Abstract
Pulmonary surfactant protein A (SP-A) is involved in innate immunity in the lung. In this study we investigated the interaction of SP-A with different serotypes of lipopolysaccharide (LPS) on the regulation of inflammatory cytokines in vitro. In the human monocytic cell line, THP-1, combining SP-A with lipid A or rough LPS further enhanced lipid A- or rough LPS-stimulated tumor necrosis factor alpha (TNF-α) mRNA levels, while SP-A-elicited increases in TNF-α mRNA levels were partially neutralized. In contrast, the combination of smooth LPS and SP-A resulted in additive effects on TNF-α mRNA levels. We also demonstrated that there was cross-tolerance between SP-A and LPS in THP-1 cells. Pretreatment of THP-1 cells with LPS modestly inhibited the response of these cells to subsequent challenge with SP-A, with regard to the production of TNF-α, whereas there was no or little effect on the production of interleukin-1β (IL-1β) and IL-8. Conversely, pretreatment of THP-1 cells with SP-A markedly increased the response to subsequent challenge with LPS with regard to the production of IL-1β and IL-8, although the production of TNF-α was modestly decreased. However, a synergistic stimulatory effect was observed when the two agents were added simultaneously to the cells. NF-κB formation was downregulated in SP-A- but not in LPS-induced tolerant cells. These results suggested that SP-A exhibits different interactions with distinct serotypes of LPS. In addition, SP-A is different from LPS with regard to the induction of cross-tolerance, and these actions may be mediated, at least in part, through different mechanisms.
The respiratory system is continually exposed to a wide array of toxic substances and infectious agents. For this reason, the lung must have a rapid, versatile, and effective first line of defense or innate immune system to defend itself during the interval required for the development of specific immunity. Because surfactant covers all of the alveolar surfaces, any inhaled pathogens must interact with surfactant before they can interact with lung cells. Therefore, it is physiologically quite important to understand the role of surfactant in host defense against respiratory infection. Recent studies have drawn attention to the probable roles of surfactant protein A (SP-A) in innate host defense and inflammatory processes of the lung (6, 8, 26, 30, 33, 48). SP-A belongs to the collectin family of C-type lectins, along with mannose-binding protein (MBP), surfactant protein D, conglutinin, and collectin-43 (7). These proteins contain collagen-like amino-terminal domains and C-terminal carbohydrate recognition domains (CRD). Collectins are involved in many aspects of host defense function, and SP-A exerts a variety of stimulatory effects on alveolar macrophages (29, 42, 48). Among its many actions, SP-A binds to some pathogens by means of its CRD, thus promoting the binding and phagocytosis of these pathogens by the macrophage (39). SP-A also stimulates the generation of oxidative activity in macrophages (3, 9, 44), immune cell proliferation (20), the production of proinflammatory cytokines (21, 22), and the increased expression of cell surface proteins (23) in a monocyte/macrophage cell line and in other cells of monocytic origin. Additional convincing evidence that SP-A plays an important role in innate immunity comes from the finding that genetically engineered SP-A-deficient mice, which have essentially normal lung structure and function, show an increased susceptibility to infection by group B streptococcus and Pseudomonas aeruginosa (24, 25).
Bacterial lipopolysaccharide (LPS) or endotoxin, a constituent of the outer membrane of gram-negative bacteria, is a potent activator of mammalian immune cells, such as macrophages (32, 41). When macrophages are activated through LPS stimulation, they produce various cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-8, and IL-12, and chemical mediators, such as prostaglandins and nitric oxide (38). These cytokines and other mediators participate in various events associated with the inflammatory response at the alveolar level (47). Gram-negative bacteria that may infect the respiratory tract include some with smooth-LPS phenotypes and others with the rough phenotype (12). Currently, contradictory observations exist about the interaction of SP-A with smooth- and rough-LPS phenotypes (16, 31). The physiological and functional significance of this interaction in vivo has not been clearly defined.
In the present study, we tested the combined biological effects of SP-A and lipid A or different LPS phenotypes on TNF-α expression by the human THP-1 monocytic cell line and compared it to the individual effects of each of these agents. We report that SP-A interacts differently with lipid A and smooth or rough LPS in the regulation of inflammatory cytokine production by these cells. Previously, we demonstrated that SP-A induces tolerance in a different manner from LPS (35). We show here that pretreatment of THP-1 cells with SP-A or LPS can induce cross-tolerance to subsequent challenge with LPS or SP-A with respect to the production of TNF-α. We believe that the induction of tolerance by SP-A may be partially due to an impaired activation of NF-κB.
MATERIALS AND METHODS
Cell culture.
THP-1 cells were purchased from American Type Culture Collection (Manassas, Va.) and grown in suspension in complete RPMI 1640 (Sigma Chemical, St. Louis, Mo.) culture medium with 0.05 mM β-mercaptoethanol and 10% heat-inactivated fetal calf serum (FCS; Summit Biotechnology, Ft. Collins, Colo.) at 37°C in a humidified incubator with a 5% CO2 atmosphere. Because the properties of the THP-1 cell change after prolonged periods in culture, cells were discarded and replaced by early frozen stocks after ∼25 passages. We used 10−8 M 1,25-dihydroxycholecalciferol (vitamin D3) (Biomol Research Laboratories, Plymouth Meeting, Pa.) to differentiate the cells at a starting density of 5 × 105 cells/ml for 72 h. After differentiation, the cells were washed once with cold phosphate-buffered saline (PBS) to remove vitamin D3 and then resuspended in complete RPMI 1640 medium with 10% FCS at 2 × 106 cells/ml in 2 ml (4 × 106 cells per treatment) in 24-well culture plates (Fisher Scientific, Pittsburgh, Pa.). Cells were then incubated in the presence or absence of SP-A and/or LPS for designated periods of time. Smooth LPS (Escherichia coli O55:B5), rough LPS (E. coli J5), and lipid A from Salmonella enterica serotype Minnesota Re595 (Sigma Chemical) were used in this study.
