTTC-3′); primer set 3 (within cdtA), DS27 (5′-TGTAAATCCTTTGGGGCGTTC-3′) and DS19 (5′-GCAAAAGTTGCCAAACTCTAG-3′); primer set 4 (within cdtB), DS16 (5′-CACAGAAAGCAAATGGAGTGTTAG-3′) and DS8 (5′-CGCTAGTTGGAAAAACCACTC-3′); primer set 5 (within cdtC), DS9 (5′-CAACTCCTACTGGAGATTTGAAAG-3′) and DS23 (5′-AAAGGGGTAGCAGCTGTTAA-3′). When using the Stratagene kit, PCR was performed with the initial denaturation step for 2 min at 94°C, followed by 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min. RT-PCR mixtures lacking reverse transcriptase were included as negative controls. When using the MasterAmp RT-PCR kit from Epicentre Technologies, RT-PCR was performed with an initial incubation step at 60°C for 20 min, followed by 30 cycles as described above. The initial 60°C step was omitted in the negative controls. Chromosomal DNA isolated from 81-176 was used as the positive control for the PCR with both kits. Aliquots of RT-PCR mixtures were electrophoresed on 1.2% agarose gels.
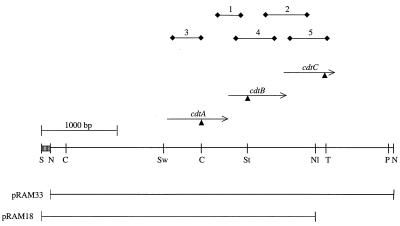
FIG. 1.
Restriction map of the 4.5-kb NcoI chromosomal fragment of the C. jejuni CH5 chromosome containing the cdt genes. The NcoI fragment was cloned into pACYC184 to generate pRAM6, into pLITMUS28 to generate pRAM12, and into pRY111 (41) to generate pRAM33. The 3.5-kb NlaV/SacI fragment, which extends 1.5 kb upstream of cdtA through the first 303 bp of cdtC, was cloned into pRY111 (41) to generate pRAM18. Triangles, insertion sites of the Kmr cassette from pILL600 (21); arrows, positions of primer sets used in RT-PCR. Primer set 1 spans cdtA and cdtB; primer set 2 spans cdtB and cdtC; primer sets 3, 4, and 5 are intragenic to cdtA, cdtB, and cdtC, respectively. Restriction enzyme sites: C, ClaI; N, NcoI; Nl, NlaIV; P, PstI; S, ScaI; St, StuI; Sw, SwaI; T, TfiI. Striped box, multiple cloning site of pLITMUS 28.
Construction of CDT mutants and transconjugants.
The kanamycin resistance (Kmr) cassette from pILL600 (21) was inserted into restriction endonuclease sites within each of the three CDT genes as seen in Fig. 1 in either pRAM12 or various subclones. The orientation of the cassette in each mutant was determined by DNA sequencing with primers that read out from both ends of the cassette (41). Plasmid DNAs containing the insertionally inactivated alleles were transformed into 81-176 by natural transformation, and mutants were selected on kanamycin (38). Transformants were characterized by Southern blotting to confirm that the plasmid DNA had integrated via a double crossover and that no vector sequences were present (data not shown).
Construction of MBP fusions of the three CDT subunits.
A portion of each CDT gene from CH5 was fused to the 3′ end of the gene encoding maltose binding protein (MBP; Mr, 42,698) of E. coli using either pMAL-p2 (cdtA and cdtC) or pMAL-c2 (cdtB). A PCR product encoding part of cdtA was generated using primers cdtA-f (5′-GGAATTCACTCCTATTACCCCACCTTTA-3′) and cdtA-r (5′-GGAATTCTCAAGGTTTTGCGGTAAAAGGCGG-3′), which correspond to bp 208 to 228 and 760 to 780, respectively, of the cdt coding region with an EcoRI site at the 5′ end of each primer. In addition, a TCA codon was added to the cdtA-r primer after the EcoRI site to produce the stop codon TGA. This resulted in an MBP-CdtA fusion protein with a predicted Mr of 64,173, which included 191 amino acids of CdtA (residues 70 to 260). The cdtB fusion was constructed with cdtB-f (5′-GTTAGTACTTGGAATTTGCAAGGC-3′) and cdtB-r (5′-CGCGGATCCTCAAAGCGGTGGAGTATAGGTTTG-3′). These primers correspond to bp 73 to 90 and 697 to 717, respectively, of the cdtB coding region with a ScaI restriction site added to the 5′ end of the cdtB-f primer and a BamHI site and stop codon added in the cdtB-r primer. The MBP-CdtB fusion protein had a predicted Mr of 65,975 and included 215 amino acids of CdtB (amino acid residues 25 to 239). The PCR product for cdtC was amplified with cdtC-f (5′-GGAATTCGCCTTTGCAACTCCTACTGGA-3′) and cdtC-r (5′-GGAATTCTCAAGGTGGGGTTATAATCATTAGTTC-3′), which correspond to bp 43 to 63 and 520 to 543, respectively, of the cdt coding region. These primers included an EcoRI site at the 5′ ends of both primers and a stop codon in the cdtC-r primer. The resulting fusion protein had a predicted Mr of 61,605 and included 167 amino acids (residues 15 to 181) of CdtC. PCRs for all three constructions were performed for 30 cycles using pRAM12 as the template under the following conditions: 95°C for 1 min, 50°C for 1 min, and 72°C for 1 min. Both cdtA and cdtC PCR products were digested with EcoRI and then cloned into the EcoRI site of pMAL-p2. The cdtB PCR product was digested with BamHI and ScaI and ligated to pMAL-c2, which had been digested with XmnI and BamHI. All transformants were screened by hybridization with appropriate DNA probes for each gene to identify positive clones. Plasmid DNAs isolated from probe-positive transformants were sequenced to confirm the appropriate junction between the MBP gene and cdt using primers which read from within the appropriate cdt gene. Large-scale expression and purification of the fusion proteins were done as recommended by New England Biolabs.
