Abstract
In vitro studies have shown that enterotoxigenic Escherichia coli (ETEC) strains are capable of invading cultured epithelial cells derived from the human ileum and colon. Two separate invasion loci (tia and tib) have previously been isolated from the classical ETEC strain H10407. The tia locus has been shown to direct the synthesis of Tia, a 25-kDa outer membrane protein. Tia is sufficient to confer the adherence and invasion phenotypes on laboratory stains of E. coli, suggesting that this protein is an adhesin and invasin. Here we report the purification of Tia and characterize its biological activity. Tia was purified by electroelution of outer membrane proteins that had been separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Purified Tia was labeled with biotin and then shown to bind to HCT8 human ileocecal epithelial cells in a specific and saturable manner. Polyclonal anti-Tia antiserum blocked this binding. These results show that Tia acts as an adhesin. Polyclonal anti-Tia antiserum also inhibited invasion of recombinant E. coli bearing tia clones, indirectly suggesting that Tia may also act as an invasin. We predict Tia to contain eight transmembrane amphipathic β-sheets with four loops that are exposed on the surface of the bacterial cell. A peptide corresponding to 19 residues in one of the four predicted surface-exposed loops inhibits Tia-mediated epithelial cell invasion. Seeding HCT8 cells on wells coated with purified Tia reduced Tia-mediated epithelial cell invasion. Together, these results indicate that Tia is an invasin and adhesin that binds a specific receptor on HCT8 cells.
Diarrheal disease is a persistent problem in developing countries. Enterotoxigenic Escherichia coli (ETEC) is a leading cause of this disease, and is responsible for about 600 million cases of diarrhea worldwide, annually resulting in over 1.2 million deaths, 700,000 of which occur in children under the age of 5 years (35). ETEC infections are initiated by the consumption of contaminated food or drink. The bacteria then colonize the small intestine via fimbrial colonization factor antigens. Subsequent to colonization, the bacteria release heat-stable and/or heat-labile enterotoxins that lead to a net secretion of fluid and diarrhea (28). However, human and animal challenge studies performed with ETEC strains that no longer produce heat-stable or heat-labile enterotoxins indicate that enterotoxins may not be exclusively required for diarrhea (21, 30, 31, 34). This result suggests the presence of previously uncharacterized enterotoxins or other virulence factors in ETEC strains.
Many diarrheal pathogens are capable of penetrating intestine epithelial cells. Although there is currently no direct evidence that epithelial cell invasion occurs during ETEC pathogenesis in humans, it has been found that ETEC strains are capable of invading epithelial cell lines derived from the human ileocecum and colon (10). Additionally, intestinal biopsy samples taken from ETEC-infected piglets have shown intracellular bacteria (27), supporting the possibility that epithelial cell invasion may occur in vivo during human infections. Two separate chromosomally encoded invasion loci (tia and tib) have been cloned from the human-specific classical ETEC strain H10407 (10, 11, 13). These loci direct nonadherent and noninvasive laboratory strains of E. coli to adhere to and invade cultured human intestinal epithelial cells.
The adherence and invasion phenotypes of the tia locus are conferred by a single gene (tia) that directs the synthesis of Tia, a 25-kDa outer membrane protein. Transformation of Tia-expressing plasmids into laboratory strains of E. coli allows these strains to adhere to and invade cultured human ileocecal and colonic epithelial cells (13). This result suggests that Tia acts as an adhesin and as an invasin. In support of this hypothesis, deletion of tia from the parent ETEC strain reduces the ability of H10407 to adhere to and invade epithelial cells to about 25% of the wild-type level (13). Additionally, Tia shares homology with Ail and Hra-1. Ail is an afimbrial adhesin and invasin that has been identified in pathogenic Yersinia species (25), and Hra-1 is an afimbrial adhesin identified in a porcine ETEC strain (24). However, direct evidence of the ability of Tia to act as an adhesin and invasin has not been shown. We report here the purification of the Tia protein and demonstrate the ability of purified Tia to bind human intestine epithelial cells, and thereby act as an adhesin. Additionally, we show that anti-Tia polyclonal antibodies block Tia-mediated epithelial cell invasion, indirectly suggesting that Tia may act as an invasin as well as an adhesin. Furthermore, these activities appear to result from the interaction between Tia and a specific receptor(s) on the surface of the host cell.
MATERIALS AND METHODS
Bacterial strains, tissue culture cells, and culture conditions.
ETEC strain H10407 (12) (serotype 078:H11; colonization factor antigen I [CFA]) was the parent strain from which the tia gene was cloned. Laboratory E. coli strains HB101 (4) and DH5α (15) were used as nonadherent and noninvasive recipients of tia-containing plasmids. Organisms were grown in Luria broth (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl per liter; pH 7.6) at 37°C and 200 rpm unless otherwise indicated. Ampicillin was added to growth medium at a final concentration of 100 μg per ml. HCT8 (ATCC CCL 244) human ileocecal epithelial cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 1 mM sodium pyruvate, and 2 mM l-glutamine (HCT8 medium). HCT8 cells were grown at 37°C in a 6% CO2 environment.
Membrane fractionation.
Outer membrane fractions were isolated as previously described (11, 32). Luria broth cultures (500 ml) of E. coli DH5α(pET125) were grown overnight with shaking at 37°C. Plasmid pET125 contains the tia gene under the transcriptional control of its native promoter (13). The overnight cultures were harvested by centrifugation and then lysed by two passages through a French press. Inner and outer membranes were separated by sucrose density ultracentrifugation.
Purification of Tia.