For cross-tolerance experiments, vitamin D3-differentiated THP-1 cells were pretreated for 24 h with SP-A (0 or 50 μg/ml) or LPS (E. coli O55:B5, 0 or 0.1 ng/ml). These pretreatment exposures to SP-A or LPS were designated as SP-A1 or LPS1. Following this incubation, the cells were carefully washed with cold PBS and then challenged with the other agent, either LPS (0 or 0.1 ng/ml) or SP-A (0 or 50 μg/ml), designated LPS2 or SP-A2, respectively, for an additional 2-h period.
Preparation of SP-A.
SP-A was prepared from the bronchoalveolar lavage fluid of alveolar proteinosis patients using a preparative isoelectric focusing protocol that we have previously described in detail (46). With this method the protein is not exposed to organic solvents, detergents, or sulfhydryl reducing agents. The purified protein was examined by two-dimensional gel electrophoresis and silver staining and was found to be >99% pure (21). Endotoxin content was determined with the QCL-1000 Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.). This test indicated an average endotoxin level in our SP-A samples of <3 pg of LPS/mg of SP-A.
THP-1 cell RNA preparation and hybridization.
RNA was prepared, and the relative amounts of specific mRNAs were quantitated by hybridization. RNA blots were hybridized with 32P-labeled TNF-α, IL-1β, IL-8, and β-actin probes as previously described (36).
Following the final wash, the blots were partially dried, and exposures were done at −80°C with Kodak X-Omat XAR film (Rochester, N.Y.) and two intensifying screens (DuPont-New England Nuclear). Levels of mRNAs for specific targets were quantitated on the X-ray films by laser densitometry and normalized using blots hybridized for β-actin mRNA.
EMSA and supershift analysis. (i) Nuclear extract preparation and EMSA.
Conditions for the isolation of nuclear extracts and electrophoretic mobility shift assay (EMSA) are identical to those described previously (18).
(ii) Supershift analysis.
In order to identify the specific NF-κB components, we incubated nuclear extracts with the radiolabeled DNA fragment for 15 min and then added 1 μl (200 μg in 0.1 ml) of TranCruz antibodies to the subunits p50, p65, or c-Rel (Santa Cruz Biotechnology, Santa Cruz, Calif.). The reaction was continued for 30 min at room temperature. The mixture was then subjected to electrophoresis as described elsewhere (18). The gel was dried and exposed to X-ray film (Kodak) between two intensifying screens at −80°C for various intervals. Each experiment was performed at least three times with different preparations of nuclear extracts.
Statistical analysis.
RNA values given for each mRNA were the means of triplicate densitometric readings of RNA blots. SigmaStat statistical software (Jandel Scientific, San Rafael, Calif.) was used to analyze the data, and results were considered significantly different when the P value was <0.05.
RESULTS
Interaction of SP-A with LPS or lipid A on TNF-α mRNA levels.
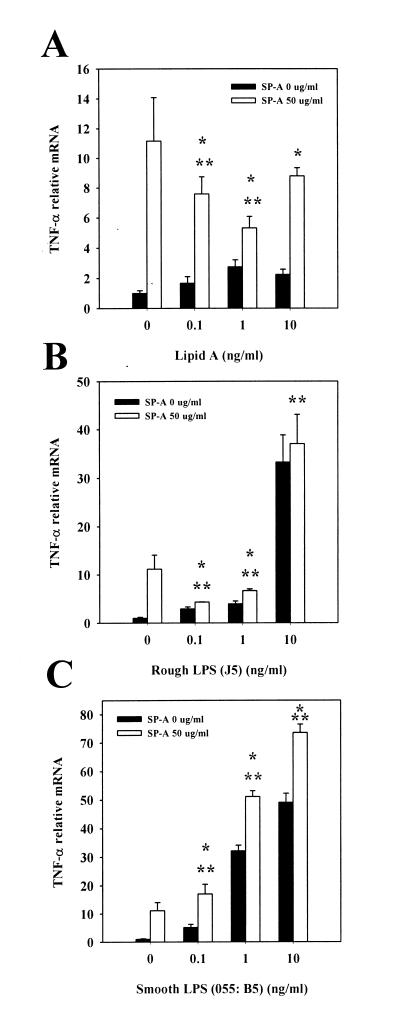
Our objective was to determine whether SP-A interacts differently with different serotypes of LPS and thus elicits distinct cellular responses. To accomplish this, THP-1 cells were differentiated with vitamin D3 and incubated with lipid A or different serotypes of LPS (in the range of 0.1 to 10 ng/ml) in the presence or absence of SP-A (50 μg/ml) for 2 h, and the relative TNF-α mRNA levels were compared. SP-A itself induced TNF-α expression in these cells. This finding is consistent with our previously reported observations (21, 22, 36, 46). In the absence of SP-A, lipid A in the range of 0.1 to 10 ng/ml induced weak but significant TNF-α expression. When the cells were incubated with lipid A in the presence of SP-A, lipid A-induced TNF-α expression was enhanced over levels seen with lipid A alone. However, the levels of induction of TNF-α resulting from combined treatment were less than those resulting from treatment with SP-A alone when either 0.1 or 1 ng of lipid A per ml in combination with 50 μg of SP-A per ml was used (Fig. 1A). When rough LPS (J5) was used with a similar treatment schedule, a similar pattern was observed, although there were greater differences between groups (Fig. 1B). In contrast, when the cells were incubated with smooth LPS (E. coli O55:B5) in the presence of 50 μg of SP-A per ml, the TNF-α mRNA levels induced by smooth LPS were increased above the levels resulting from treatment with smooth LPS or SP-A alone (Fig. 1C). In order to verify that the above results were not limited to the specific serotypes of LPS studied, some other serotypes of smooth LPS (Salmonella serotype Minnesota; Sigma) or rough LPS (Salmonella serotype Minnesota Re595; Sigma) were also included in this study. Similar results were obtained by using the same experimental protocol (data not shown).