Generation of polyclonal antisera against each CDT subunit.
Rabbit polyclonal antiserum against each MBP-CDT fusion protein was made commercially in adult New Zealand White rabbits by Harlan Bioproducts Inc. (Indianapolis, Ind.). Antisera against the CdtA, CdtB, and CdtC fusion proteins were designated ALM1, ALM2, and ALM3, respectively. Each of the three antisera was capable of neutralizing CDT activity in culture supernatants at a 1:1 dilution.
Isolation of membrane fractions.
Membrane fractions were purified by the method of Logan and Trust (23) as previously described (14), except that the membrane pellets were washed twice in 0.2 M glycine, pH 2.2, to dissociate flagellum filaments and then twice in 20 mM Tris-HCl (pH 7.4) to neutralize the pH. Protein concentrations were determined by protein assay (Bio-Rad, Hercules, Calif.).
SDS-PAGE and immunoblot analysis.
Proteins were analyzed on duplicate sodium dodecyl sulfate–12.5% polyacrylamide gel electrophoresis gels by the method of Laemmli (22). Equivalent amounts of protein in each sample, as determined by Bio-Rad protein assay, were loaded onto each gel. One gel was stained by Coomassie blue to confirm that the proteins were loaded equivalently, and the other gel was immunoblotted with anti-CDT antisera. ALM1, ALM2, and ALM3 antisera were used at dilutions of 1:10,000, 1:2,500, and 1:2,500, respectively. The secondary antibody, alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Caltag Laboratories, Burlingame, Calif.) was used at dilution of 1:5,000. Western blots were developed with nitroblue tetrazolium and 5-bromo-1-chloro-3-indolylphosphate in N,N-dimethylformamide (Promega, Madison, Wis.).
Antibody inhibition assays.
Filter-sterilized culture supernatants (1 μg of protein) were incubated for 30 min at 37°C with serial dilutions of ALM1, ALM2, and ALM3 antisera prior to IL-8 or CDT assays. Controls were the serum of each rabbit prior to immunization (preimmune sera).
Statistical analyses.
Experimental results from independent tests were presented as mean IL-8 induction (in picograms per milliliter) ± one standard deviation. Mean values of IL-8 induction were compared by using two-tailed t tests; sample variance determinations were based on F test analysis.
Nucleotide sequence accession number.
The nucleotide sequence of the cdt genes of C. jejuni CH5 has been deposited in GenBank under accession no. 159497.
RESULTS
Distinct mechanisms of IL-8 induction from INT407 cells by C. coli and C. jejuni.
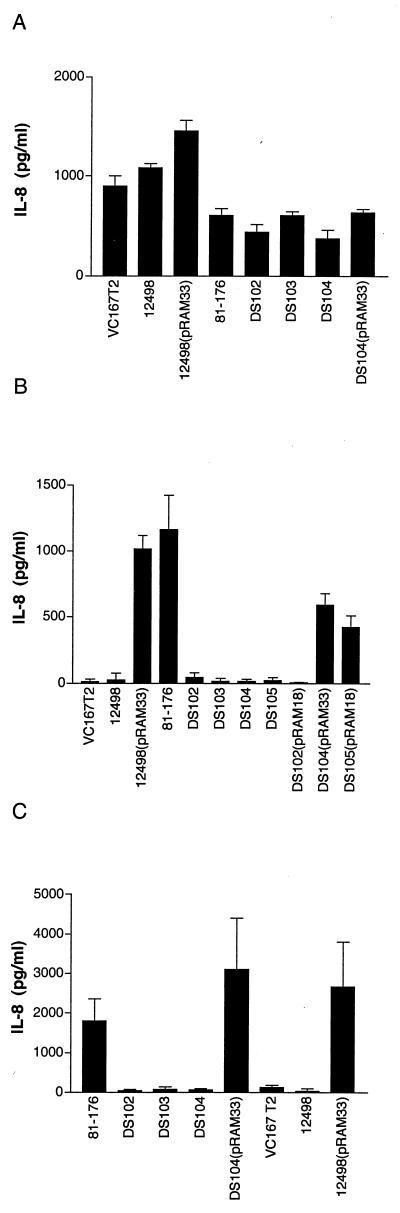
We have previously shown that viable C. jejuni can mediate IL-8 secretion from intestinal epithelial monolayers (14). Figure 2A shows that live cells of C. coli VC167 T2 mediated the secretion of 879 ± 107 pg of IL-8/ml compared to 606 ± 72 pg of IL-8/ml by C. jejuni 81-176. Another C. coli strain, 12498, mediated the secretion of 1,082 ± 43 pg of IL-8/ml. Both VC167 and 12498 strains are invasive for INT407 cells (data not shown).
