Abstract
Simple Summary
One of the traits considered in livestock production is the gain of muscle mass, which is one of the factors responsible for governing the growth of skeletal muscles. Muscle hypertrophy involves the proliferation and differentiation of muscle cells and the maturation of muscle fibers. Advances in animal genetics and breeding rely on identifying hub genes, understanding ontological features, and identifying pathways of gene activity. Therefore, the aim of this study was to identify the hub genes and mechanisms involved in skeletal muscle maturation and hypertrophy in livestock species (Bos taurus, Ovis aries, and Sus scrofa). The hub genes identified in this study can be used to better identify and improve the growth and maturation of skeletal muscles and, as a result, enhance meat quality characteristics for breeding purposes in the mentioned species.
Abstract
The aim of the current study was to identify the major genes and pathways involved in the process of hypertrophy and skeletal muscle maturation that is common for Bos taurus, Ovis aries, and Sus scrofa species. Gene expression profiles related to Bos taurus, Ovis aries, and Sus scrofa muscle, with accession numbers GSE44030, GSE23563, and GSE38518, respectively, were downloaded from the GEO database. Differentially expressed genes (DEGs) were screened out using the Limma package of R software. Genes with Fold Change > 2 and an adjusted p-value < 0.05 were identified as significantly different between two treatments in each species. Subsequently, gene ontology and pathway enrichment analyses were performed. Moreover, hub genes were detected by creating a protein–protein interaction network (PPI). The results of the analysis in Bos taurus showed that in the period of 280 dpc–3-months old, a total of 1839 genes showed a significant difference. In Ovis aries, however, during the period of 135dpc–2-months old, a total of 486 genes were significantly different. Additionally, in the 91 dpc–adult period, a total of 2949 genes were significantly different in Sus scrofa. The results of the KEGG pathway enrichment analysis and GO function annotation in each species separately revealed that in Bos taurus, DEGs were mainly enriched through skeletal muscle fiber development and skeletal muscle contraction, and the positive regulation of fibroblast proliferation, positive regulation of skeletal muscle fiber development, PPAR signaling pathway, and HIF-1 signaling pathway. In Ovis aries, DEGs were mainly enriched through regulating cell growth, skeletal muscle fiber development, the positive regulation of fibroblast proliferation, skeletal muscle cell differentiation, and the PI3K-Akt signaling, HIF-1 signaling, and Rap1 signaling pathways. In Sus scrofa, DEGs were mainly enriched through regulating striated muscle tissue development, the negative regulation of fibroblast proliferation and myoblast differentiation, and the HIF-1 signaling, AMPK signaling, and PI3K-Akt signaling pathways. Using a Venn diagram, 36 common DEGs were identified between Bos taurus, Ovis aries, and Sus scrofa. A biological pathways analysis of 36 common DEGs in Bos taurus, Ovis aries, and Sus scrofa allowed for the identification of common pathways/biological processes, such as myoblast differentiation, the regulation of muscle cell differentiation, and positive regulation of skeletal muscle fiber development, that orchestrated the development and maturation of skeletal muscle. As a result, hub genes were identified, including PPARGC1A, MYOD1, EPAS1, IGF2, CXCR4, and APOA1, in all examined species. This study provided a better understanding of the relationships between genes and their biological pathways in the skeletal muscle maturation process.
Keywords: hypertrophy, gene expression, gene ontology, muscle development
1. Introduction
Muscles make up meat, one of the most desirable animal products, and meat represents a major source of animal protein in the human diet (both skeletal muscle and visceral meat). Muscle meat is mainly composed of skeletal muscle fibers and various amounts of connective and adipose tissue, as well as small amounts of epithelial and nervous tissue, which play a role in its quantitative and qualitative properties [1]. The fundamental mechanism underlying livestock growth is the accumulation of muscle and adipose tissue [2]. Being the largest tissue in the body, skeletal muscle represents one of the most important complexes of biologically active tissues in mammals [3]. Elucidating the mechanism(s) of muscle growth and the intrinsic properties of the muscle may provide information that allows improvements in the quality of meat in production [4]. Therefore, it is necessary to know the molecular background that regulates meat growth and metabolism.
Skeletal muscle development is mainly regulated by the function of muscle precursor cells—myoblasts [5]—and is a complex process that can be divided into prenatal and postnatal stages. Livestock muscle is developed in two main stages: the prenatal phase—proliferation (hyperplasia—an increase in number of cells) as well as myotube formation, and the postnatal phase—muscle growth by hypertrophy (an increase in the size of muscle fibers) [6]. Skeletal muscle matures during late gestation at approximately 105 days in sheep [7], 210 days in cattle [8], and 114 days in pigs [4].
As mentioned above, muscle development is characterized by hyperplasia and hypertrophy. Hypertrophy is an increase in the size of skeletal muscle as a result of an increase in the diameter and length of already-formed fibers. Satellite cells proliferation (population of former myoblasts that did not fuse into myotubes) is the main cause of hyperplasia and skeletal muscle regeneration during postnatal life. Skeletal muscle stem cells reside in the spaces between muscle fibers’ sarcolemma and their basal lamina. The satellite cells are able to self-renew, proliferate, and fuse with existing muscle fibers as a prerequisite of muscle hypertrophy. All above-mentioned stages of myogenesis are key periods for skeletal muscle growth and development, and they directly affect muscle growth potential in meat production [9].
Analysis of transcriptomic data is a tool of great importance that allows identification of the key differentially expressed genes (DEGs) at each time point of the fetal and postnatal muscle development and to investigate the associated regulatory factors [3]. A complex gene network regulates the quality of meat and carcass [10]. Therefore, the identification of relationships between genes and carcass quality traits is critical to understanding animal development directly influencing meat composition. Additionally, identifying the underlying mechanisms and pathways determining the quantity and quality of muscle and marbling in carcasses is critical to better address demands of customers for prime quality meat products [1].
The aim of the current study was to identify the key genes and pathways involved in the maturation and hypertrophy of the skeletal muscle of Bos taurus, Ovis aries, and Sus scrofa species using comprehensive bioinformatics analysis.
2. Materials and Methods
2.1. Preparation of Data
The Gene Expression Omnibus (GEO) database was used as a source of expression profile collection. Three gene expression profiles, including GSE44030, GSE23563, and GSE38518, derived from Bos taurus, Ovis aries, and Sus scrofa, respectively, were downloaded from the GEO database. The dataset GSE44030 contained 20 biological samples of Bos taurus muscle with expression data from 60 days postconception (dpc) up to 3 months of age. The dataset GSE23563 contained 40 biological samples of Ovis aries skeletal muscle (70-, 85-, 100-, 120-, 135-days postconception, birth, and 1 month, 2 months of age). The dataset GSE38518 contained 24 biological samples of skeletal muscle from Sus scrofa at different developmental stages (35, 63, 91 dpc, and adult samples). Raw data of 280 dpc and 3 months in Bos taurus, 135 dpc and 2 months in Ovis aries, and 91 dpc and adult state in Sus scrofa were taken into consideration during a targeted analysis to investigate biomarkers and biological pathways of skeletal muscle maturation and hypertrophy stage (Table 1).
Table 1.
Accession numbers of Bos taurus, Ovis aries, and Sus scrofa skeletal muscle tissue expression profiles.
| Species | Experimental Group | Accession Numbers |
|---|---|---|
| Bos taurus | 280 dpc |
GSM1077020 GSM1077030 |
| 3 month |
GSM1077021 GSM1077031 |
|
| Ovis aries | 135 dpc |
GSM578053 GSM578054 GSM578055 |
| 2 month |
GSM578050 GSM578051 GSM578052 |
|
| Sus scrofa | 91 dpc |
GSM944491 GSM944492 GSM944493 |
| adult |
GSM944494 GSM944495 GSM944496 |
2.2. Differentially Expressed Genes
The interactive web tool GEO2R was applied to identify DEGs between the samples. (http://www.ncbi.nlm.nih.gov/geo/geo2r/) (accessed on 1 January 2013) [11]. GEO2R is an effective online tool mostly used to compare two sets of samples in most GEO series to identify genes with differential expression under the same experimental conditions. The Limma package was used for differential expression analysis in this web tool. Criteria for DEGs identification were Fold Change > 2 and adjusted p-value ≤ 0.05. Common genes between three species were identified using a Venn diagram.