FIG. 1.
Interaction of SP-A with LPS or lipid A on TNF-α mRNA levels. Vitamin D3-differentiated THP-1 cells were incubated with lipid A (A), rough LPS (J5) (B), or smooth LPS (E. coli O55:B5) (C) at the indicated concentrations in the absence (solid bars) or presence (open bars) of SP-A (50 μg/ml) for 2 h. Cells were then harvested and processed for TNF-α mRNA analysis by dot blotting, and relative mRNA levels were quantified by densitometry. ∗, P < 0.05 versus cells treated with lipid A or LPS without SP-A; ∗∗, P < 0.05 versus cells treated with 50 μg of SP-A per ml alone. The data are representative of one experiment out of the three or four experiments performed.
Effect of pretreatment with SP-A or LPS on cytokine mRNA levels.
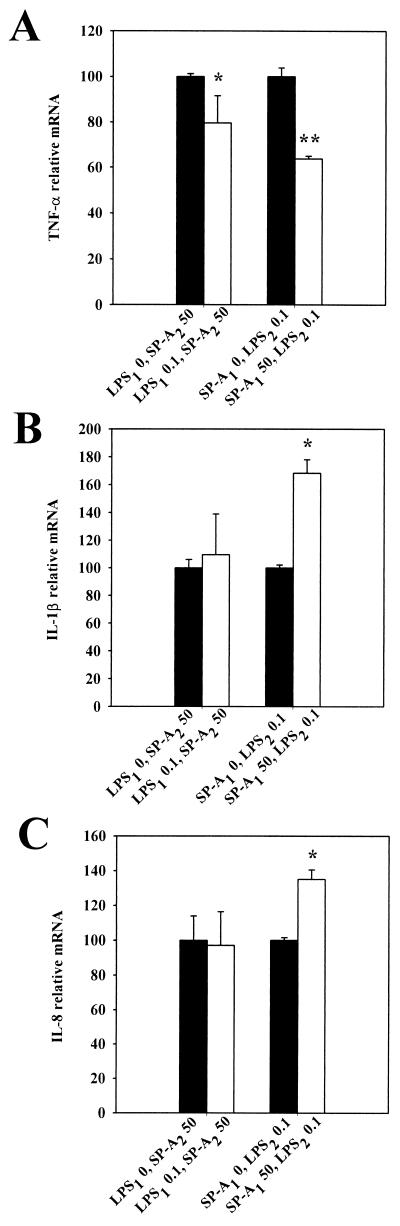
The phenomenon of LPS-induced tolerance is well described (50). Previous studies in our laboratory have shown that tolerance is not specific to LPS and that SP-A can also induce tolerance in THP-1 cells (36). We therefore investigated whether there was cross-tolerance between SP-A and LPS. In the present study, vitamin D3-differentiated THP-1 cells were pretreated for 24 h with 50 μg of SP-A per ml, 0.1 ng of LPS (E. coli O55:B5) per ml, or medium alone. The cells were then “cross-treated,” challenging them with either 0.1 ng of LPS or 50 μg of SP-A per ml for an additional 2-h period, and the relative cytokine mRNA levels were compared (Fig. 2). Pretreatment with LPS resulted in a modest inhibition of TNF-α (∼20%) in response to a subsequent challenge with SP-A compared to pretreatment with medium alone (Fig. 2A). There was little or no effect on production of IL-1β and IL-8 (Fig. 2B and C). Conversely, after pretreatment of THP-1 cells with SP-A, the production of TNF-α after a subsequent LPS challenge was decreased by ∼36% (Fig. 2A). However, there were marked increases in the production of IL-1β and IL-8 (∼68 and ∼34%, respectively) compared to cells not pretreated with SP-A (Fig. 2B and C). Thus, there is cross-tolerance between SP-A and LPS with regard to TNF-α expression but not with the other cytokines.
FIG. 2.
Effect of pretreatment with SP-A or LPS on cytokine mRNA levels. THP-1 cells were pretreated for 24 h with smooth LPS (E. coli O55:B5) (LPS1, 0 [solid bars] or 0.1 ng/ml [open bars]) or SP-A (SP-A1, 0 [solid bars] or 50 μg/ml [open bars]), washed, and then challenged with SP-A (SP-A2, 50 μg/ml) or LPS (LPS2, 0.1 ng/ml) for an additional 2 h. Cells were harvested and processed for TNF-α (A), IL-1β (B), and IL-8 (C) mRNA analysis by dot blotting, and relative mRNA levels were quantified by densitometry. ∗, P < 0.05 versus cells pretreated with 0 ng/ml LPS1; ∗∗, P < 0.05 versus cells not treated with SP-A (SP-A1). The data are representative of one experiment out of the three experiments performed.
Effect of combination of SP-A and LPS on cytokine mRNA levels.