Outer membranes were isolated from E. coli DH5α(pET125) as described above. Purified outer membranes (800 μg) were treated with Laemmli sample buffer at 98°C for 10 min and then separated by discontinuous sodium dodecyl sulfate–13% polyacrylamide gel electrophoresis (SDS–13% PAGE) for 24 h at 70 V. After electrophoresis, proteins were eluted using the Bio-Rad Whole Gel Eluter (WGE). Briefly, polyacrylamide gels were overlaid on the WGE and the individual bands were eluted into separate fractions in 60 mM Tris–40 mM 3-[cyclohexylamino]-1-propanesulfonic acid (CAPS) (pH 9.4) buffer containing 1% SDS at 250 mA for 1 h. After collection by vacuum harvesting, individual fractions were analyzed by SDS–13% PAGE. Tia-containing fractions were pooled, concentrated in dialysis tubing against sucrose, and then dialyzed against phosphate-buffered saline (PBS), pH 7.4. The concentration of the purified Tia was determined using the Pierce Micro BCA protein assay. Purified Tia was treated with urea as follows: 3 μg of purified Tia was brought to 6 M urea and then incubated with Laemmli sample buffer prior to analysis by SDS-PAGE as described above.
The same procedure was used to purify OmpW from the E. coli DH5α outer membrane. OmpW is a 21-kDa outer membrane protein produced by E. coli strains, including DH5α. The function of OmpW is unknown, although recently it has been shown to act as a receptor for colicin S4 (26, 30). OmpW was used as a control for binding assays.
Antiserum.
The immune and preimmune sera used in these assays were a generous gift from J. M. Fleckenstein. The immune serum was prepared by immunization of rabbits with a urea-solubilized suspension of a His-tagged Tia fusion protein that had been subjected to nickel-chelation affinity chromatography (J. M. Fleckenstein, unpublished data). We prepared the sera for these experiments by first absorbing them with E. coli HB101 and then subjecting them to affinity purification. E. coli HB101 absorption was performed as follows. HB101 was grown overnight in Luria broth. One milliliter of the overnight culture was harvested by centrifugation (16,000 × g for 1 min at room temperature), resuspended in 0.5 ml of PBS, mixed with 0.5 ml of antiserum, and then incubated at room temperature for 1 h. After incubation, bacteria were removed by two 1-min room temperature centrifugations at 16,000 × g. Immunoglobulin G (IgG) antibodies from the absorbed rabbit polyclonal antisera were affinity purified on protein G columns (MabTrap G II; Pharmacia) according to the manufacturer's recommendation. Absorbed and affinity-purified immunoglobulins were dialyzed against PBS overnight at 4°C. The protein concentration of the dialyzed sample was adjusted to 25 mg/ml (as determined by the Micro BCA assay).
Binding assays.
Purified Tia, purified OmpW, and bovine serum albumin (BSA) (Roche Molecular Biochemicals) were labeled with biotin using the reagent d-biotinoyl-ɛ-aminocaproic acid-N-hydroxylsuccinimide ester (Roche) following the manufacturer's recommendations. The biotin reagent was dissolved at 1 mg/ml in dimethylformamide and then incubated with each protein at a molar ratio of 10:1 (biotin-protein) and a volume ratio of 1:50 (biotin-protein) for 4 h at 4°C. After labeling, the proteins were dialyzed against PBS overnight at 4°C. The concentration of biotin-labeled protein was determined by the Pierce Micro BCA protein assay, and biotinylation was confirmed by dot blot analysis. HCT8 monolayers were prepared for binding assays by seeding approximately 105 cells in each well of a 96-well plate and then incubating the plates overnight at 37°C in a 6% CO2 environment. After overnight growth, monolayers were washed once with Earle's balanced salts solution (EBSS) and then fixed at room temperature in 0.25% glutaraldehyde in EBSS for 10 min. After fixation, the monolayers were washed twice with PBS containing 0.1% Tween 20 (PBS-T) and then blocked with FBS for 1 h at 37°C. The fixed and blocked monolayers were then washed thrice with PBS-T prior to the addition of biotin-labeled protein at various concentrations in a final volume of 60 μl of PBS containing 0.1% Tween 20. Labeled proteins were incubated with the monolayers for 1 h at 37°C and then washed thrice with PBS-T. After washing, 60 μl of a 1:20,000 dilution (in PBS-T) of peroxidase-conjugated streptavidin (Roche) was added to each well and then incubated at room temperature for 1 h. After washing thrice with PBS-T, 100 μl of a peroxidase substrate (ABST system; Roche) was added to each well, and color development was measured at 405 nm in an enzyme-linked immunosorbent assay reader.
In competition assays, 3 pmol of biotinylated Tia was mixed with 0.0, 0.2, 0.4, 1.0, 2.0, 3.0, or 4.0 pmol of unlabeled Tia. Each mixture was brought to a final volume of 60 μl and a final concentration of 0.1% Tween 20. After incubation with fixed HCT8 cell monolayers at 37°C for 1 h, the wells were washed and bound Tia was detected as described above.
When examining the effects of rabbit polyclonal anti-Tia antiserum and preimmune serum on the binding of purified Tia to HCT8 cells, serum was added simultaneously with 3 pmol of labeled Tia to reach the indicated serum dilutions in a final total volume of 60 μl. The binding assay was then performed as described above.
Invasion assays.
Bacterial invasion of epithelial cells was measured as protection from the bactericidal antibiotic gentamicin (19). Invasion assays were performed as previously described (10). Briefly, approximately 5 × 106 log-phase CFU were added to HCT8 monolayers (approximately 3.5 × 105 cells in 24-well tissue culture plates), which were then incubated at 37°C for 4 h in a 6% CO2 atmosphere. The actual inoculum for each experiment was determined by quantitative plate count. After being washed, the infected monolayers were incubated for an additional 1 h in tissue culture medium containing gentamicin. The infected monolayers were washed and then lysed in 1% Triton X-100 in deionized water, and the bacteria were quantified by plate count. Invasion is expressed as the percentage of organisms surviving exposure to gentamicin relative to the number of bacteria added to the tissue culture wells at the beginning of the assay. Since the results of invasion assays are variable on a daily basis, the datum points presented in the figures are average values (± standard deviation) from triplicate wells of a single experiment and correlate with values obtained in replicate experiments that were performed at least three times.
