Abstract
Three genes involved in biosynthesis of the lipooligosaccharide (LOS) core of Campylobacter jejuni MSC57360, the type strain of the HS:1 serotype, whose structure mimics GM2 ganglioside, have been cloned and characterized. Mutation of genes encoding proteins with homology to a sialyl transferase (cstII) and a putative N-acetylmannosamine synthetase (neuC1), part of the biosynthetic pathway of N-acetylneuraminic acid (NeuNAc), have identical phenotypes. The LOS cores of these mutants display identical changes in electrophoretic mobility, loss of reactivity with cholera toxin (CT), and enhanced immunoreactivity with a hyperimmune polyclonal antiserum generated against whole cells of C. jejuni MSC57360. Loss of sialic acid in the core of the neuC1 mutant was confirmed by fast atom bombardment mass spectrometry. Mutation of a gene encoding a putative β-1,4-N-acetylgalactosaminyltransferase (Cgt) resulted in LOS cores intermediate in electrophoretic mobility between that of wild type and the mutants lacking NeuNAc, loss of reactivity with CT, and a reduced immunoreactivity with hyperimmune antiserum. Chemical analyses confirmed the loss of N-acetylgalactosamine (GalNAc) and the presence of NeuNAc in the cgt mutant. These data suggest that the Cgt enzyme is capable of transferring GalNAc to an acceptor with or without NeuNAc and that the Cst enzyme is capable of transferring NeuNAc to an acceptor with or without GalNAc. A mutant with a nonsialylated LOS core is more sensitive to the bactericidal effects of human sera than the wild type or the mutant lacking GalNAc.
Campylobacter jejuni and Campylobacter coli are among the most prevalent causes of bacterial diarrhea in the world (15, 31). These organisms are antigenically complex, as demonstrated by the fact that there are >70 serotypes based on heat-stable (HS) antigens (34) and >100 serotypes based on the heat-labile serotyping scheme (26). Campylobacters contain sialic acid moieties both in lipooligosaccharide (LOS) core structures (3–6, 29) and in posttranslational modifications on flagellin (9).
Structural analyses of a limited number of campylobacter strains has revealed LOS-like variability in the outer core (28, 29). Moreover, the outer cores of strains from multiple serogroups contain sialic acid moieties in structures which mimic human gangliosides. This molecular mimicry is thought to result in an autoimmune response responsible for the association of some campylobacter serotypes with Guillain-Barré syndrome (GBS) (1, 28, 29). However, the biological function of sialylated LOS to pathogenesis of gastroenteritis by C. jejuni has not been examined experimentally.
Campylobacter spp. are capable of endogenous biosynthesis of sialic acid (3–6, 9, 29). The genome of C. jejuni NCTC 11168 contains multiple genes which encode proteins with similarity to prokaryotic enzymes involved in biosynthesis of sialic acid, N-acetylneuraminic acid (NeuNAc) (33). For example, NCTC 11168 has three copies of genes encoding proteins with sequence similarity to sialic acid synthases (25), the enzymes which condense N-acetylmannosamine (ManNAc) and phosphenolpyruvate to form NeuNAc. Mutation of neuB1 (cj1141) resulted in loss of NeuNAc from the LOS core in NCTC 11168 (25). Mutations in neuB2 and neuB3 had no affect on LOS but affected the apparent Mr of flagellin on sodium dodecyl sulfate-polyacrylamide gels and resulted in loss of motility, respectively (25). In addition, Gilbert et al. have described two distinct sialyl transferase activities and a β-1,3-N-acetylgalactosaminyltransferase (GalNAc transferase) in an HS:19 isolate from a GBS patient (16). Herein, we describe a set of genes responsible for NeuNAc biosynthesis in C. jejuni MSC57360, the type strain of the HS:1 serogroup, which has been shown to contain an LOS core which mimics GM2 ganglioside in structure (5), as seen in Fig. 1A. Moreover, we demonstrate that loss of NeuNAc from the LOS core of MSC57360 affects the immunogenicity of the core and the serum sensitivity of the bacterium.
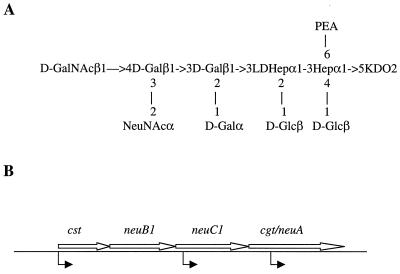
FIG. 1.
(A) Structure of the LOS core of MSC57360 (5). Abbreviations: PEA, O-phosphoethanolamine; KDO, 3-deoxy-d-manno-2-octulosonic acid; LDHep, l-glycero-d-manno-heptose; Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine; NeuNAc, N-acetylneuraminic acid. (B) Sialic acid locus of MSC57360. The lengths of the following ORFs were as indicated: cst, 881 bp; neuB1, 1,029 bp; neuC1, 1,113 bp; and cgt-neuA, 1,608 bp. The position of insertion of a chloramphenicol resistance (Cmr) cassette is indicated by the arrows below the line. The insertion into neuC1 was constructed by insertion of the Cmr cassette into a unique NdeI site which is located 118 bp into the ORF. The insertions into cst and cgt-neuA were constructed by in vitro transposition, and the position was determined by DNA sequence analysis, as described in Materials and Methods. The position of the insertion into cst was 12 bp into the ORF, and the insertion into the cgt-neuA gene was located 396 bp into the ORF. In all three mutants the Cmr cassette was inserted in the same orientation as the genes are transcribed.
MATERIALS AND METHODS
Strains and growth conditions.
C. jejuni MSC57360 (5) was a gift from John Penner, University of Toronto. C. jejuni strains were routinely grown on Mueller-Hinton (MH) agar, supplemented with kanamycin (50 μg/ml) or chloramphenicol (15 μg/ml) when appropriate at 37°C in a microaerobic environment. Escherichia coli XL-1 Blue was the host for λ ZAP Express, and DH5α was the host for routine cloning. E. coli strains were grown on Luria agar, supplemented with ampicillin (50 μg/ml), kanamycin (50 μg/ml), or chloramphenicol (20 μg/ml) when appropriate.
Molecular cloning.