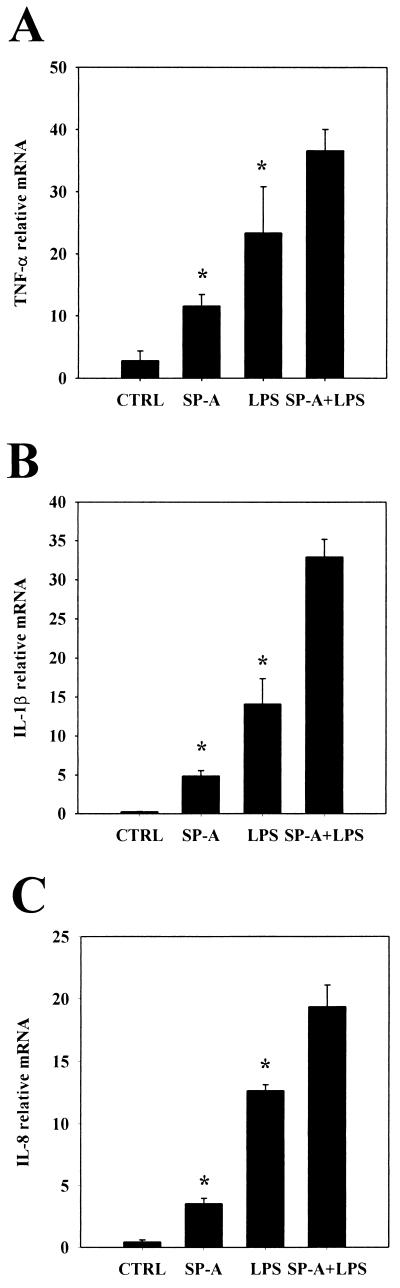
We then tested the combined biological effects of SP-A and LPS on cytokine mRNA levels from THP-1 cells. We chose to use the same LPS serotype (E. coli O55:B5) and the same dosage of LPS (0.1 ng/ml) or of SP-A (50 μg/ml) as those used in the cross-tolerance experiments. Both SP-A and LPS stimulated the production of proinflammatory cytokines, including TNF-α, IL-1β, and IL-8, by THP-1 cells. These results are similar to those that we have published previously (21, 22, 36, 46). When both SP-A and LPS were added together to the cultured cells, all three cytokine mRNA levels were further increased compared to the effects of each agent separately (Fig. 3). These results indicate that the combination of SP-A and LPS resulted in an additive stimulatory effect on these cells.
FIG. 3.
Effect of combination of SP-A and LPS on cytokine mRNA levels. THP-1 cells were incubated with medium (control, CTRL), SP-A (50 μg/ml), smooth LPS (E. coli O55:B5, 0.1 ng/ml), or combined SP-A (50 μg/ml) and LPS (0.1 ng/ml) for 2 h. Cells were then harvested and processed for TNF-α (A), IL-1β (B), and IL-8 (C) mRNA analysis by dot blotting, and relative mRNA levels were quantified by densitometry. ∗, P < 0.05 versus cells treated with SP-A plus LPS. The data are representative of one experiment out of the three or five experiments performed.
Effect of preincubation of SP-A or LPS on activation of NF-κB.
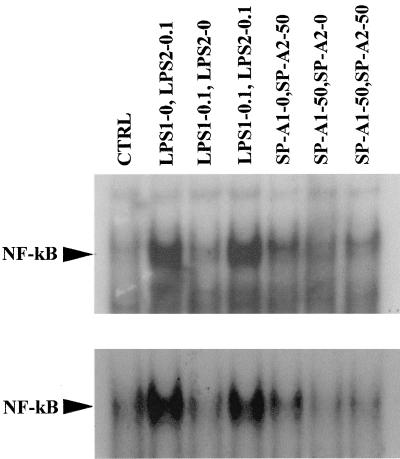
NF-κB is an important transcription factor for immune and inflammatory responses and plays a pivotal role in the regulation of cytokine production. Several studies have shown downregulation of NF-κB activation and DNA binding in LPS-induced tolerance (40). Previously, we reported that both SP-A and LPS can induce tolerance in THP-1 cells but that each produces different patterns of cytokine production (36), and we and others have also shown that SP-A or LPS stimulate production of proinflammatory cytokines via the activation of NF-κB in these cells (1, 18). Thus, studies were undertaken to determine whether impairment of NF-κB activation is involved in SP-A- or LPS-induced tolerance. This was done by performing EMSA using nuclear extracts from THP-1 cells that were preexposed to SP-A or LPS for 24 h, followed by subsequent SP-A or LPS challenge for an additional 1-h period. Figure 4 depicts two representative experiments. NF-κB DNA binding was decreased in SP-A tolerant cells after a subsequent SP-A challenge. Quantification by densitometry showed that, in challenged cells, complex formation was inhibited by ∼40% compared to cells treated with a single dose of SP-A. In contrast, pretreatment with LPS did not have any effects on NF-κB DNA binding after a similar challenge with LPS.
FIG. 4.
Effect of preincubation of SP-A or LPS on activation of NF-κB. THP-1 cells were pretreated for 24 h with smooth LPS (E. coli O55:B5) (LPS1, 0 or 0.1 ng/ml), SP-A (SP-A1, 0 or 50 μg/ml), washed, and then challenged with LPS (LPS2, 0 or 0.1 ng/ml) or SP-A (SP-A2, 0 or 50 μg/ml) for an additional 1 h. The cells were subsequently harvested, and nuclear extracts were prepared. EMSA analysis was then performed using a consensus NF-κB probe. The position of the NF-κB band is marked. Autoradiographs of representative gels are shown.
NF-κB supershift assay.
