Abstract
Cell invasion by the protozoan parasite Trypanosoma cruzi involves activation of host signaling pathways and the recruitment and fusion of lysosomes at the parasite entry site. A major signaling pathway regulating invasion of fibroblasts, epithelial cells, and myoblasts involves mobilization of Ca2+ from intracellular stores and requires the activity of a T. cruzi serine peptidase, oligopeptidase B (OPB). Deletion of the OPB gene results in a marked defect in trypomastigote virulence, consistent with a greatly reduced cell invasion capacity. Here we show that uptake by macrophages, on the other hand, is largely independent of OPB expression and sensitive to inhibition of by cytochalasin D. The residual invasion capacity of OPBnull trypomastigotes in fibroblasts still involves lysosome recruitment, although in a significantly delayed fashion. Transient elevations in intracellular Ca2+ concentrations were observed in host cells exposed to both wild-type and OPBnull trypomastigotes, but the signals triggered by the mutant parasites were less vigorous and delayed. The capacity of triggering elevation in host cell cyclic AMP (cAMP), however, was unaltered in OPBnull trypomastigotes. Modulation in cAMP levels preferentially affected the residual cell invasion capacity of OPBnull parasites, suggesting that this signaling pathway can play a dominant role in promoting cell invasion in the absence of the major OPB-dependent pathway.
Microbial pathogens have developed a remarkable variety of different strategies to disrupt or exploit mammalian cell processes in order to invade, survive, and propagate in their hosts. Signaling between pathogens and host cells has emerged as a key regulatory feature during mammalian cell invasion, as exemplified by enteric bacterial pathogens (11, 15). However, in contrast to bacteria, which often utilize host cell actin-driven uptake mechanisms, larger pathogens such as protozoa exhibit quite distinct and unusual infection strategies (1). Trypanosoma cruzi, the causative agent of Chagas' disease in humans, is a protozoan parasite capable of invading a large variety of cell types in its vertebrate host. Previous work from our laboratory revealed that invasion of many cell types by T. cruzi is independent of host actin polymerization and involves recruitment and fusion of host cell lysosomes at the site of parasite attachment (2, 25, 30, 32).
The directional movement and localized fusion of lysosomes at the T. cruzi attachment site suggested that a signal of parasite origin was locally transduced in host cells. This hypothesis was reinforced when trypomastigotes, the infective T. cruzi life cycle stages, were shown to activate phospholipase C and to trigger IP3-mediated Ca2+ release from host cell intracellular stores (24, 31). Characterization of this signaling pathway revealed that a parasite serine peptidase oligopeptidase B (OPB), is required for the generation of a soluble factor that triggers intracellular free Ca2+ concentration ([Ca2+]i) transients in mammalian cells (4–6).
Deletion of the T. cruzi OPB gene severely impairs the ability of trypomastigotes to invade mammalian cells and to establish infections in mice, without affecting parasite growth rates, differentiation, motility, or protein synthesis. The invasion defect of OPBnull trypomastigotes is associated with their inability to mobilize Ca2+ from thapsigargin-sensitive stores in mammalian host cells (6). Unlike wild-type (WT) parasites, the diminished invasion capacity of the OPBnull parasites, (about 25 to 30% of WT levels) was found to be refractory to pretreatment with thapsigargin, a drug that depletes intracellular Ca2+ stores (6). These data are consistent with the hypothesis that T. cruzi OPB functions in the generation of a Ca2+ signaling agonist for mammalian cells. This was directly demonstrated by reconstitution of the Ca2+ signaling activity in soluble extracts of OPBnull trypomastigotes with recombinant OPB (6). Interestingly, the residual level of host cell invasion by the OPBnull mutants was completely abolished when host cells were pretreated with the Ca2+ chelator MAPTA-AM, suggesting that the OPBnull trypomastigotes retain a requirement for host cell Ca2+ elevation for invasion (6).
In addition to Ca2 signaling, T. cruzi trypomastigotes (but not the noninfective epimastigote forms) trigger elevation in host cell cyclic AMP (cAMP) levels. Furthermore, inhibition of host cell adenylyl cyclase inhibits parasite invasion, whereas stimulation of cAMP production enhances it (23). Modulation in cAMP levels was also found to affect Ca2+-dependent exocytosis of lysosomes, similar to what has been reported for other Ca2+-regulated secretory pathways (23). Taken together with the observation that both T. cruzi entry and lysosome exocytosis are enhanced by disruption of the host cell actin cytoskeleton (23), these findings point to important functional parallels between this parasite's unusual cell invasion mechanism and Ca2+-regulated exocytosis (17, 23, 26).
The goal of the present study was to investigate the mechanisms underlying the residual capacity for cell invasion by the OPBnull trypomastigotes. Since deletion of the OPB gene abolishes the ability of T. cruzi to mobilize Ca2+ from host cell intracellular stores (6), it became important to determine if the cAMP signaling pathway was also affected by this mutation, and if cAMP levels differentially affected the invasion capacity of OPBnull and WT parasites. We also investigated the role of lysosome recruitment in the residual infectivity of the OPBnull mutants, and compared the kinetics of [Ca2+]i transient generation in host cells by live OPBnull and WT trypomastigotes by digital fluorescence microscopy analysis.
MATERIALS AND METHODS
Materials.
Pertussis toxin (PTx), 3-isobutyl-1-methylxanthine (IBMX), isoproterenol hydrochloride (ISO) and MDL-12,330A were from Calbiochem; cytochalasin D, 4′,6′-diamidino-2-phenylindole (DAPI), leupeptin, probenecid, and 8-bromoadenosine-3′,5′-cyclic monophosphate (8-Br-cAMP) were from Sigma. Fluo-3-AM and pluronic acid were from Molecular Probes. 2-[3H]adenine was from Amersham Life Science, and ZFA-FMK was from Enzyme Systems Products.
Mammalian cells and parasites.