2.3. Biological Processes and KEGG Pathways Analysis
An overrepresentation enrichment analysis (ORA) was performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) version 2021 (https://david.ncifcrf.gov/) (accessed on 1 December 2021) [12]. The DAVID database was utilized to conduct gene ontology (GO) analysis on biological processes, molecular functions, and cellular components, as well as pathway enrichment analysis using the Kyoto Encyclopedia of Genes and Genomes (KEGG). Gene ontology terms and KEGG pathways of the co-expressed DEGs were identified using the above online tool with a false discovery rate (FDR) of 5% to gain a better understanding of the biological mechanisms involved in skeletal muscle maturation and hypertrophy.
2.4. Network Analysis
The Search Tool for the Retrieval of Interacting Genes (STRING) version 11.5 (https://string-db.org) (accessed on 12 August 2021) was used to explore protein–protein interaction (PPI) networks and potential DEG interactions [13]. The protein–protein interaction networks of DEGs were derived from validated experiments [14]. A combined interaction score of >0.4 was considered significant. The PPI networks were visualized using Cytoscape software (http://www.cytoscape.org) (accessed on 12 August 2021) [15]. The p-value ≤ 0.05 was considered to indicate a statistically significant difference. The topology scores of the nodes in the PPI network were estimated based on closeness centrality, betweenness centrality, and degree centrality.
3. Results
3.1. Analysis of Differential Gene Expression
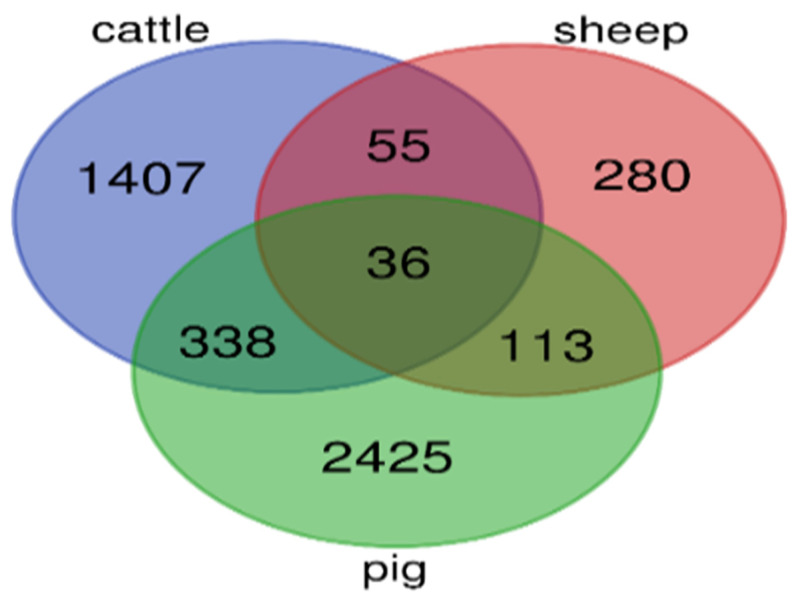
The analysis revealed that a total of 1839 genes were significantly different between the 280 dpc and 3-month stages in Bos taurus, of which 1107 genes were upregulated and 732 genes were downregulated. In Ovis aries, a total of 486 DEGs were identified between the stage of 135 dpc and 2 months, of which 240 genes were upregulated and 246 genes were downregulated. In Sus scrofa, a total of 2949 genes were significantly different between the stage of 91 dpc and adult, of which 1383 genes were upregulated and 1566 genes were downregulated. Common genes between three species were identified using a Venn diagram (Figure 1). The names of these genes identified between the three species are shown in the Supplementary Materials. Among them, 36 genes were common between Bos taurus, Ovis aries, and Sus scrofa muscle (Table 2).
Figure 1.
Number of common genes identified between three species using Venn diagram.
Table 2.
Common genes among Bos taurus, Ovis aries, and Sus scrofa.
| Genes |
|---|
| CXCR4, DTNBP1, GCLM, THRB, PPARGC1A, FST, LXN, TYMS, RPL9, HBB, ARG2, JUP, UCP3, COL1A2, PHKB, ISG15, DGAT2, APOA1, PYGM, PLIN2, MYOD1, NAMPT, POSTN, EPAS1, ME1, CKAP4, MID1IP1, JAG1, SDC4, ILF3, AIMP2, EEF1A1, IGF2, MB, DDIT3, BCAT1 |
3.2. Biological Processes and KEGG Pathways Analysis
DEGs were used as the inputs of the online software of our DAVID pathway analyses. DAVID is helpful in explaining the genome-scale datasets by translating the data collection into biological meanings. First, different expression genes for each animal were analyzed separately. The results of the GO analysis in Bos taurus revealed that DEGs were significantly enriched, with 68 significant biological processes (p-value ≤ 0.05). The most important biological processes in which DEGs were related to the maturation and hypertrophy of skeletal muscle development are listed in Table 3. Among the more significant biological processes, the processes connected with the myogenesis of Bos taurus were skeletal muscle fiber development and skeletal muscle contraction.
Table 3.
Biological processes associated with skeletal-muscle-maturation- and hypertrophy-related DEGs in Bos taurus.
| Category | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| BP | GO:0030324 | Lung development | 0.005712 | 13 |
| BP | GO:0030517 | Negative regulation of axon extension | 0.008625 | 5 |
| BP | GO:0048741 | Skeletal muscle fiber development | 0.011764 | 7 |
| BP | GO:0003009 | Skeletal muscle contraction | 0.023003 | 6 |
| BP | GO:0048146 | Positive regulation of fibroblast proliferation | 0.02471 | 9 |
| BP | GO:0048743 | Positive regulation of skeletal muscle fiber development | 0.02639 | 4 |
| BP | GO:0045662 | Negative regulation of myoblast differentiation | 0.029267 | 6 |
| BP | GO:0045663 | Positive regulation of myoblast differentiation | 0.029267 | 6 |
| BP | GO:0007179 | Transforming growth factor-beta receptor signaling pathway | 0.033284 | 11 |
DEGs—differentially expressed genes; BP—biological process; p-value ≤ 0.05.
For Ovis aries, the results of our enrichment analysis indicated 85 significant biological processes (p-value ≤ 0.05) associated to DEGs. The most important processes related to the final steps of skeletal muscle development—maturation and fiber hypertrophy—are shown in Table 4. Among them, the highest significance was assigned to the regulation of cell proliferation and growth.
Table 4.
Biological processes associated with skeletal-muscle-maturation- and hypertrophy-related DEGs in Ovis aries.
| Category | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| BP | GO:0042127 | Regulation of cell proliferation | 0.001109 | 9 |
| BP | GO:0030307 | Positive regulation of cell growth | 0.002954 | 7 |
| BP | GO:0042104 | Positive regulation of activated T cell proliferation | 0.002995 | 5 |
| BP | GO:0046697 | Decidualization | 0.003547 | 4 |
| BP | GO:0033690 | Positive regulation of osteoblast proliferation | 0.029267 | 4 |
| BP | GO:0001938 | Positive regulation of endothelial cell proliferation | 0.009497 | 6 |
| BP | GO:0048662 | Negative regulation of smooth muscle cell proliferation | 0.009759 | 4 |
| BP | GO:0001558 | Regulation of cell growth | 0.012546 | 5 |
| BP | GO:0048741 | Skeletal muscle fiber development | 0.014303 | 4 |
| BP | GO:0043568 | Positive regulation of insulin-like growth factor receptor signaling pathway | 0.020239 | 3 |
| BP | GO:0048146 | Positive regulation of fibroblast proliferation | 0.022068 | 5 |
| BP | GO:0042102 | Positive regulation of T cell proliferation | 0.032177 | 5 |
| BP | GO:0035914 | Skeletal muscle cell differentiation | 0.048064 | 5 |
DEGs—differentially expressed genes; BP—biological process; p-value ≤ 0.05.
Genes with different expressions in Sus scrofa skeletal muscle were analyzed for functional enrichment. The results revealed that these genes were linked to 106 significant biological processes (p-value ≤ 0.05), of which the most important in light of muscle maturation and hypertrophy—meat quality traits—are presented in Table 5. In this analysis, the most important biological processes are linked with cell proliferation, muscle tissue maturation, and cell cycle regulation.
Table 5.
