Abstract
Simple Summary
It is well-established that the heart regulates systemic lipid metabolism, but the exact molecular mechanisms remain largely unclear. A high-fat diet (HFD) can lead to systemic lipid overload and a range of cardiac dysfunctions such as arrhythmias, fibrillation and reduced contractility. Although pharmacological treatment is often effective, there are always side effects. Accordingly, exercise training represents an effective alternative intervention to medication. In this experiment, we established a high-fat Drosophila model by HFD feeding and conducted exercise training intervention to investigate whether the exercise intervention altered the expression of the target gene (mtp), thus affecting systemic lipid metabolism and cardiac function. Our results suggest that specific knockdown of mtp mitigates HFD-induced elevation of systemic triglycerides and protects cardiac contractility to some extent. Further analysis showed that exercise training could upregulate the expression of mtp to restore dysregulated systemic lipid metabolism and cardiac function induced by HFD. Overall, we explored the important role of mtp in systemic lipid metabolism and cardiac function, providing new insights for future clinical studies related to lipotoxic cardiomyopathy and the potential use of exercise training to treat abnormal lipid metabolism and cardiac dysfunction due to HFD.
Abstract
Current evidence suggests that the heart plays an important role in regulating systemic lipid homeostasis, and high-fat diet (HFD)-induced obesity is a major cause of cardiovascular disease, although little is known about the specific mechanisms involved. Exercise training can reportedly improve abnormal lipid metabolism and cardiac dysfunction induced by high-fat diets; however, the molecular mechanisms are not yet understood. In the present study, to explore the relationship between exercise training and cardiac mtp in HFD flies and potential mechanisms by which exercise training affects HFD flies, Drosophila was selected as a model organism, and the GAL4/UAS system was used to specifically knock down the target gene. Experiments revealed that HFD-fed Drosophila exhibited changes in body weight, increased triglycerides (TG) and dysregulated cardiac contractility, consistent with observations in mammals. Interestingly, inhibition of cardiac mtp expression reduced HFD-induced cardiac damage and mitigated the increase in triglycerides. Further studies showed that in HFD +w1118, HFD + Hand > w1118, and HFD+ Hand > mtpRNAi, cardiac mtp expression downregulation induced by HFD was treated by exercise training and mitochondrial β-oxidation capacity in cardiomyocytes was reversed. Overall, knocking down mtp in the heart prevented an increase in systemic TG levels and protected cardiac contractility from damage caused by HFD, similar to the findings observed after exercise training. Moreover, exercise training upregulated the decrease in cardiac mtp expression induced by HFD. Increased Had1 and Acox3 expression were observed, consistent with changes in cardiac mtp expression.
Keywords: Drosophila, mtp, cardiac dysfunction, exercise training, lipid metabolism, β-oxidation
1. Introduction
Unhealthy eating habits cause an excessive accumulation of lipids in the body (i.e., “obesity”), which represents a high-risk factor for harmful effects on metabolism and heart function and can lead to the development of a range of diseases such as heart disease, type 2 diabetes and cancer. After hydrolysis into glycerol and fatty acids, fats are packaged in the endoplasmic reticulum of small intestinal cells as chylomicrons (CMs) [1,2] and the liver cells as very low-density lipoproteins (VLDLs) [3]. Microsomal triglyceride transfer protein (mtp) is involved in the assembly of lipoproteins, transferring lipids to lipoprotein B (apoB) [4], which allows apoB to fold correctly and assemble primitive lipoprotein particles involved in systemic lipid metabolism [5]. An increasing body of evidence suggests that mice lacking mtp in the liver or intestine exhibit systemic or specific reductions in plasma triglyceride and cholesterol levels [6,7]. Studies on humans have shown that mtp is associated with lipid droplets in brown and white fat and plays an important role in lipid droplet formation and/or turnover [8]. In addition, mtp is composed of α and β subunits, and mitochondrial β-oxidation is the main metabolic system for the catabolism of fatty acids, generating the main energy source for various cellular processes. β-oxidation catalyzes fatty acids through four reactions (dehydration, oxidation, hydration, and thiolysis) to produce acetyl coenzyme A. Importantly, mtp catalyzes the last three steps of β-oxidation of long-chain fatty acids. The heart can influence mitochondrial β-oxidation by regulating the expression of mtp, which affects systemic lipid metabolism.
Exercise training is recognized as one of the most effective treatments for obesity and cardiovascular disease. In mouse studies, exercise training improved HFD-induced glucose intolerance and insulin resistance while increasing acetylcholine levels, ChAT activity and PKC activity [9]. It could also inhibit inflammation in the adipose tissue of obese mice induced by a high-fat diet [9,10]. Several human metabolic diseases, including obesity, impaired glucose tolerance and type 2 diabetes, have been advocated for prevention and treatment with exercise training [11,12,13,14].
Unlike other animal models such as Mice and Zebrafish, Drosophila has a short life cycle (from egg to adult in about 10 days), produces many offspring (it can produce 30 generations a year, is convenient to feed and can be used for genome editing. More importantly, sequencing and annotation of the Drosophila genome have shown that genes involved in the normal development of several organs, including the heart, are highly conserved and less redundant than in vertebrates. Moreover, it has been shown that a large number of human disease genes have Drosophila counterparts and that their hearts exhibit developmental and functional similarities to the vertebrate heart [15,16,17]. In addition, several models of HFD-induced obesity have been successfully developed in Drosophila, encapsulating the distinctive features of human obesity and diabetes [18].
An increasing body of evidence from recently published studies has confirmed that the heart plays an important role in regulating systemic lipid metabolism [19]. Animal experiments showed that cardiac MED13 expression regulation could control systemic lipid metabolism [20]. However, the specific mechanisms warrant further exploration. By establishing a Drosophila model of HFD and conducting cardiac function measurements [21,22,23], we investigated how exercise training affects HFD-induced cardiac disorders and abnormalities in lipid metabolism. Our findings suggest that specific knockdown of mtp in the heart reduces the elevation of systemic TG and alleviates HFD-induced cardiac dysfunction. In addition, in HFD + w1118, HFD + Hand > w1118, and HFD + Hand > mtpRNAi, exercise training could restore decreased cardiac mtp expression induced by HFD and improve mitochondrial β-oxidation capacity in cardiomyocytes. Our findings improve current understanding of the role of mtp on systemic lipid metabolism and cardiac function and provide new insights for future clinical studies related to lipotoxic cardiomyopathy and the potential use of exercise training to treat abnormal lipid metabolism and cardiac dysfunction induced by HFD.