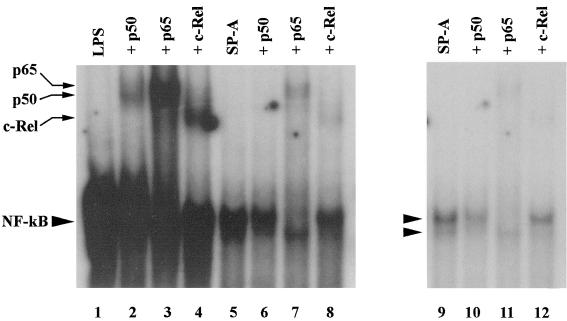
To confirm that the labeled complex was NF-κB, supershift assays were performed using antibodies to the NF-κB subunits p50, p65, and c-Rel. We have previously demonstrated that the NF-κB complexes in THP-1 cells do not contain p52 and RelB subunits (unpublished observations). The results of this analysis are shown in Fig. 5. Lanes 1 to 4 contain nuclear extracts from LPS-treated cells, and lanes 5 to 8 and lanes 9 to 12 have extracts from SP-A-treated cells. Cells were treated with LPS or SP-A for 1 h before being harvested for nuclear extract preparation. The NF-κB bands obtained were identical to those seen in tolerized cells after challenge (Fig. 4). In each case, an aliquot of nuclear extract-oligonucleotide mixture was incubated with the indicated antibody for 30 min and then analyzed by electrophoresis. The autoradiograph with lanes 1 to 8 has been heavily exposed to better demonstrate the supershifted bands. Treatment of nuclear extracts with anti-p50 antibody recognized p50 in both complexes (lanes 2 and 6). Anti-p65 antibody supershifted and reduced the intensity of the upper complex but did not affect the lower complex (lanes 3 and 7). Antibody to c-Rel supershifted and reduced the intensity of the lower complex but did not affect the upper complex (lanes 4 and 8). Lanes 9 to 12 depict a light exposure of lanes 5 to 8 to better demonstrate the NF-κB doublet and the changes in it that occur during supershifting. Therefore, NF-κB-binding complexes containing p50-p65 (upper complex) and p50–c-Rel (lower complex) are induced upon stimulation of vitamin D3-differentiated THP-1 cells with either SP-A or LPS. These results are similar to those obtained by Cordle et al. (5).
FIG. 5.
Antibody supershifts of NF-κB bands. Supershift assays were performed by incubating the nuclear extract-oligonucleotide mixtures with antibodies to p50, p65, and c-Rel, followed by EMSA. Extracts were prepared from THP-1 cells that had been treated with either 50 μg of SP-A per ml or 0.1 ng of LPS nuclear extract-oligonucleotide mixtures per ml with or without the various antibodies as indicated, and the positions of bands corresponding to each antibody are marked. A heavily exposed autoradiograph of a representative gel (lanes 1 to 8) is shown to demonstrate supershifted bands. A lighter exposure of lanes 5 to 8 is shown in lanes 9 to 12 to demonstrate the NF-κB doublet and the effects of supershifting on the NF-κB complex.
DISCUSSION
The lungs are continually exposed to ambient air that contains significant numbers of bacteria and other pathogens. Because surfactant covers the entire alveolar surface, inhaled pathogens must come in contact with surfactant before they can interact with target cells or immune cells. Moreover, both SP-A and LPS are often present in the lung during infection and the resulting inflammatory processes, and both are capable of modulating immune response in the lung. Therefore, it is important to understand the interaction between SP-A and LPS and the role of SP-A in host defense against respiratory infection.
Structurally, LPS consists of lipid A, a relatively conserved core oligosaccharide and a terminal polysaccharide of variable length and composition that comprises the O-specific antigen domain (32, 34). It has been suggested that SP-A preferentially binds to the lipid A domain of rough forms of LPS and to purified lipid A in vitro (16, 43). The binding of purified rough LPS is calcium independent and is not inhibited by competing saccharides but is inhibited or partially reversed by lipid A. In the present study we have shown that SP-A stimulates TNF-α production in THP-1 cells, as we reported earlier (21, 22, 36). Lipid A alone slightly induced TNF-α production. However, when SP-A and lipid A (in the range of 0.1 to 1 ng/ml) were both added to the cells, the stimulatory effect of SP-A was diminished. These results support the notion that SP-A binds to the lipid A moiety and that this binding partially neutralizes SP-A's activity.
Previous studies have reported that SP-A binds to LPS from the Re mutant of Salmonella serotype Minnesota and from the J5 mutant of E. coli in a calcium- and concentration-dependent manner but did not bind to E. coli O111, a form of smooth LPS (42). SP-A has also been shown to bind and cause aggregation of lipid A and rough LPS (Re595) but not of smooth LPS (O26:B6) (43, 35). Consistent with these findings, SP-A binds to certain rough but not smooth strains of E. coli and opsonizes them and enhances their phagocytosis and killing (31). Because the respiratory tract is a frequent target of a number of gram-negative bacteria that express a rough LPS phenotype, characterizing the interaction between SP-A and rough LPS is important for understanding the innate immune processes in the alveolar lumen. Our data show that both SP-A and rough LPS (J5) individually, as well as the combination of the two, stimulate production of TNF-α by THP-1 cells. However, LPS (J5) at low doses (0.1 to 1 ng/ml) partially neutralizes SP-A's activity. These results are similar to those obtained by the combination of SP-A and lipid A.
In contrast, the interaction of SP-A and smooth LPS generates different cellular responses. The combination of SP-A and smooth LPS (O55:B5) produces higher levels of TNF-α compared tests done with either substance alone. Blau et al. also reported that both SP-A and LPS (E. coli O55:135), as well as the combination of the two, stimulated production of nitrite by alveolar macrophages (3). The mechanism by which SP-A specifically enhances smooth LPS-induced TNF-α expression is not clear at present. It has been suggested that SP-A may interfere with the functions of serum components such as LPS-binding protein (LBP) (13), soluble CD14 (10), and septin (49), which have been shown to enhance LPS-induced cellular responses. Another possible interpretation for the action of SP-A is that SP-A may exert a role similar to that of LBP, forming a complex of SP-A and LPS that enhances the effect of LPS on the synthesis and secretion of cytokines such as TNF-α by alveolar macrophages. Thus, interactions between SP-A and LPS suggest that under appropriate conditions the interaction between these molecules could modulate cellular activation and signal pathways involving LPS. Such effects could theoretically involve competition for binding by other LBPs or altered presentation of LPS to inflammatory cells.