Biological processes associated with skeletal-muscle-maturation- and hypertrophy-related DEGs in Sus scrofa.
| Category | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| BP | GO:0008284 | Positive regulation of cell proliferation | 0.003942 | 47 |
| BP | GO:0007507 | Heart development | 0.007053 | 26 |
| BP | GO:0048469 | Cell maturation | 0.009036 | 10 |
| BP | GO:0000082 | G1/S transition of mitotic cell cycle | 0.009406 | 12 |
| BP | GO:0016202 | Regulation of striated muscle tissue development | 0.016587 | 4 |
| BP | GO:0048147 | Negative regulation of fibroblast proliferation | 0.019795 | 7 |
| BP | GO:0045662 | Negative regulation of myoblast differentiation | 0.021486 | 8 |
| BP | GO:0030308 | Negative regulation of cell growth | 0.022828 | 20 |
| BP | GO:0008286 | Insulin receptor signaling pathway | 0.032387 | 11 |
| BP | GO:0001822 | Kidney development | 0.042236 | 11 |
| BP | GO:0009791 | Postembryonic development | 0.042236 | 14 |
| BP | GO:0043588 | Skin development | 0.049737 | 8 |
DEGs—differentially expressed genes; BP—biological process; p-value ≤ 0.05.
Then, among the genes with different expressions, 36 common genes in Bos taurus, Ovis aries, and Sus scrofa were identified. Further analysis revealed that these genes were linked to 325 biological processes (p-value ≤ 0.05). The most significant biological processes are listed in Table 6, along with the genes assigned to them. The most significant skeletal muscle maturation and hypertrophy processes are: adipose tissue development, myoblast differentiation, and the regulation of muscle cell differentiation.
Table 6.
Biological processes associated with skeletal-muscle-maturation- and hypertrophy-related DEGs common for Bos taurus, Ovis aries, and Sus scrofa.
| Category | Description | Genes | p-Value |
|---|---|---|---|
| BP | Adipose tissue development | DGAT2, NAMPT | 2.06 × 10−4 |
| BP | Myoblast differentiation | JAG1, EPAS1 | 3.26 × 10−4 |
| BP | Regulation of muscle cell differentiation | MYOD1, IGF2 | 0.001805571 |
| BP | Positive regulation of multicellular organismal process | EPAS1, FST, CXCR4, PPARGC1A | 0.003315863 |
| BP | Insulin receptor signaling pathway | NAMPT, IGF2 | 0.007640329 |
| BP | Skin development | JAG1, COL1A2 | 0.009114935 |
| BP | Positive regulation of skeletal muscle fiber development | MYOD1 | 0.010752747 |
| BP | Regulation of skeletal muscle fiber development | MYOD1 | 0.014311969 |
| BP | Negative regulation of muscle cell differentiation | IGF2 | 0.014311969 |
| BP | Positive regulation of myoblast fusion | MYOD1 | 0.017858741 |
| BP | Positive regulation of skeletal muscle tissue development | MYOD1 | 0.017858741 |
| BP | Skeletal muscle cell differentiation | MYOD1 | 0.019627472 |
| BP | Myotube cell development | MYOD1 | 0.019627472 |
| BP | Regulation of myoblast fusion | MYOD1 | 0.021393106 |
| BP | Cardiac cell development | JAG1 | 0.023155649 |
| BP | Positive regulation of insulin receptor signaling pathway | IGF2 | 0.023155649 |
| BP | Aorta development | JAG1 | 0.02491506 |
| BP | Pulmonary valve development | JAG1 | 0.031922176 |
| BP | Positive regulation of myoblast differentiation | MYOD1 | 0.031922176 |
| BP | Myotube differentiation | MYOD1 | 0.03714533 |
| BP | Regulation of cell population proliferation | JAG1, JUP, NAMPT, IGF2 | 0.047332288 |
| BP | Positive regulation of muscle cell differentiation | MYOD1 | 0.047509573 |
| BP | Cellular response to epidermal growth factor stimulus | EEF1A1 | 0.049226369 |
DEGs—differentially expressed genes; BP—biological process; p-value ≤ 0.05.
The results of the KEGG pathway analysis in Bos taurus showed that DEGs were significantly enriched, with 22 pathways (p-value ≤ 0.05). The most significant pathways are listed in Table 7.
Table 7.
KEGG pathway analysis of the maturation- and hypertrophy-related DEGs and proportional p-values identified in Bos taurus.
| Databases | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| KEGG pathway | bta03008 | Ribosome biogenesis in eukaryotes | 2.35 × 10−7 | 25 |
| KEGG pathway | bta03320 | PPAR signaling pathway | 0.002589 | 16 |
| KEGG pathway | bta04066 | HIF-1 signaling pathway | 0.004774 | 19 |
Genes with different expression in Ovis aries skeletal muscle were analyzed for the KEGG pathway. The most important pathways are presented in Table 8.
Table 8.
KEGG pathway analysis of the maturation- and hypertrophy-related DEGs and proportional p-values identified in Ovis aries.
| Databases | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| KEGG pathway | oas04512 | ECM–receptor interaction | 4.35 × 10−6 | 15 |
| KEGG pathway | oas04151 | PI3K-Akt signaling pathway | 8.79 × 10−5 | 29 |
| KEGG pathway | oas04510 | Focal adhesion | 9.02 × 10−5 | 21 |
| KEGG pathway | oas04660 | T-cell receptor signaling pathway | 0.001049 | 12 |
| KEGG pathway | oas04066 | HIF-1 signaling pathway | 0.010325 | 10 |
| KEGG pathway | oas04015 | Rap1 signaling pathway | 0.020892 | 15 |
For Sus scrofa, the results of the pathway indicated 53 significant pathways (p-value ≤ 0.05) associated to DEGs. The most significant pathways are listed in Table 9.
Table 9.
KEGG pathway analysis of the maturation- and hypertrophy-related DEGs and proportional p-values identified in Sus scrofa.
| Databases | Gene Set | Description | p-Value | Count |
|---|---|---|---|---|
| KEGG pathway | ssc04910 | Insulin signaling pathway | 8.92 × 10−5 | 40 |
| KEGG pathway | ssc04066 | HIF-1 signaling pathway | 1.11 × 10−4 | 32 |
| KEGG pathway | ssc05205 | Proteoglycans in cancer | 1.59 × 10−4 | 52 |
| KEGG pathway | ssc04510 | Focal adhesion | 2.42 × 10−4 | 52 |
| KEGG pathway | ssc04068 | FoxO signaling pathway | 0.0012 | 37 |
| KEGG pathway | ssc04152 | AMPK signaling pathway: | 0.001827 | 33 |
| KEGG pathway | ssc04919 | Thyroid hormone signaling pathway | 0.002029 | 32 |
| KEGG pathway | ssc04512 | ECM–receptor interaction | 0.00208 | 25 |
| KEGG pathway | ssc05410 | Hypertrophic cardiomyopathy (HCM) | 0.01255 | 22 |
| KEGG pathway | ssc04151 | PI3K-Akt signaling pathway | 0.026508 | 68 |
Then, KEGG pathway analysis was performed between the three species. The list of the most significant pathways are listed in Table 10.
Table 10.
KEGG pathways common for Bos taurus, Ovis aries, and Sus scrofa.
| Databases | Description | Gene | p-Value |
|---|---|---|---|
| KEGG pathway | PPAR signaling pathway | ME1, APOA1, PLIN2 | 3.18 × 10−4 |
| KEGG pathway | Insulin signaling pathway | PYGM, PHKB, PPARGC1A | 0.001903 |
| KEGG pathway | ECM–receptor interaction | COL1A2, SDC4 | 0.00109943 |
| KEGG pathway | Insulin resistance | PYGM, PPARGC1A | 0.016151 |
3.3. PPI Network Construction and Hub Gene Identification
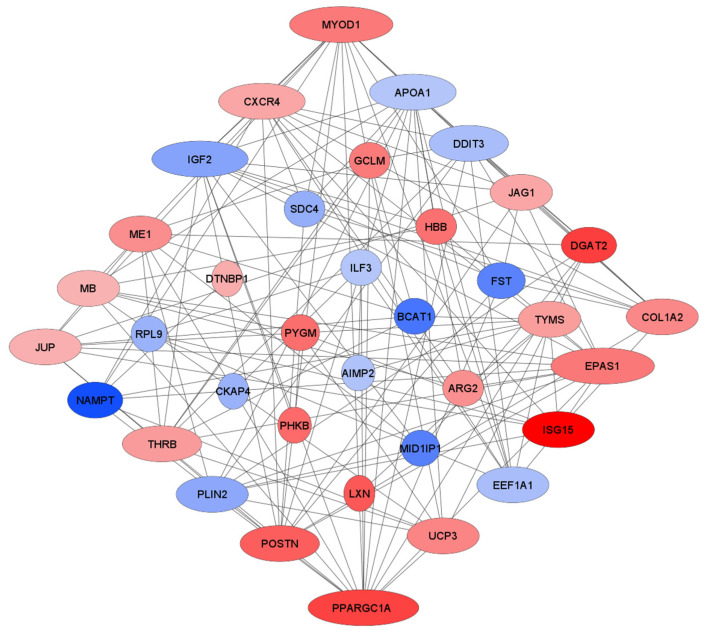
The PPI network was created using the 36 common DEGs by String database and Cytoscape software. Not-connected and partially connected proteins were omitted (Figure 2). Six key hub genes, including PPARGC1A, MYOD1, EPAS1, IGF2, CXCR4, and APOA1, were reported in Bos taurus, Ovis aries, and Sus scrofa after the network was analyzed based on degree. Among them, the most important was the peroxisome proliferator-activated receptor gamma coactivator 1-alpha, which manifested the highest closeness centrality, betweenness centrality, and degree centrality (Table 11).