2. Materials and Methods
2.1. Drosophila Strains and Husbandry
w1118 Drosophila were obtained from the Bloomington Drosophila Stock Center, and UAS-mtpRNAi Drosophila from the Drosophila Center, Tsinghua University. To reduce mtp expression in the heart, we used Hand-Gal4 (cardiomyocyte-specific driver BL48396). The normal food diet (NFD) consisted of a combination of yeast, maize and starch; the high-fat food diet (HFD) was made from 30% coconut oil mixed with the 70% volume of NFD [24,25]. All fruit flies were fed with NFD for five days, then high-fat group fruit flies were fed HFD for five days.
Hand-Gal4 male flies were crossed with w1118 female flies and female flies of the F1 generation were collected within 12 h of eclosion. These flies and virgin flies of w1118 underwent different interventions (NFD +w1118, HFD + w1118, HFD + E + w1118, NFD + Hand > w1118, HFD + Hand > w1118, and HFD + E + Hand > w1118 (E is for exercise)). Hand-Gal4 male flies were crossed with UAS-mtpRNAi female flies, and female flies from the F1 generation were eclosion was collected within 12 h of eclosion. These flies were defined as cardiomyocyte-specific knockdown of mtp groups and were subjected to different interventions (NFD + Hand > mtpRNAi, HFD + Hand > mtpRNAi and HFD + E + Hand > mtpRNAi). A total of 1800 virgin flies that were 10 days old were obtained (200 in each group). All flies were placed in transparent glass tubes (20–30 per tube). NFD-fed flies were placed in a constant temperature and humidity incubator (25 °C, 50% humidity, 12 h day-night cycle). HFD-fed flies were reared in an environment of 21–22 °C to prevent part of the high-fat food from melting and causing the flies to stick to the food and die.
2.2. Heart Function Tests
Based on previous studies, flies were anesthetized with FlyNap (Triangle Biotechnology, Shanghai, China), and the dissected fly hearts were preserved in oxygenated saline. The heartbeats were captured using an EM-CCD high-speed camera (130 fps, 30-s video), and heart rate, cardiac period, arrhythmia, contractility, shortening fraction, etc., were analyzed using semi-automatic optical heartbeat analysis software (SOHA) [26,27].
2.3. Body Weight and Triglyceride (TG) Measurement
An electronic microbalance (Shimadzu, AUW220D, Kyoto Japan) was used to weigh the fruit flies and record the weight of each fly for analysis.
TG concentration was measured using a triglyceride (TG) assay kit (Nanjing Jiancheng Bioengineering Institution, Nanjing, China) according to the manufacturer’s instructions [28].
2.4. Quantitative Real-Time Fluorescence PCR (qPCR)
Fifty hearts were placed into 1 mL of Trizol reagent lysis solution for homogenization and RNA extraction. Trizol was used to extract the organic solvent, and 10 μg total RNA was purified using oligo (dT) synthesized from total RNA with superscript II reverse transcriptase (Invitrogen). qPCR amplification reactions were performed in triplicates by mixing 1 μL of RT product with 10 μL of SYBR qPCR Mastermix (TaKaRa) containing the appropriate PCR primers. Thermal cycling and fluorescence monitoring were performed in an ABI7300 (Applied Biosystems, United States) using the following PCR conditions: (30 s at 95 °C, 5 s at 95 °C, and 30 s at 60 °C) × 40. Values were normalized with rp49. Primers used were as follows: rp49(the reference gene)F: 5′-CTAAGCTAGTCGCACAAATAGG-3′R; 5′-AACTTCTTAGAATCCGGTAGGG-3′mtpF; 5-ACGGAAATCCAGCAGAACACT-3′R:5′-ATACGTAAAGCCAACGGCCA-3′Acox3F; 5′-ACTTCCGTAGCGGACCTTTAG-3′R:5-GCAGAAGATAGTAGGGGTTCCA-3Had1F; 5-CTAAATAGCAAGGTACGCGGC-3′R; 5-GATAGTAGGGCCGTAGCGATAA-3′.
2.5. Oil Red O Dye Staining
Flies were anesthetized and dissected in ice-cold PBS. The ventral cuticle was removed from the abdomen using microscissors, and the viscera and genitalia were excised, leaving intact abdominal fat bodies attached to the dorsal cuticle. The plates were fixed with 4% paraformaldehyde for 20 min and washed three times with PBS for 10 min each time. The oil Red O solution (3:2 for staining solution and distilled water) was filtered, then incubated at room temperature for 30 min and washed three times with PBS. The photographs were taken under a Leica stereomicroscope, and after processing the images using Adobe Photoshop, the staining area of oil red was calculated using ImageJ software (ImageJ2,LOCI, Bethesda, MD, USA) [29].
2.6. Sports Training Devices and Programs
Consistent with previously established protocols, all fruit flies were fed under NFD conditions for 5 days, and those in the locomotor training group were trained from day 6 for 5 days, ending with training on day 10. Drosophila exercised for 1.5 h per day for 5 days [30,31,32]. During the locomotor training intervention, Drosophila spent 1.5 h in a glass tube without food. The fruit flies in the locomotor training control group were also placed in the glass tubes without food for 1.5 h, thus ensuring that all flies were in the same environment to avoid affecting their feeding rate [33].
2.7. Statistical Analysis
Figures were generated using GraphPad Prism6 software. Analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS) version 21.0 (SPSSInc., Chicago, IL, USA) [30,31,32]. All data are expressed as SEM ± mean. One-way ANOVA was used to identify differences between NFD, HFD and HFD + E groups of Drosophila with the same genetic background. Independent sample t-tests were used to identify differences between the two groups. A two-tailed p-value < 0.05 was statistically significant.
