Abstract
Adaptive natural killer (NK) cells are long-lived and exhibit properties of immunologic memory against cytomegalovirus (CMV). We previously reported that expansion of adaptive NK cells after CMV reactivation in recipients of allogeneic hematopoietic cell transplantation (HCT) was associated with lower AML relapse. Here, we examined the impact of adaptive NK cell expansion in a cohort of 110 individuals who underwent autologous HCT (AHCT) for lymphoid malignancies (lymphoma or multiple myeloma). In this cohort, higher absolute numbers of adaptive NK cells (>1.58/μL) at day 28 post-AHCT were associated with significantly decreased relapse risk in myeloma patients. No significant association was seen in lymphoma. Further stratification of myeloma patients by CMV serostatus revealed that the strong protective effects of adaptive NK cells occur only in CMV seropositive individuals. These findings suggest that strategies to increase adaptive NK cells after AHCT may be a therapeutic option in multiple myeloma.
Keywords: Adaptive NK cell, cytomegalovirus, autologous transplant, multiple myeloma
INTRODUCTION
Natural killer (NK) cells are innate lymphocytes that reconstitute early after hematopoietic cell transplantation (HCT). Early lymphocyte recovery (before day 30) after either autologous [1–7] or allogeneic HCT is protective in several hematologic malignancies including Hodgkin’s lymphoma [8], non-Hodgkin’s lymphoma (NHL) [9] multiple myeloma (MM) [6, 7], and acute myeloid leukemia (AML) [10–14]. NK cells recover faster than T cells [15], and early recovery of NK cells is associated with decreased transplant-related mortality after allogeneic transplant for AML [14] and acute lymphoblastic leukemia [16]. Yet, lymphocyte maturation and function post-HCT is often delayed. NK cell effector functions are lower after HCT when compared to healthy donors [17, 18].
Adaptive NK cells are a functionally robust subset that mediate protective memory responses following cytomegalovirus (CMV) infection, [19–21] and display methylation signatures similar to CD8+ T cells [22, 23]. These cells preferentially expand after CMV reactivation and typically express the maturation marker CD57 and the activating receptor NKG2C [24]. Compared to conventional NK cells, adaptive NK cells are long-lived, and studies in human disease states indicate that they are self-renewing [25, 26]. Adaptive NK cells are also relatively resistant to suppressive agents in the tumor microenvironment such as myeloid derived suppressor cells [27] and T regulatory cells [28], both of which are involved in the pathogenesis of lymphomas [29, 30] and multiple myeloma [31, 32]. Multiple myeloma, in particular, is responsive to treatments that enhance NK cell function [33, 34].
We previously studied adaptive NK cell reconstitution in patients with AML after reduced intensity conditioning (RIC) and allogeneic HCT. We found that CMV reactivation and subsequent expansion of adaptive NK cells was associated with a significant reduction in relapse [35, 36]. Adaptive NK cells also secreted more interferon-γ when cultured with leukemic cell targets than conventional NK cells [35]. CMV infection also has long-term effects on the reconstitution of T cells after transplant [37, 38], though this has been better described after allogeneic than autologous HCT. In this study we examined autologous HCT (AHCT) recipients with lymphoid malignancies and examined the impact of early adaptive NK cell expansion on post-AHCT outcomes by disease and CMV serology.
METHODS
Patients:
We included adult patients with a diagnosis of multiple myeloma, NHL, or Hodgkin lymphoma treated with their first AHCT at the University of Minnesota from 2010 to 2014. Blood was collected at days 28, 60, 90, 180, and 365 post-transplant. CMV serostatus was assessed prior to conditioning using enzyme-linked immunosorbent assays, with CMV IgG antibody levels > 10.0 EU/mL considered seropositive. After transplant, we assessed weekly for CMV reactivation through day 90 by quantitative real-time polymerase chain reaction. Negativity was considered no viral copies detected. Cytogenetic risk in multiple myeloma patients was determined by the Mayo stratification of myeloma and risk adapted therapy (mSMART) 2013 guidelines [39].
Outcomes:
The primary outcome was relapse at two years post-AHCT. Treatment-related mortality (TRM) at three years was 3% (95% CI, 1–7), so results for progression free survival and relapse were similar. We evaluated overall survival (OS) at three years as a secondary outcome stratified by low and high adaptive NK cell absolute counts.
Data collection:
The University of Minnesota Blood and Marrow Transplant program prospectively collected data regarding patient characteristics and outcomes. Patients signed consent for data analysis. The University of Minnesota Institutional Review Board approved all protocols. Patients were treated in accordance with the Declaration of Helsinki.
