Abstract
Simple Summary
The safety of long-term PPI use has increasingly raised concerns. We conducted a case-control study to explore the associations of PPI use with female cancer risks in specific age groups. Overall, PPI use was significantly associated with decreased risks of breast, cervical, endometrial, and ovarian cancers. PPIs were associated with a significant decrease in breast and ovarian cancer risks in 20–64-year-old users and a reduction in cervical and endometrial cancer risks in those aged 40–64 years. We hope that our findings based on real-world big data can provide researchers and clinicians with some possible insights. Further clinical studies are needed to elucidate the effects of PPIs on female cancers.
Abstract
Background: Firm conclusions about whether long-term proton pump inhibitor (PPI) drug use impacts female cancer risk remain controversial. Objective: We aimed to investigate the associations between PPI use and female cancer risks. Methods: A nationwide population-based, nested case-control study was conducted within Taiwan’s Health and Welfare Data Science Center’s databases (2000–2016) and linked to pathologically confirmed cancer data from the Taiwan Cancer Registry (1979–2016). Individuals without any cancer diagnosis during the 17 years of the study served as controls. Case and control patients were matched 1:4 based on age, gender, and visit date. Conditional logistic regression with 95% confidence intervals (CIs) was applied to investigate the association between PPI exposure and female cancer risks by adjusting for potential confounders such as the Charlson comorbidity index and medication usage (metformin, aspirin, and statins). Results: A total of 233,173 female cancer cases were identified, consisting of 135,437 diagnosed with breast cancer, 64,382 with cervical cancer, 19,580 with endometrial cancer, and 13,774 with ovarian cancer. After matching each case with four controls, we included 932,692 control female patients. The number of controls for patients with breast cancer, cervical cancer, endometrial cancer, and ovarian cancer was 541,748, 257,528, 78,320, and 55,096, respectively. The use of PPIs was significantly associated with reduced risk of breast cancer and ovarian cancer in groups aged 20–39 years (adjusted odds ratio (aOR): 0.69, 95%CI: 0.56–0.84; p < 0.001 and aOR: 0.58, 95%CI: 0.34–0.99; p < 0.05, respectively) and 40–64 years (aOR: 0.89, 95%CI: 0.86–0.94; p < 0.0001 and aOR: 0.87, 95%CI: 0.75–0.99; p < 0.05, respectively). PPI exposure was associated with a significant decrease in cervical and endometrial cancer risks in the group aged 40–64 years (with aOR: 0.79, 95%CI: 0.73–0.86; p < 0.0001 and aOR: 0.72, 95%CI: 0.65–0.81; p < 0.0001, respectively). In contrast, in elderly women, PPI use was found to be insignificantly associated with female cancers among users. Conclusions: Our findings, based on real-world big data, can depict a comprehensive overview of PPI usage and female cancer risk. Further clinical studies are needed to elucidate the effects of PPIs on female cancers.
Keywords: proton pump inhibitor, cancer risk, breast cancer, cervical cancer, endometrial cancer, ovarian cancer
1. Introduction
Cancer is the leading cause of premature death and disability worldwide, particularly in women, with more than one out of every six deaths due to cancer [1]. Breast cancer has become the most prevalent of all female cancers, consisting of 12% of all new annual cancer cases globally. It is reported as the main cause of death among women [2,3]. Breast cancer is followed by cervical and ovarian cancers as common cancers, with 0.6 million and 313,959 new cases, respectively, diagnosed in 2020 [4,5]. Ovarian cancer was reported to be associated with the highest mortality of gynecological malignancies because of its silent development and advanced stage at diagnosis [6,7,8]. Endometrial cancer is the sixth most common cancer in women. In 2020, there were more than 417,000 new cases of endometrial cancer, and it was the 15th most common cancer overall [9].
The use of proton pump inhibitor (PPI) medications has rapidly increased in recent years because of their effectiveness in treating gastroesophageal reflux disease and peptic ulcer disease. Since their introduction in the late 1980s, millions of people have been using these drugs continuously or for long-term periods [10]. Studies have investigated the appropriateness and judiciousness of taking PPIs in the hospital and outpatient practices [11,12]. In addition, research on the association between female cancer risks such as breast, cervical, endometrial, and ovarian cancers and PPI use has been proposed. Studies have shown inconsistent results. Some suggested associations of PPIs with a decreased risk of breast cancer [13,14,15], whereas some concluded no significant association of their use with breast and endometrial cancers [16]. Some evidence has indicated that PPIs could suppress the growth of breast, cervical, endometrial, and ovarian cancer cells in vitro and in vivo [17,18,19]. Thus, the safety of long-term PPI use has increasingly raised concerns [20].
In Taiwan in 2019, cancers of the uterine body, ovaries and other adnexa, and cervix uteri ranked fifth, seventh, and ninth, respectively, among female cancers with the highest incidence rates, with a median age of incidence at 56, 54, and 57 years, respectively [21]. The incidence rates of uterine body and ovary cancers peaked at 50 and 60 years, while cervix uteri cancer climbed with age until 80 years [21]. However, to our knowledge, no studies have been conducted on the risks of female cancers among PPI users and included stratification by age. Therefore, this study aims to explore the associations of PPI use with female cancer risks in specific age groups.