In peptide inhibition experiments, each peptide was dissolved in HCT8 medium at a concentration of 5 mM. Fifteen minutes prior to the inoculation of the monolayers, the dissolved peptides were added to the HCT8 cells to a final concentration of 500 μM. At this concentration, the peptides did not affect the pH of the tissue culture medium. Invasion assays then were performed as described above. In receptor sequestration assays, 5 μg (25 μl of a 0.2-μg/μl solution in PBS) of purified Tia, purified OmpW, or BSA was used to coat the wells of the 24-well plate. After an overnight incubation at 4°C, the wells were washed with EBSS and then exposed to UV light in a laminar-flow hood for 30 min prior to seeding the coated wells with HCT8 cells. Invasion assays were performed using the coated wells as described above.
For antibody inhibition experiments, rabbit polyclonal antisera were absorbed with E. coli HB101 and affinity purified as described above. It was necessary to affinity purify the antibodies since heat-inactivated serum inhibited bacterial growth, as determined by viability counts of bacteria grown under invasion conditions (data not shown). Affinity-purified IgG did not inhibit bacterial viability (data not shown). In antibody inhibition experiments, the absorbed and affinity-purified antibodies were added (to reach the indicated dilutions) directly to the tissue culture medium bathing the HCT8 monolayers 5 min prior to the inoculation of the wells.
Protein electrophoresis.
SDS-PAGE was performed under denaturing conditions by the method of Laemmli (20). Gels were stained for proteins with Coomassie blue. The protein concentration of samples was determined by the Bradford method (5) using a kit from Bio-Rad. For immunoblotting, proteins separated by electrophoresis were transferred to nitrocellulose (33) at 65 V for 1 h and then blocked with casein filler solution (2% casein and 0.1% sodium azide in 10 mM Tris-buffered saline [pH 7.4]). Blocked filters were probed for 1 h with absorbed and affinity-purified polyclonal rabbit anti-Tia antiserum (1:1,000 dilution in casein filler), washed in Tris-buffered saline containing 0.05% Triton X-100, incubated for 1 h with peroxidase-conjugated protein A (1:5,000 dilution in casein filler; Sigma), rewashed, and then detected with BM Blue precipitating peroxidase substrate (Roche).
Amino acid analysis.
The locations of amphipathic β-sheets and transmembrane domains were determined by using TopPret II 1.3 software (7).
Amino-terminal sequencing of proteins.
To confirm their identities, the purified Tia and OmpW proteins were sequenced by Midwest Analytical, Inc. (St. Louis, Mo.), using the Edman degradation method (9). The amino-terminal sequence of our purified Tia (DESKTGFYVT) matched that previously published for this protein (13). The amino-terminal sequence of our purified 21-kDa outer membrane protein (HEAGEFFMRA) matched that of OmpW.
RESULTS
Tia purification.
When expressed in recombinant E. coli strains, the Tia protein confers the ability to adhere to and invade epithelial cells grown in culture. Deletion of tia from ETEC strain H10407 reduces epithelial cell adherence and invasion by this strain to about 25% of the wild-type level (13). These results indicate that Tia is an adhesin and invasin. To directly test its biological activity, we purified Tia from the outer membranes of E. coli DH5α bearing the Tia-expressing plasmid pET125, which constitutively expresses the tia gene (13). We purified Tia from a recombinant laboratory strain of E. coli rather than from the parent strain because H10407 expresses Tia only when grown under adherence or invasion assay conditions (E. A. Elsinghorst and K. Park, unpublished data). Therefore, to obtain the quantity of Tia required for our experiments, E. coli DH5α(pET125) was used as the source of Tia-containing outer membranes.
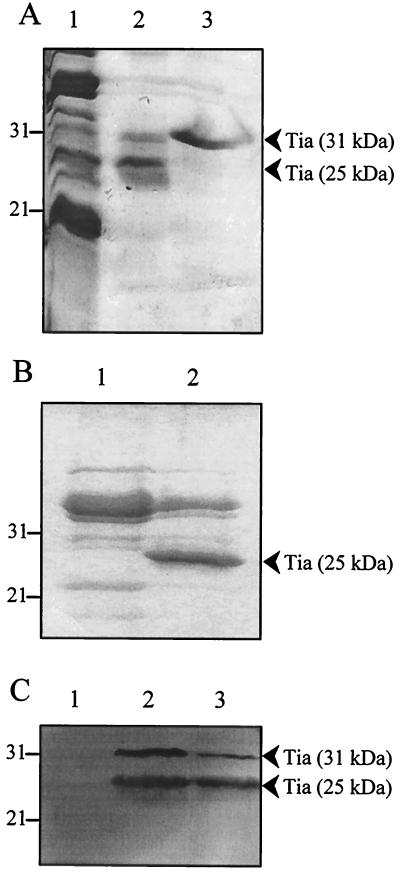
We employed several different techniques to purify Tia from outer membranes, including preparative isoelectric focusing, continuous-elution native PAGE, and denaturing PAGE. The Tia purification technique we describe here is the electroelution of outer membrane proteins separated by SDS-PAGE. In this purification technique, isolated Tia-containing outer membranes were subjected to discontinuous SDS-PAGE using gels that had been cast with a single well that ran the length of the gel. After electrophoresis, the separated proteins were electroeluted from the gel into individual fractions using a Bio-Rad WGE. Each fraction was then analyzed by SDS-PAGE to identify those fractions that contained Tia. Electroeluter fractions containing Tia always contained a 31-kDa contaminant protein (Fig. 1A). All other attempted purification methods yielded the same contaminating protein in fractions containing Tia (data not shown). Because it was consistently found along with Tia, regardless of purification method, we hypothesized that the contaminating protein might actually be a form of Tia. Therefore, we treated Tia-containing electroeluter fractions with urea, followed by standard denaturing treatment using Laemmli sample buffer. As a result of this treatment, the 25-kDa Tia band migrated solely to the 31-kDa form during SDS-PAGE (Fig. 1A). This result suggests that SDS denaturation allows Tia to retain some tertiary structure and that treatment with both urea and SDS fully denatures the protein to a form that migrates with an apparent mass of 31,000 Da. After treatment with urea and SDS, only a single band was observed by SDS-PAGE, indicating that Tia had been purified to apparent homogeneity. In the absence of plasmid pET125, E. coli DH5α does not produce an outer membrane protein with a mass similar to that of Tia (Figure 1B and reference 13), supporting the conclusion that the electroeluted Tia was homogeneous.
