Abstract
To investigate the impact of mono- and di-β-galactose moieties in tumor uptake and photodynamic therapy (PDT) efficacy, HPPH [3-(1′-hexyloxy)ethyl-3-devinylpyropheophorobide-a], the meso pyropheophorbide-a [3-ethyl-3-devinyl-pyropheophorbide-a], and the corresponding 20-benzoic acid analogs were used as starting materials. Reaction of the intermediates containing one or two carboxylic acid functionalities with 1-aminogalactose afforded the desired 172- or 20(4′)- mono- and 172, 20(4′)-di galactose conjugated photosensitizers (PSs) with and without a carboxylic acid group. The overall lipophilicity caused by the presence of galactose in combination with either an ethyl or (1′-hexyloxy)ethyl side chain at position-3 of the macrocycle made a significant difference in in vitro uptake by tumor cells and photoreaction upon light exposure. Interestingly, among the PSs investigated, compared to HPPH 1 the carbohydrate conjugates 2 and 11 in which β-galactose moieties are conjugated at positions 172 and 20(4′) of meso-pyro pheophorbide-a showed similar in vitro efficacy in FaDu cell lines, but in SCID mice bearing FaDu tumors (head & neck) Ps 11 gave significantly improved long-term tumor cure.
Keywords: Photodynamic therapy, Photosensitizer, Cancer imaging
1. Introduction
Since the approval of Photofrin for the treatment of cancer by photodynamic therapy (PDT), a wide variety of porphyrins and non-porphyrin-based photosensitizers have been reported [1–4]. Efforts have also been made to investigate the tumor targeting ability of photosensitizers by conjugating certain small molecules and peptides to target receptors known for their over-expression in tumors [5,6]. Among these targeting moieties, carbohydrates-especially β-galactose have gained attention [7–11]. The other strategy, which has also made a significant impact in developing improved agent(s) for PDT is the structure activity relationship (SAR) approach, and some of the agents, e. g. HPPH (Photochlor) [12], Foscan [13], Tookad [14], Talaporphin [15], Photobac [16], benzoporphyrin derivative (BPD) [17] and (PC4, a Phthalocyanine) [18] are at various stages of human clinical trials for the use in oncologic and non-oncological applications. Another approach, which has been equally useful in selecting effective agents before evaluating them for in vivo efficacy is to define the cell-specificity of PSs in various tumor cell types, especially PS uptake and retention [19].
Most of the porphyrin-based compounds currently being investigated for the use in PDT show poor water solubility. The common substituents that have been introduced in tetrapyrrolic systems (porphyrins, phthalocyanines or expanded porphyrins) are amine, carboxylic acid, sulfonic acid and ethylene glycol side chains, which do not show much impact in water solubility [20,21]. However, some of the glycosylated porphyrin systems may improve the hydrophilic nature by attaching the sugar moiety at the peripheral positions of the macrocycle. In general, (Glycan-protein) lectin-type receptors are highly expressed in some type of malignant cells, which specifically interact with carbohydrate-conjugated tetrapyrroles [22]. Among certain proteins the galectins are highly conserved carbohydrate recognition domain and exhibit high affinity for β–galactoside [23]. Galectins are known to involve in the modulation of cell adhesion [24], cell growth [25], immune response [26] and angiogenesis [27] and changes in their expression play a critical role in tumor progression.
In our attempt to enhance the efficacy and selectivity of HPPH [3-(1′-hexyloxy)ethyl-3-devinylpyropheophorbide-a], currently under human clinical trials[1,28,29], was conjugated with a series of mono- di- and tetrasaccharides, with the goal to target carbohydrate-binding proteins, especially galectin-1 and galectin-3. Among the compounds investigated HPPH-Gal in which a β-galactose moiety was conjugated with HPPH at position 172 showed the best efficacy [30]. The increased activity (both in vitro and in vivo) could not be confirmed on the basis of its binding to cellular galectins. However, the overall lipophilicity and the lack of transport by the ABCG2 transporter may contribute to improved efficacy [31].
In a parallel study, to determine the effect of substituents at various peripheral positions of the HPPH moiety, the 20-phenyl substituted analogs were of particular interest due to their higher tumor-cell selectivity. The meso-phenyl substituted analog provides a unique opportunity not only to introduce the targeting moieties at two different positions (17 and 20) of the molecule, but also to investigate the impact of overall charge in tumor cell-uptake and retention. Therefore, in present study, a series of HPPH-based PSs containing mono- and di-galactose moiety with and without the presence of hexyl ether side chain at position 3(1′) of the macrocycle (4–6 & 9–11) were synthesized. The in vitro PDT efficacy of these compounds were compared with known HPPH analogs 1–3 & 7,8 [30] with and without the hexyl ether side chain [31].
2. Results and discussion
2.1. Chemistry
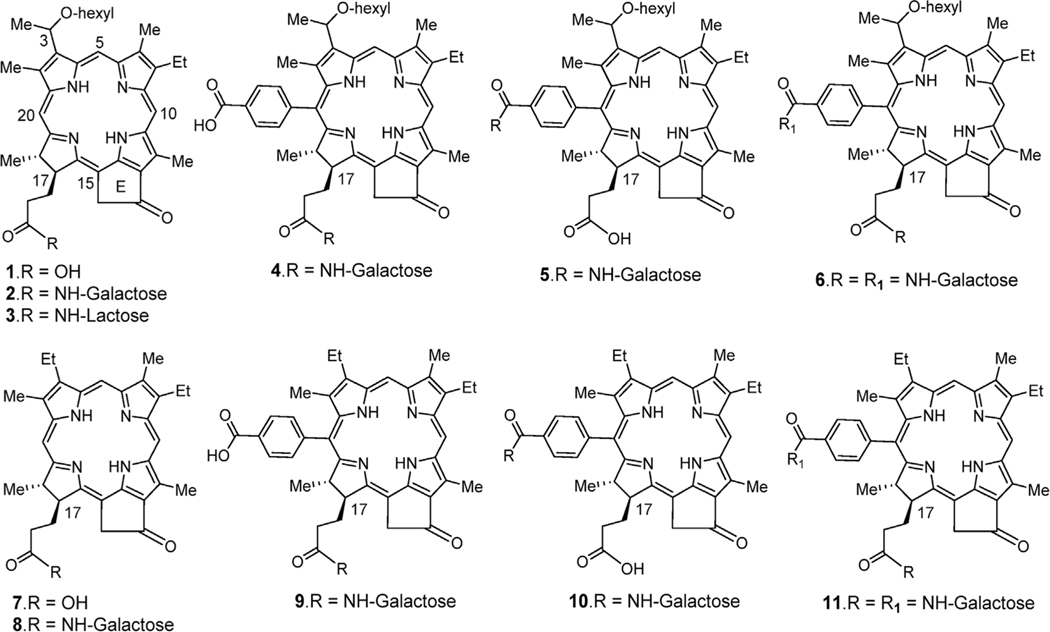
The photosensitizers investigated herein (Fig. 1) were prepared either from HPPH 1, methyl pyropheophorbide-a 12 or methyl meso pyropheophorbide-a 13 (Scheme 1) obtained from chlorophyll-a by following the reported methods [32].
Fig. 1.
Structures of photosensitizer-carbohydrate conjugates investigated in current study.
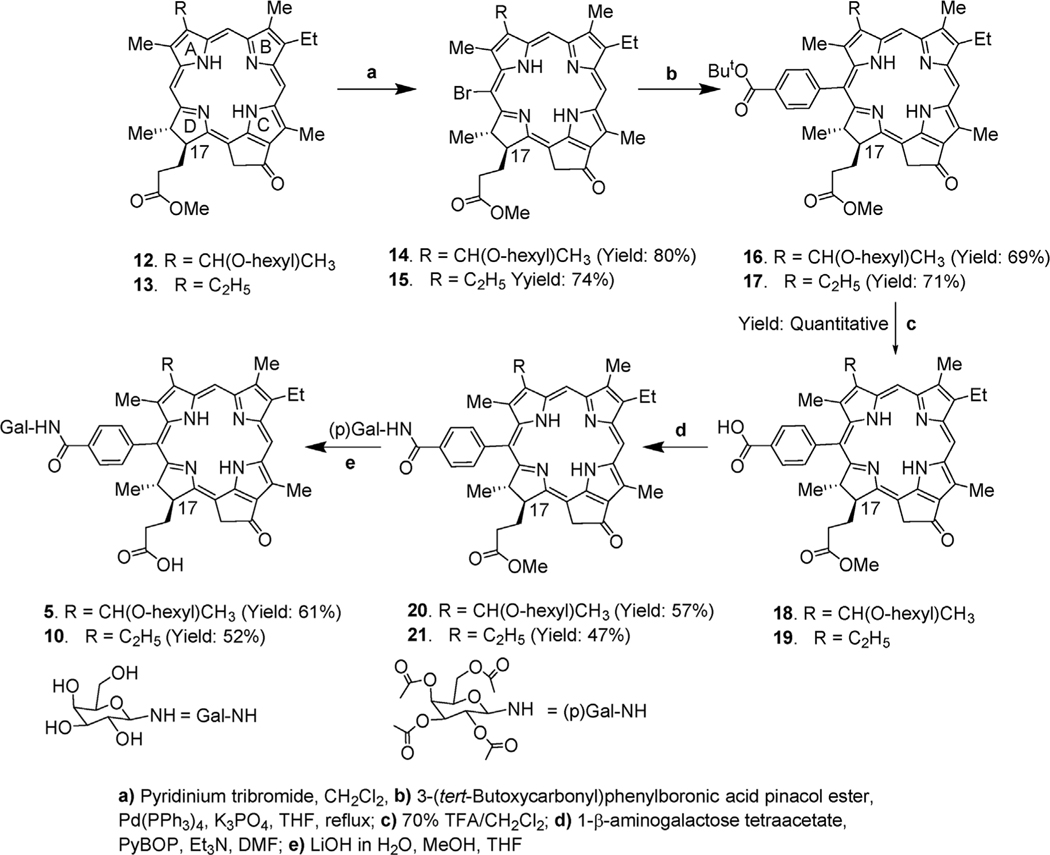
Scheme 1.
Synthesis of 20-substituted galactose conjugates of pyropheophorbide-a analogs.
2.1.1. Synthesis of 20-phenyl-β-galactose conjugated pyropheopheophorbides
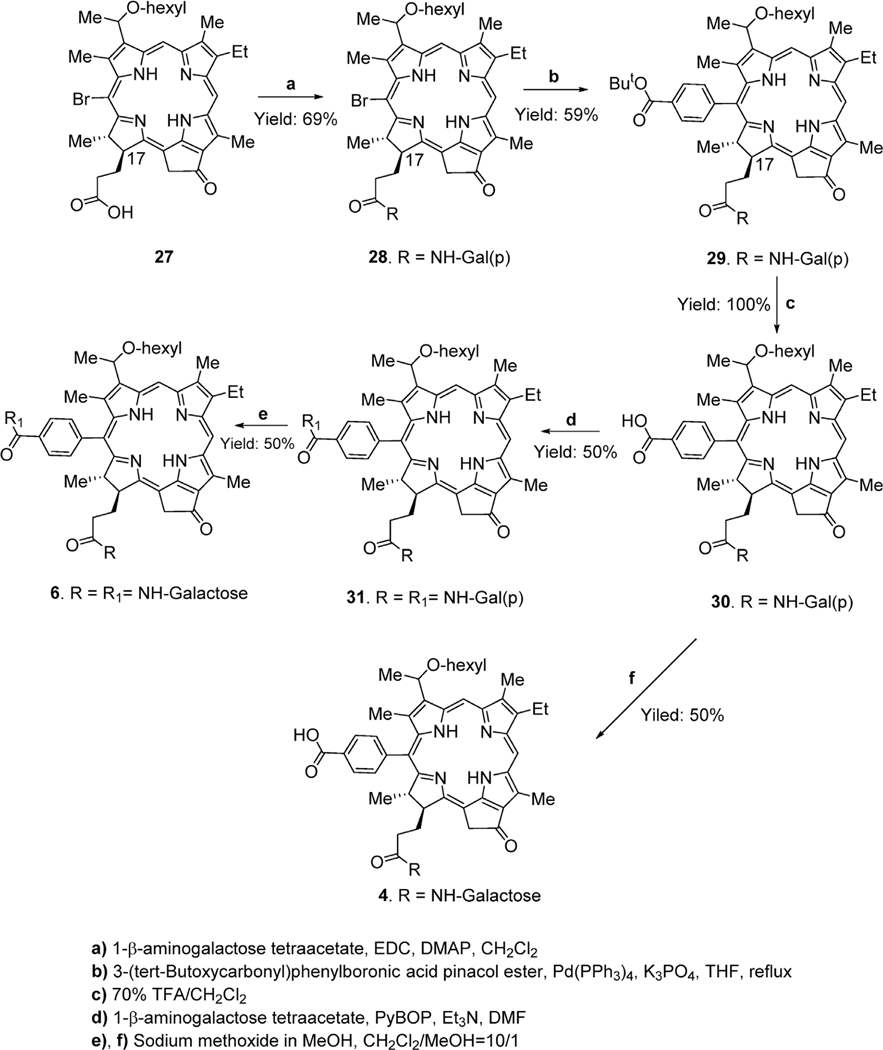
For the synthesis of the title compounds, 12 and 13 were separately reacted with pyridinium tribromide [33], the 20-bromo derivatives 14 and 15 were then converted into 20-phenyl-p-tert-butoxy analogs 16 and 17 by reacting with 4-(tert-butoxycarbonyl) phenylboronic acid pinacol ester and tetrakis(triphe-nylphosphine)palladium as catalyst [33]. Further treatment of these intermediates with trifluoroacetic acid (TFA) at room temperature gave the desired carboxylic acid chlorins 18 and 19. These chlorins were reacted with 1-β-aminogalactose tetraacetate, and the galactose protected compounds 20 and 21 were isolated in 57% and 47% yield, which on reacting with aqueous lithium hydroxide at room temperature gave PSs 5 (61% yield) and 10 (52% yield) respectively. (Scheme 1).
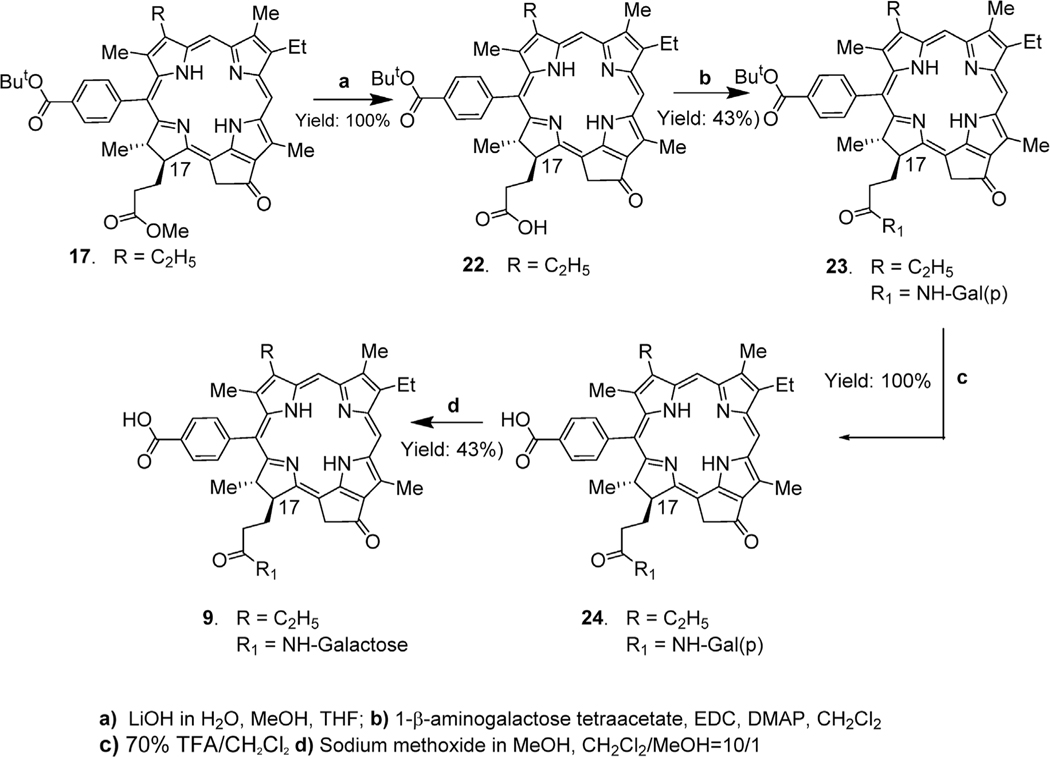
2.1.2. Synthesis of 20-phenyl-p-carboxylic acid 172-β-galactose conjugated meso pyropheophorbides
Compound 17 containing a methyl ester functionality was hydrolyzed to the corresponding carboxylic acid 22 under mild basic conditions in quantitative yield, on reacting with aqueous lithium hydroxide at room temperature. Compound 22 was then reacted with 1- β–aminogalactose tetraacetate and the carbohydrate conjugate 23 thus obtained in 43% yield on treating with TFA at room temperature afforded 20-phenyl-p-carboxylic acid analog 24 in quantitative yield. In the last step of synthesis, reaction of 24 with sodium methoxide gave the desired 20-phenyl-β-galactose conjugate 9 in 43% yield (Scheme 2).
Scheme 2.
Synthesis of 17-substituted galactose conjugate of 20-benzoic acid substituted meso pyropheophorbide-a.