Identification of immune cells:
Peripheral blood mononuclear cells (PBMCs) were isolated from blood by density centrifugation and were cryopreserved with 5% DMSO. Samples were thawed and rested overnight prior to flow cytometry. Immune receptor repertoires were determined by flow cytometry on an LSRII (BD Biosciences). PBMCs were analyzed for NK cells (CD3-CD56+), and adaptive (CD56dimCD57+NKG2C+) NK cells using fluorescently conjugated antibodies: anti-CD3 (Invitrogen; MHCD0317), anti-CD56 (BD Biosciences; 335791), anti-NKG2C (R&D; FAB138P), anti-CD57 (Biolegend; 322316). Data analysis was performed using FlowJo 9.3.2 software (TreeStar).
Statistical Analyses:
Absolute counts of different cell subsets were compared between the CMV seropositive and seronegative groups after transplant using Wilcoxon rank sum test. The optimal cut-point in absolute numbers of adaptive NK cells at day 28 associated with the largest absolute Wald test statistic of the dichotomized covariate in the univariate Fine-Gray regression was used to differentiate patients with high absolute numbers of adaptive NK cells versus low absolute counts. To avoid inflated Type I error caused by the multiple tests performed when searching for the optimal cut-point, we conducted a sensitivity analysis using the 2-fold cross-validation method [40]. Specifically, we randomly selected half of the sample as the training set (subset 1) to determine the cut-point, which was then used for defining the high/low group for the other half of the sample (subset 2); we then repeated this process by switching the 2 subsets. The data set was analyzed using the competing risks regression [41] and the estimated hazard ratio (HR) and p-values reported. Baseline characteristics of the ‘high’ and ‘low’ adaptive NK cell groups were compared using Chi-square test for categorical variables except for cases with frequency ≤ 5, when Fisher’s exact test was used. Two-sided T-tests were used for continuous variables except for stem cell dose, when Wilcoxon rank sum (Mann Whitney U) test was used.
Clinical analyses were restricted to patients who survived free-of-relapse beyond day 28 using landmark analysis. For overall survival (OS), Kaplan-Meier curves and log-rank tests were used for univariate comparisons, and Cox regression was used for multivariable analysis. For competing risk endpoints including relapse, TRM, cumulative incidence curves and Gray’s test were used for univariate analysis and Fine-Gray regression for multivariable analysis. Covariates adjusted in multivariable regression analysis included age, CMV serostatus, disease status prior to transplant, and Karnofsky performance status (KPS). Subgroup analyses were performed by CMV serostatus and disease groups. All tests were two-sided, and p-values < 0.05 were considered statistically significant. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R 3.6.1 (R Core Team).
RESULTS
We examined the records of 227 adult patients with lymphoma or multiple myeloma and excluded patients who had relapse prior to day 28 (n=4). Of the remaining 223 patients, only 110 patients had a blood sample at day 28. We elected to study the relationship of adaptive NK cells at day 28 with relapse given prior reports of decreased relapse with robust early lymphocyte recovery [1, 4, 8, 9]. None of the 110 patients reactivated CMV after AHCT. Patient, disease and transplant characteristics at the time of transplant are summarized for multiple myeloma patients (n=56) in Table 1 and lymphoma patients (n=54) in Table 2.
Table 1.
Characteristics of patients with multiple myeloma by low vs high content of adaptive NK cells (NKG2C+CD57+) at day 28 after AHCT.
