Abstract
We determined previously that lipoproteins of Borrelia burgdorferi stimulate inflammatory and anti-inflammatory cytokines (interleukin-10 [IL-10]) in monocytes. IL-10 could have an effect on innate and acquired immune responses to B. burgdorferi and influence the magnitude of the infectious inoculum and disease outcome. To understand the mechanism(s) of IL-10 action during early infection, when innate immunity expressed chiefly by skin macrophages is key, we investigated the effect of exogenous and endogenous IL-10 on the production of the macrophage-derived cytokines IL-6, IL-1β, IL-12, and tumor necrosis factor alpha (TNF-α). We used the THP-1 human monocytic cell line and recombinant lipidated OspA (L-OspA) as the model target cell and stimulant, respectively. To determine the kinetics of cytokine production by THP-1 cells, we stimulated them with L-OspA and also with heat-killed B. burgdorferi cells (HBb) and lipopolysaccharide (LPS). Exogenous IL-10 dampened production of inflammatory cytokines, as elicited by lipoproteins. The inhibition of endogenous IL-10 function by anti-IL-10 antibody reduced the production of IL-12 and IL-6 but not that of IL-1β and TNF-α. An inspection of the kinetics of cytokine production clarified this finding. TNF-α was produced prior to, and IL-β was produced at the same time as, IL-10, whereas IL-6 and IL-12 were produced later. HBb, LPS, and L-OspA yielded similar kinetics of cytokine production. This result reinforces the notion that lipoproteins are the functional molecules in HBb and perhaps in vivo. It indicates also that signaling pathways utilized by LPS and lipoproteins may be extensively shared. The results are consistent with a major role played by IL-10 in controlling the initial phase of infection with this spirochete.
Borrelia burgdorferi, the etiologic agent of Lyme disease, is spread to humans and other mammals through the bite of infected Ixodes ticks (12). The spirochete can invade multiple organs (5, 59) and persist in them for a long time (46, 67). Spirochetal persistence in the tissues has been associated with severe pathology (15, 20, 67) and both acute and chronic inflammatory conditions (50, 59). The mechanism(s) that drives the infection to the various disease states is still not fully understood. On the one hand, there is evidence that persistence of the spirochete or its antigens in the tissues is required for pathology to ensue. On the other, there is evidence that suggests that autoimmunity also may play a part (29, 32, 58). It is now well documented that B. burgdorferi lipoproteins can directly elicit inflammatory responses both in vitro and in vivo (11, 26, 27, 47, 52, 57, 66). These results strongly support the paradigm that pathogenesis of Lyme disease requires spirochetal persistence.
With the growing realization that cytokines play a key role in inflammation, research on cytokine-mediated inflammatory reactions in Lyme borreliosis is receiving great attention. Studies conducted to date have revealed that B. burgdorferi and its lipoproteins can induce in a variety of cell types the release of proinflammatory cytokines such as interleukin-1β (IL-1β) (31, 35), IL-6 (2, 11, 30, 31, 43, 52, 60, 62), IL-12 (42), and tumor necrosis factor alpha (TNF-α) (11, 17, 31, 53). These proinflammatory cytokines generally have potent effector functions that overlap extensively with each other to bring about the many components of inflammation, e.g., tissue necrosis (13, 64), chemotaxis of cellular infiltrates, induction of collagenase and prostaglandin secretion by synovial fibroblasts and chondrocytes (16, 21), bone resorption, and cartilage destruction (3, 63), as well as a plethora of microbicidal effector mechanisms (48, 61).
An important negative regulator of proinflammatory cytokines is the anti-inflammatory cytokine IL-10 (44). IL-10 is secreted under different conditions of immune activation by a variety of cell types, including T cells, B cells, and monocytes/macrophages (44). In vitro studies have shown that IL-10 suppresses the release and function of IL-1β, IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor, and IL-12 (14, 19, 23), thereby suggesting a normal endogenous feedback mechanism for the control of immune responses and inflammation. Additionally, in vivo studies have shown the direct suppressive effect of IL-10 on proinflammatory responses (4, 34, 41).
It has been demonstrated that B. burgdorferi is capable of inducing the production of IL-10 in vitro in mononuclear cells present in peripheral blood (26, 31) and synovial fluid (68). Yin et al. (68) also showed that IL-10 can be produced concomitantly with Th1 cytokines when synovial fluid mononuclear cells from infected patients are incubated with sonicated proteins of B. burgdorferi. The same study showed that this endogenously produced IL-10 can inhibit both Borrelia-specific lymphocyte proliferation and TNF-α and gamma interferon (IFN-γ) production. Studies also have shown a novel subset of T cells in human Lyme disease patients that secrete in vitro both IL-10 and IFN-γ in the presence of exogenous IL-12 (51). Recently we showed that within the peripheral blood mononuclear cell compartments of uninfected human and nonhuman primates, the cell type that transcribes the IL-10 gene in response to heat-killed B. burgdorferi cells (HBb) is the monocyte. HBb can similarly induce an up-regulation of IL-1β and IL-6 (but not of IL-4 and IFN-γ) transcription in these cells, as well as in the human monocytic cell line THP-1 (27). This study also provided evidence that the monocyte produces both pro- and anti-inflammatory cytokines in response to purified lipoproteins of B. burgdorferi.
We had hypothesized that IL-10 induced by B. burgdorferi lipoproteins could contribute to the control of inflammation in Lyme disease, such that spirochetes would be endowed with a means of controlling the inflammation that they themselves are able to induce (26, 27, 49). The viability of this hypothesis was recently illustrated by Brown et al. (11). These investigators demonstrated that C57BL/6J mice deficient in IL-10 (IL-10−/−) developed more severe arthritis yet harbored 10 times fewer spirochetes in the joints than wild-type C57BL/6J animals.