3. Results
3.1. HFD Causes Abnormal Lipid Metabolism and Cardiac Dysfunction in Drosophila
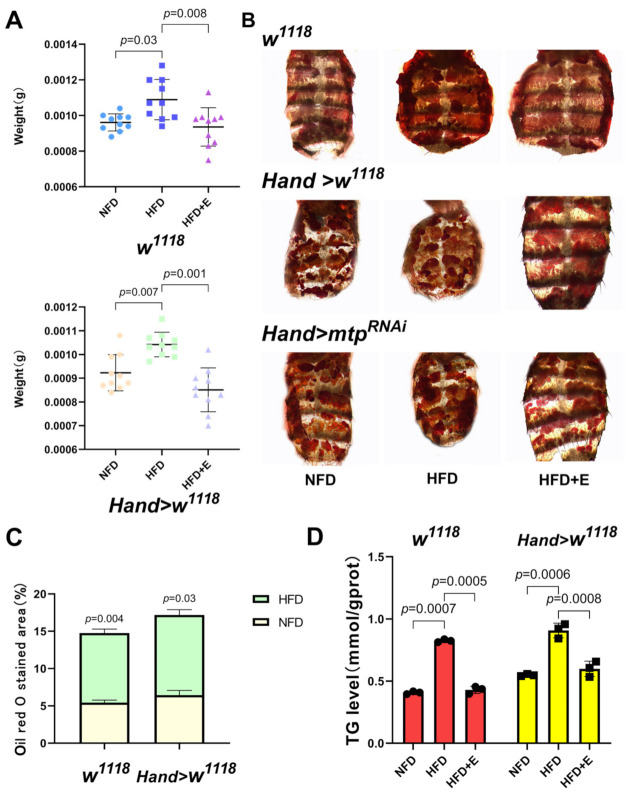
First, we established a Drosophila high-fat model. Female Drosophila were fed on NFD for 5 days and then on HFD for 5 days [24], and morphological photographs of Drosophila were taken (Figure 1A). Compared to the NFD group, the HFD-fed Drosophila exhibited a larger body size and a bulging abdomen with significantly higher body weight than the NFD group (Figure 2A). Moreover, the wing and ovary sizes of NFD and HFD flies were comparable. These findings suggested lipid uptake was responsible for the apparent abdominal bulge in HFD flies (Figure 1B–D). In addition, the oil red O staining area (Figure 2B,C) and TG content (Figure 2D) of abdominal fat were significantly greater in the HFD group of Drosophila than in the NFD group, demonstrating that our high fat protocol successfully induced a model of diet-induced obesity in Drosophila.
Figure 1.
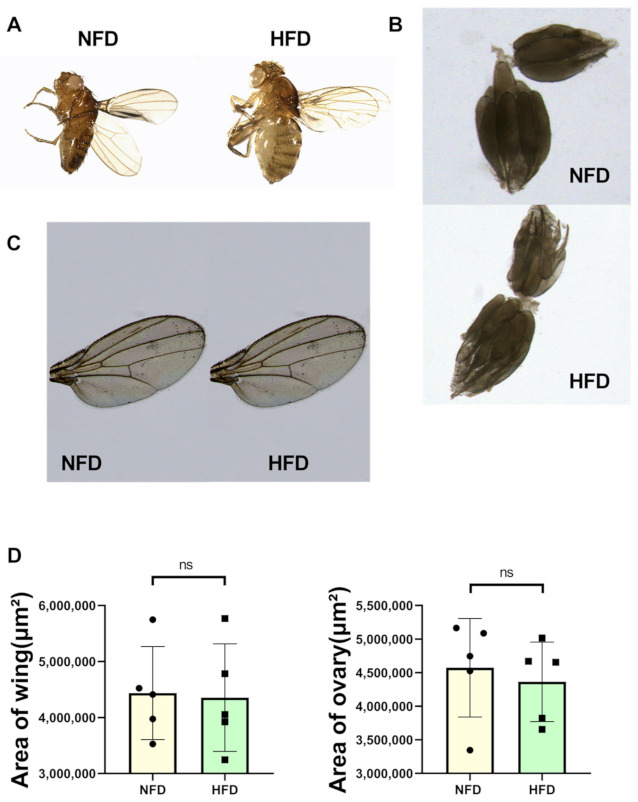
The w1118 group and Hand > w1118 group exhibited obesity after 5d of HFD feeding. (A) Morphological pictures of 10-day-old Drosophila under NFD and HFD feeding conditions, with HFD Drosophila being larger and having a protruding abdomen. (B) Photographs of ovaries of 10-day-old NFD and HFD flies. (C) Photographs of wings of 10-day-old NFD and HFD flies. (D) Comparison of wing size and ovary size between 10-day-old NFD and HFD flies (N = 5) using independent sample t-test.
Figure 2.
(A) Body weights were obtained by weighing the 10-day-old flies on an electronic microbalance, N = 10, with a significant increase in body weight in Drosophila after HFD feeding compared to the NFD group but a decrease in body weight after the exercise intervention. (B) ORO abdominal staining of each group of the 10-day-old flies under different treatment conditions. (C) ORO staining in the abdomen of Drosophila. N = 5. The area of ORO staining in the abdomen of Drosophila was larger in the HFD group compared to the NFD group. (D) Whole-body TG levels in 10-day-old Drosophila. Whole-body TG levels were significantly higher in the HFD group than in the NFD group. N = 10, repeated three times. One-way ANOVA was used for (A,D). An independent sample t-test was used for (C).
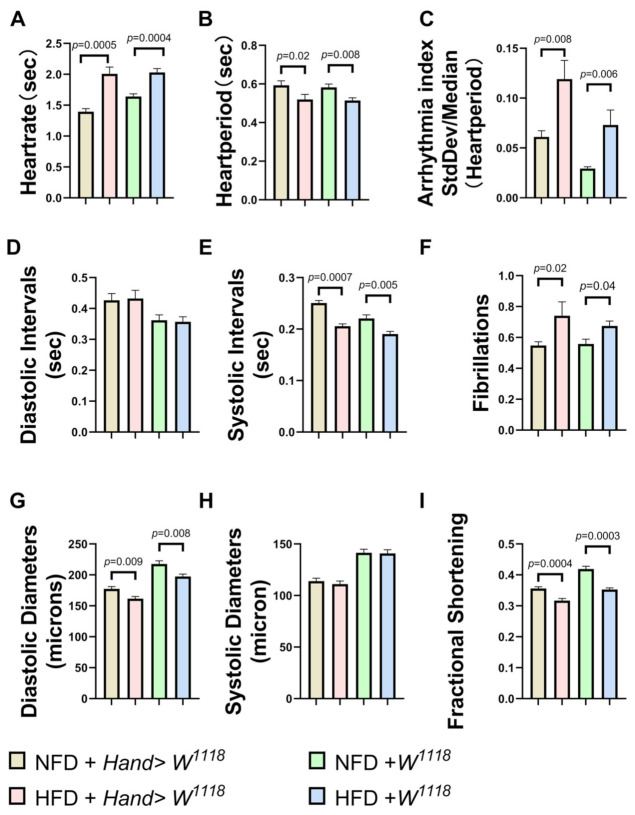
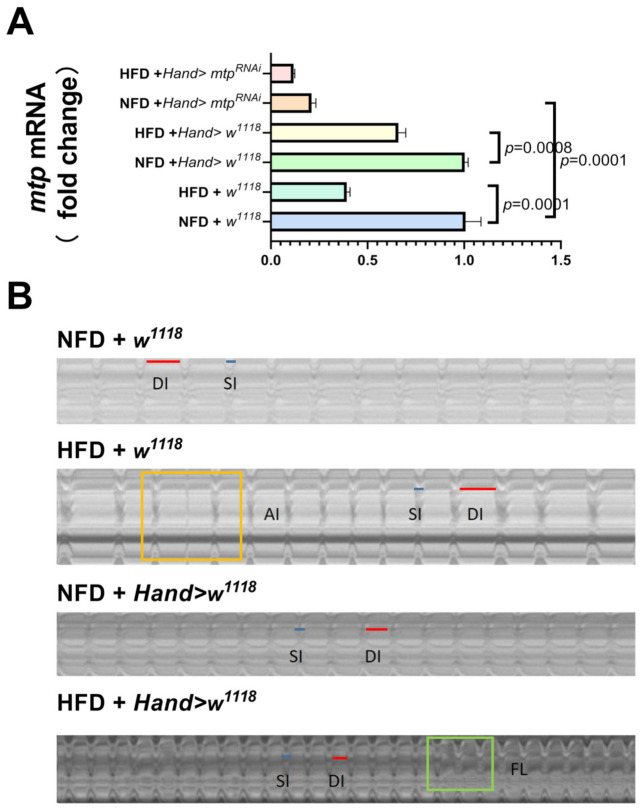
To investigate whether cardiac mtp is involved in obesity-induced cardiac dysfunction, we measured various cardiac function parameters and mtp mRNA expression in Drosophila NFD and Drosophila HFD, including heart rate (HR), cardiac cycle or heart period (HP), arrhythmia (AI), diastolic interval (DI) and systolic interval (SI), fibrillation (FL), diastolic diameter (DD), systolic diameter (SD), and fractional shortening (FS). We found varying degrees of increase in HR, AI and FL (Figure 3A,C,F) under HFD compared to NFD. It is well-established that fibrillation is the most common sustained arrhythmia and a high-risk factor for morbidity and mortality due to obesity [34]. Significant decreases in HP,SI, DD and FS (Figure 3B,E,G,I) and diastolic diameter and fractional shortening indicated a decrease in myocardial contractility in Drosophila. No significant differences were observed in DI and SD (Figure 3D,H). This finding suggests that HFD can lead to an increased heart rate in Drosophila and a concomitant increase in the frequency of arrhythmias and fibrillations, shorter