| Overall (N = 56) |
Day-28 NKG2C+CD57+ low1
(N = 12) |
Day-28 NKG2C+CD57+ high2
(N = 44) |
P-value3 | |
|---|---|---|---|---|
| Age, median (range) | 61.6 (33.7–76.0) | 61.9 (33.7–76.0) | 61.4 (34.2–73.1) | 0.90 |
| Female, n (%) | 29 (52%) | 9 (75%) | 20 (45%) | 0.10 |
| Race, n (%) | 0.49 | |||
| Caucasian | 41 (73%) | 10 (83%) | 31 (70%) | |
| African American | 6 (11%) | 0 | 6 (14%) | |
| Other/unknown | 9 (16%) | 2 (17%) | 7 (16%) | |
| CMV seropositive, n (%) | 35 (63%) | 8 (67%) | 27 (61%) | >0.99 |
| Cytogenetic risk, n (%) | 0.41 | |||
| High | 9 (16%) | 4 (33%) | 5 (11%) | |
| Intermediate | 9 (16%) | 1 (8%) | 8 (18%) | |
| Standard | 35 (63%) | 7 (58%) | 28 (64%) | |
| Unknown | 3 (5%) | 0 | 3 (7%) | |
| Disease status, n (%) | >0.99 | |||
| CR1 | 11 (20%) | 2 (17%) | 9 (20%) | |
| PR | 45 (80%) | 10 (83%) | 35 (80%) | |
| HCT-CI, n (%) | 0.39 | |||
| High risk | 14 (25%) | 4 (33%) | 10 (23%) | |
| Intermediate risk | 14 (25%) | 4 (33%) | 10 (23%) | |
| Low risk | 25 (45%) | 3 (25%) | 22 (50%) | |
| Unknown | 3 (5%) | 1 (8%) | 2 (5%) | |
| Karnofsky performance score at baseline, n (%) | 0.26 | |||
| <90 | 14 (25%) | 1 (8%) | 13 (30%) | |
| ≥90 | 42 (75%) | 11 (92%) | 31 (70%) | |
| Stem cell dose (CD34+ 106/kg), mean (SD) | 7.7 (5.5) | 9.8 (5.7) | 7.1 (5.3) | 0.05 |
| Maintenance lenalidomide | 0.18 | |||
| Yes | 40 (71%) | 8 (67%) | 32 (73%) | |
| No | 13 (23%) | 2 (17%) | 11 (25%) | |
| Unknown | 3 (5%) | 2 (17%) | 1 (2%) | |
| Transplant year, n (%) | 0.52 | |||
| 2010–2011 | 30 (54%) | 5 (42%) | 25 (57%) | |
| 2012–2014 | 26 (46%) | 7 (58%) | 19 (43%) | |
| Follow-up time, (years) median (range) | 3.0 (0.2–4.4) | 3.0 (0.7–4.4) | 3.0 (0.2–4.3) | 0.75 |
Low: Adaptive NK cells at Day 28 < 1.53 adaptive NK cells/μL Cutpoint determined by Fine-Gray regression model without adjusting for other covariates.
High: Adaptive NK cells at Day 28 ≥ < 1.53 adaptive NK cells/μL.
Chi-square was used for categorical variables except for cases with frequency ≤ 5 when Fisher’s exact test was used; T-test used for continuous variables except stem cell dose and follow-up time, when Wilcoxon rank sum (Mann Whitney U) test was used.
Abbreviations: n number, CMV cytomegalovirus, CR1 first complete response, PR partial response, HCT-CI hematopoietic cell transplant comorbidity index
Table 2.
Patient, disease and transplant characteristics of patients with lymphoma by low vs high content of adaptive NK cells (NKG2C+CD57+) at day 28 after AHCT.
| Overall (N = 54) |
Day-28 NKG2C+CD57+ low1
(N = 11) |
Day-28 NKG2C+CD57+ high2
(N = 43) |
P-value3 | |
|---|---|---|---|---|
| Age, median (range) | 55.1 (18.9–72.4) | 52.6 (22.4–70.3) | 55.4 (18.9–72.4) | 0.84 |
| Female, n (%) | 23 (43%) | 4 (36%) | 19 (44%) | 0.74 |
| Race, n (%) | 0.74 | |||
| Caucasian | 46 (85%) | 9 (82%) | 37 (86%) | |
| African American | 2 (4%) | 0 | 2 (5%) | |
| Other/unknown | 6 (11%) | 2 (18%) | 4 (9%) | |
| Diagnosis, n (%) | 0.71 | |||
| Non-Hodgkin lymphoma | 40 (74%) | 9 (82%) | 31 (72%) | |
| Aggressive B cell | 22 (41%) | 6 (54%) | 16 (37%) | |
| Indolent B cell | 1 (1%) | 0 | 1 (2%) | |
| T cell lymphoma | 2 (4%) | 0 | 2 (4%) | |
| Mantle cell lymphoma | 15 (28%) | 3 (27%) | 12 (28%) | |
| Hodgkin lymphoma | 14 (26%) | 2 (18%) | 12 (28%) | |
| CMV seropositive, n (%) | 34 (63%) | 6 (55%) | 28 (65%) | 0.73 |
| Disease status, n (%) | 0.31 | |||
| >=CR2 | 17 (31%) | 2 (18%) | 15 (35%) | |
| CR1 | 12 (22%) | 1 (9%) | 11 (26%) | |
| Progressive/relapsed | 12 (22%) | 4 (36%) | 8 (19%) | |
| PR | 13 (24%) | 4 (36%) | 9 (21%) | |
| HCT-CI, n (%) | 0.37 | |||
| High risk | 11 (20%) | 4 (36%) | 7 (16%) | |
| Intermediate risk | 18 (33%) | 3 (27%) | 15 (35%) | |
| Low risk | 25 (46%) | 4 (36%) | 21 (49%) | |
| Karnofsky performance score at baseline, n (%) | 0.33 | |||
| <90 | 6 (11%) | 0 | 6 (14%) | |
| ≥90 | 48 (89%) | 11 (100%) | 37 (86%) | |
| Stem cell dose (CD34+ 106/kg), mean (SD) | 5.9 (3.4) | 6.4 (5.0) | 5.8 (2.7) | 0.75 |
| Conditioning regimen | 0.95 | |||
| Cy/TBI | 35 (65%) | 7 (64%) | 28 (65%) | |
| BEAM/CBV | 19 (35%) | 4 (36%) | 15 (35%) | |
| Follow-up time, (years) median (range) | 3.1 (0.2–4.5) | 3.7 (0.4–4.4) | 2.8 (0.2–4.5) | 0.16 |
Low: Absolute count of NKG2C+CD57+ at Day 28 < < 1.53 adaptive NK cells/μL. This cutpoint was determined by Fine-Gray regression model without adjusting for other covariates.
