Abstract
The invasiveness and virulence of Shigella spp. are largely due to the expression of plasmid-encoded virulence factors, among which are the invasion plasmid antigens (Ipa proteins). After infection, the host immune response is directed primarily against lipopolysaccharide (LPS) and the virulence proteins (IpaB, IpaC, and IpaD). Recent observations have indicated that the Ipa proteins (IpaB, IpaC, and possibly IpaD) form a multiprotein complex capable of inducing the phagocytic event which internalizes the bacterium. We have isolated a complex of invasins and LPS from water-extractable antigens of virulent shigellae by ion-exchange chromatography. Western blot analysis of the complex indicates that all of the major virulence antigens of Shigella, including IpaB, IpaC, and IpaD, and LPS are components of this macromolecular complex. Mice or guinea pigs immunized intranasally with purified invasin complex (invaplex), without any additional adjuvant, mounted a significant immunoglobulin G (IgG) and IgA antibody response against the Shigella virulence antigens and LPS. The virulence-specific response was very similar to that previously noted in primates infected with shigellae. Guinea pigs (keratoconjunctivitis model) or mice (lethal lung model) immunized intranasally on days 0, 14, and 28 and challenged 3 weeks later with virulent shigellae were protected from disease (P < 0.01 for both animal models).
Shigellosis is a leading cause of human diarrheal disease. Each year millions of cases occur, particularly in developing countries, with over 1 million cases resulting in death (15). The constant emergence of antibiotic resistance in Shigella spp. (12), even to the newest antibiotics, underscores the need for an effective vaccine to help control Shigella disease. Vaccine strategies must consider the need for protection against four species of Shigella (S. flexneri, S. sonnei, S. dysenteriae, and S. boydii) with over 45 different serotypes, and also enteroinvasive Escherichia coli (EIEC), as cross-protection is not significant between the species. Historically, successful Shigella vaccines have emphasized presentation of lipopolysaccharide (LPS) in a manner that will elicit protection. Such vaccines include live attenuated vaccines (25, 32) and delivery of Shigella LPS or O polysaccharides with carriers such as proteosomes (28), tetanus toxoid (6), or ribosomes (16). Of these vaccine approaches, only the live attenuated vaccines utilize the native invasiveness of the shigellae to deliver the LPS and other antigens to the mucosal immune system, presumably via the follicle-associated epithelium (33). The residual pathogenicity of the attenuated vaccine strains may limit this approach unless further attenuation is achieved (7).
The pathogenesis of Shigella is attributed to the organism's ability to invade, replicate intracellularly, and spread intercellularly within the colonic epithelium. The invasion of host cells by Shigella spp. is a complex multifactorial event in which many different bacterial proteins are involved. Many of the genes for key Shigella virulence proteins are located on a 140-MDa plasmid and are conserved in all Shigella spp. Several of the plasmid-encoded proteins, called the invasion plasmid antigens (IpaA, IpaB, IpaC, and IpaD) (3), are essential virulence factors. Upon contact or attachment to host cells, the Shigella invasins induce a phagocytic event which results in engulfment and internalization of the bacterium by the host cell (21). Recent reports have identified protein complexes consisting of either IpaB and IpaC (23) or IpaB, IpaC, and IpaD (43) in the growth medium of Shigella cultures. The components of this complex are involved in the invasion process and are released upon contact with host cells (43) by a type III secretion apparatus (22). Roles for individual proteins in the complex range from induction of apoptosis by IpaB (5) to attachment to host cells and actin polymerization by IpaC (20, 40). It has been proposed that the IpaB-IpaC complex integrates into the host cell membrane, forming a channel by which other Shigella proteins gain entry into the host cell (33).
Involvement in the early host-pathogen interaction and the induction of phagocytosis suggests that the invasins or the invasin complex might be a critical target that the immune response may attempt to neutralize or inhibit. In theory, the conserved sequences and immunologic cross-reactivity of the Ipa proteins of all Shigella species may enable a vaccine containing the Ipa proteins to be effective against more than one Shigella species. Previously it has been shown that IpaB, IpaC, and IpaD, along with LPS, are major antigens recognized by Shigella-infected individuals (17, 26, 31, 39). Monkeys or humans infected with Shigella produce antibodies predominantly to IpaB and IpaC (26) and at lower frequency to IpaA, IpaD, and VirG (IcsA; another plasmid-encoded virulence protein which is required for intercellular spreading). One of the best antigen preparations for measuring the immune response to virulence components of Shigella is the water-extractable antigen (13, 17, 26) which is capable of measuring the antibody response to IpaB, IpaC, IpaD, and VirG. It is not clear if antibodies reactive with the water extract bind to individual soluble proteins or bind to a native virulence structure within the water extract.
To better understand the immunology of shigellosis and the role of the invasin complex in protective immunity, we have developed a method for purifying a native invasin-LPS complex from water-extractable antigens of intact invasive shigellae. This isolated invasin complex (invaplex), which contains the major antigens of virulent Shigella, is shown to be immunogenic when delivered by a mucosal route without the need for any additional adjuvant. Furthermore, guinea pigs and mice immunized with invaplex are protected from disease upon challenge with virulent Shigella.
MATERIALS AND METHODS
Bacterial growth and strains.
The Shigella strains used in these studies, all part of the Walter Reed Army Institute of Research collection, were Shigella flexneri 5 M90T-W (a virulent [Vir+] strain), S. flexneri 5 M90T-55 (Vir−), and S. flexneri 2a 2457T. S. flexneri 5 INC103 is a clinical isolate from Peru kindly provided by C. Fernandez-Prada.
Isolated red Shigella colonies grown on Congo red tryptic soy agar plates (29) were used to inoculate 50 ml of Penassay (antibiotic medium 3; Difco Laboratories, Detroit, Mich.) broth at 37°C. After 4 h of growth, 10 ml of the log-phase culture was added to each liter of prewarmed (37°C) Penassay broth. The 1-liter cultures were incubated overnight at 37°C in a shaking incubator.
Water extraction of Shigella proteins.
A modification of the original water extraction procedure described by Oaks et al. (26) was used to prepare the material from which the Shigella invasin complex was isolated. Typically, 4 liters of an overnight culture of virulent shigellae was used for one batch of water extract. The bacterial cells were collected by centrifugation, suspended in sterile, deionized water (0.45-μm-pore-size membrane filtered) at a volume of 50 ml per liter of overnight culture, and then incubated at 37°C in a shaking water bath (ca. 200 rpm) for 2 h. After extraction with water, the cells were collected by centrifugation at 16,000 × g for 30 min at 4°C. The supernatant was collected and centrifuged at 100,000 × g for 1 h at 4°C to pellet membrane fragments. All 100,000 × g supernatants for a single batch of water extract were pooled and stored at −70°C. The water extract was maintained on ice when possible, and protease inhibitors were not used during the procedure.
Characterization of water extract.
The total protein content of each batch of water extract was measured by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.). Water extract was analyzed for the presence of IpaB and IpaC by Western blot or spot blot assays using monoclonal antibodies (MAbs) specific for IpaB (2F1) and IpaC (2G2) (24). Only water extracts that were positive for these Ipa proteins were used for invasin complex purification.