Normal rat kidney (NRK) fibroblasts and J774 cells were maintained in Dulbecco minimal essential medium supplemented with 10% fetal bovine serum (DMEM–10% FBS) at 37°C in a humidified atmosphere containing 5% CO2. OPBnull trypomastigotes were generated by targeted replacement of the two alleles of the OPB gene as described earlier (6). Y strain WT and OPNnull tissue culture trypomastigotes were maintained by weekly passages on LLCMK2 cells as described previously (3). Epimastigotes were grown and maintained in liver infusion tryptose medium as described by Nogueira and Cohn (21).
T. cruzi cell invasion assays and immunofluorescence.
Mammalian cells were plated at a density of 2.5 × 104 cells/cm2 in DMEM–10% FBS on 12-mm round coverslips placed in 6-cm plastic tissue culture dishes and grown for 48 h at 37°C in a humidified atmosphere containing 5% CO2. Coverslips with attached cells were washed briefly with 2% FBS–DMEM and transferred to 3.5-cm dishes immediately prior to incubation for 1 h at 37°C with purified trypomastigotes at 5 × 107 parasites/ml. For cell pretreatments, drugs were added at the following final concentrations: PTx, 0.4 μg/ml; cytochalasin D, 10 μM; 8-Br-cAMP, 1 mM; IBMX, 500 μM; isoproterenol hydrochloride, 10 μM. Infected cells were washed 3 times with cold phosphate-buffered saline (PBS), fixed in 2% (wt/vol) paraformaldehyde–PBS, and the number of intracellular parasites was determined by immunofluorescence as described previously (32). At least 200 host cells from randomly chosen microscopic fields were analyzed for each experimental point. For lysosome-parasite colocalization, infected cells were labeled with the LY1C6 monoclonal antibody against rat Lamp-1, as described previously (32).
Time-lapse fluorescence microscopy.
Changes in the [Ca2+]i of NRK cells exposed to WT or OPBnull mutant trypomastigotes were continuously measured at the single cell level, using time-lapse fluorescence video microscopy in an Axiovert 135 microscope (Carl Zeiss, Inc.). A total of 80 μl of trypomastigotes at a concentration of 108 parasites/ml in PBS containing 10 μM leupeptin (to inhibit any OPB-dependent Ca2+-signaling activity released from damaged parasites) was added to NRK cells (preloaded for 1 h with 5 μM fluo-3-AM, 0.05% pluronic acid, and 0.5 mM probenecid) 30 s after the initiation of the time-lapse recording. Fluorescent images were collected with a digital camera (Orca II; Hamamatsu) through a 25× objective lens at time-lapse intervals of 2 s, using a computer-controlled shutter system (MetaMorph; Universal Imaging). Total duration of the recording was 600 s.
Determination of intracellular cAMP levels.
Confluent monolayers of NRK cells in six-well dishes were prelabeled for 24 h with 2-[3H]adenine (23 Ci/mmol or 5 μCi/ml). After labeling, cells were preincubated in 500 μl of 1 mM IBMX for 10 min at 37°C, followed by the addition of the indicated drugs or parasites in 500 μl of Ringer's BSA buffer (14) and incubated for 30 min at 37°C. After drug or parasite treatment, cells were washed three times with Ringer's BSA, and reactions terminated by the addition of 1 ml of 5% trichloroacetic acid containing 1 mM ATP and 1 mM cAMP per well. Acid-soluble nucleotides were separated on ion-exchange columns as described previously (28). In the experiments with live T. cruzi, labeled cells were exposed to 5 × 107 trypomastigotes or epimastigotes for 30 min at 37°C.
RESULTS
Host cell invasion by T. cruzi OPBnull trypomastigotes is refractory to pertussis toxin treatment of host cells.
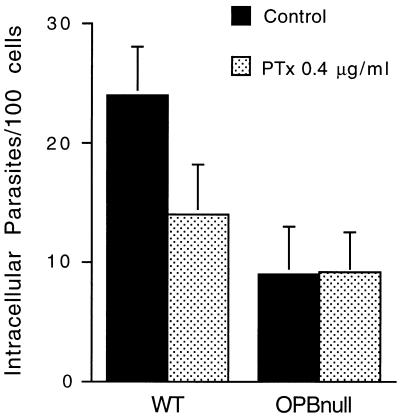
Our previous studies established that the T. cruzi serine hydrolase OPB is involved in the activation of a host cell signaling pathway that is a key event in the infection process (6). Consistent with the demonstration that the signaling pathway activated by OPB involves IP3 generation and mobilization of Ca2+ from intracellular stores (24), the residual invasion capacity of the OPBnull mutants was found to be refractory to thapsigargin pretreatment of host cells (6). In order to extend this observation and to verify if the signaling pathway involved in intracellular Ca2+ mobilization was effectively abolished in the OPBnull mutants, we investigated the effect of PTx treatment on the susceptibility of NRK cells to invasion by WT and OPBnull trypomastigotes. PTx catalyzes the ADP ribosylation of Gαi and Gαo, uncoupling these G-α subunits from their receptors and blocking signal transduction (16). Previous results showed, similarly to what is observed after thapsigargin treatment, that both mobilization of Ca2+ from intracellular stores and infection of NRK fibroblasts by T. cruzi trypomastigotes are inhibited when the host cells are pretreated with PTx (31). In Fig. 1, we show that host cell pretreatment with PTx has no effect on the invasion of OPBnull trypomastigotes, whereas it reduces the entry of WT parasites by ca. 40%. These results suggest that deletion of the OPB gene results in complete loss of the ability to signal host cells via the putative pertussis toxin-sensitive G-coupled receptor pathway, which generates IP3 and mobilizes Ca2+ from intracellular stores (24, 31).
FIG. 1.
PTx treatment of host cells does not affect the residual cell invasion capacity of OPBnull trypomastigotes. NRK fibroblasts were pretreated or not with 0.4 mg Ptx per ml overnight at 37°C. After treatment, the toxin was removed and the cells were exposed to WT or OPBnull trypomastigotes for 30 min. The data represent the average of triplicates ± the standard deviation (SD).
