Abstract
Transmission-blocking vaccines are one strategy for controlling malaria, whereby sexual-stage parasites are inhibited from infecting mosquitoes by human antibodies. To evaluate whether the recently cloned Plasmodium vivax proteins Pvs25 and Pvs28 are candidates for a transmission-blocking vaccine, the molecules were expressed in yeast as secreted recombinant proteins. Mice vaccinated with these proteins adsorbed to aluminum hydroxide developed strong antibody responses against the immunogens, although for Pvs28, this response was genetically restricted. Antisera against both recombinant Pvs25 and Pvs28 recognized the corresponding molecules expressed by cultured sexual-stage parasites isolated from patients with P. vivax malaria. The development of malaria parasites in mosquitoes was completely inhibited when these antisera were ingested with the infected blood meal. Pvs25 and Pvs28, expressed in Saccharomyces cerevisiae, are as yet the only fully characterized transmission-blocking vaccine candidates against P. vivax that induce such a potent antiparasite response.
Malaria is a disease caused by infection with protozoan parasites of the genus Plasmodium, of which four species infect humans. It produces up to 500 million new infections and 2 million deaths every year (34). Plasmodium vivax, one of the human malaria parasites, is responsible for the most prevalent form of recurrent malaria (25). Although the mortality rate is considerably lower than that for P. falciparum infection, vivax malaria causes significant morbidity for hundreds of millions of residents throughout Asia and South America. Chemotherapy and vector control have been insufficient to control the disease because parasite strains resistant to antimalarial drugs and mosquito vectors resistant to insecticides have emerged (9, 22, 23).
Plasmodium has a complicated life cycle, and efforts are being made to develop antiparasite vaccines against each stage (4, 6, 10, 11). One strategy is transmission-blocking vaccines designed to induce an immune response in the human host that will block the parasites' infectivity to the mosquito vector and consequently prevent spread of the parasite between humans. This strategy is thought to have important applications in low-transmission areas by preventing transmission from new foci of infection and in controlling escape mutants from vaccines targeted to other stages of the parasite life cycle. The target molecules of a transmission-blocking vaccine are those expressed by sexual-stage malaria parasites or by the digestive organs of the mosquito (11, 24). Antibodies raised against these molecules block the development of malaria parasites in the mosquito host.
Candidate molecules for a transmission-blocking vaccine against P. falciparum have been cloned (12, 17, 33). Among them, ookinete surface proteins Pfs25 and Pfs28 are well characterized and proven to confer transmission-blocking immunity in experimental animals (1, 2, 7, 12). Their homologues have recently been cloned from other species of malaria (5, 13, 29, 30). They all have similar structures composed of four tandem epidermal growth factor (EGF)-like domains, putatively anchored to the parasite surface by a glycosylphosphatidylinositol (GPI) moiety.
Basic studies using P. vivax have been limited because of it is less lethal than P. falciparum and difficult to maintain in in vitro cultures. There are only a few reports describing the candidate molecules for a transmission-blocking vaccine against P. vivax (20, 21, 26). Recently, Tsuboi and colleagues reported the successful isolation of the Pvs25 and Pvs28 genes from P. vivax (31). In this study, we have expressed Pvs25 and Pvs28 as recombinant proteins in Saccharomyces cerevisiae and demonstrated that they are potent candidates for a transmission-blocking vaccine against P. vivax malaria.
MATERIALS AND METHODS
Constructs.
The genes encoding Pvs25 from Ala23 to Leu195 and Pvs28 from Lys23 to Ser214 were amplified by PCR. P. vivax genomic DNA from the Salvador I strain was used, along with the forward and reverse primer pairs 5′-TATAGC GCTAGCGCCGTCACGGTATACACC–5′-TACAGAGGGCCCAAGGCAT ACATTTTTCTC for Pvs25 and 5′-CACACCGCTAGCAAAGGTCACCGCGGAGACC–5′-TCCGTTGGGCCCACTGTAAGCTGCTCCTGT for Pvs28. The reaction conditions for all PCRs were 94°C for 2 min, then 3 cycles of denaturation at 94°C, annealing at 42°C, and elongation at 72°C for 1 min each, followed by 25 cycles of annealing at 62°C. Ligation was performed with digested PCR products and plasmid YEpRPEU-3 using the NheI and ApaI restriction sites.
YEpRPEU-3 is an yeast episomal plasmid (27) encoding a secretory α-factor sequence terminating in lysine-arginine-(glutamate-alanine)2, the cleavage site of the KEX2 yeast protease, on the 5′ end of a multiple cloning site. Downstream of this site is hexahistidine sequence followed by a termination codon. Thus, all resulting recombinant proteins have an EAEAS N terminus and a GPHHHHHH C terminus (AS and GP are derived from the NheI and ApaI sequences). Recombinant protein production is under the ethanol-inducible ADH2 promoter 5′ to the α-factor sequence. Plasmid retention is maintained by tryptophan selection. The DNA sequences of all constructs were confirmed using a fluorescence-based automated DNA sequencer (ABI377; PE Applied Biosystems, Norwalk, Conn.).
Host cells and fermentation.
The resulting constructs were used to transform S. cerevisiae VK1 (haploid, trp1d lys2-801 pep4−:ura). Transformed yeast cells were screened for secretion of histidine-tagged protein by patch test as described elsewhere (14). A positive colony was expanded and used for fermentation.