High: Absolute count of NKG2C+CD57+ at Day 28 ≥ < 1.53 adaptive NK cells/μL.
Chi-square test was used for categorical variables except for cases with frequency ≤ 5, in which case Fisher’s exact test was used; T-test was used for continuous variables except for stem cell dose and follow-up time, in which case, Wilcoxon rank sum test was used.
Abbreviations: n number, CMV cytomegalovirus, CR2 second complete response, CR1 first complete response, PR partial response, HCT-CI hematopoietic cell transplant comorbidity index, Cy cyclophosphamide, TBI total body irradiation, BEAM carmustine etoposide cytarabine and melphalan, CBV cyclophosphamide BCNU and etoposide
The median age in multiple myeloma patients was 61.6 years, and 63% were CMV seropositive. All myeloma patients received high dose melphalan prior to AHCT. At the time of AHCT, 80% of multiple myeloma patients were in partial remission, and 20% were in complete remission. Of the 56 multiple myeloma patients, 71% received lenalidomide maintenance and 35 had standard risk cytogenetics by mSMART criteria [39]. None of the patients in this study started lenalidomide maintenance prior to day 28. The median age of lymphoma patients was 55.1 years, and 63% were CMV seropositive. NHL patients made up 74% of the lymphoma cohort, and the largest subgroup of these was aggressive B cell lymphoma. The majority of lymphoma patients (n = 35) received cyclophosphamide and total body irradiation conditioning (65%).
The frequencies of NK cell subsets were compared in blood between CMV seropositive and seronegative patients at days 28, 60, 90, 180, and 365 (Figure 1). The median number of adaptive NK cells was higher in CMV seropositive patients at day 90 (n = 110, p < 0.01) and day 180 (n = 73, p < 0.01) compared to CMV seronegative patients (Figure 1A). The median number of conventional NK cells was higher in CMV seronegative patients at day 28 (p < 0.05) but similar at all other time points (Figure 1B). Absolute T cell counts were higher in CMV seropositive patients at all timepoints (p < 0.01, data not shown).
Figure 1. Immune reconstitution after autologous transplant by CMV serostatus.

Dot plots by time point and CMV serostatus showing interquartile range (IQR) indicating the 25th and 75th percentiles; the line inside the box indicates the median; outliers are shown as dots. n = the number of patients with a blood sample at a given time point. A. Adaptive (CD56dimCD57+NKG2C+) NK cells by time point. B. Conventional NK cells by time point. *p < 0.05, **p < 0.01, ***p < 0.001
Transplant outcomes were examined in the 110 individuals who had lymphocyte phenotype data at day 28 stratified by levels of adaptive NK cells. We determined the optimal cut-point associated with the greatest difference in relapse risk and divided patients into “high adaptive NK cells” (≥ 1.53 adaptive NK cells/μL; n = 87) versus “low adaptive NK cells” groups (< 1.53 adaptive NK cells/μL; n = 23). The cross-validation result confirmed that the dichotomized adaptive NK cells variable was significantly associated with the risk of relapse (high vs. low HR 0.51, 95% CI 0.30–0.89, p = 0.018). We found no significant differences in patient characteristics stratified by day 28 adaptive NK cell groups (Table 1 and 2). In the overall group (n = 110), relapse at 2 years in the high adaptive NK cell group was 30% (95% CI, 21–40) compared to 61% (95% CI, 37–78) in the low adaptive NK cell group (p = 0.014) (Figure 2A). OS at 3 years was similar in patients with high and low adaptive NK cell counts (p = 0.94).
Figure 2. Cumulative incidence of relapse by absolute adaptive NK cell numbers at Day 28.