Restriction enzymes and modifying enzymes were purchased from New England Biolabs (Beverly, Mass.) and used as recommended by the supplier. MSC57360 genes were cloned from a partial Sau3A library constructed in λ ZAP Express (Stratagene, La Jolla, Calif.). The library was probed with a PCR product specific for cj1142 of NCTC 11168 (see below) which had been purified by agarose gel electrophoresis and labeled by random priming (Boehringer Mannheim, Indianapolis, Ind.) with [32P]dCTP (New England Nuclear, Boston, Mass.). Positive clones were plaque purified, rehybridized, and, once a pure population of positive phage was obtained, excised to the phagemid pBK-CMV, according to the instructions of the manufacturer.
The primers used to amplify the neuC1 gene of MSC57360, designed based on the sequence of cj1142 (33), were NEU2-F, 5′-GGTGATAGAGTGGAGCCTTTAGCTG-3′, and NEU2-B, 5′-GTCAGTTCTACCATCTTGTCTTGAACC-3′. The PCR conditions used were 30 cycles of 94°C for 1 min, 49°C for 1 min, and 72°C for 1 min. The 630-bp product was sequenced using the same primers to confirm the identity of the product.
DNA sequence analysis.
Plasmid DNAs were sequenced on both strands using terminator chemistries and Taq cycle sequencing kits from Perkin-Elmer Applied Biosystems (Foster City, Calif.) and analyzed on an Applied Biosystems model 377 DNA sequencer. Custom primers were synthesized on an Applied Biosystems model 292 DNA-RNA synthesizer. Sequences were assembled using Sequencher (Gene Codes Corporation, Ann Arbor, Mich.) and analyzed using MacVector (Oxford Molecular, Oxford, United Kingdom). DNA and protein searches were performed using BLAST analyses via the National Center for Biotechnology Information, Bethesda, Md., and the Sanger Genomic Sequencing Site (http://www.sanger.ac.uk/Projects/C. jejuni).
Site-specific mutagenesis of campylobacter genes.
The neuC1 mutant was constructed by insertion of a campylobacter chloramphenicol resistance cassette (50) into an NdeI site as indicated in Fig. 1. The orientation of the Cmr cassette was confirmed by PCR to be in the same orientation as the target gene. All other mutants were constructed using the in vitro Tn5-based transposition system (17) in which the Cmr cassette from pRY109 was first cloned into EZ::TN pMOD transposon vector (Epicentre, Madison, Wis.). The transposon was PCR amplified with primers specified by Epicentre and used in an in vitro transposition system with the target plasmid DNAs, pMSC203 and pMSC209. The reaction was transformed into E. coli DH5α by standard methods, and plasmid DNAs from individual transformants were sequenced using primers which read out from within the Cmr cassette (48) to determine the insertion point and orientation with respect to transcription of the target gene. Selected insertions were transferred into C. jejuni MSC57360 by natural transformation (19) with selection on MH agar supplemented with 15 μg of chloramphenicol per ml.
Complementation in trans.
The complete 4.2-kb insert in the pBK-CMV (the excision plasmid of λ ZAP Express)-based plasmid, pMSC209, was subcloned using sites bracketing the insert in the multiple cloning site (EcoRI-SalI) into the Kmr campylobacter shuttle plasmid, pRY107 (50). This insert, which extended from 1,058 bp upstream of the start of cst to 138 bp into the start of cgt, contained all of cst, neuB1, and neuC1. This plasmid, termed pMSC1420, was conjugatively mobilized from DH5α (RK212.2) (11) into MSC57360 neuC1 with selection on trimethoprim (10 μg/ml), chloramphenicol (20 μg/ml), and kanamycin (50 μg/ml) as previously described (19).
Purification of LOS.
Biomass of C. jejuni MSC57360 and mutant strains, which had been grown as described above, was subjected to hot phenol-water extraction (49). Subsequently, the crude LOS from the water phase of extracts was purified by enzymatic treatments with RNase A, DNase II, and proteinase K and by ultracentrifugation, as described previously (30).
Thin-layer chromatography analysis and chemical characterization of LOS.
The purified LOS preparations from C. jejuni MSC57360 and the neuC1 and cgt-neuA1 mutants were tested for reaction with the ganglioside-binding ligands of cholera toxin (CT) using a thin-layer chromatography-immunostaining technique with peroxidase conjugates of both ligands according to the procedure of Prendergast et al. (38). For chemical analysis, NeuNAc was detected and characterized as its peracetylated methyl ketoside methyl ester derivative, which was obtained after acidic methanolysis (1 M HCl, 86°C, 1 h) of LOS and peracetylation under the conditions described previously (30). Analysis of these derivatives was performed by gas-liquid chromatography (GLC) using a Hewlett-Packard 5890 series II gas chromatograph equipped with an HP-5 fused-silica capillary column and temperature program of 170°C for 3 min, increasing to 260°C at 3°C/min, and by GLC-mass spectrometry (MS) using the same chromatograph attached to a mass selective detector (model 5971A). Bound NeuNAc was estimated colorimetrically in a modified Ehrlich reaction assay (9) and also quantitated by determination of peracetylated methyl ketoside methyl esters in GLC. The methylated core oligosaccharides were examined in fast atom bombardment (FAB)-MS. First, core oligosaccharide was liberated from LOS by mild acid hydrolysis with 1% acetic acid at 100°C for 1 h and isolated by gel permeation chromatography, and the oligosaccharides (400 to 500 μg) were methylated (6). Subsequently, the FAB-MS spectra of the permethylated sample in methanol (1 to 2 μl) were recorded using an instrument equipped with an Ion Tech saddle field gun under the conditions described previously (6). Interpretations of positive ion mass spectra of permethylated derivatives were as used in earlier studies (4–6).
Generation of polyclonal antiserum against whole cells of MSC57360.
The experiments were conducted according to the principles set forth previously (8a). A New Zealand White rabbit was immunized intramuscularly with whole cells of C. jejuni MSC57360 which had been inactivated in 0.5% formaldehyde and washed extensively in phosphate-buffered saline (PBS). For the first immunization the antigen was adjuvanted with Freund's complete adjuvant. For a second boost, given 2 weeks after the first immunization, the antigen was mixed with Freund's incomplete adjuvant. The animal was exsanguinated 2 weeks after the second immunization.
Electrophoresis and Western blotting.