Figure 2.
PPI network of differentially expressed genes of skeletal muscle in Bos taurus, Ovis aries, and Sus scrofa. DEGs—differentially expressed genes; Blue—downregulated DEGs; Red—upregulated DEGs. The node size is proportional to the degree bigger than 12. The bigger size of the node means a higher engagement of protein in the developmental process of skeletal muscle in Bos taurus, Ovis aries, and Sus scrofa.
Table 11.
Top six hub genes ranked by degree method in Bos taurus, Ovis aries, and Sus scrofa.
| Betweenness | Degree | Gene Name | Gene | |
|---|---|---|---|---|
| 0.625 | 174.5703 | 15 | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha | PPARGC1A |
| 0.59322 | 76.05031 | 14 | Myoblast determination protein 1 | MYOD1 |
| 0.59322 | 73.11194 | 14 | Endothelial PAS domain protein 1 | EPAS1 |
| 0.546875 | 99.93942 | 13 | Insulin-like growth factor 2 | IGF2 |
| 0.555556 | 49.89188 | 12 | C-X-C chemokine receptor type 4 | CXCR4 |
| 0.564516 | 65.64227 | 12 | Apolipoprotein AI | APOA1 |
4. Discussion
Skeletal muscle development represents a complex process, which involves the alteration of the growth of muscle cells, as well as the differentiation and maturation of muscle fiber, including hypertrophy—an increase in the size of muscle fibers [16]. Muscle hyperplasia (an increase in number of cells) and hypertrophy (muscle fiber growth in terms of length and diameter) both depend on the proliferation of myoblasts [17]. The contribution of hyperplasia to muscle growth is limited to prenatal or a limited time after birth, while muscle growth depends more on hypertrophy [18]. The present study was conducted to identify the key genes and mechanisms involved in the maturation and hypertrophy of skeletal muscle common for livestock species, namely Bos taurus, Ovis aries, and Sus scrofa. The results of the analysis showed a total of 1839, 486, and 2949 differentially expressed genes in a comparison of pre- and postnatal muscle in Bos taurus, Ovis aries, and Sus scrofa, respectively (Figure S1, Supplementary Materials). Using BP and pathway analysis identification can help in the understanding of the essential mechanisms involved in skeletal muscle development, especially muscle maturation. The results of the KEGG pathway enrichment analysis and GO function annotation in all examined species revealed that DEGs were mainly enriched in skeletal muscle fiber development, skeletal muscle contraction, the positive regulation of fibroblast proliferation and skeletal muscle fiber development processes, ribosome biogenesis in eukaryotes, and the PPAR and HIF-1 signaling pathways in Bos taurus (Table 3 and Table 7); the regulation of cell growth, skeletal muscle fiber development, the positive regulation of fibroblast proliferation and skeletal muscle cell differentiation processes and ECM receptor interactions, and the PI3K-Akt, HIF-1, Rap1 signaling pathways in Ovis aries (Table 4 and Table 8); and the regulation of striated muscle tissue development, the negative regulation of fibroblast proliferation and myoblast differentiation processes and insulin signaling, the HIF-1, FoxO, AMPK signaling pathways, and thyroid hormone signaling pathways in Sus scrofa (Table 5 and Table 9).
Common genes for the skeletal muscle development process were identified by differential expression between the three species. In this research, we identified 36 genes responsible for the maturation and hypertrophy of skeletal muscle in Bos taurus, Ovis aries, and Sus scrofa (Figure 1, Table 2). Finally, the results of the GO and KEGG analyses revealed that common genes were mainly enriched in the biological processes and signaling pathways, of which the most important are myoblast differentiation; the differentiation of muscle cells; the positive regulation of skeletal muscle fiber development processes and PPAR; insulin; the ECM–receptor interaction; and insulin resistance (Table 6 and Table 10).
In this investigation, we used some bioinformatics tools to identify genes among of the common genes that play a key role in skeletal muscle maturation. Using the CytoNCA plugin for network analysis led us to finding some hub genes with significant expression changes that can be identified as biomarkers. Network analysis showed that six genes (PPARGC1A, MYOD1, EPAS1, IGF2, CXCR4, and APOA1) have a high degree of centrality, and they are referred to as hub genes because they are crucial for development in each species (Figure 2 and Table 11).
4.1. Myogenesis-Related Processes
The involvement of DEGs in the aforementioned processes and pathways seems to be a prerequisite of proper myogenesis, finally leading to muscle fiber maturation and hypertrophy. The top three biological processes associated with skeletal muscle maturation and hypertrophy are shown in Table 6.
Myoblast differentiation, including its regulation, is governed by multiple factors that order this multistep process to withdraw from the cell cycle and induce myoblast differentiation and fusion into multinucleated myotubes, which finally gives rise to muscle fibers [19]. Myogenic regulatory factors, such as myogenic differentiation 1 (MYOD1), are restrictedly expressed prenatally in somite-derived myogenic progenitor cells and their derived myoblasts, and are important in postnatal satellite cells regulation [20]. The development of muscle fibers occurs when myotubes mature, extend, and merge with other myoblasts and satellite cells [21]. The regulation of this phenomenon is an ordered multistep process that leads to skeletal muscle fiber growth [19], which is crucial for further muscle fiber maturation and hypertrophy. In our research, common genes related to the myoblast differentiation process were identified and are shown in Table 6. Among them, JAG1, EPAS1, MYOD1, and IGF2 are also mentioned as hub genes crucial for myogenesis that are common for all three examined species (Figure 2, Table 6 and Table 11).
During myogenesis, the adipose tissue development begins, followed by an accumulation of fat in myofibers and between muscle bundles, which increases with age [22] Myoblasts and intramuscular adipocytes are derived from mesenchymal stem cells, which are able to differentiate into myogenic and non-myogenic cell lines. In cattle, adipogenic progenitor cells begin to differentiate into preadipocytes at ~180 days of gestation [23]. It is also known that skeletal muscle satellite cells can acquire the features of adipocytes (dysdifferentiation), express adipocyte-specific genes, and accumulate lipids [24]. This suggests that muscle cells and adipocytes interplay during growth and that such early events influence skeletal muscle adipogenesis, influencing intramuscular fat content and muscle structure [25]. In the present analysis, two adipogenesis-related genes were found as common genes, namely DGAT2 and NAMPT (Table 6). The common activity of all aforementioned genes in Bos taurus, Ovis aries, and Sus scrofa muscle may indicate their key involvement in its maturation, including myofibers maturation, hypertrophy, and the gradual increase in intramuscular and intermuscular fat.
4.2. Common KEGG Pathways
As mentioned above, four KEGG pathways are common for the skeletal muscle development of Bos taurus, Ovis aries, and Sus scrofa, with PPAR and insulin signaling and insulin resistance pathways shown as the most statistically significant. Peroxisome proliferator-activated receptors (PPARs) are a class of nuclear receptors that play important roles in development and energy metabolism, the regulation of satellite cell proliferation, skeletal muscle regeneration, and the increase in muscle fiber type (oxidative or glycolytic) [26]. The PPARs have major impacts on muscle homeostasis, with PPARγ directly implicated in lipid deposition in muscle [27]. PPARs can also affect insulin signaling by multiple mechanisms. The insulin signaling pathway is responsible for glucose and lipid homeostasis, as well as proliferation and differentiation and protein synthesis/degradation, thus regulating muscle growth and hypertrophy. It is well known that insulin has an anabolic effect on growing skeletal muscle [28,29,30,31]. Moreover, PPAR-γ can also enhance insulin resistance by decreasing the production of proinflammatory mediators [32]. Numerous studies have confirmed that insulin resistance accompanied by mitochondrial dysfunction might suppress protein synthesis to induce the loss of skeletal muscle mass, and that it is also involved in regulationing myogenic differentiation [33].