Host cell actin polymerization is not required for residual invasion by OPBnull trypomastigotes.
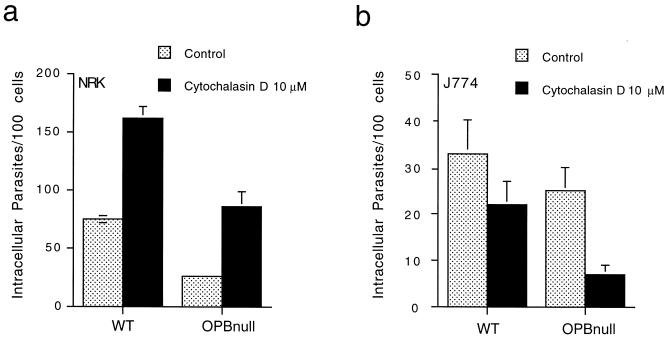
We next investigated the role of the host cell actin cytoskeleton in the residual invasion capacity of the signaling-deficient OPBnull parasites. Earlier experiments established that disruption of the host cell actin cytoskeleton with cytochalasin D significantly facilitates invasion of fibroblasts and epithelial cells by WT T. cruzi (32). This effect was interpreted as a removal of the barrier posed by the cortical actin cytoskeleton to lysosome recruitment and fusion, required for cell entry by WT parasites in these cell types (23, 25, 32). Here we compared the effect of cytochalasin D treatment on the susceptibility to invasion by OPBnull and WT trypomastigotes in fibroblasts and in a phagocytic cell line. Inhibition of OPBnull invasion by cytochalasin D in fibroblasts would be an indication of an alternative uptake mechanism, dependent on host cell actin polymerization and not involving lysosome recruitment. The results obtained with the macrophage cell line would complement these findings, since in macrophages T. cruzi uptake would be expected to occur primarily by phagocytosis and thus require an intact actin cytoskeleton. Figure 2 shows that pretreatment of NRK cells with cytochalasin D enhanced invasion by OPBnull and WT (Fig. 2a) to a similar extent (66 and 55%, respectively), which is consistent with what was previously reported for WT T. cruzi invasion (32). On the other hand, cytochalasin D significantly inhibited infection of J774 macrophages by OPBnull parasites (70%), whereas infection by WT trypomastigotes was reduced to a lesser extent (33%) (Fig. 2b). These results suggest that the residual invasion mechanism of the signaling-deficient OPBnull trypomastigotes may also involve lysosome recruitment, which is facilitated by disruption of the actin cytoskeleton.
FIG. 2.
Cytochalasin D enhances invasion of NRK cells and inhibits invasion of J774 macrophages by WT and OPBnull trypomastigotes. Cells were pretreated with 10 μM cytochalasin D for 10 min at 37°C and then exposed to parasites for 1 h (a, NRK) or 30 min (b, J774). The data represent the average of triplicates ± the SD.
OPBnull trypomastigotes recruit host cell lysosomes with a delayed kinetics.
Several lines of evidence indicate that T. cruzi requires lysosome recruitment to invade mammalian cells (23, 25, 32), and that a major component of this invasion mechanism is mediated by OPB-dependent signaling (6). The results discussed above show that the residual invasion capacity of signaling-defective OPBnull trypomastigotes, although significantly less efficient than WT parasites, still has properties consistent with a requirement for lysosome mobilization. To directly investigate this issue, we determined the kinetics of association between trypomastigotes and lysosomes in NRK fibroblasts following short-term infections.
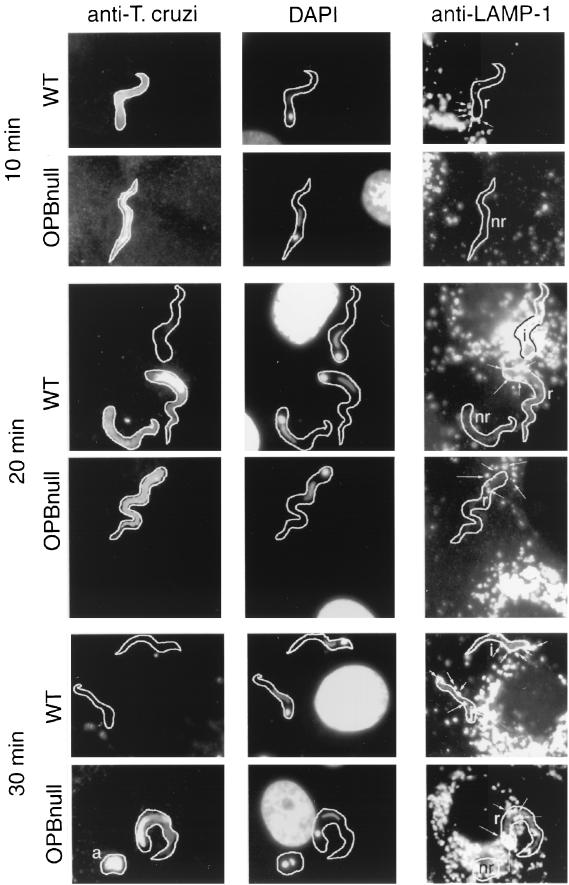
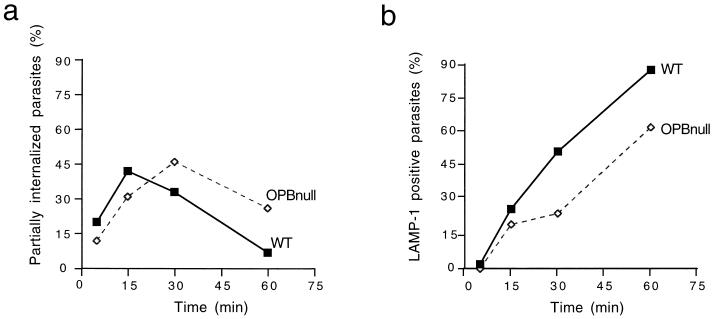
Figure 3 shows representative immunofluorescence images obtained following 10-, 20-, and 30-min time courses of T. cruzi infection of NRK cells. Extracellular (attached) parasites were visualized by immunofluorescence staining using antibodies against T. cruzi added prior to cell permeabilization, and antibodies to the lysosomal glycoprotein Lamp-1 added after cell permeabilization were used to localize host cell lysosomes. This labeling procedure revealed that the residual invasion mechanism of OPBnull trypomastigotes involves, similar to WT, an initial stage of lysosome clustering at the parasite attachment site, followed by fusion and formation of a parasite-containing intracellular vacuole which stains positive for lysosomal markers. However, whereas WT trypomastigotes attached to host cells were frequently found associated with lysosomes in the earlier time points, lysosome recruitment by OPBnull parasites showed a delayed pattern (Fig. 3). Quantification of this process (Fig. 4a) showed that the highest percentage of extracellular parasites associated with lysosomes (partially internalized) was reached after 15 min with WT and only after 30 min with OPBnull parasites. The number of completely internalized parasites for the same time course experiment was also determined, revealing a larger number of intracellular WT parasites in relation to OPBnull in all time points (Fig. 4b).