Campylobacter whole cells were digested with proteinase K (21) and electrophoresed on either 16% Tricine gels (Novex, San Diego, Calif.) or SDS–12.5% PAGE gels. LOS cores were visualized by silver staining (Bio-Rad, Hercules, Calif.). Horseradish peroxidase-labeled CT (Calbiochem, La Jolla, Calif.) was used at a final concentration of 1 μg/ml and was detected with 3,3′,5,5′-tetramethylbenzidine (Sigma, St. Louis, Mo.). Rabbit polyclonal antibody (described above) was used at a final dilution of 1:500 and detected with goat anti-rabbit immunoglobulin G (Caltag, Burlingame, CA).
Flagellin purification.
Flagellin was purified from campylobacter strains as described by Power et al. (37).
IEF of flagellins.
Isoelectric focusing (IEF) was performed using ampholytes with a pI range of 4 to 6 (Biolyte4/6; Bio-Rad) as described previously (8).
Serum sensitivity assays.
Serum sensitivity assays were done by a modification of the method of Blaser et al. (8). C. jejuni strains were grown overnight in MH biphasic cultures at 37°C, washed in PBS, pH 7.4, and adjusted to a concentration of 106 CFU/ml. Campylobacter cells (100-μl aliquots) were incubated in pools of human sera diluted to a final concentration of 10% in PBS for 30 and 60 min at 37°C. Controls consisted of bacteria incubated in PBS. Serum controls consisted of pooled human sera which had been heated to 56°C for 45 min to inactivate complement. Following the incubation period, CFU were enumerated on MH agar.
Statistical analyses.
Individual results from serum sensitivity assays were compared by using two-tailed t tests assuming equal variance between test samples.
Nucleotide sequence accession number.
The DNA sequences described here have been deposited in GenBank under accession number AF257460.
RESULTS
Identification and characterization of a set of sialic acid biosynthetic genes in MSC57360.
PCR primers were designed based on cj1142, annotated in the genome sequence of NCTC 11168 as neuC1 (see Materials and Methods), and a PCR product of the predicted size was generated from C. jejuni MSC57360 DNA. Direct DNA sequencing of the PCR product confirmed that the DNA encoded a predicted protein with significant sequence similarity to the siaA gene product of Neisseria menigitidis (11, 41), as well as lower scores to the neuC gene product of E. coli K1 (51; data not shown). This PCR product was used as a probe to clone the full-length gene from a λ ZAP Express library of MSC57360. Several overlapping clones were identified, and two, pMSC209 and pMSC203, were used as templates for DNA sequence analysis. The results confirmed that homologs of the Neisseria pathway for sialic acid biosynthesis were located on these clones in an apparent operon, as seen in Fig. 1B. Moreover, the gene order is identical to that described for the HS:2 strain, NCTC 11168 (25, 33).
The open reading frames (ORFs) found on the MSC57360 clones are summarized in Table 1. The first gene in the operon encodes a predicted protein of 35.1 kD with 100% identity to cj1140 from NCTC 11168, which was annotated by Parkhill et al. (33) as an unknown. However, the gene product also shows 53% identity and 69% similarity to a predicted protein encoded by the cstII gene of C. jejuni OH4384, which was shown by Gilbert et al. to be a bifunctional sialyl transferase, capable of adding NeuNAc by an α-2,3 linkage to d-galactose and by an α-2,8 linkage to NeuNAc (16). The MSC57360 protein also shows 32% identity and 33% similarity to CstI, an α-2,3 sialyl transferase also found in C. jejuni OH4384 (16).
TABLE 1.
Homology of predicted proteins of MSC57360 ORFs
| ORF | Size (amino acids) | Gene(s) | Protein homolog (straina) | % Identity (% similarity) | Proposed function | Reference(s) |
|---|---|---|---|---|---|---|
| 1 | 294 | cst | cj1140 (11168) | 100 (100) | Unknown | 33 |
| CstII (OH4384) | 53 (69) | 2,3- and 2,8-sialyl transferase | 16 | |||
| CstI (OH4384) | 32 (33) | 2,3-sialyl transferase | 16 | |||
| 2 | 343 | neuB1 | cj1141 (11168) | 99 (99) | NeuNAc synthase | 25, 33 |
| orf8a (OH4384) | 76 (86) | NeuNAc synthase | 16 | |||
| 3 | 371 | neuC1 | cj1142 (11168) | 100 | ManNAc synthesis | 33 |
| orf9a (OH4384) | 65 (76) | ManNAc synthesis | 16 | |||
| 4 | 536 | cgt neuA | cj1143 (11168) | 97 (97) | Fusion of CgtA and CMP-NeuNAc synthetase | 33 |
| CgtA (OH4384) | 53 (69b) | Cgt | 16 | |||
| NeuA (OH4384) | 68 (83c) | CMP-NeuNAc synthetase | 16 |
11168 refers to C. jejuni NCTC 11168.
Homology to CgtA is confined to the amino terminal 280 amino acids.
Homology to NeuA is confined to the carboxy terminal 218 amino acids.
The second ORF, which overlaps cst by 16 bp, encodes a predicted protein of 38.4 kDa that differs from cj1141 of NCTC 11168 by only 2 amino acids. This protein was designated neuB1 by Parkhill et al. (33) based on its homology to the NeuNAc synthase of N. menigitidis, SiaC (11, 41). The corresponding enzyme in E. coli K1 is known as NeuB (43), from which the nomenclature was derived. The MSC57360 NeuB1 protein also shows 76% identity and 86% similarity to the corresponding enzyme from OH4384 (16).
ORF3 starts at the same base pair that ORF2 stops at and encodes a predicted protein of 42.7 kDa which shows 100% identity to cj1142 or NeuC1, designated by Parkhill et al. (33) as a putative N-acetylglucosamine(GlcNAc)-6-phosphate 2-epimerase–GlcNAc-6-phosphatase based on the high level of homology to the corresponding enzyme, SiaA (11, 35, 41), in N. menigitidis (43% identity and 63% similarity). This enzyme is involved in biosynthesis of ManNAc, the precursor of NeuNAc (35). The MSC57360 and NCTC 11168 proteins show 65% identity and 76% similarity to the corresponding protein in OH4384 (16).