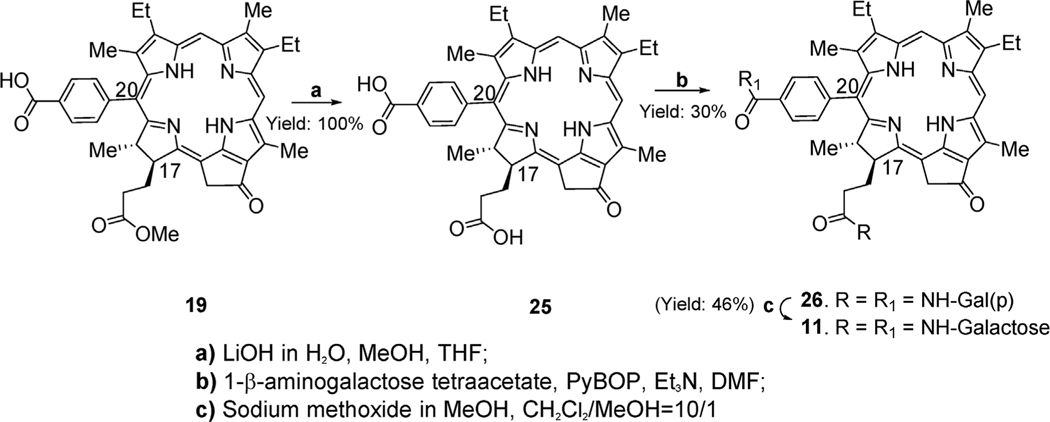
2.1.3. Synthesis of 172- and 20-phenyl-β-galactose conjugates meso pyropheophorbides
20-Phenyl-p-carboxylic acid meso pyropheophorbide-a methyl ester 19 was first converted into corresponding 17, 20-dicarboxylic acid 25 in quantitative yield on mild base hydrolysis. The dicarboxylic acid derivative was then reacted with 1- β–aminogalactose tetraacetate to provide 172, 20-disubstituted acetylated galactose derivate 26. Finally, after deprotection of galactose, moieties with sodium methoxide, the desired di-β–galactose meso pyropheophorbide 11 was isolated in 46% yield (Scheme 3).
Scheme 3.
Synthesis of 172, 20-disubstituted galactose conjugate of meso pyropheophorbide-a.
2.1.4. Synthesis 172- and 20-phenyl-β-galactose conjugated 3-(1′-hexyloxy) ethyl-3-devinylpyropheo- phorbide-a
For the preparation of di-β-galactose HPPH analog, a similar approach as discussed for compound 11 was followed. In brief, 20-bromo HPPH 27 prepared by following our own methodology [25,26] was first converted into 17–β-amino-galactose tetraacetate 28 in 69% yield. It was then reacted with 4-(tert-butoxycarbonylphenyl)boronic acid pinacol ester in the presence of palladium catalyst, and the intermediate 29 was obtained in 59% yield. The tert-butyl ester functionality was removed under acidic condition and the corresponding carboxylic acid analog 30 was obtained in quantitative yield. It was again reacted with 1- β-amino-galactose tetraacetate to produce compound 31, which, as well as compound 30, on deprotection under basic conditions gave the desired bis- and mono-galactose HPPH analogs 6 and 4 respectively, each in 50% yield (Scheme 4). All the intermediates and final products were characterized by NMR, UV-vis and mass spectrometry analysis, and the purity of the galactose derivatives was also confirmed by HPLC analyses.
Scheme 4.
Synthesis of 17-substituted and 17, 20-disubstituted galactose conjugates of HPPH (Photochlor).
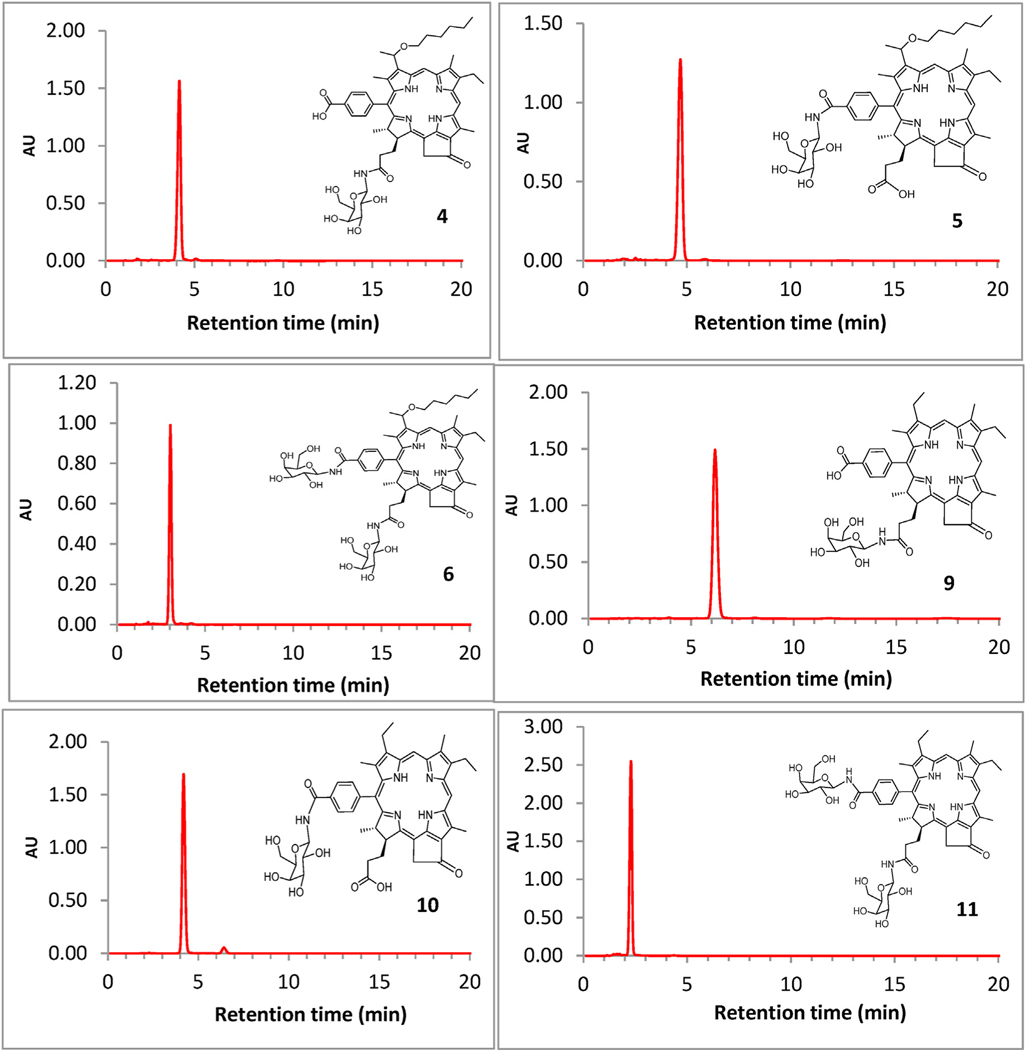
2.1.5. HPLC analysis of carbohydrate conjugates
HPLC chromatograms for compounds 4, 5, 6, 9, 10 and 11 were obtained using a Waters Delta 600 System consisting of: a 600 Controller, 600 Fluid Handling Unit and a 996 Photodiode Array Detector. A Waters Symmetry C18 column (4.6 × 150 mm, 5 μm particle size) was used to investigate the purity of the compounds before biological evaluation. An isocratic mobile phase consisting of 0.5% (v/v) acetic acid in methanol, at a flow rate of 1.0 ml/min was used. Absorption range was selected between 350 and 800 nm; data were processed at 362 nm. The purity ascertained by HPLC, and retention times of the conjugates are depicted in Fig. 2 and Table 1 respectively.
Fig. 2.
HPLC chromatograms of photosensitizer-galactose conjugates 4, 5, 6,9,10 and 11. For details (HPLC system, column, eluting solvents, absorption wavelength) see the text.
Table 1.
Retention time and the purity of carbohydrate conjugates determined by HPLC analysis.
| Compound | 4 | 5 | 6 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|
| Retention time (min) | 4.15 | 4.69 | 3.03 | 6.17 | 4.18 | 2.29 |
| Purity, % | 96.07 | 97.32 | 96.06 | 97.11 | 95.45 | 96.97 |
The 1H and 13C NMR spectra (1D and 2D) are consistent with the proposed structures. However, the 13C spectrum of 4 exhibits some unusual features that are worth noting. Each of the carbons of 4 is expected to generate up to two signals in the 1D 13C spectrum, because 4 exists as two diastereomers due to the chiral center at position 31. Nearly all carbons of 4 were observed as either one or two peaks, exactly as expected. However, four carbons exhibited unexpected, additional peaks. Each of these four carbons produced either three or four roughly equally spaced peaks. These appear at: 198.63, 198.61, 198.59, and 198.57 ppm for 131-C (i.e., the keto C=O); 149.22, 149.18, and 149.15 ppm for 14-C; 131.33, 131.30, 131.27, and 131.24 ppm for 13-C; and 107.29, 107.27, 107.24, and 107.21 ppm for 15-C. HMBC data firmly assign three of these four carbons as indicated above. The assignment of the cluster of peaks at ~131.3 ppm is less certain, but HMBC data indicate that the 13-position carbon is a likely source of these signals.
All of these four carbons are members of the photosensitizer E-ring. The only E-ring carbon that failed to generate observable signals that exceeded the expected number of peaks is 132-C, which could not be observed at all in the 1D 13C spectrum due to severe overlap with the solvent (CD3OD) resonance. This carbon may, in fact, generate additional (unexpected) peaks. If it does, however, these would simply not be observable in the 1D carbon spectrum because of the solvent interference. The HSQC spectrum shows a clear cross peak at the proton and carbon chemical shifts expected for the 132methylene group, but HSQC is not able to resolve the individual carbon resonances that compose this cross peak, preventing a peak count for this carbon. The location of these four carbons suggests that some kind of heterogeneity associated with the E-ring is responsible for producing the “extra” peaks. Interestingly, no extra peaks were observed in the 13C spectra of any of the other compounds examined here. Even protected conjugates 29 and 30, which are pre-cursors of 4, fail to exhibit the additional peaks despite the fact that each of these is structurally similar to 4.
These facts lead us to the following hypothesis. Hydrogen bonding – from a hydroxyl group of the unprotected galactose to the E-ring’s keto oxygen – may be responsible for the presence of the unexpected carbon resonances observed in 4. The additional carbon peaks could arise due to the presence of two states of each diastereomer: hydrogen bound, and non-hydrogen bound. This would account for our observation of up to four peaks for each of the affected carbons. The lack of extra peaks in 29 and 30 is consistent with this hypothesis because the acetyl-protected galactose moieties in these two precursors lack the hydroxyl groups required for hydrogen bonding to the keto oxygen. The localized nature of this effect (E-ring only) is also consistent with the hydrogen bonding scenario because the influence on carbon chemical shifts is likely to be greatest near the site of the hydrogen bond.
Although plausible, the hydrogen bond explanation fails to account for some of our observations. No other mono- or di-galactose-containing conjugate examined here exhibits the same effect in its E-ring carbons. One might expect 6 and 9 to behave like 4 because each also has an unprotected galactose at position-17, and (relative to 4) each has a similar general structure. Conjugate 6 differs from 4 only in its additional galactose linked through position-20. Conjugate 9 differs from 4 in its lack of the O-hexyl group at position-3 [1]. To be consistent with our hydrogen bonding explanation, we must assume that the O-hexyl group and second galactose moiety somehow prevent hydrogen bond formation, which would account for the behavior of 6 and 9. However, we recognize that the unusual behavior of the E-ring carbons of conjugate 4 may be due to some other source. For example, it might be due to two different ring conformations, or two different stacking geometries. Each of these could manifest itself in two distinct structural states (per diastereomer) resulting in two distinct magnetic environments (per diastereomer) in the vicinity of the E-ring. This could account for the number of carbon peaks observed, up to four per E-ring carbon. Proof of any of these proposed explanations would obviously require further studies.
2.2. Biological studies
2.2.1. In vitro PDT efficacy
The PDT efficacy of the galactose conjugated compounds was investigated in FaDu, and Colon 26 tumor cell lines known for overexpression of galectin-3. A standard MTT assay [34] was performed to determine the IC50 value and percentage cell viability of each compound at variable concentrations after exposing to 1 J/cm2 of light at 665 nm. Briefly, cells were seeded into a 96 well plate at 5000 cells per well and allowed to adhere. Photosensitizer was added to the media at two-fold dilutions with a top concentration of 1600 nM and incubated with cells for 24 h. After incubation, the cells were washed with media exposed to appropriate wavelength of light (1 J/cm2). At 48 h after PDT, the MTT assay was performed, and cell viabilities was determined. The IC50 values of all the compounds are listed in Table 2.
Table 2.
Comparative in vitro photosensitizing efficacy (IC50 values) of photo sesitizers.
| Photosensitizer | IC50 Values FaDu cells | IC50 Values Colon 26 cells |
|---|---|---|
|
| ||
| 1 | 0.044 ± 0.005 μM | 0.023 ± 0.001 μM |
| 2 | 0.055 ± 0.006 μM | 0.019 ± 0.003 μM |
| 4 | 0.195 ± 0.020 μM | 0.266 ± 0.012 μM |
| 5 | 0.161 ± 0.009 μM | 0.092 ± 0.037 μM |
| 6 | 0.109 ± 0.017 μM | 0.177 ± 0.029 μM |
| 9 | 0.066 ± 0.010 μM | 0.113 ± 0.005 μM |
| 10 | 0.128 ± 0.013 μM | 0.068 ± 0.010 μM |
| 11 | 0.039 ± 0.006 μM | 0.034 ± 0.002 μM |
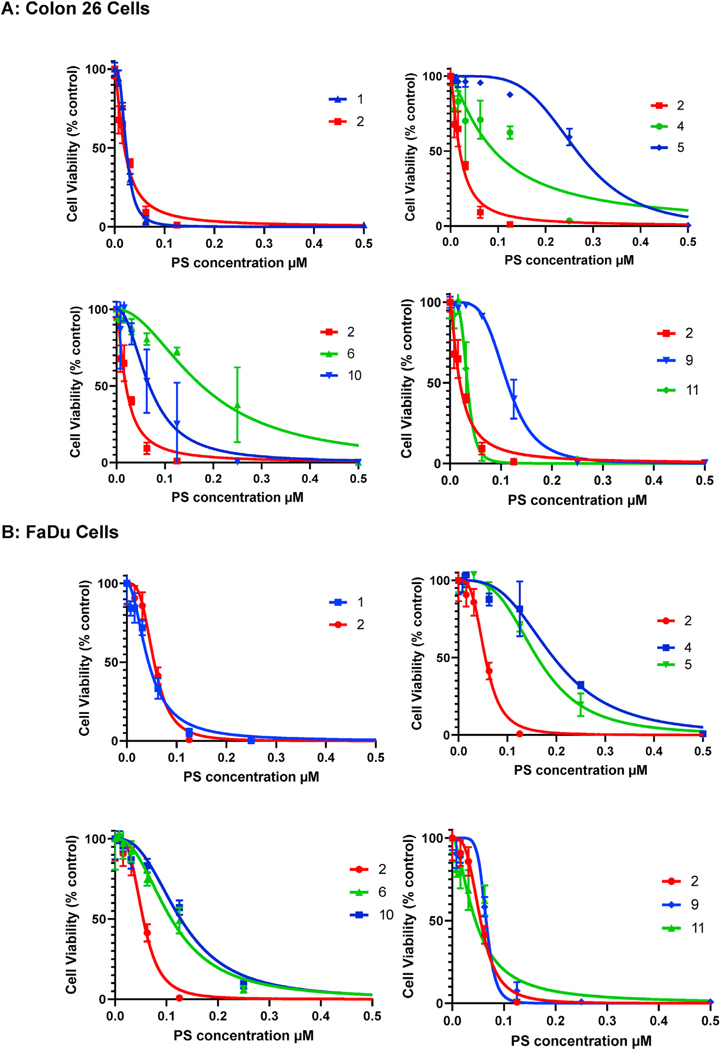
The PDT efficacy of galactose analogs varied depending on tumor cell type, but compound 2 in which the galactose moiety conjugated at position 172 of HPPH and the di-galactose analog of mesopyropheophorbide-a 11 showed significant efficacy (percentage cell survival) in both cell types. The efficacy of PS 11 was reduced on replacing the ethyl group at position 3 with 3 (1′-hexyloxy)ethyl substituent. The in vitro efficacy (percentage cell survival) results of PS 2–6 and 9–11 in both FaDu and Colon26 cell lines are shown in Fig. 3.
Fig. 3.
The in vitro photosensitizing efficacy (MTT assay) of PS-carbohydrate conjugates in FaDu and Colon 26 tumor cells under similar treatment parameters. The PDT efficacy pf PSs was compared at variable concentrations and single light dose: 1 J/cm2 at 665 +/5 nm. Among the compounds evaluated, PSs 2 and 11 were almost equally effective in both cell line.