FPLC (fast protein liquid chromatography).
Ion-exchange chromatography was used to isolate invasin complex fractions from water extract. A 5-ml anion-exchange HiTrap Q (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.) column was equilibrated with 20 mM Tris-HCl (Sigma Chemical Co., St. Louis, Mo.), pH 9.0 (buffer A), at ambient temperature. Prior to loading, Tris-HCl (0.2 M, pH 9.0) was added to the water extract sample to a final concentration of 20 mM, after which 20 to 150 ml (approximately 8 to 80 mg of total protein) of the water extract was loaded onto the column at a flow rate of 2 ml/min. After loading, the column was washed with at least 6 column volumes of buffer A. All elutions were carried out with step gradients of 24% buffer B followed by a 50% buffer B step, and finally the column was washed with 100% buffer B (1 M NaCl in 20 mM Tris-HCl, pH 9.0). Protein passing through the column was monitored at 280 nm and recorded via the PowerChrom data acquisition and analysis software (ADInstruments, Mountain View, Calif.) for the Macintosh computer operating system. Two-milliliter fractions were collected in polypropylene tubes and immediately placed at −70°C. Buffer steps were changed after the optical density at 280 nm (OD280) returned to baseline for the previous buffer. The buffer B diluent was 20 mM Tris-HCl, pH 9.0. After washing with 100% buffer B, the column was reequilibrated with buffer A before the next run. Each column used in these studies was dedicated to a specific serotype and strain of Shigella.
Each fraction was analyzed by spot blotting for the presence of IpaC and IpaB. Fractions (usually one or two) containing the greatest amount of IpaB and IpaC in 24% buffer B were pooled, as were peak Ipa protein fractions in 50% buffer B, resulting in invaplex 24 and invaplex 50, respectively, for a run. Invaplex 24 and invaplex 50 run pools, once determined to be relatively similar with respect to IpaB, IpaC, and IpaD content (determined by Western blotting), LPS content (determined by silver stain analysis of gels [see below]), and total protein composition, were combined, identified as a particular lot of invaplex 24 or invaplex 50, and stored at −80°C.
Water extract and LPS ELISAs.
Antigens used in enzyme-linked immunosorbent assays (ELISAs) include water extracts from Vir+ (M90T-W) and Vir− (M90T-55) strains of S. flexneri 5 (26, 38) (referred to as Vir+ and Vir− water extracts) and also purified LPS from either S. flexneri 2a or S. flexneri 5. Antigen was diluted in carbonate coating buffer (0.2 M carbonate, pH 9.8) and was added to polystyrene 96-well antigen plates (Dynex Technologies, Inc., Chantilly, Va.) at a concentration of 1 μg/well. Primary antibody was diluted in casein (2% casein in a Tris-saline buffer, pH 7.5) and incubated with the antigen-coated plates for 2 h. After four washes in phosphate-buffered saline (10.75 mM sodium phosphate, 145 mM NaCl) with 0.05% Tween 20, plates were probed with commercial anti-guinea pig immunoglobulin G (IgG), anti-mouse IgG, or anti-mouse IgA conjugated with alkaline phosphatase (Kirkegaard & Perry, Gaithersburg, Md.). The ELISA substrate was para-nitrophenyl phosphate (1 mg/ml in 10% diethanolamine buffer, pH 9.8, containing MgCl2 [0.1 mg/ml] and 0.02% sodium azide). The OD was measured at 405 nm on a Molecular Devices (Menlo Park, Calif.) ELISA plate reader.
Electrophoresis and Western blotting.
Western blot analyses were performed as previously described (26). Antisera used for Western blots included MAbs to IpaB (2F1), IpaC (2G2), and IpaD (16F8) (24, 38) and a monkey convalescent serum pool (M213) which contains antibodies to all Ipa proteins (IpaA, IpaB, IpaC, and IpaD) and VirG. Guinea pig sera used for Western blots were from animals immunized with invaplex 24 or invaplex 50; these sera were collected on days 0 (prebleed) and 42 (14 days postimmunization) and were diluted 1/300.
Silver staining (36) was used to stain LPS in samples treated with proteinase K (PK; Gibco-BRL, Bethesda, Md.) prior to loading on gels (11).
Immunogenicity and protective capacity of invaplex 24 and invaplex 50.
The ability of the invaplex fractions to promote an immune response in BALB/cByJ mice was tested in groups of five mice. Each mouse was immunized intranasally with 5 μg of invaplex 24 or invaplex 50 from S. flexneri 2a or S. flexneri 5 on days 0, 14, and 28. Saline was used to immunize control animals. A total antigen volume of 25 μl was delivered in 5 to 6 small drops applied to the external nares with a micropipette. Blood was taken by tail bleed from all mice on days 0, 28, and 42.
Three weeks after the final immunization with either S. flexneri 2a invaplex 24, invaplex 50, or saline, mice (15 per group) were challenged intranasally with a lethal dose of S. flexneri 2a 2457T (107 CFU/30 μl) as described for the mouse lung model (18). The mouse challenge dose was prepared from a frozen lot of S. flexneri 2a that had been harvested during the log phase of growth, which is the time of optimal invasiveness for shigellae, and then stored in liquid nitrogen (E. V. Oaks, unpublished data). Prior to intranasal immunization or challenge, mice were anesthetized with a mixture of ketamine hydrochloride (40 mg/kg) (Ketaset, Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and xylazine (12 mg/kg) (Rompun; Bayer Corp., Shawnee Mission, Kans.).
In immunogenicity and protection experiments, guinea pigs (Hartley strain; Charles River) (four to five per group) were immunized intranasally with either invaplex 24 or invaplex 50 (25 μg/dose). Diluent (0.9% saline) was used to immunize control animals. In the dose-response experiment, guinea pigs were immunized as above with either 5, 10, 25, 50, or l00 μg of protein. The antigen was applied to the external nares with a micropipette in a total volume of 50 μl per nostril. In all studies with guinea pigs, animals were immunized on days 0, 14, and 28. Guinea pigs were bled from the lateral ear vein on day 0, day 28, day 42, and 14 days after challenge (day 56). Prior to intranasal immunization, guinea pigs were anesthetized with a mixture of ketamine (40 mg/kg) and xylazine (4 mg/kg).
Three weeks after the third immunization, guinea pigs were challenged intraocularly with S. flexneri 5 INC103 (3.6 × 108 CFU) or S. flexneri 2a 2457T (6.0 × 108 CFU) and observed daily for 5 days for the occurrence of keratoconjunctivitis. The degree of inflammation and keratoconjunctivitis was scored as previously described (10). Eyes with complete or partial protection at day 5 were considered protected.
Statistical analysis. Statistical computations were performed with the Statview program (SAS Institute Inc., Cary, N.C.). The Fisher exact test was used for protection experiments, and the Wilcoxon signed rank test was used for analysis of serological data. Linear regression was used for analysis of dose-response experiment data.