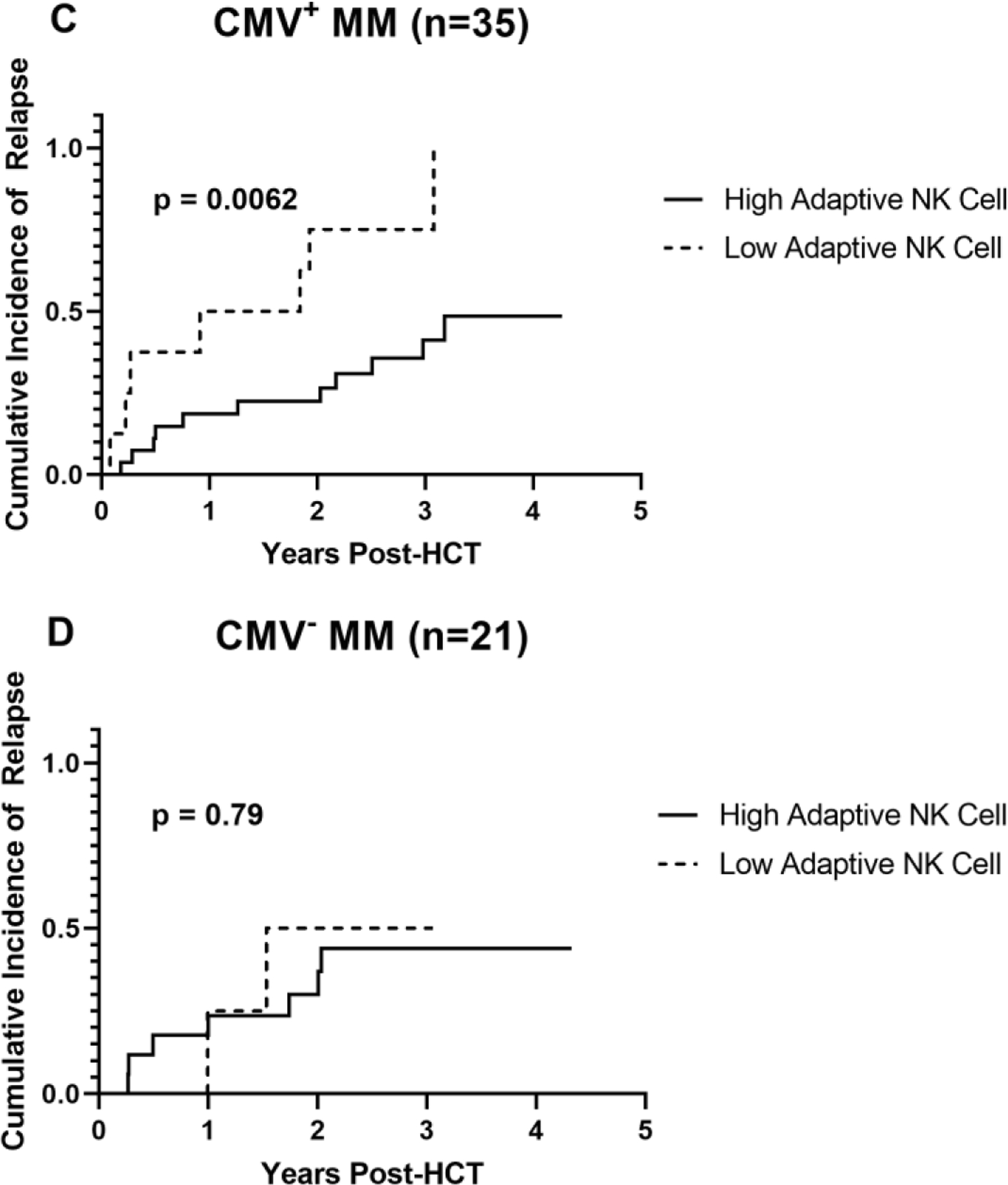
A. Relapse by high vs low adaptive NK cell counts at day 28 in the cohort of 110 individuals with either multiple myeloma or lymphoma. B. Relapse by high vs low adaptive NK cell counts at day 28 in 56 individuals with multiple myeloma. C. Relapse by high vs low adaptive NK cell numbers at day 28 in 35 CMV seropositive individuals with multiple myeloma. D. Relapse by high vs low adaptive NK cell numbers at day 28 in 21 CMV seronegative individuals with multiple myeloma. p-values were determined using Gray tests.