2.2.2. A difference in mitochondrial and lysosomal localization of HPPH and the corresponding carbohydrate analogs
The importance of subcellular localization of a photosensitizer and its impact on the destruction of tumor cells by reactive oxygen species (mainly singlet oxygen) produced after exposing the tumors/cells with an appropriate wavelength of light has been a subject of discussion from a long time [35,36]. However, it is now well accepted that most of the porphyrin-based mainly localize in mitochondria and lysosomes [37]. Depending on the type of PS, both diffusion and endocytosis play an important role in cell-uptake. It is now also well accepted that apoptosis [38] and necrosis [39] are the two main mechanisms of cell death. The in vivo studies have suggested that vascular shutdown play crucial role in reducing the survivability of tumor cells as it starves them to nutrients and oxygen [40]. However, direct cell cytotoxicity has not been ruled [41] out in less extent, and the overall mechanism of cell death by PDT is complex. In any event PSs localize either in mitochondria or lysosomes (or both) are reported to be effective, and the tumor specificity seems to depend on over all lipophilicity, charge and the nature of substituents present at peripheral position of the PDT agent.
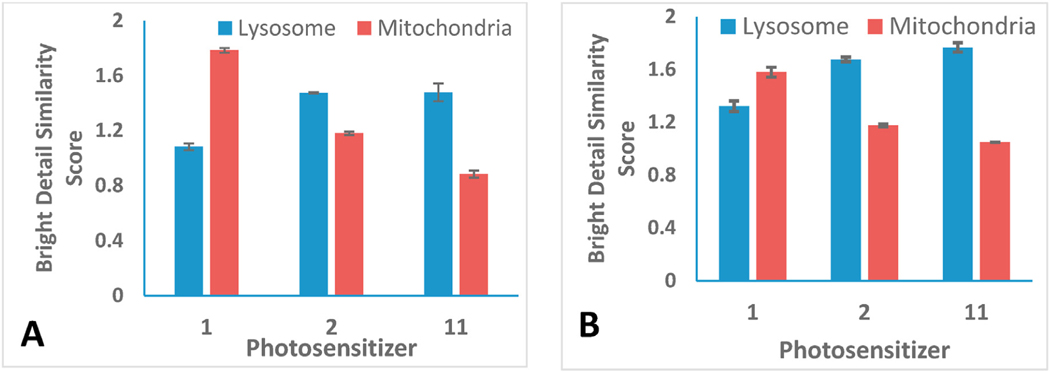
In our present study, we compared the subcellular localization characteristics of HPPH 1, with its mono- and di-galactose conjugates 2 and 11 respectively. Cells were plated in 6 well plate and allowed to adhere before 1 μM of PS 1 was added. After 24 h, Lysotracker Green DND-26 and Mitotracker Red CmxRos were added to enable fluorescent labeling of the lysosome or mitochondria, respectively. The images were analyzed using Amnis IDEAS v6.2 where the bright detail similarity score was generated by comparing co-localization between the photosensitizer and the organelle Results depicted in Fig. 4 indicate that PS 2 (non-galactose analog) shows the preference to mitochondria over the lysosome, whereas the mono- and di-galactose analogs show preference to lysosome with almost similar localization pattern.
Fig. 4.
Localization of Photosensitizer 1, 2 and 11 in FaDu (A) and Colon 26 cells (B). Photosensitizer (I μM) was added to cells with mitotracker red or lysotracker Green then imaged using Imagestream MkII. In both cell lines, the addition of a galactose moiety induces enhanced uptake in subcellular localization from mitochondria to lysosome. For images of cells labeled with Lysotracker GreenDND-26, Mitotracker Red CmxRos and the PSs (HPPH, 2 &11), see Figs. S43–S45 (Supplementary Material Information).
2.2.3. Comparative tumor uptake and PDT efficacy PSs 1, 2 and 11
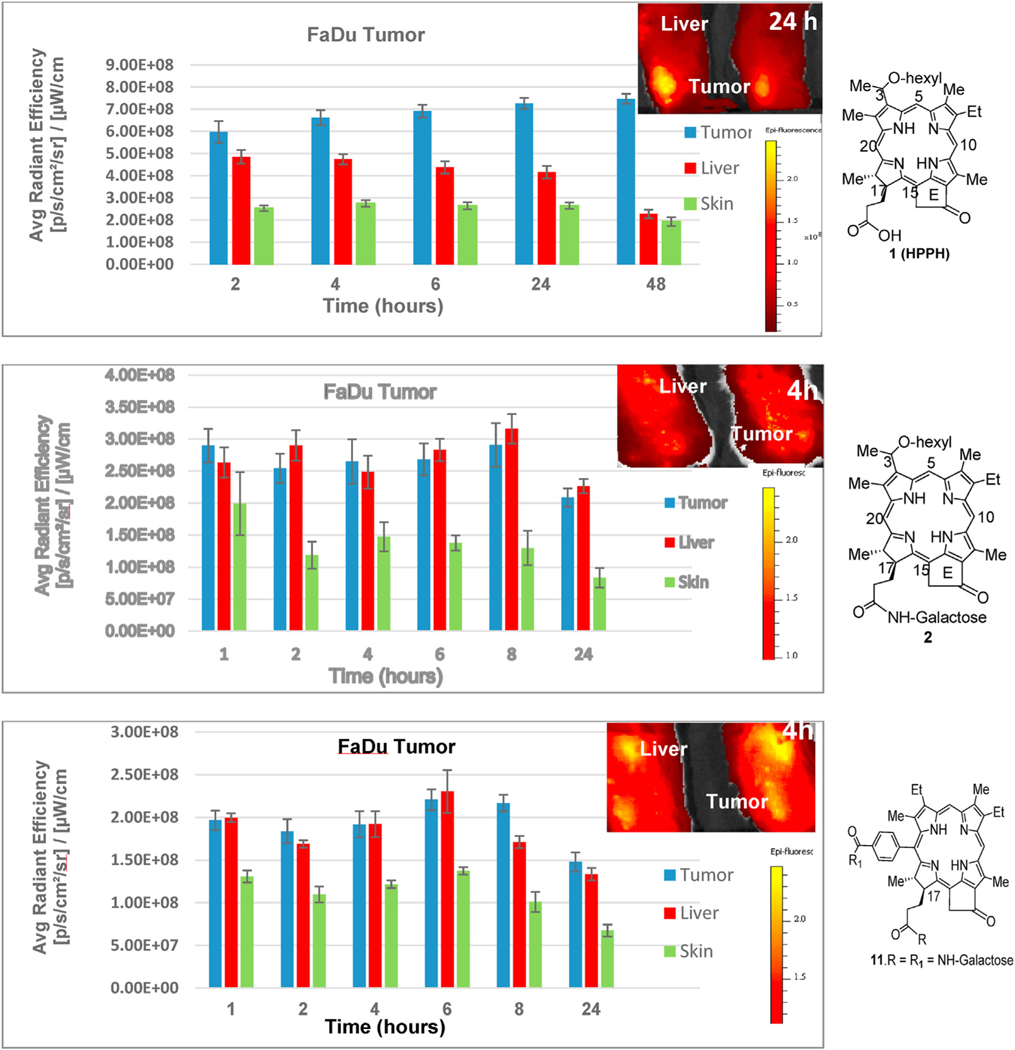
We have previously shown that conjugation of β-galactose at position 172- HPPH enhances PDT efficacy of mice bearing BALB/c tumors. In present study, the tumor-uptake and PDT efficacy of HPPH 1 was compared with the corresponding mono- and di-b-galactose conjugates 2 and 11 (at a dose of 0.47 mmol/kg) in SCID mice bearing FaDu tumors. and imaged at various time points using IVIS Spectrum. The fluorescence intensity of tumor, liver and skin was measured at variable time points and the results are shown in Fig. 5. Interestingly, both β-galactose conjugated PSs in SCID mice bearing FaDu tumors showed similar pattern of uptake in tumor, liver and skin from 1 to 8 h post-injection. However, compared to 2, the uptake of PS 11 in tumor was slightly higher at 8 h. A significant clearance from tumor, liver and skin at 24 h post-injection was observed by both the PSs. In contrast to carbohydrate analogs, the uptake and biodistribution characteristics of HPPH was significantly different. For example, the tumor-uptake increased with time, and maximum uptake was observed at 24 h post injection, with a significant clearance from both liver and skin. The high uptake of mono and di-galactose analogs in liver could be due to hepatic uptake and metabolism of galactose moiety (see Fig. 6).
Fig. 5.
PS uptake: SCID mice bearing FaDu tumors (3 mice/group) were injected with PS 1 (HPPH), 2 and 11 at a dose of 0.47 μmol/kg. The tumor vs liver and skin (adjacent to tumor) uptake were measured by fluorescence at variable timepoints using IVIS spectrum (excitation wavelength: 665 nm, emission: > 720 nm). Tumor vs liver and skin uptake was determined based on the fluorescence intensity measured by IVIS Spectrum, and was plotted against time. Tumor Images with PS 1, 2 & 3 (2/3 mice/group) are shown at the time of their optimal uptake in tumors.
Fig. 6.
Comparative In vivo anti-cancer activity of (A): HPPH 1, (B) PS 2 and (C): PS11 in SCID mice bearing FaDu tumors (head & neck) under similar PS dose: 0.47 μmol/kg, and light dose (665 nm, 135 J/cm2, 75 mW/cm2). In case of PS1 injected mice, the tumors were exposed to light at 24 h and for PSs 2 and 11 at 8 h post-injection of the PS (the timepoint of maximal PS uptake). Tumor growth was measured. Among the compounds, PS 11 (di-β-galactose analog) showed the best anticancer activity. Note: None of the mice after the PDT treatment. The mice with tumor regrowth (>400 mm3) were euthanized following the IACUC approved animal protocol.
2.2.4. Comparative in vivo PDT efficacy of HPPH (1) with mono- and di-galactose conjugates (2 and 11)
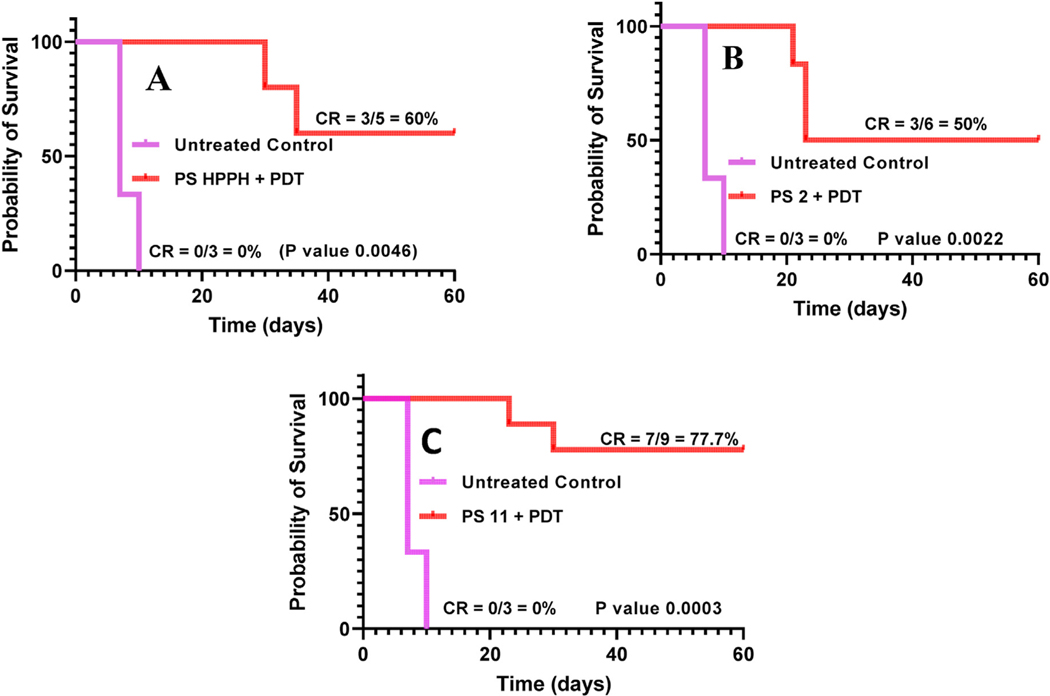
The SCID mice bearing FaDu (Head & Neck tumors) were selected to study the antitumor activity of PSs 1, 2 and 11. When the tumors were of treatment size (4–5 mm in diameter), the mice were injected with PS formulated in 1% Tween 80/5% dextrose solution at a dose of 0.47 μmol/kg. The tumors were exposed to light (665 nm, 135 J/cm2, 75 mW/cm2) at 24 h post-injection for PS 1 and 8 h post-injection for the galactose analogs 2 and 11 (due to their optimal tumor uptake at those time points), and tumor growth was measured daily. The PS 2 and 11 injected intravenously in SCID mice bearing FaDu tumors gave complete response (CR) 50% (3/6 mice were tumor free on day 60) and 77.5% (7/9 mice were tumor free on day 60) respectively on day 60, whereas HPPH, at similar drug and light dose was 60% effective (3/5 mice were tumor free on day 60). The statistical evaluation of the survival curves was analyzed by Mantel-Cox test.
3. Conclusions
In this study, a series of pyropheophorbides containing β-galactose moiety at position-17 [2] and/or position-20(4′) with variable lipophilicity by altering the substituents at position-3 were synthesized. The compounds were characterized by NMR (1H & 13C), and the purity was ascertained by HPLC. These compounds were insoluble in water and formulated in 1% Tween 80/5% dextrose (D5W) solution [27] The in vitro PDT efficacy of the conjugates was determined in two tumor cell lines: FaDu (head & neck) and Colon16. In both cell lines, PS 2 in which galactose moiety was conjugated at position-17 [2] of HPPH and compound 11 containing β-galactose moieties at positions 172 and 20(4′) of meso pyropheophorbide-a showed similar efficacy than the non-galactose analog 1 (HPPH). The PS 1 (HPPH) and the corresponding carbohydrate analogs 2 and 11 showed significant tumor uptake in mice bearing FaDu tumors. However, as expected compared to PS 1, the PSs 2 and 11 showed higher uptake in liver, which could be due to higher hepatic uptake/metabolism of β-galactose. Among the compounds tested for in vivo efficacy, the di- β-galactose conjugate 11 gave the best long-term tumor cure in SCID mice bearing FaDu tumors (7/9 mice = 77.7% tumor free on day 30). The higher efficacy of PS 11 over 2 could be due to (a) the difference in biodistribution of the PS across the tumor, (b) the consequences of PDT (immunological impact) after light treatment, and (c) the role of ABCG 2 pump in efflux of the PS (with and without the galactose moiety. These studies using a larger group of mice are currently underway, and hope will help to address these questions. Compared to PS 1 (HPPH), the carbohydrate analogs 4,5,6, and 10 were significantly less effective than the non-carbohydrate analog 1 in vitro, and were not evaluated for in vivo efficacy.
3.1. Experimental section
3.1.1. Chemistry
Chemicals used for the synthesis were purchased from Aldrich and Synthose and were reagent grade unless otherwise specified. All reactions were carried out in heat gun-dried glassware under an atmosphere of argon and magnetic stirring. Thin layer chromatography (TLC) was performed on pre-coated silica gel sheets (layer thickness 0.2 mm). Column chromatography was performed using silica gel 60 (70–230 mesh) purchased from Merk. Preparative TLC was also used for the purification of compounds (Analtec, 20 × 20 cm, silica gel GF, 1000 μm). Purity of the compounds was ascertained by TLC, NMR and HPLC. All compounds including the intermediates were >95% pure. UV-visible spectra were recorded on an UV-visible spectrophotometer using DCM and MeOH as solvents. Mass spectrometry analyses were performed at the Mass Spectrometry Facility, University of Buffalo, NY.
NMR data were acquired at 28 °C on a Bruker Avance III HD NMR spectrometer equipped with a 9.4 T narrow-bore magnet, a 5-mm BBO Z-gradient probe, and Topspin 3.2 software.
Frequencies for 1H and 13C observations were 400 MHz and 100 MHz, respectively. Chemical shifts were calibrated to the residual solvent resonance and are reported relative to tetramethylsilane (TMS) at 0.00 ppm. 1H multiplicities are reported as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad.
3.1.2. General procedure for the synthesis of compounds 16, 17, and 29 (Suzuki coupling)
To the solution of starting material (1 eq.) and K3PO4 (6 eq.) in 15 ml of dry THF, 4-(tert-butoxycarbonyl)phenylboronic acid pinacol ester (12 eq.) and Pd (PPh3)4 (0.3 eq.) were added. After refluxing overnight under Ar atmosphere, the reaction mixture was cooled to room temperature and filtered through celite to remove excess salts. Solvent was removed under reduced pressure, residue was redissolved in DCM (50 ml), washed with sat. NaHCO3/H2O/Brine (50 ml x 1 each) and dried over Na2SO4. After filtration, solvent was evaporated and resulting crude product was purified by silica column chromatography, using Ethyl Acetate: Hexane (1:4) as eluent for compounds 16 and 17. Compound 29 was purified by three separate runs of preparative TLC using silica plates and the following eluents: (1) 4% MeOH in DCM, (2) 3% MeOH in DCM, and (3) 3.5% MeOH in DCM.
3.1.3. General procedure for deprotection of tert-butyl esters for synthesis of compounds 18, 19, 24, and 30
Starting material (15–25 mg) was stirred in 3 ml of 70% solution of trifluoroacetic acid in DCM for 25 min under Ar atmosphere at room temperature. The reaction mixture was then diluted with 20 ml of DCM, washed with H2O (4 × 20 ml, until wash water pH > 6.5), dried over Na2SO4, and filtered. After removing the solvent, clean product was obtained in quantitative yield.