FIG. 1.
Purification of Tia. (A) Coomassie blue-stained SDS–13% PAGE of purified Tia. Lanes: 1, outer membranes from E. coli DH5α(pET125); 2, pooled WGE fractions containing Tia, showing 25- and 31-kDa forms of Tia; 3, Tia-containing WGE fraction treated with urea prior to SDS-PAGE, showing only the 31-kDa form of Tia. (B) Coomassie-blue stained SDS–13% PAGE of outer membranes prepared from E. coli DH5α bearing either pHC79 (the vector for plasmid pET125) (lane 1) or the Tia-expressing plasmid pET125 (lane 2). (C) Western blot performed with absorbed and IgG-affinity-purified rabbit polyclonal anti-Tia antibodies. Lanes: 1, outer membranes isolated from E. coli DH5α(pHC79); 2, purified Tia; 3, outer membranes isolated from E. coli DH5α(pET125). Molecular mass standards (in kilodaltons) are indicated to the left of the panels.
To confirm that this purified protein was indeed homogeneous Tia, we subject the preparation to amino-terminal sequencing. The resulting sequence (DESKTGFYVT) was strong and clean, indicating a single pure protein, and was an exact match with the known amino-terminal sequence of Tia (13). Additionally, both the 25- and 31-kDa bands observed in the purified Tia preparation were recognized by absorbed and affinity-purified rabbit polyclonal anti-Tia antiserum (Fig. 1C). The Tia antiserum also showed that the 31-kDa form of Tia is present in the outer membranes of E. coli DH5α containing plasmid pET125 (Fig. 1C).
We used the same electroelution technique to purify the E. coli OmpW protein to apparent homogeneity (data not shown). OmpW is a 21-kDa minor outer membrane protein of unknown function. This protein served as an outer membrane protein control for the epithelial cell binding and receptor sequestration experiments described below.
Tia is an adhesin.
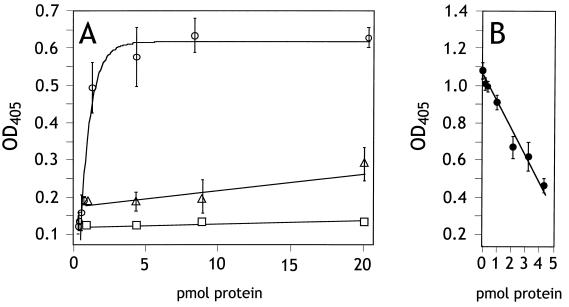
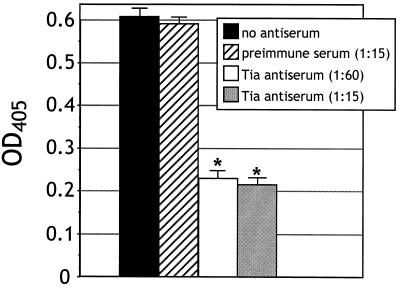
Since Tia-expressing bacteria adhere to cultured epithelial cells, we were interested in determining if purified Tia could bind epithelial cells in vitro. Therefore, the purified Tia was labeled with biotin and then incubated with monolayers of glutaraldehyde-fixed and FBS-blocked HCT8 ileocecal epithelial cells. After washing, the Tia that remained bound to monolayers was detected using peroxidase-conjugated streptavidin. Binding of Tia to epithelial cells was concentration dependent and saturated at approximately 5 pmol (Fig. 2A), suggesting that Tia bound to some specific receptor(s) that was limited in number. To support this conclusion, increasing concentrations of unlabeled Tia were mixed with 3 pmol of labeled Tia. Three pmol of unlabeled Tia was enough to decrease binding of labeled Tia by 50% (Fig. 2B). The ability of unlabeled Tia to compete with labeled protein for binding to epithelial cells indicates the presence of a limited number of Tia binding sites, such as a specific receptor. Tia failed to bind to FBS-blocked tissue culture wells that did not contain HCT8 cells. As controls, BSA and purified OmpW were biotinylated and used in binding assays. Neither protein demonstrated a specific interaction with HCT8 cells, as only background levels of binding were observed (Fig. 2A). These results showed that epithelial cell binding was a property of Tia. Polyclonal rabbit anti-Tia antiserum, but not preimmune serum, blocked Tia binding activity by 65% (Fig. 3).
FIG. 2.
Binding of purified proteins to HCT8 monolayers. Bound biotinylated protein was detected by peroxidase-coupled streptavidin and a chromogenic peroxidase substrate. Color development in each well was measured at 405 nm. (A) Saturation curves performed with 0.0, 0.04, 0.2, 0.4, 1.0, 4.0, 8.0, and 20.0 pmol of biotin-labeled protein/well. Symbols: ○, Tia; □, BSA; ▵, OmpW. (B) Competition curve performed at a constant concentration of biotin-labeled Tia (3 pmol) plus 0.0, 0.2, 0.4, 1.0, 2.0, 3.0, or 4.0 pmol of unlabeled Tia. Binding experiments were performed at least three times. The extent of biotin labeling varied from day to day, resulting in significant differences in color development. Therefore, the curve shown for each protein represents the result of one experiment performed in triplicate, with the range indicated by bars.