FIG. 2.
IL-8 induction from INT407 cells. The data represent the means and standard deviations of 3 to 14 experiments. (A) Induction of IL-8 by whole cells of Campylobacter spp. Live cells of C. jejuni or C. coli were added to INT407 cells at a multiplicity of infection of 100:1 as described in Hickey et al. (14). Medium controls induced 50 ± 45 pg of IL-8/ml. (B) INT407 cells were inoculated with 1 μg of membrane proteins from Campylobacter strains. IL-8 release was measured by enzyme-linked immunosorbent assay after 24 h of incubation at 37°C. Medium controls induced 46 ± 56 pg of IL-8/ml. (C) INT407 cells were inoculated with supernatants of Campylobacter cultures grown in 25 mM DOC. DOC supernatants were dialyzed against water, concentrated 100-fold, and filter sterilized as described in Materials and Methods. Controls, which consisted of uninoculated MH medium plus DOC dialyzed and concentrated in the same manner as the samples, induced 48 ± 35 pg of IL-8/ml.
Figure 2B shows that 1 μg of membrane proteins isolated from C. jejuni 81-176 mediated the secretion of 1,164 ± 260 pg of IL-8/ml. In contrast, C. coli VC167 T2 and C. coli 12498 membranes were negative in the IL-8 assay (12 ± 18 and 26 ± 52 pg of IL-8/ml, respectively).
Generation of site-specific mutations in each of the cdt genes of C. jejuni 81-176.
Independent research in this laboratory had indicated that CDT activity was associated with membranes in C. jejuni but not C. coli (see below). The cdt genes were cloned and sequenced from C. jejuni CH5 as described in Materials and Methods. DNA sequence analysis revealed that the predicted CDT proteins of CH5 are identical to those of C. jejuni 81-176 (30) and NCTC 11168 (27). Site-specific mutations were generated in each gene by insertion of a Kmr cassette at the restriction sites indicated in Fig. 1 and transformed into C. jejuni 81-176 as described in Materials and Methods. The 81-176 mutant strains harboring the Kmr cassette in the same orientation as cdtA, cdtB, and cdtC are transcribed were named DS105, DS102, and DS103, respectively. The cassette was also inserted into the cdtA gene in the same site as that for DS105 but in the opposite orientation to generate mutant DS104. Supernatants of all four mutants had no detectable CDT activity, which has been reported for similar insertional mutant C. jejuni cdt genes (40).
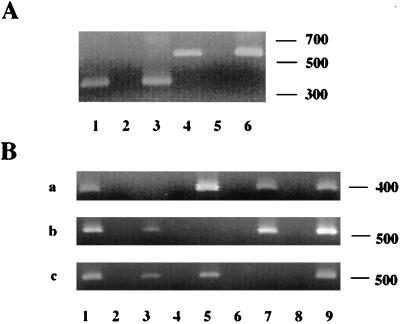
RT-PCR experiments were done using mRNA isolated from the mutants to examine the effect of the Kmr cassette on the transcription of downstream genes. Primers which spanned the cdtA and cdtB genes (DS18 and DS15 or primer set 1; Fig. 1) and the cdtB and cdtC genes (DS21 and DS14 or primer set 2) were used in RT-PCRs with total RNA isolated from C. jejuni 81-176. The predicted 370- and 575-bp products were generated with primer sets 1 and 2, respectively, indicating that all three genes are cotranscribed in a single mRNA, as previously assumed (29, 40) (Fig. 3A, lanes 1 and 4). When mRNA from the cdtA mutant DS104 was used, no RNA specific for either cdtB or cdtC was detected with intragenic primer pairs 4 and 5, respectively (data not shown), suggesting a polar effect when the Kmr cassette is inserted in the orientation opposite to that from which the genes are transcribed. However, RNAs specific for cdtB and cdtC were detected in the other cdtA mutant, DS105 (Fig. 3B, b and c, lane 3), and cdtC mRNA was detected in DS102 (Fig. 3B, c, lane 5), confirming the predicted lack of polarity when the cassette is inserted in the same direction as the target gene is transcribed (32).
FIG. 3.
RT-PCR analyses. Negative controls were RT-PCR assays done without reverse transcriptase; positive controls were PCR assays done using the same primer sets but with DNA as the template. The sequences of primers are given in Materials and Methods, and the positions of primer sets are shown schematically in Fig. 1. (A) RT-PCR analysis of wild-type 81-176. Lane 1, RT-PCR with primer set 1; lane 2, primer set 1 negative control; lane 3, primer set 1 positive control; lane 4, RT-PCR with primer set 2; lane 5, primer set 2 negative control; lane 6, primer set 2 positive control. Ten microliters of 50-μl reaction mixtures were run on 1.2% agarose gels. (B) RT-PCR analysis of 81-176 wild-type and mutant strains. (a) Primer set 3, 20 μl of 100-μl reaction mixtures (Stratagene kit); (b) primer set 4, 10 μl of 50-μl reaction mixtures (EpiCentre kit); (c) primer set 5, 20 μl of 100-μl reaction mixtures (Stratagene kit). Lane 1, 81-176 RNA; lane 2, 81-176 negative control; lane 3, DS105 RNA; lane 4, DS105 negative control; lane 5, DS102 RNA; lane 6, DS102 negative control; lane 7, DS103 RNA; lane 8, DS103 negative control; lane 9, 81-176 positive control.