3.1.4. General procedure for the synthesis of the compounds 20, 21, 26, and 31
Starting material (1 eq.), 1-β-amino-galactose tetraacetate (2.5 eq.) and benzotriazol-1-yloxytris(dimethylamino) phosphonium hexafluorophosphate (2.5 eq.) were dissolved in dry DMF (7 ml) under Ar atmosphere. To this solution, triethylamine (2.5 eq.) was added, and the reaction mixture was stirred overnight (for compounds 26 and 31 for 32 h) at room temperature. After this, 20 ml of DCM was added to the reaction mixture, and it was washed with H2O (3 × 20 ml) and dried over Na2SO4. After filtration, the solvent was removed under reduced pressure. The resulting residue was purified by three separate runs of preparative TLC using silica plates and the following eluents: (1) 4% MeOH in DCM, and (2 & 3) 3% MeOH in DCM.
3.1.5. General procedure for the synthesis of the compounds 5, 10, 22, and 25
Starting material (1 eq.) was dissolved in 10–20 ml of a degassed (3 times, vacuum – Ar) mixture of MeOH and THF (1:1). LiOH monohydrate (15–25 eq.) was dissolved in 5–10 ml of H2O and degassed (3 times, vacuum – Ar). Then, the solution of LiOH was added to the solution of starting material and the reaction mixture was degassed (5 times, vacuum – Ar) and stirred overnight at room temperature. The reaction mixture was then neutralized with a cold (0 °C) 5% aqueous solution of CH3COOH. The product was extracted from the reaction mixture with DCM (50 ml). DCM solution was washed with H2O (5 × 50 ml). The organic layer was then dried over Na2SO4, filtered, and solvent was removed under reduced pressure. Yield was quantitative for compounds 22 and 25. Compounds 5 and 10, however, required purification by silica column chromatography, using gradient 30–90% MeOH in DCM as eluent.
3.1.6. General procedure for the synthesis of the compounds 23 and 28
Starting material (1 eq.), 1-β-amino-galactose tetraacetate (2 eq.), 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (2 eq.), and 4-Dimethylaminopyridine (2.2 eq.) were dissolved in dry DCM (15 ml) and stirred overnight under Ar atmosphere. After this, the reaction mixture was diluted with DCM (15 ml) and washed with H2O (3 × 30 ml). Then, the organic layer was dried over Na2SO4, filtered, and the solvent was evaporated under reduced pressure. The product was purified by three separate runs of preparative TLC using silica plates and the following eluents: (1) 3% MeOH in DCM, (2) 4% MeOH in DCM, and (3) 3% MeOH in DCM.
3.1.7. General procedure for the deacetylation of galactose moieties for obtaining compounds 4, 6, 9, and 11
Starting material (1 eq.) was dissolved in a mixture of dry DCM (10 ml) and MeOH (1.0 ml). Sodium methoxide in MeOH (0.5 M, 250 μL) was added to this solution, and the reaction mixture was stirred for 25 min under Ar atmosphere at room temperature. It was then neutralized with a solution of 5% acetic acid in DCM and diluted with DCM to 50 ml total volume. The reaction mixture was then washed with H2O (3 × 50 ml), dried over Na2SO4, filtered, and solvents were evaporated under vacuum. The crude product was purified by silica column chromatography, using gradient 35–95% MeOH in DCM as eluent.
Compound 4.
14 mg of starting photosensitizer 30 were used for reaction by following the method described above, and the title compound was obtained in 50% yield (6 mg). UV-Vis λmax (MeOH): 671 (rel. intensity 0.149), 615 (0.026), 548 (0.044), 513 (0.037), 412 (0.319); 1H NMR (400 MHz, CD3OD, δ ppm): 10.07/10.03 (1H, s, PS 5-H), 9.15/9.01 (1H, s, 10-H), 8.41 (1H, dd, J = 7.8, 1.7 Hz, 203a-H), 8.25 (1H, dd, J = 7.8, 1.7 Hz, 203b-H), 8.18 (1H, dd, J = 7.8, 1.7 Hz, 202a-H), 7.66/7.65 (1H, dd, J = 7.8, 1.7 Hz, 202b-H), 5.85/5.84 (1H, q, J = 6.8 Hz, 31-H), 5.12 (1H, d, J = 20.1 Hz, 132-CHH), 5.11/5.08 (1H, d, J = 20.1 Hz, 132-CHH), ~4.81 (1H, d, J ~8 Hz, Gal 1-H), 4.34/4.33 (1H, q, J = 7.2 Hz, 18-H), 4.10 (1H, m, 17-H), 3.834/3.832 (1H, dd, J = 1.1, ~3 Hz, Gal 4-H), ~3.72/3.59 (1H, m, −OCHH(CH2)4CH3), ~3.56–3.69 (2H, m, Gal 6-CH2), ~3.65/3.38 (1H, m, −OCHH(CH2)4CH3), ~3.57 (1H, m, Gal 5-H), ~3.48/3.40 (2H, m, 8-CH2CH3), ~3.45 (1H, m, Gal 3-H), ~3.43 (1H, m, Gal 2-H), ~3.32/3.25 (3H, s, 12-CH3), 3.19/3.16 (3H, s, 7-CH3), ~2.48 (1H, m, 17-CH2CHH−), 2.40/2.39 (3H, s, 2-CH3), 2.33 (1H, m, 17-CHHCH2−), 2.17/1.99 (3H, d, J = 6.7 Hz, 31-CH3), ~2.01 (1H, m, 17-CHHCH2−), ~1.96 (1H, m, 17-CH2CHH−), ~1.72/1.63 (1H, m, −OCH2CHH(CH2)3CH3), ~1.72/1.53 (1H, m, −OCH2CHH(CH2)3CH3), 1.58/1.53 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.42/1.34 (1H, m, −O(CH2)2CHH(CH2)2CH3), ~1.42/1.23 (1H, m, −O(CH2)2CHH(CH2)2CH3), ~1.17/1.06 (2H, m, −O(CH2)4CH2CH3), ~1.17/1.03 (2H, m, −O(CH2)3CH2CH2CH3), 1.08/1.05 (3H, d, J = 7.1 Hz, 18-CH3), 0.69/0.56 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3; 13C NMR (100 MHz, CD3OD, δ ppm): 198.63/198.61/198.59/198.57#, 176.4/176.3, 175.1, 174.1, 162.2/162.1, 155.44/155.36, 152.9/152.7, 149.22/149.18/149.15#, 146.0/145.9, 144.11/144.09, 143.0/142.7, 140.8/140.7, 139.6/139.5, 139.23/139.21, 137.7/137.6, 135.23/135.14, 135.21/134.77, 134.90/134.66, 133.0/132.9, 131.33/131.30/131.27/131.24#, 130.5, 129.9, 129.3/129.2, 113.5, 107.29/107.27/107.24/107.21#, 104.4/104.3, 99.8/99.7, 81.6/81.5, 78.2, 75.8/75.7, 74.2/74.1, 71.2, 70.6/70.3, 70.4, 62.5, 53.2, 49.6*, ~48.9*, 33.7/33.6, 32.8/32.6, 31.3/31.2, 31.12/31.05, 27.2/27.1, 25.3/24.9, 23.6/23.5, 21.4, 20.0/19.9, 17.65/17.61, 14.2/14.1, 14.2/14.0, 11.61/11.56, 11.31/11.28. # See Results & Discussion. * From HSQC. HRMS (ESI) calculated for C52H64N5O10[MH+] 918.4653, found 918.47026. HPLC retention time: 4.15 min (see Supporting Information, Fig. S43, purity >96.0%).
Compound 5.
16 mg of starting photosensitizer 20 were used for reaction by following the method described above, and the title compound was obtained in 61% yield (8 mg). UV-Vis λmax (MeOH): 670 (rel. intensity 0.252), 613 (0.045), 549 (0.076), 515 (0.049), 413 (0.541); 1H NMR (400 MHz, CD3OD, δ ppm): 10.13/10.08 (1H, s, PS 5-H), 9.03 (1H, br s, 10-H), 8.38 (1H, dd, J = 7.9, 1.9 Hz, 203a-H), 8.19 (2H, m, 202a-H & 203b-H), 7.74 (1H, m, 202b-H), 5.85/5.84 (1H, q, J = 6.7 Hz, 31-H), 5.280/5.277 (1H, d, J = 9.1 Hz, Gal 1-H), 4.95 (1H, d, J = 20.2 Hz, 132-CHH), ~4.80* (1H, m, 132-CHH), 4.11/4.09 (1H, q, J = 7.2 Hz, 18-H), 4.01 (1H, d, J = 3.2 Hz, Gal 4-H), 3.92 (1H, t, J = 9.3 Hz, Gal 2-H), ~3.77–3.88 (2H, m, Gal 6-CH2), ~3.87 (1H, m, 17-H), ~3.79 (1H, m, Gal 5-H), ~3.61–3.74/3.59,3.43 (2H, m, −OCH2(CH2)4CH3), ~3.69 (1H, m, Gal 3-H), ~3.46 (2H, m, 8-CH2CH3), 3.23/3.21 (3H, s, 7-CH3), ~3.18/3.16 (3H, br s, 12-CH3), 2.39/2.38 (3H, s, 2-CH3), ~2.26 (1H, m, 17-CH2CHH−), 2.14/2.02 (3H, d, J = 6.7 Hz, 31-CH3), ~1.84 (2H, m, 17-CHHCHH−), 1.64–1.78/1.49–1.70 (2H, m, −OCH2CH2(CH2)3CH3), 1.56 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.47 (1H, m, 17-CHHCH2−), ~1.30–1.48/1.20–1.40 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.16/1.04 (4H, m, −O(CH2)3(CH2)2CH3), 0.89 (3H, m, 18-CH3), 0.68/0.58 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3); 13C NMR (100 MHz, CD3OD, δ ppm): 198.3/198.2, 177.8, 173.22/173.20, 170.8, 162.7/162.2, 155.27/155.26, 152.9, 149.0, 146.18/146.02, 146.01, 143.2/142.9, 140.4, 139.6, 137.7, 135.8, 135.2, 134.7, 134.1/134.0, 133.7, 131.3, 129.6, 128.8, 128.47/128.45, 112.53/112.50, 107.1, 104.5, 100.1, 82.5, 78.6, 75.9, 74.3/74.1, 71.3, 70.7, 70.6/70.4, 62.7, 53.38/53.36, 49.4*, 49.1*, 32.8, 32.7, 31.3/31.2, 30.8, 27.2/27.1, 25.2/24.9, 23.6/23.5, 21.3, 19.98/19.96, 17.7, 14.5/14.1, 14.23/14.19, 11.49/11.48, 11.34/11.31. * From HSQC. HRMS (ESI) calculated for C52H64N5O10 [MH+] 918.4653, found 918.4704. HPLC retention time: 4.69 min (see Supporting Information, Fig. S43, purity >97.3%).
Compound 6.
13 mg of starting photosensitizer 31 were used for reaction by following the method described above, and the title compound was obtained in 50% yield (5 mg). UV-Vis λmax (MeOH): 671 (rel. intensity 0.107), 616 (0.020), 547 (0.034), 515 (0.024), 412 (0.237); 1H NMR (400 MHz, 95:5 CD3OD/CDCl3, δ ppm): 10.11/10.07 (1H, s, PS 5-H), 9.49/9.48 (1H, s, 10-H), 8.38 (1H, d, J = 7.9 Hz, 203a-H), 8.34/8.33 (1H, dd, J = 7.9, 1.5 Hz, 202a-H), 8.204/8.198 (1H, dd, J = 7.9, ~1.6 Hz, 203b-H), 7.78/7.76 (1H, dd, J = 8.0, 1.6 Hz, 202b-H), 5.84/5.83 (1H, q, J = 6.8 Hz, 31-H), 5.27 (1H, d, J = 9.1 Hz, 20-Gal 1-H), 5.18 (2H, m, 132-CH2), ~4.81* (1H, d, J ~ 9 Hz, 17-Gal 1-H), 4.304/4.300 (1H, q, J = 7.1 Hz, 18-H), 4.13 (1H, m, 17-H), 4.01 (1H, d, J ~ 3.4 Hz, 20-Gal 4-H), 3.89 (1H, t, J = 9.2 Hz, 20-Gal 2-H), ~3.84 (1H, m, 17-Gal 4-H), 3.76–3.86 (2H, m, 20-Gal 6-CH2), ~3.78 (1H, m, 20-Gal 5-H), ~3.62–3.72/3.57,3.38 (2H, m, −OCH2(CH2)4CH3), ~3.69 (1H, dd, J = 9.4, 3.4 Hz, 20-Gal 3-H), ~3.67 (2H, m, 8-CH2CH3), 3.56–3.69 (2H, m, 17-Gal 6-CH2), 3.564/3.560 (3H, s, 12-CH3), ~3.56 (1H, m, 17-Gal 5-H), ~3.48 (1H, m, 17-Gal 3-H), ~3.41 (1H, m, 17-Gal 2-H), 3.244/3.242 (3H, s, 7-CH3), ~2.49 (1H, m, 17-CH2CHH−), ~2.38 (1H, m, 17-CHHCH2−), 2.38/2.37 (3H, s, 2-CH3), ~2.20 (1H, m, 17-CHHCH2−), 2.14/1.98 (3H, d, J = 6.7 Hz, 31-CH3), ~2.05 (1H, m, 17-CH2CHH−), 1.68 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.71/1.60 (2H, m, −OCH2CH2(CH2)3CH3), ~1.42/1.33 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.18/1.06 (4H, m, −O(CH2)3(CH2)2CH3), 1.03/1.02 (3H, d, J = 7.0 Hz, 18-CH3), 0.71/0.59 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3); 13C NMR (100 MHz, 95:5 CD3OD/CDCl3, δ ppm): 198.6, 176.2, 173.3*, 170.6, 162.0*, 155.4, 153.0, 149.4*, 146.1, 146.0, 143.0/142.7*, 140.5*, 139.8, 137.7*, 135.7*, 134.9*, 134.6*, 134.0*, 133.7*, 131.4*, 129.77/129.74, 128.9, 128.3, 112.4, 107.33/107.32, 104.81/104.78, 100.0, 82.3, 81.4, 78.4, 78.0, 75.8, 75.6, 74.0, 71.2, 71.1, 70.6/70.3, 70.5, 70.2, 62.6, 62.4, 53.3, 49.4*, 49.3*, 33.9, 32.7/32.6, 31.2/31.1, 30.9*, 27.1/27.0, 25.2/24.9, 23.5/23.4, 21.3, 20.1, 17.7, ~14.2, 14.2/14.1, 11.9, 11.4. * From HSQC or HMBC. HRMS (ESI) calculated for C58H75N6O14 [MH+] 1079.5341, found 1079.5372. HPLC retention time: 3.03 min (see Supporting Information, Fig. S43, purity >96.0%).
Compound 9.
14 mg of starting photosensitizer 24 were used for reaction by following the method described above, and the title compound was obtained in 43% yield (5 mg). UV-Vis λmax (MeOH): 665 (rel. intensity 0.105), 608 (0.020), 546 (0.032), 512 (0.026), 412 (0.245); 1H NMR (400 MHz, CD3OD, δ ppm): 9.43 (1H, s, 10-H), 9.30 (1H, s, 5-H), 8.41 (1H, dd, J = 7.9, 1.7 Hz, 203a-H), 8.25 (1H, dd, J = 7.8, 1.7 Hz, 203b-H), 8.20 (1H, dd, J = 7.9, 1.7 Hz, 202a-H), 7.67 (1H, dd, J = 7.8, 1.7 Hz, 202b-H), 5.20, 5.17 (2H, ABq, JAB = 20.3 Hz, 132-CH2), 4.81* (1H, Gal 1-H), 4.33 (1H, q, J = 7.1 Hz, 18-H), 4.15 (1H, dd, J ~ 8.8, 2.9 Hz, 17-H), 3.83 (1H, d, J ~ 2.7 Hz, Gal 4-H), 3.79 (2H, q, J = 7.7 Hz, −CH2CH3), 3.59–3.69 (4H, m, Gal 6-CH2 & −CH2CH3), 3.56 (3H, s, 12-CH3), ~3.55 (1H, m, Gal 5-H), 3.39–3.47 (2H, m, Gal 2-H & Gal 3-H), 3.20 (3H, s, 7-CH3), ~2.50, ~2.43, ~2.14, ~2.03 (each 1H, 4 × m, 17-CH2CH2−), 2.32 (3H, s, 2-CH3), 1.66 (3H, t, J = 7.6 Hz, −CH2CH3), 1.65 (3H, t, J = 7.6 Hz, −CH2CH3), 1.09 (3H, d, J = 7.0 Hz, 18-CH3); 13C {1H} NMR (100 MHz, CD3OD, δ ppm): 198.7, 176.4, 174.8, 174.5, 161.8, 155.7, 152.5, 149.4, 146.1, 145.3, 144.3, 141.8, 139.4, 137.3, 136.6, 135.1, 133.5, 133.0, 131.2, 130.9, 130.5, 129.8, 129.0, 113.3, 107.3, 104.6, 97.2, 81.5, 78.2, 75.8, 71.2, 70.4, 62.5, 53.1, 49.6*, 49.2*, 36.5, 33.8, 21.4, 20.1, 20.0, 17.7, 17.1, 14.2, 11.7, 11.0. * From HSQC. MS (ESI) calculated for C46H52N5O9[MH+] 818.3765, found 818.3756. HPLC retention time: 6.17 min (see Supporting Information, Fig. S44, purity >97.1%).
Compound 10.