Protein purification.
The fermentation supernatant was recovered by tangential microfiltration and concentrated to 300 ml by tangential ultrafiltration with a 1,000-molecular-weight-cutoff YD10 spiral hollow fiber filter (Amicon, Beverly, Mass.) and then continuously dialyzed by tangential diafiltration with 1.5 liters of 2× phosphate-buffered saline (PBS), pH 7.4. The retentate was incubated with nickel-nitrilotriacetic acid (Ni-NTA) agarose with shaking at 4°C. After overnight incubation, the suspension was transferred to a column, and the resin was washed with 2× PBS, pH 7.4, with 2× PBS, pH 6.8, and with 1× PBS, pH 6.4. The protein was eluted from the resin by using 0.25 M sodium acetate (pH 4.5) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Size exclusion chromatography was then performed using a 16/60 Superdex-75 column (Pharmacia, Uppsala, Sweden). Fractions containing the protein of interest were pooled, and the protein concentration was determined by a bicinchoninic acid assay (Pierce, Rockford, Ill.). Recombinant protein identity was confirmed by N-terminal sequencing and electron spray mass spectroscopy (Structural Biology Section, Mass Spectroscopy Laboratory, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH).
Animals and immunization.
Female 6- to 8-week-old BALB/c (H-2d), C57BL/6 (H-2b), A/J (H-2a), B10.BR (H-2k), CAF1 (H-2d/a), BALB.B (H-2b), and C57BL/10 (H-2b) mice (Jackson Laboratory, Bar Harbor, Maine) were used. Mice were injected intraperitoneally with 500 μl of PBS containing 50 μg of immunogen adsorbed with 800 μg of alum (aluminum hydroxide; Superfos Biosector, Vedbeak, Denmark) four times at 3-week intervals. Sera were collected before vaccination and 2 weeks after the third and fourth immunizations. All animal studies were done in compliance with NIH guidelines and under the auspices of an Animal Care and Use Committee-approved protocol.
Enzyme-linked immunosorbent assay (ELISA).
Serum antibodies to Pvs25 and Pvs28 were assayed as described previously (2). Briefly, flat-bottom 96-well microtiter plates (Immulon 4; Dynex Technology Inc., Chantilly, Va.) were coated with antigen at 4°C overnight. The saturating concentration of antigen was determined to be 200 ng of Pvs25 or 100 ng of Pvs28 per well. The plates were blocked with 1% skim milk (Difco, Detroit, Mich.) in TBS-T (0.05% Tween 20 in Tris-buffered saline) for 1 h at room temperature. Mouse sera were serially diluted in blocking buffer. One hundred microliters of diluted serum was added to antigen-coated wells in duplicate and incubated for 2 h at room temperature. After extensive washing with TBS-T, the plates were incubated with 100 μl of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) for 1 h. Bound antibodies were visualized by adding 100 μl of the substrate solution (p-nitrophenyl phosphate Sigma 104 substrate; Sigma Chemical Co., St. Louis, Mo.). The absorbance at 410 nm was read with a microplate reader. Antibody titers were calculated at 0.2 absorbance units, twofold greater than that of the matched dilution of adjuvant-vaccinated mouse sera.
Ookinete culture.
P. vivax gametocytes for ookinete culture were obtained under informed consent from patients admitted to the Malaria Clinic in Mae Sod, Thailand. Blood samples were collected in heparinized syringes by venipuncture from gametocyte-positive patients as determined by Giemsa-stained blood smears. Five milliliters of blood diluted with 20 ml of suspension activation buffer (10 mM Tris [pH 7.4], 170 mM NaCl, 10 mM glucose) was passed through a sterile a CF11 (Whatman, Clifton, N.J.) column to remove leukocytes. After centrifugation of the filtrate, the supernatant was removed and the pellet was resuspended with 10 ml of PBS containing 100 μM xanthurenic acid. After incubation for 45 min at room temperature, the mixture was layered onto 47% Percoll (Pharmacia)–/RPMI 1640 and then centrifuged at 500 × g for 15 min. The gametocyte-rich fraction at the interface was collected and washed three times with suspension activation buffer. Finally, the pellet was resuspended with 1 ml of culture medium (RPMI 1640 supplemented with hypoxanthine [50 μg/ml], 25 mM HEPES, 20% heat-inactivated fetal bovine serum, 24 mM NaHCO3, heparin [10 U/ml], penicillin [5 U/ml], and streptomycin [5 mg/ml], pH 7.8), and incubated for 24 h at 37°C. The cultured parasites were used for Western blotting and immunofluorescence microscopic analyses (IFA) as previously described (32).
Transmission-blocking assay.
Peripheral blood was collected from a splenectomized chimpanzee infected with the Salvador I strain of P. vivax. Blood was collected in heparin. Less than 1 h after collection, blood was centrifuged and the plasma was removed. Mixtures were made of packed erythrocytes and mouse antiserum against Pvs25 or Pvs28. Mixtures were immediately placed in feeding apparatuses and sequentially offered to four mosquito species. The first, Anopheles freeborni mosquitoes, were allowed to feed for 8 min. The apparatuses were then lifted, and cages of A. stephensi, A. gambiae, and A. farauti mosquitoes were similarly allowed to feed. Mosquitoes were then individually separated to remove unfed mosquitoes. Mosquitoes were incubated at 25°C in an incubator. Dissections were made at 6 to 7 days after feeding, and oocysts formed in the midgut were counted by microscopy.