cardiac periods and decreased pumping capacity of the heart (shortened fractions and diastolic diameters). Indeed, increased levels of fat and circulating blood lipids lead to cardiac dysfunction. For example, increased expression of lipid transport proteins in the heart leads to elevated lipids in cardiac myocytes with cardiac dysfunction [35]; cardiac pathological remodeling (i.e., hypertrophy and fibrosis) occurs during chronic HFD feeding, which affects systolic and diastolic function in the hearts of obese mice [36], consistent with findings of previous studies of lipotoxic cardiomyopathy [37]. Moreover, we found a significant decrease in mtp expression in the hearts of Drosophila in the HFD group compared to the NFD group (Figure 4A). These results suggest that increased dietary fat leads to obesity and cardiac dysfunction in Drosophila, which may be related to reduced mtp in cardiac myocytes (Figure 4A,B).
Figure 3.
NFD and HFD groups of 10-day-old Drosophila M-mode ECG, with quantitative analysis of HR, HP, AI, DI, SI, FL, SD, DD, FS. Compared to NFD, w1118 and Hand > w1118 under HFD conditions, HR, AI and FL (A,C,F) were significantly upregulated. Moreover, HP, SI, DD and FS (B,E,G,I) decreased significantly, while DI and SD (D,H) did not show significant differences. All Drosophila were virgin flies, and the sample sizes for the two groups of NFD and HFD were N = 28 and N = 30, respectively. All p values were from independent samples t-tests.
Figure 4.
(A) Relative expression levels of mtp in Drosophila cardiomyocytes. mtp expression in Drosophila cardiomyocytes was significantly higher in the NFD group than in the HFD group. The test sample consisted of about 50 isolated hearts from the 10-day-old virgin flies. (B) M-mode ECGs under different treatments, the blue line is the systolic interval, and the red line is the diastolic interval; arrhythmia is marked in a yellow rectangle and fibrillation in a green rectangle. The intercept time for each group of M-mode ECGs was 10 s. In (A), one-way ANOVA was used to identify differences between the strains of Drosophila. LSD was used for post hoc testing.
3.2. Specific Knockdown of mtp in the Heart Alleviates HFD-Induced Cardiac Dysfunction, Similar to the Effect of Exercise Training
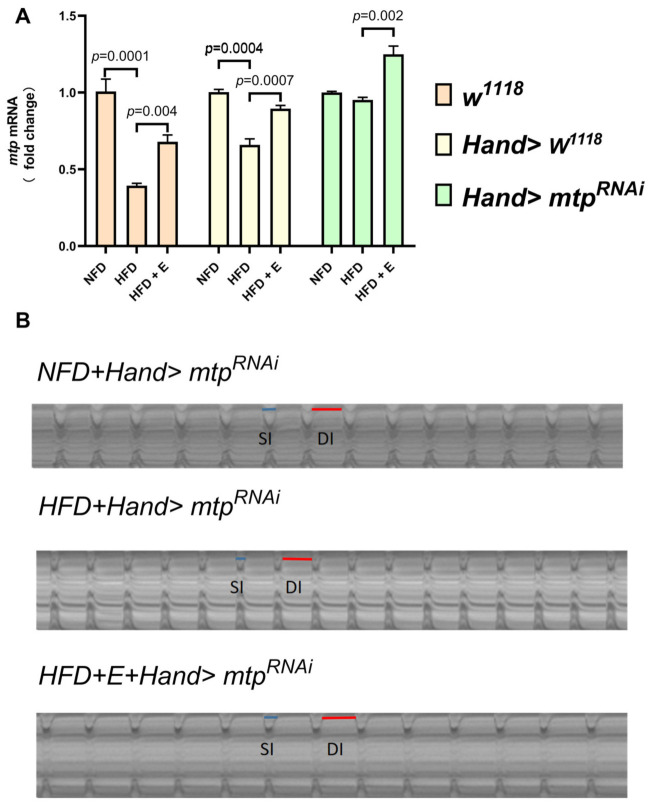
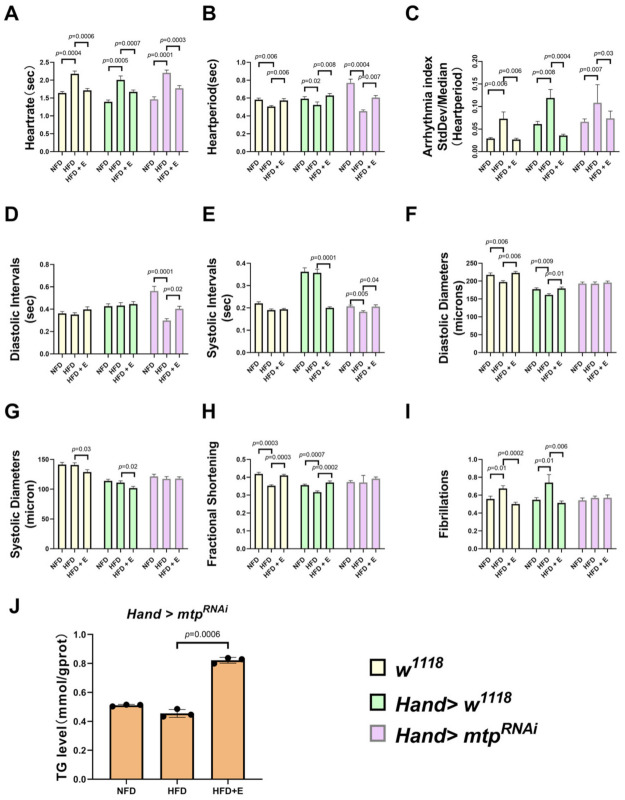
We subjected high-fat Drosophila to five days of exercise and then measured mtp mRNA expression levels, cardiac function and whole-body triglyceride content. The results showed that exercise training significantly increased cardiac mtp expression in all groups of HFD Drosophila (Figure 5A). After exercise training, compared to HFD + w1118 and HFD + Hand > w1118, HFD + E+w1118 and HFD + E+Hand > w1118 Drosophila showed slower heart rate, increased cardiac period, decreased arrhythmia index, increased diastolic diameter, decreased systolic diameter, reduced fibrillation and increased shortening fraction (Figure 6A–C,F–I), with significant improvements in both cardiac rhythm and systolic function, effectively treating HFD-induced cardiac dysfunction. Exercise training also significantly reduced whole-body TG levels in HFD + w1118 and HFD + Hand > w1118 Drosophila, demonstrating that exercise training could upregulate mtp expression in cardiac myocytes while modulating whole-body lipid levels and reducing whole-body TG, thereby protecting the heart from HFD-induced dysfunction.