14 mg of starting photosensitizer 21 were used for reaction by following the method described above, and the title compound was obtained in 52% yield (6 mg). UV-Vis λmax (MeOH): 667 (rel. intensity 0.101), 612 (0.019), 546 (0.029), 511 (0.020), 412 (0.230); 1H NMR (400 MHz, CD3OD, δ ppm): 9.22 (1H, s, PS 5-H), 9.13 (1H, s, 10-H), 8.37 (1H, dd, J = 7.9, 1.9 Hz, 203a-H), 8.23 (1H, dd, J = 7.9, 1.6 Hz, 202a-H), 8.17 (1H, dd, J = 7.9, 1.9 Hz, 203b-H), 7.69 (1H, dd, J = 7.9, 1.6 Hz, 202b-H), 5.27 (1H, d, J = 9.1 Hz, Gal 1-H), 5.04 (2H, m, 132-CH2), 4.19 (1H, q, J = 7.1 Hz, 18-H), 4.01 (1H, d, J = 3.2 Hz, Gal 4-H), 3.97 (1H, dd, J ~ 9.4, 3.2 Hz, 17-H), 3.93 (1H, t, J = 9.3 Hz, Gal 2-H), 3.76–3.88 (2H, m, Gal 6-CH2), 3.78 (1H, m, Gal 5-H), 3.74 (2H, q, J = 7.7 Hz, 3-CH2CH3), 3.69 (1H, dd, J = 9.6, 3.4 Hz, Gal 3-H), 3.44 (2H, q, J = 7.7 Hz, 8-CH2CH3), 3.37 (3H, s, 12-CH3), 3.12 (3H, s, 7-CH3), ~2.34 (1H, m, 17-CH2CHH−), 2.29 (3H, s, 2-CH3), ~2.11 (1H, m, 17-CHHCH2−), ~1.96 (1H, m, 17- CH2CHH−), ~1.79 (1H, m, 17-CHHCH2−), 1.64 (3H, t, J = 7.6 Hz, 3-CH2CH3), 1.57 (3H, t, J = 7.6 Hz, 8-CH2CH3), 0.94 (3H, d, J = 7.1 Hz, 18-CH3); 13C {1H} NMR (100 MHz, CD3OD, δ ppm): 198.6, 179.8, 173.9, 170.8, 162.4, 155.6, 152.5, 149.4, 146.2, 146.1, 145.4, 141.5, 139.4, 137.3, 136.4, 135.8, 135.1, 133.8, 132.9, 131.2, 129.2, 128.7, 128.4, 112.3, 107.2, 104.7, 97.5, 82.5, 78.6, 75.9, 71.3, 70.7, 62.7, 53.7, 49.6*, 49.2*, 34.8, 32.0, 21.3, 20.1, 20.0, 17.7, 17.2, 14.4, 11.7, 11.0. * From HSQC. HRMS (ESI) calculated for C46H52N5O9 [MH+] 818.3765, found 818.3753. HPLC retention time: 4.18 min (see Supporting Information, Fig. S44, purity >95.4%).
Compound 11.
12 mg of starting photosensitizer 26 were used for reaction by following the method described above, and the title compound was obtained in 46% yield (5 mg). UV-Vis λmax (MeOH): 667 (rel. intensity 0.097), 610 (0.012), 545 (0.024), 514 (0.014), 412 (0.224); 1H NMR (400 MHz, 99:1 CD3OD/CDCl3, δ ppm): 9.27 (1H, s, 10-H), 9.20 (1H, s, PS 5-H), 8.36 (1H, dd, J = 7.9, 1.9 Hz, 203a-H), 8.25 (1H, dd, J = 7.9, 1.6 Hz, 202a-H), 8.17 (1H, dd, J = 7.9, 1.9 Hz, 203b-H), 7.69 (1H, dd, J = 7.9, 1.6 Hz, 202b-H), 5.28 (1H, d, J = 9.1 Hz, 20-Gal 1-H), ~5.1 (2H, m, 132-CH2), ~4.82 (1H, d, J ~ 9 Hz, 17-Gal 1-H), 4.20 (1H, q, J = 7.0 Hz, 18-H), 4.04 (1H, dd, J ~ 8.9, 3.7 Hz, 17-H), 4.01 (1H, d, J = 3.3 Hz, 20-Gal 4-H), 3.90 (1H, t, J = 9.3 Hz, 20-Gal 2-H), ~3.85 (1H, m, 17-Gal 4-H), ~3.76–3.87 (2H, m, 20-Gal 6-CH2), ~3.78 (1H, m, 20-Gal 5-H), ~3.71 (2H, m, 3-CH2CH3), ~3.69 (1H, m, 20-Gal 3-H), ~3.58–3.70 (2H, m, 17-Gal 6-CH2), ~3.57 (1H, m, 17-Gal 5-H), ~3.54 (2H, m, 8-CH2CH3), 3.49 (1H, dd, J = 9.5, 3.2 Hz, 17-Gal 3-H), 3.47 (3H, s, 12-CH3), 3.43 (1H, dd, J ~ 9.4, 8.9 Hz, 17-Gal 2-H), 3.13 (3H, s, 7-CH3), ~2.44 (1H, m, 17-CH2CHH−), ~2.31 (1H, m, 17-CHHCH2−), 2.27 (3H, s, 2-CH3), ~2.07 (1H, m, 17-CHHCH2−), ~2.03 (1H, m, 17-CH2CHH), 1.61 (6H, t, J = 7.5 Hz, 3-CH2CH3& 8-CH2CH3), 0.97 (3H, d, J = 7.0 Hz, 18-CH3); 13C {1H} NMR (100 MHz, 99:1 CD3OD/CDCl3, δ ppm): 198.6, 176.2, 173.7, 170.6, 161.6, 155.6, 152.4, 149.4, 146.1, 146.0, 145.3, 141.5, 139.4, 137.2, 136.3, 135.6, 134.9, 133.7, 132.8, 131.2, 129.2, 128.8, 128.2, 112.1, 107.0, 104.7, 97.4, 82.3, 81.4, 78.4, 77.9, 75.8, 75.6, 71.2, 71.0, 70.5, 70.3, 62.6, 62.4, 53.2, ~49.4*, ~48.8*, 33.9, 30.9, 21.3, 20.0, 19.9, 17.7, 17.1, 14.3, 11.8, 11.1. * From HSQC. HRMS (ESI) calculated for C52H63N6O13[MH+] 979.4453, found 979.4444. HPLC retention time: 2.29 min (see Supporting Information, Fig. S44, purity >96.9%)
Compound 16.
40 mg of starting photosensitizer 14 were used for reaction by following the method described above, and the title compound was obtained in 69% yield (31 mg). UV-Vis λmax (CH2Cl2): 670 (rel. intensity 0.303), 615 (0.050), 550 (0.099), 514 (0.059), 417 (0.728); 1H NMR (400 MHz, CDCl3, δ ppm): 10.17/10.12 (1H, s, 5-H), 9.554/9.547 (1H, s, 10-H), 8.41 (1H, dd, J = 7.9, 1.8 Hz, 203a-H), 8.26/8.25 (1H, dd, J = 7.9, 1.8 Hz, 203b-H), 8.21/8.20 (1H, dd, J = 7.9, ~1.8 Hz, 202a-H), 7.73/7.71 (1H, dd, J = 7.9, 1.7 Hz, 202b -H), 5.830/5.825 (1H, q, J = 6.7 Hz, 31-H), 5.22 (2H, m, 132-CH2), 4.28/4.26 (1H, q, J = 7.1 Hz, 18-H), 4.11 (1H, dd, J = 8.4, 3.3 Hz, 17-H), 3.739/3.736 (2H, q, J = 7.7 Hz, 8-CH2CH3), 3.703/3.698 (3H, s, 12-CH3), 3.67@ (1H, t, J = 6.7 Hz, −OCH2(CH2)4CH3), 3.591/3.587 (3H, s, −COOCH3), 3.55@ (0.5H, dt, J = 9.0, 6.5 Hz, −OCHH(CH2)4CH3), 3.45@ (0.5H, dt, J = 9.0, 6.9 Hz, −OCHH(CH2)4CH3), 3.310/3.306 (3H, s, 7-CH3), ~2.56 (1H, m, 17-CH2CHH−), ~2.47 (1H, m, 17-CHHCH2−), 2.37/2.36 (3H, s, 2-CH3), ~2.24 (1H, m, 17-CHHCH2−), ~2.22 (1H, m, 17-CH2CHH−), 2.14/2.00 (3H, d, J = 6.7 Hz, 31-CH3), 1.761 (9H, s, −COOC(CH3)3), ~1.76/~1.65 (2H, m, −OCH2CH2(CH2)3CH3), 1.740/1.738 (3H, t, J = 7.7 Hz, 8-CH2CH3), ~1.22–1.48 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.24/~1.16 (4H, m, −O(CH2)3(CH2)2CH3), 1.05/1.04 (3H, d, J = 7.0 Hz, 18-CH3), 0.80/0.73 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.51/−1.57 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.2, 173.4, 171.12/171.09, 165.7, 160.2/160.1, 153.7, 151.6, 148.4, 145.6, 144.6/144.5, 141.8/141.4, 139.25/139.14, 139.14/139.11, 136.83/136.82, 134.5/134.3, 133.9/133.6, 132.7/132.6, 132.2/132.0, 131.7, 131.28/131.26, 129.4, 129.0, 128.9/128.8, 111.0, 106.3, 103.75/103.70, 99.25/99.17, 81.6, 72.9/72.8, 69.7/69.5, 52.24/52.21, 51.6, 48.6, 48.30/48.26, 31.75/31.65, 31.23/31.21, 30.3/30.1, 29.8, 28.3 (3C), 26.1/26.0, 25.0/24.6, 22.6/22.5, 21.0, 19.5, 17.4, 14.1/13.8, 14.0/13.9, 12.1, 11.3. Note: @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C51H63N4O6 [MH+] 827.4748, found 827.4793.
Compound 17.
40 mg of starting photosensitizer 15 were used for reaction by following the method described above, and the title compound was obtained in 71% yield (33 mg). UV-Vis λmax (CH2Cl2): 667 (rel. intensity 0.306), 612 (0.053), 547 (0.082), 516 (0.055), 416 (0.736); 1H NMR (400 MHz, CDCl3, δ ppm): 9.50 (1H, s, 10-H), 9.37 (1H, s, 5-H), 8.39 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.25 (1H, dd, J = 7.8, 1.8 Hz, phenyl H), 8.17 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 7.71 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 5.20, 5.18 (2H, ABq, JAB = 19.9 Hz, 132-CH2), 4.22 (1H, q, J = 7.1 Hz, 18-H), 4.09 (1H, dd, J ~ 8.3, 3.5 Hz, 17-H), 3.79 (2H, q, J = 7.6 Hz, 3-CH2CH3), 3.71 (2H, q, J = 7.7 Hz, 8-CH2CH3), 3.68 (3H, s, 12-CH3), 3.57 (3H, s, −COOCH3), 3.29 (3H, s, 7-CH3), ~2.54 (1H, m, 17-CH2CHH−), ~2.45 (1H, m, 17-CHHCH2−), 2.31 (3H, s, 2-CH3), ~2.14–2.27 (2H, m, 17-CHHCHH−), 1.76 (9H, s, −COOC(CH3)3), 1.72 (3H, t, J = 7.7 Hz, 8-CH2CH3), 1.65 (3H, t, J = 7.6 Hz, 3-CH2CH3), 1.05 (3H, d, J = 7.1 Hz, 18-CH3), −1.45 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.2, 173.4, 171.4, 165.8, 159.8, 153.8, 151.1, 148.5, 145.6, 144.7, 144.0, 140.2, 138.8, 136.1, 135.0, 134.3, 132.1, 131.7, 131.6, 131.0, 129.3, 128.8, 128.6, 110.8, 106.2, 103.9, 96.8, 81.5, 52.1, 51.6, 48.6, 48.3, 31.2, 29.9, 28.3 (3C), 21.1, 19.50, 19.46, 17.4, 16.8, 14.1, 12.1, 11.3. HRMS (ESI) calculated for C45H51N4O5 [MH+] 727.3859, found 727.3869.
Compound 18.
25 mg of starting photosensitizer 16 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (23 mg). UV-Vis λmax (CH2Cl2): 669 (rel. intensity 0.276), 613 (0.039), 548 (0.074), 515 (0.042), 416 (0.687); 1H NMR (400 MHz, CDCl3, δ ppm): 10.18/10.14 (1H, s, 5-H), 9.57/9.56 (1H, s, 10-H), 8.59 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.43/8.42 (1H, dd, J = 7.8, 1.9 Hz, phenyl H), 8.33/8.32 (1H, dd, J = 7.8, ~2.0 Hz, phenyl H), 7.83/7.80 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 5.849/5.846 (1H, q, J = 6.8 Hz, 31-H), 5.24 (2H, m, 132-CH2), 4.31/4.29 (1H, q, J = 7.1 Hz, 18-H), 4.15 (1H, dd, J ~ 8.2, 3.1 Hz, 17-H), 3.74 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.714/3.710 (3H, s, 12-CH3), 3.69@ (1H, t, J = 6.7 Hz, −OCH2(CH2)4CH3), 3.600/3.597 (3H, s, −COOCH3), 3.57@ (0.5H, dt, J = 9.1, 6.4 Hz, −OCHH(CH2)4CH3), 3.47@ (0.5H, dt, J = 9.1, 6.8 Hz, −OCHH(CH2)4CH3), 3.313/3.312 (3H, s, 7-CH3), ~2.57 (1H, m, 17-CH2CHH−), ~2.49 (1H, m, 17-CHHCH2−), 2.40/2.39 (3H, s, 2-CH3), ~2.18–2.34 (2H, m, 17-CHHCHH−), 2.15/2.02 (3H, d, J = 6.7 Hz, 31-CH3), ~1.76/1.66 (2H, m, −OCH2CH2(CH2)3CH3), 1.744/1.742 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.23–1.49 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.24/~1.17 (4H, m, −O(CH2)3(CH2)2CH3), 1.08/1.07 (3H, d, J = 7.1 Hz, 18-CH3), 0.80/0.74 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.49/−1.55 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.2, 173.4, 170.9, 170.8/170.7, 160.2/160.1, 153.8, 151.7, 148.5, 147.2, 144.64/144.63, 142.0/141.6, 139.24/139.22, 139.16/139.04, 136.94/136.92, 134.9/134.7, 133.8/133.5, 132.6/132.5, 132.4/132.3, 131.33/131.32, 130.2, 129.63/129.61, 129.2, 129.0, 110.6, 106.3, 103.91/103.87, 99.5/99.4, 73.0/72.9, 69.8/69.6, 52.31/52.28, 51.7, 48.7, 48.35/48.32, 31.8/31.7, 31.29/31.27, 30.3/30.1, 29.9, 26.1/26.0, 25.0/24.6, 22.6/22.5, 21.02/21.01, 19.5, 17.4, 14.1/13.8, 14.0/13.9, 12.2, 11.4. Note: @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C47H55N4O6 [MH+] 771.4122, found 771.4109.
Compound 19.
25 mg of starting photosensitizer 17 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (23 mg). UV-Vis λmax (CH2Cl2): 668 (rel. intensity 0.346), 614 (0.065), 549 (0.093), 514 (0.062), 417 (0.751); 1H NMR (400 MHz, CDCl3, δ ppm): 9.52 (1H, s, meso H), 9.40 (1H, s, meso H), 8.58 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 8.41 (1H, dd, J = 7.8, 1.7 Hz, phenyl H), 8.28 (1H, dd, J = 7.9, 1.5 Hz, phenyl H), 7.80 (1H, dd, J = 7.9, 1.5 Hz, phenyl H), 5.23, 5.21 (2H, ABq, JAB = 20.0 Hz, 132-CH2), 4.26 (1H, q, J = 7.1 Hz, 18-H), 4.12 (1H, dd, J ~ 8.4, 3.5 Hz, 17-H), 3.82 (2H, q, J = 7.6 Hz, 3-CH2CH3), 3.72 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.69 (3H, s, 12-CH3), 3.59 (3H, s, −COOCH3), 3.30 (3H, s, 7-CH3), ~2.55 (1H, m, 17-CH2CHH−), ~2.48 (1H, m, 17-CHHCH2−), 2.33 (3H, s, 2-CH3), ~2.16–2.32 (2H, m, 17-CHHCHH−), 1.72 (3H, t, J = 7.6 Hz, 8-CH2CH3), 1.67 (3H, t, J = 7.6 Hz, 3-CH2CH3), 1.08 (3H, d, J = 7.1 Hz, 18-CH3), −1.41 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.3, 173.4, 171.1, 170.9, 159.8, 153.8, 151.2, 148.5, 147.2, 144.8, 144.2, 140.2, 138.9, 136.2, 135.0, 134.8, 132.5, 131.3, 131.1, 130.1, 129.6, 128.9, 128.8, 110.5, 106.3, 104.0, 97.1, 52.2, 51.7, 48.6, 48.4, 31.3, 29.9, 21.0, 19.5 (2C), 17.4, 16.9, 14.1, 12.1, 11.3. HRMS (ESI) calculated for C41H43N4O5 [MH+] 671.3233, found 671.3210.
Compound 20.