RESULTS
Isolation and characterization of invaplex 24 and invaplex 50.
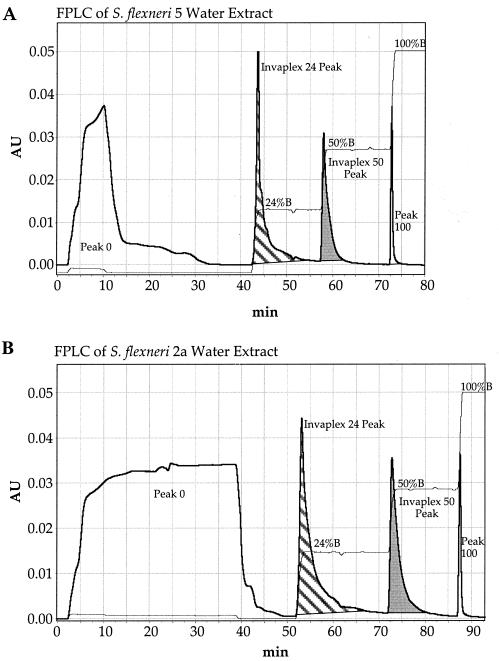
In preliminary experiments, S. flexneri water extract material was fractionated on an FPLC ion-exchange column by elution with continuous 0 to 1.0 M NaCl gradients in 20 mM Tris, pH 9.0 (0 to 100% buffer B). It was found that the IpaB and IpaC consistently eluted in two peaks near 24% buffer B and 50% buffer B (data not shown). Therefore, step gradients of 24% buffer B, 50% buffer B, and a final wash at 100% buffer B were used in all subsequent experiments. Typical FPLC chromatographs of water extracts from S. flexneri 5 and S. flexneri 2a eluted with step gradients are shown in Fig. 1 (peak 0 [0% buffer B] represents protein that did not bind to the HiTrap Q anion-exchange column). Each fraction was evaluated by spot blotting using IpaB and IpaC MAbs (data not shown). The fractions containing most of the IpaB and IpaC activity were in the invaplex 24 and invaplex 50 peaks. Undetectable or very low amounts of IpaB and IpaC were in peaks 0 (0% buffer B) and 100 (100% buffer B). This FPLC profile is reproducible in that identical results were obtained with the same batches of water extract, with different batches of water extract, and for both serotypes of S. flexneri used in this study (Fig. 1). Typical yields of invaplex 24 and invaplex 50 are approximately 2 to 3 mg and 1 to 2 mg, respectively, per liter of original Shigella culture.
FIG. 1.
FPLC ion-exchange chromatography of water-extracted proteins prepared from S. flexneri 5 (A) and S. flexneri 2a (B). Water extracts (8 mg of total protein) were separated on 5-ml columns of anion-exchange resin HiTrap Q (Pharmacia). Two tracings are plotted: OD280 (Arbitrary units [AU]; thick line), which shows the four protein peaks (peak 0, invaplex 24, invaplex 50, and peak 100); and conductivity (thin line), representative of percent buffer B (1 M NaCl in 20 mM Tris-HCl, pH 9.0) and clearly showing the 24, 50, and 100% buffer B steps. The flow rate was 2.0 ml/min, and 2-ml fractions were collected throughout the run. The invaplex 24 and invaplex 50 peak fractions were collected and used in further experiments. All fractions were analyzed for IpaC and IpaB content by spot blotting. Quantities of IpaC and IpaB were greatest in the invaplex 24 and invaplex 50 peak fractions.
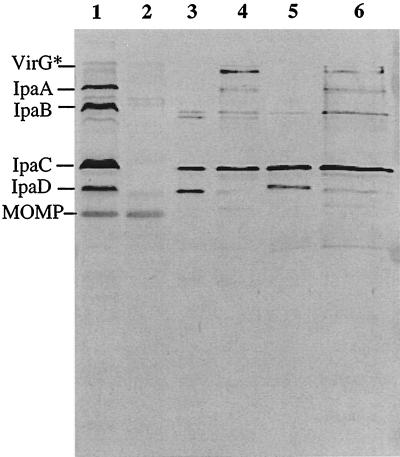
Western blot analysis detected the presence of IpaB, IpaC, and IpaD in the invaplex fractions using convalescent serum (Fig. 2) or MAbs (data not shown). One difference between the invaplex 24 and invaplex 50 preparations is that most invaplex 24 samples contained more IpaD than did the corresponding invaplex 50 preparations (Fig. 2). Using convalescent monkey serum, it was found that invaplex 50 also contained IpaA and VirG* (a truncated form of the 120-kDa VirG [IcsA] protein) (Fig. 2) (40). VirG* has not been detected in invaplex 24 fractions by the methods used. Additional proteins were present in the invaplex preparations, but their identities are not known (Fig. 3).
FIG. 2.
Western blot analysis of invaplex preparations from S. flexneri 5 and S. flexneri 2a. Samples run on the gel are S. flexneri 5 whole cell lysate, Vir+ (lane 1), S. flexneri 5 whole cell lysate, Vir− (lane 2), invaplex 24 from S. flexneri 5 (lane 3), invaplex 50 from S. flexneri 5 (lane 4), invaplex 24 from S. flexneri 2a (lane 5), and invaplex 50 from S. flexneri 2a (lane 6). The blot was probed with pooled monkey convalescent serum M213. The pooled convalescent serum reacts with all of the Ipa proteins (IpaA, IpaB, IpaC, and IpaD) and also VirG*. Each lane containing invaplex preparations was loaded with 3 μg of total protein.
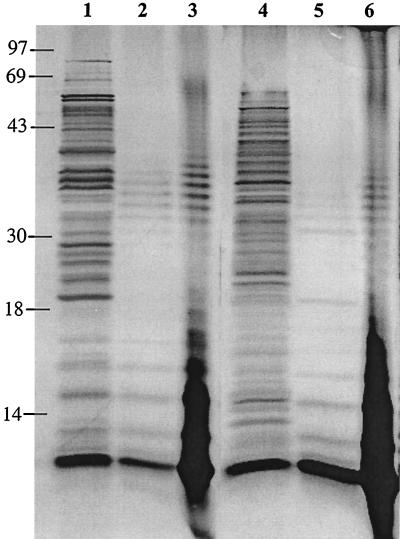
FIG. 3.
Silver stain showing protein and LPS content in invaplex 24 and invaplex 50 preparations before treatment with PK and LPS content after PK treatment. Samples on the gel are invaplex 24 from S. flexneri 2a (lanes 1 [no PK] and 2 [with PK]), 10 μg of purified S. flexneri 2a LPS (lanes 3 and 6), and invaplex 50 from S. flexneri 2a (lanes 4 [no PK] and 5 [with PK]). Bands remaining after PK treatment are LPS bands which run from the very low molecular size core (at the bottom of the gels) to larger forms of LPS containing O side chains. Each lane containing invaplex preparations was loaded with 10 μg protein or protein equivalent in PK-treated samples. Sizes are indicated in kilodaltons.