RESULTS
Cloning of Pvs25 and Pvs28.
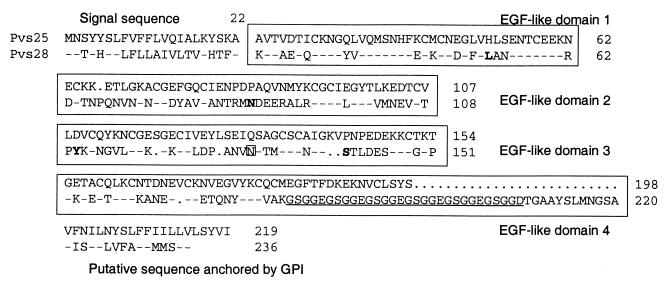
Pvs25 and Pvs28 share the typical family characteristics of the homologous proteins of other Plasmodium parasites. They have a signal sequence at the N terminus, four tandem EGF-like domains, and a C-terminal putative GPI attachment sequence (Fig. 1). At the amino acid level they have about 40% homology to both each other and the other members of this family. Pvs28 has a possible N-glycosylation site in the third EGF-like domain and a unique pentad amino acid GSGG(E/D) repeat in the fourth EGF-like domain. The Pvs28 cloned here from Salvador I DNA differs from the published Salvador I sequence (31) at positions Leu52, Asn87, Tyr110, and Ser140, replacing Met, Asp, Asn, and Thr, respectively. At least two of these differences have been identified as dimorphic polymorphisms: L/M and S/T at positions 52 and 140 (T. Tsuboi et al., unpublished data). Pvs25 and Pvs28 genes without the signal sequences and GPI linkers were cloned into the yeast expression plasmid YEpRPEU-3.
FIG. 1.
Structures of Pvs25 and Pvs28. Each protein has a signal sequence, four EGF-like domains, and a sequence moiety for the putative attachment of a GPI anchor, as well as 22 (Pvs25) or 20 (Pvs28) conserved cysteines. Numbers indicate the sequence positions of amino acids. In Pvs28, bold letters indicate amino acids that differ from the reported sequence, and the boxed asparagine in the third EGF domain indicates a putative N-linked glycosylation site. GSGG(E/D) repeats in Pvs28 are underlined. Identical (-) and absent (•) amino acids are also shown.
Expression and purification of recombinant Pvs25 and Pvs28.
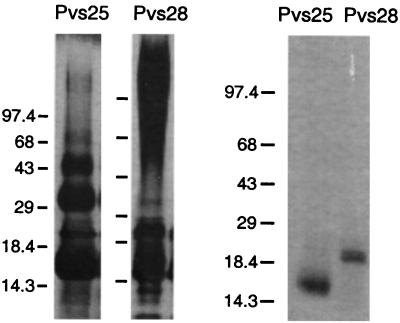
Recombinant proteins were purified from the yeast culture medium by Ni-NTA chromatography (Fig. 2). Recombinant Pvs28 contained a large amount of glycosylated protein that ran as a smear in the high-molecular-weight range. When a synthetic variant of Pvs28 was made with a Gln substituted for Asn130, the smear disappeared (data not shown). Pvs25, on the other hand, tended to polymerize. After size exclusion chromatography, the purified proteins were analyzed by N-terminal sequencing and mass spectroscopy for protein identification (Table 1). Pvs25 had a single N-terminal sequence which matched the predicted the first amino acid, indicating that this protein was full length, as it was purified based on a C-terminal hexahistidine sequence. Mass spectroscopic analysis also showed a sharp peak at 20,500.6 Dal. This peak was 23.6 Da less than the predicted molecular mass, explained by the formation of disulfide bonds between the 22 cysteines of Pvs25. By contrast, Pvs28 had multiple N-terminal sequences and multiple peaks in mass spectroscopy. This presumably occurred due to cleavage by yeast proteases. These analyses also confirmed that the higher-weight molecules found in the Pvs25 Ni-NTA eluant were polymerized forms of the recombinant protein (data not shown).
FIG. 2.
Coomassie blue-stained SDS-polyacrylamide gels of recombinant Pvs25 and Pvs28. Recombinant proteins tagged with hexahistidine were purified with Ni-NTA agarose from yeast culture supernatant (left) followed by size exclusion chromatography (right). Five microliters of Ni-NTA agarose eluant (left) or 5 μg of purified protein (right) was loaded in each lane. The purified proteins shown were used for further experiments. Sizes of the molecular weight markers are shown on the left in kilodaltons.
TABLE 1.
N-terminal sequencing and mass spectroscopy of recombinant proteins
| Protein | Positiona | N-terminal amino acid sequenceb | Mol wt
|
|
|---|---|---|---|---|
| Predicted | Observed | |||
| Pvs25 | 1 | EAEASAVTVDTI | 20,524.2 | 20,500.6 |
| Pvs28 | 1 | EAE(A)(S)KV(T)A(E)T(Q) | 21,533.9 | 21,619.3 |
| 10 | ETQ(C)(K)(N)G(Y)V(V)Q(M) | 20,646.9 | 20,496.4 | |
| 12 | (Q)(C)KNGYV(V)(Q)(M)SN | 20,416.7 | 19,533.0 | |
Position of the first sequenced amino acid with reference to the predicted sequence of the mature secreted recombinant proteins.