FIG. 3.
Kinetics of lysosome recruitment by WT and OPBnull trypomastigotes in NRK fibroblasts. NRK cells were exposed to 108 WT or OPBnull trypomastigotes per ml for 10, 20, or 30 min at 37°C. Immunofluorescence was performed using (i) anti-T. cruzi antibodies prior to cell permeabilization to detect extracellular parasites (left column), (ii) DAPI to detect NRK and parasite nuclei (center column) or (iii) anti-Lamp-1 monoclonal antibody after cell permeabilization to detect host cell lysosomes (right column). Lysosomes recruited to the sites of parasite attachment are indicated by arrows. i, Intracellular parasites; r, extracellular parasites recruiting lysosomes; nr, extracellular parasites not recruiting lysosomes.
FIG. 4.
OPBnull trypomastigotes recruit lysosomes and invade cells in a delayed pattern. NRK cells were exposed to WT or OPBnull trypomastigotes for the indicated periods of time. Parasites attached to the cells and partially internalized were stained with an anti-T. cruzi antibody; recruitment of lysosomes was visualized by staining with an anti-Lamp-1 monoclonal antibody after cell permeabilization, and the total number of parasites was determined by nuclear staining with DAPI. (a) Percent parasites recruiting lysosomes = (number of anti-T. cruzi-Lamp-1 double-positive parasites/total parasites) × 100. (b) Percent internalized parasites = (Lamp-1-positive parasites/total parasites) × 100.
These results suggest that even though OPBnull trypomastigotes appear to be able to induce recruitment and fusion of lysosomes at their invasion site, the process is clearly delayed in relation to WT parasites. This finding explains the significantly lower levels of invasion by OPBnull trypomastigotes observed at a given time point.
[Ca2+]i transients triggered in NRK fibroblasts by OPBnull trypomastigotes are delayed.
Previous results suggested that the alternative signaling mechanism leading to invasion of OPBnull parasites also involved [Ca2+]i transients, because parasite entry is abolished when host cells are loaded with the Ca2+ chelator MAPTA-AM (6). However, OPBnull trypomastigotes do not contain the OPB-dependent soluble agonist present in WT parasites, which triggers Ca2+ release from thapsigargin-sensitive intracellular stores (6). We therefore hypothesized that [Ca2+]i transients, perhaps derived from an alternative source of Ca2+, might be generated during the interaction of live OPBnull trypomastigotes with mammalian cells.
In order to initially characterize the pattern of Ca2+ signaling triggered by live trypomastigotes, we performed time-lapse fluorescence imaging of NRK fibroblasts loaded with the Ca2+ sensitive dye fluo-3-AM. Figure 5 illustrates a time-lapse sequence in which a localized zone of [Ca2+]i elevation was detected at the site of attachment of a live WT trypomastigote. Figure 5a and e show phase-contrast images corresponding to the beginning and end, respectively, of the time lapse sequence. In panels b, c, and d are representative frames of the variation in fluo-3 fluorescence intensity detected in the host cell a few seconds after the parasite was stably attached. A localized elevation of [Ca2+]i was observed at the site of trypomastigote attachment, a pattern consistent with the stimulus originating from the parasite. Other cells in the vicinity without attached trypomastigotes did not show any [Ca2+]i transients during a 500-s observation period (not shown). This single cell response pattern is distinct from the overall [Ca2+]i elevation observed in the majority of cells when WT trypomastigote soluble extracts are added to fluo-3-loaded NRK fibroblasts (6). We thus proceeded to image fluo-3-AM-loaded NRK cells exposed to WT and OPBnull trypomastigotes at the single cell level and to register the time point after contact with the parasites in which individual cells responded with an [Ca2+]i elevation.
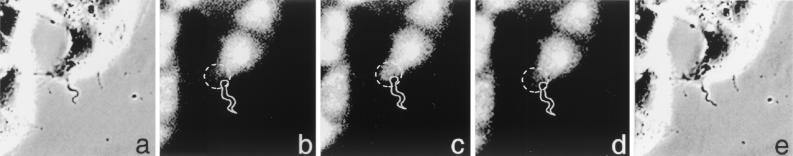
FIG. 5.
Localized Ca2+ signaling response generated by T. cruzi trypomastigotes in NRK cells. (a) Phase-contrast image of a stably attached trypomastigote at the beginning of the time lapse recording. (b, c, and d) Fluorescence images of fluo-3-loaded NRK cells at 39, 41, and 43 s, respectively, after initiation of the time-lapse recording, showing a localized transient [Ca2+]i elevation at the site of parasite attachment. (e) Phase-contrast image obtained after finalization of the time-lapse recording. Dashed circles indicate the region of the cell were a localized [Ca2+]i elevation occurred as a consequence of parasite attachment.