LPS content of invaplex preparations was evaluated by silver staining PK-treated samples on polyacrylamide gels. Figure 3 shows silver-stained gels of invaplex 24 and invaplex 50 preparations from S. flexneri 2a before and after PK treatment. A typical LPS core at the bottom of the gel was found in invaplex 24 and invaplex 50 preparations. In addition, LPS bands of gradually increasing molecular size (representing repeat units of the O polysaccharide added onto the core) were also present.
Immunogenicity and safety of invaplex 24 and invaplex 50 in mice.
Mice were immunized with invaplex 24 or invaplex 50 in order to determine the immunogenicity and safety of the preparations. Death or visible side effects due to toxicity (such as ruffled fur or lethargy) did not occur in mice immunized with 5 or 10 μg of invaplex 24 or invaplex 50 prepared from S. flexneri 2a or S. flexneri 5.
Invaplex 50 from S. flexneri 2a or S. flexneri 5 elicited significant IgA and IgG levels against the Ipa-containing water extract antigen and frequently against the water extract antigen prepared from plasmid-free, avirulent shigellae (Tables 1 and 2). The invaplex 24 antigen consistently stimulated an IgA and/or IgG response which was virulence specific, in that it reacted predominantly with the water extract antigen prepared from virulent shigellae (Tables 1 and 2).
TABLE 1.
Immune response to LPS and water extract in mice immunized with invaplex 24 or invaplex 50 from S. flexneri 2aa
| Immunization | Day | Median ELISA reading (range)
|
|||||
|---|---|---|---|---|---|---|---|
|
S. flexneri 2a LPS
|
Water extract
|
||||||
| Vir+
|
Vir−
|
||||||
| IgA | IgG | IgA | IgG | IgA | IgG | ||
| Invaplex 24 | 0 | 0.066 (0.056–0.070) | 0.032 (0.027–0.039) | 0.056 (0.051–0.171) | 0.021 (0.020–0.029) | 0.052 (0.046–0.098) | 0.019 (0.019–0.020) |
| 28 | 0.268 (0.219–0.386)* | 0.078 (0.067–0.111)* | 0.282 (0.157–0.689)* | 0.929 (0.413–1.302)* | 0.075 (0.040–0.102) | 0.061 (0.036–0.127)* | |
| 42 | 1.776 (0.838–2.052)* | 0.461 (0.233–0.589)* | 0.638 (0.431–2.065)* | 1.485 (1.267–1.869)* | 0.069 (0.050–0.108) | 0.210 (0.121–0.262)* | |
| Invaplex 50 | 0 | 0.070 (0.063–0.072) | 0.037 (0.033–0.040) | 0.063 (0.057–0.090) | 0.024 (0.023–0.024) | 0.048 (0.045–0.058) | 0.021 (0.020–0.022) |
| 28 | 0.121 (0.090–0.133)* | 0.043 (0.037–0.052) | 0.121 (0.091–0.213)* | 0.244 (0.125–0.626)* | 0.056 (0.046–0.071) | 0.186 (0.078–0.326)* | |
| 42 | 0.365 (0.192–0.834)* | 0.062 (0.050–0.102)* | 0.511 (0.270–0.878)* | 1.194 (0.415–1.654)* | 0.126 (0.080–0.167) | 0.615 (0.268–0.883)* | |
Mice were immunized intranasally three times with S. flexneri 2a-derived invaplex 24 or invaplex 50. The serum IgA or IgG reactive with S. flexneri 2a LPS and water extract (Vir+ or Vir−) was determined by ELISA. Blood was taken from all mice before treatment (day 0), after the second immunization (day 28), and after three immunizations (day 42). Values represent the median (25/75 percentile) for each group of mice (n = 10). Values significantly greater (P < 0.05, Wilcoxon signed rank test) than for the day 0 serum are indicated by asterisks. Sera were diluted 1/180 for IgA analysis and 1/360 for IgG analysis.
TABLE 2.
Immune response to LPS and water extract in mice immunized with invaplex 24 or invaplex 50 from S. flexneri 5
| Immunization | Day | Median ELISA reading (range)
|
|||||
|---|---|---|---|---|---|---|---|
|
S. flexneri 5 LPS
|
Water cxtract
|
||||||
| Vir+
|
Vir−
|
||||||
| IgA | IgG | IgA | IgG | IgA | IgG | ||
| Invaplex 24 | 0 | 0.036 (0.035–0.037) | 0.026 (0.025–0.038) | 0.031 (0.03–0.033) | 0.020 (0.019–0.022) | 0.040 (0.034–0.042) | 0.023 (0.021–0.023) |
| 28 | 0.038 (0.035–0.050) | 0.036 (0.026–0.071) | 0.097 (0.084–0.163)* | 0.136 (0.113–0.137)* | 0.035 (0.035–0.042) | 0.030 (0.025–0.051)* | |
| 42 | 0.056 (0.038–0.245) | 0.077 (0.059–0.238)* | 0.598 (0.408–0.692)* | 0.827 (0.487–0.957)* | 0.043 (0.039–0.135) | 0.059 (0.050–0.259)* | |
| Invaplex 50 | 0 | 0.033 (0.033–0.037) | 0.027 (0.026–0.034) | 0.044 (0.040–0.045) | 0.020 (0.020–0.022) | 0.046 (0.044–0.046) | 0.023 (0.022–0.024) |
| 28 | 0.045 (0.043–0.121)* | 0.056 (0.032–0.174)* | 0.054 (0.049–0.066)* | 0.075 (0.060–0.095)* | 0.069 (0.060–0.087)* | 0.112 (0.085–0.192)* | |
| 42 | 1.415 (0.307–1.821)* | 1.315 (0.455–1.554)* | 0.286 (0.189–0.882)* | 0.351 (0.276–0.548)* | 0.603 (0.373–0.954)* | 0.555 (0.411–0.725)* | |
Mice were immunized intranasally three times with S. flexneri 5-derived invaplex 24 or invaplex 50. The serum IgA or IgG reactive with S. flexneri 5 LPS and water extract (Vir+ or Vir−) was determined by ELISA. Blood was taken from all mice before treatment (day 0), after the second immunization (day 28), and after three immunizations (day 42). Values represent the median (25/75 percentile) for each group of mice (n = 5). Values significantly greater (P < 0.05, Wilcoxon signed rank test) than for the day 0 serum are indicated by asterisks. Sera were diluted 1/180 for IgA analysis and 1/360 for IgG analysis.
Significant increases in IgA and IgG to homologous LPS occurred in mice immunized with S. flexneri 2a Invaplex 24, S. flexneri 2a Invaplex 50, and also S. flexneri 5 Invaplex 50 (Tables 1 and 2). S. flexneri 5 Invaplex 24 did not stimulate significant increases of IgA to LPS in mice.
Immunogenicity and safety of S. flexneri invaplex 24 and invaplex 50 vaccines in guinea pigs.