IL-10 could have an effect on both the innate and acquired immune responses to B. burgdorferi and could influence the magnitude of the infectious inoculum as well as disease outcome. To begin to understand the mechanism(s) of IL-10 action during the early phase of infection, when innate immunity expressed chiefly by skin macrophages should play an important role, we investigated the effect of both exogenous (added) and endogenous IL-10 on the production of the macrophage-derived pro-inflammatory cytokines IL-6, IL-1β, IL-12, and TNF-α. We used the THP-1 human monocytic cell line and purified recombinant lipidated OspA (L-OspA) as the model target cell and stimulant, respectively. THP-1 cells were used to ensure consistent results, as it is difficult to obtain the large quantities of human peripheral blood monocytes that were required to conduct the experiments presented in this paper. Although L-OspA appears not to be expressed by B. burgdorferi in the early phases of infection (6, 18, 50), the use of this (or any other) lipoprotein as a model is justified in so far as its immunological effects are elicited by the lipid, not the protein, moiety. The lipid moiety is likely shared by all B. burgdorferi lipoproteins. We also stimulated the THP-1 cells with lipopolysaccharide (LPS) and HBb. LPS was used as a positive control for monocyte responses, and the B. burgdorferi cells were used to allow for inferences on whether lipoproteins were the principal stimulants of monocytes/macrophages in this spirochete. Finally, the kinetics of cytokine production by THP-1 cells upon stimulation with L-OspA, LPS, or HBb also was determined. The results are discussed in terms of the possible role played by IL-10, as induced by B. burgdorferi lipoproteins, in controlling the initial phase of infection with this spirochete.
MATERIALS AND METHODS
Bacteria and lipoproteins.
The JD1 strain of B. burgdorferi sensu stricto was used throughout. HBb were prepared as previously described (26). HBb instead of viable organisms were used in order to have a consistent batch of organisms for all studies. Recombinant L-OspA and unlipidated OspA (U-OspA) were the same as already reported (26, 27) and were obtained from John Dunn, Brookhaven National Laboratories, Brookhaven, N.Y. The L-OspA preparation contained less than 0.25 endotoxin unit per mg of protein, as assessed by Limulus amebocyte assay (Associates of Cape Cod, Woods Hole, Mass.). LPS from Escherichia coli strain 026:B6 was from Sigma Chemical Company (St. Louis, Mo.).
Cell culture and stimulation of cytokine production.
The THP-1 monocyte cell line was the same as described previously (16) and was obtained from the American Type Culture Collection (Manassas, Va.). THP-1 cells were pretreated with 0.05 μM 1α,25-dihydroxyvitamin D3 (Calbiochem-Nova Biochem International, La Jolla, Calif.) for 48 h (36) prior to being used in this study. For dose-response studies, THP-1 cells at 106/ml were stimulated with various concentrations of HBb (105/ml to 108/ml) and L-OspA, U-OspA, and LPS (0.01, 0.1, 1, and 10 μg/ml) for 48 h. Cultures were subsequently centrifuged at 400 × g for 10 min at 4°C, and cell-free supernatants were collected and stored at −70°C until they were used.
Effect of IL-10 on cytokine production.
To study the effect of exogenous IL-10 on IL-1β, IL-6, IL-12 (p40), and TNF-α production, THP-1 cells were stimulated with 1 μg of L-OspA or LPS per ml in the presence or absence of various concentrations (0.01, 0.1, 1, 10, and 100 ng/ml) of human recombinant IL-10 (rIL-10) (catalog no. 19701V; PharMingen, San Diego, Calif.). To determine the effect of endogenous IL-10 on cytokine production, cells were stimulated with L-OspA or LPS in the presence or absence of a neutralizing rat anti-human IL-10 antibody (Ab) (catalog no. 18550D; PharMingen) at 10, 20, and 40 μg/ml. Normal rat immunoglobulin G1 (catalog no. 20610D) (PharMingen) was used as control. All cultures were incubated for 48 h, after which time cell-free supernatants were collected following centrifugation at 400 × g for 10 min at 4°C. Supernatants were aliquoted and stored at −70°C until they were used.
Measurement of cytokine concentrations.
Cytokines were measured as described previously (27). IL-6, IL-10, IL-12 (p40), and TNF-α (PharMingen) and IL-1β (Biosource International, Camarillo, Calif.) in culture supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using paired cytokine-specific monoclonal Abs according to the manufacturer's instructions.
Kinetics of cytokine production.
For the study of kinetics of cytokine production, cell-free supernatants were collected after THP-1 cells had been stimulated for 1 h (TNF-α) and 2, 8, 16, 24, 48, 72, 96, 120, 144, and 168 h. The concentrations of stimulants used were 107/ml for HBb and 1 μg/ml for L-OspA, U-OspA, and LPS.
Statistics.
Student's t test was used to compare the data. Significance was established at a P level of 0.05.
RESULTS
Levels of cytokine production as a function of stimulant concentration.