2. Methods
2.1. Data Source
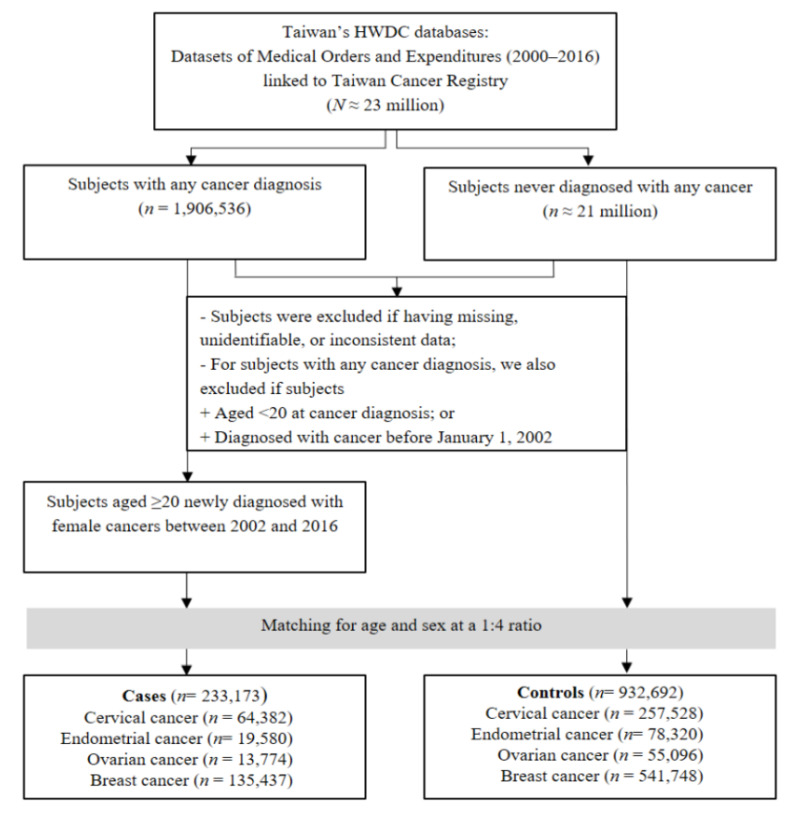
We used Taiwan’s Health and Welfare Data Science Center (HWDC) databases, from which we retrieved medication and diagnosis data (2000–2016) and linked these to pathologically confirmed cancer data from the Taiwan Cancer Registry (TCR) (1979–2016) (Figure 1). The TCR is a population-based cancer registry standardizing medical definitions and terminology as well as codes and procedures of the registry’s reporting system that tracks patients with a cancer diagnosis. The HWDC is a centralized data repository administered by Taiwan’s Ministry of Health and Welfare (MOHW) that stores de-identified claims data of beneficiaries of the National Health Insurance (NHI) [22]. Taiwan’s NHI, as compulsory health insurance, covers 99.8% of the Taiwanese residents, accounting for over 23 million people [23]. The diagnoses of diseases in this study were identified from the validated International Classification of Diseases, Clinical Modification, Ninth Revision [ICD-9-CM] codes [22,23]. We obtained ethical approval for this study from the Joint Institutional Review Board of Taipei Medical University (TMU-JIRB), Taipei, Taiwan (approval number: N202003609).
Figure 1.
Workflow of the case-control study design.
2.2. Study Population
We conducted a case-control study using incident cases by extracting all newly diagnosed individuals with female cancers (e.g., ICD-9-CM codes 174 for breast, 180 for cervical, 182 for endometrial, and 183 for ovarian cancer) between 1 January 2002 and 31 December 2016. Those eligible patients were confirmed by the TCR database and defined as cases, and the date of a cancer diagnosis was defined as the index date. Individuals without any cancer diagnosis during the 17 years of the study served as controls. We randomly selected four controls among individuals for each case. Propensity score matching was applied for age, month, and year of diagnosis. Afterward, controls were assigned an index date corresponding to the diagnosis date of each case [24]. We excluded patients with cancer who were younger than 20 years of age and those who had missing, unidentifiable, or inconsistent data in this study.
2.3. PPI Exposure
Information about patients’ medications was retrieved from the prescription claims in the HWDC database. We collected medication information including drug codes, drug names, dispensing data, and the total daily dose for each prescription. PPIs were classified using Anatomical Therapeutic Chemical (ATC) code A02BC (see Appendix A). PPI exposure was analyzed only before the cancer diagnosis (e.g., index date). We considered whether patients had ever been exposed to PPI medications or not. Thus, patients with PPI prescriptions prescribed for at least 60 days within two years before the index date were classified as PPI users. In addition, patients who had never been prescribed any PPIs were defined as non-users.
2.4. Confounding Factors
The propensity score was determined using logistic regression, as proposed by Rosenbaum and Rubin (1983) [25], to estimate the probabilities of patients between cancer (case) and non-cancer (control) groups, as shown in Table 1. A number of potential confounding factors were included in the study. The known or suspected use of drugs can modify the risk of cancers or influence carcinogenic effects, including metformin (ATC, A10BA02) [26,27,28], aspirin (ATC, B01AC06) [28,29,30], and statins (ATC, C10AA) [31] (Table 1). Exposure to those drugs was defined if they were prescribed for at least two months (e.g., 60 days) within two years before the index date.
Table 1.