Guinea pigs immunize intranasally with S. flexneri 2a invaplex 24 or invaplex 50 showed no visible signs of toxicity such as fur ruffling, lethargy, or diarrhea after immunization with doses ranging from 5 to 100 μg. In addition, guinea pigs immunized with either S. flexneri 2a invaplex 24 or invaplex 50 gained weight at a rate comparable to that for guinea pigs treated with normal saline (data not shown).
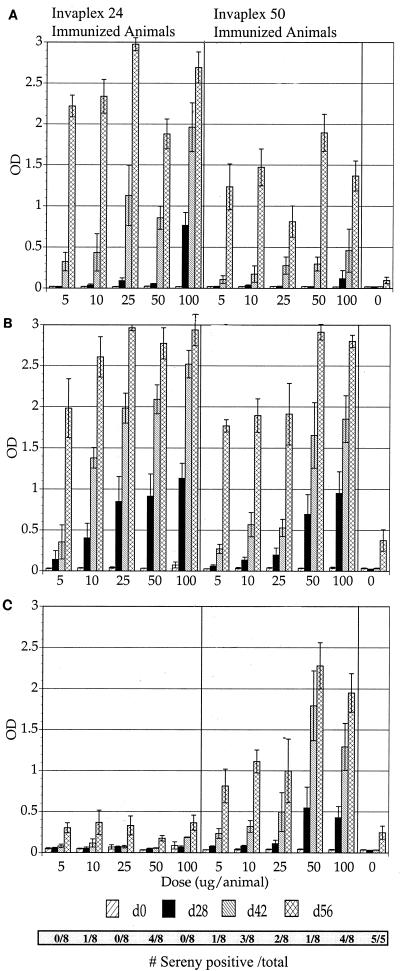
Dose-response experiments were conducted to determine the immunogenicity of the S. flexnei 2a invaplex 24 and invaplex 50 vaccines in guinea pigs. Guinea pigs (four per group) were immunized with either 0, 5, 10, 25, 50, or 100 μg of invaplex per dose. Each animal was immunized intranasally three times at 2-week intervals. LPS and Vir+ and Vir− water extracts were used as ELISA antigens to measure the antibody response generated after immunization with different doses of invaplex (Fig. 4). Regression analysis indicated that there was a positive correlation between the ELISA antibody levels and the quantity of antigen used in the immunization. This was true for the antibody levels detected after three doses of invaplex 24 for both the LPS (r > 0.9, P < 0.05) and Vir+ water extract (r > 0.8, P < 0.05) ELISA. Similar correlations were observed for invaplex 50 immunized guinea pigs for LPS (r > 0.8, P < 0.05) and Vir+ water extract (r > 0.9, P < 0.05). In the invaplex 50-immunized group, there was also a correlation between antibody levels to the Vir− water extract antigen and the dose of antigen used for immunization.
FIG. 4.
Antibody response to LPS and water extract in guinea pigs immunized with different doses of invaplex 24 or invaplex 50 vaccine. Five groups of guinea pigs (four animals per group) were immunized with 0, 5, 10, 25, 50, or 100 μg of either S. flexneri 2a invaplex 24 or invaplex 50 per dose. Guinea pigs were immunized intranasally on days 0, 14, and 28; blood was collected on days 0, 28, 42, and 56. The animals were challenged on day 49. The serum IgG response was measured by ELISA against S. flexneri 2a LPS (top), Vir+ water extract (B), and Vir− water extract (C). In each panel, the buffer control animal data are on the for right. The bars represent mean OD405 ± standard error of the mean for each group of four guinea pigs. The horizontal bar at the bottom gives the number of Sereny test-positive eyes over the number of eyes challenged for a particular group.
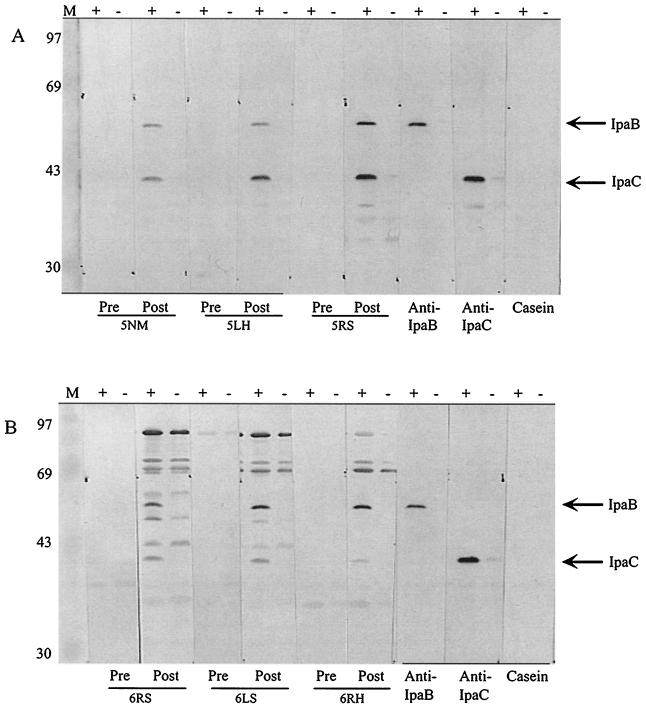
At higher doses of invaplex, antibody levels to LPS and water extract were detected after only two doses (Fig. 4). At the 100-μg dose, a positive anti-LPS response was present after two doses of vaccine, whereas at lower doses a positive anti-LPS response was not detectable until after three immunizations. Positive antibody responses against the Vir+ water extract were evident in all dose groups for invaplex 24 after just two doses, while for invaplex 50 a positive antibody response to the Vir+ water extract was evident in animals immunized with two invaplex doses of 25 μg or more. As noted above for mice, invaplex 24, at all doses, also produced a striking virulence-specific antibody response in guinea pigs, whereas invaplex 50 produced antibodies reactive with both Vir+ and Vir− water extract antigens. The invaplex 50 response to both virulence-associated antigens and antigens not encoded by the virulence plasmid occurred at all doses. Western blot analysis of invaplex 24 or invaplex 50-immunized guinea pigs revealed that the invaplex 24-induced virulence-specific response was directed primarily at IpaB and IpaC (Fig. 5A). Invaplex 50-immunized animals produced antibodies to IpaB and IpaC and also other proteins with approximate molecular sizes of 70, 72, and 85 kDa (Fig. 5B). The 70-, 72-, and 85-kDa proteins were not virulence specific.
FIG. 5.
Western blot analysis of sera collected from guinea pigs immunized with S. flexneri 2a invaplex 24 (A) or S. flexneri 2a invaplex 50 (B). Whole cell lysates of S. flexneri 5 M90T-W (+) or S. flexneri 5, M90T-55 (−) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to nitrocellulose. Strips containing both + and − samples were incubated with sera collected prior to immunization (Pre) and after three intranasal immunizations (25 μg/dose) with Invaplex (Post); numbers below are individual guinea pig numbers. MAbs 2F1 (anti-IpaB) and 2G2 (anti-IpaC) were used to identify the IpaB and IpaC polypeptides. A negative control strip was incubated with casein alone in place of primary antibody. Molecular mass markers are indicated in kilodaltons on the left.