The data presented here are somewhat contradictory to the findings of Sano et al., who reported that SP-A inhibited mRNA expression and secretion of TNF-α induced by smooth LPS (O26:B6), but that rough LPS (Re)-induced TNF-α expression was unaffected by SP-A (35). There are several possible explanations for the apparent discrepancies. For example, in these two studies, SP-A was purified by different methods, and its activity may be dependent on the method of isolation (27, 37, 44). Moreover, the doses of LPS used in these two different studies varied tremendously, suggesting a fundamental difference in the culture system. There is some evidence that responses to high doses of LPS such as those used by Sano et al. (35) do not depend on CD14, while low doses do (38). Both soluble CD14 and LBP are necessary for neutrophil responses to low concentrations of smooth LPS, but the presence of LBP alone is sufficient to elicit a response to rough LPS (14). In addition, the means by which cells are activated to elaborate cytokines is also important. We have found that under conditions similar to those that we used for the THP-1 cells, U937 cells do not respond to SP-A stimulation and the responses to LPS are significantly less than those of THP-1 cells (unpublished observations).
Pretreatment of cells or animals with LPS renders them resistant to a subsequent LPS challenge. This phenomenon is referred to as tolerance (50). Previous studies in our laboratory have shown that tolerance is not specific to LPS; SP-A can also induce tolerance in THP-1 cells, though the patterns of cytokine alteration are distinct (36). We extended our earlier studies to explore whether there is cross-tolerance between these two molecules. Pretreatment of THP-1 cells with LPS modestly inhibited the response of these cells to subsequent treatment with SP-A, with regard to the production of TNF-α, compared to cells not pretreated with LPS. There was little or no effect on production of IL-1β and IL-8. Conversely, pretreatment of THP-1 cells with SP-A resulted in unique changes in cytokine release upon subsequent LPS stimulation. Specifically, TNF-α was decreased, while IL-1β and IL-8 production were significantly augmented. However, when both SP-A and LPS were added simultaneously to the cells, an additive stimulatory effect was observed. Production of all three cytokines studied was remarkably enhanced. These results clearly demonstrate that there exists a cross-tolerance between SP-A and LPS.
There are other reports available on cross-tolerance. For example, LPS and IL-1 were found to induce a state of cross-tolerance against each other, as judged by transcription factor activation (28). Another study showed that TNF-α injection in vivo resulted in hyporesponsiveness to the lethal effects of a subsequent dose of LPS, though TNF-α did not appear to mimic LPS in vitro (4). Most recently, Mendez and colleagues demonstrated that prior sublethal hemorrhage made rats tolerant and imparted a significant survival benefit and reduction in pulmonary vascular injury after a subsequent LPS challenge. Circulating TNF levels were also reduced in tolerant animals (29). Taken as a whole, these experiments illustrate cross-tolerance between LPS and a number of other stimuli or treatments, supporting the idea that tolerance is a nonspecific phenomenon.
The molecular mechanisms leading to LPS tolerance have been well studied but are still not completely delineated. LPS tolerance is not accompanied by decreased expression of CD14 (41). Investigations of the role of NF-κB in LPS tolerance using a human monocytic cell line (Mono Mac 6) showed a predominance of p50 homodimers, rather than the more common p50-p65 heterodimeric form of NF-κB (17). The p50 homodimeric form of NF-κB is thought to have a markedly impaired ability to stimulate cytokine genes. However, we found that in the THP-1 cell line there is no alteration of NF-κB activation in LPS-induced tolerant cells, though impairment of NF-κB activation occurs in SP-A-induced tolerant cells. Furthermore, resolution of the NF-κB complex in our gel shift assays showed that NF-κB exists as p50-p65 and p50–c-Rel heterodimers rather than as p50 homodimers. Other studies in LPS-tolerant peritoneal macrophages also showed that NF-κB was not downregulated (19, 45). Differences in NF-κB heterodimeric composition or some degree of IκB binding to NF-κB in tolerant cells may account for the altered electrophoretic mobility of NF-κB in the tolerant cells. Furthermore, in addition to effects on NF-κB, LPS tolerance also involves inactivation of mitogen-activated protein kinase family cascades, including extracellular signal-regulated kinase, p38, or c-Jun N-terminal kinase (40). Thus, multiple LPS-activated cascades are suppressed in LPS-tolerant macrophages.
The potential physiological relevance of SP-A tolerance or SP-A/LPS cross-tolerance is not clear at present. SP-A is always present in the alveolar lumen and increased SP-A has been associated with a variety of lung disease states, such as AIDS-related pneumonia, sarcoidosis, hypersensitivity pneumonitis, and asbestosis (11, 15, 26). We speculate that SP-A-induced tolerance or cross-tolerance to LPS may be a protective mechanism that prevents damage to the lung by avoiding excessive inflammation, as has been postulated for LPS tolerance (50).
In summary, there is an interaction between SP-A and LPS in vitro. Both SP-A and LPS have a stimulatory effect on TNF-α production by THP-1 cells. Distinct results are found when SP-A and different serotypes of LPS are simultaneously added to the cells. In addition, both SP-A and LPS (E. coli O55:B5) can induce a state of cross-tolerance against each other with respect to TNF-α production, while alterations of IL-1β and IL-8 are different in LPS–SP-A- or SP-A–LPS-induced cross-tolerance. The finding that NF-κB formation is downregulated in SP-A- but not in LPS-induced tolerant cells suggests that the mechanisms responsible for SP-A or LPS tolerance, at least in part, are different. It is difficult at present to speculate on the biological implications of such an SP-A–LPS interaction in vivo, and further studies are required to understand its effect on the overall cytokine balance at the onset and during the inflammatory response in the lung.