We next looked at relapse incidence in each disease cohort. In the cohort of patients with multiple myeloma (n = 56), the cumulative incidence of relapse at 2 years in the high adaptive NK cell group was 25% (95% CI, 13–39) compared to 67% (95% CI, 31–87) in the low adaptive NK cell group (p = 0.020) (Figure 2B). When we further subdivided the multiple myeloma patients into CMV seropositive (n = 35) and seronegative (n = 21), we observed that relapse rates were markedly lower in CMV seropositive multiple myeloma patients with relapse at 2 years of 22% (95% CI, 9–40) in the high adaptive NK cell group compared to 75% (95% CI 23–94) in the low adaptive NK cell group (p = 0.0062) (Figure 2C). There was no effect of adaptive NK cells in CMV seronegative multiple myeloma patients (Figure 2D). In the multivariable analysis including disease status, age, cytogenetic risk, and Karnofsky performance status (KPS), high adaptive NK cells were independently associated with significantly lower relapse risk (HR 0.12, 95% CI 0.03–0.80, p = 0.003) (Table 3). We also looked at adaptive NK cells at day 28 as a continuous variable in the multivariable analysis with disease status, age, cytogenetic risk, and Karnofsky performance status. Here we saw that adaptive NK cells as a continuous variable remained significant but the effect size was smaller (HR 0.98, 95% CI 97–99%, p = 0.002).
Table 3.
Details of multivariable landmark analysis result for relapse for adaptive NK cells
| Hazard Ratio | 95% CI | p value | |
|---|---|---|---|
| CMV+ MM group (n = 35) | |||
| NKG2C+ CD57+ NK cells at day+28 post AHCT (high vs. low) | 0.12 | 0.03, 0.48 | 0.003 |
| Age | 1.02 | 0.95, 1.10 | 0.60 |
| Karnofsky performance score | 0.96 | 0.86, 1.06 | 0.40 |
| Disease status1 (reference: CR1) | |||
| PR | 3.71 | 1.00,13.78 | 0.05 |
| Cytogenetic Risk (reference: standard) | |||
| Intermediate | 3.95 | 0.79, 19.70 | 0.09 |
| High | 3.00 | 0.84, 10.76 | 0.09 |
Two patients with missing comorbidity and 3 patients with missing risk were excluded. Age and Karnofsky performance score are continuous variables.
Abbreviations: n number, CMV cytomegalovirus, CR1 first complete response, PR partial response
We saw no effect based on adaptive NK cell numbers at day 28 in the NHL cohort (p = 0.30) or in CMV seropositive NHL patients (p = 0.31). There was no stratifying effect seen based on adaptive NK cell numbers at day 28 in the Hodgkin lymphoma cohort (p = 0.75), and the small sample size of this group prevented further subdivision by CMV serostatus.
DISCUSSION
In our prior work in the allogeneic HCT setting, we found that CMV reactivation and subsequent expansion of adaptive NK cells in AML patients who had received reduced intensity conditioning (RIC) was associated with lower rates of relapse [35, 36]. In those studies, adaptive NK cell numbers were low for months following transplant and only began to increase in CMV seropositive individuals at six months, with the highest expansions occurring in individuals who reactivated CMV. We also saw that recipients exhibiting low adaptive NK cell frequencies (< 5%) prior to transplant with RIC also exhibited low adaptive NK cell frequencies of reconstituting adaptive NK cells after CMV reactivation. Patients who received myeloablative conditioning regimens had lower adaptive NK cell levels than patients who received RIC, indicating that conditioning regimen strongly influences adaptive NK cell reconstitution. We also saw that high frequencies of recipient-derived, adaptive NK cells prior to transplant were associated with high frequencies of donor-derived adaptive NK cells after CMV reactivation [36] showing that host factors also impact adaptive NK cell reconstitution in the allogeneic setting.
In this study of AHCT for lymphoid malignancies, the patients expanded adaptive NK cells by day 28 despite the absence of clinical CMV reactivation. This earlier expansion of adaptive NK cells in the autologous setting is likely multifactorial and includes the absence of immunosuppressive medications used in the allogeneic setting, differences in conditioning regimens, and the lack of graft-versus-host responses in AHCT. Homeostatic expansion of adaptive NK cells after autologous transplant is at least in part driven by subclinical elevations in CMV antigen in the previously exposed recipient and endogenous IL-15 production as the patient recovers from lymphodepletion [42].
Here we showed that higher adaptive NK cell numbers are associated with decreased relapse in multiple myeloma but not in lymphoma. Multiple myeloma cells are sensitive to NK cell mediated killing [43] but less is known about the role of adaptive NK cells against myeloma. Myeloma cells express high levels of HLA-E [44], the ligand for the inhibitory receptor NKG2A expressed on conventional NK cells. Adaptive NK cells that express high levels of NKG2C express low levels of NKG2A [45]. This difference in inhibitory receptor expression may explain the strong anti-myeloma relapse effect compared to lymphoma cells where expression of ligands that influence NK cell function is not as well characterized. The lack of effect in lymphoma may also be attributed to stronger impact of outcomes related to pre-transplant disease status or disease histology. One limitation of this relatively small study was the significant heterogeneity among lymphoma patients.