In our analysis, the PPAR signaling pathway was represented by three common genes, namely ME1, APOA1, and PLIN2 (Table 10). The insulin signaling pathway and insulin resistance were also the pathways indicated as common for Bos taurus, Ovis aries, and Sus scrofa, with the activity of PYGM, PHKB, and PPARGC1A genes similar for all species (Table 10). The interplay of the above-mentioned genes belonging to both pathways seems to be vital for the muscle development of Bos taurus, Ovis aries, and Sus scrofa.
4.3. Hub Genes
The network analysis performed on the batch of genes common for three livestock species revealed six hub genes governing the maturation and hypertrophy of skeletal muscle: PPARGC1A, MYOD1, EPAS1, IGF2, CXCR4, and APOA1 (Figure 2, Table 11).
The first of them, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A), was identified as the hub gene with the highest degree of connectivity. PGC-1a (aka PPARGC1A) was reported to be a necessary control factor in skeletal muscle development, adaptation to exercise, the transcriptional control of genes responsible for angiogenesis, fatty acid oxidation, oxidative phosphorylation, mitochondrial biogenesis, and muscle fiber type composition and transition to a slow-twitch muscle type [34,35,36]. PPARGC1A is necessary for the proper myogenic differentiation of C2C12 cells, acting as a mediator of mitochondria biogenesis in the emerging myotubes [37]. Moreover, a decrease in PPARGC1A expression is accompanied by reactive species generation and mitochondrial damage, which finally results in inefficient differentiation [38]. It also appears to play a protective role against atrophy-linked skeletal muscle deterioration [39]. According to our findings, this gene is involved in the positive regulation of multicellular organismal processes, insulin signaling and the insulin resistance pathway (Table 10), and the processes governing the balance between cell division and differentiation, which determine organ size in multicellular organisms and also influence the balance between skeletal muscle hypertrophy and atrophy [40].
One of the muscle regulatory factors and also a hub gene (Table 11), MYOD1 is expressed in developing skeletal muscle [20]. The expression of the MYOD1 gene, a major transcriptional regulator of myogenesis, is detectable in proliferating myoblasts, activated satellite cells, and myocytes [41]. It also promotes the transcription of p21 and myogenin, which allows cells to exit the cell cycle and stop the proliferation of differentiated myocytes [42,43]. MYOD1 activity with MYF5 is required for skeletal muscle growth, hypertrophy, and regeneration, which are processes strongly dependent on the activation of satellite cells. The regulation of muscle cell differentiation is the key determinant of the frequency, rate, and extent of skeletal muscle development. It is well known and also confirmed in our analysis that MYOD1, one of the other high-grade hub genes, is related to the regulation of muscle cell differentiation, skeletal muscle fiber development, myoblast fusion, myotube cell development, and other processes mentioned in Table 6 and Table 10.
The next identified hub gene is endothelial PAS domain protein 1 (EPAS 1; known as hypoxia inducible factor 2A (HIF2A) (Table 11). HIF2A is a protein that, in an HIF complex, plays an important role in the ability of tissue to adapt to changing oxygen levels [44]. It also regulates the insulin signaling pathway [45], which, as mentioned earlier, has a significant role in metabolism, growth, reproduction, and aging. Our results showed that HIF2A is related to the myoblast differentiation and positive regulation of multicellular organismal processes. The available literature data indicates that HIF2A induces the quiescence and self-renewal of satellite cells, which are hypoxic in the niche, and blocks their myogenic differentiation [46]; however, some studies indicate that HIF2A is preferentially expressed in postdifferentiation myoblasts. Its pharmacological inhibition accelerates muscle regeneration by increasing satellite cells proliferation and differentiation [46,47]. However, some authors observed that the deficiency of HIF1A and HIF2A delayed muscle regeneration by reducing the number of satellite cells [47,48]. HIF2A stimulates the expression of genes encoding antioxidant enzymes, suppressing aberrant ROS accumulation, and its deficiency leads to severely striated muscle damage [44]. It may also modulate glucose metabolism, probably indirectly by PPAR [49]. HIF2A influences skeletal muscle myofiber types and metabolic capacity, encodes an oxidative slow-twitch muscle program in the skeletal muscle, and controls the glycolytic and oxidative metabolism of skeletal muscle fibers together with HIF1A protein [50]. Moreover, HIF2A activation in the skeletal muscle can induce angiogenesis [51]. HIF2A appears to be a potent regulator of processes vital for skeletal muscle development by playing a significant role in controlling, directly or indirectly, processes of proliferation and differentiation, the self-renewal of satellite cells, ROS control, glucose metabolism, and vascularization.
Insulin-like growth factor 2 (IGF2) is a hub gene (Table 11). According to our findings, the gene has been associated to the regulation of muscle cell differentiation, positive regulation of the insulin receptor signaling pathway, and regulation of cell population proliferation (Table 6 and Table 10). IGF2 was previously discovered to regulate the postnatal growth of skeletal muscle and internal organs [52]. IGF2 is a myogenesis regulator and autocrine factor that initiates myoblast differentiation in vitro and supports muscle fiber formation—the fusion of mononucleotide precursor cells into multinucleated mature cells [53,54,55]. IGF2 and MYOD1 are co-regulated during myogenesis, which affects the progression of myogenic differentiation [56]. The anabolic actions of IGF1 and IGF2 on skeletal muscle are closely related and are mediated by the same receptor, namely the type 1 IGF receptor (IGF1R; [57]). IGF2 released by differentiating myoblasts can enhance the transcriptional properties of MYOD1 [58]. Zhu et al. [59] demonstrated that IGF2 deficiency in myotubes leads to impaired mitochondria function, manifested by a reduced content of mitochondrial protein, an imbalance of fission/fusion, and impaired biogenesis. The authors proposed that mitochondrial defects may occur through the IGF2-SIRT1-PGC1α(PPARGC1A) pathway [59].
The penultimate hub gene identified as a common gene for Bos taurus, Ovis aries, and Sus scrofa skeletal muscle maturation and hypertrophy is C-X-C chemokine receptor type 4 (CXCR4), a motility-stimulating chemokine receptor [60]. Both the chemokine SDF1 (also known as CXCL12) secreted by myofibers and its primary receptor CXCR4 promote developmental myogenesis as well as muscle regeneration. SDF1 attracts CXCR4-positive satellite cells, stimulating cell migration, promoting myoblast fusion with existing myofibers, and inducing angiogenesis in regenerating muscles [61,62]. Vasyutina et al. [63] indicated that CXCR4-dependent signals also control survival. The CXCR4/SDF1 axis is involved in cell migration, which is also essential for skeletal muscle repair. Furthermore, as indicated by Ref. [64], the overexpression of CXCR4/SDF1 can protect cachectic muscle from wasting by increasing the fiber area by 20%. Despite the well-established role of CXCR4/SDF1 in embryonic muscle development and muscle regeneration, the function of this pathway during adult myogenesis remains to be fully elucidated [65]. Two other hub genes, EPAS1/HIF2A and PPARGC1A, have already been mentioned as being involved in this process. The interactions of CXCR4, HIF2A, and PPAR are documented in the literature data, proving that the HIF2A transcription regulation of CXCR4 is involved in macrophage migration and chemotaxis [66], as well as the PPAR-related downregulation of CXCR4 gene expression in cancer cells [67].
The last hub gene identified in this study is apolipoprotein A1 (APOA1). APOA1 is the major apolipoprotein component of the high-density lipoprotein (HDL), which is involved in cholesterol transport, the regulation of lipid metabolism and transport, and mitochondria biogenesis and metabolism [68]. It is endogenously expressed by skeletal muscle cells [69]. In the absence of insulin, APOA1 increases glucose uptake into skeletal muscle cells and increase glucose consumption in skeletal muscles, resulting in improved glucose tolerance [70,71]. Finally, it was also proved that APOA1 increases glucose disposal in skeletal muscle, thus supporting a role for HDL in reducing insulin resistance [72]. Moreover, the results of Liu et al. [73] suggest that APOA1 may be useful as a molecular marker of intramuscular fat (IMF) deposition in chickens. Moreover, the APOA1 gene was proposed by Ref. [74] as one of the genes responsible for the IMF content in high marbling samples of longissimus muscle in cattle. In another IMF biomarkers identification study, APOA1 was clustered to a fat deposition group of genes, with the PPAR signaling and ECM–receptor interaction signaling pathways being shown as the most important [75], which corresponds well with the results obtained in our analysis (Table 6 and Table 10).