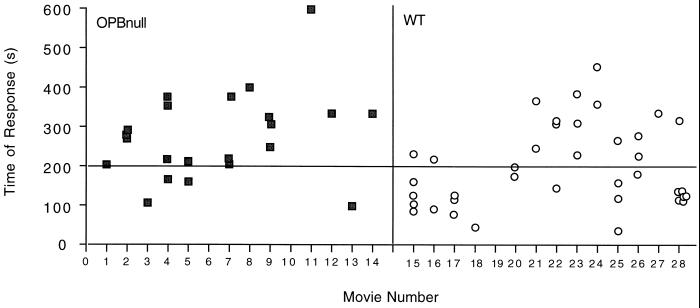
Time-lapse imaging experiments showed that live OPBnull trypomastigotes are still able to induce a transient [Ca2+]i increase in NRK fibroblasts. We analyzed a total of 28 independent time-lapse experiments in order to compare the kinetics of the signaling triggered by WT and OPBnull trypomastigotes. Table 1 shows that the number of NRK fibroblasts responding with a transient [Ca2+]i increase to OPBnull trypomastigotes is 39% less than the number of cells responding to WT trypomastigotes during a 10-min period. Figure 6 shows the response kinetics of individual cells after exposure to OPBnull or WT trypomastigotes. A total of 47% of the NRK cells exposed to WT parasites responded with a [Ca2+]i elevation within 200 s, whereas only 17% of the cells responded to OPBnull trypomastigotes in the same time interval (Table 1). In addition, the fraction of experiments in which only one or no cells responded was 32% (nine experiments) for OPBnull trypomastigotes, versus 10.5% (three experiments) for WT trypomastigotes (Table 1, Fig. 6). The P value obtained in a Student's unpaired t test of the mean response time of cells exposed to WT or OPB null parasites was 0.0074, thus confirming that the difference between the two groups is highly significant. These findings are consistent with the results of the lysosome recruitment time course experiments (Fig. 3 and 4), and suggest that OPB null trypomastigotes trigger less-vigorous Ca2+ signaling in NRK cells, which leads to less efficient lysosome recruitment and invasion compared to WT parasites.
TABLE 1.
[Ca2+]i transients generated by OPBnull and WT T. cruzi trypomastigotes in NRK fibroblasts
| Parasite strain | No. of movies | Total RCa | No. of movies with RC of ≤1 (%) | tr <200 s (%)b |
|---|---|---|---|---|
| WT | 14 | 38 | 3 (10.5) | 18 (47) |
| OPBnull | 14 | 23 | 9 (32) | 4 (17) |
RC, total number of cells responding to trypomastigotes with a [Ca2+]i transient. Movies with an RC of ≤1 represent the number of individual movies in which only one or no cell responded.
tr, time elapsed from addition of trypomastigotes to the moment when the first [Ca2+]i transient was detected. A tr of <200 s represents the number of cells that responded in the initial 200 s after exposure to the parasites.
FIG. 6.
OPBnull trypomastigotes trigger delayed Ca2+ signaling in NRK cells. WT (■) or OPBnull (□) trypomastigotes were added to NRK cells preloaded with the Ca2+-sensitive dye fluo-3. Time-lapse images were acquired, and the time frame in which each individual [Ca2+]i elevation occurred was determined. The plot shows the total number of responsive cells in each individual movie for a recording period of 600 s.
OPBnull trypomastigotes retain the capacity to elevate host cell cAMP levels, and their invasion capacity is preferentially affected by cAMP modulation.
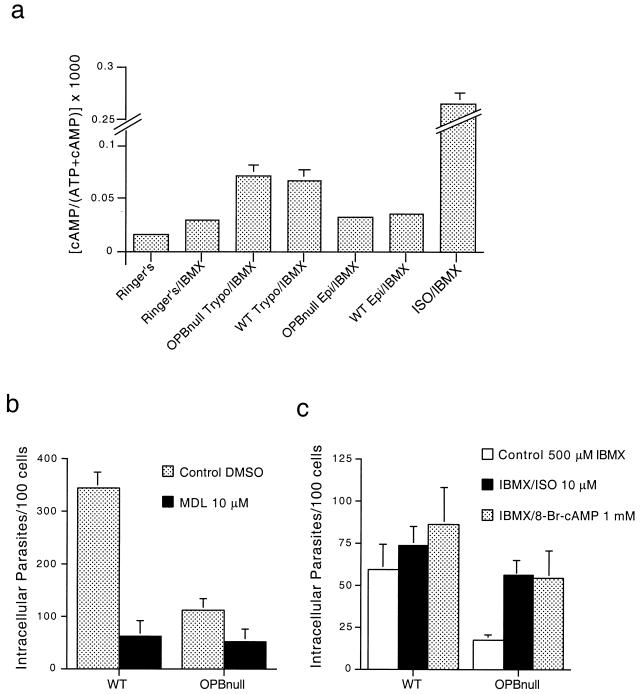
Previous studies demonstrated that WT trypomastigotes stimulate cAMP production in NRK cells and that host cell invasion can be modulated by agents that alter intracellular cAMP levels (23). We investigated if deletion of the OPB gene, in addition to abolishing the parasite's capacity to mobilize Ca2+ from intracellular stores, also affected its ability to elevate cAMP. Figure 7a shows that WT and OPBnull trypomastigotes induce similar levels of intracellular cAMP elevation after an exposure period of 30 min. The cAMP values measured under these conditions are clearly in the linear range of detection, since treatment with isoproterenol, an agonist that elevates cAMP through stimulation of the β-adrenergic receptor, induces a significantly higher response (Fig. 7a). Also similarly to what is observed with WT parasites, exposure of NRK cells to OPBnull epimastigotes does not result in elevated cAMP (Fig. 7a). These results indicate that the OPB-dependent signaling pathway that controls Ca2+ mobilization from intracellular stores is independent from the cAMP-stimulatory pathway. Both pathways, however, are detected only in the infective trypomastigote forms and are therefore likely to play important roles in the cell invasion process.
FIG. 7.