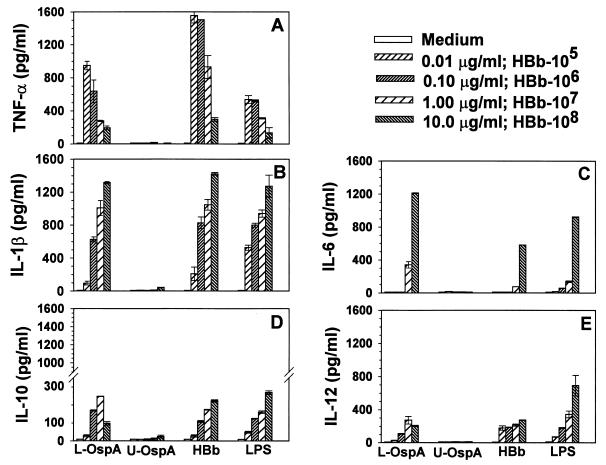
Experiments were first conducted to determine the concentrations of L-OspA, HBb, and LPS required to stimulate THP-1 cells to produce detectable and optimal levels of IL-1β, IL-6, IL-12, TNF-α, and IL-10. U-OspA and medium alone served as negative controls. Cytokines were quantified in 48-h cultures using specific ELISAs. L-OspA, HBb, and LPS induced cytokine production in a dose-dependent manner. The concentrations of IL-1β and IL-6 were augmented with increasing concentrations of L-OspA, HBb, and LPS (Fig. 1B and C). With the exception of L-OspA at 10 μg/ml, IL-10 and IL-12 levels were also gradually augmented with increasing concentrations of HBb and LPS (Fig. 1D and E). In contrast, the concentration of TNF-α decreased with increasing concentrations of these stimulants (Fig. 1A). All cytokines except IL-6 were detectable at stimulant concentrations of 0.01 μg/ml, albeit at low levels except for TNF-α. This cytokine was produced at relatively high levels (1,600 pg/ml) in response to a dose as low as 0.01 μg of L-OspA or LPS per ml and 105 HBb per ml. HBb at 105/ml did not stimulate IL-1β, IL-6, IL-12, or IL-10 production, a result previously observed by us for IL-6 and IL-10 (26). Neither U-OspA at any of the concentrations used nor medium alone induced significant production of any cytokine. Based on the results of this study, 1 μg of L-OspA, U-OspA, or LPS per ml and 107 HBb per ml were chosen as optimal concentrations for stimulation of cytokine production in subsequent experiments.
FIG. 1.
Dose-response analysis of stimulants required to induce optimal levels of TNF-α, IL-1β, IL-6, and IL-10 production. Vitamin D3-treated THP-1 cells (106/ml) were incubated for 48 h with supplemented medium (RPMI), various concentrations of HBb (heat-killed spirochetes of the JD1 strain), L-OspA, U-OspA, and LPS. TNF-α, IL-1β, IL-6, and IL-10 in the cell-free supernatants were quantified by Ab capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. Each bar represents the mean ± standard deviation for duplicate cultures. Data are representative of those from two separate experiments.
Effect of exogenous IL-10 on the concentrations of IL-12, IL-6, IL-1β, and TNF-α induced by L-OspA and LPS stimulation.
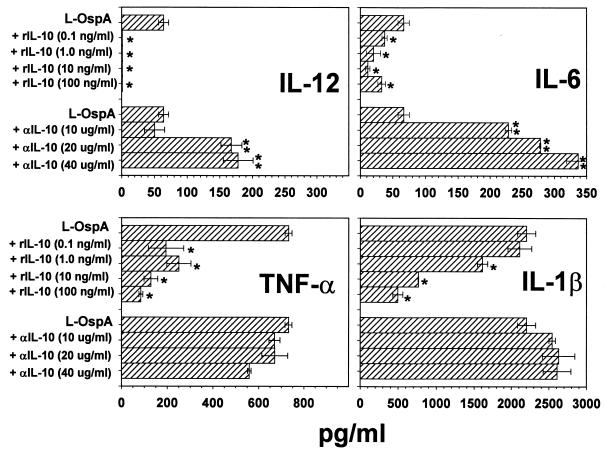
We examined whether exogenous IL-10 added at the time of stimulation was able to affect the concentration of IL-β, IL-6, IL-12, or TNF-α measured 48 h after L-OspA or LPS stimulation. This time point was chosen because preliminary kinetic studies of cytokine production in this system showed that at 48 h poststimulation (PS), cytokine concentrations either had reached a plateau or were changing at a relatively low rate. THP-1 cells were incubated with stimulants in the absence or presence of rIL-10 at concentrations of 0.1, 1, 10, and 100 ng/ml. All tested cytokines were below the detection limits in the absence of stimulants and in the presence of rIL-10 alone (data not shown). IL-12 was significantly reduced (P < 0.007) in supernatants of L-OspA-treated cells with as little as 0.1 ng of added rIL-10 per ml and remained essentially undetectable at the higher concentrations of added rIL-10 (Fig. 2). Concentrations of IL-6 and TNF-α also were significantly diminished (P < 0.04) in the presence of all concentrations of exogenous IL-10 (Fig. 2). IL-1β was not significantly reduced in the presence of 0.1 ng of rIL-10 per ml but was significantly decreased (P < 0.02) with 1 to 100 ng of added rIL-10 per ml (Fig. 2). Our results indicate that exogenous IL-10 affects the production (transcription, processing, and/or export) of inflammatory cytokines by THP-1 cells when these cells are stimulated with L-OspA. Similar results were obtained for each cytokine when LPS was used as the stimulant (data not shown).
FIG. 2.