Baseline characteristics of cases and controls for female cancers.
| Characteristics | Cases (with Cancer) (n = 233,173) |
Controls (without Cancer) (n = 932,692) |
p-Value |
|---|---|---|---|
| Age | |||
| Mean ± SD | 52.05 ± 12.75 | 52.05 ± 12.74 | 1 |
| 20–39 years, n (%) | 36,968 (15.85) | 147,872 (15.85) | 1 |
| 40–64 years, n (%) | 156,120 (66.96) | 624,480 (66.96) | 1 |
| ≥65 years, n (%) | 40,085 (17.19) | 160,340 (17.19) | 1 |
| Comorbid conditions, n (%) | |||
| Myocardial infarction | 421 (0.18) | 1921 (0.21) | 0.015 |
| Congestive heart failure | 3202 (1.37) | 14,451 (1.55) | <0.0001 |
| Peripheral vascular disease | 1551 (0.67) | 7319 (0.78) | <0.0001 |
| Cerebrovascular disease | 8985 (3.85) | 41,794 (4.48) | <0.0001 |
| Dementia | 1583 (0.68) | 7660 (0.82) | <0.0001 |
| Chronic pulmonary disease | 6538 (2.8) | 29,240 (3.14) | <0.0001 |
| Rheumatic disease | 3140 (1.35) | 15,928 (1.71) | <0.0001 |
| Peptic ulcer disease | 25,949 (11.13) | 120,765 (12.95) | <0.0001 |
| Liver disease | 12,912 (5.54) | 59,355 (6.36) | <0.0001 |
| Diabetes | 29,356 (12.59) | 141,444 (15.17) | <0.0001 |
| Hemiplegia or paraplegia | 352 (0.15) | 1742 (0.19) | <0.001 |
| Renal disease | 4876 (2.09) | 22,314 (2.39) | <0.0001 |
| CCI | |||
| Mean ± SD | 0.47 ± 0.92 | 0.53 ±0.94 | |
| Other drugs, n (%) | |||
| Metformin | 21,758 (9.33) | 106,760 (11.46) | <0.0001 |
| Aspirin | 15,508 (6.65) | 73,217 (7.91) | <0.0001 |
| Statin | 17,244 (7.40) | 86,129 (9.26) | <0.0001 |
CCI, Charlson comorbidity index.
The competing risks could confound the chance of cancer; thus, we identified those comorbidities that might be associated with mortality based on diagnostic codes from outpatient and hospitalization data. Charlson comorbidities were included in the analysis, except for cancer. Those diseases were considered if patients were administrated at least twice two years before the index date.
2.5. Statistical Analysis
Conditional logistic regression with 95% confidence intervals (CIs) was applied to investigate the association between PPI exposure and cancer risk. The models were adjusted for those potential confounding factors in Table 1 and stratified by different age groups (e.g., young age, 20–39; middle-aged, 40–64; elderly, more than 65 years; and overall age groups). All data management was performed using SAS v.9.4 software (SAS Institute Inc., Cary, NC, USA). Statistical analysis was 2-sided, and a p-value < 0.05 indicated statistical significance.
3. Results
3.1. Baseline Characteristics
We identified 1,906,536 patients newly diagnosed with cancer from 2000 to 2016. A total of 233,173 patients newly diagnosed with a female cancer at an age of 20 years or older between 2002 and 2016 were included as cases; these included 135,437 patients diagnosed with breast cancer, 64,382 with cervical cancer, 19,580 with endometrial cancer, and 13,774 with ovarian cancer (Figure 1). After matching each case with four controls for age and sex, we included a total of 932,692 control female patients, and the number of controls for patients with breast, cervical, endometrial, and ovarian cancers was 541,748, 257,528, 78,320, and 55,096, respectively. Both cancer cases and controls had an average age of 52.05 years (Table 1). The 40–64 age group predominated in all four cancers, accounting for 66.96%. The prevalence of peptic ulcer disease (25,949/233,173) and diabetes (120,765/932,692) was the highest in the case group and was lower than the figures for control group by 1.82% and 2.09%, respectively. The frequency of metformin, aspirin, and statin use in the case group was lower than that in the control group by 2.13%, 1.26%, and 1.86%, respectively.
3.2. Associations of PPI Use with Overall Female Cancers
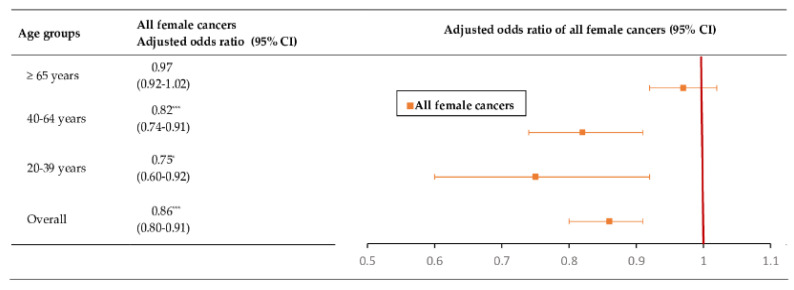
Figure 2 demonstrates the association between PPI use and female cancers among different age groups. Overall, PPI use was associated with a statistically significantly reduced risk of female cancers (adjusted odds ratio (aOR): 0.86, 95%CI: 0.80–0.91; p < 0.0001). The decrease in female cancer risk was found to be significantly associated with PPI users aged 20–39 years (aOR: 0.75, 95%CI: 0.60–0.92; p < 0.05) and 40–64 years (aOR: 0.82, 95%CI: 0.74–0.91; p < 0.0001); however, there was no significant association between PPI use and female cancer risk among those aged 65 years and older.
Figure 2.
The association of PPI use with overall risk of all female cancers by age groups with adjusted odds ratios. CI, confidence interval. Footnote: * p < 0.05, *** p < 0.0001.