All three CDT proteins are membrane associated in C. jejuni.
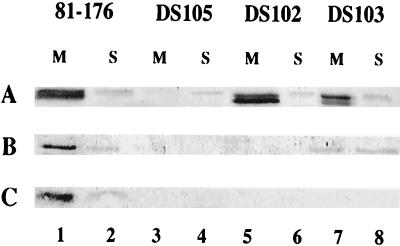
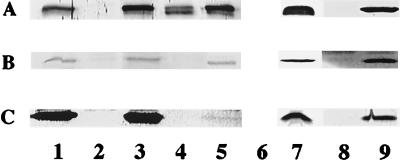
81-176 cells were separated into membrane and soluble fractions and examined by Western blotting with antisera against recombinant forms of the proteins: ALM1 (anti-CdtA), ALM2 (anti-CdtB), and ALM3 (anti-CdtC). These data are shown in Fig. 4. The CdtA band of 81-176 (Fig. 4A, lanes 1 and 2) appears as a doublet localized predominantly to the membrane fraction (lane 1). This band is missing in fractions from the nonpolar cdtA mutant DS105 (Fig. 4A, lanes 3 and 4). The band seen in the soluble fraction of 81-176 detected with ALM1 (A, lane 2) likely represents a cross-reacting protein since it is also present in the cdtA mutant (lane 4). The CdtA doublet was also localized to the membrane fractions of the cdtB mutant, DS102 (lane 5), and the cdtC mutant, DS103 (lane 7). The observed protein, which migrates at an apparent molecular mass of approximately 28 kDa, correlates well with CdtA, which has a predicted molecular mass of 27.6 kDa.
FIG. 4.
Cellular localization of CDT proteins. Membrane and soluble fractions of 81-176 and CDT mutants (10 μg of protein) were immunodetected with ALM1 (A), ALM2 (B), and ALM3 (C) antisera. The strains are noted above their respective lanes, which contain either membrane (M) or soluble (S) protein fractions. Lanes 1 and 2, 81-176; lanes 3 and 4, DS105; lanes 5 and 6, DS102; lanes 7 and 8, DS103.
ALM2 identified a protein with an approximate molecular mass of 27 kDa in the wild-type membrane fractions (Fig. 4B, lane 1) and, to a lesser degree, in the soluble fraction (lane 2), and this protein was not found in the corresponding fractions of the cdtB mutant, DS102 (lanes 3 and 4, respectively). The apparent molecular mass of CdtB correlates well with the predicted size of 27.1 kDa. The CdtB protein was also apparent in the cdtC mutant, DS103, but, in contrast to the predominant membrane location of CdtB in the wild type, in DS103, CdtB appeared to be in approximately equal amounts in membrane and soluble fractions (lanes 7 and 8, respectively). Surprisingly, no CdtB was observed in either membrane (lane 3) or soluble fractions (lane 4) of the nonpolar cdtA mutant, DS105.
ALM3 detected a protein with an approximate molecular mass of 19 kDa in the membrane fraction of the wild-type (Fig. 4C, lane 1), and a minor amount of this protein was visualized in the soluble fraction (lane 2). This protein was also missing in both the soluble (lane 8) and membrane fractions (lane 7) from the cdtC mutant, DS103. The predicted molecular mass of mature CdtC is 19.1 kDa, which corresponds well with the observed molecular mass. Surprisingly, CdtC was not observed in membrane or soluble fractions of either DS105 (lanes 3 and 4) or DS102 (lanes 5 and 6).
Membrane-associated induction of IL-8 by C. jejuni is mediated by CDT.
Membranes from DS105, which lacked all three CDT subunits, induced levels of IL-8 comparable to those induced by medium-alone controls (Fig. 2B). Membranes from DS104 also lacked all three CDT subunits (Fig. 5, lane 6) and showed no IL-8 induction (Fig. 2B). Similarly, membranes from DS102, which synthesized CdtA only, and DS103, which synthesized CdtA and CdtB, induced levels of IL-8 comparable to those induced by medium-alone controls.
FIG. 5.
Complementation in trans. Membrane fractions were immunodetected with ALM1 (A), ALM2 (B), and ALM3 (C). Lane 1, C. jejuni 81-176; lane 2, DS105; lane 3, DS105(pRAM18); lane 4, DS102; lane 5, DS102(pRAM18); lane 6, DS104; lane 7, DS104(pRAM33); lane 8, C. coli 12498; lane 9, C. coli 12498(pRAM33).