Residual cell invasion by OPBnull trypomastigotes is modulated by cAMP. (a) Intracellular levels of cAMP were measured in IBMX-pretreated NRK cells after exposure to WT and OPBnull trypomastigotes and epimastigotes or exposure to isoproterenol. Infection of NRK cells by OPBnull trypomastigotes was quantitated after pretreatment of the host cells with different cAMP modulators: b, 10 μM MDL-12,330A for 30 min at 37°C; and c, 10 μM isoproterenol or 1 mM 8-Br-cAMP for 30 min at 37°C. The data represent the average of triplicate determinations ± the SD.
To obtain insight into the role of the cAMP signaling pathway in T. cruzi invasion, we investigated the effect of agents that modulate host cell cAMP levels on susceptibility to infection by WT or OPBnull mutant trypomastigotes. Pretreatment of NRK cells with the adenylyl cyclase inhibitor MDL-12,330A reduces invasion by both WT and OPBnull trypomastigotes (Fig. 7b), whereas exposure to isoproterenol or 8-Br-cAMP (a membrane-permeant analogue of cAMP) stimulates infection by both parasite types (Fig. 7c). Interestingly, whereas the stimulatory effect of isoproterenol and 8-Br-cAMP on susceptibility to WT trypomastigotes invasion is small (20 to 25% increase), a significantly stronger effect is observed for invasion by OPBnull trypomastigotes (69% increase), with the numbers of intracellular parasites reaching levels similar to those normally obtained with WT parasites. These results suggest that in the absence of the major OPB-dependent signaling pathway, cAMP stimulation plays an important role in the lysosome-mediated residual invasion phenotype observed in the OPBnull parasites.
DISCUSSION
Recent studies have provided extensive evidence that activation of signaling cascades in host cells plays a central role in the T. cruzi cell invasion mechanism (5, 20, 34). Mobilization of Ca2+ from intracellular stores has been specifically implicated, since cell loading with Ca2+ chelators and Ca2+ store depletion by thapsigargin effectively inhibit trypomastigote entry (7, 24, 31). An investigation of the T. cruzi Ca2+ signaling capacity revealed that the infective trypomastigotes forms contain a soluble factor capable of triggering intracellular free Ca2+ transients in several mammalian cell types and that production of this factor requires the activity of a parasite serine peptidase, OPB (4, 5, 6, 24, 31). Deletion of the T. cruzi OPB gene by targeted replacement resulted in a significant attenuation of the parasite's virulence for mice and in a 60 to 70% reduction in their ability to invade mammalian cells. Since OPBnull trypomastigotes do not contain the OPB-dependent soluble Ca2+ signaling factor, the retention of a residual invasion capacity in the mutant parasites suggested the existence of an alternative mechanism for host cell entry (6).
The major pathway utilized by T. cruzi to invade several epithelial and fibroblast cell lines was shown to involve lysosome recruitment and fusion at the parasite attachment site (23, 25, 32). Experimental conditions that affect lysosome distribution or that affect fusion of lysosomes with the plasma membrane significantly interfere with trypomastigote invasion (23, 25, 26). Several lines of evidence support the idea that Ca2+-regulated lysosome exocytosis may be a ubiquitous process that is subverted by T. cruzi as a mechanism for cell invasion. Regulated exocytosis of granules with lysosomal characteristics has been more frequently described in cells of the hematopoietic lineage, such as platelets, mast cells, neutrophils, and cytotoxic lymphocytes (22). Recent evidence indicates, however, that intracellular free Ca2+ elevations can trigger exocytosis of conventional lysosomes in many cell types and that the process may be regulated by a transmembrane protein with Ca2+-binding properties, synaptotagmin VII (19, 26). Taken together, the available evidence suggests that activation of host cell signaling pathways and Ca2+ elevation at the site of parasite attachment results in localized lysosome recruitment, docking, and fusion, events that contribute to formation of the T. cruzi-containing intracellular vacuole.
The low levels of cell invasion observed when host cells are exposed to OPBnull trypomastigotes are not affected by depletion of intracellular Ca2+ stores with thapsigargin, while invasion by WT parasites is greatly reduced (6). Here we show that host cell pretreatment with PTx, a condition previously shown to inhibit infection by WT T. cruzi (31), also does not affect invasion by OPBnull trypomastigotes. These findings reinforce the hypothesis that OPBnull trypomastigotes lost the capacity to activate the G protein-dependent host cell signaling pathway that leads to IP3 formation and Ca2+ mobilization from intracellular stores and that has been directly linked to lysosome recruitment (5, 6). Such observations raised the possibility that the residual invasion capacity of OPBnull mutants might be dependent on a completely different mechanism, not involving lysosome recruitment.
One possibility was that this alternative invasion mechanism might resemble phagocytosis, requiring host cell actin polymerization. Our data, however, show that invasion of NRK cells by OPBnull trypomastigotes is enhanced by host cell pretreatment with cytochalasin D, similarly to what is observed with WT parasites. However, in J774 macrophages cytochalasin D has a more pronounced inhibitory effect on infection by OPBnull than WT parasites suggesting that in phagocytic cells the main route of internalization of the mutant parasites involves actin polymerization. Taken together with the results obtained with PTx treatment, the effect of cytochalasin D indicates that the invasion pathway utilized by OPBnull trypomastigotes is independent of OPB-mediated signaling but still involves a mechanism that is facilitated by depolymerization of host cell actin microfilaments. The apparent more important role of phagocytosis in the uptake of OPBnull parasites by macrophages suggests that the actin-independent pathway utilized for invasion may be less vigorous in these mutants, resulting in a larger fraction of the parasites being engulfed instead of driving their own internalization.
We thus proceeded to directly verify if lysosome recruitment was involved in the cell entry process of OPBnull trypomastigotes. Time course experiments revealed that lysosomes are recruited to the sites of both WT and OPBnull trypomastigote attachment and that lysosomal markers are similarly gradually incorporated into the nascent parasitophorous vacuoles. However, a marked difference was observed in the kinetics of the process: lysosome recruitment and host cell internalization were significantly slower with OPBnull trypomastigotes. Taken together with the observation that in macrophages OPBnull trypomastigotes appear to be taken up predominantly by an actin polymerization-dependent phagocytic mechanism, these results suggest that the invasion capacity of the mutants, although similar to WT with respect to lysosome recruitment, is much less vigorous.