The start of ORF4 overlaps with the stop codon of ORF3 and encodes a predicted protein of 62.5 kDa. This protein shows 97% identity with cj1143 from NCTC 11168 (33) over the full length (536 amino acids). Protein cj1143 was annotated as a CMP-NeuNAc synthetase by Linton et al. (25). However, the homology of cj1143 and ORF4 of MSC57360 to known CMP-NeuNAc synthetases is limited to the carboxy-terminal 218 amino acids. This region also shows 67% identity and 80% similarity to a putative CMP-NeuNAc synthetase described in OH4384 (16) and 38% identity and 57% similarity to a known CMP-NeuNAc synthetase from Hemophilus ducreyi (42). The N-terminal 280 amino acids of ORF4 shows 44 to 45% identity and 54 to 55% similarity to two β-1,4-N-acetylgalactosaminyltransferase (Cgt) enzymes (GalNAc transferases) from OH4384 and another C. jejuni HS:19 isolate (16). Thus, this ORF in both MSC57360 and NCTC 11168 appears to represent a fusion of the cgt and neuA1 genes.
Insertional mutagenesis of MSC57360 LOS genes.
A Cmr cassette (50) was inserted as a SmaI-ended fragment into a unique NdeI site within neuC1 which had been blunted by treatment with Klenow enzyme. This plasmid, called pMSC203::Cm was used to transform MSC57360. Subsequent mutations into cst and cgt-neuA were generated in E. coli DH5α by in vitro transposition of a Cmr cassette (50) as described in Materials and Methods. The position and orientation of the transposon insertions into individual plasmids was determined by DNA sequence analysis, and selected insertions were transformed into MSC57360. All C. jejuni transformants were characterized by PCR to confirm that the insert had integrated via double crossover (data not shown).
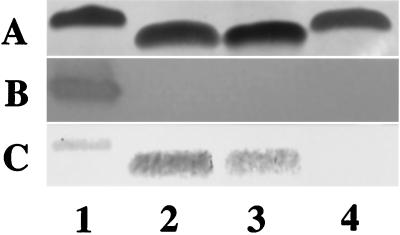
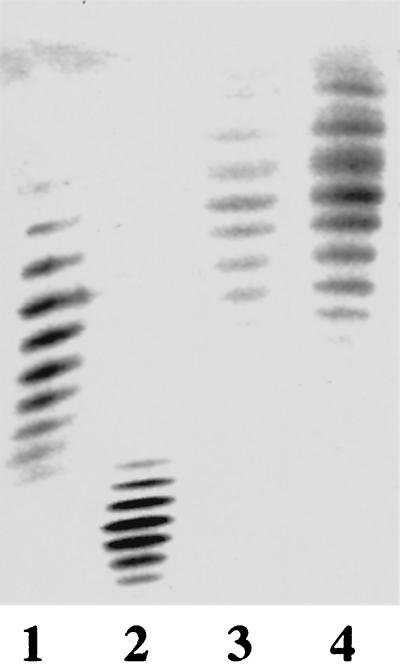
Proteinase K-treated whole cells from MSC57360 and the mutants were electrophoresed on Tricine gels and silver stained to visualize LOS cores. The results, shown in Fig. 2A, indicate that the mobility of the cores of cst (lane 2) and neuC1 (lane 3) mutants were identical to one another but were reduced in apparent Mr compared to the wild type (lane 1). The cgt-neuA mutant (lane 4) displayed an intermediate mobility between that of the wild type and the cst and neuC1 mutants. Figure 2B shows the reaction of the LOS cores with CT; all three mutants have lost reactivity with CT. Similar loss of reactivity with CT was observed with purified LOS (data not shown). Figure 2C shows an immunoblot of the same whole-cell digests which have been immunodetected with a polyclonal rabbit antiserum generated against whole cells of MSC57360. The results indicate that the cst (lane 2) and neuC1 (lane 3) mutants showed enhanced immunoreactivity compared to wild-type MSC57360 (lane 1). The LOS core of the cgt-neuA mutant, however, was not detected at the antibody dilution used (lane 4).
FIG. 2.
Comparison of LOS of MSC57360 and mutants. Proteinase K-digested whole-cell preparations were electrophoresed on 16% Tricine gels and silver stained (A), reacted with CT (final concentration, 1 μg/ml) (B), or immunodetected with polyclonal rabbit antiserum against whole cells of MSC57360 (final dilution, 1:500) (C). Lane 1, MSC57360; lane 2, MSC57360 cst; lane 3, MSC57360 neuC1; lane 4, MSC57360 cgt. The apparent Mr of the LOS core of wild-type MSC57360 on Tricine gels is approximately 9.2 kDa.
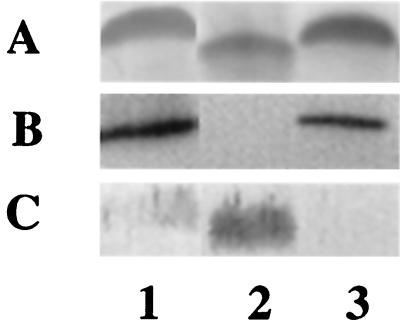
To confirm that the insertion into neuC1 was not exerting a polar effect on cgt-neuA, a Kmr shuttle plasmid (50) (pMSC1420) containing the cst, neuB1, and neuC1 genes was transferred into the neuC1 mutant. As seen in Fig. 3, the mobility of the core was restored to that of the wild type, CT binding was restored, and the immunoreactivity with the anti-MSC57360 antibody was reduced (lanes 3).
FIG. 3.
Complementation of MSC57360 neuC1 in trans. Plasmid pMSC1420 was conjugally mobilized from E. coli DH5α (RK212.2) into MSC57360 neuC1. Proteinase K-digested whole-cell preparations were electrophoresed on 16% Tricine gels and silver stained (A), reacted with CT (final concentration, 1 μg/ml) (B) or immunodetected with polyclonal rabbit antiserum against whole cells of MSC57360 (final dilution, 1:500) (C). Lane 1, MSC57360; lane 2, MSC57360 neuC1; lane 3, MSC57360 neuC1 (pMSC1420).
Chemical characterization of the LOS core of the neuC1 and cgt-neuA mutants of MSC57360.