When viable cells were used in the IL-8 assay, there were no significant differences in IL-8 release between 81-176 and any of the cdt mutants (Fig. 2A), indicating that induction of IL-8 by 81-176 via internalization into epithelial cells (14) is independent of CDT expression. This is also consistent with the ability of whole cells of the two C. coli strains, which are CDT negative by both bioassay and DNA hybridization (data not shown), to induce IL-8 (see below).
Complementation in trans.
DS104 was complemented in trans with pRAM33, a Cmr shuttle vector containing all of the cdt genes (Fig. 1). DS104(pRAM33) exhibited CDT activity and synthesized all three subunits (Fig. 5, lane 6). Membrane fractions from DS104(pRAM33) induced 590 ± 88 pg of IL-8/ml, as shown in Fig. 2B. Similarly, as mentioned above, membranes from the two C. coli strains, VC167 and 12498, which are CDT negative by both bioassay and DNA hybridization (data not shown), failed to induce IL-8 release (Fig. 2B). Membrane preparations of both C. coli VC167 T2 and 12498 failed to react with ALM1, ALM2, or ALM3 antisera. Results for membranes of 12498 are seen in Fig. 5, lane 8. However, when the C. jejuni CDT genes were transferred into C. coli 12498 on pRAM33, all three CDT proteins could be detected in membrane fractions by immunoblotting, as shown in Fig. 5, lane 9. These same membrane fractions from 12498(pRAM33) induced 1,014 ± 105 pg of IL-8/ml (Fig. 2B).
DS105 was also complemented in trans with pRAM18, containing all of cdtA and cdtB and the first 303 bp of cdtC (Fig. 1). Western blot analysis of DS105(pRAM18) (Fig. 5, lane 3) indicated that CdtA, CdtB, and CdtC proteins were present at levels comparable to those for wild-type 81-176 (lane 1) as determined by comparison of dilutions of membrane preparations of the same protein concentration. The levels of CDT activity in membrane preparations of DS105(pRAM18) were also equivalent to that for wild-type 81-176, and membrane preparations from DS105(pRAM18) induced 424 ± 85 pg of IL-8/ml (Fig. 2B).
In DS102(pRAM18) (Fig. 5, lane 5) the levels of CdtA and CdtB in the membrane fraction were comparable to those seen in wild-type 81-176 (lane 1). However, the levels of CdtC in DS102(pRAM18) were consistently lower than those seen in either the wild type (Fig. 5C, lane 1) or DS102(pRAM18) (Fig. 5C, lane 3). CDT activity could be detected in membrane fractions of DS102(pRAM18), but at levels that were approximately fourfold lower than those seen with wild-type 81-176 as determined by serial dilution in four independent assays. Membrane preparations from DS102(pRAM18) produced IL-8 levels comparable to those produced by medium-alone controls (Fig. 2B).
Titration of CDT and IL-8 activity in membranes of C. jejuni 81-176.
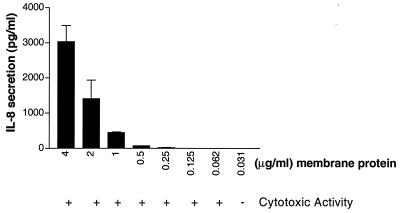
Membrane preparations of 81-176 which had been adjusted to 4 μg of protein/ml were serially diluted, and the CDT activity and IL-8 activities were compared. The results, shown in Fig. 6, indicated that the amount of IL-8 released is proportional to the amount of membrane protein added to the assay mixture. When 4 μg of protein/ml was added, IL-8 activity was 3,036 pg/ml, but when 0.5 μg of total protein/ml was used, IL-8 was barely detectable (71 pg/ml). CDT cytotoxic activity, however, could be detected with ≥62 ng of membrane protein.
FIG. 6.
Comparison of end point dilutions of 81-176 membrane proteins in the CDT and IL-8 assays. Membrane proteins from 81-176 were serially diluted and added to HeLa cells for CDT assay and to INT407 cells to measure IL-8 release. The amount of IL-8 released is indicated. These data represent the means and standard deviations of four independent experiments. The medium control induced 42 ± 34 pg of IL-8/ml.
Growth of C. jejuni in DOC causes release of CDT and IL-8 activity.
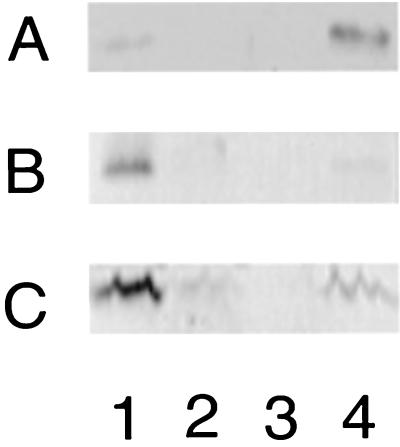
CdtA is thought to be a lipoprotein (40), and sodium deoxycholate (DOC) is known to solubilize lipoproteins from membranes (37). 81-176 was grown overnight in MH media with or without DOC (25 mM), and the location of the CDT proteins was determined by immunoblotting with the ALM antisera. Figure 7 shows that when 81-176 was grown without DOC, all three CDT subunits were found in the membrane (lane 1) and no CDT proteins were detected in the supernatant (lane 3). However, when the bacteria were grown in the presence of DOC, CdtA and CdtB proteins were no longer detectable in membranes and CdtC levels were greatly reduced (lane 2). Instead, all three CDT proteins were found in concentrated supernatants of DOC-grown cells (lane 4). Similarly, CDT activity in membranes was reduced >100-fold when the bacteria were grown in the presence of DOC and the highest level of CDT activity was found in the supernatants (data not shown).