Five amino acids (EAEAS) are derived from vector sequences. Amino acids in parentheses were not detected.
Immunogenicity of recombinant Pvs25 and Pvs28.
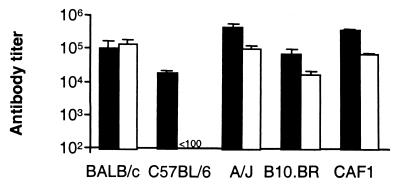
To assess the immunogenicity of these proteins, we vaccinated several strains of mice with Pvs25 or Pvs28 and tested the resultant sera in ELISAs (Fig. 3). Serum from all strains of mice vaccinated with Pvs25 recognized the immunogen, although there was a 23-fold difference in mean antibody titers between the highest (A/J)- and lowest (C57BL/6)-responding groups (465,012.3 ± 150,833.7 and 19,648.4 ± 2,729.1, respectively; arithmetic mean ± standard deviation from four mice). Strikingly, vaccination with Pvs28 did not induce an antibody response in C57BL/6 mice. However, other strains of mice developed anti-Pvs28 antibodies between titers of 17,608.3 ± 4,539.9 (B10.BR mice) and 140,022.1 ± 66,121.9 (BALB/c mice). Serum from prevaccinated mice and control mice vaccinated with adjuvant alone showed no antibody response (data not shown). Consistent with the antibody responses, splenic T cells from all vaccinated mice except C57BL/6 mice vaccinated with Pvs28 proliferated in response to the immunizing antigen (data not shown).
FIG. 3.
Immunological responses of mice vaccinated with Pvs25 or Pvs28, as determined by ELISA. Several strains of mice were vaccinated with Pvs25 (filled columns) or Pvs28 (open columns). Serum samples collected 2 weeks after the third vaccination were serially diluted, and ELISAs were performed on plates coated with the immunizing antigen. Sera from preimmune mice and mice injected with alum alone did not recognize these proteins (data not shown). Antibody titers were calculated as the serum dilution giving an optical density of 0.2. Results are expressed as the arithmetical mean value from four mice.
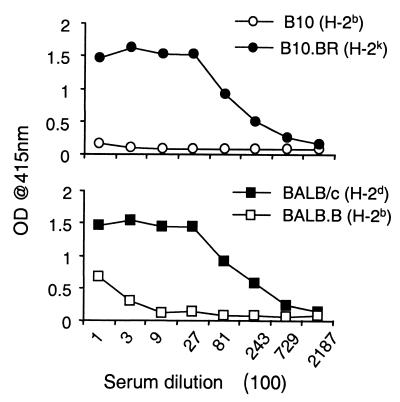
B- and T-cell responses to Pvs28 were detected in B10.BR mice, which are H-2 congenic with C57BL/10 mice. Except for a few loci, C57BL/10 mice are genetically identical to the nonresponding C57BL/6 mice, suggesting that nonresponsiveness is linked with the H-2b haplotype. To test this possibility, two sets of congenic mice strains, BALB/c - BALB.B and C57BL/10-B10.BR, were vaccinated with Pvs28 (Fig. 4). As expected, like C57BL/6 mice, C57BL/10 mice could not develop antibody responses, and BALB.B mice bearing H-2b were also impaired in antibody response.
FIG. 4.
Impaired immune responses against recombinant Pvs28 in mice with an H-2b haplotype. Two sets of congenic strains, B10 (top) and BALB background (bottom), were immunized with Pvs28, and antibody responses of the sera to Pvs28 were analyzed. Sera were collected from five mice and pooled 2 weeks after the third immunization. Experimental details are as for Fig. 3. OD, optical density.
Biological activity of antisera against Pvs25 or Pvs28.
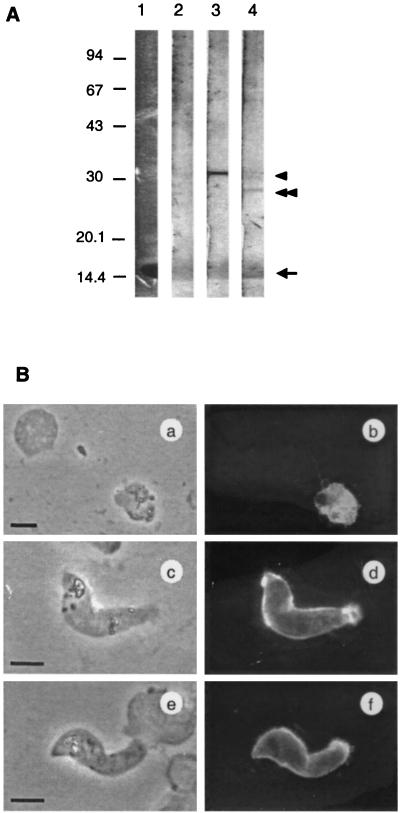
Western blotting and IFA using cultured parasites isolated from P. vivax malaria patients were performed. As shown in Fig. 5A, anti-Pvs25 and anti-Pvs28 antisera raised in mice recognized approximately 30-kDa and 28-kDa molecules, respectively, in the lysates from 24-h-cultured P. vivax parasites under nonreduced conditions. The deduced molecular masses of Pvs25 and Pvs28 are 21.8 and 22.4 kDa. However, the antisera to both proteins recognized molecules of slightly larger size. This is consistent with findings with other malarial antigens that have both complex globular EGF domains and GPI moieties (e.g., MSP-119, predicted size of 11 kDa and SDS-PAGE-determined size of 19 kDa). We could also detect a faint band migrating at the size of Pvs25 in strips stained with anti-Pvs28, suggesting that there was a cross-reactivity between Pvs25 and Pvs28. This cross-reactivity was also observed when the immunoblotting was performed against recombinant antigens (data not shown). Control sera obtained from mice vaccinated with PfMSP-119 secreted from yeast showed no reaction. Furthermore, IFA revealed that both anti-Pvs25 and anti-Pvs28 antisera stained the surface of mature ookinetes (Fig. 5B, d and f). The culture used here contained parasites at various developmental stages. Anti-Pvs25 antiserum stained a wide range of parasites from zygotes to mature ookinetes (Fig. 5B, b and d); by contrast, anti-Pvs28 stained mainly retort to mature ookinetes and only faintly recognized zygotes at the same dilution as the anti-Pvs25 antiserum (data not shown). These findings are consistent with previous reports demonstrating that the P25 family of proteins, to which Pvs25 belongs, are expressed at the early stage of parasite development in mosquito vectors relative to the P21/28 family, to which Pvs28 belongs (18). This discrepancy in the timing of protein expression between Pvs25 and Pvs28 may also explain the observed differential intensity of staining in the Western blots (strong staining with anti-Pvs25 antiserum and faint staining with anti-Pvs28 antiserum). Neither of these antisera stained asexual-stage parasites by Western blotting or IFA, and again control serum did not stain any parasites (data not shown).