20 mg of starting photosensitizer 18 were used for reaction by following the method described above, and the title compound was obtained in 57% yield (16 mg). UV-Vis λmax (CH2Cl2): 668 (rel. intensity 0.906), 612 (0.143), 545 (0.269), 513 (0.177), 416 (2.182); 1H NMR (400 MHz, CDCl3, δ ppm): 10.17/10.13 (1H, s, PS 5-H), 9.562/9.555 (1H, s, 10-H), 8.253/8.249 (1H, dd, J = 7.9, ~1.6 Hz, 202a-H), 8.18/8.17 (1H, dd, J = 7.9, 2.1 Hz, 203a-H), 8.04/8.03 (1H, dd, J = 7.9, 2.0 Hz, 203b-H), 7.76/7.73 (1H, dd, J = 7.9, 1.7 Hz, 202b-H), 7.34 (1H, d, J = 9.1 Hz, amide NH), 5.81 (1H, q, J = 6.7 Hz, 31-H), 5.58 (1H, dd, J = 9.2, 8.7 Hz, Gal 1-H), 5.57 (1H, d, J = 3.1 Hz, Gal 4-H), 5.37 (1H, dd, J = 10.4, 8.7 Hz, Gal 2-H), 5.32 (1H, dd, J = 10.3, 3.1 Hz, Gal 3-H), ~5.21 (2H, m, 132-CH2), ~4.18–4.32 (4H, m, 18-H, Gal 5-H & Gal 6-CH2), 4.12 (1H, dd, J = 8.1, 3.3 Hz, 17-H), 3.74 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.703/3.699 (3H, s, 12-CH3), 3.66@ (~1H, t, J = 6.8 Hz, −OCH2(CH2)4CH3), 3.570/3.566 (3H, s, −COOCH3), 3.54@ (~0.5H, dt, J = 9.0, 6.4 Hz, −OCHH(CH2)4CH3), 3.44@ (~0.5H, dt, J = 9.1, 6.8 Hz, −OCHH(CH2)4CH3), 3.299/3.297 (3H, s, 7-CH3), ~2.50 (2H, m, 17-CHHCHH−), 2.32/2.31 (3H, s, 2-CH3), ~2.25 (1H, m, 17-CHHCH2−), 2.220/2.218, 2.20, 2.11, 2.07 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~2.19 (1H, m, 17-CH2CHH−), 2.13/1.99 (3H, d, J = 6.7 Hz, 31-CH3), ~1.74/1.64 (2H, m, −OCH2CH2(CH2)3CH3), 1.734/1.731 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.21–1.47 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.24 (1H, br s, core NH), ~1.23/1.15 (4H, m, −O(CH2)3(CH2)2CH3), 1.03/1.02 (3H, d, J = 7.0 Hz, 18-CH3), 0.79/0.72 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.53/−1.59 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 173.4, 172.17/172.16, 170.8, 170.4, 170.0, 169.8, 167.02/167.00, 160.2/160.0, 153.7, 151.7, 148.4, 145.7, 144.6, 142.0/141.6, 139.21/139.07, 139.19, 136.9, 135.0/134.8, 133.8/133.4, 132.7/132.5, 132.61/132.59, 132.4/132.2, 131.4/131.3, 129.2, 127.18/127.15, 126.7, 110.5, 106.3, 103.9/103.8, 99.43/99.40, 79.5, 72.91/72.86, 72.5, 70.8, 69.8/69.6, 68.8, 67.3, 61.2, 52.3/52.2, 51.6, 48.7, 48.32/48.28, 31.7/31.6, 31.20/31.18, 30.3/30.1, 29.8, 26.1/26.0, 25.0/24.6, 22.6/22.5, 21.05/21.04, 20.98, 20.8, 20.65, 20.62, 19.5, 17.4, 14.03/ 13.74, 13.98/13.90, 12.2, 11.3. @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C61H74N5O14 [MH+] 1100.5232, found 1100.5211.
Compound 21.
20 mg of starting photosensitizer 19 were used for reaction by following the method described above, and the title compound was obtained in 47% yield (14 mg). UV-Vis λmax (CH2Cl2): 669 (rel. intensity 0.643), 614 (0.102), 543 (0.192), 515 (0.126), 415 (1.558); 1H NMR (400 MHz, CDCl3, δ ppm): 9.51 (1H, s, 10-H), 9.38 (1H, s, PS 5-H), 8.23 (1H, dd, J = 7.9, 1.6 Hz, 202a-H), 8.17 (1H, dd, J = 7.9, 1.8 Hz, 203a-H), 8.04 (1H, dd, J = 7.9, 1.8 Hz, 203b-H), 7.75 (1H, dd, J = 7.9, 1.6 Hz, 202b-H), 7.36 (1H, d, J = 9.1 Hz, amide NH), 5.55–5.62 (2H, m, Gal 1-H & Gal 4-H), 5.38 (1H, dd, J = 10.3, 9.0 Hz, Gal 2-H), 5.33 (1H, dd, J = 10.3, 3.2 Hz, Gal 3-H), 5.20, 5.18 (2H, ABq, JAB = 20.0 Hz, 132-CH2), 4.21–4.27 (3H, m, Gal 5-H & Gal 6-CH2), 4.19 (1H, q, J = 7.1 Hz, 18-H), 4.10 (1H, dd, J = 8.4, 3.5 Hz, 17-H), 3.79 (2H, q, J = 7.7 Hz, −CH2CH3), 3.71 (2H, q, J = 7.7 Hz, −CH2CH3), 3.68 (3H, s, 12-CH3), 3.56 (3H, s, −COOCH3), 3.29 (3H, s, 7-CH3), ~2.51 (1H, m, 17-CH2CHH−), ~2.46 (1H, m, 17-CHHCH2−), 2.26 (3H, s, 2-CH3), ~2.23 (1H, m, 17-CHHCH2−), 2.22, 2.21, 2.12, 2.08 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~2.18 (1H, m, 17-CH2CHH−), 1.72 (3H, t, J = 7.6 Hz, −CH2CH3), 1.65 (3H, t, J = 7.6 Hz, −CH2CH3), 1.32 (1H, br s, core NH), 1.04 (3H, d, J = 7.0 Hz, 18-CH3), −1.45 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 173.4, 172.1, 171.1, 170.4, 170.0, 169.8, 167.1, 159.7, 153.8, 151.2, 148.5, 145.8, 144.8, 144.1, 140.2, 138.8, 136.2, 134.94, 134.88, 132.61, 132.56, 131.2, 131.1, 128.8, 127.1, 126.7, 110.3, 106.3, 104.0, 97.0, 79.5, 72.5, 70.8, 68.8, 67.3, 61.2, 52.1, 51.6, 48.6, 48.4, 31.2, 29.9, 21.03, 21.01, 20.75, 20.64, 20.61, 19.49, 19.45, 17.4, 16.9, 14.0, 12.1, 11.3. HRMS (ESI) calculated for C55H60N5O13 [MH+] 1000.4344, found 1000.4349.
Compound 22.
25 mg of starting photosensitizer 17 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (24 mg). UV-Vis λmax (CH2Cl2): 669 (rel. intensity 0.313), 615 (0.047), 546 (0.096), 514 (0.059), 417 (0.724); 1H NMR (400 MHz, CDCl3, δ ppm): 9.47 (1H, s, meso H), 9.34 (1H, s, meso H), 8.38 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 8.23 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.15 (1H, dd, J = 7.9, 1.6 Hz, phenyl H), 7.70 (1H, dd, J = 7.9, 1.6 Hz, phenyl H), 5.19, 5.16 (2H, ABq, JAB = 19.9 Hz, 132-CH2), 4.22 (1H, q, J = 7.1 Hz, 18-H), 4.09 (1H, m, 17-H), 3.77 (2H, q, J = 7.6 Hz, 3-CH2CH3), 3.69 (2H, q, J = 7.7 Hz, 8-CH2CH3), 3.64 (3H, s, 12-CH3), 3.27 (3H, s, 7-CH3), ~2.56 (1H, m, 17-CH2CHH−), ~2.44 (1H, m, 17-CHHCH2−), ~2.12–2.32 (2H, m, 17-CHHCHH−), 2.29 (3H, s, 2-CH3), 1.73 (9H, s, −COOC(CH3)3), 1.70 (3H, t, J = 7.7 Hz, 8-CH2CH3), 1.64 (3H, t, J = 7.6 Hz, 3-CH2CH3), 1.04 (3H, d, J = 7.1 Hz, 18-CH3), −1.44 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.4, 176.9, 171.4, 165.8, 159.6, 153.9, 151.2, 148.5, 145.6, 144.8, 144.1, 140.3, 138.8, 136.1, 135.1, 134.3, 132.1, 131.7, 131.6, 131.0, 129.4, 128.8, 128.6, 110.8, 106.3, 103.9, 96.8, 81.5, 52.0, 48.5, 48.3, 30.8, 29.5, 28.3 (3C), 21.1, 19.5, 19.4, 17.4, 16.8, 14.1, 12.1, 11.3. HRMS (ESI) calculated for C44H49N4O5 [MH+] 713.3703, found 713.3689.
Compound 23.
24 mg of starting photosensitizer 22 were used for reaction by following the method described above, and the title compound was obtained in 43% yield (15 mg). UV-Vis λmax (CH2Cl2): 670 (rel. intensity 0.389), 615 (0.058), 543 (0.119), 514 (0.073), 416 (0.897); 1H NMR (400 MHz, CDCl3, δ ppm): 9.51 (1H, s, 10-H), 9.39 (1H, s, PS 5-H), 8.42 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.24 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.19 (1H, dd, J = 7.9, 1.6 Hz, phenyl-H), 7.70 (1H, dd, J = 7.9, 1.6 Hz, 202b-H), 6.03 (1H, d, J = 9.3 Hz, amide NH), 5.38 (1H, d, J = 3.3 Hz, Gal 4-H), 5.22, 5.19 (2H, ABq, JAB= 19.8 Hz, 132-CH2), 5.16 (1H, t, J = 9.3 Hz, Gal 1-H), 5.07 (1H, dd, J = 10.3, 3.4 Hz, Gal 3-H), 4.94 (1H, dd, J = 10.3, 9.3 Hz, Gal 2-H), 4.22 (1H, q, J = 7.0 Hz, 18-H), 4.13 (1H, dd, J = 8.0, 3.6 Hz, 17-H), 3.94–4.07 (3H, m, Gal 5-H & Gal 6-CH2), 3.80 (2H, q, J = 7.6 Hz, −CH2CH3), 3.72 (2H, q, J = 7.7 Hz, −CH2CH3), 3.67 (3H, s, 12-CH3), 3.29 (3H, s, 7-CH3), ~2.47, ~2.38, ~2.20, ~1.90 (each 1H, 4 × m, 17-CH2CH2−), 2.31 (3H, s, 2-CH3), 2.05, 1.97, 1.95, 1.91 (each 3H, 4 × s, 4 × CH3C(=O)O−), 1.75 (9H, s, −C(=O)OC(CH3)3), 1.72 (3H, t, J = 7.6 Hz, −CH2CH3), 1.65 (3H, t, J = 7.6 Hz, −CH2CH3), 1.32 (1H, br s, core NH), 1.05 (3H, d, J = 7.1 Hz, 18-CH3), −1.46 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 172.3, 171.4, 171.3, 170.3, 170.0, 169.7, 165.8, 159.5, 153.8, 151.1, 148.5, 145.6, 144.8, 144.1, 140.3, 138.8, 136.1, 135.1, 134.4, 132.1, 131.7, 131.6, 131.1, 129.4, 128.8, 128.7, 110.8, 106.3, 103.9, 96.9, 81.5, 78.5, 72.2, 70.8, 68.4, 67.1, 61.0, 52.0, 48.6, 48.3, 33.2, 30.0, 28.4 (3C), 21.1, 20.7, 20.6, 20.52, 20.49, 19.51, 19.47, 17.4, 16.8, 14.1, 12.1, 11.3. HRMS (ESI) calculated for C58H68N5O13 [MH+] 1042.4814, found 1042.4578.
Compound 24.
15 mg of starting photosensitizer 23 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (14 mg). UV-Vis λmax (CH2Cl2): 669 (rel. intensity 0.413), 613 (0.077), 547 (0.111), 514 (0.081), 416 (0.902); 1H NMR (400 MHz, CDCl3, δ ppm): 9.56 (1H, s, 10-H), 9.43 (1H, s, PS 5-H), 8.60 (1H, dd, J = 7.9, 1.7 Hz, 203a-H), 8.38 (1H, dd, J = 7.8, 1.7 Hz, 203b-H), 8.33 (1H, dd, J = 7.9, ~1.5 Hz, 202a-H), 7.80 (1H, dd, J = 7.8, ~1.5 Hz, 202b-H), 6.14 (1H, d, J = 9.2 Hz, amide NH), 5.39 (1H, d, J = 3.3 Hz, Gal 4-H), 5.24, 5.206 (2H, ABq, JAB = 19.8 Hz, 132-CH2), 5.210 (1H, t, J = 9.4 Hz, Gal 1-H), 5.14 (1H, dd, J = 10.3, 3.4 Hz, Gal 3-H), 4.96 (1H, dd, J = 10.2, 9.3 Hz, Gal 2-H), 4.24 (1H, q, J ~ 7.1 Hz, 18-H), 4.14 (1H, dd, J ~ 8.3, 3.1 Hz, 17-H), 3.98–4.09 (3H, m, Gal 5-H & Gal 6-CH2), 3.82 (2H, q, J = 7.5 Hz, 3-CH2CH3), 3.74 (2H, q, J = 7.7 Hz, 8-CH2CH3), 3.70 (3H, s, 12-CH3), 3.31 (3H, s, 7-CH3), ~2.50, (1H, m, 17-CHHCH2−), ~2.47 (1H, m, 17-CH2CHH−), 2.33 (3H, s, 2-CH3), ~2.28 (1H, m, 17-CHHCH2−), 2.07, 1.99, 1.97, 1.92 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~1.94 (1H, m, 17-CH2CHH), 1.73 (3H, t, J = 7.6 Hz, 8-CH2CH3), 1.67 (3H, t, J = 7.5 Hz, 3-CH2CH3), ~1.3 (1H, br s, core NH), 1.06 (3H, d, J = 6.9 Hz, 18-CH3), −1.43 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.0, 172.5, 171.3, 171.2, 170.3*, 170.0, 169.9, 169.3, 159.8*, 148.7*, 146.9, 144.7, 144.3, 140.3*, 138.9, 136.1, 135.2, 134.7, 132.8, 131.5, 131.2, 130.3, 129.4, 129.0, ~110.5*, 106.5*, 104.0, 97.1, 78.5, 72.2, 70.8, 68.4, 67.1, 61.0, 52.5, 48.6, 48.1, 33.5, 29.7, 21.1, 20.7, 20.6, 20.5 (2C), 19.5 (2C), 17.4, 16.9, 14.1, 12.2, 11.3. See spectrum & notes in Supporting Information regarding three missing 13C peaks. * From HMBC. MS (ESI) calculated for C54H60N5O13[MH+] 986.4, found 986.4; HRMS (ESI) calculated for C54H59N5O13Na[MNa+] 1008.4007, found 1008.4020.
Compound 25.
20 mg of starting photosensitizer 19 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (19 mg). UV-Vis λmax (CH2Cl2): 668 (rel. intensity 0.375), 615 (0.056), 547 (0.115), 514 (0.071), 417 (0.869); 1H NMR (400 MHz, CD3OD, δ ppm): 9.21 (1H, s, 5-H), 9.10 (1H, s, 10-H), 8.41 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.22 (1H, dd, J = 7.9, 1.8 Hz, phenyl H), 8.09 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 7.59 (1H, dd, J = 7.9, 1.7 Hz, phenyl H), 5.00, 4.88 (2H, ABq, JAB= 20.0 Hz, 132-CH2), 4.08 (1H, q, J = 7.2 Hz, 18-H), 3.93 (1H, dd, J = 9.7, 2.9 Hz, 17-H), 3.72 (2H, q, J = 7.6 Hz, −CH2CH3), ~3.42 (2H, m, −CH2CH3), 3.33 (3H, s, ring CH3), 3.10 (3H, s, ring CH3), ~2.35, ~2.01, ~1.99, ~1.65 (each 1H, 4 × m, 17-CH2CH2−), 2.24 (3H, s, 2-CH3), 1.63 (3H, t, J = 7.6 Hz, −CH2CH3), 1.55 (3H, t, J = 7.6 Hz, −CH2CH3), 0.91 (3H, d, J = 7.1 Hz, 18-CH3); 13C {1H} NMR (100 MHz, CD3OD, δ ppm): 198.4, 176.8, 173.5, 169.9, 161.7, 155.6, 152.5, 149.2, 147.0, 146.1, 145.5, 141.4, 139.4, 137.3, 136.3, 135.7, 133.7, 132.9, 132.0, 131.1, 130.7, 130.1, 129.3, 112.3, 107.1, 104.7, 97.5, 53.2, 49.6*, 49.1*, 32.1, 30.6, 21.2, 20.1, 19.9, 17.6, 17.1, 14.2, 11.6, 11.0. * From HSQC. HRMS (ESI) calculated for C40H41N4O5 [MH+] 657.3077, found 657.3070.
Compound 26.