FIG. 7.
Effect of growth in DOC on localization of CDT proteins. Shown are Western blots of membrane fractions and 100-fold-concentrated supernatants of wild-type 81-176 cultured with or without DOC (25 mM) and immunodetected with ALM1 at a dilution of 1:10,000 (A), ALM2 at a dilution of 1:2,500 (B), and ALM3 at a dilution of 1:2,500 (C). Lane 1, 81-176, membrane fraction of cells grown without DOC; lane 2, 81-176, membrane fraction of cells grown with DOC; lane 3, 81-176, supernatant from cells grown without DOC; lane 4, 81-176 supernatant from cells grown in DOC. The amount of protein loaded was 2 μg per lane.
Figure 2C shows that concentrated culture supernatants from DOC-grown cultures of C. jejuni 81-176 which were concentrated 100-fold mediated the release of 1,800 ± 555 pg of IL-8/ml from intestinal epithelial cells. Concentrated supernatants from cells grown without DOC were negative (data not shown). Figure 2C also shows that DOC supernatants from cdt mutants DS102, DS103, and DS104 were negative. However, DOC supernatants from DS104(pRAM33) produced over 3,000 pg of IL-8/ml. Similarly, supernatants from DOC-grown C. coli strains VC167 and 12498, lacking CDT, did not induce release of IL-8. However, DOC supernatants of C. coli 12498(pRAM33) released 2,668 ± 1,142 pg of IL-8/ml.
Antibodies to each of the three CDT subunits inhibit IL-8 activity in DOC supernatants.
ALM1, ALM2, and ALM3 antisera each demonstrated inhibitory effects on the ability of DOC supernatants to induce IL-8 liberation and CDT activity. All three antisera completely inhibited CDT activity at dilutions of 1:2 and 1:4. At dilutions of 1:8, ALM1 antiserum no longer inhibited CDT activity. ALM2 and ALM3 antisera showed some variation between triplicate experiments but showed ≤75% inhibition of CDT cytotoxic activity at dilutions of 1:8. Preimmune sera from the same rabbits did not inhibit CDT activity at a 1:1 dilution.
IL-8 release was more sensitive to the neutralization effects of the three antisera, consistent with the requirement for more CDT protein. Preimmune sera from the rabbits which generated ALM1 and ALM2 antisera showed low-level inhibition of IL-8 induction, but the postimmune sera showed significant increases in titer. Thus, treatment with ALM1 at a dilution of 1:1,024 produced 75% of wild-type IL-8 activity (a 64-fold increase over that for the preimmune serum) and ALM2 showed inhibition at dilutions up to 1:128 (a 32-fold increase over that for preimmune serum). The preimmune serum from the rabbit which generated ALM3 showed no inhibition at a dilution of 1:1; ALM3 antiserum inhibited IL-8 release at a dilution of 1:512.
DISCUSSION
The data presented here indicate that C. jejuni can induce IL-8 from intestinal epithelial cells by two distinct mechanisms. The first, as previously described (14), requires that live bacterial cells adhere and/or invade eukaryotic epithelial cells. Moreover, the two strains of C. coli examined here are also able to induce IL-8 by what appears to be a similar mechanism. However, IL-8 can also be induced by membrane proteins of C. jejuni strains but not membrane proteins of the two C. coli strains used. Although we previously reported that C. jejuni membrane preparations were inactive in the IL-8 assay (14), removal of the abundant flagellin protein by glycine solubilization of flagella allowed for detection of IL-8 activity, likely by allowing addition of more CDT proteins. Genetic studies also confirmed the existence of at least two distinct mechanisms of cytokine release by 81-176. CDT mutants remained invasive and capable of inducing IL-8 during the internalization process. However, membranes of mutants defective in any of the three CDT subunits lost the ability to induce IL-8. Although Shenker et al. (35) have shown that CdtB from A. actinomycetemcomitans is sufficient for cytotoxic activity, the genetic studies reported here with C. jejuni suggest that CdtC is necessary for (or at least enhances) cytotoxic activity, since a strain with wild-type levels of CdtA and CdtB but reduced levels of CdtC showed a reduced cytotoxic effect. This same strain failed to induce any detectable IL-8, suggesting that CdtC is required for IL-8 release, as well. However, given the relative insensitivity of the IL-8 assay compared to that of the cytotoxin assay, we cannot exclude the possibility that all three CDT subunits are necessary for IL-8 induction. The genetic studies described here, which are the first that measure expression of individual CDT subunits and not simply cytotoxic activity, have revealed new information about CDT subunit interactions. Thus, there is an apparent interdependency of the protein subunits for translation, or CdtB and CdtC must interact with each other and/or CdtA in order to avoid degradation or be transported to the membrane or both.