FIG. 5.
Immunological reactivity of antisera to clinically isolated P. vivax. (A) Western blotting analysis of cultured P. vivax. Protein extracts from cultured parasites isolated from patients were size fractionated by SDS-PAGE under nonreducing conditions, and proteins were transferred to a polyvinylidene difluoride filter. Filter strips were incubated with anti-PfMSP-119 (lane 2), anti-Pvs25 (lane 3), or anti-Pvs28 (lane 4) mouse antiserum. Pvs25 (arrowhead) or Pvs28 (double arrowhead) were stained by antiserum against Pvs25 or Pvs28. Nonspecific staining was observed at around 15 kDa (arrow) coincident with an abundance of protein as determined by Coomassie blue staining (lane 1). Positions of standard molecular size markers are indicated in kilodaltons on the left. (B) IFA of cultured P. vivax sexual-stage parasites. Ice-acetone-fixed parasites were stained with anti-Pvs25 (a to d) or anti-Pvs28 (e and f) mouse antiserum from CAF1 mice. IFA (a, c, and e) and phase-contrast (b, d, and f) images of identical fields are shown. Bars = 5 μm.
Finally, the transmission-blocking activity of these mouse antisera was analyzed (Table 2). The assay system used was based on membrane feeding to mosquitoes using peripheral blood obtained from a chimpanzee infected with the P. vivax Salvador I strain (28) (identical to that from which the genes encoding Pvs25 or Pvs28 were cloned). Four species of susceptible anopheline mosquitoes developed oocysts in their midguts after ingestion of a P. vivax-infected blood meal mixed with control serum through a membrane-feeding apparatus. In contrast, oocyst formation was dramatically suppressed when anti-Pvs25 or -Pvs28 antisera from CAF1 mice were mixed with the infected blood. Anti-Pvs25 antiserum completely prevented oocyst formation in all mosquitoes. Of all mosquitoes fed with anti-Pvs28 antiserum, only one became infected, and that with a single oocyst. To further evaluate the transmission-blocking activity of these sera, we repeated this experiment using serial dilutions (Table 3). Again both anti-Pvs25 and anti-Pvs28 antisera significantly reduced transmission of parasites to mosquitoes up to the maximum dilution tested (1:32) (P < 0.01 with Mann-Whitney test). Blocking by anti-Pvs25 antiserum was much more effective at 1:8 or 1:32 dilution compared with that by anti-Pvs28 antiserum.
TABLE 2.
Transmission-blocking activities of sera from mice vaccinated with Pvs25 or Pvs28
| Expta | No. of oocyst-positive mosquitoes/no. dissected
|
|||
|---|---|---|---|---|
| A. freeborni | A. stephensi | A. gambiae | A. farauti | |
| 1 | ||||
| Control serum | 44/44 (67.4 ± 29.4b) | 35/35 (63.0 ± 32.4) | 29/29 (21.8 ± 25.2) | 17/17 (34.2 ± 22.7) |
| Anti-Pvs25 antiserum | 0/71 | 0/38 | 0/44 | 0/17 |
| Anti-Pvs28 antiserum | 0/61 | 0/38 | 0/25 | 0/17 |
| 2 | ||||
| Control serum | 13/13 (362.6 ± 95.8) | 11/11 (327.8 ± 51.4) | 13/13 (124.1 ± 89.0) | NDc |
| Anti-Pvs25 antiserum | 0/40 | 0/54 | 0/33 | ND |
| Anti-Pvs28 antiserum | 0/38 | 1/67d | 0/33 | ND |
Sera and infected blood cells were mixed in a ratio of 1:1 or 1:2 in experiment 1 or 2, respectively. Control serum was obtained from mice injected with alum alone.
Geometric mean number of oocysts per mosquito midgut ± standard deviation.