Effect of exogenous IL-10 and anti-IL-10 Ab on IL-12, IL-6, IL-1β, and TNF-α production. Vitamin D3-treated THP-1 cells (106/ml) were incubated with L-OspA (1 μg/ml) in the presence or absence of various concentrations of human rIL-10 and anti-IL-10 Ab (αIL-10). After 48 h, specific cytokines in cell-free supernatants were quantified by Ab capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. ∗, significantly different from value for cells incubated with L-OspA alone (P < 0. 007, P < 0.04, and P < 0.02 for IL-12, IL-6 and TNF-α, and IL-1β, respectively); ∗∗, significantly different from value for cells incubated with L-OspA alone (P < 0.01). P values were calculated by use of Student's t test. Each bar represents the mean ± standard deviation for duplicate cultures. Data are representative of those from three and two separate experiments for rIL-10 and anti-IL-10, respectively.
Effect of endogenous IL-10 on the concentrations of IL-12, IL-6, IL-1β, and TNF-α induced by L-OspA and LPS stimulation.
To investigate if endogenously produced IL-10 could affect the concentration of L-OspA- or LPS-induced inflammatory cytokines, neutralizing human anti-IL-10 Ab (10, 20, or 40 μg/ml) was added to THP-1 cell cultures. The concentrations of all tested cytokines were below the detection limits in the presence of anti-IL-10 alone (data not shown). Anti-IL-10 Ab significantly affected (P < 0.01) the L-OspA-induced concentrations of IL-12 and IL-6 in an Ab dose-dependent manner (Fig. 2). In contrast, concentrations of IL-1β and TNF-α produced by L-OspA stimulation were essentially unchanged at all Ab doses (P > 0.05) (Fig. 2). Similar results were obtained for each cytokine when LPS was used as the stimulant (not shown).
Further experiments were conducted to determine the specificity of the inhibitory effect of IL-10 on IL-12, IL-6, IL-1β, and TNF-α production, using isotype-matched control Ab as well as other appropriate controls. As already observed, 10 ng of rIL-10 per ml markedly reduced the concentrations of IL-12, IL-6, IL-1β, and TNF-α in L-OspA cultures. Additionally, anti-IL-10 Ab (20 μg/ml) again failed to significantly enhance (P > 0.05) the concentration of either IL-1β or TNF-α but markedly enhanced the concentrations of IL-12 and IL-6 as induced by L-OspA (data not shown). Moreover, when both exogenous rIL-10 and anti-IL-10 Ab were added at the same time to stimulated THP-1 cultures, the Ab completely neutralized the inhibitory effect of rIL-10 on IL-6 and IL-1β levels and partially (up to 78%) neutralized the effect on IL-12 and TNF-α levels. Isotype control Ab (20 μg/ml) did not affect the concentration of any of the tested cytokines (data not shown).
Kinetics of IL-6, IL-1β, TNF-α, IL-10, and IL-12 production.
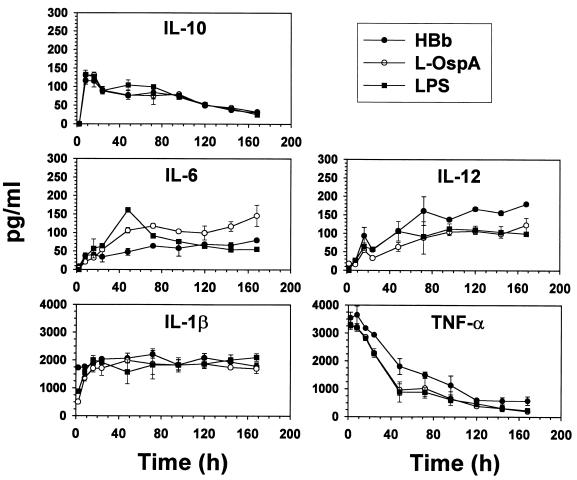
The kinetics of cytokine production by THP-1 cells was determined using HBb, L-OspA, U-OspA, and LPS as stimulants. Cytokine concentrations were determined at 2, 8, 16, 24, 48, 72, 96, 120, 144, and 168 h after addition of the stimulants to the cultures. Neither U-OspA nor medium alone induced the production of any cytokines at any time (data not shown). In preliminary experiments we had noticed that TNF-α was produced earlier than all other cytokines. Hence, this cytokine was also quantified at 1 h PS. The concentrations of all of the cytokines studied increased rapidly within the first 20 h of stimulation with HBb, L-OspA, or LPS. After this time, cytokine concentrations either continued to increase at a lesser rate (IL-6 and IL-12), remained essentially constant (IL-1β), or declined gradually (IL-10 and TNF-α). The concentration of TNF-α was detectable within 1 h PS, reached its peak value at 2 h after this time, and declined sharply thereafter. The concentration of IL-1β reached its peak value between 10 and 20 h PS and was at roughly half that value at about 2 h PS (Fig. 3). The IL-10 concentration peaked between 8 and 16 h PS and declined thereafter. The concentrations of both IL-6 and IL-12 increased more slowly than those of the previous three cytokines and reached a plateau by 48 h PS.
FIG. 3.
Kinetics of cytokine production by THP-1 cells in response to HBb (107/ml) (heat-killed spirochetes of the JD1 strain), L-OspA (1 μg/ml), and LPS (1 μg/ml). Vitamin D3-treated THP-1 cells (106/ml) were incubated with stimulants, and cell-free supernatants were collected at 2, 8, 16, 24, 48, 72, 96, 120, 144, and 168 h after addition of stimulants to THP-1. TNF-α, IL-1β, IL-6, and IL-10 in supernatants were quantified by Ab capture ELISA. The lower limit of detection of the ELISA was 15 pg/ml. Each bar represents the mean ± standard deviation for duplicate cultures. All tested cytokines were below detection limits in the absence of stimulants. Data are representative of those from three separate experiments.