3.3. Associations of PPI Use with Breast, Cervical, Endometrial, and Ovarian Cancers
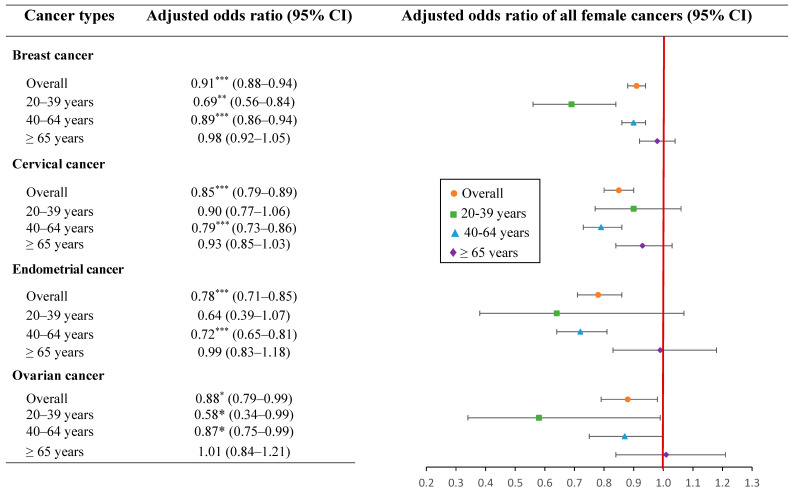
Figure 3 presents the breast, cervical, endometrial, and ovarian cancer risks among PPI users by age group. The use of PPIs was significantly associated with a reduced risk of breast and ovarian cancers in the groups aged 20–39 years (aOR: 0.69, 95%CI: 0.56–0.84; p < 0.001 and aOR: 0.58, 95%CI: 0.34–0.99; p < 0.05, respectively) and 40–64 years (aOR: 0.89, 95%CI: 0.86–0.94; p < 0.0001 and aOR: 0.87, 95%CI: 0.75–0.99; p < 0.05, respectively). PPI exposure was associated with a significant decrease in cervical and endometrial cancer risks in the group aged 40–64 years (aOR: 0.79, 95%CI: 0.73–0.86; p < 0.0001 and aOR: 0.72, 95%CI: 0.65–0.81; p < 0.0001, respectively). In addition, PPI use was associated with a lower risk of breast, cervical, endometrial, and ovarian cancers among females aged ≥65 years, but no significant association was found between these cancer risks and PPI use. Likewise, there was no statistically significant association between PPI use in the 20–39 age group and cervical and endometrial cancer risks.
Figure 3.
The association of PPI use with breast, cervical, endometrial, and ovarian cancer risks by age groups with adjusted odds ratios. CI, confidence interval. Footnote: * p < 0.05, ** p < 0.001, *** p < 0.0001.
4. Discussion
4.1. Main Findings
In this case-control study, we demonstrated an association between PPI use and risks of female cancers. PPI use was associated with a significant decrease in breast and ovarian cancers in users aged 20–64 years and a reduction in cervical and endometrial cancer risks in those aged 40–64 years. In contrast, in elderly women, our findings found that there was an insignificant association between PPI users and female cancer risks.
4.2. Biological Plausibility
4.2.1. Breast Cancer
As found in this study, the use of PPIs was linked to a lower risk of breast cancer. Probable mechanisms have been proposed to elucidate the potential antitumor activity of PPIs. First, the inhibition of the H+/K+-ATPase might contribute to the build-up of protons inside cells to reduce the intracellular pH, which prevents the development of breast cancer cells [32,33]. The association between esomeprazole, a PPI, and melanoma cell inhibition through a caspase-dependent pathway involving cytosolic acidification and alkalinization of the tumor pH was indicated in an in vitro study [34]. In addition, PPIs can directly suppress cancer development by targeting tumor-specific T cell-originated protein kinase through a proton pump-independent mechanism [35]. Second, PPIs also act as vacuolar H+-ATPase (V-ATPase) inhibitors, suggesting that they might affect the tumor acidic microenvironment and prevent the extracellular signal that controls the activity of kinase 1/2, Akt/Src kinases, and pyruvate kinase M2 from being phosphorylated. As a result, cancer cell growth might be inhibited or undergo apoptosis [36,37,38]. Ihraiz et al. (2020) investigated the effects of PPIs in three breast cancer cell lines including MCF-7, T47D, and MDA-MB-231 and revealed that PPI treatment was significantly associated with a decrease in breast cancer cells [15].
4.2.2. Cervical Cancer
Little evidence has indicated the direct mechanism of the association between PPI use and cervical cancer; however, regarding the treatment mechanism, PPI use increases the effectiveness of treatment, especially in resistant forms [19]. One of the major mechanisms might be that PPIs directly inhibit the V-ATPase, which plays a vital role in pumping protons across the plasma membrane and across the membranes of numerous intracellular compartments at the cellular level [19,39]. An in vitro study demonstrated the inhibition of the V-ATPase via siRNA or that PPIs might enhance the chemosensitivity of paclitaxel in cervical cancer cells [19]. Another mechanism is via fast intracellular acidification and activation of the caspase enzymes [37]. Lee et al. (2011) showed that omeprazole, a PPI, has the ability to mitigate the resistance of tumor cells to chemotherapy, by altering the process of the transfer of lysosomes, and activate programmed cell death mechanisms. In addition, other studies have indicated that a mixture of esomeprazole and amygdalin can suppress the development of cervical cancer cells in vitro [40,41].
4.2.3. Endometrial Cancer
The results highlighted that PPI use was statistically significantly associated with a decreased risk of endometrial cancer. There are two probable mechanisms that may explain our finding. First, PPIs induce chemosensitization and/or have an impact because of direct pH-dependent antitumor activity that might mediate the beneficial effects of PPIs [33]. Previous studies have indicated the possible antitumor impacts of PPIs [42,43,44]. Second, PPI treatment inhibits proliferation of cancer by inhibiting V-ATPases residing in the plasma membrane, including intracellular acidification and alkalization of the tumor microenvironment, which have a chemopreventive impact [45]. Numerous in vitro and in vivo studies have investigated the impacts of V-ATPase activity on several cancers such as pancreatic, breast, cervical, and prostate cancers and malignant melanomas [19,41,45,46,47]. It is therefore conceivable that PPIs block the V-ATPase to ultimately enhance cytotoxicity and apoptosis.