Membranes of C. coli strains which naturally lack CDT are unable to induce IL-8, but membranes from the same C. coli strain containing a shuttle plasmid encoding the C. jejuni cdt operon induced IL-8 activity. Although a recent report indicates that most C. coli strains also contain CDT genes related to those of C. jejuni (10), CDT was not detected in the two C. coli strains used in this study by either the cytotoxin assay, DNA probing (data not shown), or Western blotting with the ALM antisera. It remains to be determined if CDTs from bacteria other than C. jejuni are capable of IL-8 induction, but our preliminary data indicate that membrane fractions of E. coli DH5α containing a clone of E. coli CDT (33) are also capable of inducing release of IL-8 from INT407 cells (data not shown).
Growth in physiological levels of DOC (2.5 mM) caused release of CDT into the supernatant. Growth of C. jejuni in this same level of DOC has previously been shown to result in synthesis of a pilus-like structure required for virulence (8). Thus, it appears that passage of C. jejuni through the bile-rich small intestine would both affect synthesis of these structures and enhance the release of CDT. However, it remains to be determined if DOC causes release of CDT by solubilization of lipoproteins (37) or by a more-specific mechanism such as that reported for the release of Ipa proteins from Shigella spp. (31).
CDTs exert numerous effects on eukaryotic target cells. All CDTs have been shown to arrest epithelial cells in the G2/M phase of the cell cycle (2, 7, 26, 35, 40), and for E. coli CDT there is an accumulation of actin stress fibers concomitant with the cessation of cell division (2). In addition, CDT from H. ducreyi has been shown to arrest human T cells in the G2/M phase and to induce apoptosis (12). Similarly, Shenker et al. (35) showed that although CDT from A. actinomycetemcomitans arrested the cell cycle of both HeLa cells and human T cells, T cells were fivefold more sensitive to the toxic effects than epithelial cells. These results suggested that the primary effect of CDT in H. ducreyi and A. actinomycetemcomitans infections may be immunosuppression of the host T-cell response. In this report we demonstrate that C. jejuni CDT, in addition to arresting epithelial cells in the G2/M phase of the cell cycle, also induces release of the proinflammatory cytokine IL-8. The IL-8 studies have been done with INT407 cells, which were previously used in studies of C. jejuni IL-8 induction (14), although similar results were obtained with HeLa cells (data not shown). Both HeLa and INT407 cells appear more sensitive to the cytotoxic affects than to the IL-8-inducing effects of CDT (data not shown). The observation of a proinflammatory component to CDT is consistent with earlier reports that inflammatory infiltrates were present in the submucosa of rabbit ileum injected with either live cultures or culture supernatants of CDT-producing E. coli strains (5). Moreover, Johnson and Lior also noted inflammatory responses to CDTs in the rabbit permeability factor assay and in rat ileal loops (17, 18). We are currently examining the role of CDT in inflammatory diarrhea in animal models.
ACKNOWLEDGMENTS
This work was supported by Naval Medical Research and Development Command work unit no. 61102AS13O1291.
We thank Isabelle Walker for excellent technical assistance and Alison O'Brien for C. coli strain 12498.
T.E.H. and A.L.M. contributed equally to this work.
REFERENCES
- 1.Albert M J, Faruque S M, Faruque A S, Bettelheim K A, Neogi P K, Bhuiyan N A, Kaper J B. Controlled study of cytolethal distending toxin-producing Escherichia coli infections in Bangladeshi children. J Clin Microbiol. 1996;34:717–719. doi: 10.1128/jcm.34.3.717-719.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bacon D J, Alm R A, Burr D H, Hu L, Kopecko D J, Ewing C P, Trust T J, Guerry P. Involvement of a plasmid in virulence of Campylobacter jejuni 81-176. Infect Immun. 2000;68:4384–4390. doi: 10.1128/iai.68.8.4384-4390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black R E, Levine M M, Clements M L, Hughes T P, Blaser M J. Experimental Campylobacter jejuni infections in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 5.Bouzari S, Vatsala B R, Varghese A. In vitro adherence property of cytolethal distending toxin (CLDT) producing EPEC strains and effect of the toxin on rabbit intestine. Microb Pathog. 1992;12:153–157. doi: 10.1016/0882-4010(92)90118-8. [DOI] [PubMed] [Google Scholar]
- 6.Comayras C, Tasca C, Peres S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cortes-Bratti X, Chaves-Olarte E, Lagergard T, Thelesam M. The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell cycle arrest in the G2 phase. J Clin Investig. 1999;103:107–115. doi: 10.1172/JCI3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doig P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyigor A, Dawson K A, Langlois B E, Pickett C L. Cytolethal distending toxin genes in Campylobacter jejuni and Campylobacter coli isolates: detection and analysis by PCR. J Clin Microbiol. 1999;37:1646–1650. doi: 10.1128/jcm.37.5.1646-1650.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelfanova V, Hansen E J, Spinola S M. Cytolethal distending toxin of Haemophilus ducreyi induces apoptotic death of Jurkat T cells. Infect Immun. 