DISCUSSION
The completion of the sequence of the B. burgdorferi genome has permitted new insights into the spirochete's immunobiology. The revelation that this organism has the potential of expressing more than 105 lipoprotein genes, roughly 11% of its genome's coding capacity (24), and the recent finding that lipoproteins can elicit not only inflammatory but also anti-inflammatory mediators, e.g., IL-10 from monocytes (27), have added support to the contention that lipoproteins are crucial virulence factors in Lyme disease pathogenesis.
IL-10, as elicited by lipoproteins from cells such as the macrophage, may have an initial effect on the establishment of a B. burgdorferi infection, when innate immunity is at play, and a subsequent effect on disease outcome, when acquired immune responses and perhaps even autoimmunity are the main protagonists. Here we have focused on the effect of IL-10 on the macrophage response to lipoproteins. We view this scenario as a model of the initial phase of infection, when acquired immunity has not yet developed and when the contribution of sensitized lymphocytes to the prevailing cytokine pattern need not be factored in. Other cells of the innate immune system also known to produce IL-10 in response to stimulants such as LPS are Langerhans and dendritic cells. However, relative to macrophages, a much lower proportion of these cells (0 to 5%) synthesizes IL-10 (as well as TNF-α, IL-6, and IL-12) in response to LPS (40) and thus possibly also in response to bacterial lipoproteins. Hence, the main source of “innate IL-10” in the initial phase of a B. burgdorferi infection in humans is likely the monocyte/macrophage.
Our results show that IL-10 can significantly dampen the production (or enhance the uptake and binding) of inflammatory cytokines, as elicited from macrophages by lipoproteins. Although we have not formally demonstrated it in this paper, we will assume henceforth in this discussion that IL-10 influences the transcription, processing, and/or release (all grouped under the term production, for short), not the uptake and binding, of inflammatory cytokines by macrophages. This assumption is based on the parallelism we observed between the effects of L-OspA and LPS on cytokine concentrations measured at 48 h PS and as a function of time PS. In view of this parallelism and since IL-10, as induced by LPS, is known to affect the transcription, and hence the production, of inflammatory cytokines also elicited by LPS (19), our assumption is reasonable, notwithstanding the differences in LPS and lipoprotein receptors, i.e., Tlr4 for LPS (8) versus Tlr2 for lipoproteins (33, 39).
While production of the four proinflammatory cytokines we studied was diminished by the addition of exogenous IL-10 to the cultures, the inhibition of endogenous IL-10 function by added anti-IL-10 Ab seemed to reduce production only of IL-12 and IL-6 and not of IL-1β and TNF-α. An inspection of the kinetics of cytokine production helps to clarify this apparent contradiction. Both TNF-α and IL-β were chiefly produced prior to (TNF-α) or roughly at the same time as (IL-β) IL-10, whereas IL-6 and IL-12 were produced later. Hence, by the time the endogenously produced IL-10 was available in the culture medium for antibody binding, both TNF-α and IL-β already had been released.
Both the kinetic and dose-response attributes of TNF-α production in response to L-OspA, HBb, or LPS merit discussion. As already noted by others, monocytes/macrophages produce TNF-α shortly after stimulation with either LPS (19) or lipoproteins (31). In our study, the concentration of TNF-α peaked within the first hour PS, whereas those of all other cytokines, including IL-10, peaked or reached a plateau at between 8 and 40 h PS. TNF-α is a potent neutrophil chemoattractant and inducer of phagocytosis and cytotoxicity in both neutrophils and macrophages and thus plays a key role in initiating and facilitating the innate immune response against bacterial pathogens (1, 45). Even the reactivity of γδ T cells, a cell type which probably influences early innate host responses, is triggered by TNF-α (38). In view of this early and copious production of TNF-α, our notion that B. burgdorferi lipoproteins facilitate the establishment of infection by stimulating the production of IL-10 in macrophages would seem ill-founded. However, the promotion of bactericidal effects is only one aspect of the function of TNF-α, for this cytokine is also essential in inducing the production of IL-10 by macrophages. In fact, TNF-α has been shown to have a major enhancing effect on IL-10 mRNA production by monocytes. It induces a 20- to 120-fold increase over baseline production. In contrast, IL-1α, IL-1β, IL-6, granulocyte-macrophage colony-stimulating factor, transforming growth factor β, and IFN-γ have little effect (<3-fold). Moreover, TNF-α also augments LPS-induced IL-10 secretion (65). Once again, it is possible that the same is true for lipoprotein-induced TNF-α and IL-10 production. Hence, if TNF-α was not secreted in the first place, the putative beneficial effects that IL-10 may have on the establishment of a B. burgdorferi infection might be greatly diminished.
Another unique aspect of the kinetics of TNF-α production was that, unlike with the concentrations of the other cytokines we measured, which either reached a plateau (IL-6, IL-12, and IL-β) or decreased very slowly thereafter, the concentration of TNF-α decreased in a biphasic fashion, swiftly between 1 and 45 h PS and more gradually after that time. The apparent decrease in TNF-α concentration may be explained as follows. The THP-1 cell line can be induced to express and release both TNF-α receptors, TNF-R55 and TNF-R75, upon exposure to LPS (28). Proteolytic cleavage of both receptors and release of the corresponding TNF-binding proteins, TNF-R55-BP and TNF-R75-BP, can be mediated by TNF-α itself (7). TNF-α is functionally inactivated by TNF-R55-BP and TNF-R75-BP (6). The apparent decrease in TNF-α concentration may be due to its binding to the soluble receptors. As a consequence, TNF-α may have failed to bind to either the capture or detecting Abs in the sandwich ELISA that we employed for TNF-α quantification.