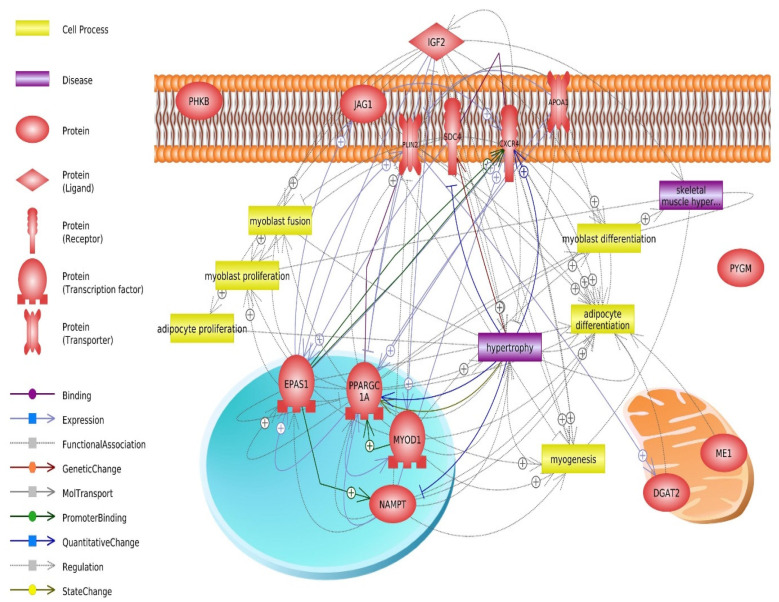
Muscle fiber hypertrophy produced by satellite cell activation, proliferation, differentiation, and fusion with existing fibers was the predominant contributor to postnatal muscle growth and meat quality [76]. With the use of previous findings, we have depicted the genes that play a pivotal role in the above-mentioned processes, namely the hub genes shared by the three ruminant species we studied. The aforementioned six hub genes can be used as biomarkers for breeding programs because of their relationship to the maturation and hypertrophy of skeletal muscle, as well as their role in the deposition of intramuscular fat. Their interaction can be the basis for directing the development of skeletal muscles in accordance with the declared demands of breeders or consumers. The summary of the relevance of the identified genes and related processes is presented in Figure 3.
Figure 3.
Pathway studio visualization of gene relevance network.
5. Conclusions
Our investigation of transcriptome profiles in the muscle tissues of Bos taurus, Ovis aries, and Sus scrofa showed that common DEGs up-regulated and down-regulated between the described species. Biological pathways were found to be directly related to muscle growth and hypertrophy. The progress of gene annotation studies will allow more precise knowledge representation, which in turn will generate more informative results from data analyses. The results may provide novel insights into targets that can be used for future investigations of underlying molecular mechanisms. Future research should focus on these important genes and pathways in order to definitively pinpoint advantageous biological targets for analyzing skeletal muscle maturation and hypertrophy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12243471/s1, Table S1. Name of common genes identified between 3 species using Venn diagram; Table S2. Name of common genes identified between Bos Taurus and Ovis aries using Venn diagram; Table S3. Name of common genes identified between Bos taurus and Sus scrofa using Venn diagram; Table S4. Name of common genes identified between Sus scrofa and Ovis aries using Venn diagram. Table S5. Genes ranked by degree method in cattle. Table S6. Genes ranked by degree method in sheep. Table S7. Genes ranked by degree method in pig. Figure S1. Differentially expressed genes with blue and red colors has been shown, blue showing low expression and red showing greater expression. The node size is proportional to the degree bigger than 12. The bigger size of the node means higher engagement of protein in the developmental process of skeletal muscle in cattle. Figure S2. Differentially expressed genes with blue and red colors has been shown, blue showing low expression and red showing greater expression. The bigger size of the node means higher engagement of protein in the developmental process of skeletal muscle in sheep. Figure S3. Differentially expressed genes with blue and red colors has been shown, blue showing low expression and red showing greater expression. The bigger size of the node means higher engagement of protein in the developmental process of skeletal muscle in pigs.
Author Contributions
Conceptualization, M.M. and T.S.; methodology, F.M. and Z.R.; software, T.S., F.M., and Z.R.; validation, M.M., T.S., F.M., and Z.R.; formal analysis, M.M., T.S., F.M. and Z.R.; investigation, M.M., T.S., and Z.R.; resources, M.M. and T.S.; data curation, M.M., T.S., and Z.R.; writing—original draft preparation, F.M.; writing—review and editing, M.M., T.S., and Z.R.; visualization, M.M., and T.S.; supervision, M.M. and Z.R.; project administration, M.M.; funding acquisition, M.M. and T.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This research was carried out on publicly available data deposited in the NCBI GEO database. Therefore, the approval of the ethics committee was not required.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Shahid Bahonar University of Kerman, the Vice Chancellor for Research and Technology (Grant number: G-311/8719).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Silva-Vignato B., Coutinho L.L., Cesar A.S.M., Poleti M.D., Regitano L.C.A., Balieiro J.C.C. Comparative muscle transcriptome associated with carcass traits of Nellore cattle. BMC Genom. 2017;18:506. doi: 10.1186/s12864-017-3897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes R.D.C., Silva S.D.L., Carvalho M.E., Rezende F.M.D., Pinto L.F.B., Santana M.H.D.A., Stella T.R., Meirelles F.V., Rossi Júnior P., Leme P.R., et al. Protein synthesis and degradation gene SNPs related to feed intake, feed efficiency, growth, and ultrasound carcass traits in Nellore cattle. Genet. Mol. Res. 2013;12:2923–2936. doi: 10.4238/2013.August.12.8. [DOI] [PubMed] [Google Scholar]
- 3.Shi T., Xinyue W., Zhida Z., Wenping H.U., Zhang L. Integrated analysis of miRNA-mediated ceRNA networks in ovine skeletal muscle development. Res. Sq. 2020 doi: 10.21203/rs.3.rs-18319/v1. [DOI] [Google Scholar]
- 4.Picard B., Berri C., Lefaucheur L., Molette C., Sayd T., Terlouw C. Skeletal muscle proteomics in livestock production. Brief. Funct. Genom. 2010;9:259–278. doi: 10.1093/bfgp/elq005. [DOI] [PubMed] [Google Scholar]
- 5.Shahjahan M. Skeletal muscle development in vertebrate animals. Asian J. Med. Biol. Res. 2015;1:139–148. doi: 10.3329/ajmbr.v1i2.25592. [DOI] [Google Scholar]
- 6.Sandoval C., Wu G., Smith S.B., Dunlap K.A., Satterfield M.C. Maternal nutrient restriction and skeletal muscle development: Consequences for postnatal health. Adv. Exp. Med. Biol. 2020;1265:153–165. doi: 10.1007/978-3-030-45328-2_9. [DOI] [PubMed] [Google Scholar]
- 7.Du M., Zhu M.J. Applied Muscle Biology and Meat Science. CRC Press; Boca Raton, FL, USA: 2009. Fetal programming of skeletal muscle development; pp. 81–96. [DOI] [Google Scholar]
- 8.Du M., Tong J., Zhao J., Underwood K.R., Zhu M., Ford S.P., Nathanielsz P.W. Fetal programming of skeletal muscle development in ruminant animals. J. Anim. Sci. 2010;88:E51–E60. doi: 10.2527/jas.2009-2311. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadabadi M., Bordbar F., Jensen J., Du M., Guo W. Key Genes Regulating Skeletal Muscle Development and Growth in Farm Animals. Animals. 2021;11:835. doi: 10.3390/ani11030835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponsuksili S., Du Y., Hadlich F., Siengdee P., Murani E., Schwerin M., Wimmers K. Correlated mRNAs and miRNAs from co-expression and regulatory networks affect porcine muscle and finally meat properties. BMC Genom. 2013;14:533. doi: 10.1186/1471-2164-14-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis S., Meltzer P.S. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 12.Sherman B.T., Hao M., Qiu J., Jiao X., Baseler M.W., Lane H.C., Imamichi T., Chang W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update) Nucleic Acids Res. 2022;50:W216–W221. doi: 10.1093/nar/gkac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szklarczyk D., Franceschini A., Wyder S., Forslund K., Heller D., Huerta-Cepas J., Simonovic M., Roth A., Santos A., Tsafou K.P. STRING v10: Protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschini A., Szklarczyk D., Frankild S., Kuhn M., Simonovic M., Roth A., Lin J., Minguez P., Bork P., Von Mering C. STRING v9. 1: Protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2012;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu W., Chen D., Li Y., Wu P., Zhang J., Liu L. Muscle fiber differentiation and growth patterns during hyperplasia and hypertrophy in the ricefield eel regulated by myogenic regulatory factors. N. Am. J. Aquac. 2018;80:180–186. doi: 10.1002/naaq.10025. [DOI] [Google Scholar]