4.2.4. Ovarian Cancer
Regarding ovarian cancer risk in PPI users, our findings indicated a decreased risk of ovarian cancer in PPI users. Some possible mechanisms support our finding. First, the acidic microenvironment of cancer cells has been shown to be correlated with cancer aggressiveness, including increased invasiveness, angiogenesis, metastasis, and chemoresistance [48]. Furthermore, extracellular acidity suppresses the activity of cytotoxic T lymphocytes and natural killer cells, consequently decreasing antitumor defenses [49]. Tumors have adapted to acidic microenvironments through overexpression of proton pumps, which extrude protons out from the intracellular space of tumor cells. Earlier studies have demonstrated this phenomenon in numerous cell lines, including ovarian adenocarcinomas [19,50]. Second, the inhibition of V-ATPases reduces the acidity of the tumor microenvironment, which slows cell proliferation and triggers tumor cell apoptosis. Hence, PPIs might have antitumor activity and enhance the effectiveness of antitumor therapy through V-ATPase inhibition [51,52]. An in vitro study highlighted that omeprazole, a PPI, enhanced the impact of chemotherapeutic agents on chemoresistant epithelial ovarian cancer and clear cell carcinoma by reducing the acidic tumor microenvironment [17]. Furthermore, Lee et al.’s study (2015) revealed that elevated expression of V-ATpase mRNA was found to be significantly associated with poor survival in patients with ovarian cancer. Third, PPIs could potentially inhibit fatty acid synthase (FASN) using the crystal structure of FASN thioesterase, inducing apoptosis in chemosensitive and platinum-resistant ovarian cancer cells. PPI inhibition of FASN has been shown in in vitro and in vivo studies [53,54,55].
Interestingly, our results revealed that a significantly reduced risk of breast cancer was observed in PPI users aged 20–64 years, which was consistent with the findings of previous case-control studies [13,56,57]. In addition, evidence from an age stratification analysis in a cohort study indicated that the benefit increased with age, especially among older PPI users aged 50–65 years [58]. An Icelandic population-based case-control study, nevertheless, found no significant association between PPI use and breast cancer. This inconsistency could be due to the study population, sample size, and adjusted confounders [45]. Likewise, the decrease in ovarian cancer risk was significant in 20–64-year-old PPI users. Indeed, a previous study indicated that PPIs directly bind to the active site and inhibit FASN thioesterase, providing a crucial foundation for repositioning PPIs as anticancer treatments [53]. Regarding long-term and high-dose PPI treatment that has been demonstrated to be well tolerated in patients with few side effects, repositioning PPIs as anticancer medications will unlikely be associated with increased toxicity [10,59]. In terms of cervical and endometrial cancers, our findings showed that PPI drug use was associated with a significantly decreased cancer risk in females aged 40–64 years. Ballinger et al. (2022) conducted an observational study and clinical trials on postmenopausal women and demonstrated that taking PPI medications was not related to endometrial cancer; however, they found a trend in decreased risk with increasing PPI potency. Inconsistencies between our study and previous observational studies might be attributed to the study period, number of subjects, and adjusted confounders [16].
5. Strengths and Limitations
Our study possesses a number of strengths. This study features its high-quality registry data. Not only were all PPIs in Taiwan recorded in the HWDC databases, but cancer cases were also identified based on the Taiwan Cancer Registry database where all cancer diagnoses have been confirmed by pathology. Furthermore, the extensive database consisting of 23 million patients’ claims data enabled us to stratify subjects by age. To our knowledge, this current study is the first to involve a subgroup age analysis for female cancers and PPIs. Although a previous relevant study stratified individuals by age, it analyzed the association of PPI use with breast cancer risk rather than the risk of cervical, endometrial, and ovarian cancer risk [58].
We acknowledge that our study has its limitations. First, this study revealed associations instead of causality between PPIs and cancer risks. This study preliminarily showed potential cancer medication signals for clinicians or researchers to conduct and determine their causality or mechanisms in the future” to this sentence “This study preliminarily showed potential PPI medication signals and female cancer risks for clinicians or researchers to conduct and determine their causality or mechanisms in the future. Second, patient’ lifestyles, medication adherence, PPI dosage, and laboratory data were not provided in the HWDC database. Despite the unavailability of such data, all cancer diagnoses in our study were confirmed based on pathological reports, and the large sample size of millions of individuals could mitigate the impact caused by the lack of adherence data. Third, some of the established risk factors were not extracted from the HWDC database, such as hormone replacement therapy, oral contraception, obesity (e.g., BMI), HPV infection or vaccination, hypertension, hyperinsulinemia, and number of pregnancies/infertility, etc. Fourth, the other limitation is the retrospective format of the study. Finally, the results of this study cannot be generalized to other populations.
6. Conclusions
Overall, PPI use was significantly associated with decreased risks of breast, cervical, endometrial, and ovarian cancers. PPIs were associated with a significant decrease in breast and ovarian cancer risks in 20–64-year-old users and a reduction in cervical and endometrial cancer risks in those aged 40–64 years. Notably, our results should be interpreted with concern, because they demonstrate associations but not causality between PPI use and female cancer risks. We hope that our findings based on real-world big data can provide researchers and clinicians with some possible insights. Further clinical studies are needed to elucidate the effects of PPIs on female cancers.