Unlike with IL-1β, IL-6, and IL-10, whose concentrations measured at 48 h PS increased in response to increasing concentrations of stimulants, the concentration of TNF-α diminished. The maximal concentration of TNF-α was attained at the lowest concentration of stimulants. In fact, the highest concentration of TNF-α was observed at a THP-1-to-spirochete cell ratio of 10:1 (Fig. 1A). One would expect, as with the other cytokines, that TNF-α transcription should have been enhanced with increasing stimulant concentration, and most likely this is what happened. However, since we measured the TNF-α concentration at 48 h PS, the putative effect of the release of TNF-R55-BP and TNF-R75-BP must be taken into account as well. We submit that, as the transcription of TNF-α increased, in parallel with the increase in stimulant concentration, the enhanced availability of TNF-α promoted the release of larger amounts of TNF-R55-BP and TNF-R75-BP. As a consequence, less TNF-α was detected in the medium at 48 h PS.
Stimulation of THP-1 cells with HBb and L-OspA yielded similar kinetics of cytokine production. This further reinforces the notion that lipoproteins are the functionally active molecules in the HBb preparation and perhaps in vivo. Recently, it was shown that stimulation of monocytes by intact spirochetes occurs, as with pure lipoproteins, via CD14 (56). Hence, by inference, lipoproteins are the probable stimulants of monocytes/macrophages on live organisms. The responses to LPS and L-OspA that we observed also were notably analogous. This indicates that signaling pathways utilized by both stimulants may be extensively shared. It is already known that LPS and lipoproteins share CD14 as a coreceptor on cells of the myeloid lineage, while their respective receptors, namely T1r-4 and T1r-2, differ (8, 27, 33, 39, 47, 57, 66).
We have hypothesized here that IL-10, as elicited from macrophages by spirochetal lipoproteins, facilitates the establishment of a B. burgdorferi infection. To this end, IL-10 would contribute to down-regulate inflammatory and microbicidal effector mechanisms of the innate immune response. The observations we have made using THP-1 cells and L-OspA as a model are consistent with this notion. Interestingly, a similar role has been attributed to factors in tick saliva (10, 37, 54, 55). These factors, conveyed by the tick to the site of inoculation during spirochetal transmission, may act in synergy with spirochetal lipoproteins and usher spirochetes through the early localized phase of infection. Later, as the spirochetes disseminate and acquired immunity develops, both residual tick saliva- and lipoprotein-induced IL-10 may continue to influence the disease's natural history, by tilting the balance of the T-cell response towards a Th2 type (10, 22, 25, 37, 69) and, as posited by Brown et al. (11) and Giambartolomei et al. (26), by curbing inflammation.
ACKNOWLEDGMENTS
We thank John Dunn (Brookhaven National Laboratory) for purified recombinant OspA. We also thank Christie Trew for her excellent secretarial help.
Special thanks go to the Department of Biotechnology, India, for providing a Senior Overseas Associateship to P. K. Murthy. This work was supported by grants U50/CCU606604 from the Centers for Disease Control and Prevention and RR00164 from the National Center for Research Resources, National Institutes of Health.
REFERENCES
- 1.Abraham S N, Arock M. Mast cells and basophils in innate immunity. Semin Immunol. 1998;10:373–381. doi: 10.1006/smim.1998.0140. [DOI] [PubMed] [Google Scholar]
- 2.Akins D R, Purcell B K, Mitra M, Norgard M V, Radolf J D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993;61:1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend P M, Dayer J M. Inhibition of the production and effects of interleukin-1 and tumor necrosis factor-α in rheumatoid arthritis. Arthritis Rheum. 1995;38:151–160. doi: 10.1002/art.1780380202. [DOI] [PubMed] [Google Scholar]
- 4.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk H D, Docke W D. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W, Persing D H, Armstrong A L, Peeples R A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991;139:263–273. [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold S W, Fikrig E, Bockenstedt L K, Persing D H. Circumvention of outer surface protein A immunity by host-adapted Borrelia burgdorferi. Infect Immun. 1995;63:2255–2261. doi: 10.1128/iai.63.6.2255-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bemelmans M H, van Tits L J, Buurman W A. Tumor necrosis factor: function release and clearance. Crit Rev Immunol. 1996;16:1–11. doi: 10.1615/critrevimmunol.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- 8.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 9.Bjornberg F, Lantz M, Olsson I, Gullberg U. Mechanisms involved in the processing of the p55 and the p75 tumor necrosis factor (TNF) receptors to soluble receptor forms. Lymphokine Cytokine Res. 1994;13:203–211. [PubMed] [Google Scholar]
- 10.Brossard M, Wikel S K. Immunology of interactions between ticks and hosts. Med Vet Entomol. 1997;11:270–276. doi: 10.1111/j.1365-2915.1997.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown J P, Zachary J F, Teuscher C, Weis J J, Wooten M. Dual role of interleukin 10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect Immun. 1999;67:5142–5150. doi: 10.1128/iai.67.10.5142-5150.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burgdorfer W A, Barbour G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease: a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 13.Casey L C, Balk R A, Bone R C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–778. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cassatella M A, Meda L, Bonora A, Ceska M, Constantin G. Interleukin-10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–2211. doi: 10.1084/jem.178.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle P K. Lyme disease. In: Manning S, editor. Pathogenesis of lyme disease. St. Louis, Mo: Mosby Year Book Co.; 1993. pp. 179–183. [Google Scholar]