20 mg of starting photosensitizer 25 were used for reaction by following the method described above, and the title compound was obtained in 30% yield (12 mg). UV-Vis λmax (CH2Cl2): 670 (rel. intensity 0.374), 615 (0.059), 545 (0.111), 514 (0.073), 417 (0.901); 1H NMR (400 MHz, CDCl3, δ ppm): 9.52 (1H, s, 10-H), 9.40 (1H, s, PS 5-H), 8.24 (1H, dd, J = 7.8, 1.6 Hz, 202a-H), 8.19 (1H, dd, J = 7.9, 1.8 Hz, 203a-H), 8.03 (1H, dd, J = 7.9, 1.8 Hz, 203b-H), 7.73 (1H, dd, J = 7.9, 1.6 Hz, 202b-H), 7.43 (1H, d, J = 9.0 Hz, 20-amide NH), 5.95 (1H, d, J = 9.2 Hz, 17-amide NH), 5.59 (1H, t, J = 9.0 Hz, 20-Gal 1-H), 5.57 (1H, d, J ~ 3.2 Hz, 20-Gal 4-H), 5.38 (1H, dd, J = 10.3, 8.8 Hz, 20-Gal 2-H), 5.37 (1H, d, J = 3.4 Hz, 17-Gal 4-H), 5.32 (1H, dd, J = 10.3, 3.2 Hz, 20-Gal 3-H), 5.21, 5.19 (2H, ABq, JAB = 20.0 Hz, 132-CH2), 5.17 (1H, t, J = 9.3 Hz, 17-Gal 1-H), 5.07 (1H, dd, J = 10.3, 3.4 Hz, 17-Gal 3-H), 4.90 (1H, dd, J = 10.3, 9.3 Hz, 17-Gal 2-H), 4.18–4.27 (3H, m, 20-Gal 5-H & 20-Gal 6-CH2), 4.21 (1H, q, J ~ 7.1 Hz, 18-H), 4.15 (1H, dd, J = 7.3, 3.9 Hz, 17-H), 3.96–4.04 (3H, m, 17-Gal 5-H & 17-Gal 6-CH2), 3.80 (2H, q, J = 7.6 Hz, 3-CH2CH3), 3.72 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.68 (3H, s, 12-CH3), 3.30 (3H, s, 7-CH3), 2.50 (1H, m, 17-CHHCH2−), ~2.31 (1H, m, 17-CH2CHH−), 2.27 (3H, s, 2-CH3), 2.23, 2.20, 2.11, 2.07, 2.02, 1.98, 1.94, 1.91 (each 3H, 8 × s, 8 × CH3C(=O)O−), ~2.21 (1H, m, 17-CHHCH2−), 1.84 (1H, m, 17-CH2CHH), 1.72 (3H, t, J = 7.6 Hz, 8-CH2CH3), 1.65 (3H, t, J = 7.6 Hz, 3-CH2CH3), ~1.3 (1H, br s, core NH), 1.03 (3H, d, J = 7.0 Hz, 18-CH3), −1.47 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 172.3, 172.1, 171.19, 171.16, 170.4, 170.3, 170.0, 169.9, 169.8, 169.7, 167.2, 159.4, 153.8, 151.2, 148.4, 145.6, 144.8, 144.2, 140.2, 138.9, 136.2, 135.0, 134.9, 132.7, 132.6, 131.3, 131.1, 128.8, 127.3, 126.7, 110.3, 106.3, 104.0, 97.1, 79.5, 78.4, 72.5, 72.1, 70.8, 70.7, 68.7, 68.4, 67.3, 67.0, 61.2, 60.9, 52.1, 48.6, 48.2, 33.1, 29.9, 21.1 (2C), 20.8, 20.67, 20.66, 20.64, 20.62, 20.50, 20.49, 19.51, 19.47, 17.4, 16.9, 14.1, 12.1, 11.3. HRMS (ESI) calculated for C68H78N6O21Na [MNa+] 1337.5118, found 1337.5147.
Compound 28.
40 mg of starting photosensitizer 27 were used for reaction by following the method described above, and the title compound was obtained in 69% yield (26 mg). UV-Vis λmax (CH2Cl2): 673 (rel. intensity 0.498), 615 (0.063), 551 (0.144), 518 (0.084), 415 (1.048); 1H NMR (400 MHz, CDCl3, δ ppm): 10.233/10.225 (1H, s, 5-H), 9.579 (1H, s, 10-H), 6.00 (1H, q, J = 6.6 Hz, 31-H), ~5.98 (1H, m, amide NH), 5.38 (1H, d, J = 3.3 Hz, Gal 4-H), ~5.26 (2H, m, 132-CH2), 5.18/5.17 (1H, t, J = 9.3 Hz, Gal 1-H), 5.060/5.058 (1H, dd, J = 10.3, 3.3 Hz, Gal 3-H), 4.930/4.925 (1H, dd, J = 10.2, 9.4 Hz, Gal 2-H), 4.88/4.87 (1H, q, J = 7.0 Hz, 18-H), 4.31 (1H, m, 17-H), ~4.04 (2H, m, Gal 6-CH2), 3.98 (1H, m, Gal 5-H), 3.73 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.52–3.72 (2H, m, −OCH2(CH2)4CH3), 3.68 (3H, s, ring CH3), 3.62 (3H, s, ring CH3), 3.31 (3H, s, 7-CH3), 2.55 (1H, m, 17-CHHCH2−), 2.41 (1H, m, 17-CH2CHH−), 2.23 (1H, m, 17-CHHCH2−), 2.16/2.11 (3H, d, J = 6.7 Hz, 31-CH3), 2.052/2.049, 1.99, 1.94, 1.90 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~1.82 (1H, m, 17-CH2CHH−), ~1.74 (2H, m, −OCH2CH2(CH2)3CH3), 1.72 (3H, t, J = 7.6 Hz, 8-CH2CH3), 1.59/1.58 (3H, d, J = 6.9 Hz, 18-CH3), ~1.28–1.46 (2H, m, −O(CH2)2CH2(CH2)2CH3), 1.20 (4H, m, −O(CH2)3(CH2)2CH3), 0.77/0.75 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.81/−1.85 (1H, s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 195.9, 172.3, 171.6/171.5, 171.2, 170.3, 169.9, 169.7, 160.6/160.5, 153.5, 152.2, 148.0, 144.6, 142.2/142.0, 139.74/139.72, 138.2/138.1, 137.3, 133.3/133.2, 132.9/132.8, 131.7, 129.7, 106.7, 103.83/103.80, 99.67/99.65, 94.5/94.4, 78.4, 73.2/73.1, 72.2, 70.8, 69.8/69.7, 68.3, 67.1, 61.1, 51.8, 51.6, 48.6, 32.7, 31.73/31.70, 30.24/30.19, 29.9, 26.1/26.0, 25.0/24.9, 22.6/22.5, 20.8, 20.7, 20.6, 20.51, 20.48, 19.5, 17.4, 17.04/16.97, 13.95/13.93, 12.2, 11.3. HRMS (ESI) calculated for C53H67N5O12Br [MH+] 1044.3970, found 1044.3998.
Compound 29.
30 mg of starting photosensitizer 28 were used for reaction by following the method described above, and the title compound was obtained in 59% yield (19 mg). UV-Vis λmax (CH2Cl2): 670 (rel. intensity 0.181), 615 (0.027), 547 (0.054), 513 (0.033), 415 (0.427); 1H NMR (400 MHz, CDCl3, δ ppm): 10.17/10.12 (1H, s, PS 5-H), 9.55 (1H, s, 10-H), 8.43 (1H, dd, J = 7.9, 1.8 Hz, 203a-H), 8.25/8.24 (1H, dd, J = 7.8, 1.8 Hz, 203b-H), 8.22/8.21 (1H, dd, J = 8.0, 1.6 Hz, 202a-H), 7.71/7.68 (1H, dd, J = 7.9, 1.7 Hz, 202b-H), 6.03/6.01 (1H, d, J = 9.2 Hz, amide NH), 5.82/5.81 (1H, q, J = 6.7 Hz, 31-H), 5.38 (1H, d, J = 3.3 Hz, Gal 4-H), ~5.22 (2H, m, 132-CH2), 5.17/5.16 (1H, t, J = 9.3 Hz, Gal 1-H), 5.072/5.070 (1H, dd, J = 10.3, 3.3 Hz, Gal 3-H), 4.942/4.935 (1H, dd, J = 10.3, 9.3 Hz, Gal 2-H), 4.26/4.24 (1H, q, J = 7.2 Hz, 18-H), 4.15 (1H, m, 17-H), 3.94–4.07 (3H, m, Gal 5-H & Gal 6-CH2), 3.74 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.69 (3H, s, 12-CH3), 3.67@ (~1H, t, J = 6.6 Hz, −OCH2(CH2)4CH3), 3.54@ (~0.5H, dt, J = 9.1, 6.4 Hz, −OCHH(CH2)4CH3), 3.44@ (~0.5H, dt, J = 9.0, 6.8 Hz, −OCHH(CH2)4CH3), 3.30 (3H, s, 7-CH3), ~2.49 (1H, m, 17-CHHCH2−), ~2.39 (1H, m, 17-CH2CHH−), 2.36/2.35 (3H, s, 2-CH3), 2.21 (1H, m, 17-CHHCH2−), 2.13/1.99 (3H, d, J = 6.6 Hz, 31-CH3), 2.053/2.049, 1.97, 1.95, 1.93 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~1.91 (1H, m, 17-CH2CHH−), ~1.76/1.64 (2H, m, −OCH2CH2(CH2)3CH3), 1.75 (9H, s, −C(=O)OC(CH3)3), 1.73 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.2–1.5 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.23/1.17 (2H, m, −O(CH2)4CH2CH3), ~1.23/1.16 (2H, m, −O(CH2)3CH2CH2CH3), ~1.22 (1H, br s, core NH), 1.031/1.025 (3H, d, J = 7.0 Hz, 18-CH3), 0.79/0.73 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.54/−1.60 (1H, s, core NH); 13C NMR (100 MHz, CDCl3, δ ppm): 196.1, 172.3, 171.29/171.28, 171.0, 170.3, 169.9, 169.7, 165.7, 159.9/159.7, 153.8, 151.6, 148.4, 145.6, 144.60/144.58, 141.9/141.5, 139.3, 139.2/139.1, 136.88/136.86, 134.5/134.3, 133.8/133.5, 132.7/132.6, 132.2/132.0, 131.7, 131.31/131.30, 129.5, 129.0, 128.9/128.8, 110.9, 106.3, 103.8/103.7, 99.3/99.2, 81.6, 78.5, 72.9/72.8, 72.2, 70.7, 69.8/69.5, 68.4, 67.1, 61.0, 52.2/52.1, 48.6, 48.21/48.17, 33.27/33.25, 31.8/31.7, 30.3/30.1, 30.0, 28.3 (3C), 26.1/26.0, 25.0/24.6, 22.6/22.5, 21.1/21.0, 20.7, 20.6, 20.51, 20.49, 19.5, 17.4, 14.1/13.8, 14.0/13.9, 12.1, 11.3. @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C64H80N5O14 [MH+] 1142.5702, found 1142.5747.
Compound 30.
15 mg of starting photosensitizer 29 were used for reaction by following the method described above, and the title compound was obtained in quantitative yield (14 mg). UV-Vis λmax (CH2Cl2): 671 (rel. intensity 0.577), 615 (0.085), 547 (0.170), 514 (0.108), 415 (1.355); 1H NMR (400 MHz, CDCl3, δ ppm): 10.19/10.15 (1H, s, PS 5-H), 9.56 (1H, s, 10-H), 8.64 (1H, dd, J = 7.9, 1.7 Hz, 203a-H), 8.41/8.40 (1H, dd, J = 7.8, 1.8 Hz, 203b-H), 8.373/8.368 (1H, dd, J = 7.9, 1.8 Hz, 202a-H), 7.81/7.78 (1H, dd, J = 7.9, 1.7 Hz, 202b-H), 6.16/6.14 (1H, d, J = 9.2 Hz, amide NH), 5.85/5.84 (1H, q, J = 6.7 Hz, 31-H), 5.40 (1H, d, J = 3.2 Hz, Gal 4-H), ~5.24 (2H, m, 132-CH2), 5.224/5.218 (1H, t, J = 9.3 Hz, Gal 1-H), 5.140/5.136 (1H, dd, J = 10.3, 3.3 Hz, Gal 3-H), 4.97/4.96 (1H, dd, J = 10.3, 9.3 Hz, Gal 2-H), 4.29/4.27 (1H, q, J = 7.1 Hz, 18-H), 4.17 (1H, m, 17-H), ~3.97–4.09 (3H, m, Gal 5-H & Gal 6-CH2), 3.74 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.693 (3H, s, 12-CH3), 3.688@ (~1H, t, J = 6.8 Hz, −OCH2(CH2)4CH3), 3.57@ (~0.5H, dt, J = 9.1, 6.4 Hz, −OCHH(CH2)4CH3), 3.47@ (~0.5H, dt, J = 9.0, 6.8 Hz, −OCHH(CH2)4CH3), 3.31 (3H, s, 7-CH3), ~2.50 (2H, m, 17-CHHCHH−), 2.40/2.39 (3H, s, 2-CH3), ~2.29 (1H, m, 17-CHHCH2−), 2.15/2.02 (3H, d, J = 6.7 Hz, 31-CH3), 2.061/2.057, 1.99, 1.974/1.973, 1.952/1.950 (each 3H, 4 × s, 4 × CH3C(=O)O−), ~1.93 (1H, m, 17-CH2CHH), ~1.77/1.66 (2H, m, −OCH2CH2(CH2)3CH3), 1.74 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.22–1.49 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.25/1.17 (4H, m, −O(CH2)3(CH2)2CH3), 1.059/1.055 (3H, d, J = 7.1 Hz, 18-CH3), 0.80/0.74 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.51/−1.57 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 172.6, 171.37/171.36, 170.8, 170.3, 169.99, 169.96, 169.8, 159.9/159.8, 153.7, 151.6, 148.5, 146.9, 144.7/144.6, 142.0/141.6, 139.22/139.20, 139.19/139.06, 136.94/136.92, 134.8/134.6, 133.8/133.5, 132.8/132.6, 132.5/132.3, 131.3, 130.3, 129.51/129.49, 129.2, 129.1, 110.6, 106.4, 103.91/103.87, 99.5/99.4, 78.5, 72.95/72.88, 72.3, 70.8, 69.8/69.6, 68.4, 67.1, 61.0, 52.51/52.49, 48.6, 48.1, 33.5, 31.8/31.7, 30.3/30.1, 29.8, 26.1/26.0, 25.0/24.6, 22.6/22.5, 21.08/21.07, 20.7, 20.6, 20.5 (2C), 19.5, 17.4, 14.1/13.8, 14.0/13.9, 12.2, 11.4. @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C60H72N5O14[MH+] 1086.5076, found 1086.5063;
Compound 31.
20 mg of starting photosensitizer 30 were used for reaction by following the method described above, and the title compound was obtained in 50% yield (13 mg). UV-Vis λmax (CH2Cl2): 671 (rel. intensity 0.241), 614 (0.036), 546 (0.074), 513 (0.046), 415 (0.557); 1H NMR (400 MHz, CDCl3, δ ppm): 10.18/10.13 (1H, s, PS 5-H), 9.561/9.559 (1H, s, 10-H), 8.27/8.26 (1H, dd, J = 7.9, ~1.6 Hz, 202a-H), 8.21/8.20 (1H, dd, J = 7.9, ~2.0 Hz, 203a-H), 8.03/8.02 (1H, dd, J = 7.9, 2.0 Hz, 203b-H), 7.73/7.70 (1H, dd, J = 7.9, 1.7 Hz, 202b-H), 7.43/7.42 (1H, d, J = 9.1 Hz, 20-amide NH), 5.97/5.96 (1H, d, J = 9.3 Hz, 17-amide NH), 5.813/5.807 (1H, q, J = 6.7 Hz, 31-H), 5.58 (1H, dd, J = 9.2, 8.7 Hz, 20-Gal 1-H), 5.57 (1H, d, J ~ 3.2 Hz, 20-Gal 4-H), 5.373 (1H, dd, J = 10.3, 8.7 Hz, 20-Gal 2-H), 5.366 (1H, d, J ~ 3.3 Hz, 17-Gal 4-H), 5.32 (1H, dd, J = 10.3, 3.2 Hz, 20-Gal 3-H), ~5.22 (2H, m, 132-CH2), 5.17/5.16 (1H, t, J = 9.3 Hz, 17-Gal 1-H), 5.070/5.066 (1H, dd, J = 10.4, 3.4 Hz, 17-Gal 3-H), 4.90/4.89 (1H, dd, J = 10.4, 9.3 Hz, 17-Gal 2-H), ~4.18–4.28 (4H, m, 18-H, 20-Gal 5-H & 20-Gal 6-CH2), 4.16 (1H, m, 17-H), ~3.94–4.04 (3H, m, 17-Gal 5-H & 17-Gal 6-CH2), 3.74 (2H, q, J = 7.6 Hz, 8-CH2CH3), 3.70 (3H, s, 12-CH3), 3.67@ (~1H, t, J = 6.7 Hz, −OCH2(CH2)4CH3), 3.55@ (~0.5H, dt, J = 9.0, 6.4 Hz, −OCHH(CH2)4CH3), 3.44@ (~0.5H, dt, J = 9.0, 6.8 Hz, −OCHH(CH2)4CH3), 3.30 (3H, s, 7-CH3), 2.51 (1H, m, 17-CHHCH2−), ~2.32 (1H, m, 17-CH2CHH−), 2.32/2.31 (3H, s, 2-CH3), 2.230/2.227, 2.20, 2.11, 2.07, 2.023/2.020, 1.976/1.975, 1.939, 1.924/1.922 (each 3H, 8 × s, 8 × CH3C(=O)O−), ~2.22 (1H, m, 17-CHHCH2−), 2.13/1.99 (3H, d, J = 6.7 Hz, 31-CH3), 1.85 (1H, m, 17-CH2CHH−), ~1.75/1.64 (2H, m, −OCH2CH2(CH2)3CH3), 1.73 (3H, t, J = 7.6 Hz, 8-CH2CH3), ~1.21–1.46 (2H, m, −O(CH2)2CH2(CH2)2CH3), ~1.26 (1H, br s, core NH), ~1.24/1.16 (4H, m, −O(CH2)3(CH2)2CH3), 1.02/1.01 (3H, d, J = 7.0 Hz, 18-CH3), 0.79/0.72 (3H, distorted t, J ~ 7 Hz, −O(CH2)5CH3), −1.55/−1.61 (1H, br s, core NH); 13C {1H} NMR (100 MHz, CDCl3, δ ppm): 196.1, 172.3, 172.1, 171.2, 170.81/170.79, 170.4, 170.34/170.33, 170.0, 169.9, 169.8, 169.7, 167.1, 159.7, 153.8, 151.7, 148.4, 145.6, 144.7/144.6, 142.0/141.6, 139.23/139.07, 139.20, 137.0/136.9, 135.0/134.8, 133.7/133.4, 132.72/132.71, 132.67/132.54, 132.4/132.3, 131.4, 129.17/129.16, 127.4/127.3, 126.7, 110.4, 106.3, 103.91/103.86, 99.5/99.4, 79.5, 78.4, 72.93/72.86, 72.5, 72.1, 70.8, 70.7, 69.8/69.6, 68.8, 68.4, 67.3, 67.0, 61.2, 60.9, 52.2, 48.7, 48.2, 33.1, 31.8/31.7, 30.3/30.1, 29.9, 26.1/26.0, 25.0/24.7, 22.6/22.5, 21.1 (2C), 20.75, 20.69, 20.66, 20.64, 20.62, 20.5 (2C), 19.5, 17.4, 14.04/13.74, 13.98/13.91, 12.1, 11.3. Note: @ See spectrum & notes in Supporting Information. HRMS (ESI) calculated for C74H91N6O22 [MH+] 1415.6186, found 1415.6445.