We saw a more significant effect of adaptive NK cells with relapse when we compared adaptive NK cells at day 28 as a categorical variable (high versus low) compared to adaptive NK cells at day 28 as a continuous variable. There was a wide range of adaptive NK cell levels at day 28 in CMV seropositive patients and positive CMV serostatus itself was not protective of relapse. Our results using a data specific cut-point analysis suggest that a relatively low level of adaptive NK cells is sufficient to confer protection from relapse. We hypothesize that robust reconstitution of NK cells early after transplant allows for improved immune surveillance and control of the plasma cells that persist after melphalan chemotherapy.
We saw no difference in the OS at three years in patients with high versus low adaptive NK cell counts. The similar OS despite significant differences in relapse indicates that patients who relapsed were successfully salvaged with subsequent therapies.
The highest levels of adaptive NK cells after AHCT were detected in CMV seropositive individuals as early as day 28 after transplantation, and these differences were enhanced at the later time points (day 60 and 100). These adaptive NK cells potentially contribute to immune-mediated disease control and protection from relapse post-AHCT in multiple myeloma patients. Interventions to increase numbers of adaptive NK cells might provide a novel approach to improving myeloma outcomes after AHCT.
ACKNOWLEDGEMENTS:
This work was supported by NIH grants P01 CA111412 (JSM), P01 CA65493 (JSM), R35 CA197292 (JSM), K99/R00 HL123638 (FC), and T32 2T32HL007062 (AM). Statistical analysis was performed with Biostatistics Shared Resource of the University of Minnesota Masonic Cancer Center, supported by NIH/NCI grant P30CA07759.
Footnotes
Declarations of Interest: None
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References:
- 1.Porrata LF, et al. , Early lymphocyte recovery predicts superior survival after autologous stem cell transplantation in non-Hodgkin lymphoma: a prospective study. Biol Blood Marrow Transplant, 2008. 14(7): p. 807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joao C, et al. , Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant, 2006. 37(9): p. 865–71. [DOI] [PubMed] [Google Scholar]
- 3.Porrata LF, et al. , Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation for patients with primary systemic amyloidosis. Clin Cancer Res, 2005. 11(3): p. 1210–8. [PubMed] [Google Scholar]
- 4.Porrata LF, et al. , Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia, 2002. 16(7): p. 1311–8. [DOI] [PubMed] [Google Scholar]
- 5.Porrata LF, et al. , Prolonged survival associated with early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant, 2001. 28(9): p. 865–71. [DOI] [PubMed] [Google Scholar]
- 6.Porrata LF, et al. , Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood, 2001. 98(3): p. 579–85. [DOI] [PubMed] [Google Scholar]
- 7.Rueff J, et al. , Lymphocyte subset recovery and outcome after autologous hematopoietic stem cell transplantation for plasma cell myeloma. Biol Blood Marrow Transplant, 2014. 20(6): p. 896–9. [DOI] [PubMed] [Google Scholar]
- 8.Porrata LF, et al. , Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin’s disease. Br J Haematol, 2002. 117(3): p. 629–33. [DOI] [PubMed] [Google Scholar]
- 9.Porrata LF, et al. , Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant, 2004. 33(3): p. 291–8. [DOI] [PubMed] [Google Scholar]
- 10.Damlaj M, et al. , Lymphocyte recovery is an independent predictor of relapse in allogeneic hematopoietic cell transplantation recipients for acute leukemia. World J Transplant, 2017. 7(4): p. 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YJ, et al. , Early lymphocyte recovery predicts superior overall survival after unmanipulated haploidentical blood and marrow transplant for myelodysplastic syndrome and acute myeloid leukemia evolving from myelodysplastic syndrome. Leuk Lymphoma, 2013. 54(12): p. 2671–7. [DOI] [PubMed] [Google Scholar]
- 12.Han DK, et al. , Implication of early lymphocyte recovery after allogeneic hematopoietic stem cell transplantation in children with leukemia. Yonsei Med J, 2013. 54(1): p. 62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke MJ, et al. , Early lymphocyte recovery and outcomes after umbilical cord blood transplantation (UCBT) for hematologic malignancies. Biol Blood Marrow Transplant, 2011. 17(6): p. 831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhlmann L, et al. , Lymphocyte subset recovery and outcome after T-cell replete allogeneic hematopoietic SCT. Bone Marrow Transplant, 2011. 46(10): p. 1357–62. [DOI] [PubMed] [Google Scholar]
- 15.Kanakry CG, et al. , Origin and evolution of the T cell repertoire after posttransplantation cyclophosphamide. JCI Insight, 2016. 1(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minculescu L, et al. , Early Natural Killer Cell Reconstitution Predicts Overall Survival in T Cell-Replete Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant, 2016. 22(12): p. 2187–2193. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen S, et al. , NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood, 2005. 105(10): p. 4135–42. [DOI] [PubMed] [Google Scholar]