- 16.Dayer J M, De Rochemonteix B, Burrus B, Demczuk S, Dinarello C A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986;77:645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Defosse D L, Johnson R C. In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun. 1992;60:1109–1113. doi: 10.1128/iai.60.3.1109-1113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Silva A M, Telford S R, 3rd, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Waal Malefyt R, Abrams J, Bennett B, Figdor C, de Vries J E. Interleukin-10 inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.England J D, Bohm R P, Jr, Roberts E D, Philipp M T. Mononeuropathy multiplex in rhesus monkeys with chronic Lyme disease. Ann Neurol. 1997;1:375–384. doi: 10.1002/ana.410410313. [DOI] [PubMed] [Google Scholar]
- 21.Evequoz V, Bettens F, Kristensen F, Trechsel U, Stadler B M, Dayer J M, De Weck A L, Fleisch H. Interleukin 2-independent stimulation of rabbit chondrocyte collagenase and prostaglandin E2 production by an interleukin 1-like factor. Eur J Immunol. 1984;14:490–495. doi: 10.1002/eji.1830140603. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira B R, Silva J S. Saliva of Rhipicephalus sanguineus tick impairs T cell proliferation and IFN-gamma-induced macrophage microbicidal activity. Vet Immunol Immunopathol. 1998;64:279–293. doi: 10.1016/s0165-2427(98)00135-4. [DOI] [PubMed] [Google Scholar]
- 23.Fiorentino D F, Zlotnik A, Mosmann T R, Howard M H, OíGarra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [Google Scholar]
- 24.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 25.Ganapamo F, Rutti B, Brossard M. Immunosuppression and cytokine production in mice infested with Ixodes ricinus ticks: a possible role of laminin and interleukin-10 on the in vitro responsiveness of lymphocytes to mitogens. Immunology. 1996;87:259–263. doi: 10.1046/j.1365-2567.1996.450512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giambartolomei G H, Dennis V A, Philipp M T. Borrelia burgdorferi stimulates the production of interleukin-10 in peripheral blood mononuclear cells from uninfected humans and rhesus monkeys. Infect Immun. 1998;66:2691–2697. doi: 10.1128/iai.66.6.2691-2697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giambartolomei G H, Dennis V A, Philipp M T. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1999;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glaser K B, Pease I, Li J, Morgan D W. Enhancement of the surface expression of tumor necrosis factor alpha (TNF-alpha) but not the p55 TNF-alpha receptor in the THP-1 monocytic cell line by matrix metalloprotease inhibitors. Biochem Pharmacol. 1999;57:291–302. doi: 10.1016/s0006-2952(98)00300-1. [DOI] [PubMed] [Google Scholar]
- 29.Gross D M, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy Z A, Field J A, Steere A C, Huber B T. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 1998;281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 30.Habicht G S, Katona L I, Benach J L. Cytokines and the pathogenesis of neuroborreliosis: Borrelia burgdorferi induces glioma cells to secrete interleukin-6. J Infect Dis. 1991;164:568–574. doi: 10.1093/infdis/164.3.568. [DOI] [PubMed] [Google Scholar]
- 31.Haupl T, Landgraf S, Netusil P, Biller N, Capiau C, Desmons P, Hauser P, Burmester G R. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 32.Hemmer B, Gran B, Zhao Y, Marques A, Pascal J, Tzou A, Kondo T, Cortese I, Bielekova B, Straus S E, McFarland H F, Houghten R, Simon R, Pinilla C, Martin R. Identification of candidate T-cell epitopes and molecular mimics in chronic Lyme disease. Nat Med. 1999;5:1375–1382. doi: 10.1038/70946. [DOI] [PubMed] [Google Scholar]
- 33.Hirschfeld M, Kirschning C J, Schwandner R, Wesche H, Weis J H, Wooten R M, Weis J J. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J Immunol. 1999;163:2382–2386. [PubMed] [Google Scholar]
- 34.Joosten L A, LuhBberts E, Durez P, Helsen M M, Jacobs M J, Goldman M, van den Berg W B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–260. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 35.Kenefick K B, Lim L C I, Alder J D, Schmitz J L, Czuprynski C J, Schell R F. Induction of interleukin-1 release by high- and low-passage isolates of Borrelia burgdorferi. J Infect Dis. 1993;167:1086–1092. doi: 10.1093/infdis/167.5.1086. [DOI] [PubMed] [Google Scholar]
- 36.Kitchens R L, Munford R S. Enzymatically deacylated lipopolysaccharide (LPS) can antagonize LPS at multiple sites in the LPS recognition pathway. J Biol Chem. 1995;270:9904–9910. doi: 10.1074/jbc.270.17.9904. [DOI] [PubMed] [Google Scholar]
- 37.Kopecky J, Kuthejlove M, Pechova J. Salivary gland extract from Ixodes ricinus ticks inhibits production of interferon-gamma by the upregulation of interleukin-10. Parasite Immunol. 1999;21:351–356. doi: 10.1046/j.1365-3024.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 38.Lahn M, Kalataradi H, Mittelstadt P, Pflum E, Vollmer M, Cady C, Mukasa A, Vella A T, Ikle D, Harbeck R, O'Brien R, Born W. Early preferential stimulation of gamma delta T cells by TNF-α. J Immunol. 1998;160:5221–5230. [PubMed] [Google Scholar]