ACKNOWLEDGMENTS
This work was supported by National Heart, Lung, and Blood Institute grant HL-54683.
We thank Todd M. Umstead and Jill Hayden for excellent technical assistance.
REFERENCES
- 1.Antal J M, Divis L T, Erzurum S C, Wiedemann H P, Thomassen M J. Surfactant suppresses NF-κB activation in human monocytic cells. Am J Respir Cell Mol Biol. 1996;14:374–379. doi: 10.1165/ajrcmb.14.4.8600942. [DOI] [PubMed] [Google Scholar]
- 2.Blackwell T S, Blackwell T R, Christman J W. Induction of endotoxin tolerance depletes nuclear factor-κB and suppresses its activation in rat alveolar macrophages. J Leukoc Biol. 1997;62:885–891. doi: 10.1002/jlb.62.6.885. [DOI] [PubMed] [Google Scholar]
- 3.Blau H, Riklis S, Van Iwaarden J F, McCormack F X, Kalina M. Nitric oxide production by rat alveolar macrophages can be modulated in vitro by surfactant protein A. Am J Physiol. 1997;272:L1198–L1204. doi: 10.1152/ajplung.1997.272.6.L1198. [DOI] [PubMed] [Google Scholar]
- 4.Cavaillon J M, Pitton C, Fitting C. Endotoxin tolerance is not a LPS-specific phenomenon: partial mimicry with IL-1, IL-10 and TGF-β. J Endotoxin Res. 1994;1:21–29. [Google Scholar]
- 5.Cordle S R, Donald R, Read M A, Hawiger J. Lipopolysaccharide induces phosphorylation of MAD3 and activation of c-Rel and related NF-κB proteins in human monocytic THP-1 cells. J Biol Chem. 1993;268:11803–11810. [PubMed] [Google Scholar]
- 6.Crouch E. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol. 1998;19:177–210. doi: 10.1165/ajrcmb.19.2.140. [DOI] [PubMed] [Google Scholar]
- 7.Day A J. The C-type carbohydrate recognition domain (CRD) superfamily. Biochem Soc Trans. 1994;22:83–88. doi: 10.1042/bst0220083. [DOI] [PubMed] [Google Scholar]
- 8.Eggleton P, Reid K B M. Lung surfactant proteins involved in innate immunity. Curr Opin Immunol. 1999;11:28–33. doi: 10.1016/s0952-7915(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 9.Fraher L J, Borron P, Watson P, DeSousa D, Kiesel M, Veldhuizen R A W, Lewis J F, Possmayer F. Surfactant associated protein A (SP-A) stimulates nitric oxide synthase (iNOS) in the mouse macrophage line J774. Am J Respir Crit Care Med. 1997;155:A804. [Google Scholar]
- 10.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J. 1999;13:1455–1476. doi: 10.1183/09031936.99.13614779. [DOI] [PubMed] [Google Scholar]
- 12.Griffiss J M, Schneider H, Mandrell R E, Yamasaki R, Jarvis G A, Kim J J, Gibson B W, Hamadeh R, Apicella M A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1998;10(Suppl. 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- 13.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hailman E, Vasselon T, Kelley M, Busse L A, Hu M C, Lichenstein H S, Detmers P A, Wright S D. Stimulation of macrophages and neutrophils by complexes of lipopolysaccharide and soluble CD14. J Immunol. 1996;156:4384–4390. [PubMed] [Google Scholar]
- 15.Hermans C, Bernard A. Lung epithelial-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1996;159:646–678. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 16.Kalina M, Blau H, Riklis S, Kravtsov V. Interaction of surfactant protein A with bacterial lipopolysaccharide may affect some biological functions. Am J Physiol. 1995;268:L144–L151. doi: 10.1152/ajplung.1995.268.1.L144. [DOI] [PubMed] [Google Scholar]
- 17.Kastenbauer S, Ziegler-Heitbrock H W L. NF-κB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect Immun. 1999;67:1553–1559. doi: 10.1128/iai.67.4.1553-1559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koptides M, Umstead T M, Floros J, Phelps D S. Surfactant protein A activates NF-κB in the THP-1 monocytic cell line. Am J Physiol. 1997;273:L382–L388. doi: 10.1152/ajplung.1997.273.2.L382. [DOI] [PubMed] [Google Scholar]
- 19.Kraatz J, Clair L, Rodriguez J L, West M A. Macrophage endotoxin signal transduction in sepsis: Endotoxin pretreatment upregulates nuclear factor κB and inhibits mitogen activated protein kinase. Surg Forum. 1997;48:53–55. [Google Scholar]
- 20.Kremlev S G, Umstead T M, Phelps D S. Effects of surfactant protein A and surfactant lipids on lymphocyte proliferation in vitro. Am J Physiol. 1994;267:L357–L364. doi: 10.1152/ajplung.1994.267.4.L357. [DOI] [PubMed] [Google Scholar]
- 21.Kremlev S G, Phelps D S. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol. 1994;267:L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- 22.Kremlev S G, Umstead T M, Phelps D S. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. Am J Physiol. 1997;272:L996–L1004. doi: 10.1152/ajplung.1997.272.5.L996. [DOI] [PubMed] [Google Scholar]
- 23.Kremlev S G, Phelps D S. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am J Physiol. 1997;272:L1070–L1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 24.LeVine A M, Kurak K E, Bruno M D, Stark J M, Whitsett J A, Korfhagen T R. Surfactant protein A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- 25.LeVine A M, Kurak K E, Wright J R, Watford W T, Bruno M D, Ross G F, Whitsett J A, Korfhagen T R. Surfactant protein A binds group B streptococcus enhancing phagocytosis and clearance from lungs of surfactant protein-A-deficient mice. Am J Respir Cell Mol Biol. 1999;20:279–286. doi: 10.1165/ajrcmb.20.2.3303. [DOI] [PubMed] [Google Scholar]