Vaccination with invaplex 24 and invaplex 50 and challenge of animals with virulent shigellae.
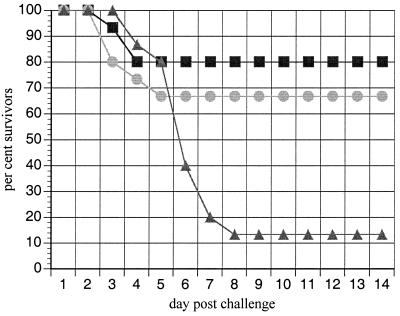
A significant level of protection against lethal challenge was achieved in mice immunized with S. flexneri 2a invaplex 24 or invaplex 50 (Fig. 6). Invaplex-immunized mice lost weight upon challenge, but by days 3 to 4 they began to recover and gain weight whereas control mice soon died. Similar levels of protection were afforded by invaplex 24 (12 of 15 mice survived) and invaplex 50 (10 of 15 mice survived).
FIG. 6.
Protection of mice from lethal lung challenge by immunization with either invaplex 24 or invaplex 50. Three groups of 15 mice were immunized with either S. flexneri 2a invaplex 24 (squares), S. flexneri 2a invaplex 50 (circles), or buffer (triangles). Mice were immunized intranasally at 2-week intervals with 5 μg of invaplex administered at each immunization. Three weeks after the final immunization, all animals were intranasally challenged with 107 CFU of S. flexneri 2a 2457T. Percent survivors is plotted for each of 14 days postchallenge. P values, calculated by the Fisher exact test, are 0.001 for invaplex 24-immunized mice and 0.008 for invaplex 50-immunized mice.
Using the guinea pig keratoconjunctivitis model, animals immunized intranasally with either invaplex 24 or invaplex 50 from S. flexneri 2a or S. flexneri 5 were significantly protected against homologous challenge (Table 3). After challenge, the animals all showed a tremendous boost in antibody levels to the water extract and LPS, indicating a successful priming by the invaplex 24 and invaplex 50 vaccines (Fig. 4). Control guinea pigs immunized with buffer diluent did not produce antibodies to any of the Shigella antigens tested. Upon challenge, these animals produced much lower levels of antibodies (measured at 7 days postinfection) than did those animals first immunized with invaplex 24 or invaplex 50 vaccine and then challenged.
TABLE 3.
Protection in the guinea pig keratoconjunctivits model using invaplex 24 or invaplex 50 vaccine prepared form virulent S. flexneri
| Vaccine treatmenta | No. positive/no. challengedb | Pc |
|---|---|---|
| S. flexneri 2a invaplex 24 | 2 /18 | <0.001 |
| S. flexneri 2a invaplex 50 | 3 /16 | <0.001 |
| None (buffer control) | 15 /15 | |
| S. flexneri 5 invaplex 24 | 1 /8 | 0.003 |
| S. flexneri 5 invaplex 50 | 0 /10 | <0.001 |
| None (buffer control) | 9 /10 |
Guinea pigs were immunized intranasally three times (on days 0, 14, and 28) with 25 μg of invaplex preparation per dose and challenged on day 49. Animals were infected with 3 × 108 to 6 × 108 CFU of the challenge agent, which was the same strain as that used to prepare the invaplex vaccine.
Animals were considered positive for disease if they had on day 5 a 2+ or 3+ rating (keratoconjunctivitis without purulence or severe keratoconjunctivitis with purulence) based on the scale developed by Hartman et al. (10).
Determined by the Fisher exact test.
DISCUSSION
The protective immune response which is necessary to prevent future Shigella infections is not completely understood. In a natural infection, the shigellae have direct extra- and intracellular interactions with M cells, macrophages, dendritic cells, lymphocytes, and the colonic epithelium during the course of infection (33, 42). The inflammatory and specific immune responses produced as a result of the pathogen-host interactions are likely directed at essential virulence components in an attempt to neutralize and eliminate the pathogen. The resulting immunity offers protection against future infection with the homologous serotype (8, 9). In addition to the LPS antibody response, the infected host also responds quite vigorously against the Shigella invasins (13, 26, 27, 31). Most infected individuals produce antibodies to IpaB and IpaC and, at a lower frequency, to IpaD, IpaA, and VirG; in fact, it is not unusual to see an antibody response directed at only the invasins and LPS. Although the function of antibodies to the Ipa proteins is not entirely understood, it is possible to inhibit the invasiveness of Shigella or EIEC with anti-Ipa IgA found in colostrum (4) or MAbs to IpaC or IpaB (24, 34). More recently, a MAb to the carboxy-terminal end of IpaC exhibited inhibition of IpaC-induced actin polymerization in permeabilized host cells (40). Epitope mapping of IpaC has indicated that monkeys responding to three epitopic regions are less likely to develop severe disease (37), and one of these three epitope regions (region III) colocalizes with the actin polymerization domain (40). Even so, it has not been possible to correlate protective immunity with a specific antibody response to any of the invasins as measured by Western blot analyses or ELISAs. In contrast, numerous studies have concluded that LPS is an essential vaccine component (8, 9, 19, 28, 30), but it is also clear that LPS delivered by itself is not protective (1, 19). This suggests that presentation of LPS in a manner which elicits a protective immune response comparable to natural infection is necessary for a successful Shigella vaccine. A Shigella vaccine which stimulates antibody production to both LPS and the Ipa proteins might more completely mimic the natural immune response.
In this study, a novel method has been developed for isolating a macromolecular complex containing the major known virulence factors and immunogens from intact, viable, virulent shigellae. We refer to this structure as the invasin complex, or invaplex. It has been possible to isolate two forms of the invaplex, called invaplex 24 and invaplex 50, by FPLC ion-exchange chromatography from S. flexneri 2a and S. flexneri 5. Both forms contain the invasins (IpaB, IpaC, and IpaD) and LPS, but IpaA and VirG* (41), a truncated form of VirG, were found only in Invaplex 50. Other unidentified proteins were also present in both invaplex preparations.
The relationship between the invaplex and Ipa protein complexes recently described (23, 35) has not been determined. Although both structures contain the Ipa proteins, one clear difference is the presence of LPS in the invaplex but not in the IpaB-IpaC complex (23). Interestingly, Kadurugamuwa and Beveridge (14) have shown that gentamicin-induced microvesicles of shigellae contain LPS and the Ipa proteins but the predominant proteins are the major outer membrane proteins (MOMPs). The invaplex 24 and invaplex 50 preparations do not have detectable levels of the MOMPs. Sizing experiments indicate that the LPS and invasins of the invaplex comigrate at a molecular size greater than that of thyroglobulin, which is about 669 kDa (K. R. Turbyfill and E. V. Oaks, unpublished data). The invaplex is very likely derived from the outer membrane surface in that it contains LPS and IpaD, both surface-exposed components of the outer membrane (22, 38), yet it does not contain detectable quantities of the MOMPs which are integral membrane proteins. Furthermore, the solubility of the invaplex in aqueous buffers, without the need for detergents or urea, suggests that the complex may exist in a hydrophilic environment, possibly at the periphery of the shigella cell. The IpaB-IpaC complex described by Menard et al. is released into the medium from shigellae by a type III secretory event (22, 23). However, the staging area for the secreted IpaB-IpaC complex is not known.