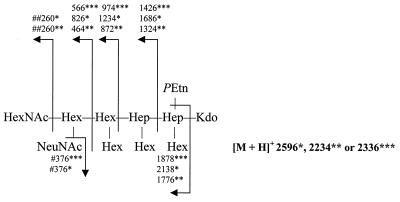
Upon methanolysis followed by peracetylation of LOS of wild-type MSC57360 and cgt-neuA, the peracetylated methyl ketoside methyl ester derivative of NeuNAc was detected by GLC and combined GLC-MS. The NeuNAc derivative from the LOS was identical in all parameters in GLC-MS to authentic NeuNAc which underwent the same derivatization procedure. Unlike these LOSs, NeuNAc was not detected in the neuC1 mutant LOS when a colorimetric assay was used or when more-sensitive detection by GLC-MS was utilized. Furthermore, to confirm the loss of NeuNAc from the LOS of this strain, core oligosaccharides were liberated from LOSs of wild-type MSC5730 and the neuC1 mutant, methylated, and subsequently analyzed in FAB-MS. As shown in Fig. 4, the permethylated core oligosaccharides of wild-type MSC57360 possessed a pseudomolecular ion, m/z = 2596 [M + H]+, and the mass spectrum included daughter ions indicative of sialylation, particularly m/z = 376. In contrast, the mass spectrum of the neuC1 mutant lacked the latter ion, and the pseudomolecular ion, m/z = 2234 [M + H]+, was sufficiently less because of the absence of NeuNAc. The results, therefore, support the loss of NeuNAc from the neuC1 mutant. Furthermore, FAB-MS analysis of the methylated core oligosaccharide of cgt-neuA mutant LOS yielded a pseudomolecular ion, m/z = 2336 [M + H]+, which was sufficiently less than that of wild-type MSC57360 because of the absence of an N-acetylhexosamine (HexNAc) residue. Consistent with this, the mass spectrum lacked the daughter ion m/z = 260 but contained daughter ions indicative of sialylation, including m/z = 376. Moreover, NeuNAc was detected by GLC-MS analysis of cgt-neuA mutant LOS after methanolysis and peracetylation as described above. Thus, the core oligosaccharide of cgt-neuA mutant LOS lacks terminal HexNAc but is sialylated.
FIG. 4.
Analysis of positive-ion FAB-MS for permethylated core oligosaccharide from LOS of C. jejuni MSC57360 and mutants. Numbers refer to m/z values for ions. Abbreviations: HexNAc, N-acetylhexosamine; Hex, hexose; Hep, heptose; Kdo, 3-deoxy-d-manno-2-octulosonic acid. The pseudomolecular ion and daughter ions observed for the oligosaccharide of MSC57360 are indicated by a single asterisk, whereas those of mutants in neuC1 and cgt-neuA are indicated by two and three asterisks, respectively. The ion for NeuNAc, indicated by a single pound sign, was absent from the neuC1 mutant, and that for HexNAc indicated by two pound signs was absent in the cgt-neuA mutant.
Effect of sia mutations of MSC57360 flagellin.
Flagellins of Campylobacter spp. have been shown to contain sialic acid, which affects the glycoform pattern in IEF gels (9, 18). Flagellins were purified from MSC57360 wild type and the cst, neuC1, and cgt-neuC1 mutants, and the IEF patterns were examined. Figure 5 shows that there was no difference in the IEF pattern of flagellin from wild-type MSC57360 (lane 3) and the neuC1 mutant (lane 4). Similarly, there was no difference in the IEF pattern of flagellins isolated from either the cst or cgt-neuA mutants (data not shown), indicating that these MSC57360 genes are not involved in biosynthesis of the posttranslational modifications of flagellin. Flagellin from C. coli VC167 and a ptmB mutant encoding a CMP-NeuNAc synthetase (18) are shown for comparison. Interestingly, the wild-type flagellins from VC167 and MSC57360 display markedly distinct IEF patterns, suggesting differences in the posttranslational modifications of these proteins.
FIG. 5.
Comparison of IEF patterns of flagellins of VC167 T2 and MSC57360. Flagellins were electrophoresed on IEF gels of pI 4 to 6 and stained with Coomassie blue. Lane 1, VC167 T2; lane 2, VC167 T2 ptmB; lane 3, MSC57360; lane 4, MSC57360 neuC1.
Loss of sialic acid in LOS results in increased serum sensitivity.
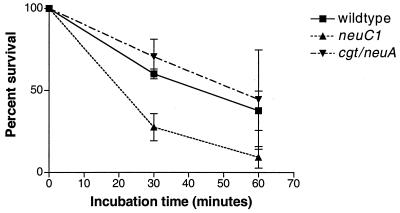
Figure 6 compares the sensitivities of wild-type MSC57360 and the neuC1 and cgt-neuA mutants to normal human sera. Bacteria were incubated with normal human serum and the same serum which had been heated to inactivate complement. Bacterial counts were determined at 0, 30, and 60 min of incubation. (Results are given as means ± standard errors.) After 30 min of incubation the cgt-neuA mutant showed serum sensitivity (70% ± 10.0% survival) comparable to that of the wild type (60% ± 3.0% survival; P = 0.07), but the survival of the neuC1 mutant (27% ± 8.0%) was significantly reduced compared to the wild type (P = 0.0001). After 60 min of incubation, survival of the wild type and cgt-neuA was reduced to 37% ± 12.0% and 44% ± 30.0%, respectively. Survival of the neuC1 mutant was 9% ± 6.0% (P = 0.01). Heat inactivation of the serum pools resulted in loss of all bactericidal activity (data not shown).
FIG. 6.
Comparison of serum resistance of MSC57360 and mutants. C. jejuni strains were incubated in the presence of 10% normal serum for 0, 30, and 60 min at 37°C, and the percent viable cells remaining were enumerated by plate count. The data represent the mean and standard error (error bars) of three experiments with the cgt mutant and five experiments with the wild type and the neuC1 mutant.
DISCUSSION
Sialic acid is an important surface component of a number of bacterial pathogens. The similarity of the polysialic acid capsules of E. coli K1 and meningococci with the embryonic form of the neural cell adhesion molecule is thought to be responsible for the poor immunogenicity of these neuropathogens (13). Moreover, sialylated capsules and LOS are known to render bacteria resistant to complement killing (14, 36, 39, 44–47) and can affect bacterial interactions with neutrophils (40, 47) and epithelial cells (44, 45). Although considerable attention has focused on the relationship of the sialylated LOS cores of C. jejuni and the development of GBS (1, 28), the function of sialylation in the pathogenesis of diarrheal diseases has not been considered. In an effort to begin to elucidate this role, we have generated mutations affecting the core of the type strain of the HS:1 serogroup, which has a defined LOS core structure with GM2 ganglioside mimicry.