- 17.Simó I., Faggiani M., Fernandez D.A., Sciara A.A., Arranz S.E. The cellular basis of compensatory muscle growth in the teleost Odontesthes bonariensis. J. Exp. Biol. 2022;225:jeb242567. doi: 10.1242/jeb.242567. [DOI] [PubMed] [Google Scholar]
- 18.Costa T.C., Gionbelli M.P., Duarte M.d.S. Fetal programming in ruminant animals: Understanding the skeletal muscle development to improve meat quality. Anim. Front. 2021;11:66–73. doi: 10.1093/af/vfab061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langlois S., Cowan K.N. Regulation of skeletal muscle myoblast differentiation and proliferation by pannexins. Protein Rev. 2016;925:57–73. doi: 10.1007/5584_2016_53. [DOI] [PubMed] [Google Scholar]
- 20.Asfour H.A., Allouh M.Z., Said R.S. Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Exp. Biol. Med. 2018;243:118–128. doi: 10.1177/1535370217749494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nesvadbova M., Borilova G. Molecular regulation of skeletal muscle tissue formation and development. Vet. Med. 2018;63:489–499. doi: 10.17221/7/2018-VETMED. [DOI] [Google Scholar]
- 22.Sadkowski T., Ciecierska A., Majewska A., Oprządek J., Dasiewicz K., Ollik M., Wicik Z., Motyl T. Transcriptional background of beef marbling—Novel genes implicated in intramuscular fat deposition. Meat Sci. 2014;97:32–41. doi: 10.1016/j.meatsci.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Shen X., Tang J., Ru W., Zhang X., Huang Y., Lei C., Cao H., Lan X., Chen H. CircINSR regulates fetal bovine muscle and fat development. Front. Cell Dev. Biol. 2021;8:615638. doi: 10.3389/fcell.2020.615638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vettor R., Milan G., Franzin C., Sanna M., De Coppi P., Rizzuto R., Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am. J. Physiol. Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 25.Hocquette J.-F., Gondret F., Baéza E., Médale F., Jurie C., Pethick D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal. 2010;4:303–319. doi: 10.1017/S1751731109991091. [DOI] [PubMed] [Google Scholar]
- 26.Angione A.R., Jiang C., Pan D., Wang Y.-X., Kuang S. PPARδ regulates satellite cell proliferation and skeletal muscle regeneration. Skelet. Muscle. 2011;1:33. doi: 10.1186/2044-5040-1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manickam R., Duszka K., Wahli W. PPARs and microbiota in skeletal muscle health and wasting. Int. J. Mol. Sci. 2020;21:8056. doi: 10.3390/ijms21218056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Neill B.T., Lauritzen H.P.M.M., Hirshman M.F., Smyth G., Goodyear L.J., Kahn C.R. Differential role of insulin/IGF-1 receptor signaling in muscle growth and glucose homeostasis. Cell Rep. 2015;11:1220–1235. doi: 10.1016/j.celrep.2015.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhoads R.P., Baumgard L.H., El-Kadi S.W., Zhao L.D. Physiology and Endocrinology Symposium: Roles for insulin-supported skeletal muscle growth. J. Anim. Sci. 2016;94:1791–1802. doi: 10.2527/jas.2015-0110. [DOI] [PubMed] [Google Scholar]
- 30.Schiaffino S., Dyar K.A., Ciciliot S., Blaauw B., Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 31.Rommel C., Bodine S.C., Clarke B.A., Rossman R., Nunez L., Stitt T.N., Yancopoulos G.D., Glass D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 32.Zand H., Morshedzadeh N., Naghashian F. Signaling pathways linking inflammation to insulin resistance. Diabetes Metab. Syndr. Clin. Res. Rev. 2017;11:S307–S309. doi: 10.1016/j.dsx.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Luo W., Ai L., Wang B.-F., Zhou Y. High glucose inhibits myogenesis and induces insulin resistance by down-regulating AKT signaling. Biomed. Pharmacother. 2019;120:109498. doi: 10.1016/j.biopha.2019.109498. [DOI] [PubMed] [Google Scholar]
- 34.Eivers S.S., McGivney B.A., Gu J., MacHugh D.E., Katz L.M., Hill E.W. PGC-1α encoded by the PPARGC1A gene regulates oxidative energy metabolism in equine skeletal muscle during exercise. Anim. Genet. 2012;43:153–162. doi: 10.1111/j.1365-2052.2011.02238.x. [DOI] [PubMed] [Google Scholar]
- 35.Ruas J.L., White J.P., Rao R.R., Kleiner S., Brannan K.T., Harrison B.C., Greene N.P., Wu J., Estall J.L., Irving B.A., et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotelnikova E., Shkrob M.A., Pyatnitskiy M.A., Ferlini A., Daraselia N. Novel approach to meta-analysis of microarray datasets reveals muscle remodeling-related drug targets and biomarkers in Duchenne muscular dystrophy. PLoS Comput. Biol. 2012;8:e1002365. doi: 10.1371/journal.pcbi.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sin J., Andres A.M., Taylor D.J.R., Weston T., Hiraumi Y., Stotland A., Kim B.J., Huang C., Doran K.S., Gottlieb R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy. 2016;12:369–380. doi: 10.1080/15548627.2015.1115172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman F.A., Quadrilatero J. Mitochondrial network remodeling: An important feature of myogenesis and skeletal muscle regeneration. Cell. Mol. Life Sci. 2021;78:4653–4675. doi: 10.1007/s00018-021-03807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang C., Ji L.L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012;1271:110. doi: 10.1111/j.1749-6632.2012.06738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahmann I., Bröhl D., Zyrianova T., Isomura A., Czajkowski M.T., Kapoor V., Griger J., Ruffault P.-L., Mademtzoglou D., Zammit P.S., et al. Oscillations of MyoD and Hes1 proteins regulate the maintenance of activated muscle stem cells. Genes Dev. 2019;33:524–535. doi: 10.1101/gad.322818.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yagi M., Ji F., Charlton J., Cristea S., Messemer K., Horwitz N., Di Stefano B., Tsopoulidis N., Hoetker M.S., Huebner A.J., et al. Dissecting dual roles of MyoD during lineage conversion to mature myocytes and myogenic stem cells. Genes Dev. 2021;35:1209–1228. doi: 10.1101/gad.348678.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pajalunga D., Crescenzi M. Restoring the cell cycle and proliferation competence in terminally differentiated skeletal muscle myotubes. Cells. 2021;10:2753. doi: 10.3390/cells10102753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu M., Yang G., Chen Y., Zhou X., Chen H., Li M., Yu K., Zhang X., Xie S., Zhang Y., et al. CEP2 attenuates myoblast differentiation but does not affect proliferation. Int. J. Biol. Sci. 2015;11:99–108. doi: 10.7150/ijbs.8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majmundar A.J., Wong W.J., Simon M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wada T. Transcription factor EPAS1 regulates insulin signaling pathway. Yakugaku Zasshi J. Pharm. Soc. Japan. 2007;127:143–151. doi: 10.1248/yakushi.127.143. [DOI] [PubMed] [Google Scholar]
- 46.Xie L., Yin A., Nichenko A.S., Beedle A.M., Call J.A., Yin H. Transient HIF2A inhibition promotes satellite cell proliferation and muscle regeneration. J. Clin. Investig. 2018;128:2339–2355. doi: 10.1172/JCI96208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X., Yang S., Wang C., Kuang S. The hypoxia-inducible factors HIF1α and HIF2α are dispensable for embryonic muscle development but essential for postnatal muscle regeneration. J. Biol. Chem. 2017;292:5981–5991. doi: 10.1074/jbc.M116.756312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malila Y., Thanatsang K., Arayamethakorn S., Uengwetwanit T., Srimarut Y., Petracci M., Strasburg G.M., Rungrassamee W., Visessanguan W. Absolute expressions of hypoxia-inducible factor-1 alpha (HIF1A) transcript and the associated genes in chicken skeletal muscle with white striping and wooden breast myopathies. PLoS ONE. 2019;14:e0220904. doi: 10.1371/journal.pone.0220904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimba S., Wada T., Hara S., Tezuka M. EPAS1 promotes adipose differentiation in 3T3-L1 cells. J. Biol. Chem. 2004;279:40946–40953. doi: 10.1074/jbc.M400840200. [DOI] [PubMed] [Google Scholar]