FIG. 3.
Anti-Tia antiserum blocks Tia binding to HCT8 cells. IgG from E. coli HB101-absorbed rabbit preimmune or polyclonal anti-Tia antiserum was affinity purified on a protein G column. Three picomoles of purified and biotinylated Tia was added to HCT8 monolayers simultaneously with the indicated dilutions of absorbed and purified rabbit preimmune or polyclonal immune serum. Bound protein was detected by peroxidase-coupled streptavidin and a chromogenic peroxidase substrate. Color development was measured at 405 nm. Antibody inhibition experiments were performed at least three times. The extent of biotin labeling varied from day to day, resulting in significant differences in color development. Therefore, the values shown for each condition represent the results of one experiment performed in triplicate. Statistically significant (P < 0.001) effects of antibody treatments, as determined by analysis of variance, are indicated (∗).
Anti-Tia antiserum inhibits invasion.
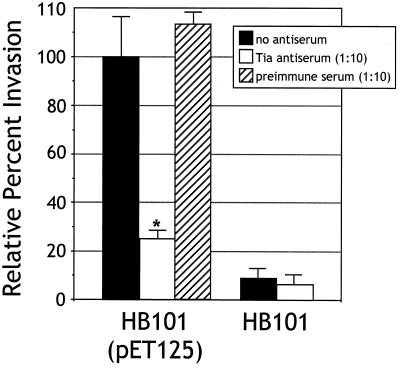
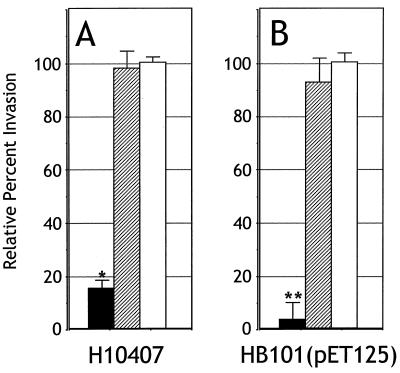
The results obtained from the binding experiments show that Tia acts as an adhesin. To address the question of whether Tia mediates invasion, IgG antibodies from preabsorbed polyclonal anti-Tia antiserum were purified on a protein G column and then added to standard invasion assays. Antibodies were added to the wells of a 24-well plate containing confluent HCT8 cells prior to inoculation of those wells with log-phase bacteria. A 1:10 dilution of anti-Tia antibodies decreased invasion by recombinant E. coli HB101 carrying Tia-expressing plasmids by about 75%, approaching the background levels observed by HB101 alone (Fig. 4). Antibodies from absorbed and affinity-purified preimmune serum had no effect on Tia-mediated invasion. These results suggest that Tia acts as an invasin as well as an adhesin. However, these results do not prove that Tia is an invasin, since the effect of anti-Tia antibodies might be indirect.
FIG. 4.
Anti-Tia antibody inhibits invasion of HCT8 cells. Invasion assays were performed in the absence of antibodies (no antiserum) or in the presence of absorbed and affinity-purified IgG from rabbit preimmune serum or polyclonal anti-Tia antiserum. All percent invasion values are expressed relative to the invasion of E. coli HB101(pET125) in the absence of antiserum, which represents 100% invasion. Data are shown as an average of three replicates performed in triplicate. The average percent invasion of HCT8 cells by E. coli HB101(pET125) in the absence of antiserum was 0.40% (the percent of the inoculum recovered after gentamicin treatment). HB101 is included as a control to show the background level of invasion observed with this laboratory strain of E. coli. The statistically significant (P < 0.005) effect of antibody treatments, as determined by analysis of variance, is indicated (∗).
Peptide inhibition.
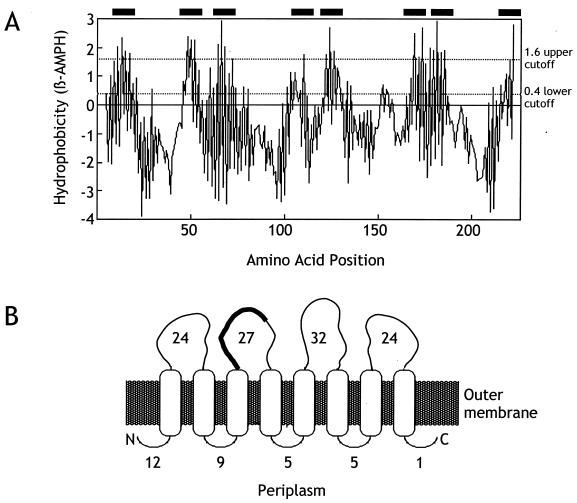
We were interested in predicting how Tia might be folded in the bacterial outer membrane. Therefore, we used TopPred software to analyze the Tia amino acid sequence. TopPred predicted eight certain transmembrane amphipathic sheets within the protein (Fig. 5). Previously reported homology searches revealed that Tia has limited homology with Ail, an adhesin and invasin identified in Yersinia species (13, 25). One region of homology spans residues 78 to 96 of Tia in which 11 of 19 amino acids either are identical or represent conserved changes compared with Ail. The corresponding sequence of amino acids in Ail was predicted to reside in a loop that is exposed on the bacterial cell surface (2). In Tia, we predict that these 19 amino acids are also surface exposed (Fig. 5).
FIG. 5.
Proposed Tia topology. (A) Prediction of amphipathic β-sheets. The amino acid sequence corresponding to mature Tia (i.e., Tia as found in the outer membrane after cleavage of its signal sequence [13]) was analyzed for the presence of amphipathic β-sheets (full window 10) using TopPret software. Filled bars along the top of the figure indicate membrane-spanning β-sheets predicted as certain (1.6 upper cutoff). (B) Model of proposed folding of Tia in the outer membrane. The number adjacent to each exposed loop indicates the number of amino acid residues predicted to be in each corresponding loop. The 19-amino-acid region of the second outward-facing loop that is homologous to Ail is indicated by a thick black line.