Abbreviations
| aOR | Adjusted odds ratio |
| ATC Classification | Anatomical Therapeutic Chemical classification |
| CCI | Charlson comorbidity index |
| CI | Confidence interval |
| FASN | Fatty acid synthase |
| HWDC | Health and welfare data science |
| ICD-9-CM | International Classification of Diseases, 9th revision, Clinical Modification |
| MOHW | Ministry of Health and Welfare |
| NHI | National health insurance |
| PPI | Proton pump inhibitor |
| TMU-JIRB | Taipei Medical University—Joint Institutional Review Board |
| TCR | Taiwan Cancer Registry |
| V-ATPase | Vacuolar H+-ATPase |
Appendix A
Table A1.
The PPIs classification using Anatomical Therapeutic Chemical (ATC) code A02BC.
| ATC Code | Name | Available in Taiwan |
|---|---|---|
| A02BC01 | omeprazole | 1995~ |
| A02BC02 | pantoprazole | 1998~ |
| A02BC03 | lansoprazole | 2004~ |
| A02BC04 | rabeprazole | 2000~ |
| A02BC05 | esomeprazole | 2002~ |
| A02BC06 | dexlansoprazole | 2004~ |
| A02BC07 | dexrabeprazole | Not Available |
| A02BC08 | vonoprazan | Not Available |
| A02BC09 | tegoprazan | Not Available |
Author Contributions
Conceptualization, N.T.H.N., C.-W.H., C.-H.W., M.-C.L., J.C.H., M.-H.H., U.I., P.-A.N., and H.-C.Y.; methodology, N.T.H.N., C.-W.H., C.-H.W., U.I., P.-A.N., and H.-C.Y.; formal analysis, N.T.H.N., C.-W.H., C.-H.W., P.-A.N., and H.-C.Y.; writing—original draft preparation, N.T.H.N., C.-W.H., C.-H.W., M.-C.L., J.C.H., M.-H.H., U.I., P.-A.N., and H.-C.Y.; writing—review and editing, N.T.H.N., C.-W.H., C.-H.W., M.-C.L., J.C.H., M.-H.H., U.I., P.-A.N., and H.-C.Y.; visualization, N.T.H.N., P.-A.N., and H.-C.Y. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study’s ethical review was approved by Joint Institutional Review Board of Taipei Medical University (TMU-JIRB), Taipei, Taiwan (approval number: N202003609).
Informed Consent Statement
Informed consent was impossible and waived because all the data analyzed in this study were de-identified.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from databases of Health and Welfare Data Science Center and are available with the permission of Taiwan’s Ministry of Health and Welfare.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research is sponsored in part by the National Science and Technology Council (NSTC) under grant NSTC 110-2320-B-038-029-MY3 and NSTC 111-2321-B-038-004, and the Ministry of Education in Taiwan.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer. [(accessed on 25 September 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer.
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Breast Cancer Facts and Statistics. [(accessed on 25 September 2022)]. Available online: https://www.breastcancer.org/facts-statistics.
- 4.Zhang S., Xu H., Zhang L., Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2020;32:720–728. doi: 10.21147/j.issn.1000-9604.2020.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A., Pius C., Nabeel M., Nair M., Vishwanatha J.K., Ahmad S. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019;8:7018–7031. doi: 10.1002/cam4.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lheureux S., Gourley C., Vergote I., Oza A.M. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Barroilhet L., Behbakht K., Berchuck A. Ovarian Cancer, Version 2 2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 9.Endometrial Cancer Statistics. [(accessed on 25 September 2022)]. Available online: https://www.wcrf.org/cancer-trends/endometrial-cancer-statistics/
- 10.Thomson A.B., Sauve M.D., Kassam N., Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J. Gastroenterol. 2010;16:2323–2330. doi: 10.3748/wjg.v16.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotman S.R., Bishop T.F. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS ONE. 2013;8:56060. doi: 10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zink D.A., Pohlman M., Barnes M., Cannokn M.E. Long-term use of acid suppression started inappropriately during hospitalization. Aliment. Pharmacol. Ther. 2005;21:1203–1209. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen C.H., Lee C.Z., Lin Y.C., Kao L.T., Lin H.C. Negative Association of Proton Pump Inhibitors with Subsequent Development of Breast Cancer: A Nationwide Population-Based Study. J. Clin. Pharmacol. 2019;59:350–355. doi: 10.1002/jcph.1329. [DOI] [PubMed] [Google Scholar]
- 14.Kamal H., Sadr-Azodi O., Engstrand L., Brusselaers N. Association between Proton Pump Inhibitor Use and Biliary Tract Cancer Risk: A Swedish Population-Based Cohort Study. Hepatology. 2021;74:2021–2031. doi: 10.1002/hep.31914. [DOI] [PubMed] [Google Scholar]
- 15.Ihraiz W.G., Ahram M., Bardaweel S.K. Proton pump inhibitors enhance chemosensitivity, promote apoptosis, and suppress migration of breast cancer cells. Acta Pharm. 2020;70:179–190. doi: 10.2478/acph-2020-0020. [DOI] [PubMed] [Google Scholar]
- 16.Ballinger T.J., Djuric Z., Sardesai S., Hovey K., Andrews C., Braskey T.M. Proton Pump Inhibitor Use and Obesity-Associated Cancers in the Women’s Health Initiative. Cancer Epidemiol Biomark. Prev. 2022;31:1511. doi: 10.1158/1055-9965.EPI-22-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y.Y., Jeon H.K., Hong J.E., Cho Y.J., Ryu J.Y., Choi J.J. Proton pump inhibitors enhance the effects of cytotoxic agents in chemoresistant epithelial ovarian carcinoma. Oncotarget. 2015;6:35040–35050. doi: 10.18632/oncotarget.5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J., Shi X.Y., Li Z.M., Pan X.H., Li Z.L., Che Y. Proton pump inhibitors can reverse the YAP mediated paclitaxel resistance in epithelial ovarian cancer. BMC Mol. Cell Biol. 2019;20:49. doi: 10.1186/s12860-019-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song T., Jeon H.K., Hong J.E., Choi J.J., Kim T.J., Choi C.H. Proton Pump Inhibition Enhances the Cytotoxicity of Paclitaxel in Cervical Cancer. Cancer Res. Treat. 2017;49:595–606. doi: 10.4143/crt.2016.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cancer Registry Annual Report, 2019, Taiwan. [(accessed on 25 September 2022)]; Available online: https://www.hpa.gov.tw/Pages/List.aspx?nodeid=269.