ND, not determined.
One oocyst was detected.
TABLE 3.
Comparison of transmission-blocking activities of anti-Pvs25 and anti-Pvs28 antisera
| Seruma | Dilutionb | Geometric mean no. of oocysts | Infectivityc | No. of infected mosquitoes/no. of dissected |
|---|---|---|---|---|
| Control | 1:2 | 136.8 ± 60.6 | 100 | 20 /20 |
| Anti-Pvs25 | 1:2 | 0 | 0 | 0 /33 |
| 1:8 | 0 | 0 | 0 /42 | |
| 1:32 | 3.31 ± 6.9 | 2.4 | 26 /34 | |
| Anti-Pvs28 | 1:2 | 0.91 ± 0.5 | 0.7 | 5 /47 |
| 1:8 | 4.82 ± 12.9 | 3.5 | 17 /23 | |
| 1:32 | 56.46 ± 31.9 | 41.3 | 22 /22 |
Sera from CAF1 mice were fed to A. stephensi.
Mouse sera were diluted with chimpanzee serum and then mixed 1:1 with P. vivax-infected erythrocytes from a chimpanzee.
Calculated as the geometric mean oocyst number of the test group divided by the mean oocyst number of the control group times 100.
DISCUSSION
This is the first report describing fully characterized candidate molecules for a transmission-blocking vaccine against the human malaria parasite P. vivax. The vaccination of mice with recombinant Pvs25 and Pvs28 produced antisera that recognized the corresponding molecules expressed by P. vivax sexual-stage parasites and, for Pvs25, completely blocked the transmission of parasites to mosquito vectors.
Both Pvs25 and Pvs28 have four cysteine-rich EGF-like domains. Such domains are known to be involved in protein-protein interactions that depend on the conformational structures formed by disulfide bonds (19). The recombinant proteins produced in this report were expressed in yeast, where posttranslational protein modifications (e.g., addition of sugars or formation of disulfide bonds) can occur. The mass spectroscopic analyses of the recombinant molecules that we produced suggest that all cysteines in recombinant Pvs25 formed disulfide bonds and released protons (Table 1). These molecules also show a shift in apparent molecular mass between reduced and nonreduced SDS-PAGE (data not shown). Furthermore, vaccination with these proteins induced biologically active antibodies in terms of recognition of parasite protein and blocking of parasite transmission. As previous reports have demonstrated that a proper conformation is required to induce transmission-blocking antibodies (3), these data taken together suggest that these recombinant may duplicate the native structure of the parasite molecules. Thus, the discrepancies in mobility on SDS-PAGE shown between recombinant (Fig. 2) and parasite (Fig. 5) proteins are probably due to the absence of a GPI moiety in the recombinants rather than conformational changes.
Recombinant Pvs25 is highly immunogenic in all strains of mice tested and induced both T- and B-cell responses. For recombinant Pvs28 the results were very similar, with the exception of mice with an H-2b-haplotype, which were unable to respond. This failure of Pvs28 to induce immune responses in H-2b-bearing mice was likely due to the failure of major histocompatibility complex class II molecules (specifically the I-Ab molecule) of this strain to present T-cell epitopes from Pvs28. Hence, vaccination with Pvs28 failed to activate helper T cells and consequently B-cell antibody production. The development of a very small anti-Pvs28 response in BALB/B mice suggests that factors other than H-2b may be involved as well. This major histocompatibility complex-linked unresponsiveness is a problem that will need to be addressed in future vaccine development.
For malaria in general, antigenic variation observed in target molecules may make it difficult to develop an effective vaccine against hepatic and erythrocytic parasites. However, unlike other malaria vaccines, the target molecules for transmission-blocking vaccines are expressed by the parasite in the mosquito vector. As such, these molecules are thought not to be exposed to the human immune system and so avoid immune selection pressure. Indeed, Pfs25 and Pfs28 display minimal variation between different field isolates (8, 15). The Western blotting and IFA results shown here, that antisera against Pvs25 or Pvs28 cloned from the Central or South America-derived Salvador I strain of P. vivax could recognize the corresponding molecules expressed by parasites isolated from patients in Thailand, support the concept that transmission-blocking vaccines may avoid the problem of antigenic variation. However, polymorphisms have been found in Pvs25 and Pvs28 isolated from different areas where malaria is endemic (31). Most of these polymorphisms are conservative point mutations, although variation in the number of C-terminal repeats was observed with Pvs28; the cause of this variation is not known. The questions of whether Pvs25 or Pvs28 are expressed in parasites circulating in human blood, such as gametocytes, and whether the antisera to the recombinant proteins will also inhibit the transmission of field-isolated parasites to mosquito vectors in the wild remain to be answered.
The actual mechanisms responsible for blocking the transmission of the parasite to the mosquito are elusive. One possibility is that these antisera interfere with molecules crucial to parasite growth, especially those involved in the transition from zygote to ookinete. Certainly monoclonal or polyclonal antibody against Pfs25, the P. falciparum homologue of Pvs25, inhibits the transformation of zygotes to mature ookinetes in vitro (16). Another possibility is that the development of the ookinete to an oocyst, especially the invasion by ookinetes into the epithelial cells of the mosquito midgut, is blocked. The former may occur in the early stages development in the mosquito, and the latter may occur in the late steps. The results of IFA (Fig. 5B) show that anti-Pvs25 serum stained intracellular regions of zygotes and anti-Pvs28 stained the surface of ookinetes, suggesting that antibodies against Pvs25 and Pvs28 may play different roles in blocking parasite development.