The similarity in biological activity between Tia and Ail suggested that the 19-amino-acid region of homology might be involved in Tia function. The predicted surface exposure of these sequences supported this hypothesis. To test this possibility, we synthesized two peptides: a Tia peptide (GYDFYQHYNVPVRTEVEFY) that corresponded to residues 78 to 96 of Tia and a scrambled peptide (QTYRPFVGYEVYDYFHVNE) that contained the same residues but in a random order. These peptides were added to the tissue culture medium bathing HCT8 monolayers prior to the inoculation of those monolayers with log-phase ETEC strain H10407 or E. coli HB101 bearing the Tia-expressing plasmid pET125. The presence of the Tia peptide inhibited invasion by these two strains by over 80% compared to untreated wells or wells treated with the scrambled peptide (Fig. 6), indicating that this region of Tia may be involved in its biological activity.
FIG. 6.
Peptide inhibition of Tia-mediated invasion. Tia peptide (solid bars), scrambled peptide (hatched bars), or no peptide (open bars) was added to HCT8 monolayers to a final concentration of 500 μM prior to the addition of log-phase bacteria. Standard invasion assays were then performed. Data are shown as an average of three replicates performed in triplicate. (A) Invasion of HCT8 cells by ETEC strain H10407 relative to untreated wells receiving no peptide, which represents 100% invasion (percent invasion by H10407 in no peptide wells was 0.88%). (B) Invasion of HCT8 cells by E. coli HB101(pET125) relative to untreated wells receiving no peptide, which represents 100% invasion [percent invasion by HB101(pET125) in no peptide wells was 3.12%]. E. coli HB101 without a Tia-expressing plasmid had an invasion percentage (with or without Tia-specific or scrambled peptide) of 0.32% ± 0.06% relative to E. coli HB101(pET125). Statistically significant effects of peptide treatments as determined by analysis of variance are indicated by ∗ (P < 0.05) and ∗∗ (P < 0.005).
The Tia receptor can be sequestered.
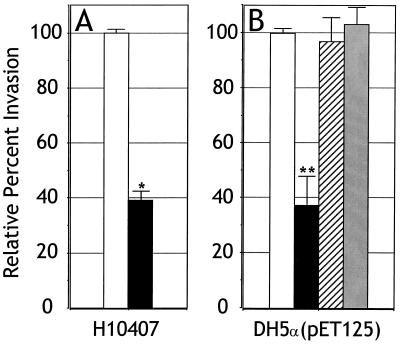
The saturation curve observed during Tia binding to HCT8 cells suggested that a specific receptor might be recognized by this protein. To provide additional evidence that a specific molecule might be involved in Tia binding, receptor sequestration experiments were performed. HCT8 cells were seeded into tissue culture wells that had been coated with BSA, purified OmpW, or purified Tia. If Tia binds to a specific receptor, coating the plastic surface with Tia might sequester that receptor to the basolateral surface of the epithelial cell. Such sequestration would effectively reduce the abundance of that receptor on the apical surface of the cell, thereby inhibiting Tia-mediated bacterial invasion. Coating wells with Tia decreased invasion efficiency by E. coli DH5α containing Tia-expressing plasmids, as well as the invasion efficiency of the wild-type ETEC strain H10407 (Fig. 7). Coating the wells with BSA or OmpW had no effect on invasion efficiency, supporting the hypothesis that Tia binds to a specific receptor.
FIG. 7.
The Tia receptor can be sequestered. Tissue culture wells were coated with purified Tia (solid bars), purified OmpW (hatched bars), or BSA (stippled bars) or left untreated (open bars) prior to seeding with HCT8 cells. Invasion assays were then performed with the coated wells. Data are shown as an average of three replicates. (A) Invasion of HCT8 cells by ETEC strain H10407 relative to untreated wells representing 100% invasion (percent invasion by H10407 in untreated wells was 0.60%). (B) Invasion of HCT8 cells by E. coli DH5α(pET125) relative to untreated wells, which represents 100% invasion [percent invasion by E. coli DH5α(pET125) in untreated wells was 1.03%]. E. coli DH5α without a Tia-expressing plasmid had an invasion percentage (with or without coating of wells) of 1.26% ± 0.07% relative to E. coli DH5α(pET125). Statistically significant effects of treatments as determined by analysis of variance are indicated by ∗ (P < 0.005) and ∗∗ (P < 0.05).
DISCUSSION
It has previously been shown that the tia locus of ETEC strain H10407 directs the synthesis of a 25-kDa outer membrane protein, Tia. Recombinant laboratory strains of E. coli bearing Tia-expressing plasmids are capable of adhering to and invading cultured human intestine epithelial cells (10, 13). Deletion of tia from the H10407 genome reduces the ability of H10407 to adhere to and invade epithelial cells to about 25% of the wild-type level (13). The residual invasion activity present in H10407 tia deletion mutants is due to the presence of the tib locus, as double tia-tib deletion mutants are noninvasive (E. A. Elsinghorst, unpublished data). The tib locus also contributes to the residual HCT8 epithelial cell adherence present in tia deletion mutants: H10407 tia-tib double deletion mutants retain only about 10% of the H10407 wild-type HCT8 cell adherence activity (E. A. Elsinghorst, unpublished data). H10407 produces a fimbrial adhesin (CFA/I) that may contribute to the adherence activity observed in tia-tib deletion mutants.