- 21.Hsieh C.Y., Su C.C., Shao S.C., Sung S.F., Lin S.J., Kao Yang Y.H. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019;11:349–358. doi: 10.2147/CLEP.S196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Insurance Administration, MOHAW, Taiwan; [(accessed on 25 September 2022)]. Universal Health Coverage in Taiwan. Available online: https://www.nhi.gov.tw/English/Content_List.aspx?n=4D7051840BF42F52&topn=ED4A30E51A609E49. [Google Scholar]
- 23.ICD-9-CM and ICD-10-CM/PCS Mapping Table. National Health Insurance Administration, MOHAW; Taipei, Taiwan: 2020. [Google Scholar]
- 24.Grimes D.A., Schulz K.F. Compared to what? Finding controls for case-control studies. Lancet. 2005;365:1429–1433. doi: 10.1016/S0140-6736(05)66379-9. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum P.A., Rubin B.D. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biom. J. 1983;70:41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 26.Gadducci A., Biglia N., Tana R., Cosio S., Gallo M. Metformin use and gynecological cancers: A novel treatment option emerging from drug repositioning. Crit. Rev. Oncol. Hematol. 2016;105:73–83. doi: 10.1016/j.critrevonc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Lee D.Y., Lee T.S. Associations between metabolic syndrome and gynecologic cancer. Obstet. Gynecol. Sci. 2020;63:215–224. doi: 10.5468/ogs.2020.63.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y.H., Wang P.H., Chen P.N., Yang S.F., Hsiao Y.H. Molecular and Cellular Mechanisms of Metformin in Cervical Cancer. Cancers. 2021;13:2545. doi: 10.3390/cancers13112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Zhao J., Chen X., Zhang F., Li X. Aspirin use and endometrial cancer risk: A meta-analysis and systematic review. Ann. Transl. Med. 2020;8:461. doi: 10.21037/atm.2020.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D., Bai B., Xi Y., Wang T., Zhao Y. Is aspirin use associated with a decreased risk of ovarian cancer? A systematic review and meta-analysis of observational studies with dose-response analysis. Gynecol. Oncol. 2016;142:368–377. doi: 10.1016/j.ygyno.2016.04.543. [DOI] [PubMed] [Google Scholar]
- 31.Markowska A., Antoszczak M., Markowska J., Huczyński A. Statins: HMG-CoA Reductase Inhibitors as Potential Anticancer Agents against Malignant Neoplasms in Women. Pharmaceuticals. 2020;13:422. doi: 10.3390/ph13120422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan-Yu L., Sun L.N., Zhang X.H., Li Y.Q., Yu L., Yuan Z.Q.Y. A Review of the Novel Application and Potential Adverse Effects of Proton Pump Inhibitors. Adv. Ther. 2017;34:1070–1086. doi: 10.1007/s12325-017-0532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang B.Y., Zhang J., Wang J.L., Sun S., Wang Z.H., Wang L.P. Intermittent high dose proton pump inhibitor enhances the antitumor effects of chemotherapy in metastatic breast cancer. J. Exp. Clin. Cancer Res. 2015;34:85. doi: 10.1186/s13046-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marino M.L., Fais S., Djavaheri-Mergny M., Villa A., Meschini S., Lozupone F. Proton pump inhibition induces autophagy as a survival mechanism following oxidative stress in human melanoma cells. Cell Death Dis. 2010;1:87. doi: 10.1038/cddis.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh W., Sleptsova-Freidrich I., Petrovic N. Use of proton pump inhibitors as adjunct treatment for triple-negative breast cancers. An introductory study. J. Pharm. Pharm. Sci. 2014;17:439–446. doi: 10.18433/J34608. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y., Chen M., Huang S., Zou X. Pantoprazole inhibits human gastric adenocarcinoma SGC-7901 cells by downregulating the expression of pyruvate kinase M2. Oncol. Lett. 2016;11:717–722. doi: 10.3892/ol.2015.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Milito A., Lessi E., Logozzi M., Lozupone F., Spada M., Marino M.L. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007;67:5408–5417. doi: 10.1158/0008-5472.CAN-06-4095. [DOI] [PubMed] [Google Scholar]
- 38.Fais S., De Milito A., You H., Qin W. Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 2007;67:10627–10630. doi: 10.1158/0008-5472.CAN-07-1805. [DOI] [PubMed] [Google Scholar]
- 39.Mizunashi K., Furukawa Y., Katano K., Abe K. Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone resorption in humans. Calcif. Tissue Int. 1993;53:21–25. doi: 10.1007/BF01352010. [DOI] [PubMed] [Google Scholar]
- 40.Nishi T., Forgac M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 2002;3:94–103. doi: 10.1038/nrm729. [DOI] [PubMed] [Google Scholar]
- 41.Larsson H., Mattson H., Sundell G., Carlsson E. Animal pharmacodynamics of omeprazole. A survey of its pharmacological properties in vivo. Scand. J. Gastroenterol. Suppl. 1985;108:23–35. doi: 10.3109/00365528509095817. [DOI] [PubMed] [Google Scholar]