Based on the data presented here, we have elected in the first instance to concentrate our vaccine development efforts on recombinant Pvs25. It gives a higher yield in in vitro expression systems, an important consideration for a vaccine to be used in developing countries. As a recombinant protein, it is better characterized, with no apparent posttranslational modifications and an appropriate secondary structure. The antigenic variation of Pvs25 in field isolates appears to be more limited than for Pvs28 (31). Vaccination of mice with Pvs25 does not appear to be genetically restricted, and the production of transmission-blocking antibodies appears more potent. If the complete transmission-blocking activity observed here can be reproduced in primate models or human trials, these vaccine candidates may be powerful weapons in malaria control.
ACKNOWLEDGMENTS
This work was supported in part by Grant-in-Aid for Scientific Research 11670242 and Grants-in-Aid for Scientific Research on Priority Areas 08281104 and 11147220 from the Ministry of Education, Science, Sports and Culture, Japan, and a grant from the Japan-U.S. Cooperative Medical Science Program. This investigation also received financial support from the UNDP/World Bank/WHO Special Program for Research and Training in Tropical Diseases.
We thank Carole A. Long for critical comment, Richard L. Shimp and Rossane L. Hearn for technical assistance, and Yanling Zhang for protein purification, Mark Garfield and Carl Hammer (Structural Biology Section and Mass Spectroscopy Laboratory, NIAID/NIH) for protein identification, and Mary Kiganda and Brian Kristal for animal handling.
REFERENCES
- 1.Barr P J, Green K M, Gibson H L, Bathurst I C, Quakyi I A, Kaslow D C. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J Exp Med. 1991;174:1203–1208. doi: 10.1084/jem.174.5.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy P E, Kaslow D C. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect Immun. 1997;65:1109–1113. doi: 10.1128/iai.65.3.1109-1113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy P E, Pimenta P, Kaslow D C. Pgs28 belongs to a family of epidermal growth factor-like antigens that are targets of malaria transmission-blocking antibodies. J Exp Med. 1993;177:505–510. doi: 10.1084/jem.177.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engers H D, Godal T. Malaria vaccine development: current status. Parasitol Today. 1998;14:56–64. doi: 10.1016/s0169-4758(97)01184-8. [DOI] [PubMed] [Google Scholar]
- 5.Fried M, Gwadz R W, Kaslow D C. Identification of two cysteine-rich, lipophilic proteins on the surface of Plasmodium knowlesi ookinetes: Pks20 and Pks24. Exp Parasitol. 1994;78:326–330. doi: 10.1006/expr.1994.1034. [DOI] [PubMed] [Google Scholar]
- 6.Good M F, Kaslow D C, Miller L H. Pathways and strategy for developing a malaria blood-stage vaccine. Annu Rev Immunol. 1998;16:57–87. doi: 10.1146/annurev.immunol.16.1.57. [DOI] [PubMed] [Google Scholar]
- 7.Gozar M M G, Price V L, Kaslow D C. Saccharomyces cerevisiae-secreted fusion proteins Pfs25 and Pfs28 elicit potent Plasmodium falciparum transmission-blocking antibodies in mice. Infect Immun. 1998;60:59–64. doi: 10.1128/iai.66.1.59-64.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hafalla J C R, Santiago M L O, Pasay M C J, Ramirez B L, Gozar M M G, Saul A, Kaslow D C. Minimal variation in the Pfs28 ookinete antigen from Philippine field isolates of Plasmodium falciparum. Mol Biochem Parasitol. 1997;87:97–99. doi: 10.1016/s0166-6851(97)00042-x. [DOI] [PubMed] [Google Scholar]
- 9.Harinasuta T, Suntharasamai P, Viravan C. Chloroquine-resistant falciparum malaria in Thailand. Lancet. 1965;2:657–660. doi: 10.1016/s0140-6736(65)90395-8. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman S L, Rogers W O, Carucci D J, Venter J C. From genomics to vaccines: malaria as model system. Nat Med. 1998;4:1351–1353. doi: 10.1038/3934. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow D C. Progress towards transmission-blocking vaccine. In: Good M F, Saul A J, editors. Molecular immunological considerations in malaria vaccine. London, England: CRC Press; 1994. pp. 209–244. [Google Scholar]
- 12.Kaslow D C, Quankyi I A, Syin C, Raum M B, Keister D B, Coligan J E, McCutchan T F, Miller L H. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature. 1988;333:74–76. doi: 10.1038/333074a0. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow D C, Syin C, McCutchan T F, Miller L H. Comparison of the primary structure of the 25 kDa ookinete surface antigens of Plasmodium falciparum and Plasmodium gallinaceum reveal six conserved regions. Mol Biochem Parasitol. 1989;33:283–287. doi: 10.1016/0166-6851(89)90090-x. [DOI] [PubMed] [Google Scholar]
- 14.Kaslow D C, Shiloach J. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV25H expressed in yeast and purified using nickel-NTA agarose. Bio Technology. 1994;12:494–499. doi: 10.1038/nbt0594-494. [DOI] [PubMed] [Google Scholar]