The association of Tia with bacterial adherence and invasion suggests that this protein acts as an adhesin and invasin. To gain direct evidence for its biological activity, we purified Tia from recombinant E. coli bearing a Tia-expressing plasmid. We used a recombinant E. coli strain for Tia purification because we have found that Tia is expressed by the parent ETEC strain only when grown under adherence or invasion assay conditions (Elsinghorst and Park, unpublished data). Therefore, to purify sufficient quantities of Tia for these studies, we utilized a laboratory strain of E. coli bearing a plasmid that constitutively expresses Tia (13). We confirmed the purified protein to be Tia by amino-terminal sequencing and immunoblotting. When examined by SDS-PAGE, purified Tia migrated as two bands with apparent masses of 25 and 31 kDa. Upon treatment with SDS and urea, the purified Tia migrated as a single band with an apparent mass of 31 kDa. A Tia homolog (referred to as Omp21) has been identified in Comamonas acidovorans. Purified preparations of this 21-kDa outer membrane protein also exhibited multiple bands (at 21 and 24 kDa) upon examination by SDS-PAGE (1), suggesting that such migration patterns may be a characteristic of Tia and its homologs.
Purified Tia bound to cultured human ileocecal epithelial cells in a specific and saturable manner. Such binding is not an intrinsic property of all outer membrane proteins, as purified OmpW failed to adhere to HCT8 cells. These results show that Tia is an adhesin. The ability of Tia to act as an adhesin is in agreement with its known 67% homology with Hra-1 (13). Hra-1 is an afimbrial adhesin that can agglutinate human and animal erythrocytes and human colonic epithelial cells (24). Although these experiments do not provide direct evidence that Tia is an invasin, the ability of polyclonal anti-Tia antiserum to inhibit Tia-mediated epithelial cell invasion suggests that this protein is an invasin. This conclusion is supported by the observations that a peptide corresponding to Tia residues 78 through 96 blocked Tia-mediated invasion of HCT8 cells and that plating HCT8 cells in wells coated with purified Tia inhibited invasion. However, the effects of these treatments on the invasion of HCT8 cells by Tia-expressing bacteria could be indirect. Therefore, additional experiments will be needed to prove that Tia acts directly as an invasin.
Under the conditions employed in these experiments, Tia binding to HCT8 cells saturated at approximately 5 pmol, suggesting that this protein recognizes some specific molecule that is limited in abundance in the plasma membrane of the epithelial cells. In support of this conclusion, purified unlabeled Tia effectively competed with purified labeled Tia for binding to epithelial cells. Additionally, coating tissue culture wells with Tia prior to seeding with HCT8 cells reduced the efficiency of Tia-mediated invasion. These results indicate that Tia binds to a molecule that can be sequestered to the basolateral surface of the epithelial cell. Such a molecule could be referred to as a Tia receptor. Additional supporting evidence for a specific Tia receptor comes from our inhibition experiments in which a Tia peptide blocked Tia-mediated invasion. Previous work has shown that Tia-expressing recombinant E. coli will invade only certain epithelial cell lines. The cell line specificity displayed by these recombinant strains is identical to that displayed by the wild-type parent ETEC strain, H10407 (13). These findings suggest that the Tia receptor is present only in specific cell lines and regions of the intestinal epithelium. We are currently performing experiments to determine the identity and distribution of this receptor.
Analysis of the Tia amino acid sequence allowed us to predict that this protein contains eight outer membrane spanning amphipathic β-sheets. Based on the predicted structure of other outer membrane proteins (17), the eight Tia amphipathic β-sheets are likely to associate as a β-barrel. This predicted tertiary structure results in the presence of four loops that are exposed on the surface of the microorganism. One of these loops contains the 19-amino-acid Ail homologous sequence that appears to be involved in Tia biological activity based on our peptide inhibition experiments. Additional experiments will be needed to determine if other regions of Tia are involved in its biological activity.
We purified Tia under denaturing conditions using the ionic detergent SDS to solubilize Tia-containing outer membranes. Interestingly, SDS solubilization did not appear to completely denature Tia. The use of an additional denaturing agent, such as urea, was required in combination with SDS to completely denature the protein. We performed our experiments with purified Tia that had been denatured only with SDS. The ability of Tia to retain some folded structure in the presence of SDS may have allowed the protein to remain biologically active. Alternatively, the binding activity of Tia may not be dependent on the maintenance of a particular tertiary structure, as might be suggested by the ability of a synthetic peptide to block Tia-mediated invasion of HCT8 cells. Although our purified Tia does retain binding ability, it is possible that partial denaturation with SDS interferes with the full biological activity of the protein. Consequently, there may be differences in the biological activity of our purified Tia compared to native Tia found in the outer membrane of H10407.
Colonization of the intestine epithelial mucosa is a key ETEC virulence mechanism. Most ETEC strains have been shown to possess fimbrial adhesins (i.e., CFAs) that are thought to be responsible for the initial adherence of the pathogen to the intestinal epithelium, and it is known that these CFAs are required for disease (3, 6, 8, 14, 22). In addition to fimbrial adhesins, some ETEC strains are capable of producing afimbrial adhesins (23), but the role of these adhesins in the disease process has not been established. Tia appears to be another type of afimbrial adhesin produced by some strains of ETEC. In other enteric pathogens, afimbrial adhesins are thought to mediate an intimate adherence that allows the more effective delivery of toxins and other effector molecules to the host cell (16, 18). In the case of ETEC infections, CFA-mediated colonization might allow a more intimate contact directed by afimbrial adhesins. This intimate contact might allow the most efficient and effective delivery of ETEC enterotoxins. In the laboratory, Tia can direct epithelial cell invasion. Although epithelial cell invasion currently has not been demonstrated during human ETEC infections, the ability of animal-specific ETEC strains to penetrate the intestinal epithelium in vivo (27) supports a possible role for this activity in the disease process. Invasion directed by Tia might contribute to the development of diarrheal disease through uncharacterized mechanisms. We are currently conducting experiments to determine the role of Tia in the pathogenesis of human ETEC infections.