Run-to-run consistency of invaplex 24 and invaplex 50 isolated from S. flexneri was very reproducible. In addition, the simplicity of the invaplex purification and the solubility in aqueous buffer lends itself to rapid purification on a large scale and adaptation to biological systems. Yields of the invaplex preparations were approximately 2 to 3 mg for invaplex 24 and 1 to 2 mg for invaplex 50 for each liter of starting Shigella culture.
Our studies indicate that low doses (5 μg in mice and 5 to 25 μg in guinea pigs) of either invaplex 24 or invaplex 50 are immunogenic using intranasal immunizations. Successful immunizations (eliciting a serum antibody response and protection) with the invaplex 24 or invaplex 50 preparation did not require an adjuvant. Neither invaplex preparation resulted in any visible side effects such as death, weight loss, or lethargy in the immunized mice or guinea pigs. The slight fur ruffling noted in mice has resolved within 1 day. Serum IgA and IgG responses to LPS and protein antigens were produced in invaplex-immunized animals.
In dose-response experiments in guinea pigs using S. flexneri 2a invaplex 24 and invaplex 50, it was possible to stimulate a measurable antibody response to LPS and water extract with three 5-μg doses. Higher doses (25, 50, and 100 μg) in guinea pigs generated a positive antibody response to water extract after two intranasal doses. In all cases, animals immunized with invaplex 24 or invaplex 50 showed a dramatic increase in antibody levels to LPS and water extract after challenge 3 weeks postimmunization with virulent shigellae of the same serotype as the invaplex vaccine. This rapid boost in titer (postchallenge blood was collected 1 week after challenge) was a result of the effective priming of the immune system by the invaplex vaccines.
Interestingly, invaplex 24 produced a virulence-specific antibody response in both guinea pigs and mice very similar to that produced in monkeys or humans infected with Shigella spp. (26, 39). Western blot analysis of immunized guinea pigs indicated that the majority of antibody was directed at IpaB and IpaC. Invaplex 50 stimulated a serum antibody response which was not virulence specific (as measured by the water extract ELISA), suggesting that non-plasmid-encoded antigens are in the invaplex 50 and are capable of stimulating an antibody response. Western blot analysis indicates that 70-, 72-, and 85-kDa proteins (non-plasmid encoded) were recognized in invaplex 50-immunized guinea pigs. Even so, invaplex 50 preparations did stimulate antibodies to virulence proteins such as IpaC and IpaB, as determined by Western blot analysis of immune sera.
Guinea pigs immunized intranasally with invaplex 24 or invaplex 50 from S. flexneri 2a or S. flexneri 5 were protected from severe keratoconjunctivitis upon challenge with the homologous organism. The level of protection ranged from complete to partial protection. Invaplex 24 and invaplex 50 also protected mice from a lethal challenge of S. flexneri 2a. Although many of the immunized mice lost weight during the first 2 to 3 days after challenge, they began to recover by day 4, whereas control mice continued to lose weight. The level of protection afforded by the invaplex vaccines in guinea pigs or mice is comparable to that generated by live attenuated vaccines or other subunit vaccines targeting LPS (10, 19). However, unlike other subcellular vaccines such as proteosomes (19, 28), ribosomes prepared from avirulent shigellae (16), or O-polysaccharide conjugates (6), the Invaplex vaccines were capable of stimulating antibodies against the invasins. Previous outer membrane protein-LPS vaccines described by Adamus et al., which were delivered subcutaneously with complete Freund's adjuvant, were likely deficient in the invasins due to the detergents used in vaccine preparation (1). Live attenuated vaccines, such as S. flexneri 2a SC602, which is an icsA deletion mutant, are capable of stimulating antibodies to the virulence proteins but only at a low frequency unless higher, reactogenic doses are given (7).
Successful mucosal vaccination and protection with invaplex indicates that an effective immune response to LPS and other Shigella antigens, such as the Ipa proteins, was produced in immunized animals. The availability of a subunit preparation derived from shigellae which contains the major antigens and virulence factors, possibly in the form of an intact virulence structure, provides a novel approach to Shigella vaccines. Furthermore, the lack of apparent toxicity of the Invaplex vaccines suggests that higher doses are possible and that combining invaplex preparations from multiple Shigella serotypes will make a multivalent vaccine extremely likely. Preliminary studies have successfully produced Invaplex vaccine preparations from all species of Shigella and also EIEC (K. R. Turbyfill, E. V. Oaks, and A. B. Hartman, Abstr. 100th Gen. meet. Am. Soc. Microbiol., abstr. E-103, 2000).
Delivering subunit vaccines by the mucosal route (intranasal, oral, etc) is difficult and not very effective unless suitable mucosal adjuvants are used (2). The potent immune response generated by the invaplex preparations without any additional adjuvant and the known capacity of the Ipa proteins to interact with host cells, and in particular immune cells (33), suggest that the invaplex may be able to enhance the immune response to coadministered antigens, somewhat like cholera toxin. In fact, studies evaluating adjuvanticity have indicated that invaplex 24 and invaplex 50 stimulate an IgG subclass response to ovalbumin comparable to that of cholera toxin (E. V. Oaks, K. R. Turbyfill, W. D. Picking, and W. Picking, abstr. 100th Gen. meet. Am. Soc. Microbiol. abstr. E-58, 2000). The capacity of invaplex to serve as a vaccine and a mucosal adjuvant will make the construction of a vaccine against multiple mucosal pathogens feasible.
ACKNOWLEDGMENTS
We thank Larry Hale for encouragement and support for this project. We also thank E. Cenizal, J. Martinez, C. Vogel, and A. Wallis for superb technical assistance.