- 50.Badin P.-M., Sopariwala D.H., Lorca S., Narkar V.A. Muscle Arnt/Hif1β is dispensable in myofiber type determination, vascularization and insulin sensitivity. PLoS ONE. 2016;11:e0168457. doi: 10.1371/journal.pone.0168457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niemi H., Honkonen K., Korpisalo P., Huusko J., Kansanen E., Merentie M., Rissanen T.T., Andre H., Pereira T., Poellinger L. HIF-1α and HIF-2α induce angiogenesis and improve muscle energy recovery. Eur. J. Clin. Investig. 2014;44:989–999. doi: 10.1111/eci.12333. [DOI] [PubMed] [Google Scholar]
- 52.Younis S., Schönke M., Massart J., Hjortebjerg R., Sundström E., Gustafson U., Björnholm M., Krook A., Frystyk J., Zierath J.R. The ZBED6–IGF2 axis has a major effect on growth of skeletal muscle and internal organs in placental mammals. Proc. Natl. Acad. Sci. USA. 2018;115:E2048–E2057. doi: 10.1073/pnas.1719278115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antoniou A., Mastroyiannopoulos N.P., Uney J.B., Phylactou L.A. miR-186 inhibits muscle cell differentiation through myogenin regulation. J. Biol. Chem. 2014;289:3923–3935. doi: 10.1074/jbc.M113.507343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duan C., Ren H., Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: Roles in skeletal muscle growth and differentiation. Gen. Comp. Endocrinol. 2010;167:344–351. doi: 10.1016/j.ygcen.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Zanou N., Gailly P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013;70:4117–4130. doi: 10.1007/s00018-013-1330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borensztein M., Monnier P., Court F., Louault Y., Ripoche M.-A., Tiret L., Yao Z., Tapscott S.J., Forné T., Montarras D., et al. Myod and H19-Igf2 locus interactions are required for diaphragm formation in the mouse. Development. 2013;140:1231–1239. doi: 10.1242/dev.084665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kalista S., Schakman O., Gilson H., Lause P., Demeulder B., Bertrand L., Pende M., Thissen J.-P. The type 1 insulin-like growth factor receptor (IGF-IR) pathway is mandatory for the follistatin-induced skeletal muscle hypertrophy. Endocrinology. 2012;153:241–253. doi: 10.1210/en.2011-1687. [DOI] [PubMed] [Google Scholar]
- 58.Wilson E.M., Rotwein P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. J. Biol. Chem. 2006;281:29962–29971. doi: 10.1074/jbc.M605445200. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Y., Gui W., Tan B., Du Y., Zhou J., Wu F., Li H., Lin X. IGF2 deficiency causes mitochondrial defects in skeletal muscle. Clin. Sci. 2021;135:979–990. doi: 10.1042/CS20210128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bae G.-U., Gaio U., Yang Y.-J., Lee H.-J., Kang J.-S., Krauss R.S. Regulation of myoblast motility and fusion by the CXCR4-associated sialomucin, CD164. J. Biol. Chem. 2008;283:8301–8309. doi: 10.1074/jbc.M706730200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brzoska E., Kowalewska M., Markowska-Zagrajek A., Kowalski K., Archacka K., Zimowska M., Grabowska I., Czerwińska A.M., Czarnecka-Góra M., Stremińska W., et al. Sdf-1 (CXCL12) improves skeletal muscle regeneration via the mobilisation of Cxcr4 and CD34 expressing cells. Biol. Cell. 2012;104:722–737. doi: 10.1111/boc.201200022. [DOI] [PubMed] [Google Scholar]
- 63.Vasyutina E., Stebler J., Brand-Saberi B., Schulz S., Raz E., Birchmeier C. CXCR4 and Gab1 cooperate to control the development of migrating muscle progenitor cells. Genes Dev. 2005;19:2187–2198. doi: 10.1101/gad.346205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinelli G.B., Olivari D., Re Cecconi A.D., Talamini L., Ottoboni L., Lecker S.H., Stretch C., Baracos V.E., Bathe O.F., Resovi A. Activation of the SDF1/CXCR4 pathway retards muscle atrophy during cancer cachexia. Oncogene. 2016;35:6212–6222. doi: 10.1038/onc.2016.153. [DOI] [PubMed] [Google Scholar]
- 65.Bobadilla M., Sainz N., Abizanda G., Orbe J., Rodriguez J.A., Páramo J.A., Prósper F., Pérez-Ruiz A. The CXCR4/SDF1 axis improves muscle regeneration through MMP-10 activity. Stem Cells Dev. 2014;23:1417–1427. doi: 10.1089/scd.2013.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imtiyaz H.Z., Williams E.P., Hickey M.M., Patel S.A., Durham A.C., Yuan L.-J., Hammond R., Gimotty P.A., Keith B., Simon M.C. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Investig. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rovito D., Gionfriddo G., Barone I., Giordano C., Grande F., De Amicis F., Lanzino M., Catalano S., Andò S., Bonofiglio D. Ligand-activated PPARγ downregulates CXCR4 gene expression through a novel identified PPAR response element and inhibits breast cancer progression. Oncotarget. 2016;7:65109. doi: 10.18632/oncotarget.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van der Vorst E.P.C. Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and Other Body Fluid Proteins. Springer; Berlin/Heidelberg, Germany: 2020. High-density lipoproteins and apolipoprotein A1; pp. 399–420. [DOI] [PubMed] [Google Scholar]
- 69.Zhang H., Wang Y., Li J., Yu J., Pu J., Li L., Zhang H., Zhang S., Peng G., Yang F., et al. Proteome of skeletal muscle lipid droplet reveals association with mitochondria and apolipoprotein aI. J. Proteome Res. 2011;10:4757–4768. doi: 10.1021/pr200553c. [DOI] [PubMed] [Google Scholar]
- 70.Drew B.G., Duffy S.J., Formosa M.F., Natoli A.K., Henstridge D.C., Penfold S.A., Thomas W.G., Mukhamedova N., de Courten B., Forbes J.M., et al. High-density lipoprotein modulates glucose metabolism in patients with type 2 diabetes mellitus. Circulation. 2009;119:2103–2111. doi: 10.1161/CIRCULATIONAHA.108.843219. [DOI] [PubMed] [Google Scholar]
- 71.Fritzen A.M., Domingo-Espín J., Lundsgaard A.-M., Kleinert M., Israelsen I., Carl C.S., Nicolaisen T.S., Kjøbsted R., Jeppesen J.F., Wojtaszewski J.F.P., et al. ApoA-1 improves glucose tolerance by increasing glucose uptake into heart and skeletal muscle independently of AMPKα2. Mol. Metab. 2020;35:100949. doi: 10.1016/j.molmet.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang S., Tabet F., Cochran B.J., Torres L.F.C., Wu B.J., Barter P.J., Rye K.-A. Apolipoprotein AI enhances insulin-dependent and insulin-independent glucose uptake by skeletal muscle. Sci. Rep. 2019;9:1350. doi: 10.1038/s41598-018-38014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu J., Fu R., Liu R., Zhao G., Zheng M., Cui H., Li Q., Song J., Wang J., Wen J. Protein profiles for muscle development and intramuscular fat accumulation at different post-hatching ages in chickens. PLoS ONE. 2016;11:e0159722. doi: 10.1371/journal.pone.0159722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen D., Li W., Du M., Cao B. Adipogenesis, fibrogenesis and myogenesis related gene expression in longissimus muscle of high and low marbling beef cattle. Livest. Sci. 2019;229:188–193. doi: 10.1016/j.livsci.2019.09.032. [DOI] [Google Scholar]
- 75.Li J., Xing S., Zhao G., Zheng M., Yang X., Sun J., Wen J., Liu R. Identification of diverse cell populations in skeletal muscles and biomarkers for intramuscular fat of chicken by single-cell RNA sequencing. BMC Genom. 2020;21:752. doi: 10.1186/s12864-020-07136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Q., Zhang Y., Chen Y., Yang N., Wang X.-J., Zhu D. Systematic identification of genes involved in divergent skeletal muscle growth rates of broiler and layer chickens. BMC Genom. 2009;10:87. doi: 10.1186/1471-2164-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.