To further understand the basis for the delayed lysosome recruitment phenotype, we investigated the capacity of OPBnull trypomastigotes to trigger intracellular Ca2+ transients in NRK cells. As discussed above, previous studies demonstrated that extracts of OPBnull trypomastigotes are deficient in the OPB-dependent factor that mobilizes Ca2+ from host cell intracellular stores (6). Since the process of lysosome recruitment appears to be directly linked to intracellular Ca2+ elevations, the delayed lysosome recruitment phenotype of OPBnull parasites was predicted to also require Ca2+, perhaps mobilized from an alternative source. Direct observations of the interaction between live trypomastigotes and NRK cells loaded with Ca2+-sensitive dye confirmed this prediction: OPBnull trypomastigotes are still capable of triggering [Ca2+]i transients, although the process is clearly delayed in relation to what is observed with WT parasites. The more potent response induced by WT trypomastigotes allowed us to visualize events in which the [Ca2+]i transient generated was clearly localized at the parasite attachment site. The responses triggered by OPBnull trypomastigotes, although still detectable at the single cell level, were significantly less intense, precluding detection of a localized response. Although the lack of effect of thapsigargin treatment strongly suggests that OPBnull trypomastigotes are not mobilizing Ca2+ from host cell intracellular stores, the alternative source of Ca2+ utilized is currently unknown. One interesting possibility is that the “stretch”-induced Ca2+ channels that have been detected on the plasma membrane of many cell types (18) might be involved. The vigorous motility exhibited by T. cruzi trypomastigotes is not affected by deletion of the OPB gene, so it is conceivable that active motility after host cell attachment could result in the opening of such channels and in Ca2+ influx from the extracellular medium. Our observations are consistent with a scenario in which the delayed Ca2+ transients triggered by OPBnull trypomastigotes would be the result of Ca2+ influx through plasma membrane channels, whereas the more intense response observed with WT parasites would also include Ca2+ mobilization from intracellular stores, through the PTx-sensitive, IP3-mediated signaling pathway that is dependent on the OPB-generated Ca2+ agonist.
Previous work in our laboratory showed that host cell cAMP levels modulate both Ca2+-dependent lysosome exocytosis and lysosome-mediated T. cruzi invasion, again highlighting the interesting similarities between these two processes (23). Although the exact mechanism by which cAMP regulates lysosome exocytosis is not known, it is possible that cAMP-dependent protein kinase A mediates phosphorylation of vesicle membrane components involved in docking and fusion events (10) or that the effects observed are due to phosphorylation-dependent disassembly of cortical cytoskeleton components (8, 12, 27) or modulation of microtubule-dependent vesicular transport (9, 13, 33). We showed previously that membrane-permeant analogs of cAMP enhance T. cruzi entry into NRK fibroblasts and that the infective trypomastigotes are able to stimulate cAMP production in host cells (23, 32). Here we found that OPBnull trypomastigotes retain the capacity of elevating host cell cAMP and that the residual invasion capacity of these parasites can be restored close to WT levels by host cell treatment with drugs that stimulate cAMP production. These results indicate that T. cruzi is able to trigger at least two independent signaling pathways that facilitate lysosomal recruitment and fusion, a process required for successful invasion of fibroblasts by both WT and OPBnull parasites. The finding that cAMP levels have a more marked influence on the invasion of OPBnull trypomastigotes suggests that cAMP is modulating the invasion process independently of the major IP3-dependent signaling pathway that mobilizes Ca2+ from intracellular stores. Although cAMP can affect a number of different cellular mechanisms, it is noteworthy that elevation in cAMP levels induces dispersion of lysosomes from the perinuclear area to the cell periphery, the site where T. cruzi invasion occurs (14, 29). It is thus conceivable that when the number of lysosomes in the proximity of the plasma membrane is increased, Ca2+ influx through plasma membrane channels may provide a sufficient stimulus for lysosome exocytosis and for parasite entry to occur.
ACKNOWLEDGMENTS
This work was supported by an NIH grant and a Burroughs-Wellcome Molecular Parasitology Scholar Award to N.W.A. and by a fellowship from the South African Foundation for Research Development to R.M.
We are very grateful to C. Berlot (Physiology Department, Yale University) for help with cAMP determinations and to H. Tan for excellent technical assistance.