Figure 5.
Effects of inhibition of cardiac mtp and exercise training on mRNA expression in Drosophila. All samples (N = 50 per group) were virgin flies of 10 days. (A) The cardiac mRNA expression levels of Drosophila under different treatments, on the one hand, HFD decreased the mRNA expression levels of cardiac mtp in the w1118 and Hand > w1118 groups; on the other hand, HFD did not significantly affect the mRNA expression levels of cardiac mtp in Hand > mtpRNAi Drosophila. In contrast, exercise training in Drosophila with HFD upregulated the mRNA expression levels of cardiac mtp in all Drosophila of HFD. (B) M-mode ECG of Drosophila in the Hand > mtpRNAi group, the blue line is the systolic interval, and the red line is the diastolic interval. In (A), one-way ANOVA was used to identify differences between strains of Drosophila NFD, HFD and HFD +E. LSD was used for post hoc testing.
Figure 6.
Effect of inhibition of cardiac mtp and exercise training on cardiac function and Hand > mtpRNAi whole-body TG levels in Drosophila. (A–I) Under different treatments, cardiac function assays included HR, HP, AI, DI, SI, DD, SD, FS and FL. NFD + w1118, HFD + w1118, HFD + E+w1118, NFD + Hand > w1118, HFD + Hand > w1118, HFD + E + Hand > w1118, NFD + Hand > mtpRNAi, HFD + Hand > mtpRNAi, and HFD + E + Hand > mtpRNAi. Sample sizes N = 26, 28, 24, 27, 28, 19, 34, 22 and 22. All samples were virgin flies of 10 days. (J) Whole-body TG levels of Hand > mtpRNAi Drosophila. All samples were virgin flies of 10 days, N = 10, and measurements were repeated three times. Whole-body TG levels were significantly higher in Drosophila in the HFD + E group compared to the HFD group. One-way ANOVA was used to identify differences between the strains of Drosophila NFD, HFD and HFD + E. LSD was used for post hoc testing.
HFD deteriorated cardiac function in Drosophila and caused a significant decrease in mRNA levels of mtp in the heart. Next, to investigate the relationship between HFD-induced cardiomyopathy and cardiomyocyte mtp, we targeted KD against mtp in cardiomyocytes and assessed whole-body TG levels in Drosophila with HFD. We found no significant difference in mRNA levels of mtp between NFD + Hand > mtpRNAi and HFD + Hand > mtpRNAi (Figure 5A). Moreover, the knockdown of mtp in the heart prevented an increase in systemic TG levels (Figure 6J), suggesting that mtp in cardiomyocytes plays an important role in regulating systemic lipid metabolism. Interestingly, no significant differences were found between the NFD + Hand > mtpRNAi group and the HFD + Hand > mtpRNAi group in DD, SD, FS and FL (Figure 6F–I); and differences were found in HR, HP, AI, DI and SI (Figure 6A–E) during M-mode ECG. These results suggest that NFD + Hand > mtpRNAi and HFD + Hand > mtpRNAi differed significantly, indicating that HFD mainly affected the cardiac rhythmicity of Hand > mtpRNAi. Compared to the control HFD + w1118 and HFD + Hand > w1118 groups, knocking down the cardiac mtp of HFD + Hand > mtpRNAi could reduce the damage of myocardial contractile function by HFD to some extent (Figure 5B). Knockdown of the cardiac mtp alone yielded effects similar to exercise training in treating cardiomyopathy in Drosophila with HFD, whereas the HFD + Hand > mtpRNAi group of Drosophila showed a significant increase in cardiac mtp expression (Figure 5A) and systemic TG levels (Figure 6J) after exercise training. We also found a decrease in HR (Figure 6A) and AI (Figure 6C) and an increase in HP, DI and SI (Figure 6B,D,E), indicating that the abnormal heart rhythm caused by HFD was significantly improved and the cardiac function of Drosophila was restored.