The genetic locus of MSC57360 described here is involved in biosynthesis of LOS cores, as are the corresponding genetic loci described for HS:19 and HS:2 strains (16, 25). Mutation of the neuC1 and cst genes resulted in identical phenotypes of LOS cores, each with the same change in electrophoretic mobility, loss of reactivity with CT, and enhanced immunoreactivity with a polyclonal antibody against whole cells of the strain. Chemical analyses of the core of the neuC1 mutant confirmed the loss of NeuNAc. The loss of sialic acid in the core of the cst mutant suggests that, unlike the situation described in the GBS isolate OH4384, MSC57360 does not contain a second copy of a sialyl transferase with α-2,3-sialyltransferase activity. Moreover, BLASTP analysis suggests that NCTC 11168 also contains a single sialyl transferase with homology to those described in OH4384 (16).
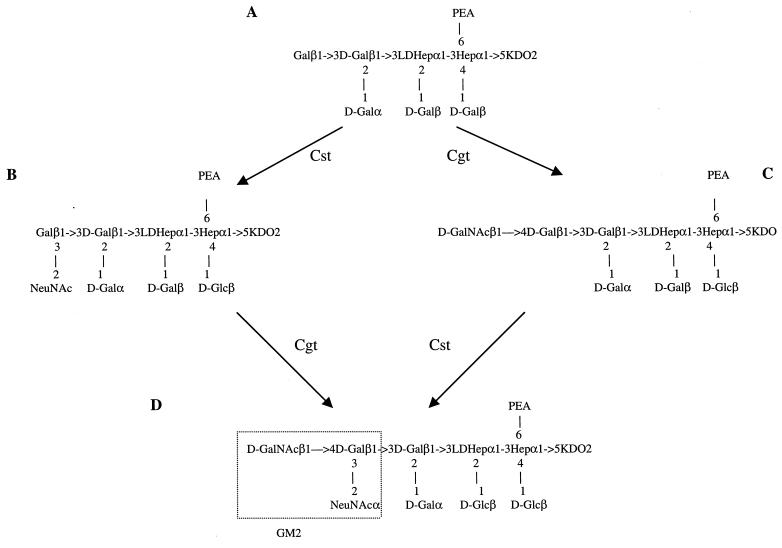
Both C. jejuni NCTC 11168 and MSC57360 have a gene which appears to be a fusion of genes encoding Cgt and a CMP-NeuNAc synthetase. Although this ORF was annotated by Parkhill et al. as a CMP-NeuNAc synthetase (33), the protein appears to function in MSC57360 as a GalNAc transferase. The core mobility displayed by a mutant in this gene was intermediate between that of the wild type and the cst and neuC1 mutants, suggesting that the cgt mutant core was still sialylated, and FAB-MS analyses confirmed the loss of GalNAc and the presence of sialic acid. This is in contrast to the data of Gilbert et al. (16) who reported that the galactosyltransferase activity of Cgt from OH4384 was specific for a sialylated acceptor. In MSC57360 it appears that the Cgt enzyme can transfer GalNAc to a nonsialylated acceptor, and, conversely, Cst can transfer NeuNAc to a core lacking GalNAc. If sialic acid were added to a precursor structure (Fig. 7A), there would exist an intermediate structure which is identical to the core of the type strain of HS:2 (6) (Fig. 7B). This structure, which is also the predicted core of the cgt mutant, would be expected to be poorly immunogenic. If the GalNAc were added to the core first, there would be no ganglioside mimicry in the intermediate (Fig. 7C), and it would be expected to be immunogenic, similar to the core of the cst mutant. The presence of antibodies in polyclonal antisera generated against whole cells of MSC57360 suggests that such immunogenic intermediate structures are present in low amounts in the population of LOS cores.
FIG. 7.
Schematic of alternate pathways in the synthesis of the core of MSC57360 (D). Shown are different intermediate structures which could be generated during biosynthesis of the core of MSC57360 depending upon the order in which the sialyl transferase, Cst, and the GalNAc transferase, Cgt, react with the precursor structure (A). Structure B is the same as the core structure of the type strain of HS:2 (6). Structure C corresponds to the predicted core of the cst mutant.
Interestingly, mutation of ORF4, which is a fusion of cgt (16) and neuA, results in the loss of GalNAc but not NeuNAc from the LOS core. This suggests that the fusion protein has either lost CMP-NeuNAc synthetase activity or that there are additional copies of genes encoding enzymes with the same function. Indeed, NCTC 11168, in addition to containing cj1143 (neuA1), contains two other copies of neuA alleles, cj1311 (neuA2) and cj1331 (neuA3 or ptmB). The neuA3 or ptmB allele has been shown to be involved in posttranslational modification of flagellin of C. coli VC167 (17) (Fig. 5), but the role of this gene in LOS biosynthesis in VC167, whose core is uncharacterized, remains open. Clearly, the role of the multiple neuA alleles in Campylobacter spp. requires additional study.
The presence of NeuNAc in the LOS core of MSC57360 results in decreased immunogenicity of the core and increased resistance to serum killing by complement. In Neisseria the presence of sialic acid on LOS also results in serum resistance but reduces the ability of the bacteria to be internalized into some eukaryotic cells (44, 45). There is a tremendous range in the ability of different strains of C. jejuni to be internalized into intestinal epithelial cells (22–24, 32) as well as differences in the behavior of different strains in various animal models of virulence (7; D. Burr and P. Guerry, unpublished data). There are no reports of which we are aware on the virulence of MSC57360 in animal models, but the strain invades INT407 cells at levels below those of E. coli K-12 (data not shown). However, having established the function of these genes in a strain of known LOS core structure, we are now examining the effect of LOS sialylation on pathogenesis of virulent strains of C. jejuni.
ACKNOWLEDGMENTS
We thank John Penner for strain MSC57360, Don Burr for generation of antiserum against MSC57360, Peter Doig for advice on IEF gels, and Isabelle Walker for excellent technical assistance.
This work was supported by Naval Medical Research and Development Command Work Unit nos. 61102A3M161102BS13AK.111 and 62787A.870.A0004, by grant 1 RO1 A143559 from the National Institute of Allergy and Infectious Diseases to P.G., and by a grant from the Irish Health Research Board to A.P.M. M.M.P. is a recipient of an Irish Health Board Post-Doctoral Fellowship.