3.1.8. Method to determine in vitro PDT efficacy (MTT assay)
Cells were plated in 96 well plate between 5 × 104 and 10 × 104 cells per well. The cells were allowed to adhere to the plates then photosensitizer was added. The maximum dose was 1.6 μM and the minimum was 6.25 nM. The cells incubated with the photosensitizer for 24 h then exposed to light at the appropriate wavelength. After 48 h, the cell viability was read using an MTT assay and the results were analyzed and plotted using Graphpad Prism software.
3.1.9. Method of tumor-implantation
Colon 26 and FaDu tumors were transplanted in 6–8-week-old Female Balb/c (NCI Balb/cAnNCr) and SCID mice (Strain C.B Igh-1b Icr Tac Prkdc Scid) respectively and were maintained in the animal facility. Mice bearing an established tumor (~ 7 days after implantation) were treated with the PDT. Tumor size was measured on two axes (in millimeters; L, longest axis, and W, shortest axis) with the aid of Vernier calipers. Tumor size (mm3) was calculated by using the formula: tumor weight = 1/2 (L × W2). All studies were performed in accordance with protocols approved by the institutional animal care and use committee at Roswell Park Cancer Institute.
3.1.10. Determination of in vivo tumor uptake and PDT efficacy
SCID mice with FaDu tumors and Colon 26 tumors of 200–250 mm3 were injected intravenously (i.v) with photosensitizer PS 2 and PS 11 at dose of 0.47 μmol/kg in Tween formulations. The PS uptake in FaDu tumors was determined by fluorescence imaging using a PerkinElmer IVIS Spectrum at variable timepoints, and the maximum uptake was observed post-injection. At this timepoint the tumors were irradiated with light (fluence:135 J/cm2; fluence rate: 75 mW/cm2) for 30 min at 665 nm using a Lightwave™ laser diode. Mice were restrained without anesthesia in plexiglass holders designed to expose only the tumor and a 2–4 mm annular margin of skin to light. The tumor assessment and measurements were taken daily, then three times a week for 4 weeks, and twice a week thereafter for a total of 30 days post treatment. Tumor volume (mm2) was estimated using a formula: tumor volume = ½ (L x W2). Two axes (mm) of tumor (L, longest axis; W, shortest axis) were measured with the aid of a Vernier caliper. The complete tumor regression (CR) was defined as the inability to detect tumor by palpation at the initial site of tumor appearance for more than two-month post-therapy. The Partial tumor regression (PR) was defined as ≥ 50% reduction in initial tumor size. The edema, erythema, and scar formation in the treatment field was observed and recorded. Tumor response for each treatment was evaluated fot the tumor response.
Supplementary Material
Acknowledgments
The authors are highly thankful to NIH for the financial support (partial) through a program project grant (PO1 CA55791), Photolitec, LLC, Buffalo and the shared resources of the Roswell Park Comprehensive Center, Buffalo, NY, United States Support Grant (P30 CA016056). The authors are also thankful to Mr. Eric Jensen and Dr. Valerie A. Frerichs, Mass spectrometry Facility, SUNY, Buffalo.
ABBREVIATIONS
- PDT
photodynamic therapy
- PS
photosensitize
- HSQC
heteronuclear single quantum coherence
- ROS
Reactive oxygen species
Footnotes
Summer Research Program.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmcr.2022.100047.
References
- [1].Ethirajan M, Chen Y, Joshi P, Pandey RK, The role of porphyrin chemistry in tumor imaging and photodynamic therapy, Chem. Soc. Rev 40 (2011) 340–362. [DOI] [PubMed] [Google Scholar]
- [2].Heidi A, Hamblin AR, New photosensitizers for photodynamic therapy, Biochem. J 47 (2016) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lo P-C, Rodriguez-Morgade MS, Pandey RK, Ng DKP, Torres T, Dumoulin F, The unique features and promises of phthalocyanines as advanced photosensitizers for photodynamic therapy of cancer, Chem. Soc. Rev 49 (2020) 1041–1056. [DOI] [PubMed] [Google Scholar]
- [4].Sara RG Fernandes R. Fernandes B. Sarmento PMR Pereira JPC Tome, Photoimmunoconjugates: novel synthetic strategies to target and treat cancer by photodynamic therapy, Org. Biomol. Chem (2019), 10.1059/cBob02902d. [DOI] [PubMed] [Google Scholar]
- [5].(a) Sibrian-Vazquez M, Jensen TJ, Hammer RP, Vicente MGH, Peptidemediated cell transport of water soluble porphyrin conjugates, J. Med. Chem 49 (2006) 1364–1372; [DOI] [PubMed] [Google Scholar]; (b) Srivatsan A, Ethirajan M, Pandey SK, Dubey S, Zheng X, Liu T-H, Shibata M, Missert J, Morhgan J, Pandey RK, Conjugation of cRGD peptide to chlorophyll-a based photosensitizer (HPPH) alters its pharmacokinetics with enhanced tumor-imaging and PDT efficacy, Mol. Pharm 8 (2011) 1186–1197; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ranyuk E, Cauchon N, Klarskov K, Guerin B, van Lier JE, Phthalocyaninepeptide conjugates: receptor-targeting bifunctional agents for imaging and photodynamic therapy, J. Med. Chem 56 (2013) 1520–1534. [DOI] [PubMed] [Google Scholar]
- [6].(a) Cheruku RR, Cacaccio J, Durrani FA, Tabaczynski WA, Watson R, Marko A, Kumar R, Elkhouly ME, Fuzukumi S, Missert JR, Yao R, Sajjad M, Chandra D, Guru K, Pandey RK, Epidermal growth factor receptor-targeted multifunctional photosensitizers for bladder cancer imaging and photodynamic therapy, J. Med. Chem 62 (2019) 2598–2617; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cheruku RR, Cacaccio J, Durrani FA, Tabaczynski WA, Watson K. Siters JR Missert EC Tracy M. Dukh K. Guru RC Koya P. Kalinski H. Baumann RK Pandey J. Med. Chem 64 (2021) 741–767; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Marko AJ, Borah BM, Siters KE, Missert JR, Gupta A, Pera P, Lam MF, Pandey RK, Targeted nanoparticles for fluorescence imaging of folate receptor positive tumors, Biomolecules 10 (2020) 1651, 10.3390/biom10121651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zheng G, Graham A, Shibata M, Missert JR, Oseroff AR, Dougherty TJ, Pandey RK, Synthesis of β-galactose conjugated chlorins derived by enyne metathesis as galectin-specific photosensitizers for photodynamic therapy, J. Org. Chem 66 (2001) 8709–8716. [DOI] [PubMed] [Google Scholar]
- [8].Guolin Li, Pandey SK, Graham A, Dobhal MP, Mehta R, Chen Y, Gryshuk A, Olson K, Oseroff A, Pandey RK, Functionalization of OEP-based benzochlorins to develop carbohydrate-conjugated photosensitizers. Attempt to target-beta-galactoside-recognized proteins, J. Org. Chem 69 (2004) 158–172. [DOI] [PubMed] [Google Scholar]
- [9].Achelle S, Couleaud P, Baldeck P, Fichou M-P, Maillard P, Carbohydrate-porphyrin conjugates with two-photon absorption properties as potential photosensitizing agents for photodynamic therapy, Eur. JOC 7 (2011) 1271–1279. [Google Scholar]
- [10].Hao E, Jensen TJ, Vicente MGH, Synthesis of porphyrin-carbohydrate conjugates using “click” chemistry and their preliminary evaluation in human HEP2 cells, J. Porphyr. Phthalocyanines 13 (2009) 51–59. [Google Scholar]
- [11].Zheng X, Pandey RK, Porphyrin-carbohydrate conjugates: impact of carbohydrate moieties in photodynamic therapy (PDT), Anti Cancer Agents Med. Chem 8 (2008) 241–268. [DOI] [PubMed] [Google Scholar]
- [12].Rigual N, Shafirstein G, Cooper MT, Baumann H, Bellnier DA, Sunar U, Tracy EC, Rohrbacj DJ, Wilding G, Tan W, Sullivan M, Marzianu M, Henderson BW, Photodynamic therapy with 3-(1’-hexyloxy)ethyl-3-devinylpyropheophorbide-a, Clin. Cancer Res 19 (2013) 6605–6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cramers P, Ruevakamp M, Oppelaar H, Dalesio O, Baas P, Stewart FA, Foscan uptake and tissue distribution in relation to photodynamic therapy, Br. J. Cancer 88 (2003) 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tookad Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localized prostate cancer, BJU Int. 112 (2013) 766–774. [DOI] [PubMed] [Google Scholar]
- [15].Usuda J, Tsutsui H, Honda H, et al. , Photodynamic therapy of lung cancers based on novel photodynamic diagnosis using talaporfin sodium (NPe6) and autofluorescence bronchoscopy, Lung Cancer 58 (2007) 317–323. [DOI] [PubMed] [Google Scholar]
- [16].Patel N, Pera P, Joshi P, Dukh M, Tabaczynski WA, Siters KE, Kryman M, Cheruku RR, Durrani F, Missert JR, Watson R, Ohulchanskyy TY, Tracy E, Baumann H, Pandey RK, Highly effective dual-function near infrared (NIR) photosensitizer for fluorescence imaging and photodynamic therapy (PDT) of cancer, J. Med. Chem 59 (2016) 9774–9787. [DOI] [PubMed] [Google Scholar]
- [17].Kramer N, Miller JW, Michaud RS, Moulton RS, Hasan T, Flotte TJ, Gragoudas ES, Liposomal benzoporphyrin derivative verteporfin photodynamic therapy. Selective treatment of choroidal neovascularization in monkeys, Opthalmology 103 (1996) 427–438. [DOI] [PubMed] [Google Scholar]
- [18].Baron ED, Malbasa CL, Santo-Domingo D, Fu P, Miller JD, Hannerman KK, Hsia AH, Oleinick NL, Colussi VC, Cooper KD, Silicon phthalocyanine (Pc 4) photodynamic therapy is a safe modality for cutaneous neoplasms: results of phase 1 clinical trial, Laser Surg. Med 42 (2010) 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tracy EC, Bowmann M-J, Pandey RK, Baumann H, Cell-specific retention and action of pheophorbide-based photosensitizers in human lung cancer cells, Photochem. Photobiol 95 (2019) 846–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu L, Liu L, Li W, Chen R, Gao Y, Zhang W, Photodynamic therapy of oligoethylene glycol dendronized reduction-sensitive porphyrins, J. Mater. Chem B 3 (2015) 3062–3071. [DOI] [PubMed] [Google Scholar]
- [21].Che Y, Zhao D, Liu Y, Polysaccharide-porphyrin -fullerene supramolecular conjugates as photo-driven DNA cleavage reagents, Chem. Commun 51 (2015) 12266. [DOI] [PubMed] [Google Scholar]
- [22].Abrahamse H, Hamblin MR, New photosensitizers for photodynamic therapy, Biochem. J 473 (2016) 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rini JM, Lectin structure, Annu. Rev. Biophys. Biomol. Struct 24 (1995) 551–577. [DOI] [PubMed] [Google Scholar]
- [24].Brewer CF, Micell MC, Baum LG, Carbohydrate -modifying biocatalysts-taylor & francis eBooks, Curr. Opin. Struct. Biol 12 (2002) 616–623. [DOI] [PubMed] [Google Scholar]
- [25].Yang RY, Liu FT, Galectins in cell growth and apoptosis, Cell. Mol. Life Sci 60 (2003) 267–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tadokoro T, Ikekita M, Toda T, et al. , Involvement of galectin-3 with vascular cell adhesion molecule-1 in growth regulation of mouse BALB/3T3 cells, J. Biol. Chem 284 (2009) 35556–35563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Riss D, Jin I, Qian X, Bayliss J, Scheithauer DW, Young WF, Vidal S, Kovacs K, Raz A, Lloyd RV, Differential expression of galectin-3 in pituitary tumors, Cancer Res. 63 (2003) 2251–2255. [PubMed] [Google Scholar]
- [28].Nava HR, Allamaneni SS, Dougherty TJ, Cooper MJ, Tan W, Wilding G, Henderson BW, Photodynamic therapy (PDT) using HPPH for the treatment of precancerous lesions associated with Barrett’s esophagus, Laser Surg. Med 43 (2011) 705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dhillon SS, Demmy TL, Yendamuri S, Loewen G, Nwogu C, Cooper M, Henderson BW, A phase 1 study of light dose for photodynamic therapy (PDT) using HPPH for treatment of non-small cell carcinoma or non-small cell microinvasive bronchogenic carcinoma. A dose ranging study, J. Thorac. Oncol 11 (2016) 234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zheng X, Morgan J, Pandey SK, Chen Y, Tracy E, Baumann H, Missert JR, Batt C, Jackson J, Belliner DA, Henserson BW, Pandey RK, Conjugation of 2(1′- hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo, J. Med. Chem 52 (2009) 4306–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, Pandey RK, Oseroff AR, The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2, Clin. Cancer Res 13 (2007) 2463–2470. [DOI] [PubMed] [Google Scholar]
- [32].Pandey RK, Sumlin AB, Constantine S, Aoudia M, Potter WA, Bellnier DA, Henderson BW, Rodgers MA, Smith KM, Dougherty TJ, Alkyl ether analogs of chlorophyll-a derivatives: Part 1. Synthesis, photophysical properties and photodynamic efficacy, Photochem. Photobiol 64 (1996) 194–204. [DOI] [PubMed] [Google Scholar]
- [33].(a) Ethirajan M, Joshi P, White WH, Ohkubo K, Fukuzumi S, Pandey RK, Remarkable regioselective position-10 bromination of bacteriopyropheophorbide-a and ring-B reduced Pyropheophorbide-a, Org. Lett 13 (2011) 1956–1959; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ethirajan M, Chen P, Ohulchanskyy TY, Goswami LN, Gupta A, Srivatsan A, Dobhal MP, Missert JR, Prasad PN, Kadish K, Pandey RK, Regioselective synthesis and photophysical and electrochemical studies of 20-substituted cyanine-dye-purpurinimide conjugates. Incorporation of Ni(II) into the conjugates enhances its tumor-uptake and fluorescence-imaging ability, Chem. Eur J 19 (2013) 6670–6684. [DOI] [PubMed] [Google Scholar]
- [34].Van Meerloo J, Kaspers GJL, Cloos J, Cell sensitivity assays: the MTT assay, Methods Mol. Biol 731 (2011) 237–245. [DOI] [PubMed] [Google Scholar]
- [35].Kessel D, Correlation between subcellular localization and photodynamic efficacy, J. Porphyr. Phthalocyanines 8 (2004) 1009–1014. [Google Scholar]
- [36].Reiners JL, Agostinis P, Berg K, Oleinick NL, Kessel D, Assessing autophagy in the context of photodynamic therapy, Autophagy 6 (2010) 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Martins WK, Santos NF, Sousa Roucha C, Bacellar IOL, Tsubone TM, Viotto AC, Matsukuma AY, Abrantes ABP, Slani P, Dis LG, Baptista M, Parallel damage in mitochondria and lysosomes in an efficient way to photoinduced cell death, Autophagy 15 (2019) 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oleinick NL, Moris RL, Belichenko I, The role of apoptosis in response ro photodynamic therapy: what, where, why and how, Photochem. Photobiol. Sci 1 (2002) 1–21. [DOI] [PubMed] [Google Scholar]
- [39].Zhou C, New trends in photobiology: mechanisms of tumor necrosis induced by photodynamic therapy, J. Photochem. Photobiol. B Biol 3 (1989) 299–318. [DOI] [PubMed] [Google Scholar]
- [40].Suzuki T, Tanaka M, Sasaki M, Ichkawa H, Nishie H, Kataoka H, Vascular shutdown by photodynamic therapy using Talaporphin Sodium, Cancers 12 (2020) 2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mroz P, Yaroslavsky A, Kharkwal GB, Hamblin MR, Cell death pathways in photodynamic therapy of cancer, Cancers 3 (2011) 2516–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.