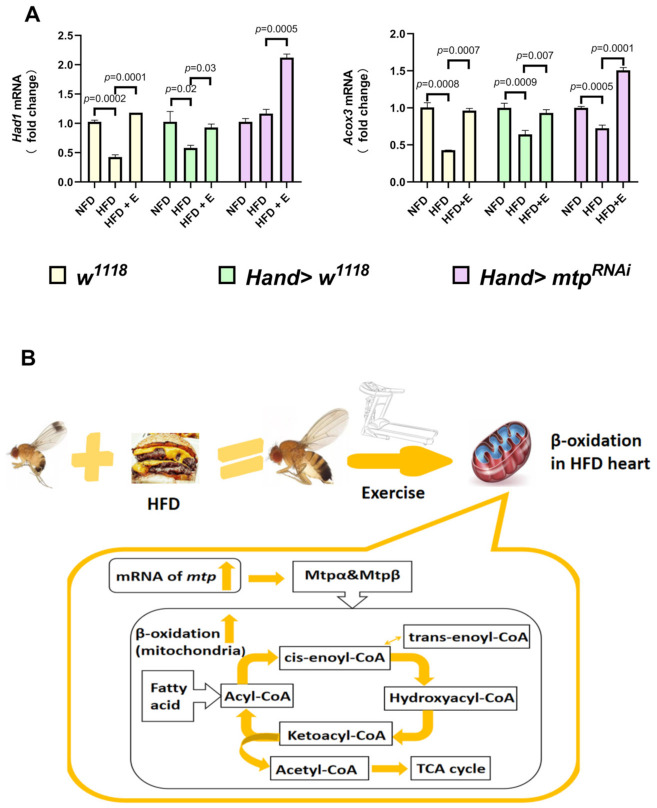
3.3. Exercise Training Upregulates Cardiomyocyte mtp Expression and Reverses the HFD-Induced Decrease in Cardiac Beta-Oxidation Capacity
It is well-established that β-oxidation catalyzes the degradation of long-chain fatty acids through four steps (dehydration, oxidation, hydration, and thiolysis) to produce acetyl coenzyme A. mtp consists of a hetero-octamer of four mtpα and four mtpβ subunits. The mtpα subunit has long-chain 3-enoyl coenzyme A hydratase and long-chain 3-hydroxy coenzyme A dehydrogenase activities, catalyzing the second and third steps, respectively; the mtpβ subunit has long-chain 3-ketoacyl coenzyme A Thiolase activity, catalyzing the fourth step. This study was based on previous approaches to β-oxidation function as assessed by acyl-coenzyme A dehydrogenase (β Hydroxy acid dehydrogenase 1 abbreviated as Had1) and 3-hydroxyacyl coenzyme A dehydrogenase (Acyl-CoA oxidase 3 abbreviated as Acox3) [38,39,40]. As seen in Figure 7A, HFD caused a decrease in Had1 expression in w1118 and Hand > w1118 Drosophila cardiomyocytes, as well as a decrease in Acox3 expression in w1118, Hand > w1118 and Hand > mtpRNAi Drosophila cardiomyocytes, indicating that HFD can cause a decrease in cardiac β-oxidation and a reduction in the ability to catalyze long-chain fatty acids, predisposing to excessive accumulation of fat in the heart. However, after exercise training, there was a general increase in Had1 and Acox3 in w1118, Hand > w1118, and Hand > mtpRNAi Drosophila cardiomyocytes, suggesting that exercise training could reverse the decrease in cardiac β-oxidation induced by HFD (Figure 7B), consistent with changes in cardiac mtp expression (Figure 5A).
Figure 7.
(A) Comparison of the expression levels of Had1 and Acox3mRNA in w1118, Hand > w1118, and Hand > mtpRNAi hearts under different treatments using one-way ANOVA with LSD for post hoc testing. Compared to the NFD groups, Had1 expression was decreased in HFD + w1118 and HFD + Hand > w1118 Drosophila cardiomyocytes, while Acox3 expression was decreased in HFD + w1118, HFD + Hand > w1118 and HFD + Hand > mtpRNAi Drosophila cardiomyocytes. Compared with the HFD groups, there was a general increase in Had1 and Acox3 in w1118, Hand > w1118, and Hand > mtpRNAi Drosophila cardiomyocytes after exercise training. All samples were virgin flies of 10 days (N = 50 per group). (B) Putative role of mtp in β-oxidation in the heart stimulated by exercise training.
4. Discussion
Unhealthy lifestyle habits, especially excessive intake of high-fat foods, represent a risk factor that predisposes to cardiovascular disease. Drosophila is widely acknowledged as an excellent model for studying cardiovascular function. More importantly, 77% of human disease genes have Drosophila counterparts, 26 of which have been associated with cardiovascular disease [15,16,17]. Exercise training is an effective means of preventing and treating obesity and associated cardiovascular disease [11,12,13,14]. Accordingly, we performed exercise training on high-fat Drosophila to explore how exercise training might impact high-fat diet-induced cardiac dysfunction.
As an important target in regulating systemic lipid metabolism, mtp, when expressed in the liver and adipose, is involved in the correct folding and assembly of downstream apoB, affecting the secretion of chylomicrons (CMs) and the synthesis of very low-density lipoproteins (VLDL) [3]. In addition, mtp in cardiac myocytes is involved in regulating systemic lipid metabolism, and knocking down mtp in the heart alone can prevent the increase in systemic TG levels caused by HFD. Furthermore, knocking down mtp in the heart can protect systolic cardiac function from HFD to a certain extent, similar to treating Drosophila with HFD by exercise training. Although it is well-recognized that the heart plays an important role in regulating systemic lipid metabolism, and previous studies have found that mtp in cardiac myocytes can regulate systemic lipid metabolism under HFD conditions, the exact mechanism remains unclear [41].
Current evidence suggests that HFD induces myocardial hypertrophy and fibrosis, reduced coronary reserve and cardiac function. Importantly, exercise limits lipid metabolism disorders, cardiac hypertrophy and fibrosis and helps prevent lipotoxic cardiomyopathy. It is well-established that exercise lowers the heart rate [42] and alleviates cardiac oxidative stress by inhibiting LOX-1 receptor expression [43]. In addition, exercise-based cardiac rehabilitation reduces hospitalization rates and cardiovascular mortality and improves the quality of life [44]. A study found that exercise-based cardiac rehabilitation reduced hospitalization rates, cardiovascular mortality and improved quality of life. Interestingly, exercise was found to mediate cardioprotection through upregulation of miR-344g-5p, which targets Hmgcs2 mRNA, and inhibits HMGCS2 upregulation, thereby suppressing lipotoxicity [45]. It has also been suggested that aerobic exercise reverses cardiac remodeling by reducing inflammation, fibrosis and apoptosis in HFD rats, to a certain extent by inhibiting P2X7R expression in cardiomyocytes [46]. Herein, we showed that exercise training had a therapeutic effect on the decrease in cardiac mtp expression caused by HFD, both in w1118, Hand > w1118 and Hand > mtpRNAi Drosophila; Had1 and Acox3mRNA expression in the heart of HFD flies was elevated to some extent after exercise training, suggesting that the ability of cardiomyocytes to catalyze long-chain fatty acid catabolism may have increased.
5. Conclusions
Overall, we provided compelling evidence that knocking down mtp in the heart could prevent an increase in systemic TG levels and protect cardiac contractility from HFD-induced damage, which is similar to performing exercise training to some extent. Moreover, exercise training upregulated the decrease in cardiac mtp expression induced by HFD. An increase in the expression of Had1 and Acox3, which are downstream of mtp, was observed, which was highly consistent with the changes in cardiac mtp. This finding suggests that the upregulation of cardiac mtp by exercise training may be an important pathway to treat HFD-induced cardiac dysfunction and abnormal lipid metabolism. It is conceivable that elevated cardiac mtp expression indirectly drives up downstream β oxidase, thereby perhaps increasing the ability of cardiomyocyte mitochondria to break down triglycerides. Given the important role of cardiomyocyte mtp in regulating systemic lipid metabolism and protecting the heart from lipotoxicity, the ameliorative effects of exercise training on cardiac mtp provide new insights for future clinical studies related to lipotoxic cardiomyopathy, especially on the potential use of exercise training to treat abnormalities in lipid metabolism and cardiac dysfunction due to HFD.