- 15.Kaslow D C, Quakyi I A, Keister D B. Minimal variation in a vaccine candidate from the sexual stage of Plasmodium falciparum. Mol Biochem Parasitol. 1989;32:101–103. doi: 10.1016/0166-6851(89)90134-5. [DOI] [PubMed] [Google Scholar]
- 16.Kaslow D C, Bathurst I C, Lensen T, Ponnuduraoi T, Barr P J, Keister D B. Saccharomyces cerevisiae recombinant Pfs25 adsorbed to alum elicits antibodies that block transmission of Plasmodium falciparum. Infect Immun. 1994;62:5576–5580. doi: 10.1128/iai.62.12.5576-5580.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kocken C H, Jansen J, Kaan A M, Beckers P J, Ponnudurai T, Kaslow D C, Konings R N, Schoenmakers J G. Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol. 1993;61:59–68. doi: 10.1016/0166-6851(93)90158-t. [DOI] [PubMed] [Google Scholar]
- 18.Kumar N, Carter R. Biosynthesis of two-stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinete. Mol Biochem Parasitol. 1985;14:127–139. doi: 10.1016/0166-6851(85)90032-5. [DOI] [PubMed] [Google Scholar]
- 19.McDonald N Q, Hendrickson W A. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993;73:421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 20.Mendis K N, Muneshinghe Y D, de Silva Y N, Keragalla I, Carter R. Malaria transmission-blocking immunity induced by natural infection of Plasmodium vivax in humans. Infect Immun. 1987;55:369–372. doi: 10.1128/iai.55.2.369-372.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Premawansa S, Peiris J S, Perera K L, Ariyaratne G, Carter R, Mendis K N. Target antigens of transmission blocking immunity of Plasmodium vivax malaria. Characterization and polymorphism in natural parasite isolates. J Immunol. 1990;144:4376–4383. [PubMed] [Google Scholar]
- 22.Rieckmann K H, Davis D R, Hutton C D. Plasmodium vivax resistance to chloroquine? Lancet. 1989;2:1183–1184. doi: 10.1016/s0140-6736(89)91792-3. [DOI] [PubMed] [Google Scholar]
- 23.Roberts D R, Andre R G. Insecticide resistance issues in vector-bore disease control. Am J Trop Med Hyg. 1994;50:21–34. doi: 10.4269/ajtmh.1994.50.21. [DOI] [PubMed] [Google Scholar]
- 24.Shahabuddine, M., S. Cociancich, and H. Zieler. The search for novel malaria transmission-blocking targets in the mosquito midgut. Parasitol. Today 14:493–497. [DOI] [PubMed]
- 25.Sharma B K, Talwar K K, Bhatnagar V, Kumar L, Ganguly N K, Mahajan R C. Recurrent anaphylaxis due to Plasmodium vivax infection. Lancet. 1979;1:1340–1341. doi: 10.1016/s0140-6736(79)91965-2. [DOI] [PubMed] [Google Scholar]
- 26.Snewin V A, Premawansa S, Kapilananda G M G, Ratnayaka L, Udagama P V, Mattei D M, Khouri E, DelGiudice G, Peiris J S M, Mendis K N, David P H. Transmission blocking immunity in Plasmodium vivax malaria: antibodies raised against a peptide block parasite development in the mosquito vector. J Exp Med. 1995;181:357–362. doi: 10.1084/jem.181.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stowers A W, Keister D B, Muratova O, Kaslow D C. A region of Plasmodium falciparum antigen Pvs25 that is the target of highly potent transmission-blocking antibodies. Infect Immun. 2000;68:5530–5538. doi: 10.1128/iai.68.10.5530-5538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan J S, Morris C L, McClure H M, Strobert E, Richardson B B, Galland G G, Goldman I F, Collins W E. Plasmodium vivax infections in chimpanzees for sporozoite challenge studies in monkeys. Am J Trop Med Hyg. 1996;55:344–349. doi: 10.4269/ajtmh.1996.55.344. [DOI] [PubMed] [Google Scholar]
- 29.Tsuboi T, Cao Y-M, Kaslow D C, Shiwaku K, Torii M. Primary structure of a novel ookinete surface protein from Plasmodium berghei. Mol Biochem Parasitol. 1997;85:131–134. doi: 10.1016/s0166-6851(96)02821-6. [DOI] [PubMed] [Google Scholar]
- 30.Tsuboi T, Kaslow D C, Cao Y-M, Shiwaku K, Torii M. Comparison of Plasmodium yoelii ookinete surface antigens with human and avian malaria parasite homologues reveals two highly conserved regions. Mol Biochem Parasitol. 1997;87:107–111. doi: 10.1016/s0166-6851(97)00049-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsuboi T, Kaslow D C, Gozar M M G, Tachibana M, Cao Y-M, Torii M. Sequence polymorphism in two novel Plasmodium vivax ookinete surface proteins, Pvs25 and Pvs28, that are malaria transmission-blocking vaccine candidates. Mol Med. 1998;4:772–782. [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuboi T, Cao Y-M, Hitsumoto Y, Yanagi T, Kanbara H, Torii M. Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect Immun. 1997;65:2260–2264. doi: 10.1128/iai.65.6.2260-2264.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson K C, Criscio M D, Kaslow D C. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol Biochem Parasitol. 1993;58:355–358. doi: 10.1016/0166-6851(93)90058-6. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. World malaria situation in 1994. Wkly Epidemiol Rec. 1997;72:285–292. [Google Scholar]