REFERENCES
- 1.Allos B M. Association between Campylobacter infection and Guillain Barre syndrome. J Infect Dis. 1997;176(Suppl. 2):S125–128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 2.Annunziato P W, Wright L F, Vann W F, Silver R P. Nucleotide sequence and genetic analysis of the neuD and neuB genes in region 2 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1995;177:312–319. doi: 10.1128/jb.177.2.312-319.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspinall G O, McDonald A G, Pang H. Structures of the O chains from lipopolysaccharides of Campylobacter jejuni serotypes O:23 and O:36. Carbohydr Res. 1992;231:13–30. doi: 10.1016/0008-6215(92)84003-b. [DOI] [PubMed] [Google Scholar]
- 4.Aspinall G O, McDonald A G, Pang H, Kurjanczyl L A, Penner J L. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structure of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barré syndrome. Biochemistry. 1994;33:241–249. doi: 10.1021/bi00167a032. [DOI] [PubMed] [Google Scholar]
- 5.Aspinall G O, McDonald A G, Raju T S, Pang H, Moran A P, Penner J L. Chemical structures of the core regions of Campylobacter jejuni serotypes O:1, O:4, O:23 and O:36 lipopolysaccharides. Eur J Biochem. 1993;213:1017–1027. doi: 10.1111/j.1432-1033.1993.tb17849.x. [DOI] [PubMed] [Google Scholar]
- 6.Aspinall G O, McDonald A G, Raju T S, Pang H, Kurjanczyk L A, Penner J L, Moran A P. Chemical structure of the core region of Campylobacter jejuni serotype O:2 lipopolysaccharide. Eur J Biochem. 1993;213:1029–1037. doi: 10.1111/j.1432-1033.1993.tb17850.x. [DOI] [PubMed] [Google Scholar]
- 7.Bacon D J, Alm R A, Burr D H, Hu L, Kopecko D J, Ewing C P, Trust T J, Guerry P. Involvement of a plasmid in the virulence of Campylobacter jejuni 81–176. Infect Immun. 2000;68:4384–4390. doi: 10.1128/iai.68.8.4384-4390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaser M J, Smith P F, Kohler P E. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis. 1985;151:227–235. doi: 10.1093/infdis/151.2.227. [DOI] [PubMed] [Google Scholar]
- 8a.Committee on the Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. NIH publication 86–23. Bethesda, Md: Institute of Laboratory Animal Resources, National Research Council; 1985. [Google Scholar]
- 9.Doig P, Kinsella N, Guerry P, Trust T J. Characterization of a posttranslational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol Microbiol. 1995;19:379–387. doi: 10.1046/j.1365-2958.1996.370890.x. [DOI] [PubMed] [Google Scholar]
- 10.Downs A, Pigman W. Qualitative and quantitative determination of sialic acids. Methods Carbohydr Chem. 1976;7:233–240. [Google Scholar]
- 11.Edwards U, Muller A, Hammerschmidt S, Gerardy-Schahn R, Frosch M. Molecular analysis of the biosynthesis pathway of the alpha-2,8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol Microbiol. 1994;14:141–149. doi: 10.1111/j.1365-2958.1994.tb01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Figurski D H, Helinski D R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finne J, Leinonen M, Makela P N. Antigenic similarities between brain components and bacteria causing meninigitis: implications for vaccine development. Lancet. 1983;ii:355–357. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald T J. Activation of the classical and alternative pathways of complement by Treponema pallidum subsp. pallidum and Treponema vincentii. Infect Immun. 1987;55:2066–2073. doi: 10.1128/iai.55.9.2066-2073.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freidman C R, Neimann J, Wegener H C, Tauxe R V. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, editors. Campylobacter. Washington, D.C.: American Society for Microbiology; 2000. pp. 121–138. [Google Scholar]
- 16.Gilbert M, Brisson J-R, Karwaski M-F, Michniewicz J, Cunningham A-M, Wu Y, Young N M, Wakarchuk W W. Biosynthesis of ganglioside mimics in Campylobacter jejuni OH4384. J Biol Chem. 2000;275:3896–3906. doi: 10.1074/jbc.275.6.3896. [DOI] [PubMed] [Google Scholar]
- 17.Goryshin I Y, Reznikoff W S. Tn5 in vitro transposition. J Biol Chem. 1998;273:7367–7374. doi: 10.1074/jbc.273.13.7367. [DOI] [PubMed] [Google Scholar]
- 18.Guerry P, Doig P, Alm R A, Burr D H, Kinsella N, Trust T J. Identification and characterization of genes required for posttranslational modification of Campylobacter coli VC167 flagellin. Mol Microbiol. 1996;19:369–378. doi: 10.1046/j.1365-2958.1996.369895.x. [DOI] [PubMed] [Google Scholar]
- 19.Guerry P, Yao R, Alm R A, Burr D H, Trust T J. Systems of experimental genetics for Campylobacter spp. Methods Enzmol. 1994;235:474–481. doi: 10.1016/0076-6879(94)35163-5. [DOI] [PubMed] [Google Scholar]
- 20.Harris L A, Logan S M, Guerry P, Trust T J. Antigenic variation of Campylobacter flagella. J Bacteriol. 1987;169:5066–5071. doi: 10.1128/jb.169.11.5066-5071.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu L, Kopecko D J. Campylobacter jejuni 81-176 associates with microtubules and dynein during invasion of human intestinal cells. Infect Immun. 1999;67:4171–4182. doi: 10.1128/iai.67.8.4171-4182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konkel M E, Hayes S F, Joens L A, Cieplak W. Characteristics of the internalization and intracellular survival of Campylobacter jejuni in human epithelial cells cultures. Microb Pathog. 1992;13:357–370. doi: 10.1016/0882-4010(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 24.Konkel M E, Jones L A. Adhesion to and invasion of HEp-2 cells by Campylobacter spp. Infect Immun. 1989;57:2984–2990. doi: 10.1128/iai.57.10.2984-2990.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linton D, Karlyshev A V, Hitchen P G, Morris H R, Dell A, Gregson N A, Wren B W. Multiple N-acetyl neuraminic acid synthetase (neuB) genes in Campylobacter jejuni: identification and characterization of the gene involved in sialylation of lipo-oligosaccharide. Mol Microbiol. 2000;35:1120–1134. doi: 10.1046/j.1365-2958.2000.01780.x. [DOI] [PubMed] [Google Scholar]