- 26.Mason R J, Greene K, Voelker D R. Surfactant protein A and surfactant protein D in health and disease. Am J Physiol. 1998;275:L1–L13. doi: 10.1152/ajplung.1998.275.1.L1. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh J C, Mervin-Blake S, Connor E, Wright J R. Surfactant protein A protects growing cells and reduces TNF-α activity from LPS-stimulated macrophages. Am J Physiol. 1996;271:L310–L319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- 28.Medvedev A E, Kopydlowski K M, Vogel S N. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 29.Mendez C, Kramer A A, Salhab K F, Valdes G A, Norman J G, Tracey K J, Carey L C. Tolerance to shock: an exploration of mechanism. Ann Surg. 1999;229:843–849. doi: 10.1097/00000658-199906000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phelps D S. Pulmonary surfactant modulation of host-defense function. App Cardiopulm Pathophysiol. 1995;5:221–229. [Google Scholar]
- 31.Pikaar J C, Voorhout W F, Van Strijp J A G, Van Iwaarden F. Opsonic activities of surfactant proteins A and D in phagocytosis of gram-negative bacteria by alveolar macrophages. J Infect Dis. 1995;172:481–489. doi: 10.1093/infdis/172.2.481. [DOI] [PubMed] [Google Scholar]
- 32.Raetz C R, Ulevitch R J, Wright S D, Sibley C H, Ding A, Nathan C F. Gram-negative endotoxin: an extraordinary lipid with profound effects on eukaryotic signal transduction. FASEB J. 1991;5:2652–2660. doi: 10.1096/fasebj.5.12.1916089. [DOI] [PubMed] [Google Scholar]
- 33.Reid K B M. Functional roles of the lung surfactant proteins SP-A and SP-D in innate immunity. Immunobiology. 1998;199:200–207. doi: 10.1016/S0171-2985(98)80027-2. [DOI] [PubMed] [Google Scholar]
- 34.Rietshel E T, Brade L, Lindner B, Zahringer U. Biochemistry of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. I. Molecular biochemistry and cellular biology. Boca Raton, Fla: CRC Press; 1992. pp. 3–41. [Google Scholar]
- 35.Sano H, Sohma H, Muta T, Nomura S, Voelker D R, Kuroki Y. Pulmonary surfactant protein A modulates the cellular response to smooth and rough lipopolysaccharides by interaction with CD14. J Immunol. 1999;163:387–395. [PubMed] [Google Scholar]
- 36.Song M, Phelps D S. Comparison of SP-A and LPS effects on the THP-1 monocytic cell line. Am J Physiol. 2000;279:L110–L117. doi: 10.1152/ajplung.2000.279.1.L110. [DOI] [PubMed] [Google Scholar]
- 37.Suwabe A, Mason R J, Voelker D R. Calcium dependent association of surfactant protein A with pulmonary surfactant: application to simple surfactant protein A purification. Arch Biochem Biophys. 1996;327:285–291. doi: 10.1006/abbi.1996.0123. [DOI] [PubMed] [Google Scholar]
- 38.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 39.Tino M J, Wright J R. Surfactant protein A stimulates phagocytosis of specific pulmonary pathogens by alveolar macrophages. Am J Physiol. 1996;270:L677–L688. doi: 10.1152/ajplung.1996.270.4.L677. [DOI] [PubMed] [Google Scholar]
- 40.Tominaga K, Saito S, Matsuura M, Nakano M. Lipopolysaccharide tolerance in murine peritoneal macrophages induces downregulation of the lipopolysaccharide signal transduction pathway through mitogen-activated protein kinase and nuclear factor-κB cascades, but not lipopolysaccharide-incorporation steps. Biochem Biophys Acta. 1999;1450:130–144. doi: 10.1016/s0167-4889(99)00037-3. [DOI] [PubMed] [Google Scholar]
- 41.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 42.Van Golde L M G. Potential role of surfactant proteins A and D in innate lung defense against pathogens. Biol Neonate. 1996;67(Suppl. 1):2–17. doi: 10.1159/000244202. [DOI] [PubMed] [Google Scholar]
- 43.Van Iwaarden J F, Pikaar J C, Storm J, Brouwer E, Verhoef J, Oosting R S, Van Golde L M G, Vanstrijp J A G. Binding of surfactant protein A to the lipid A moiety of bacterial lipopolysaccharides. Biochem J. 1994;303:407–411. doi: 10.1042/bj3030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Iwaarden J F, Teding van Berkhout F, Whitsett J A, Oosting R S, Van Golde L M G. A novel procedure for the rapid isolation of surfactant protein A with retention of its alveolar-macrophage-stimulating properties. Biochem J. 1995;309:551–555. doi: 10.1042/bj3090551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wahlstrom K, Bellingham J, Rodriguez J L, West M A. Inhibitory κBα control of nuclear factor-κB is dysregulated in endotoxin-tolerant macrophages. Shock. 1999;11:242–247. doi: 10.1097/00024382-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Wang G, Phelps D S, Umstead T M, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-α production in the THP-1 cell line. Am J Physiol. 2000;278:L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 47.Welbourn C R B, Yong Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages, and inflammatory mediators. Br J Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- 48.Wright J R. Immunomodulatory functions of surfactant. Physiol Rev. 1997;77:931–961. doi: 10.1152/physrev.1997.77.4.931. [DOI] [PubMed] [Google Scholar]
- 49.Wright S D, Ramos R A, Patel M, Miller D S. Septin: a factor in plasma that opsonizes lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992;176:719–727. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ziegler-Heitbrock H W L. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]