REFERENCES
- 1.Adamus G, Mulczk M, Witkowska D, Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980;30:321–324. doi: 10.1128/iai.30.2.321-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyaka P N, Marinaro M, Vancott J L, Takahashi I, Fujihashi K, Yamamoto M, van Ginkel F W, Jackson R J, Hiyono H, McGhee J R. Strategies for mucosal vaccine development. Am J Trop Med Hyg. 1999;60:35–45. doi: 10.4269/ajtmh.1999.60.35. [DOI] [PubMed] [Google Scholar]
- 3.Buysse J M, Stover C K, Oaks E V, Venkatesan M, Kopecko D J. Molecular cloning of invasion plasmid antigen (Ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987;169:2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonare S B, Silva M L, Trabulsi L R, Carneiro-Sampaio M M S. Inhibition of HEp-2 cell invasion by enteroinvasive Escherichia coli by human colostrum IgA. Int Arch Allergy Immunol. 1995;108:113–118. doi: 10.1159/000237127. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen D, Ashkenazi S, Green M S, Gdalevich M, Robin G, Slepon R, Yavzpori M, Orr N, Block C, Ashkenazi I, Shemer J, Taylor D N, Hale T L, Sadoff J C, Pavliakova D, Schneerson R, Robbins J B. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 7.Coster T S, Hoge C W, VanDeVerg L L, Hartman A B, Oaks E V, Venkatesan M M, Cohen D, Robin G, Fontaine-Thompson A, Sansonetti P J, Hale T L. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreccio C, Prado V, Ojeda A, Cayyazo M, Abrego P, Guers L, Levine M M. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 9.Formal S B, Oaks E V, Olsen R E, Wingfield-Eggleston M, Snoy P J, Cogan J P. The effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 10.Hartman A B, Powell C J, Shulta C L, Oaks E V, Eckels K H. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59:4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoge C W, Gambel J M, Srijan A, Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis. 1998;26:341–345. doi: 10.1086/516303. [DOI] [PubMed] [Google Scholar]
- 13.Islam D, Wretlind B, Hammarstrom L, Christensson B, Lindberg A A. Semiquantitative estimation of Shigella antigen-specific antibodies: correlation with disease severity during shigellosis. APMIS. 1996;104:563–574. doi: 10.1111/j.1699-0463.1996.tb04912.x. [DOI] [PubMed] [Google Scholar]
- 14.Kadurugamuwa J L, Beveridge T J. Delivery of the non-membrane-permeative antibiotic gentamicin into mammalian cells by using Shigella flexneri membrane vesicles. Antimicrob Agents Chemother. 1998;42:1476–1483. doi: 10.1128/aac.42.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotloff K L, Winickoff J P, Ivanoff B, Clemens J D, Swerdlow D L, Sansonetti P J, Adak G K, Levine M M. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull W H O. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]
- 16.Levenson V I, Egorova T P, Belkin Z P, Fedosova V G, Subbotina J L, Rukhadze E Z, Dzhikidze E K, Stassilevich Z K. Protective ribosomal preparation from Shigella sonnei as a parenteral candidate vaccine. Infect Immun. 1991;59:3610–3618. doi: 10.1128/iai.59.10.3610-3618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li A, Rong Z C, Ekwall E, Forsum U, Lindberg A A. Serum antibody responses against Shigella lipopolysaccharides and invasion plasmid-coded antigens in Shigella infected Swedish patients. Scand J Infect Dis. 1993;25:569–577. doi: 10.3109/00365549309008545. [DOI] [PubMed] [Google Scholar]
- 18.Mallett C P, VanDeVerg L, Collins H H, Hale T L. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine. 1993;11:190–196. doi: 10.1016/0264-410x(93)90016-q. [DOI] [PubMed] [Google Scholar]
- 19.Mallett C P, Hale T L, Kaminski R W, Larsen T, Orr N, Cohen D, Lowell G H. Intranasal or intragastric immunization with proteosome-Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun. 1995;63:2382–2386. doi: 10.1128/iai.63.6.2382-2386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquart M E, Picking W L, Picking W D. Soluble invasion plasmid antigen C (IpaC) from Shigella flexneri elicits epithelial cell responses related to pathogen invasion. Infect Immun. 1996;64:4182–4187. doi: 10.1128/iai.64.10.4182-4187.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menard R, Prevost M-C, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93:1254–1258. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills J A, Buysse J M, Oaks E V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988;56:2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noriega F R, Wang J Y, Losonsky G, Maneval D R, Hone D M, Levine M M. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;62:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oaks E V, Hale T L, Formal S B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oaks E V, Picking W D, Picking W L. Antibody response of monkeys to invasion plasmid antigen D after infection with Shigella spp. Clin Diagn Lab Immunol. 1996;3:242–245. doi: 10.1128/cdli.3.2.242-245.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orr N, Robin G, Cohen D, Arnon R, Lowell G H. Immunogenicity and efficacy of oral or intranasal Shigella flexneri 2a and Shigella sonnei proteosome-lipopolysaccharide vaccines in animal models. Infect Immun. 1993;61:2390–2395. doi: 10.1128/iai.61.6.2390-2395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Payne S M, Finkelstein R A. Detection and differentiation of iron-responsive avirulent mutants on Congo red agar. Infect Immun. 1977;18:94–98. doi: 10.1128/iai.18.1.94-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phalipon A, Kauffman M, Michetti P, Cavaillon J M, Huerre M, Sansonetti P J, Kraehenbuhl J P. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J Exp Med. 1995;182:769–778. doi: 10.1084/jem.182.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samandari T, Kotloff K L, Losonsky G A, Picking W D, Sansonetti P J, Levine M M, Sztein M B. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221–2232. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 32.Sansonetti P J, Arondel J, Fontaine A, d'Haauteville H, Bernardini M L. OmpB (osmo-regulation) and icsA (cell to cell spread) mutants of Shigella flexneri. Evaluation as vaccine candidates. Probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 33.Sansonetti P J, van Nhieu G T, Egile C. Rupture of the intestinal epithelial barrier and mucosal invasion by Shigella flexneri. Clin Infect Dis. 1999;28:466–475. doi: 10.1086/515150. [DOI] [PubMed] [Google Scholar]
- 34.Shaikh N M, Nair G B, Kumar R. Significance of the secreted form of IpaC, a 45 kDa protein of Shigella dysenteriae 1, in the invasive process as determined by monoclonal antibodies. FEMS Microbiol Lett. 1995;125:247–253. doi: 10.1111/j.1574-6968.1995.tb07365.x. [DOI] [PubMed] [Google Scholar]
- 35.Terajima J, Moriishi E, Kurata E, T, Watanabe H. Preincubation of recombinant Ipa proteins of Shigella sonnei promotes entry of non-invasive Escherichia coli into HeLa cells. Microb Pathog. 1999;26:223–230. doi: 10.1006/mpat.1999.0300. [DOI] [PubMed] [Google Scholar]
- 36.Tsai C-M, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 37.Turbyfill K R, Joseph S, Oaks E V. Recognition of three epitopic regions on invasion plasmid antigen C (IpaC) by immune sera of rhesus monkeys infected with Shigella flexneri 2a. Infect Immun. 1995;63:3927–3935. doi: 10.1128/iai.63.10.3927-3935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turbyfill K R, Mertz J A, Mallett C P, Oaks E V. Identification of epitope and surface-exposed domains of Shigella flexneri invasion plasmid antigen D (IpaD) Infect Immun. 1998;66:1999–2006. doi: 10.1128/iai.66.5.1999-2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van de Verg L L, Herrington D A, Boslego J, Lindberg A A, Levine M M. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166:158–161. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 40.Van Nhieu G T, Caron E, Hall A, Sansonetti P J. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 1999;18:3249–3262. doi: 10.1093/emboj/18.12.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkatesan M M, Buysse J M, Oaks E V. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J Bacteriol. 1992;174:1990–2001. doi: 10.1128/jb.174.6.1990-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wassef J, Keren D F, Mailloux J L. Role of M cells in initial bacterial uptake and in ulcer formation in the rabbit intestinal loop model in shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 1995;14:2461–2470. doi: 10.1002/j.1460-2075.1995.tb07243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]