- 26.Lior H, Woodward D L, Edgar J A, Larouche L J, Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat labile antigenic factors. J Clin Microbiol. 1982;15:761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masson L, Holbein B E. Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J Bacteriol. 1983;154:728–736. doi: 10.1128/jb.154.2.728-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran A P, Appelmelk B J, Aspinall G O. Molecular mimicry of host structures by lipopolysaccharides of Campylobacter and Helicobacter spp.: implications in pathogenesis. J Endotoxin Res. 1996;3:521–531. [Google Scholar]
- 29.Moran A P, Penner J L. Serotyping of Campylobacter jejuni based on heat-stable antigens: relevance, molecular basis and implications in pathogenesis. J Appl Microbiol. 1999;86:361–377. doi: 10.1046/j.1365-2672.1999.00713.x. [DOI] [PubMed] [Google Scholar]
- 30.Moran A P, Rietschel E T, Kosunen T U, Zähringer U. Chemical characterization of Campylobacter jejuni lipopolysaccharides containing N-acetylneuraminic acid and 2,3-diamino-2,3-dideoxy-d-glucose. J Bacteriol. 1991;173:618–626. doi: 10.1128/jb.173.2.618-626.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberhelman R A, Taylor D N. Campylobacter infections in developing countries. In: Nachamkin I, Blaser M J, editors. Campylobacter. Washington, D.C.: American Society for Microbiology; 2000. pp. 139–153. [Google Scholar]
- 32.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H M, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable tracts. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 34.Penner J L, Hennesy J N. Passive hemaglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen M, Fessner W D, Frosch M, Luneberg E. The siaA gene involved in capsule polysaccharide biosynthesis of Neisseria meningitidis B codes for N-acylglucosamine-6-phophate 2-epimerase. FEMS Microbiol Lett. 2000;184:161–164. doi: 10.1111/j.1574-6968.2000.tb09008.x. [DOI] [PubMed] [Google Scholar]
- 36.Platt M W, Correa N, Mold C. Growth of group B streptococci in human serum leads to increased cell surface sialic acid and decreased activation of the alternative complement pathway. Can J Microbiol. 1994;40:99–105. doi: 10.1139/m94-016. [DOI] [PubMed] [Google Scholar]
- 37.Power M E, Guerry P, McCubbin W D, Kay C M, Trust T J. Structural and antigenic characteristics of Campylobacter coli FlaA flagellin. J Bacteriol. 1994;176:3303–3313. doi: 10.1128/jb.176.11.3303-3313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prendergast M M, Lastovica A J, Moran A P. Lipopolysaccharides from Campylobacter jejuni O:41 strains associated with Guillain-Barré syndrome exhibit mimicry of GM1 ganglioside. Infect Immun. 1998;66:3649–3755. doi: 10.1128/iai.66.8.3649-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rest R F, Frangipane J V. Growth of Neisseria gonorrhoeae in CMP-N-acetylneuraminic acid inhibits nonopsonic (opacity-associated outer membrane protein-mediated) interactions with human neutrophils. Infect Immun. 1992;60:989–997. doi: 10.1128/iai.60.3.989-997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swartley J S, Stephens D S. Identification of a genetic locus involved in the biosynthesis of N-acetyl-d-mannosamine, a precursor of the (α2-8)-linked polysialic acid capsule of serogroup B Neisseria menigitidis. J Bacteriol. 1994;176:1530–1534. doi: 10.1128/jb.176.5.1530-1534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tullius M V, Munson R S, Jr, Wang J, Gibson B W. Purification, cloning, and expression of a cytidine 5′-monophosphate N-acteylneuraminic acid synthetase from Haemophilus ducreyi. J Biol Chem. 1996;271:15373–15380. doi: 10.1074/jbc.271.26.15373. [DOI] [PubMed] [Google Scholar]
- 43.Vann W F, Tavarez J J, Crowley J, Vimr E, Silver R P. Purification and characterization of the Escherichia coli K1 neuB gene product N-acetylneuraminic acid synthetase. Glycobiology. 1997;7:697–701. doi: 10.1093/glycob/7.5.697. [DOI] [PubMed] [Google Scholar]
- 44.Van Putten J P. Phase variation of lipopolysaccharide directs interconversion of invasive and immunoresistant phenotypes of Neisseria gonorrhoeae. EMBO J. 1993;12:4043–4051. doi: 10.1002/j.1460-2075.1993.tb06088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Putten J P, Robertson B D. Molecular mechanisms and implications for infection of lipooligosaccharide variation in Neisseria. Mol Microbiol. 1995;16:847–853. doi: 10.1111/j.1365-2958.1995.tb02312.x. [DOI] [PubMed] [Google Scholar]
- 46.Vogel U, Hammerschmidt S, Frosch M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med Microbiol Immunol. 1996;185:81–87. doi: 10.1007/s004300050018. [DOI] [PubMed] [Google Scholar]
- 47.Vogel U, Weinberger A, Frank R, Muller A, Kohl J, Atkinson J P, Frosch M. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect Immun. 1997;65:4022–4029. doi: 10.1128/iai.65.10.4022-4029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Y, Taylor D E. Chloramphenicol resistance in Campylobacter coli: nucleotide sequence, expression, and cloning vector construction. Gene. 1990;94:23–28. doi: 10.1016/0378-1119(90)90463-2. [DOI] [PubMed] [Google Scholar]
- 49.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–92. [Google Scholar]
- 50.Yao R, Alm R A, Trust T J, Guerry P. Construction of new Campylobacter cloning vectors and a new mutational cat cassette. Gene. 1993;130:127–130. doi: 10.1016/0378-1119(93)90355-7. [DOI] [PubMed] [Google Scholar]
- 51.Zapata G, Crowley J M, Vann W F. Sequence and expression of the Escherichia coli K1 neuC gene product. J Bacteriol. 1992;174:315–319. doi: 10.1128/jb.174.1.315-319.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zapata G, Vann W F, Aaronson W, Lewis M S, Moos M. Sequence of the cloned Escherichia coli K1 CMP-N-acetylneuraminic acid synthetase gene. J Biol Chem. 1989;264:14769–14774. [PubMed] [Google Scholar]