Acknowledgments
We thank the Key Laboratory of Physical Fitness and Exercise Rehabilitation of Hunan Province and Bloomington Drosophila Stock Center for fly stocks. We also thank Karen Ocorr and Rolf Bodmer (Sanford Burnham Institute of Neuroscience and Aging Research Center) for supporting semi-automatic optical echocardiographic analysis software.
Author Contributions
T.P. and M.D. conceived and designed the experiments and wrote the manuscript. T.P., Q.L., H.Y. and P.Z. collected the samples. T.P., M.D., R.T. and P.Z. performed the experiments. T.P. and L.Z. analyzed the data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 32071175.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giammanco A., Cefalù A.B., Noto D., Averna M.R. The pathophysiology of intestinal lipoprotein production. Front. Physiol. 2015;6:61. doi: 10.3389/fphys.2015.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussain M.M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 2014;25:200–206. doi: 10.1097/MOL.0000000000000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamilton R.L. Synthesis and secretion of plasma lipoproteins. Adv. Exp. Med. Biol. 1972;26:7–24. doi: 10.1007/978-1-4684-7547-0_2. [DOI] [PubMed] [Google Scholar]
- 4.Gordon D.A., Wetterau J.R., Gregg R.E. Microsomal triglyceride transfer protein: A protein complex required for the assembly of lipoprotein particles. Trends Cell Biol. 1995;5:317–321. doi: 10.1016/S0962-8924(00)89054-6. [DOI] [PubMed] [Google Scholar]
- 5.Wetterau J.R., Lin M.C., Jamil H. Microsomal triglyceride transfer protein. Biochim. Biophys. Acta. 1997;1345:136–150. doi: 10.1016/S0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 6.Sirtori C.R., Pavanello C., Bertolini S. Microsomal transfer protein (MTP) inhibition-a novel approach to the treatment of homozygous. Ann. Med. 2014;46:464–474. doi: 10.3109/07853890.2014.931100. [DOI] [PubMed] [Google Scholar]
- 7.Lin M., Zhao S., Shen L., Xu D. Potential approaches to ameliorate hepatic fat accumulation seen with MTP inhibition. Drug Saf. 2014;37:213–224. doi: 10.1007/s40264-014-0147-x. [DOI] [PubMed] [Google Scholar]
- 8.Love J.D., Suzuki T., Robinson D.B., Harris C.M., Johnson J.E., Mohler P.J., Jerome W.G., Swift L.L. Microsomal Triglyceride Transfer Protein (MTP) Associates with Cytosolic Lipid Droplets in 3T3-L1 Adipocytes. PLoS ONE. 2015;10:e0135598. doi: 10.1371/journal.pone.0135598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Z., Tang J., Ji K. Exercise prevents HFD-induced insulin resistance risk: Involvement of TNF-α level regulated by vagus nerve-related anti-inflammatory pathway in the spleen. Diabetol. Metab. Syndr. 2021;13:124. doi: 10.1186/s13098-021-00712-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway B., Rene A. Obesity as a disease: No lightweight matter. Obes. Rev. 2004;5:145–151. doi: 10.1111/j.1467-789X.2004.00144.x. [DOI] [PubMed] [Google Scholar]
- 11.Jensen M.D., Ryan D.H., Apovian C.M., Ard J.D., Comuzzie A.G., Donato K.A., Hu F.B., Hubbard V.S., Jakicic J.M., Kushner R.F., et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity. management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129((Suppl. S2)):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. Erratum in: Circulation 2014, 129 (Suppl. S2), S139–S140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone S., Del Buono M.G., Ozemek C., Lavie C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019;62:327–333. doi: 10.1016/j.pcad.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Lavie C.J., Arena R., Swift D.L., Johannsen N.M., Sui X., Lee D.C., Earnest C.P., Church T.S., O’Keefe J.H., Milani R.V., et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacchi E., Negri C., Zanolin M.E., Milanese C., Faccioli N., Trombetta M., Zoppini G., Cevese A., Bonadonna R.C., Schena F., et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: A randomized controlled trial (the RAED2 study) Diabetes Care. 2012;35:676–682. doi: 10.2337/dc11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams M.D., Celniker S.E., Holt R.A., Evans C.A., Gocayne J.D., Amanatides P.G., Scherer S.E., Li P.W., Hoskins R.A., Galle R.F., et al. The genome sequence of Drosophila melanogaster sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 16.Souidi A., Jagla K. Drosophila Heart as a Model for Cardiac Development and Diseases. Cells. 2021;10:3078. doi: 10.3390/cells10113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaffran S., Frasch M. Early signals in cardiac development. Circ. Res. 2002;91:457–469. doi: 10.1161/01.RES.0000034152.74523.A8. [DOI] [PubMed] [Google Scholar]
- 18.Trinh I., Boulianne G.L. Modeling obesity and its associated disorders in Drosophila. Physiology. 2013;28:117–124. doi: 10.1152/physiol.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bordicchia M., Liu D., Amri E.Z., Ailhaud G., Dessì-Fulgheri P., Zhang C., Takahashi N., Sarzani R., Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012;122:1022–1036. doi: 10.1172/JCI59701. Erratum in: J. Clin. Investig. 2012, 122, 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grueter C.E., van Rooij E., Johnson B.A., DeLeon S.M., Sutherland L.B., Qi X., Gautron L., Elmquist J.K., Bassel-Duby R., Olson E.N. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149:671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taghli-Lamallem O., Akasaka T., Hogg G., Nudel U., Yaffe D. Dystrophin deficiency in Drosophila reduces lifespan and causes a dilated cardiomyopathy phenotype. Aging Cell. 2008;7:237–249. doi: 10.1111/j.1474-9726.2008.00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ocorr K., Reeves N.L., Wessells R.J., Fink M., Chen H.S., Akasaka T., Yasuda S., Metzger J.M., Giles W., Posakony J.W., et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proc. Natl. Acad. Sci. USA. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wessells R., Fitzgerald E., Piazza N., Ocorr K., Morley S., Davies C., Lim H.Y., Elmén L., Hayes M., Oldham S., et al. d4eBP acts downstream of both dTOR and dFoxo to modulate cardiac functional aging in Drosophila. Aging Cell. 2009;8:542–552. doi: 10.1111/j.1474-9726.2009.