1999;67:6394–6402. doi: 10.1128/iai.67.12.6394-6402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris L A, Logan S M, Guerry P, Trust T J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987;169:5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey T E, Baqar S, Bourgeois A L, Ewing C P, Guerry P. Campylobacter jejuni-stimulated secretion of interleukin-8 by INT-407 cells. Infect Immun. 1999;67:88–93. doi: 10.1128/iai.67.1.88-93.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, O'Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson W M, Lior H. Cytotoxic and cytotonic factors produced by Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis. J Clin Microbiol. 1986;24:275–281. doi: 10.1128/jcm.24.2.275-281.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson W M, Lior H. Response of Chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible interpretation as heat-labile (LT) enterotoxin. FEMS Microbiol Lett. 1987;43:19–23. [Google Scholar]
- 18.Johnson W M, Lior H. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb Pathog. 1988;4:115–126. doi: 10.1016/0882-4010(88)90053-8. [DOI] [PubMed] [Google Scholar]
- 19.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ketley J M. Pathogenesis of enteric infection by Campylobacter. Microbiology. 1997;143:5–21. doi: 10.1099/00221287-143-1-5. [DOI] [PubMed] [Google Scholar]
- 21.Labigne-Roussel A, Couroux P, Tompkins L S. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Logan S M, Trust T J. Molecular identification of surface protein antigens of Campylobacter jejuni. Infect Immun. 1983;42:675–682. doi: 10.1128/iai.42.2.675-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCardell B A, Madden J M, Lee E C. Campylobacter jejuni and Campylobacter coli production of a cytotonic toxin immunologically similar to cholera toxin. J Food Prot. 1984;47:943–949. doi: 10.4315/0362-028X-47.12.943. [DOI] [PubMed] [Google Scholar]
- 25.Ohguchi M, Ishisaki A, Okahashi N, Koide M, Koseki T, Yamato K, Noghuchi T, Nishihara T. Actinobacillus actinomycetemcomitans toxin induces both cell cycle arrest in G2/M phase and apoptosis. Infect Immun. 1998;66:5980–5987. doi: 10.1128/iai.66.12.5980-5987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda J, Fukumoto M, Takeda Y, Nishibuchi M. Examination of diarrheagenicity of cytolethal distending toxin: suckling mouse response to products of cdtABC gene of Shigella dysenteriae. Infect Immun. 1997;65:428–433. doi: 10.1128/iai.65.2.428-433.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable tracts. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 28.Peres S Y, Marches O, Daigle F, Nougayrede J P, Herault F, Tasca C, De Rycke J, Oswald E. A new cytolethal distending toxin (CDT) from Escherichia coli producing CNF2 blocks HeLa cell division in G2/M phase. Mol Microbiol. 1997;24:1095–1107. doi: 10.1046/j.1365-2958.1997.4181785.x. [DOI] [PubMed] [Google Scholar]
- 29.Pickett C L. Campylobacter toxins and their role in pathogenesis. In: Nachamkin I, Blaser M J, editors. Campylobacter. Washington, D.C.: American Society for Microbiology; 2000. pp. 179–190. [Google Scholar]
- 30.Pickett C L, Pesci E C, Cottle D L, Russell G, Erdem A N, Zeytin H. Prevalence of cytolethal distending toxin production in Campylobacter jejuni and relatedness of Campylobacter spp. cdtB genes. Infect Immun. 1996;64:2070–2078. doi: 10.1128/iai.64.6.2070-2078.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pope L M, Reed K E, Payne S M. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun. 1995;63:3642–3648. doi: 10.1128/iai.63.9.3642-3648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz A, Josenhans C, Suerbaum S. Cloning and characterization of the Helicobacter pylori flbA gene, which codes for a membrane protein involved in coordinated expression of flagellar genes. J Bacteriol. 1997;179:987–997. doi: 10.1128/jb.179.4.987-997.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott D A, Kaper J B. Cloning and sequencing of the genes encoding Escherichia coli cytolethal distending toxin. Infect Immun. 1994;62:244–251. doi: 10.1128/iai.62.1.244-251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S A, Tummuru M K R, Miller G G, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shenker B J, McKay T, Datar S, Miller M, Chowhan R, Demuth D. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing G2 arrest in human T cells. J Immunol. 1999;162:4773–4780. [PubMed] [Google Scholar]
- 36.Sugai M, Kawamoto T, Peres S Y, Ueno Y, Komatsuzawa H, Fujiwara T, Kurihara H, Suginaka H, Oswald E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun. 1998;66:5008–5019. doi: 10.1128/iai.66.10.5008-5019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzagoloff A, Penefsky H S. Extraction and purification of lipoprotein complexes from membranes. Methods Enzymol. 1971;20:219–229. [Google Scholar]
- 38.Wang Y, Taylor D E. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–955. doi: 10.1128/jb.172.2.949-955.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassenaar T M. Toxin production by Campylobacter. Clin Microbiol Rev. 1997;10:466–476. doi: 10.1128/cmr.10.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- 42.Yao R, Burr D H, Guerry P. CheY mediated modulation of Campylobacter jejuni virulence. Mol Microbiol. 1997;23:1021–1032. doi: 10.1046/j.1365-2958.1997.2861650.x. [DOI] [PubMed] [Google Scholar]
- 43.Young V B, Knox K A, Schauer D B. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun. 2000;68:184–191. doi: 10.1128/iai.68.1.184-191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]