- 42.De Milito A., Canese R., Marino M.L., Borghi M., Lero M., Villa A. pH-dependent antitumor activity of proton pump inhibitors against human melanoma is mediated by inhibition of tumor acidity. Int. J. Cancer. 2010;127:207–219. doi: 10.1002/ijc.25009. [DOI] [PubMed] [Google Scholar]
- 43.Ferrari S., Perut F., Fagioli F., Brach Del Prever A., Meazza C., Parafioriti A. Proton pump inhibitor chemosensitization in human osteosarcoma: From the bench to the patients’ bed. J. Transl. Med. 2013;11:268. doi: 10.1186/1479-5876-11-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spugnini E.P., Buglioni S., Carocci F., Francesco M., Vincenzi B., Fanciulli M. High dose lansoprazole combined with metronomic chemotherapy: A phase I/II study in companion animals with spontaneously occurring tumors. J. Transl. Med. 2014;12:225. doi: 10.1186/s12967-014-0225-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hálfdánarson Ó., Fall K., Ogmundsdottir M.H., Lund S.H., Steingrímsson E., Ogmundsdottir H.M. Proton pump inhibitor use and risk of breast cancer, prostate cancer, and malignant melanoma: An Icelandic population-based case-control study. Pharmacoepidemiol. Drug Saf. 2019;28:471–478. doi: 10.1002/pds.4702. [DOI] [PubMed] [Google Scholar]
- 46.Yu M., Lee C., Wang M., Tannock I.F. Influence of the proton pump inhibitor lansoprazole on distribution and activity of doxorubicin in solid tumors. Cancer Sci. 2015;106:1438–1447. doi: 10.1111/cas.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabolić I., Brown D., Verbavatz J.M., Kleinman J. H(+)-ATPases of renal cortical and medullary endosomes are differentially sensitive to Sch-28080 and omeprazole. Am. J. Physiol. 1994;266:868–877. doi: 10.1152/ajprenal.1994.266.6.F868. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Wahab A.F., Mahmoud W., Al-Harizy R.M. Targeting glucose metabolism to suppress cancer progression: Prospective of anti-glycolytic cancer therapy. Pharmacol. Res. 2019;150:104511. doi: 10.1016/j.phrs.2019.104511. [DOI] [PubMed] [Google Scholar]
- 49.Loeffler D.A., Juneau P.L., Heppner G.H. Natural killer-cell activity under conditions reflective of tumor micro-environment. Int. J. Cancer. 1991;48:895–899. doi: 10.1002/ijc.2910480617. [DOI] [PubMed] [Google Scholar]
- 50.Luciani F., Spada M., De Milito A., Molinari A., Rivoltini L., Montinaro A. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl. Cancer Inst. 2004;96:1702–1713. doi: 10.1093/jnci/djh305. [DOI] [PubMed] [Google Scholar]
- 51.Ikemura K., Hiramatsu S., Okuda M. Drug Repositioning of Proton Pump Inhibitors for Enhanced Efficacy and Safety of Cancer Chemotherapy. Front. Pharmacol. 2017;8:911. doi: 10.3389/fphar.2017.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tozzi M., Sørensen C.E., Magni L., Christensen N.M., Bouazzi R., Buch C.M. Proton Pump Inhibitors Reduce Pancreatic Adenocarcinoma Progression by Selectively Targeting H(+), K(+)-ATPases in Pancreatic Cancer and Stellate Cells. Cancers. 2020;12:640. doi: 10.3390/cancers12030640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fako V.E., Wu X., Pflug B., Liu J.Y., Zhang J.T. Repositioning proton pump inhibitors as anticancer drugs by targeting the thioesterase domain of human fatty acid synthase. J. Med. Chem. 2015;58:778–784. doi: 10.1021/jm501543u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bauerschlag D.O., Maass N., Leonhardt P., Verburg F.A., Pecks U., Zeppernick F. Fatty acid synthase overexpression: Target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 2015;13:146. doi: 10.1186/s12967-015-0511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uddin S., Jehan Z., Ahmed M., Alyan A., Al-Dayel F., Hussain A. Overexpression of fatty acid synthase in Middle Eastern epithelial ovarian carcinoma activates AKT and Its inhibition potentiates cisplatin-induced apoptosis. Mol. Med. 2011;17:635–645. doi: 10.2119/molmed.2011.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan M.-M., Ho W.K., Yoon S.Y., Mariapun S., Hasan S.N., Lee D.S.C. A case-control study of breast cancer risk factors in 7663 women in Malaysia. PLoS ONE. 2018;13:0203469. doi: 10.1371/journal.pone.0203469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jingtao Hu W.H., Chenyu Sun C.C., Yue L. Association between the Use of Proton Pump Inhibitors with the Risk and the Prognosis of Breast Cancer: A Systematical Review. World J. Surg. 2021;4:1327. [Google Scholar]
- 58.Ding D.C., Sung F.C., Chen W., Wang J.H., Lin S.Z. Proton pump inhibitors reduce breast cancer risk in gastric ulcer patients: A population-based cohort study. Breast J. 2020;26:474–478. doi: 10.1111/tbj.13519. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y.X., Metz D.C. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from databases of Health and Welfare Data Science Center and are available with the permission of Taiwan’s Ministry of Health and Welfare.