- 39.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 40.Lore K, Sonnerborg A, Spetz A L, Anderson U, Anderson J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. J Immunol Methods. 1998;214:97–111. doi: 10.1016/s0022-1759(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 41.Lubberts E, Joosten L A, Helsen M M, van den Berg W B. Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine. 1997;10:361–369. doi: 10.1006/cyto.1997.0298. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Seiler K P, Tai K, Yang L, Woods M, Weis J J. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Weis J J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moore K, OíGarra A, de Waal Malefyt R, Vieira P, Mossman T R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 45.Nancy C A, Meierovics A L, Belosesvic M, Green S J. Tumor necrosis factor-alpha: central regulatory cytokine of macrophage antimicrobial activities. Pathobiology. 1991;59:182–184. doi: 10.1159/000163640. [DOI] [PubMed] [Google Scholar]
- 46.Nocton J J, Dressler F, Rutledge B J, Rys P N, Persing D H, Steere A C. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N Engl J Med. 1994;30:229–234. doi: 10.1056/NEJM199401273300401. [DOI] [PubMed] [Google Scholar]
- 47.Norgard M V, Arndt L L, Akins D R, Cuetty L L, Harrrich D A, Radolf J D. Activation of human monocytic cells by Treponema pallisum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides proceeds via a pathway distinct from that of lipopolysaccharide but involves the transcriptional activator NK-kappa B. Infect Immun. 1996;64:3845–3852. doi: 10.1128/iai.64.9.3845-3852.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nussler A K, Wittel U A, Nussler N C, Beger H G. Leukocytes, the Janus cells in inflammatory disease. Langenbecks Arch Surg. 1999;384:222–232. doi: 10.1007/s004230050196. [DOI] [PubMed] [Google Scholar]
- 49.Philipp M T. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 1998;6:44–47. doi: 10.1016/S0966-842X(97)01201-8. [DOI] [PubMed] [Google Scholar]
- 50.Philipp M T, Johnson B J B. Animal models of Lyme disease: pathogenesis and immunoprophylaxis. Trends Microbiol. 1994;2:431–437. doi: 10.1016/0966-842x(94)90800-1. [DOI] [PubMed] [Google Scholar]
- 51.Pohl-Koppe A, Balashov K E, Steere A C, Logigian E L, Hafler D A. Identification of a T cell subset capable of both IFN-γ and IL-10 secretion in patients with chronic Borrelia burgdorferi infection. J Immunol. 1998;160:1804–1810. [PubMed] [Google Scholar]
- 52.Radolf J D, Arndt L L, Akins D R, Curetty L L, Levi M E, Shen Y, Davis L S, Norgard M V. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytes/macrophages. J Immunol. 1995;154:2866–2877. [PubMed] [Google Scholar]
- 53.Radolf J D, Norgard M V, Brandt M E, Isaacs R D, Thompson P A, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991;147:1968–1974. [PubMed] [Google Scholar]
- 54.Schoeler G B, Manweiler S A, Wikel S K. Cytokine responses of C3H/HeN mice infested with Ixodes scapularis or Ixodes pacificus nymphs. Parasite Immunol. 2000;22:31–40. doi: 10.1046/j.1365-3024.2000.00272.x. [DOI] [PubMed] [Google Scholar]
- 55.Schoeler G B, Manweiler S A, Wikel S K. Ixodes scapularis: effects of repeated infestations with pathogen-free nymphs on macrophage and T lymphocyte cytokine responses of BALB/c and C3H/HeN mice. Exp Parasitol. 1999;92:239–248. doi: 10.1006/expr.1999.4426. [DOI] [PubMed] [Google Scholar]
- 56.Sellati T J, Bouis D A, Caimano M J, Feulner J A, Ayers C, Lien E, Radolf J D. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J Immunol. 1999;163:2049–2056. [PubMed] [Google Scholar]
- 57.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 58.Sigal L H. Lyme disease: a review of aspects of its immunology and immunopathogenesis. Annu Rev Immunol. 1997;15:63–92. doi: 10.1146/annurev.immunol.15.1.63. [DOI] [PubMed] [Google Scholar]
- 59.Steere A C. Lyme disease. N Engl J Med. 1989;21:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 60.Tai K, Ma Y, Weis J J. Normal human B lymphocytes and mononuclear cells respond to the mitogenic and cytokine-stimulatory activities of Borrelia burgdorferi and its lipoprotein OspA. Infect Immun. 1994;62:520–528. doi: 10.1128/iai.62.2.520-528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takemura R, Werb Z. Secretory products of macrophages and their physiological functions. Am J Physiol. 1984;246:C1–C9. doi: 10.1152/ajpcell.1984.246.1.C1. [DOI] [PubMed] [Google Scholar]
- 62.Tatro J B, Romero L I, Beasley D, Steere A C, Reichlin S. Borrelia burgdorferi and Escherichia coli lipopolysaccharides induce nitric oxide and interleukin-6 production in cultured rat brain cells. J Infect Dis. 1994;169:1014–1022. doi: 10.1093/infdis/169.5.1014. [DOI] [PubMed] [Google Scholar]
- 63.Vassalli P. The pathophysiology of tumor necrosis factor. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 64.Waage A, Halstensen A, Aspevik T. Association between tumor necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987;i:355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- 65.Wanidworanum C, Strobet W. Predominant role of tumor necrosis factor-alpha in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- 66.Wooten R M, Morrison T B, Weis J H, Wright S D, Thieringer R, Weis J J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 67.Yang L J, Weis H, Eichwald E, Kolbert C P, Persing D H, Weis J J. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yin Z, Braun J, Neure L, Wu P, Eggens U, Krause A, Kamradt T, Sieper J. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheumatism. 1997;40:66–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 69.Zeidner N, Mbow M L, Dolan M, Massung R, Baca E, Piesman J. Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun. 1997;65:3100–3106. doi: 10.1128/iai.65.8.3100-3106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]