ACKNOWLEDGMENTS
We thank Jim Fleckenstein for the generous gift of rabbit preimmune and polyclonal anti-Tia antisera.
This work was supported by a grant from the University of Kansas General Research Fund to E. Elsinghorst.
REFERENCES
- 1.Baldermann C, Lupas A, Lubieniecki J, Engelhardt H. The regulated outer membrane protein Omp21 from Comamonas acidovorans is identified as a member of a new family of eight-stranded β-sheet proteins by its sequence and properties. J Bacteriol. 1998;180:3741–3749. doi: 10.1128/jb.180.15.3741-3749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer K B, Miller V L. Amino acid substitutions in naturally occurring variants of ail result in altered invasion activity. J Bacteriol. 1992;174:1360–1369. doi: 10.1128/jb.174.4.1360-1369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black R. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine. 1993;11:100–106. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 4.Boyer H W, Rouland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Cassels F J, Wolf M K. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol. 1995;15:214–226. doi: 10.1007/BF01569828. [DOI] [PubMed] [Google Scholar]
- 7.Claros M G, Heijnen G. TopPret II: an improved software for membrane protein structure predictions. CABIOS. 1994;10:685–686. doi: 10.1093/bioinformatics/10.6.685. [DOI] [PubMed] [Google Scholar]
- 8.DeGraaf F K, Mooi F R. The fimbrial adhesins of Escherichia coli. Adv Microb Physiol. 1986;28:65–143. doi: 10.1016/s0065-2911(08)60237-4. [DOI] [PubMed] [Google Scholar]
- 9.Edman P. On the mechanism of phenylisothiocyanate degradation of peptides. Acta Chem Scand. 1956;10:761–768. [Google Scholar]
- 10.Elsinghorst E A, Kopecko D J. Molecular cloning of epithelial cell invasion determinants from enterotoxigenic Escherichia coli. Infect Immun. 1992;60:2409–2417. doi: 10.1128/iai.60.6.2409-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elsinghorst E A, Weitz J A. Epithelial cell invasion and adherence directed by the enterotoxigenic Escherichia coli tib locus is associated with a 104-kilodalton outer membrane protein. Infect Immun. 1994;62:3463–3471. doi: 10.1128/iai.62.8.3463-3471.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans D G, Silver R P, Evans D J, Jr, Chase D G, Gorbach S L. Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun. 1975;12:656–667. doi: 10.1128/iai.12.3.656-667.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaastra W, Svennerholm A M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 16.Hicks S, Frankel G, Kaper J B, Dougan G, Phillips A D. Role of intimin and bundle-forming pili in enteropathogenic Escherichia coli adhesion to pediatric intestinal tissue in vitro. Infect Immun. 1998;66:1570–1578. doi: 10.1128/iai.66.4.1570-1578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaehnig F. Structure predictions of membrane proteins are not that bad. Trends Biochem Sci. 1990;15:93–95. doi: 10.1016/0968-0004(90)90188-h. [DOI] [PubMed] [Google Scholar]
- 18.Kenny B, Lai L C, Findlay B B, Donnenberg M S. EspA, a protein secreted by enterpathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 19.Kihlstrom E. Infection of HeLa cells with Salmonella typhimurium 395 MS and MR10 bacteria. Infect Immun. 1977;17:290–295. doi: 10.1128/iai.17.2.290-295.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine M M. Vaccines against enterotoxigenic Escherichia coli infections. In: Levine M M, Woodrow G C, editors. New generation vaccines. New York, N.Y: Marcell Dekker; 1990. pp. 649–660. [Google Scholar]
- 23.Lindenthal C, Elsinghorst E A. Identification of a glycoprotein produced by enterotoxigenic Escherichia coli. Infect Immun. 1999;67:4084–4091. doi: 10.1128/iai.67.8.4084-4091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutwyche P, Rupps R, Cavanagh J, Warren R A, Brooks D E. Cloning, sequencing, and viscometric adhesion analysis of heat-resistant agglutinin 1, an integral membrane hemagglutinin from Escherichia coli O9:H10:K99. Infect Immun. 1994;62:5020–5026. doi: 10.1128/iai.62.11.5020-5026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller V L, Bliska J B, Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990;172:1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy M P, Herbert B R, Walsh B J, Tyler M I, Traini M, Sanchez J C, Hochstrasser D F, Williams K L, Gooley A A. Extraction of membrane proteins by differential solubilization for separation using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:837–844. doi: 10.1002/elps.1150190539. [DOI] [PubMed] [Google Scholar]
- 27.Moxley R A, Berberov E M, Francis D H, Xing J, Moayeri M, Welch R A, Baker D R, Barletta R G. Pathogenicity of an enterotoxigenic Escherichia coli hemolysin (hlyA) mutant in gnotobiotic piglets. Infect Immun. 1998;66:5031–5035. doi: 10.1128/iai.66.10.5031-5035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilsl H, Smajs D, Braun V. Characterization of colicin S4 and its receptor, OmpW, a minor protein of the Escherichia coli outer membrane. J Bacteriol. 1999;181:3578–3581. doi: 10.1128/jb.181.11.3578-3581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sack R B, Kline R L, Spira W M. Oral immunization of rabbits with enterotoxigenic Escherichia coli protects against intraintestinal challenge. Infect Immun. 1988;56:387–394. doi: 10.1128/iai.56.2.387-394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlager T A, Wanke C A, Guerrant R L. Net fluid secretion and impaired villous function induced by colonization of the small intestine by nontoxigenic colonizing Escherichia coli. Infect Immun. 1990;58:1337–1343. doi: 10.1128/iai.58.5.1337-1343.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schnaitman C A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970;104:890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamid gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanke C, Guerrant R. Small-bowel colonization alone is cause of diarrhea. Infect Immun. 1987;55:1924–1926. doi: 10.1128/iai.55.8.1924-1926.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. The world health report 1999—making a difference. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]