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Birse R.T., Choi J., Reardon K., Rodriguez J., Graham S., Diop S., Ocorr K., Bodmer R., Oldham S. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. by the TOR pathway in Drosophila. Cell Metab. 2010;12:533–544. doi: 10.1016/j.cmet.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stobdan T., Sahoo D., Azad P., Hartley I., Heinrichsen E., Zhou D., Haddad G.G. High fat diet induces sex-specific differential gene expression in Drosophila melanogaster. PLoS ONE. 2019;14:e0213474. doi: 10.1371/journal.pone.0213474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ocorr K., Akasaka T., Bodmer R. Age-related cardiac disease model of Drosophila. Mech. Ageing Dev. 2007;128:112–116. doi: 10.1016/j.mad.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fink M., Callol-Massot C., Chu A., Ruiz-Lozano P., Izpisua Belmonte J.C., Giles W., Bodmer R., Ocorr K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques. 2009;46:101–113. doi: 10.2144/000113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diop S.B., Birse R.T., Bodmer R. High Fat Diet Feeding and High Throughput Triacylglyceride Assay in Drosophila Melanogaster. J. Vis. Exp. 2017;127:56029. doi: 10.3791/56029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng L., Feng Y., Wen D.T., Wang H., Wu X.S. Fatiguing exercise initiated later in life reduces incidence of fibrillation and improves sleep quality in Drosophila. Age. 2015;37:9816. doi: 10.1007/s11357-015-9816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen D.T., Zheng L., Yang F., Li H.Z., Hou W.Q. Endurance exercise prevents high-fat-diet induced heart and mobility premature aging and dsir2 expression decline in aging Drosophila. Oncotarget. 2017;9:7298–7311. doi: 10.18632/oncotarget.23292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen D.T., Zheng L., Li J.X., Cheng D., Liu Y., Lu K., Hou W.Q. Endurance exercise resistance to lipotoxic cardiomyopathy is associated with cardiac NAD+/dSIR2/PGC-1α pathway activation in old Drosophila. Biol. Open. 2019;8:bio044719. doi: 10.1242/bio.044719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong R., Piper M.D., Wertheim B., Partridge L. Quantification of food intake in Drosophila. PLoS ONE. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., Yang S., Fu J., Liu A., Liu D., Cao S. Inhibition of endoplasmic reticulum stress prevents high-fat diet mediated atrial fibrosis and fibrillation. J. Cell Mol. Med. 2020;24:13660–13668. doi: 10.1111/jcmm.15816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu H.C., Kovacs A., Blanton R.M., Han X., Courtois M., Weinheimer C.J., Yamada K.A., Brunet S., Xu H., Nerbonne J.M., et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ. Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 36.Shao D., Kolwicz SCJr Wang P., Roe N.D., Villet O., Nishi K., Hsu Y.A., Flint G.V., Caudal A., Wang W., Regnier M., et al. Increasing Fatty Acid Oxidation Prevents High- Fat Diet-Induced Cardiomyopathy Through Regulating Parkin-Mediated Mitophagy. Circulation. 2020;142:983–997. doi: 10.1161/CIRCULATIONAHA.119.043319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guida M.C., Birse R.T., Dall’Agnese A., Toto P.C., Diop S.B., Mai A., Adams P.D., Puri P.L., Bodmer R. Intergenerational inheritance of high fat diet-induced cardiac lipotoxicity in Drosophila. Nat. Commun. 2019;10:193. doi: 10.1038/s41467-018-08128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azevedo P.S., Polegato B.F., Paiva S., Costa N., Santos P., Bazan S., Fernandes A.A.H., Fabro A., Pires V., Tanni S.E., et al. The role of glucose metabolism and insulin resistance in cardiac remodelling induced by cigarette smoke exposure. J. Cell Mol. Med. 2021;25:1314–1318. doi: 10.1111/jcmm.16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen T., Liu J., Li N., Wang S., Liu H., Li J., Zhang Y., Bu P. Mouse SIRT3 attenuates hypertrophy-related lipid accumulation in the heart through the deacetylation. PLoS ONE. 2015;10:e0118909. doi: 10.1371/journal.pone.0155173. Erratum in: PLoS ONE 2016, 11, e0155173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange K.H., Isaksson F., Juul A., Rasmussen M.H., Bülow J., Kjaer M. Growth hormone enhances effects of endurance training on oxidative muscle metabolism in. Am. J. Physiol. Endocrinol. Metab. 2000;279:E989–E996. doi: 10.1152/ajpendo.2000.279.5.E989. [DOI] [PubMed] [Google Scholar]
- 41.Lee S., Bao H., Ishikawa Z., Wang W., Lim H.Y. Cardiomyocyte Regulation of Systemic Lipid Metabolism by the Apolipoprotein B-Containing Lipoproteins in Drosophila. PLoS Genet. 2017;13:e1006555. doi: 10.1371/journal.pgen.1006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freitas P.D., Ferreira P.G., Silva A.G., Stelmach R., Carvalho-Pinto R.M., Fernandes F.L., Mancini M.C., Sato M.N., Martins M.A., Carvalho C.R. The Role of Exercise in a Weight-Loss Program on Clinical Control in Obese Adults with Asthma. A Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2017;195:32–42. doi: 10.1164/rccm.201603-0446OC. [DOI] [PubMed] [Google Scholar]
- 43.Machado M.V., Vieira A.B., da Conceição F.G., Nascimento A.R., da Nóbrega A.C.L., Tibirica E. Exercise training dose differentially alters muscle and heart capillary density and metabolic functions in an obese rat with metabolic syndrome. Exp. Physiol. 2017;102:1716–1728. doi: 10.1113/EP086416. [DOI] [PubMed] [Google Scholar]
- 44.Riahi S., Mohammadi M.T., Sobhani V., Soleimany M. Chronic effects of aerobic exercise on gene expression of LOX-1 receptor in the heart of rats fed with high fat diet. Iran J. Basic Med. Sci. 2015;18:805–812. [PMC free article] [PubMed] [Google Scholar]
- 45.Li S., Qian X., Gong J., Chen J., Tu W., Chen X., Chu M., Yang G., Li L., Jiang S. Exercise Training Reverses Lipotoxicity-induced Cardiomyopathy by Inhibiting HMGCS2. Med. Sci. Sports Exerc. 2021;53:47–57. doi: 10.1249/MSS.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 46.Chen X., Li H., Wang K., Liang X., Wang W., Hu X., Huang Z., Wang Y. Aerobic Exercise Ameliorates Myocardial Inflammation, Fibrosis and Apoptosis in High-Fat-Diet Rats by Inhibiting P2X7 Purinergic Receptors. Front Physiol. 2019;10:1286. doi: 10.3389/fphys.2019.01286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.