- 18.Foley B, et al. , NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood, 2011. 118(10): p. 2784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arase H, et al. , Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science, 2002. 296(5571): p. 1323–6. [DOI] [PubMed] [Google Scholar]
- 20.Brown MG, et al. , Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science, 2001. 292(5518): p. 934–7. [DOI] [PubMed] [Google Scholar]
- 21.Smith HR, et al. , Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc Natl Acad Sci U S A, 2002. 99(13): p. 8826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlums H, et al. , Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity, 2015. 42(3): p. 443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merino A, et al. , Chronic stimulation drives human NK cell dysfunction and epigenetic reprograming. J Clin Invest, 2019. 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovalenko EI, et al. , Identification of Human Memory-Like NK Cells. Curr Protoc Cytom, 2017. 79: p. 9 50 1–9 50 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corat MA, et al. , Acquired somatic mutations in PNH reveal long-term maintenance of adaptive NK cells independent of HSPCs. Blood, 2017. 129(14): p. 1940–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlums H, et al. , Adaptive NK cells can persist in patients with GATA2 mutation depleted of stem and progenitor cells. Blood, 2017. 129(14): p. 1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarhan D, et al. , Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res, 2016. 76(19): p. 5696–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarhan D, et al. , Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res, 2018. 6(7): p. 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amini RM, et al. , Altered profile of immune regulatory cells in the peripheral blood of lymphoma patients. BMC Cancer, 2019. 19(1): p. 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, et al. , Functional role of regulatory T cells in B cell lymphoma and related mechanisms. Int J Clin Exp Pathol, 2015. 8(8): p. 9133–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Malek E, et al. , Myeloid-derived suppressor cells: The green light for myeloma immune escape. Blood Rev, 2016. 30(5): p. 341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JN, et al. , Increased activated regulatory T cell subsets and aging Treg-like cells in multiple myeloma and monoclonal gammopathy of undetermined significance: a case control study. Cancer Cell Int, 2018. 18: p. 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frohn C, et al. , Anti-myeloma activity of natural killer lymphocytes. Br J Haematol, 2002. 119(3): p. 660–4. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi T, et al. , Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol, 2005. 128(2): p. 192–203. [DOI] [PubMed] [Google Scholar]
- 35.Cichocki F, et al. , CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia, 2016. 30(2): p. 456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cichocki F, et al. , Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight, 2019. 4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itzykson R, et al. , Cytomegalovirus shapes long-term immune reconstitution after allogeneic stem cell transplantation. Haematologica, 2015. 100(1): p. 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallez-Hawkins G, et al. , Cytomegalovirus immune reconstitution occurs in recipients of allogeneic hematopoietic cell transplants irrespective of detectable cytomegalovirus infection. Biol Blood Marrow Transplant, 2005. 11(11): p. 890–902. [DOI] [PubMed] [Google Scholar]
- 39.Mikhael JR, et al. , Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc, 2013. 88(4): p. 360–76. [DOI] [PubMed] [Google Scholar]
- 40.Lee JW, et al. , Scoring and staging systems using cox linear regression modeling and recursive partitioning. Methods Inf Med, 2006. 45(1): p. 37–43. [PubMed] [Google Scholar]
- 41.Zhou B, et al. , Competing risks regression for stratified data. Biometrics, 2011. 67(2): p. 661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porrata LF, et al. , Interleukin-15 affects patient survival through natural killer cell recovery after autologous hematopoietic stem cell transplantation for non-Hodgkin lymphomas. Clin Dev Immunol, 2010. 2010: p. 914945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah N, et al. , Phase I study of cord blood-derived natural killer cells combined with autologous stem cell transplantation in multiple myeloma. Br J Haematol, 2017. 177(3): p. 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar S, et al. , Optimal selection of natural killer cells to kill myeloma: the role of HLA-E and NKG2A. Cancer Immunol Immunother, 2015. 64(8): p. 951–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guma M, et al. , Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood, 2004. 104(12): p. 3664–71. [DOI] [PubMed] [Google Scholar]