REFERENCES
- 1.Sibley L D, Andrews N W. Cell invasion by un-palatable parasites. Traffic. 2000;1:100–106. doi: 10.1034/j.1600-0854.2000.010202.x. [DOI] [PubMed] [Google Scholar]
- 2.Andrews N W. Lysosome recruitment during host cell invasion by Trypanosoma cruzi. Trends Cell Biol. 1995;5:133–137. doi: 10.1016/s0962-8924(00)88965-5. [DOI] [PubMed] [Google Scholar]
- 3.Andrews N W, Hong K S, Robbins E S, Nussenzweig V. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp Parasitol. 1987;64:474–484. doi: 10.1016/0014-4894(87)90062-2. [DOI] [PubMed] [Google Scholar]
- 4.Burleigh B A, Andrews N W. A 120-kDa alkaline peptidase from Trypanosoma cruzi is involved in the generation of a novel Ca2+-signaling factor for mammalian cells. J Biol Chem. 1995;270:5172–5180. doi: 10.1074/jbc.270.10.5172. [DOI] [PubMed] [Google Scholar]
- 5.Burleigh B A, Caler E V, Webster P, Andrews N W. A cytosolic serine endopeptidase from Trypanosoma cruzi is required for the generation of Ca2+ signaling in mammalian cells. J Cell Biol. 1997;136:609–620. doi: 10.1083/jcb.136.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caler E V, Vaena de Avalos S, Haynes P A, Andrews N W, Burleigh B A. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J. 1998;17:4975–4986. doi: 10.1093/emboj/17.17.4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorta M L, Ferreira A T, Oshiro M E M, Yoshida N. Ca2+ signal induced by Trypanosoma cruzi metacyclic trypomastigote surface molecules implicated in mammalian cell invasion. Mol Biochem Parasitol. 1995;73:285–289. doi: 10.1016/0166-6851(94)00123-5. [DOI] [PubMed] [Google Scholar]
- 8.Downey G P, Elson E L, Schwab B D, Erzurum S C, Young S K, Worthen G S. Biophysical properties and microfilament assembly in neutrophils: modulation by cyclic AMP. J Cell Biol. 1991;114:1179–1190. doi: 10.1083/jcb.114.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epple H J, Kreusel K M, Hanski C, Schulzke J D, Riecken E O, Fromm M. Differential stimulation of intestinal mucin secretion by cholera toxin and carbachol. Pflugers Arch. 1997;433:638–647. doi: 10.1007/s004240050325. [DOI] [PubMed] [Google Scholar]
- 10.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–4. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 11.Galán J E, Bliska J B. Cross-talk between bacterial pathogens and their host cells. Annu Rev Cell Dev Biol. 1996;12:221–255. doi: 10.1146/annurev.cellbio.12.1.221. [DOI] [PubMed] [Google Scholar]
- 12.Goldman J E, Abramson B. Cyclic AMP-induced shape changes of astrocytes are accompanied by rapid depolymerization of actin. Brain Res. 1990;528:189–96. doi: 10.1016/0006-8993(90)91657-3. [DOI] [PubMed] [Google Scholar]
- 13.Hayakawa T, Bruck R, Ng O C, Boyer J L. DBcAMP stimulates vesicle transport and HRP excretion in isolated perfused rat liver. Am J Physiol. 1990;259:G727–G735. doi: 10.1152/ajpgi.1990.259.5.G727. [DOI] [PubMed] [Google Scholar]
- 14.Heuser J E. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J Cell Biol. 1989;108:855–864. doi: 10.1083/jcb.108.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ireton K, Cossart P. Interaction of invasive bacteria with host signaling pathways. Curr Opin Cell Biol. 1998;10:276–283. doi: 10.1016/s0955-0674(98)80151-8. [DOI] [PubMed] [Google Scholar]
- 16.Ismaa T P, Shine J. G-protein coupled receptors. Curr Opin Cell Biol. 1992;4:195–202. doi: 10.1016/0955-0674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- 17.Kima, P. E., B. Burleigh, and N. W. Andrews. Surface targeted lysosomal membrane glycoprotein (Lamp-1) enhances lysosome exocytosis and cell invasion by Trypanosoma cruzi. Cell. Microbiol., in press. [DOI] [PubMed]
- 18.Lee J, Ishihara A, Oxford G, Johnson B, Jacobson K. Regulation of cell movement is mediated by stretch-activated calcium channels. Nature. 1999;400:382–386. doi: 10.1038/22578. [DOI] [PubMed] [Google Scholar]
- 19.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews N W. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ming M, Ewen M E, Pereira M E A. Trypanosome invasion of mammalian cells requires activation of the TGFβ signaling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira N, Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976;143:1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page L J, Darmon A J, Uellner R, Griffiths G M. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401:146–156. doi: 10.1016/s0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez A, Martinez I, Chung A, Berlot C H, Andrews N W. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J Biol Chem. 1999;274:16754–16759. doi: 10.1074/jbc.274.24.16754. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez A, Rioult M G, Ora A, Andrews N W. A trypanosome-soluble factor induces IP3 formation, intracellular Ca2+ mobilization and microfilament rearrangement in host cells. J Cell Biol. 1995;129:1263–1273. doi: 10.1083/jcb.129.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez A, Samoff E, Rioult M G, Chung A, Andrews N W. Host cell invasion by trypanosomes requires lysosomes and microtubule/kinesin-mediated transport. J Cell Biol. 1996;134:349–362. doi: 10.1083/jcb.134.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez A, Webster P, Ortego J, Andrews N W. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rovere P, Inverardi L, Bender J R, Pardi R. Feedback modulation of ligand-engaged alpha L/beta 2 leukocyte integrin (LFA-1) by cyclic AMP-dependent protein kinase. J Immunol. 1996;156:2273–2279. [PubMed] [Google Scholar]
- 28.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 29.Schenkman S, Andrews N W, Nussenzweig V, Robbins E S. Trypanosoma cruzi invade a mammalian epithelial cell in a polarized manner. Cell. 1988;55:157–165. doi: 10.1016/0092-8674(88)90018-9. [DOI] [PubMed] [Google Scholar]
- 30.Schenkman S, Diaz C, Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991;72:76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- 31.Tardieux I, Nathanson M H, Andrews N W. Role in host cell invasion of Trypanosoma cruzi-induced cytosolic free Ca2+ transients. J Exp Med. 1994;179:1017–1022. doi: 10.1084/jem.179.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tardieux I, Webster P, Ravesloot J, Boron W, Lunn J A, Heuser J E, Andrews N W. Lysosome recruitment and fusion are early events required for trypanosome invasion of mammalian cells. Cell. 1992;71:1117–1130. doi: 10.1016/s0092-8674(05)80061-3. [DOI] [PubMed] [Google Scholar]
- 33.Tousson A, Fuller C M, Benos D J. Apical recruitment of CFTR in T-84 cells is dependent on cAMP and microtubules but not Ca2+ or microfilaments. J Cell Sci. 1996;109:1325–1334. doi: 10.1242/jcs.109.6.1325. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida N, Favoreto S, Jr, Ferreira A T, Manque P M. Signal transduction induced in Trypanosoma cruzi metacyclic trypomastigotes during the invasion of mammalian cells. Braz J Med Biol Res. 2000;33:269–278. doi: 10.1590/s0100-879x2000000300003. [DOI] [PubMed] [Google Scholar]